Abstract
BACKGROUND
Perineural invasion (PNI), as a key pathological feature of tumor spread, has emerged as an independent prognostic factor in patients with rectal cancer (RC). The preoperative stratification of RC patients according to PNI status is beneficial for individualized treatment and improved prognosis. However, the preoperative evaluation of PNI status is still challenging.
AIM
To establish a radiomics model for evaluating PNI status preoperatively in RC patients.
METHODS
This retrospective study enrolled 303 RC patients in a single institution from March 2018 to October 2019. These patients were classified as the training cohort (n = 242) and validation cohort (n = 61) at a ratio of 8:2. A large number of intra- and peritumoral radiomics features were extracted from portal venous phase images of computed tomography (CT). After deleting redundant features, we tested different feature selection (n = 6) and machine-learning (n = 14) methods to form 84 classifiers. The best performing classifier was then selected to establish Rad-score. Finally, the clinicoradiological model (combined model) was developed by combining Rad-score with clinical factors. These models for predicting PNI were compared using receiver operating characteristic curve (ROC) analysis and area under the ROC curve (AUC).
RESULTS
One hundred and forty-four of the 303 patients were eventually found to be PNI-positive. Clinical factors including CT-reported T stage (cT), N stage (cN), and carcinoembryonic antigen (CEA) level were independent risk factors for predicting PNI preoperatively. We established Rad-score by logistic regression analysis after selecting features with the L1-based method. The combined model was developed by combining Rad-score with cT, cN, and CEA. The combined model showed good performance to predict PNI status, with an AUC of 0.828 [95% confidence interval (CI): 0.774-0.873] in the training cohort and 0.801 (95%CI: 0.679-0.892) in the validation cohort. For comparison of the models, the combined model achieved a higher AUC than the clinical model (cT + cN + CEA) achieved (P < 0.001 in the training cohort, and P = 0.045 in the validation cohort).
CONCLUSION
The combined model incorporating Rad-score and clinical factors can provide an individualized evaluation of PNI status and help clinicians guide individualized treatment of RC patients.
Keywords: Radiomics, Perineural invasion, Rectal cancer, Computed tomography, Preoperative prediction, Model building
Core Tip: In this study, a radiomics model integrating Rad-score and clinical factors was developed and validated for predicting perineural invasion status in patients with rectal cancer. Radiomics features were extracted from intra- and peritumoral regions. This radiomics model showed good performance and outperformed the clinical factors. Therefore, the combined model might assist in predicting perineural invasion status and improving prognosis of patients with rectal cancer.
INTRODUCTION
Rectal cancer (RC) is one of the most common cancers of the digestive tract worldwide, with a growing morbidity[1]. In the last decade, the combination of neoadjuvant chemoradiotherapy (nCRT) and surgery has improved local control of locally advanced RC, but it does not significantly affect prognosis[2]. Different biological characteristics of RC may cause different treatment responses, risks of distant metastasis, and outcomes[3].
Recently, there is an increasing interest in perineural invasion (PNI) as a potential route of tumor spread, in addition to the well-known routes of direct extension, lymphatic metastasis, and hematogenous metastasis[4]. PNI refers to the biological process characterized by cancer cells invading the nerves and spreading along the nerve sheaths[5,6]. This process can be found in the main tumor and peritumoral area[7,8]. Previous studies have demonstrated the prognostic value of PNI in RC in terms of both recurrence and survival[9-11]. Other studies have shown that PNI can be an indicator for identifying patients who can benefit from nCRT and postoperative adjuvant chemotherapy[12,13]. Therefore, understanding PNI status in advance is helpful for clinicians to make individualized treatment plans for RC patients.
However, PNI status can only be confirmed by assessing the pathology of surgical specimens. In other words, neither biopsy nor imaging examinations [computed tomography (CT)/magnetic resonance imaging (MRI)] can accurately determine PNI status of RC[6]. Recent advances in radiomics have enabled researchers to extract numerous quantitative features from medical images and provide a comprehensive overview of heterogeneity in tumors[14]. Latterly, radiomics analysis has been used to predict PNI status in colorectal cancer[14,15]. Considering the higher incidence of PNI in RC (compared with colon cancer)[16], several researchers evaluated the performance of MRI-based radiomics for PNI prediction in RC[6,17,18]. However, the sample sizes in the previous studies were really small (PNI+: 26-32). At present, there is still a lack of CT-based radiomics research in this field. Therefore, we aimed to evaluate the predictive value of CT-based radiomics for PNI prediction in a bigger cohort of RC patients.
MATERIALS AND METHODS
Patients
The local ethics committee approved this study (document number: 1159), and the requirement of informed consent was waived because of the retrospective nature of this study.
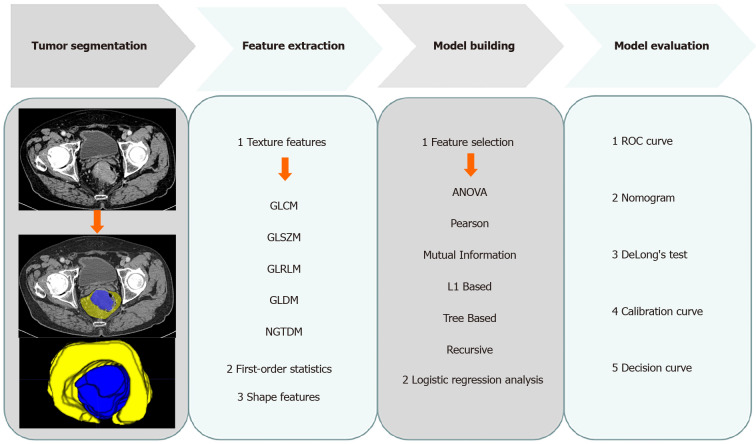
The RC patients were reviewed by browsing the radiological and pathological databases from March 2018 to October 2019. A total of 303 patients (170 men and 133 women, mean age 58.9 ± 11.7 years, age range 23-86 years) were enrolled, according to the following inclusion criteria: (1) Adults with histologically confirmed rectal adenocarcinoma; (2) Clinical materials such as enhanced CT images and tumor markers were complete; and (3) No prior therapy before CT examination. The exclusion criteria were as follows: (1) Quality of CT images was poor; (2) Patients treated without surgery; and (3) Patients with other malignant tumors besides RC. The patient recruitment pathway is shown in Figure 1. The workflow of the radiomics analysis is shown in Figure 2.
Figure 1.
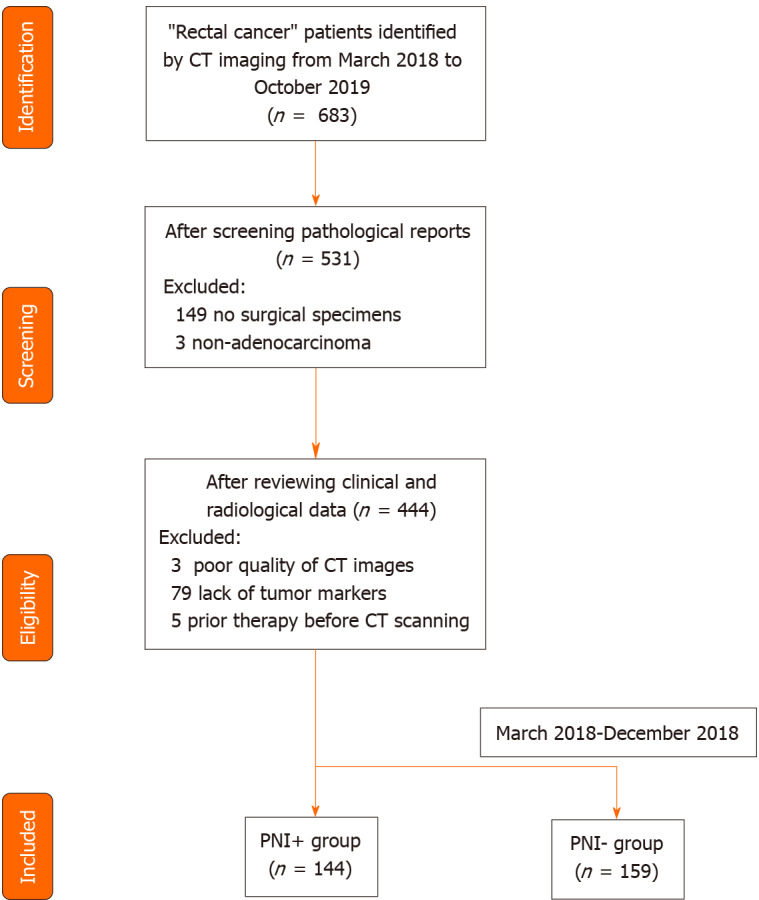
Flowchart of patients’ recruitment pathway. CT: Computed tomography; PNI: Perineural invasion.
Figure 2.
Radiomics workflow. GLCM: Gray level co-occurrence matrix; GLSZM: Gray level size zone matrix; GLRLM: Gray level run length matrix; GLDM: Gray level dependence matrix; NGTDM: Neighbouring gray tone difference matrix; ANOVA: Analysis of variance; ROC: Receiver operating characteristic curve.
The clinical and pathological data of each patient were derived from medical records. The baseline data including age, sex, tumor volume, location, tumor markers [carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, and CA125], pathological TNM stage, and histological grade are shown in Table 1.
Table 1.
Baseline characteristics of the study population
|
Characteristics
|
PNI+ (n = 144)
|
PNI
-
(n = 159)
|
P
value
|
The training set(n = 242)
|
The validation set(n = 61)
|
P
value
|
| Age (mean ± SD, yr) | 58.9 ± 11.8 | 58.9 ± 11.7 | 0.952 | 58.4 ± 11.9 | 60.8 ± 10.2 | 0.162 |
| Sex (male/female) | 77/67 | 93/66 | 0.379 | 140/102 | 30/31 | 0.223 |
| Volume (cm3) | 19.7 | 20.8 | 0.132 | 20.1 | 20.8 | 0.847 |
| Location1 | 0.075 | 0.045 | ||||
| Middle-low | 101 | 96 | 164 | 33 | ||
| High | 43 | 63 | 78 | 28 | ||
| cT stage (T1-2/T3/T4) | 25/98/21 | 57/90/12 | < 0.001 | 61/156/25 | 21/32/8 | 0.222 |
| cN stage (N0/N1/N2) | 31/52/61 | 75/59/25 | < 0.001 | 89/84/69 | 17/27/17 | 0.313 |
| Neoadjuvant therapy (+/-) | 25/119 | 37/122 | 0.203 | 51/191 | 11/50 | 0.599 |
| CEA (+/-) (positive ≥ 5 ng/mL) | 77/67 | 56/103 | 0.001 | 107/135 | 26/35 | 0.823 |
| CA19-9 (+/-) (positive ≥ 30 U/mL) | 43/101 | 35/124 | 0.119 | 64/178 | 14/47 | 0.577 |
| CA125 (+/-) (positive ≥ 24 U/mL) | 20/124 | 15/144 | 0.226 | 20/222 | 3/58 | 0.378 |
| pT stage (T1/T2/T3/T4) | 0/15/117/12 | 8/44/95/12 | < 0.001 | 6/48/171/17 | 2/11/41/7 | 0.584 |
| pN stage (0/N1/N2) | 29/74/41 | 81/56/22 | < 0.001 | 88/101/53 | 22/29/10 | 0.579 |
| Stage (Ⅰ/Ⅱ/Ⅲ) | 10/28/115 | 38/43/78 | < 0.001 | 40/48/154 | 8/14/39 | 0.731 |
| Histologic grade (G1/G2/G3) | 1/105/38 | 2/133/24 | 0.014 | 2/189/51 | 1/49/11 | 0.523 |
| Rad-score | 0.60 ± 0.19 | 0.40 ± 0.20 | < 0.001 | 0.50±0.21 | 0.49 ± 0.23 | 0.805 |
Location: Low (0-5 cm from the anal verge), middle (5.1-10 cm from the anal verge), and high (10.1-15 cm from the anal verge). This table summarized the results of cT-stage and cN-stage for the two readers. CT: Computed tomography; PNI: Perineural invasion; CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9; CA125: Carbohydrate antigen 125; cT stage: Computed tomography-reported T stage; cN stage: Computed tomography-reported N stage; pT stage: Pathological T stage; pN stage: Pathological N stage.
Reference standard for pathology
All patients with RC were diagnosed by pathology based on resected specimens. The pathological confirmatory reports were acquired from electronic medical records. PNI status was defined as positive, if (1) At least 33% of the nerve circumference was surrounded by cancer cells (without invasion of nerve sheath); or (2) Cancer cells were within any layer of the nerve sheath[6,19].
CT examination and evaluation
In our hospital, the chest-abdomen-pelvis enhanced CT is used to detect both primary and metastatic lesions for patients with clinically suspected RC. The CT scanners were restricted to Somatom Definition AS+ and Somatom Definition Flash in this study. The parameters of CT examinations are shown in the Supplementary material.
Two experienced radiologists (10 years’ and 5 years’ experience in abdominal imaging) were assigned to review CT images. The identification of patients was removed from CT images, and the readers were blinded to all clinical and pathological information. The CT-reported T stage (cT) and N stage (cN) were determined by summarizing the results of the two readers (Table 1). The inter-observer variability of cT/cN was evaluated by a weighted kappa statistics test. Because CT is limited to distinguish T1 from T2 in RC, lesions of cT1 were classified as cT2 in this study. When reviewing the CT images, the radiologists solved disagreements by discussion.
Feature extraction and model building
The stability of radiomics features was tested on 20 patients. One radiologist drew volumes of interest (VOIs) twice for evaluating intra-class correlation coefficient (intra-ICC), and the other drew VOIs once for evaluating inter- ICC. The features with ICC < 0.75 were deleted according to the commonly admitted knowledge: ICC < 0.5, poor reliability; 0.5-0.75, moderate reliability; ICC > 0.75, good reliability[20]. The main tumor and peritumoral area were separately drawn slice by slice to obtain intra- and peritumoral features (Figure 2).
The CT images were resampled to a pixel spacing of 1.0 mm in three anatomical directions. Then high- and low-pass wavelet filters, Laplacian-of-Gaussian filters, and other transformation methods such as square, square root, logarithm, exponential, gradient, lbp2d, and lbp3 were used to pre-process the original images. Radiomics features were extracted by using PyRadiomics[21]. A total of 4214 features (three types: First-order statistics, shape features, and texture features) were extracted from intra- and peritumoral regions. Z-score was used to normalize the features for eliminating the differences in the value scales. Redundant features were randomly removed by correlation analysis with a threshold of 0.48. Then the features were selected using six different methods (analysis of variance, Pearson, mutual information, L1-based, tree-based, and recursive). Subsequently, 14 different machine-learning methods were used to build 84 classifiers. The optimal parameters were adjusted to improve the area under the receiver operating characteristic (ROC) curve (AUC) of the test set and output the best classifier (Rad-score).
Statistically significant risk variables (Rad-score and clinical factors) from the univariate logistic regression analysis were then entered into a multivariate analysis for developing the clinical and combined models. A nomogram was generated for the combined model visualization, graphical evaluation of variable importance, and the calculation of predictive accuracy. ROC curve analyses were performed to assess the diagnostic performance of the models.
Statistical analysis
All statistical analyses were performed using R software (version 3.6.1), SPSS (version 21), Stata (version 15.0), and Medcalc (version 15.2.2). Differences of the factors in Table 1 were assessed by chi-square test or Fisher’s exact test, Mann-Whitney test, and t-test. AUCs of the models were compared by DeLong’s test.
RESULTS
Patient characteristics
A total of 303 RC patients (144 PNI+ and 159 PNI-) were enrolled in this study. Clinical factors such as cT/cN stage, CEA (+/-), pathological T/N stage, and grade had significant differences between PNI+ and PNI- groups (Table 1). The weighted kappa coefficients of cT and cN between two readers were 0.709 [95% confidence interval (CI): 0.630-0.789] and 0.849 (95%CI: 0.801-0.897), which showed substantial consistency for cT and almost perfect consistency for cN (0.41-0.60, moderate, 0.61-0.80, substantial and 0.81-1.00, almost perfect)[22]. There were no significant differences in sex, age, volume, location, CA19-9, and CA125 between PNI+ and PNI- groups (Table 1). The patients were randomly divided into the training cohort (n = 242) and the validation cohort (n = 61). Except for location (P = 0.045), there were no significant differences in other factors between the training and validation groups (Table 1).
Feature selection and model building
A total of 3095 features (1490 intratumoral and 1605 peritumoral) had good reliability with ICC > 0.75. After deleting redundant features, we selected only seven intratumoral and 13 peritumoral features using the L1-based method. Rad-score was established by logistic regression, as shown in Supplementary material. Rad-score was an independent risk factor for predicting PNI [odds ratio (OR) = 3.148, P < 0.001]. As for clinical factors, CEA, cT, and cN were independent risk factors for predicting PNI preoperatively (OR = 2.528, 1.636, and 1.458; P = 0.003, 0.087, and 0.001, respectively), as shown in Table 2. The factor location had a significant difference in the univariate logistic regression analysis; however, it was excluded by multivariate analysis (Table 2). Thus, the combined model (Rad-score + CEA + cT + cN) was built by multivariate logistic regression analysis. The formula of the combined model is shown in Supplementary material.
Table 2.
Risk factors selected by logistic regression analysis
|
Variables
|
Univariate
|
Multivariate
|
||
|
|
OR
|
P
value
|
OR
|
P
value
|
| Age | 1.001 | 0.961 | - | - |
| Sex | 0.787 | 0.358 | - | - |
| Volume | 1.000 | 1.000 | - | - |
| Location | 1.779 | 0.050 | 1.470 | 0.265 |
| cT stage | 2.328 | < 0.001 | 1.636 | 0.087 |
| cN stage | 1.550 | < 0.001 | 1.458 | 0.001 |
| CEA | 2.631 | < 0.001 | 2.528 | 0.003 |
| CA19-9 | 1.478 | 0.182 | - | - |
| CA125 | 0.717 | 0.484 | - | - |
| Rad-score | 3.012 | < 0.001 | 3.148 | < 0.001 |
If P value < 0.1, variables were included in the model. CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9; CA125: Carbohydrate antigen 125; OR: Odds ratio.
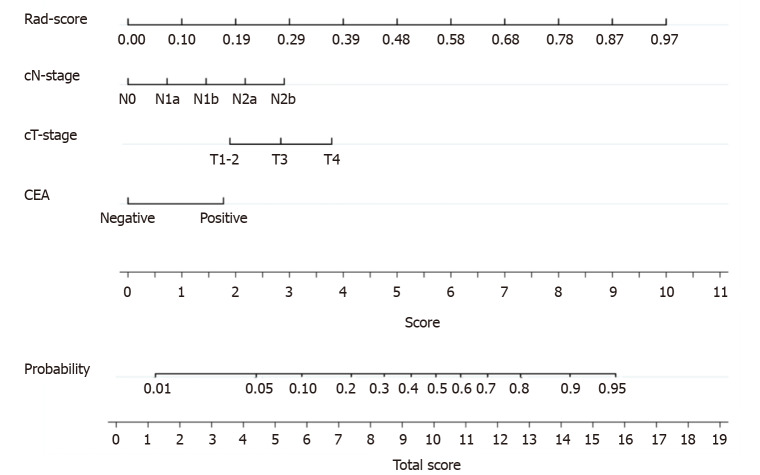
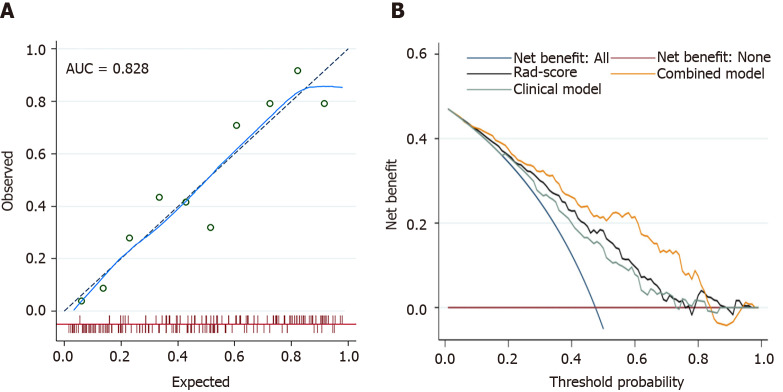
A nomogram was generated for the visualization of the combined model (Figure 3). Higher total score obtained from the nomogram is associated with greater predicted risk of PNI. The combined model had good fit according to the Hosmer-Lemeshow test (P = 0.122). In the calibration curve of the nomogram (Figure 4), the y axis represents the actual observed probability of PNI, and the x axis represents the predicted probability of PNI. A locally weighted regression line (solid line) of calibration plots is used to demonstrate the general trend of predicted risk. The model had a good agreement between the predicted and observed probability, because the solid line was close to the reference line (dotted line) in this study. This conclusion was consistent with the result of the Hosmer-Lemeshow test. However, among patients with predicted probability > 83%, the model overestimated actual risk of PNI+ (about 15% at most). The decision curve was performed to assess the clinical usefulness of the combined model in predicting PNI. The net benefit is measured on the y axis. Figure 4 shows that the combined model (nomogram) obtained more benefit than “treat all”, “treat none”, Rad-score, and the clinical model, when the threshold probability was found to be in the range of 10% to 83%.
Figure 3.
The nomogram was developed in the training cohort. Sum the points of variables in the “score” axis to get the total points. The risk of perineural invasion is the corresponding value on the “probability” axis. CEA: Carcinoembryonic antigen; cT: Computed tomography-reported T stage; cN: Computed tomography-reported N stage.
Figure 4.
The fit and usefulness evaluation of the nomogram. A: The calibration curve of the nomogram shows a good agreement between the predicted and observed risks in the training cohort; B: The decision curve demonstrates that the nomogram (combined model) obtains more benefit than “treat all”, “treat none”, Rad-score, and the clinical model, when the threshold probability is in the range of 10% to 83%. AUC: Area under the receiver operating characteristic curve.
Classification results
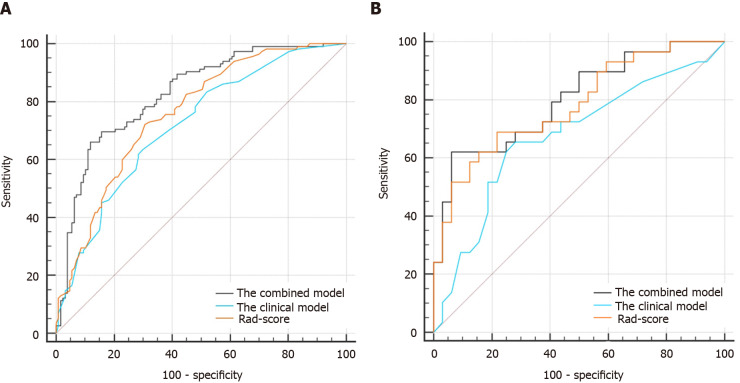
In the case of the clinical model (cT + cN + CEA), the resulting AUCs were 0.718 (95%CI: 0.657-0.774) in the training cohort and 0.674 (95%CI: 0.542-0.789) in the validation cohort. Improved performance was achieved by adding Rad-score to the clinical factors. The AUCs of the combined model (0.828; 95%CI: 0.774-0.873 in the training cohort and 0.801; 95%CI: 0.679-0.892 in the validation cohort) were higher than those of the clinical model (P < 0.001 and P = 0.045, respectively), as shown in Table 3 and Figure 5. The combined model had a higher AUC than Rad-score (AUC = 0.828 vs 0.760, P = 0.020) in the training cohort. However, there was no significant difference between the combined model and Rad-score in the validation cohort (AUC = 0.801 vs 0.782, P = 0.640).
Table 3.
Comparisons of variables and models in the training and validation cohorts
|
|
The training set
|
The validation set
|
||||||
|
|
AUC
|
SEN (%)
|
SPE (%)
|
P
value
|
AUC
|
SEN (%)
|
SPE (%)
|
P
value
|
| CEA | 0.597 (0.533-0.660) | 50.00 | 76.38 | < 0.001 | 0.635 (0.502-0.754) | 96.55 | 40.63 | 0.042 |
| cT stage | 0.613 (0.549-0.675) | 84.35 | 33.86 | < 0.001 | 0.589 (0.456-0.714) | 75.86 | 43.75 | 0.004 |
| cN stage | 0.670 (0.607-0.729) | 42.61 | 84.25 | < 0.001 | 0.684 (0.553-0.797) | 86.21 | 40.63 | 0.045 |
| Rad-score | 0.760 (0.701-0.812) | 72.17 | 69.29 | 0.020 | 0.782 (0.658-0.878) | 68.97 | 78.12 | 0.640 |
| Clinical model | 0.718 (0.657-0.774) | 63.48 | 70.08 | < 0.001 | 0.674 (0.542-0.789) | 65.52 | 71.87 | 0.045 |
| Combined model | 0.828 (0.774-0.873) | 66.09 | 88.19 | 0.801 (0.679-0.892) | 62.07 | 93.75 | ||
P value: Compared with the combined model by DeLong’s test. AUC: Area under the receiver operating characteristic curve; SEN: Sensitivity; SPE: Specificity; CEA: Carcinoembryonic antigen.
Figure 5.
The comparisons of receiver operating characteristic curves in this study. A: In the training cohort: Area under the receiver operating characteristic curve (AUC) = 0.828 for the combined model, 0.718 for the clinical model, and 0.760 for Rad-score; B: In the validation cohort: AUC = 0.801 for the combined model, 0.674 for the clinical model, and 0.782 for Rad-score.
Subgroup analysis
In the cohort of patients treated with nCRT, the AUC of the combined model was higher than that of the clinical model (AUC = 0.853 vs 0.710, P = 0.011) (Table 4). Among stage III patients, the combined model still had a higher AUC than the clinical model (AUC = 0.796 vs 0.630, P < 0.001). As for the performance among stage II patients, the combined model failed to outperform the clinical model (AUC = 0.670 vs 0.553, P = 0.098). Considering the difference between the upper third RC and middle-lower RC in prognosis[23], we performed a subgroup analysis showing that the AUC of the combined model in upper RC group (0.817; 95%CI: 0.730-0.885) was similar with that of the middle-lower RC group (0.824; 95%CI: 0.764-0.875).
Table 4.
Subgroup analyses of the models in the whole cohort
|
|
The clinical model
|
The combined model
|
P
value
|
||||
|
Subgroups
|
AUC
|
SEN (%)
|
SPE (%)
|
AUC
|
SEN (%)
|
SPE (%)
|
|
| nCRT | |||||||
| With (n = 62) | 0.710 (0.581-0.818) | 68.00 | 75.68 | 0.853 (0.740-0.930) | 68.00 | 89.19 | 0.011 |
| Without (n = 241) | 0.712 (0.650-0.768) | 48.74 | 83.61 | 0.814 (0.759-0.861) | 65.55 | 87.70 | < 0.001 |
| Stage | |||||||
| II (n = 62) | 0.553 (0.422-0.680) | 21.05 | 93.02 | 0.670 (0.538-0.784) | 36.84 | 100.00 | 0.098 |
| III (n = 193) | 0.630 (0.558-0.698) | 56.52 | 69.23 | 0.796 (0.732-0.851) | 73.04 | 79.49 | < 0.001 |
P value: Comparisons between the clinical model and combined model. AUC: Area under the receiver operating characteristic curve; SEN: Sensitivity; SPE: Specificity; nCRT: Neoadjuvant chemoradiotherapy.
DISCUSSION
In this study, a combined model showing potential for predicting PNI of RC outperformed the clinical model (AUC = 0.828 vs 0.718, P < 0.001 in the training cohort; 0.801 vs 0.674, P = 0.045 in the validation cohort), indicating that adding Rad-score to the clinical factors improved the predictive value. However, model calibration was not perfect due to the modest overestimation of PNI risk for high-risk patients.
It has been shown in recent studies that PNI is not only the simple diffusion of cancer cells along connective tissues covering the nerve sheath, but also the interaction of a variety of neurotrophic factors and chemokines between cancer cells and the surrounding microenvironment[5,6]. This process may induce cancer invasion, local recurrence, and metastasis, resulting in poor prognosis. Accurate prediction of PNI helps to evaluate prognosis of RC patients. Currently, the sole approach to determine PNI status is the pathological examination of surgical specimens. Preoperative prediction of PNI helps the formulation of individualized treatment[16,24]. For example, PNI+ patients should accept more aggressive treatment; for instance, nCRT.
In contrast to CT and MRI, radiomics may achieve desirable outcomes for predicting PNI by extracting high-throughput features that can quantify differences between tissues invisible to the naked eyes. Different MRI-based radiomics models have been reported in RC[6,17,18]. However, the sample sizes in the previous studies were small (PNI+: 26-32). In our study, a total of 144 PNI+ patients were enrolled, which increased the reliability of the conclusion.
As for the methodology of radiomics, the machine-learning methods used in our study and previous studies were similar. However, the previous studies only included the intratumoral region, ignoring the peritumoral region in which PNI can also appear[7]. There is evidence that radiomics features of peritumoral regions can offer information about biological characteristics of other tumors, such as gastric[25], breast[26], and lung[27] cancer. Different from the previous studies[6,17,18], we built the model by using both peri- and intratumoral regions, and the model had comparable AUCs with the previous MRI-based models[6,18]. The specificities of our model were 88.19% in the training cohort and 93.75% in the validation cohort, as shown in Table 3, indicating low false-positive rate (misdiagnosis rate) for detecting PNI. However, the sensitivities were low (66.09% in the training cohort and 62.07% in the validation cohort).
In terms of clinical factors, cT and cN included in our model revealed a higher risk of PNI in patients with more advanced RC, which was consistent with the conclusion of a meta-analysis[28]. As for Rad-score, it was more important than clinical factors in the prediction of PNI, due to its longer axis in the nomogram. For example, a PNI+ lesion in Figure 2 was incorrectly identified as PNI- by the clinical model (CEA = negative, cT = T3, cN = N1a) and correctly determined after adding Rad-score (0.805) to the clinical model with a total score of 12 points in the nomogram, showing a probability of 73% to be PNI+.
Referring to the 62 patients receiving nCRT, we found that AUC of the combined model was improved (0.853; 95%CI: 0.740-0.930), suggesting that this model was also suitable for patients treated with nCRT. With the consideration of individualized evaluation of RC patients with different stages, the combined model obtained a higher AUC than the clinical model for stage III patients (AUC = 0.796 vs 0.630, P < 0.001). However, the combined model failed to outperform the clinical model among stage II patients (AUC = 0.670 vs 0.553, P = 0.098), which might be caused by the small sample size of stage II patients. As for the subgroup analysis of location, the combined model had similar predictive values in upper RC group (AUC = 0.817) and in middle-lower RC group (AUC = 0.824), indicating good applicability of the model for both upper and middle-lower RC patients.
There were several limitations to this study. Firstly, bias may have existed due to the retrospective design of this study. Secondly, PNI- patients from January 2019 to October 2019 were not included, because the current PNI- patients were sufficient to complete this radiomics analysis. Thirdly, all patients were enrolled from a single institution. In the future, it is necessary to conduct a multicenter validation to extend the versatility of the radiomics model.
CONCLUSION
A combined model incorporating a radiomics signature and clinical factors was described in this study. This model can provide assistance in the individualized prediction of PNI status in patients with RC.
ARTICLE HIGHLIGHTS
Research background
Perineural invasion (PNI) has emerged as an independent prognostic factor in patients with rectal cancer (RC). The preoperative prediction of PNI status is beneficial for individualized treatment and improved prognosis.
Research motivation
Nowadays, preoperative assessment of PNI status is still challenging.
Research objectives
To build a radiomics prediction model for evaluating PNI status preoperatively in RC patients.
Research methods
We enrolled 303 RC patients in a single institution from March 2018 to October 2019. These patients were classified as the training cohort (n = 242) and validation cohort (n = 61). A large number of intra- and peritumoral radiomics features were extracted to build the Rad-score and combined model.
Research results
Our study enrolled more patients (144 PNI+ and 159 PNI-) than previous studies[6,17,18]. Rad-score was built by logistic regression analysis. The combined model was developed by combining Rad-score with computed tomography (CT)-reported T stage and N stage, and carcinoembryonic antigen. The combined model showed good performance to predict PNI status, with an area under the receiver operating characteristic curve of 0.828 [95% confidence interval (CI): 0.774-0.873] in the training cohort and 0.801 (95%CI: 0.679-0.892) in the validation cohort.
Research conclusions
The combined model incorporating Rad-score and clinical factors helps to provide an individualized PNI status evaluation.
Research perspectives
Other biological characteristics besides PNI are also related to the prognosis of RC patients; for instance, intramural lymphovascular invasion (LVI). Intramural LVI cannot be determined by magnetic resonance imaging and CT. Therefore, using radiomics or deep learning to predict intramural LVI of RC is valuable in the future.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of West China Hospital of Sichuan University (Approved No.1159).
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: Zhao YL and Huang CC are employed by the company Beijing Deepwise & League of PHD Technology Co., Ltd. The remaining authors declare no conflicts-of-interest related to this article.
Manuscript source: Unsolicited manuscript
Peer-review started: June 2, 2021
First decision: June 25, 2021
Article in press: August 11, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dulskas A, Peltrini R S-Editor: Liu M L-Editor: A P-Editor: Guo X
Contributor Information
Mou Li, Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Yu-Mei Jin, Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Yong-Chang Zhang, Department of Radiology, Chengdu Seventh People’s Hospital, Chengdu 610213, Sichuan Province, China.
Ya-Li Zhao, Department of Research Collaboration, R&D Center, Beijing Deepwise & League of PHD Technology Co., Ltd, Beijing 100080, China.
Chen-Cui Huang, Department of Research Collaboration, R&D Center, Beijing Deepwise & League of PHD Technology Co., Ltd, Beijing 100080, China.
Sheng-Mei Liu, Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Bin Song, Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China. songlab_radiology@163.com.
Data sharing statement
No additional data are available.
References
- 1.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Lombardi R, Cuicchi D, Pinto C, Di Fabio F, Iacopino B, Neri S, Tardio ML, Ceccarelli C, Lecce F, Ugolini G, Pini S, Di Tullio P, Taffurelli M, Minni F, Martoni A, Cola B. Clinically-staged T3N0 rectal cancer: is preoperative chemoradiotherapy the optimal treatment? Ann Surg Oncol. 2010;17:838–845. doi: 10.1245/s10434-009-0796-7. [DOI] [PubMed] [Google Scholar]
- 3.Meng X, Xia W, Xie P, Zhang R, Li W, Wang M, Xiong F, Liu Y, Fan X, Xie Y, Wan X, Zhu K, Shan H, Wang L, Gao X. Preoperative radiomic signature based on multiparametric magnetic resonance imaging for noninvasive evaluation of biological characteristics in rectal cancer. Eur Radiol. 2019;29:3200–3209. doi: 10.1007/s00330-018-5763-x. [DOI] [PubMed] [Google Scholar]
- 4.Kim CH, Yeom SS, Lee SY, Kim HR, Kim YJ, Lee KH, Lee JH. Prognostic Impact of Perineural Invasion in Rectal Cancer After Neoadjuvant Chemoradiotherapy. World J Surg. 2019;43:260–272. doi: 10.1007/s00268-018-4774-8. [DOI] [PubMed] [Google Scholar]
- 5.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Chen Y, Zheng D, Pang P, Zhang H, Zheng X, Liao J. Pretreatment MR-based radiomics nomogram as potential imaging biomarker for individualized assessment of perineural invasion status in rectal cancer. Abdom Radiol (NY) 2021;46:847–857. doi: 10.1007/s00261-020-02710-4. [DOI] [PubMed] [Google Scholar]
- 7.Ueno H, Hase K, Mochizuki H. Criteria for extramural perineural invasion as a prognostic factor in rectal cancer. Br J Surg. 2001;88:994–1000. doi: 10.1046/j.0007-1323.2001.01810.x. [DOI] [PubMed] [Google Scholar]
- 8.Ceyhan GO, Liebl F, Maak M, Schuster T, Becker K, Langer R, Demir IE, Hartel M, Friess H, Rosenberg R. The severity of neural invasion is a crucial prognostic factor in rectal cancer independent of neoadjuvant radiochemotherapy. Ann Surg. 2010;252:797–804. doi: 10.1097/SLA.0b013e3181fcab8d. [DOI] [PubMed] [Google Scholar]
- 9.Sun Q, Liu T, Liu P, Luo J, Zhang N, Lu K, Ju H, Zhu Y, Wu W, Zhang L, Fan Y, Liu Y, Li D, Liu L. Perineural and lymphovascular invasion predicts for poor prognosis in locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery. J Cancer. 2019;10:2243–2249. doi: 10.7150/jca.31473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng J, Sheng W, Huang D, Venook AP, Xu Y, Guan Z, Cai S. Perineural invasion in pT3N0 rectal cancer: the incidence and its prognostic effect. Cancer. 2011;117:1415–1421. doi: 10.1002/cncr.25620. [DOI] [PubMed] [Google Scholar]
- 11.Huang CM, Huang CW, Huang MY, Lin CH, Chen CF, Yeh YS, Ma CJ, Huang CJ, Wang JY. Coexistence of perineural invasion and lymph node metastases is a poor prognostic factor in patients with locally advanced rectal cancer after preoperative chemoradiotherapy followed by radical resection and adjuvant chemotherapy. Med Princ Pract. 2014;23:465–470. doi: 10.1159/000363604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikberg M, Chabok A, Letocha H, Kindler C, Glimelius B, Smedh K. Lymphovascular and perineural invasion in stage II rectal cancer: a report from the Swedish colorectal cancer registry. Acta Oncol. 2016;55:1418–1424. doi: 10.1080/0284186X.2016.1230274. [DOI] [PubMed] [Google Scholar]
- 13.Song JH, Yu M, Kang KM, Lee JH, Kim SH, Nam TK, Jeong JU, Jang HS, Lee JW, Jung JH. Significance of perineural and lymphovascular invasion in locally advanced rectal cancer treated by preoperative chemoradiotherapy and radical surgery: Can perineural invasion be an indication of adjuvant chemotherapy? Radiother Oncol. 2019;133:125–131. doi: 10.1016/j.radonc.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, He L, Dong D, Yang C, Liang C, Chen X, Ma Z, Huang X, Yao S, Tian J, Liu Z. Individualized prediction of perineural invasion in colorectal cancer: development and validation of a radiomics prediction model. Chin J Cancer Res. 2018;30:40–50. doi: 10.21147/j.issn.1000-9604.2018.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, Liu J, Wu G, Chen S, Pc FJ, Xie W, Tang W. Development and Validation of a Nomogram for Preoperative Prediction of Perineural Invasion in Colorectal Cancer. Med Sci Monit. 2019;25:1709–1717. doi: 10.12659/MSM.914900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Huang X, Sun J, Gao P, Song Y, Chen X, Zhao J, Wang Z. Prognostic value of perineural invasion in colorectal cancer: a meta-analysis. J Gastrointest Surg. 2015;19:1113–1122. doi: 10.1007/s11605-015-2761-z. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Wang Q, Guo Y, Zhang Y, Fu Y, Zhang H. Preoperative prediction of perineural invasion with multi-modality radiomics in rectal cancer. Sci Rep. 2021;11:9429. doi: 10.1038/s41598-021-88831-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YS, Qiu YJ, Zheng GH, Gong HP, Ge YQ, Zhang YF, Feng F, Wang YT. High resolution MRI-based radiomic nomogram in predicting perineural invasion in rectal cancer. Cancer Imaging. 2021;21:40. doi: 10.1186/s40644-021-00408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakst RL, Wong RJ. Mechanisms of Perineural Invasion. J Neurol Surg B Skull Base. 2016;77:96–106. doi: 10.1055/s-0036-1571835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benchoufi M, Matzner-Lober E, Molinari N, Jannot AS, Soyer P. Interobserver agreement issues in radiology. Diagn Interv Imaging. 2020;101:639–641. doi: 10.1016/j.diii.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 21.van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017;77:e104–e107. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roldán-Nofuentes JA, Amro RM. Combination of the weighted kappa coefficients of two binary diagnostic tests. J Biopharm Stat. 2018;28:909–926. doi: 10.1080/10543406.2017.1402781. [DOI] [PubMed] [Google Scholar]
- 23.Clancy C, Flanagan M, Marinello F, O'Neill BD, McNamara D, Burke JP. Comparative Oncologic Outcomes of Upper Third Rectal Cancers: A Meta-analysis. Clin Colorectal Cancer. 2019;18:e361–e367. doi: 10.1016/j.clcc.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Cienfuegos JA, Rotellar F, Baixauli J, Beorlegui C, Sola JJ, Arbea L, Pastor C, Arredondo J, Hernández-Lizoáin JL. Impact of perineural and lymphovascular invasion on oncological outcomes in rectal cancer treated with neoadjuvant chemoradiotherapy and surgery. Ann Surg Oncol. 2015;22:916–923. doi: 10.1245/s10434-014-4051-5. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, Wang H, Wu J, Chen C, Yuan Q, Huang W, Li T, Xi S, Hu Y, Zhou Z, Xu Y, Li G, Li R. Noninvasive imaging evaluation of tumor immune microenvironment to predict outcomes in gastric cancer. Ann Oncol. 2020;31:760–768. doi: 10.1016/j.annonc.2020.03.295. [DOI] [PubMed] [Google Scholar]
- 26.Braman N, Prasanna P, Whitney J, Singh S, Beig N, Etesami M, Bates DDB, Gallagher K, Bloch BN, Vulchi M, Turk P, Bera K, Abraham J, Sikov WM, Somlo G, Harris LN, Gilmore H, Plecha D, Varadan V, Madabhushi A. Association of Peritumoral Radiomics With Tumor Biology and Pathologic Response to Preoperative Targeted Therapy for HER2 (ERBB2)-Positive Breast Cancer. JAMA Netw Open. 2019;2:e192561. doi: 10.1001/jamanetworkopen.2019.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Morales J, Tunali I, Stringfield O, Eschrich SA, Balagurunathan Y, Gillies RJ, Schabath MB. Peritumoral and intratumoral radiomic features predict survival outcomes among patients diagnosed in lung cancer screening. Sci Rep. 2020;10:10528. doi: 10.1038/s41598-020-67378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID. Perineural Invasion is a Strong Prognostic Factor in Colorectal Cancer: A Systematic Review. Am J Surg Pathol. 2016;40:103–112. doi: 10.1097/PAS.0000000000000518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.