Abstract
Upper and lower motor neuron pathologies are critical to the autopsy diagnosis of amyotrophic lateral sclerosis (ALS). Further investigation is needed to determine how the relative burden of these pathologies affects the disease course. We performed a blinded, retrospective study of 38 ALS patients, examining the association between pathologic measures in motor cortex, hypoglossal nucleus, and lumbar cord with clinical data, including progression rate and disease duration, site of symptom onset, and upper and lower motor neuron signs. The most critical finding in our study was that TAR DNA-binding protein 43 kDa (TDP-43) pathologic burden in lumbar cord and hypoglossal nucleus was significantly associated with a faster progression rate with reduced survival (p < 0.02). There was no correlation between TDP-43 burden and the severity of cell loss, and no significant clinical associations were identified for motor cortex TDP-43 burden or severity of cell loss in motor cortex. C9orf72 expansion was associated with shorter disease duration (p < 0.001) but was not significantly associated with pathologic measures in these regions. The association between lower motor neuron TDP-43 burden and fast progression with reduced survival in ALS provides further support for the study of TDP-43 as a disease biomarker.
Keywords: Amyotrophic lateral sclerosis, Lower motor neuron, Motor cortex, Progression rate, Spinal cord, TAR DNA-binding protein 43 kDa (TDP-43), Upper motor neuron
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is characterized by upper motor neuron (UMN) and lower motor neuron (LMN) signs, progressive weakness, and muscle atrophy (1). Extra-motor symptoms such as cognitive dysfunction are also common (1−3), which may reflect an underlying multisystem TDP-43 proteinopathy (4, 5). ALS is characterized pathologically by the accumulation of ubiquitinated proteins, including TAR DNA-binding protein 43 kDa (TDP-43) (6), and this is thought to be detrimental to normal cellular function. TDP-43 pathology has been noted in upper and lower motor neuron pools, non-motor neurons, and glia of ALS patients (2, 7, 8), as well as within skeletal muscle (9) and axons (10). Elevated levels of TDP-43 have also been detected in the plasma of ALS patients compared with controls (11), suggesting that widespread dysregulation of RNA-binding proteins, including TDP-43, is a fundamental feature of the disease. The pathologic heterogeneity of TDP-43 across patients has been well characterized (12, 13) and a greater whole brain TDP-43 burden is associated with both cognitive impairment and C9orf72 expansion (14, 15).
TDP-43 is an informative protein that may be used to better understand how cellular pathology contributes to patient symptoms in ALS, particularly since misfolded TDP-43 may be an early marker of cellular dysfunction that appears before evident neuron loss. However, it remains unclear how TDP-43 relates to rate of disease progression and disease duration, as well as to the site of symptom onset and relative balance of UMN and LMN signs. TDP-43 burden and cell loss in UMN and LMN pools may also be useful in better understanding current models of anterograde and retrograde injury in ALS (16−18). For instance, to examine whether the burden of TDP-43 and cell loss in UMN and LMN pools strongly associates with patterns of disease onset, or other clinical features. Regarding TDP-43 burden and disease duration, few clinicopathologic studies of ALS have looked directly at this question. Several studies have suggested a surprising trend of greater whole brain TDP-43 burden with shorter disease duration in ALS (12, 19). This differs from neurodegenerative diseases where the burden of misfolded protein is typically greatest in patients with the longest disease duration (20, 21). Examining whole brain TDP-43 burden in ALS, we previously reported that a “TDP-43 severe” group had the shortest duration and fastest progression mean values (14). That study did not focus on the motor system specifically, nor the association between motor system pathologies and clinical findings.
The aim of this study was to examine the clinical associations of pathology in UMN and LMN pools in a blinded, retrospective clinicopathologic analysis of 38 autopsied ALS patients (21 men, 17 women, median age of onset 61 years, and median duration 36 months). We assessed neuronal loss in motor cortex (UMNs) and lumbar cord and hypoglossal nucleus (LMNs), as well as the inclusion density of TDP-43 pathology in the same. The addition of TDP-43 to the study design was critical because TDP-43 burden and neuron loss do not always correlate (22) and TDP-43 may be a more sensitive marker of the disease process and early cell injury (23). Independently collected clinical data were then examined to determine whether patterns of cell loss and/or TDP-43 pathology associated with salient clinical variables, such as onset site, the balance of UMN and LMN signs, and the rate of disease progression and disease duration.
MATERIALS AND METHODS
Case Identification
The patients included in the study all came to autopsy at Houston Methodist Hospital. The patients were evaluated in the clinic of 2 of the study authors specializing in neuromuscular disease. Final pathologic assessments were performed in all cases by Houston Methodist neuropathologists, confirming the clinical diagnosis of ALS. Informed written consent was previously obtained from patients or their next of kin with research permissions provided. The study was performed with the approval of the Institutional Review Board at Houston Methodist Hospital (IRB Pro00010377).
Hematoxylin and eosin (H&E)-stained material for each of the 38 ALS autopsies was reviewed by a neuropathologist (M.D.C.) to determine 3 regions of interest (ROIs) were present per case: (i) primary motor cortex, characterized by thick agranular frontal cortex with large pyramidal cells in lamina V, including Betz cells; (ii) lower motor neurons of hypoglossal nucleus; and (iii) lower motor neurons of lumbar spinal cord. Cases were excluded if the ROIs could not be sufficiently defined for both UMN and LMN pools, the orientation was insufficient for further pathologic evaluation, or there were confounding factors, such as diffuse hypoxic/ischemic injury. The list of suitable cases was then provided to 2 study authors (S.J.C., S.H.A.), who were blinded to pathologic data and details of autopsy diagnoses. These 2 authors reviewed and collated the salient clinical information per patient prior to further analyses.
Clinical Assessment
A complete clinical chart review was performed by the lead author, supervised by a neurologist and neuromuscular disease specialist (S.H.A.). Clinical variables recorded included: (i) age of onset and disease duration (months), (ii) site of disease onset (lower extremity [LE]; upper extremity [UE]; bulbar; or diffuse), (iii) UMN versus LMN symptom predominance at onset, based on first documented physical exam tone, strength, and reflexes, (iv) Appel ALS (AALS) score monthly rate of change (24), (v) C9orf72 expansion status (also see below under Pathologic Assessment), and (vi) cognitive impairment. For each patient, site of disease onset, UMN versus LMN predominance, and AALS progression rate were reviewed and agreed upon by these 2 authors.
The AALS monthly rate of change was calculated using the initial and most advanced AALS scores. If only an initial score was available (e.g. in a rapidly progressive patient), AALS rate of change was calculated using reported time of symptom onset (month 0, considered to be a normal AALS score of 30) and the initial documented AALS score. Rates were stratified into 4 progression categories (slow, 1 = <2 points per month; intermediate, 2 = 2−3.49 points per month; fast, 3 = 3.5−5.99 points per month; very fast, 4 = ≥6 points per month), or were not available (6 patients). Cognitive impairment was assessed by neuropsychological evaluation, MoCA, MMSE, or clinical descriptions. Missing data included rate of progression (6 patients), cognitive impairment status (4 patients), and disease duration (one patient).
Histological and Immunohistochemical Procedures
The autopsy procedure at our institution for ALS patients has previously been described in detail (4, 14). As described above, only cases with UMN pools in frontal (motor) cortex and LMNs in hypoglossal nucleus and lumbar cord were studied (3 blocks per patient). The staining protocol for tissue examination included H&E and full-length TDP-43 immunostain (Proteintech, 10782-2-AP, rabbit polyclonal antibody, 1:200; Rosemont, IL). Immunostaining was performed on brain tissue fixed in 20% formalin following brain removal, typically within 8–24 hours of the patient’s death per research protocol. Formalin-fixed paraffin embedded tissue was sectioned at 4 µm, mounted on charged slides and dried at 60°C. Immunostaining was performed on a BenchMark ULTRA platform (Ventana Medical Systems, Inc., Tucson, AZ) with appropriate positive and negative controls. Full-length (N-terminal) TDP-43 immunostain was used because this is very sensitive to pathologic inclusions in ALS (14), as it facilitates the identification of “pre-inclusion” pathology wherein granular cytoplasmic aggregates are detected in neurons coupled with loss of nuclear staining (25). (As described below, phospho-TDP staining was also performed in spinal cord samples to examine the correlation of inclusion pathology by both approaches.) C9orf72 expansion status was determined or confirmed by assessing dipeptide repeat proteins in the hippocampal tissue of each case using antibodies to poly-glycine-alanine (poly-GA) (mouse monoclonal, 1:200, EMD Millipore, MABN889) and poly-glycine-proline (poly-GP) (rabbit polyclonal, 1:1000, EMD Millipore, ABN455), as previously described (26).
Neuropathological Assessment: H&E and TDP-43 Pathologies
Each slide was coded with a unique pathology identifier such that the corresponding patient identification was not known to the lead author, a neuropathologist and the primary rater for H&E and TDP-43 pathologies. For each of the 3 study regions of interest (ROI), a qualitative assessment of neuron loss was made from H&E preparations and a semiquantitative assessment of TDP-43 inclusion density was made, resulting in 6 pathologic parameters per case (2 per ROI). Pathologic rating of each sample was performed blinded to all clinical and autopsy report data. Every slide and neuropathologic assessment by the lead author was subsequently reviewed with a second neuropathologist (M.D.C.) at a multi-headed microscope. Prior to statistical analyses, and blinded to clinical data, final pathologic assessments provided to the study biostatistician (L.E.P.) were agreed upon by 2 neuropathologists (S.J.C., M.D.C.). This resulted in rare modifications to initial scoring (∼6.5% of all pathology measures), typically by a single degree in the semiquantitative scales described below. Modification of a rating of no pathologic alteration in motor cortex (score 0) to mild pathology (score 1) was the most common alteration, and modifications were less common to LMN assessments or TDP-43 inclusion density measures.
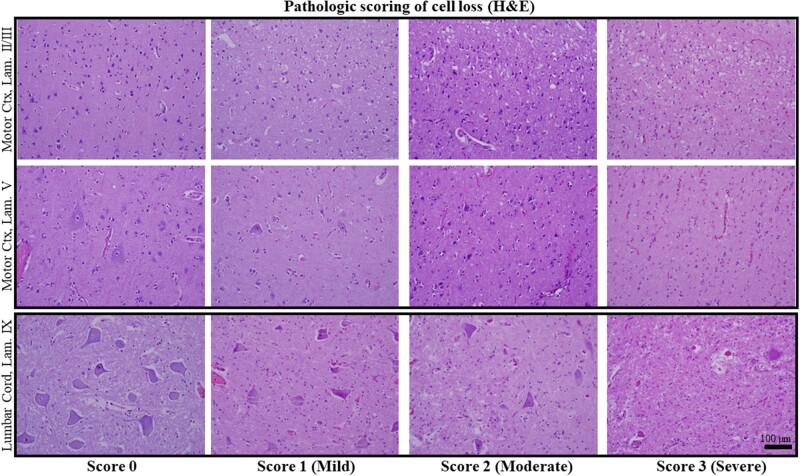
For H&E pathologies, both UMN (motor cortex) and LMN (hypoglossal nucleus, lumbar cord) ROIs were each rated on a four-point scale from no pathologic alteration (score 0) to advanced/severe pathology (score 3). For motor cortex, ratings included: no pathologic alteration (score 0); mild pathology (score 1), characterized by mild neuron loss, mild cortical atrophy, and vascular congestion; moderate pathology (score 2), characterized by moderate neuronal loss, moderate cortical atrophy and mild to moderate vacuolization of superficial cortical laminae; and severe pathology (score 3), characterized by severe, pan-cortical neuron loss, atrophy and collapse of cortex, and a spongiform appearance of cortex (Fig. 1, top and middle rows). For LMN pools, the same scoring system was used with an emphasis on residual α-motor neuron density. For LMN pools, ratings included: No pathologic alteration (score 0); mild pathology (score 1), characterized by mild LMN loss, gliosis, and early parenchymal vacuolization and vascular congestion; moderate pathology (score 2), consisting of moderate LMN loss and moderate parenchymal vacuolization, gliosis, and vascular congestion; and severe (score 3) pathology, reserved for near-total LMN loss, gliosis and severe vacuolization and rarefaction (infarct-like) (Fig. 1, bottom row).
FIGURE 1.
Pathologic scoring of cell loss in H&E-stained autopsy material. Scores ranged from no evident cell loss (score 0; left-most column) to severe cell loss (score 3; right-most column) (see “Materials and Methods” for detail). The top 2 rows show lamina II and III (top row) and lamina V (middle row) in 4 ALS patients with scores 0 − 3. In the left-most panel of the middle row (score 0), large pyramidal cells (Betz cells) in lamina V are evident and in the score 3 example this layer is depleted of neurons. The bottom row shows residual α-motor neurons in the lateral portion of Lamina IX of the lumbar cord, ranging from score 0 (a non-ALS example; no ALS case in this study was score 0) to scores 1 − 3 (study samples shown). All images taken at ×200 magnification and the scale bar in the bottom right panel applies to all images.
The density of TDP-43 inclusion pathology was assessed in study ROIs using a semiquantitative approach. For each ROI, the focus of greatest pathologic involvement was identified, and TDP-43 inclusion density was recorded as the number of total pathologic inclusions (neuronal, glial, and neuritic) in 3 successive fields at 400× magnification. For samples with extremely dense TDP-43 inclusion pathology (>30 to innumerable), the TDP-43 density value was recorded as 40 for statistical analysis (14).
To examine the relationship of N-terminal TDP-43 and phospho-TDP-43 (pTDP-43) inclusion pathology, additional staining was carried out in 35 study spinal cords. Sections were stained for phospho-TDP43 (Ser409/410) (22309-1-AP, 1:500, Proteintech), using a previously described immunostaining approach (9). Representative fields of pTDP-43 pathology in the ventral horn of spinal cord were captured at 200× magnification in cellSens software 1.13 (Olympus America, Inc., Center Valley, PA), using a DP71 camera mounted on a Olympus BX-43 Microscope. Image acquisition parameters were held constant and photomicrographs were loaded into ImageJ (27) for quantitation using a thresholding approach (28). Color thresholding (RGB mode) was used to generate a binary image from which DAB-positive, pTDP-43 inclusions were automatically counted using the analyze particles tool under the Analyze menu.
Neuropathological Assessment: UMN Versus LMN Predominant Patterns
The morphologic (H&E) and TDP-43 inclusion density measures were subsequently utilized to determine the predominant site of ALS-related pathology for each variable and patient sample. For H&E pathologies, categories included: LMN predominant cell loss (lumbar or hypoglossal pathology scores > cortex) and cortical predominant cell loss (cortex > lumbar or hypoglossal scores). Cases without a predominant site were categorized as: diffuse-severe cell loss (scores of ≥2 in UMN and LMN ROIs) and diffuse-mild cell loss (scores of ≤1 in UMN and LMN ROIs). TDP-43 inclusion density was similarly categorized as cortical predominant TDP, LMN predominant TDP, and diffuse TDP. Cases with diffuse-severe TDP pathology had a 0% difference between UMN and LMN TDP-43 values and maximal inclusion density at both sites. Cases with diffuse-mild TDP pathology had a 0% difference (4 cases) or 18% difference (one case) between UMN and LMN sites and no ROI with a density value exceeding 10.
Statistical Methods
Analysis of variance (ANOVA) and t-tests were employed to test for equality of means between various pathologic and clinical groups. Welch ANOVA and Welch t-tests were used for tests involving unequal variances. Prior to clinico-pathologic associations, it was determined that duration of disease and AALS progression rate were very strongly correlated (p < 0.01). Therefore, duration was split into 4 quartiles, which were used as the final duration/progression rate metric for statistical testing. Pearson and Spearman rank correlation were used to examine significant correlations between pathologic (H&E, N-terminal TDP-43) and clinical data and the directionality of the correlations. Chi-squared contingency table analysis was also used for tests of independence for categorical variables. All statistical tests were two-sided and a p < 0.05 was considered significant.
RESULTS
Clinical Characteristics
A summary of demographic and clinical data is provided in Table 1. Briefly, the 38 patients studied included 21 men and 17 women, hereafter referred to as ALS01 to ALS38, with a median age of 61 years at disease onset (interquartile range [IQR], 7 years; ranging from 41 to 79 years) and a median disease duration of 36 months (IQR, 34; ranging from 11 to 117 months). Six patients were designated as familial ALS (fALS) (16.2%), 31 patients as sporadic (sALS) (83.8%), and for one patient the fALS/sALS designation was not known. Nine patients had C9orf72 expansion (24.3%) as determined by premortem molecular testing and/or dipeptide repeat pathology (poly-GA, poly-GP) in autopsy samples. Thus, the molecular basis in 5 of 6 fALS patients was accounted for by C9orf72 expansion.
TABLE 1.
Clinical and Neuropathology Data in 38 Study Patients
| Variable | N (%) | Median | Other |
|---|---|---|---|
| Age at onset | 61 years | 41 − 79 years (IQR 7) | |
| Men/women | 21 (55)/17 (45) | ||
| Duration (months)* | 36 months | 11 − 117 months (IQR 34) | |
| Site of symptom onset | |||
| Lower extremity (LE) | 18 (47.4) | − | − |
| Upper extremity (UE) | 10 (26.3) | − | − |
| Bulbar | 7 (18.4) | − | − |
| Multifocal (UE, LE, and/or Bulbar) | 3 (7.9) | − | − |
| UMN vs LMN signs and symptoms† | |||
| UMN prominence | 6 (20.0) | − | − |
| LMN prominence | 11 (36.7) | − | − |
| Equal UMN and LMN prominence | 13 (43.3) | − | − |
| Familial ALS | 6 (16.2) | 62 years‡ | − |
| C9orf72 expansion# | 5 | − | − |
| Sporadic ALS | 31 (83.8) | 67 years | − |
| C9orf72 expansion | 4 | − | − |
| AALS score progression rate | |||
| Slow (<2 points per month) | 3 (9.4) | 1.33 pts/month | 83 months duration* |
| Intermediate (2 − 3.49 points per month) | 10 (31.3) | 2.37 pts/month | 54 months duration |
| Fast (3.5 − 6 points per month) | 14 (43.8) | 4.56 pts/month | 32 months duration |
| Very fast (>6 points per month) | 5 (15.6) | 9.00 pts/month | 20 months duration |
| Cognitive status$ | |||
| Impaired | 17 (50%) | − | 6 C9ORF72 patients |
| Intact | 17 (50%) | − | 3 C9ORF72 patients |
| Pathologic pattern (H&E) | |||
| Cortical predominant cell loss | 1 (2.7) | − | − |
| LMN predominant cell loss | 28 (75.7) | − | − |
| Diffuse, severe cell loss | 7 (18.9) | − | − |
| Diffuse, mild cell loss | 1 (2.7) | − | − |
| TDP-43 inclusion density | |||
| Cortical TDP predominant | 15 (40.5) | − | 67% LMN predom path+ |
| LMN TDP predominant | 12 (32.5) | − | 92% LMN predom path |
| Diffuse, severe TDP | 6 (16.2) | − | − |
| Diffuse, mild TDP | 4 (10.8) | − | − |
AALS Progression Rate and Duration (months) were significantly correlated (p < 0.01). Median duration shown by AALS score progression rate. Duration was used in statistical analyses.
UMN versus LMN predominance at onset (clinically) could not be determined in eight patients.
Median age at death.
Patients with C9orf72 expansion (N = 9) accounted for 24.3% of the study cohort.
Cognitive status was not known in four patients.
Independent of TDP predominance, the majority in both groups had LMN predominant cell loss by H&E and no correlations between inclusion density and cell loss were present in any ROI.
The earliest site of symptoms at disease onset was lower extremity in 18 patients (47.4%), upper extremity in 10 patients (26.3%), bulbar in 7 patients (18.4%), and was multifocal in 3 (7.9%). Of the 30 patients, where UMN and LMN predominance at onset could be accurately determined, 6 patients (20%) had UMN-predominant symptoms, 11 patients (36.7%) demonstrated LMN-predominant symptoms, and 13 patients (43.3%) demonstrated equal UMN and LMN signs and symptoms. None of the patients were diagnosed as having the primary lateral sclerosis or primary muscular atrophy variants of ALS.
Duration was known in all but one patient, and speed of progression could be determined in 32 patients. Three patients (9.4%) were “slow progressors” (range of monthly rate of change in AALS score, 0.96−1.67 and disease duration range, 82−117 months) and 5 patients (15.6%) were “rapid progressors” (range of monthly rate of change in AALS score, 6.7−9.4; disease duration range, 12−25 months). As described above, duration (months) and progression rate were very strongly correlated, such that quartiles of duration were used for statistical analyses. Mild cognitive impairment was present in 17 of the 34 patients (50%) where cognitive status was documented, and 35% of these cognitively impaired patients had C9orf72 expansion.
Neuropathologic Characteristics
Neuropathologic data are summarized in the bottom half of Table 1. Of 38 patients studied, LMN and UMN ROIs with H&E and TDP-43 data were available in 37 (one patient had partial ROI data). An LMN predominant cell loss pattern (H&E stain) was by far the most prevalent pattern, being seen in 28 patients (75.7%). This was followed by a diffuse pattern in 8 patients (21.6%) (7 diffuse-severe cell loss, 1 diffuse-mild cell loss). Only a single patient had cortical predominant cell loss (2.7%). Notably, this occurred in a patient with C9orf72 expansion, rapidly progressive disease, and diffuse-severe TDP pathology.
In contrast, the predominant region of TDP-43 pathology was more evenly distributed. Twelve patients (32.5%) had LMN predominant TDP pathology, 10 patients had a diffuse TDP pattern (27.0%) (4 diffuse-mild TDP, 6 diffuse-severe TDP), and 15 patients (40.5%) had cortical predominant TDP. The majority of LMN predominant TDP (92%) and cortical predominant TDP (67%) patients had LMN predominant cell loss on H&E. As such, there was no significant association between the pattern of TDP pathology and cell loss (χ2 = 2.9, p = 0.09). Neither were there correlations between TDP-43 inclusion density and H&E-based ratings of cell loss in lumbar (Spearman rho = -0.08), hypoglossal (Spearman rho = -0.17), and motor cortex (Spearman rho = 0.19) ROIs. N-terminal TDP-43 inclusion density and phospho-TDP-43 inclusion counts in ImageJ were strongly correlated across ALS spinal cords (rho = 0.47, p = 0.0041) (Spearman’s rank correlation as implemented in R [29]), supporting the use of the N-terminal TDP-43 measures for subsequent clinicopathologic associations.
As described in the “Materials and Methods”, TDP-43 inclusion density was recorded as the number of total pathologic inclusions (neuronal, glial, and neuritic) in 3 successive fields at 400× magnification. Variably dense glial cytoplasmic inclusion (GCI) pathology was noted in both motor cortex and spinal cord across patients, but GCI pathology was not enumerated separately.
Clinicopathologic Associations
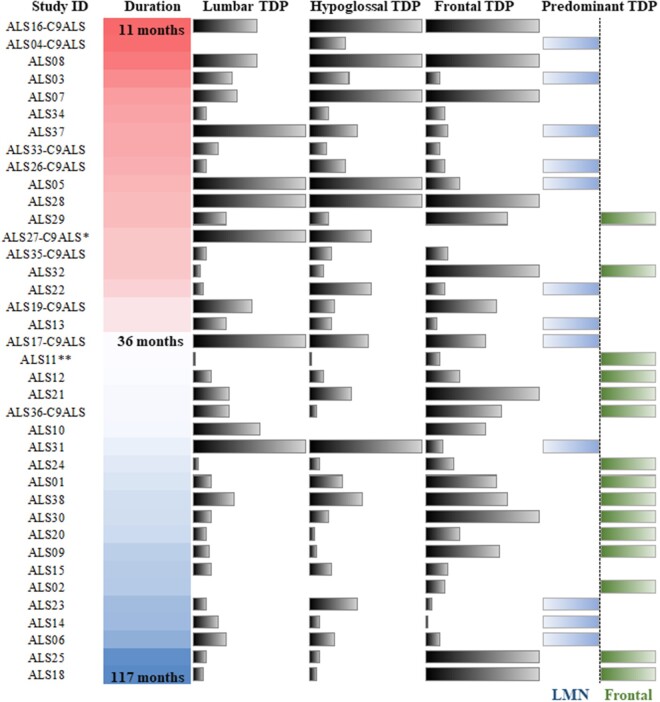
Clinicopathologic associations are summarized in Table 2. The most notable pathologic association with duration was TDP-43 inclusion density in both LMN ROIs, which was not seen for the motor cortex ROI. TDP-43 inclusion density was greatest in patients with short disease duration (n = 10; average duration 18.6 months, SD = 4.8) and lowest in slowly progressive, longer disease duration patients (n = 10; average duration 81.6 months, SD = 20.6), for both the lumbar cord (p = 0.0196) and the hypoglossal nucleus (p = 0.0118) (Fig. 2).
TABLE 2.
Groupwise Comparisons of Average Pathologic Scores
| Disease Duration (Quartiles) |
C9orf72 Expansion |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Q1 (n = 10) | Q2 (n = 9) | Q3 (n = 9) | Q4 (n = 10) | p value | No (n = 28) | Yes (n = 9) | p value |
| Months Survival (Average) | 11 − 24 (18.6) | 25 − 36 (28.4) | 37 − 58 (44.0) | 60 − 117 (81.6) | <0.0005 | 50.8 | 25.4 | <0.0005 |
| Progression Rate* | Very Fast | Fast | Intermediate | Slow | − | − | − | − |
| Age at Onset | 66.0 | 59.1 | 56.3 | 62.2 | 0.036 | 60.5 | 62.1 | 0.60 |
| H&E Pathologic Rating | ||||||||
| Lumbar Cord | 2.1 | 2.1 | 2.6 | 2.4 | 0.3981 | 2.4 | 2.0 | 0.1804 |
| Hypoglossal Nucleus | 1.9 | 2.1 | 2.1 | 2.5 | 0.3115 | 2.0 | 2.3 | 0.3555 |
| Motor Cortex | 1.4 | 1.2 | 1.4 | 1.6 | 0.7936 | 1.4 | 1.4 | 0.9583 |
| TDP-43 Density | ||||||||
| Lumbar Cord | 18.4 | 15.3 | 17.9 | 6.9 | 0.0196 | 13.3 | 19.5 | 0.2412 |
| Hypoglossal Nucleus | 23.0 | 13.6 | 14.1 | 5.7 | 0.0118 | 14.3 | 15.0 | 0.8907 |
| Motor Cortex | 16.4 | 18.9 | 21.2 | 18.1 | 0.9165 | 19.8 | 16.6 | 0.5984 |
Approximate progression rate of each duration quartile. Progression rate and duration were significantly correlated (p < 0.01) (see “Results”).
FIGURE 2.
Relationship between disease duration and predominant site of TDP-43 pathology. Disease duration, shown from 11 months (sample ALS16) to 117 months (sample ALS18) and TDP-43 burden and predominance. For Lumbar, Hypoglossal, and Frontal TDP bars, the value shown indicates the TDP-43 density for that patient and ROI. The “Predominant TDP” column indicates cases with LMN-predominant TDP (leftward bars), cortical-predominant TDP (rightward bars), or diffuse TDP pathology with no predominant site (no bar shown). Notes: Patients with C9orf72 expansion are indicated by “C9ALS” appended after the sample ID. *This sample lacked frontal TDP data. **Duration was not known for this sample, so the median duration of all samples is shown.
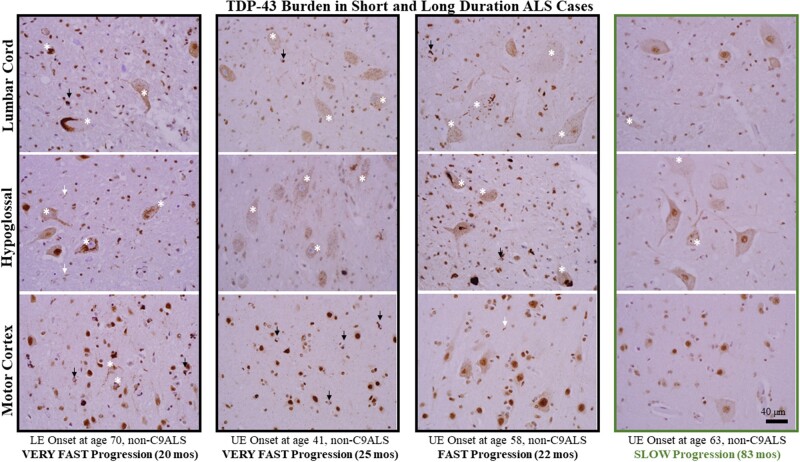
Spearman rank correlation also revealed significant, negative correlations between duration (in months) and TDP-43 inclusion density in lumbar cord (rho = -0.34, p < 0.05) and hypoglossal nucleus (rho = -0.50, p < 0.01) (i.e. greater TDP-43 inclusion density with shorter duration). Likewise, Spearman rank correlation indicated a significant, negative correlation between phospho-TDP-43 inclusion counts in ALS spinal cord and disease duration (months) (rho = -0.36, p < 0.05). TDP-43 pathology in several short- and long-lived patient examples are shown in Figure 3.
FIGURE 3.
TDP-43 burden in short and long duration ALS cases. TDP-43 burden in 3 short-lived, rapidly progressive patients (first 3 columns) and one long-lived, slowly progressive patient (right-most column). For each set of patient images, N-terminal TDP-43 staining is shown for lumbar cord (top row), hypoglossal nucleus (middle row) and motor cortex (bottom row). The images are representative of study samples and demonstrate greater TDP-43 inclusion pathology in the LMN pools of more rapidly progressive patients. The images also show the heterogeneity of neuronal pathology (white asterisks), including granular “pre-inclusions” with loss of nuclear staining (see text for detail), short neurites (white arrows), and oligodendroglial cytoplasmic inclusions (black arrows). Note that in several images the granular, pre-inclusion pathology with loss of nuclear staining is predominant (e.g. LMN pools of the patient shown in the second column with onset at age 41). All images taken at ×400 magnification and the scale bar in the bottom right panel applies to all images.
Spearman correlation indicated a strong, positive correlation of TDP-43 inclusion density between lumbar and hypoglossal ROIs (rho = 0.65, p < 0.01) but no correlation between the TDP-43 density in either LMN ROI with motor cortex. Duration quartiles did not differ by age at onset, cognitive impairment status, H&E-based measures of cell loss, or cortical TDP-43 inclusion density.
A cortical predominant TDP pattern was noted in longer-lived patients, and 13 of the 15 cases (87%) with this pattern were in the longer-lived third and fourth quartiles of duration (median survival 59 months) (χ2 = 13.3, p < 0.001) (Fig. 2). However, the cortical predominant TDP pattern did not equate to an UMN predominant clinical presentation, or a cortical predominant cell loss pattern (a rare pattern by H&E). For example, of 13 study patients with survival exceeding ≥50 months, cortical predominant TDP was seen in 9 patients (69.2%). However, features of LMN-predominant disease were seen in 6 of these patients, or there were equal UMN and LMN features (5 patients) (UMN/LMN predominance at onset was unknown for 2). None of these cases had cortical predominant cell loss by H&E, with cell loss being rated as diffuse-severe (2 patients), diffuse-mild (1 patient), or LMN predominant (10 patients).
Patients with C9orf72 expansion had a significantly shorter duration of disease (25.4 months) than those patients without C9orf72 expansion (50.8 months) (p < 0.001). However, there was no significant difference in the severity of cell loss or TDP-43 inclusion density between patients with and without C9orf72 expansion. Pathologic associations of UMN versus LMN symptom predominance at onset were less clear, in part because only 6 cases had UMN-predominant symptoms at onset and 4 of these patients (67%) had C9orf72 expansion.
Stratified by site of clinical onset—lower extremity, upper extremity, bulbar, and diffuse groups—there was no significant difference in age at onset, duration of disease, C9orf72 status, or TDP-43 inclusion density. A trend was identified, however, between severity of neuron loss in the lumbar cord ROI and lower extremity onset (p = 0.0577). Likewise, 10 of the 15 patients (67%) with severe neuron loss in lumbar cord (score 3) presented with lower extremity onset clinically, compared with 32% of cases with mild (score 1) or moderate (score 2) lumbar cord pathology (χ2 = 4.4, p = 0.037).
DISCUSSION
Here, we examined the relationship between salient clinical features of ALS with UMN and LMN neuropathologies. A major strength of the research design is that the neuropathologic analysis was performed blinded to independently collected clinical data, including disease duration, progression rate and onset site. We found that cell loss in the LMNs of lumbar spinal cord was nearly uniform across patients and that this loss exceeded that of UMN pools in nearly all cases. Moreover, we found that greater TDP-43 pathology in LMN pools was significantly associated with faster disease progression and reduced overall survival in ALS (for spinal cord this was identified using both N-terminal and phospho-TDP-43-based measures). Notably, spinal cord LMN loss did not correlate with either TDP-43 pathologic burden or disease duration. As such, the statistical association between greater TDP-43 burden and faster progression/reduced survival did not appear to be driven by clearance of pathologic protein in long-lived ALS patients with fewer residual LMNs. Further, among the 8 patients with a diffuse pattern of UMN and LMN cell loss, 83% had “fast” or “very fast” progression rate with a median survival of 30 months. This suggests that a subset of patients have more extensive and severe motor neuron loss at disease onset, and that this is not necessarily associated with C9orf72 status. In contrast to the associations identified for LMN pools, primary motor cortex (M1) cell loss was absent (n = 4) or mild (n = 20) in the majority of cases (63.2%) and an UMN-predominant pattern of cell loss was seen in only a single patient (2.6%). Likewise, TDP-43 burden in M1 had no association with disease duration or predominance of UMN or LMN findings at onset. Finally, variably dense GCI pathology was seen across the motor system regions-of-interest studied here. We have previously found GCI density in white matter is greatest in cases with the most extensive and severe TDP-43 pathology in whole brain (a “TDP43-severe” subgroup) (14). Future studies are needed to determine the clinical and pathologic associations, if any, of GCI density in UMN and LMN regions-of-interest and associated white matter tracts.
The burden of TDP-43 pathology was greater in the LMN pools of rapidly progressive ALS patients studied here. There was no association between the severity of LMN loss and TDP-43 pathology. This suggests a complex relationship between misfolded TDP-43 and cell death and dysfunction in LMN pools, potentially mediated by a pro-inflammatory environment (30), the astrocytic response to neuronal injury (31), or other processes, such as mitochondrial dysfunction (32). The paradoxical relationship of greater TDP-43 pathology with shorter disease duration contrasts with neurodegenerative diseases, such as Alzheimer disease (20) and multiple system atrophy (21), where greater pathologic burden is typically seen in the longest-lived patients. Misfolded TDP-43 and SOD1, like phospho-tau and α-synuclein, may demonstrate seeding activity that causes conformational changes in wildtype protein (33). A time-dependent, spatial spread model of ALS has been proposed (17), potentially involving prion-like mechanisms (16). It is therefore reasonable to hypothesize that TDP-43 burden in the brain would be greatest in the longest-lived ALS patients. Yet autopsy studies of long-lived ALS patients have shown TDP-43 pathology may actually be very mild and limited in anatomic distribution (34). Likewise, a recent study of military veterans with ALS and long duration (>10 years) demonstrated that the long-lived patients had less TDP-43 pathology than short-lived patients (35). A prior study by our group also found a trend between reduced patient survival and greater whole brain TDP-43 burden (14). One possible explanation for these results is heterogeneity of TDP-43 strains, as described in FTLD-TDP (36), with more virulent forms leading to a rapid clinical course and a more fulminant TDP-43 proteinopathy. Another possibility is that brain and spinal cord TDP-43 burden also reflects intrinsic cellular insults that may be more widespread in rapidly progressive patients. The finding that greater TDP-43 burden in spinal cord—and potentially in other brain areas—correlates with faster progression and reduced survival in ALS supports the proposed use of TDP-43 as a biomarker in the disease (23). In addition, the majority of patient samples studied here had a LMN-predominant pattern of cell loss, as previously reported in several older studies (37−40), all performed prior to the discovery of TDP-43. With the limitations of an autopsy-based study, this does provide some support for a retrograde model of ALS progression, wherein the initial site of injury involves LMN pools. Earlier studies found ALS patients with severe LMN loss and near normal UMN density with only rare cases having severe UMN pathology and a mildly affected cord (39)—the same result was seen here. Neither were associations seen between UMN and LMN cell loss, also consistent with earlier studies (37, 39). As described above, we also found that TDP-43 burden in LMNs significantly associated with progression speed and overall survival. Exactly how TDP-43 contributes to LMN and axonal injury in ALS is not known, but this may involve mechanisms of cytoskeletal disruption (41), sequestration of mRNA-binding proteins necessary for normal axonal function (42), or mitochondrial dysfunction with increased production of reactive oxygen species (32).
Motor cortex cell loss and TDP-43 pathology did not associate with clinical parameters, such as onset site, duration, progression rate, or predominance of UMN symptoms. Surprisingly, only 9 patients had maximal TDP-43 inclusion density in motor cortex, and only one of these cases had an UMN-predominant clinical presentation. Further, among 5 cortical predominant TDP patients with severe UMN TDP-43 and minimal LMN TDP-43 pathology, none had C9orf72 expansion, none had an UMN-predominant clinical presentation, 3 had LMN-predominant signs at presentation, and 4 had cognitive impairment. Motor cortex TDP-43 burden may therefore reflect other disease features not sufficiently captured in this study, or associate with whole brain TDP-43 burden and impaired cognition (12, 14). Along these lines, 64% of cognitively impaired, non-C9ALS patients had moderate or severe motor cortex TDP-43 burden in this study. One limitation to our assessment of motor cortex pathology is that lateral tract degeneration, using LFB-stained or similar materials, was not considered, and this may have limited our assessment of more mild forms of UMN pathology.
C9orf72 expansion is associated with greater global TDP-43 burden (12, 14, 15), a more rapid clinical course, and cognitive impairment (43, 44). Based on these associations, we hypothesized that greater TDP-43 burden would be identified in the motor system of C9ALS patients, contributing to cell toxicity in UMNs and LMNs and reduced survival. Consistent with earlier studies, we found reduced survival in C9ALS patients. However, we did not identify any differences in motor system TDP-43 burden or in the severity of motor neuron loss. C9ALS patients with rapid progression, cognitive impairment and very mild TDP-43 burden have been described, demonstrating that other pathologies may mediate progression in this form of ALS (e.g. RNA foci, poly-GA and poly-GR aggregates). Additional studies are needed to examine the burden of C9ALS-related pathologies, including RNA foci, DPRs, TDP-43, and microglial activation, in the motor system of C9ALS patients to determine which of these pathologies correlates with progression speed. Our findings would suggest this is unlikely to be TDP-43 burden alone.
In conclusion, we found that TDP-43 pathologic burden in LMN pools, but not motor cortex, is associated with faster disease progression with reduced survival in ALS. We also found that LMN cell loss exceeds motor cortex cell loss in most ALS patients and identified a trend between severity of lumbar cord LMN pathology and lower extremity onset. Future studies are needed to examine whether clearance of misfolded TDP-43 in LMN pools and blocking of dysfunctional cellular responses activated by misfolded TDP-43 would help to slow the progression of disease.
ACKNOWLEDGMENTS
We appreciate the excellent technical assistance provided by the personnel of the autopsy, histology and immunohistochemistry sections in the Department of Pathology and Genomic Medicine at Houston Methodist Hospital. We are grateful to the ALS patients and their family members for the donations that made this work possible.
DATA AVAILABILITY
Data and/or supporting microscopic images from this study are available from the corresponding author upon reasonable request.
This study was supported by Clinician-Scientist Research Award (Houston Methodist Research Institute); Investigator-initiated Research Award (ALS Association); and NIH NINDS (RF1NS118584).
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1.Ravits J, Appel S, Baloh RH, et al. Deciphering amyotrophic lateral sclerosis: What phenotype, neuropathology and genetics are telling us about pathogenesis. Amyotroph Lateral Scler Frontotemporal Degener 2013;14(Suppl 1):5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grad LI, Rouleau GA, Ravits J, et al. Clinical spectrum of amyotrophic lateral sclerosis (ALS). Cold Spring Harb Perspect Med 2017;7:a024117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringholz GM, Appel SH, Bradshaw M, et al. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 2005;65:586–90 [DOI] [PubMed] [Google Scholar]
- 4.Cykowski MD, Takei H, Schulz PE, et al. TDP-43 pathology in the basal forebrain and hypothalamus of patients with amyotrophic lateral sclerosis. Acta Neuropathol Commun 2014;2:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geser F, Brandmeir NJ, Kwong LK, et al. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol 2008;65:636–41 [DOI] [PubMed] [Google Scholar]
- 6.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006;314:130–3 [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie IR, Bigio EH, Ince PG, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 2007;61:427–34 [DOI] [PubMed] [Google Scholar]
- 8.Turk M, Haaker G, Winter L, et al. C9ORF72-ALS: p62- and ubiquitin-aggregation pathology in skeletal muscle. Muscle Nerve 2014;50:454–5 [DOI] [PubMed] [Google Scholar]
- 9.Cykowski MD, Powell SZ, Appel JW, et al. Phosphorylated TDP-43 (pTDP-43) aggregates in the axial skeletal muscle of patients with sporadic and familial amyotrophic lateral sclerosis. Acta Neuropathol Commun 2018;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onozato T, Nakahara A, Suzuki-Kouyama E, et al. Axonal TDP-43 aggregates in sporadic amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol 2016;42:561–72 [DOI] [PubMed] [Google Scholar]
- 11.Verstraete E, Kuiperij HB, van Blitterswijk MM, et al. TDP-43 plasma levels are higher in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2012;13:446–51 [DOI] [PubMed] [Google Scholar]
- 12.Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013;74:20–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi R, Tada M, Shiga A, et al. Heterogeneity of cerebral TDP-43 pathology in sporadic amyotrophic lateral sclerosis: Evidence for clinico-pathologic subtypes. Acta Neuropathol Commun 2016;4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cykowski MD, Powell SZ, Peterson LE, et al. Clinical significance of TDP-43 neuropathology in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 2017;76:402–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper-Knock J, Hewitt C, Highley JR, et al. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain 2012;135:751–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polymenidou M, Cleveland DW.. The seeds of neurodegeneration: Prion-like spreading in ALS. Cell 2011;147:498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravits J.Focality, stochasticity and neuroanatomic propagation in ALS pathogenesis. Exp Neurol 2014;262 Pt B:121–6 [DOI] [PubMed] [Google Scholar]
- 18.Piotrkiewicz M, Hausmanowa-Petrusewicz I.. Amyotrophic lateral sclerosis: A dying motor unit? Front Aging Neurosci 2013;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatima M, Tan R, Halliday GM, et al. Spread of pathology in amyotrophic lateral sclerosis: Assessment of phosphorylated TDP-43 along axonal pathways. Acta Neuropathol Commun 2015;3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobinski M, Wegiel J, Tarnawski M, et al. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol 1997;56:414–20 [DOI] [PubMed] [Google Scholar]
- 21.Cykowski MD, Coon EA, Powell SZ, et al. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 2015;138:2293–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geser F, Martinez-Lage M, Robinson J, et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol 2009;66:180–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumder V, Gregory JM, Barria MA, et al. TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: A systematic review and meta-analysis. BMC Neurol 2018;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haverkamp LJ, Appel V, Appel SH.. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 1995;118:707–19 [DOI] [PubMed] [Google Scholar]
- 25.Brandmeir NJ, Geser F, Kwong LK, et al. Severe subcortical TDP-43 pathology in sporadic frontotemporal lobar degeneration with motor neuron disease. Acta Neuropathol 2008;115:123–31 [DOI] [PubMed] [Google Scholar]
- 26.Cykowski MD, Dickson DW, Powell SZ, et al. Dipeptide repeat (DPR) pathology in the skeletal muscle of ALS patients with C9ORF72 repeat expansion. Acta Neuropathol 2019;138:667–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW.. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen EC.Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken) 2013;296:378–81 [DOI] [PubMed] [Google Scholar]
- 29.R: A Language and Environment for Statistical Computing. [Computer Program]. Vienna, Austria 2021
- 30.Appel SH, Zhao W, Beers DR, et al. The microglial-motoneuron dialogue in ALS. Acta Myol 2011;30:4–8 [PMC free article] [PubMed] [Google Scholar]
- 31.Yamanaka K, Chun SJ, Boillee S, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 2008;11:251–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang P, Deng J, Dong J, et al. TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS Genet 2019;15:e1007947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAlary L, Plotkin SS, Yerbury JJ, et al. Prion-like propagation of protein misfolding and aggregation in amyotrophic lateral sclerosis. Front Mol Neurosci 2019;12:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishihira Y, Tan CF, Hoshi Y, et al. Sporadic amyotrophic lateral sclerosis of long duration is associated with relatively mild TDP-43 pathology. Acta Neuropathol 2009;117:45–53 [DOI] [PubMed] [Google Scholar]
- 35.Spencer KR, Foster ZW, Rauf NA, et al. Neuropathological profile of long-duration amyotrophic lateral sclerosis in military Veterans. Brain Pathol 2020;30:1028–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee EB, Porta S, Michael Baer G, et al. Expansion of the classification of FTLD-TDP: Distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol 2017;134:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiernan JA, Hudson AJ.. Changes in sizes of cortical and lower motor neurons in amyotrophic lateral sclerosis. Brain 1991;114:843–53 [DOI] [PubMed] [Google Scholar]
- 38.Lawyer T Jr, Netsky MG.. Amyotrophic lateral sclerosis. AMA Arch Neurol Psychiatry 1953;69:171–92 [DOI] [PubMed] [Google Scholar]
- 39.Pamphlett R, Kril J, Hng TM.. Motor neuron disease: A primary disorder of corticomotoneurons? Muscle Nerve 1995;18:314–8 [DOI] [PubMed] [Google Scholar]
- 40.Udaka F, Kameyama M, Tomonaga M.. Degeneration of Betz cells in motor neuron disease. A Golgi study. Acta Neuropathol 1986;70:289–95 [DOI] [PubMed] [Google Scholar]
- 41.Soler-Martin C, Vilardosa U, Saldana-Ruiz S, et al. Loss of neurofilaments in the neuromuscular junction in a rat model of proximal axonopathy. Neuropathol Appl Neurobiol 2012;38:61–71 [DOI] [PubMed] [Google Scholar]
- 42.Fallini C, Bassell GJ, Rossoll W.. The ALS disease protein TDP-43 is actively transported in motor neuron axons and regulates axon outgrowth. Hum Mol Genet 2012;21:3703–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byrne S, Elamin M, Bede P, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: A population-based cohort study. Lancet Neurol 2012;11:232–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umoh ME, Fournier C, Li Y, et al. Comparative analysis of C9orf72 and sporadic disease in an ALS clinic population. Neurology 2016;87:1024–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and/or supporting microscopic images from this study are available from the corresponding author upon reasonable request.