Abstract
Background
There is scanty data in India on polycystic ovary syndrome (PCOS) from several small, undersized, convenience-based studies employing differing diagnostic criteria and reporting varied regional prevalence. It is difficult to draw clear-cut conclusions from these studies; therefore, the present multicentric, well-designed, large-scale representative countrywide epidemiological study on PCOS across India was conceived with the aim to generate the actual prevalence rates of PCOS in India with a total sample size of approximately 9000 individuals.
Objective
The primary objectives of the study are to estimate the national prevalence of PCOS in India and the burden of comorbidities and to compare the variation in efficacy of standard therapeutic modalities for metabolic dysfunction in women with PCOS.
Methods
This multicentric umbrella study consists of three different substudies. Substudy 1 will involve recruitment of women aged 18-40 years using a multistage sampling technique from randomly selected polling booths across urban and rural areas to estimate national prevalence, phenotypic variation, and risk factors among regions. Substudy 2 involves recruitment of subjects from the community pool of substudy 1 and the institutional pool for quantitation of comorbidities among women with PCOS. Substudy 3, an interventional part of the study, aims for comparison of variation in efficacies of common treatment modalities and will be conducted only at 2 centers. The eligible consenting women will be randomized in a 1:1 ratio into 2 arms through a blinding procedure. All these women will undergo clinical, biochemical, and hormonal assessment at baseline and at 3 and 6 months. The data generated will be analyzed using the reliable statistical software SPSS (version 26).
Results
The study is ongoing and is likely to be completed by April 2022. The data will be compiled and analyzed, and the results of the study will be disseminated through publications.
Conclusions
The Indian Council of Medical Research–PCOS study is the first of its kind attempting to provide accurate and comprehensive data on prevalence of PCOS in India.
Trial Registration
Clinical Trials Registry–India CTRI/2018/11/016252; ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=26366
International Registered Report Identifier (IRRID)
DERR1-10.2196/23437
Keywords: polycystic ovary syndrome, prevalence, metabolic aberrations, community pool, therapeutic modalities, India, metabolic dysfunction, phenotypic variations, ovarian morphology, PCOS epidemiology
Plain English Summary
Polycystic ovary syndrome (PCOS) is a multifactorial disorder with unknown etiology and numerous clinical manifestations in reproductive-aged women around the world. The metabolic abnormalities linked to this syndrome include type 2 diabetes, insulin resistance (IR) obesity, abnormal glucose tolerance (AGT), and cardiovascular diseases (CVD). The sketchy data and escalating prevalence of PCOS and associated comorbidities among Indian women led to organization of a multidisciplinary brainstorming meeting held September 11, 2014, at Indian Council of Medical Research (ICMR) headquarters, and the ensuing recommendations suggested formulation of the ICMR–PCOS Task Force to conduct a multicentric, multiphase study among Indian women with PCOS.
This is a multicentric, multispecialty study with a common comprehensive predesigned protocol involving 10 centers located in the 6 zones of the country. The women from the community (rural and urban) will be screened using a validated screening questionnaire, after obtaining a written informed consent from the subjects. The proforma will capture all details about menstrual cyclicity, acne, hirsutism, socioeconomic status, family history of PCOS, etc. The national prevalence will be calculated from the community pool for substudy 1. All biochemical and hormonal analysis will be done by standard assays using similar kits, reagents, and equipment. Ultrasonography (US) including magnetic resonance imaging (MRI) of the abdomen will be performed using a common approved protocol. The various comorbidities such as AGT, nonalcoholic steatohepatitis, and sleep apnea will be evaluated from both the pools (ie, hospital cohort and community cohort in substudy 2). The interventional randomized double-blind trial (ie, oral contraceptive pills [OCPs] vs metformin) will be conducted at only 2 centers for substudy 3. Apart from this, a knowledge, attitude, and practices survey of service providers in the public health system will be conducted to understand the training needs and gaps in the health system addressing PCOS management.
Introduction
Overview
Polycystic ovary syndrome, affecting approximately 116 million women worldwide, is now considered the commonest endocrinopathy of reproductive-aged women [1,2]. Stein and Leventhal initially described it as the association of bilateral ovaries with amenorrhea in 1935 [3,4]. Now, its association with a constellation of metabolic aberrations including obesity, AGT, IR, non-alcoholic fatty liver disease (NAFLD), metabolic syndrome, sleep apnea, stroke, CVD, neuropsychiatric comorbidities, microalbuminuria, mitogenesis, etc, in addition to cosmetic and reproductive dysfunction, is increasingly being recognized [5-9].
Global prevalence of PCOS among geographical zones is reported differently as 5%-10% in developed countries, 5%-30% in the United States, and 3.7%-22.5% across India [10-13]. Since there is no specific established treatment protocol available, early diagnosis and management is likely to reduce the risk of long-term complications of PCOS. The etiology of the disorder is being actively investigated; various reports speculate on the environmental and genetic factor interactions responsible [14-16]. Various small, regional, poorly designed studies using varying diagnostic criteria suggest a high prevalence of PCOS in India, seemingly running parallel to the epidemic of noncommunicable diseases, especially type 2 diabetes. There are no systematic representative data on the prevalence of PCOS or burden of its comorbidities among Indian women to date [17-31]. The existing data hint at the potential for large-scale epidemiological studies on PCOS across India to generate the actual prevalence and picture of the disease in the community. Therefore, there is an immense need to generate nationwide data on the prevalence of PCOS, including its comorbidities, besides any variations in phenotypes or treatment responses among Indian women.
To address these issues concerning PCOS among Indian women, ICMR, New Delhi, has taken the initiative to conduct a nationwide multicentric study titled “Evaluation of prevalence, regional phenotypic variation, co-morbidities, risk factors and the variations in response to different therapeutic modalities among Indian women with polycystic ovary syndrome (PCOS): A Multicenter study across India” to quantitate the burden of PCOS and its comorbidities in its phase 1.
Objectives
The objectives of the proposed study are as follows:
Primary Objectives
The primary objectives are to (1) estimate national prevalence, phenotypic variations, and risk factors of PCOS in the community; (2) estimate the burden of comorbidities such as metabolic syndrome, dyslipidemia, cardiovascular complications, NAFLD, psychiatric abnormalities, sleep apnea, AGT including type 2 diabetes, and impairment of quality of life (QOL) among known cases of PCOS; and (3) compare the variation in efficacy of standard therapeutic modalities for metabolic dysfunction in women with PCOS (2 sites, All India Institute of Medical Sciences [AIIMS] and Sher-i-Kashmir Institute of Medical Sciences [SKIMS]).
Secondary Objectives
The secondary objective is to assess knowledge and management practices among service providers in the public health sector on PCOS to develop a standard screening and training modules to facilitate management of PCOS at different levels of the public health care system.
Prerequisites for Initiation of the Study
To begin with, the study required establishment of a PCOS network comprising members from the specialties and departments of endocrinology, dermatology, community medicine, biochemistry, laboratory medicine, pediatrics, internal medicine, obstetrics and gynecology, and other relevant departments based on their expertise and interest in the area of PCOS. In addition, there will be a team of national reviewers and advisors (members of the Task Force Committee) who will guide in formulating the protocol, advising on methods of assessment, and analyzing the data on relevant aspects. In view of the relevance to the project, a cardiologist (for cardiovascular risk assessment), a gastroenterologist (for NAFLD assessment), a radiologist (for devising methodology for ovarian morphology and implications of data interpretation), a psychiatrist (for assessment of psychiatric comorbidity and QOL), a pediatrician (for assessment of prepubertal risk factors and design of interventions), a dermatologist (for hyperandrogenism assessment strategies), a molecular biologist (for genetic and epidermal differentiation complex work-up), and a biostatistician (for sampling designs and final analysis) have been incorporated in the team and committee.
The following participating centers are involved in the study (Figure 1): (1) Sher-i-Kashmir Institute of Medical Sciences, Srinagar (North); (2) Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh (North); (3) All India Institute of Medical Sciences, New Delhi (North); (4) ICMR National Institute for Research in Reproductive Health (NIRRH), Seth G S Medical College and King Edward Memorial Hospital, Grant Medical College and JJ Hospital, Mumbai (West); (5) Institute of Post Graduate Medical Education & Research (IPGMER), Kolkata (East); (6) North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences (NEIGRIHMS), Shillong (North East); (7) All India Institute of Medical Sciences, Raipur (Central); (8) Osmania Medical College, Hyderabad (South); (9) Maternal Health & Research Trust, Hyderabad (South); and (10) Government Medical College, Thiruvananthapuram (South).
Figure 1.
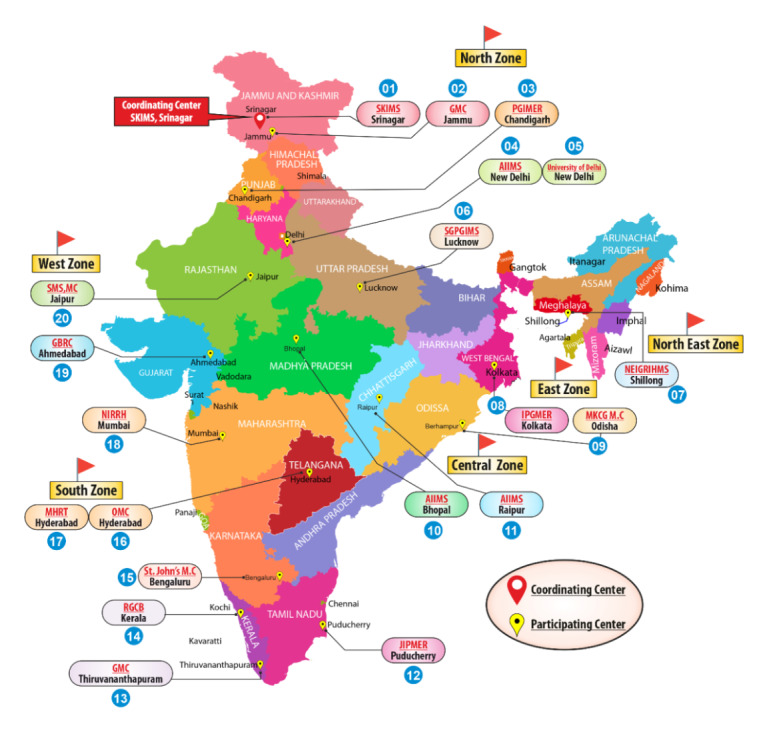
Layout of participating centers. AIIMS: All India Institute of Medical Sciences; GMC: Government Medical College; IPGMER: Institute of Post Graduate Medical Education & Research; MHRT: Maternal Health & Research Trust; NEIGRIHMS: North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences; NIRRH: National Institute for Research in Reproductive Health; OMC: Osmania Medical College; PGIMER: Postgraduate Institute of Medical Education and Research; SKIMS: Sher-i-Kashmir Institute of Medical Sciences.
Methodological Steps and Study Design
Overview
The milestone steps to proceed with estimation of national prevalence, phenotypic variation, and risk factors involve a community-based, cross-sectional survey to recruit women belonging to the age group of 18-40 years from both rural (n=500) and urban areas (n=500) across 6 zones (ie, North, South, East, West, North East, Central India). ICMR has always been a leader in developing ethical standards for any human research considering the moral, ethical, and social values and ethos of diverse populations in the country. The proposed study will be conducted according to the Helsinki Declaration of 1975 and stands approved by the Institutional Ethics Committees at all 10 study sites. A written informed consent will be obtained from the participants assuring their understanding of the purpose of the study and obligations and consequences of their participation.
Detailed Methodology: Substudies 1 and 2
Participant Selection
Women aged 18-40 years who are not pregnant or lactating and have been residing in the area for 1 year or more will be included in the study. Women who are known to have PCOS will be recorded for the purposes of PCOS prevalence and will only be evaluated in detail for substudy 2 if they are drug-naïve. Women taking oral contraceptives, steroids, antiepileptics, or other drugs known to interfere in glucose or lipid metabolism will not be evaluated in detail and will be excluded from the study.
Substudy 1: Community Pool
The study design is a two-stage, cross-sectional, community-based study.
Study Population
Women aged 18-40 years will be recruited using a multistage sampling technique involving urban and rural areas. The polling booths will be selected randomly at the coordinating center by a random selection process. This study will be conducted in 6 zones of the country involving 10 centers that are likely to represent a majority of ethnic groups.
Inclusion Criteria
The following are the criteria for inclusion: women aged 18-40 years who are permanent residents of the area (>1 year), willing to participate in the study and sign informed consent, known to have PCOS, and not on treatment.
Exclusion Criteria
Pregnant or lactating women and those with cognitive limitations, physical limitations, or both that prevented them from answering the questionnaire will be excluded from the study.
Women with a history of drug intake such as steroids, androgens, oral contraceptives, antiepileptics, or drugs known to interfere in glucose or lipid metabolism will also be excluded.
Sampling Design
A multistage cluster sampling technique is being used in this study. The first step in the sampling procedure will be to have representative data from the 6 zones (N, S, E, W, NE, and Central) of the country (Figure 1). The participating institutions from 6 zones were selected in view of location, logistics, feasibility, and the investigator profile. One urban and one rural Vidhan Sabha constituency near each institution will be selected. The choice of selection of districts and the constituencies will be given to the principal investigators at each site in order to make the study execution simpler. A rural district would mean 70% of subjects pursuing agriculture activities, while urban would mean that less than 30% have agriculture as the source of income.
After the constituencies are selected, the entire list of polling booths will be obtained from the Regional Election Commission offices. Polling booths from rural or urban constituencies will be selected to fulfill the required sample size using a random number table so that each polling booth has an equal chance to get selected. This procedure will be carried out at the coordinating center (SKIMS, Srinagar) by a third party not directly involved in the study. Next, the sites will procure the electoral roll, and voter IDs will be searched for women (aged 18-40 years) to be the potentially eligible subjects. The resulting list of women will be screened using a checklist for eligibility. If a woman is not eligible or refuses to participate in the study, the offer will go to the next woman in the list. If there is more than one eligible woman in a family, the sampling frame will also shift to the next in the voter ID list. The procedure will continue until a total of 1000 women (500 rural and 500 urban) are screened per center (Figure 2).
Figure 2.
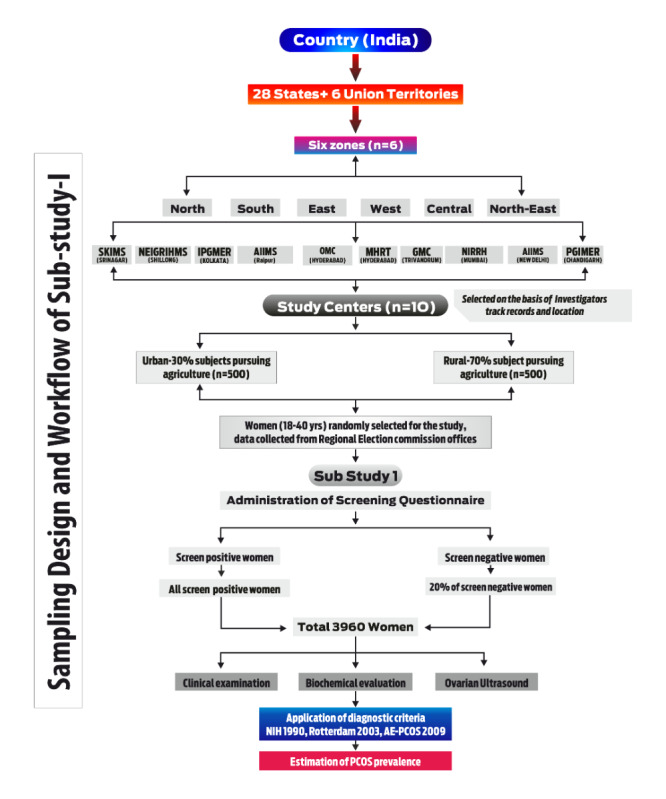
Flowchart for sampling design and workflow of the study. AE-PCOS: Androgen Excess and Polycystic Ovary Syndrome; AIIMS: All India Institute of Medical Sciences; GMC: Government Medical College; IPGMER: Institute of Post Graduate Medical Education & Research; MHRT: Maternal Health & Research Trust; NEIGRIHMS: North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences; NIH: National Institutes of Health; NIRRH: National Institute for Research in Reproductive Health; OMC: Osmania Medical College; PCOS: polycystic ovary syndrome; PGIMER: Postgraduate Institute of Medical Education and Research; SKIMS: Sher-i-Kashmir Institute of Medical Sciences.
Estimation of Sample Size for Substudy 1
Sample size was calculated, primarily to estimate the prevalence of PCOS in the country. Previously reported prevalence rates of PCOS have varied from 3.7% to 22.3% [32,33]. For the purpose of calculation of sample size, we presume the prevalence of PCOS in India to be 10%. With an assumed prevalence of 10% and an absolute error margin of 1% in a 95% 2-sided confidence interval and making allowance for about 20% refusals or nonresponse, we need to target a total of 4500 women. Further, being a cluster design and taking a design effect of 2, a total of 9000 women aged 18-40 years will be studied from 10 centers (ie, 1000 per center, except for the 2 centers in Hyderabad, which have been designated with 500 each), half from a rural community and half from an urban community at each center.
Based on a pilot study previously conducted by the chief coordinator using a similar questionnaire [34], 30% (2700/9000) of the women are expected to screen positive (likely to have PCOS) on the screening questionnaire and therefore are required to be investigated in detail. Additionally, of the 70% (6300/9000) of women expected to screen negative on the screening questionnaire, 20% (1260/6300) will require examination and investigation in detail. This will make the sample size of women requiring detailed evaluation and blood testing a total of 3960 (N=2700+1260). Keeping in view the number of centers (N=10), each participant center will screen approximately 1000 women, and a total of 440 women who will be invited for detailed examinations: 20% (140/700) of screen-negative women and all 30% (300/1000) who screen positive (Figure 2).
Evaluation Methods
Both the subgroups of women coming from the community pool and institutional pool will be evaluated as per the defined protocol for clinical, biochemical, and hormonal assessment during the second to the seventh day of the cycle. In the institutional pool, women attending endocrinology, gynecology, dermatology, or other related clinics for symptoms and signs of PCOS will be asked to report to the research staff or PCOS station for detailed evaluation as per the common protocol. Support will be obtained from the public health system for enrolling women from the community and conducting further examinations, investigations, and follow-up at their facilities in urban and rural areas. All those women who are drug-naïve will undergo clinical assessment (anthropometry, hirsutism scoring, acne, oligomenorrhea, androgenic alopecia, primary infertility), biochemical evaluation (liver function test, kidney function test, lipids, oral glucose tolerance test [OGTT]), high-sensitivity C-reactive protein (hsCRP) and cytokine tests (selected cases), 25-hydroxyvitamin D [25OHD] testing (selected cases), and hormonal estimations (thyroxine [T4], thyrotropin, luteinizing hormone [LH], follicle-stimulating hormone [FSH], prolactin [PRL], cortisol, anti-Müllerian hormone [AMH], dehydroepiandrosterone sulfate [DHEAS], 17-hydroxyprogesterone [17OHP], sex hormone–binding globulin [SHBG], serum total testosterone and insulin at 0, 30, and 120 minutes, estradiol [E2], progesterone [P4], procollagen type I N-terminal propeptide, CrossLaps, anti–thyroid peroxidase, ferritin, B12, pro–brain natriuretic peptide, and C-peptide levels). The blood samples will be transported in a cold chain to one center (SKIMS, Srinagar) for lab analysis using electrochemiluminescence immunoassay (ECLIA; Roche Diagnostics USA). Analytes such as SHBG and serum total testosterone levels will be transported to one center (SKIMS, Srinagar) for purposes of uniformity and will be analyzed in one go. In a subset of cases (n=100), total testosterone and SHBG will be assayed by ECLIA and liquid chromatography–tandem mass spectrometry in the Department of Endocrinology, AIIMS, New Delhi.
The 1990 National Institutes of Health (NIH) consensus conference criteria will be taken as the qualifying criteria. This necessitates the presence of the following 2 criteria: (1) oligoovulation or anovulation (intermenstrual interval ≥35 days, presence of ≤8 cycles per year, or both) and (2) clinical signs, biochemical evidence, or both of hyperandrogenism after ruling out disorders with similar clinical presentation such as hyperprolactinemia, Cushing syndrome, congenital adrenal hyperplasia, and androgen-secreting tumors with specific laboratory analysis (cortisol, 17OHP, and DHEAS). To compare, the 2003 Rotterdam criteria and the 2009 Androgen Excess and PCOS (AE-PCOS) Society criteria will also be employed. Therefore, US examination to satisfy the diagnosis of PCOS by these criteria will be done. A common standard operating procedure for abdominal examination (liver, pancreas, ovary, and uterus) will be adhered to with a built-in system to minimize interobserver and equipment variations. The 2003 Rotterdam criteria necessitate the presence of 2 of the following 3 criteria: (1) oligoovulation, anovulation, or both; (2) clinical signs, biochemical evidence, or both of hyperandrogenism; and (3) polycystic ovary in US. The 2009 AE-PCOS Society criteria necessitate the presence of the following criteria: (1) oligoovulation and/or anovulation (intermenstrual interval ≥35 days, presence of ≤8 cycles per year, or both) and/or polycystic ovary in US and (2) clinical signs, biochemical evidence, or both of hyperandrogenism. To exclude etiologies that could mimic PCOS like Cushing syndrome, late-onset adrenal hyperplasia, or androgen-producing neoplasm, we will exclude thyroid disorders, renal hyperplasia, hyperprolactinemia, and late-onset congenital adrenal hyperplasia by medical history and hormone tests. This will further serve to unravel various comorbidities such as AGT, metabolic syndrome, IR, cardiovascular risk, psychiatric morbidities, and impairment of QOL (Figure 3). Besides, these women will be evaluated for risk factors such as birth weight, parental history of gestational diabetes or PCOS, risk of low birth weight, precocious pubarche, prepubertal obesity, antiepileptic drug intake, familial association with lifestyle diseases, etc. Abdominal MRI for comparing ovarian morphology will also be done at a selected center (SKIMS, Srinagar) using the predesigned uniform protocol.
Figure 3.
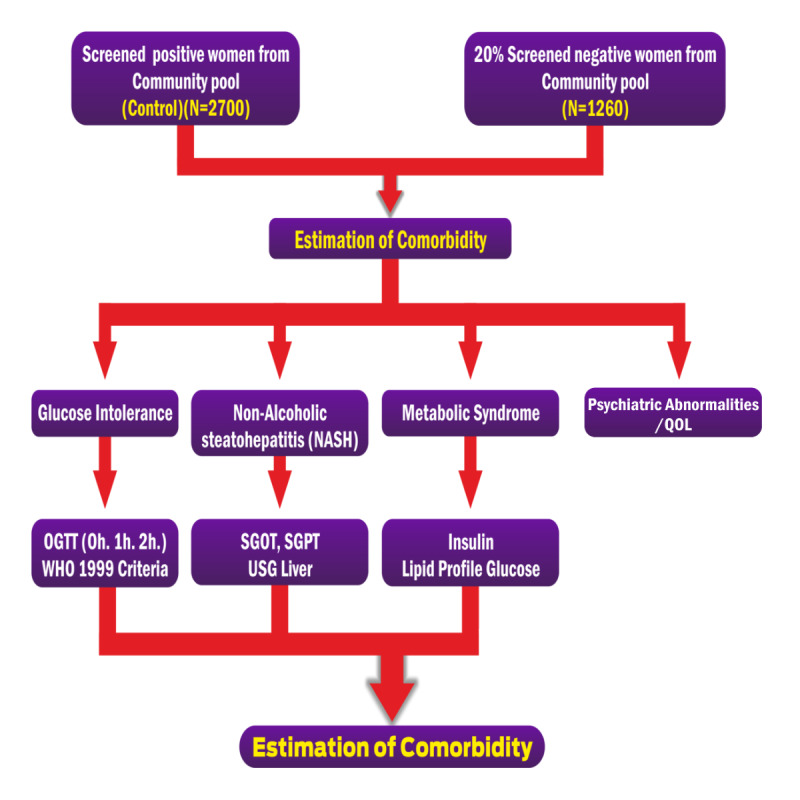
Study design and workflow chart of substudy 2. OGTT: oral glucose tolerance test; SGOT: serum glutamic oxaloacetic transaminase; SGPT: serum glutamic pyruvic transaminase; QOL: quality of life; USG: ultrasonography; WHO: World Health Organization.
Study Tool: The Questionnaire
In this study, structured questionnaires will be used for capturing information. The eligibility and screening questionnaires comprise 7 and 43 questions (part 1), respectively. The screening questionnaire comprises three sections: (1) personal details, (2) menstrual history and clinical information, and (3) history of any systemic diseases. Part 1 of the questionnaires will be filled at the field site to check eligibility and identify probable PCOS on the basis of positive responses to questions. The subjects who test positive on the questionnaire (ie, probable PCOS) and 20% of those who test negative (healthy women) will be invited to the respective institutes for detailed clinical assessment. The questionnaire stands pilot-tested and modified after several rounds of investigator meetings.
Clinical Assessment
History
The subjects will be invited to nearby institutes for detailed clinical, biochemical, hormonal, and sonography assessments. These women will be assessed for clinical detail about menstruation (regularity, duration, flow, amenorrhea), weight gain, unwanted male pattern hair growth, hair fall, infertility, etc. The details about any illness will be recorded. Psychiatric morbidity will be assessed using the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria with the Mini-International Neuropsychiatric Interview, version 4.0. The details of family pedigree will also be recorded in the predesigned questionnaire.
Examination
Anthropometric assessment including measurement of height, weight, waist circumference, skinfold thickness on dorsum of hand and triceps, and blood pressure and detailed systemic examination will be undertaken. Height and weight will be measured with subjects in light clothes and without shoes, using standard apparatus. Weight will be measured to the nearest 0.1 kg on a calibrated digital scale. Height will be measured by stadiometer by standard methodology to the nearest 0.1 cm. Waist and hip circumference will be measured to the nearest 0.5 cm with a measuring tape. The waist circumference will be defined as the point midway between the iliac crest and the costal margins (lower ribs), while hip circumference will be defined as the widest circumference over the buttocks and below the iliac crest [35]. Body mass index (BMI) will be derived as weight in kg divided by the square of height in meters. Overweight will be defined using the Asian BMI range of 23.0-24.9 kg/m2, while obesity will be defined as having a BMI equal to or greater than 25 kg/m2 [36]. Quantitation of hirsutism by a single observer by counting 9 specific body areas for the Ferriman-Gallwey score and assessments of acne vulgaris, androgenic alopecia, and acanthosis nigricans will be performed in all subjects [37].
Radiological Examination
Ultrasonography of the abdomen will be performed to evaluate the polycystic ovary morphology and grading of fatty liver (NAFLD) and nonalcoholic pancreatic steatosis. The US will be performed at all centers, and MRI of the pelvis (n=100) will be performed primarily at SKIMS, Srinagar, to compare US and standardization of US technique by 2 independent radiologists. MRI will be done as per a randomized selection proportional to the subjects at the site.
Laboratory Evaluation
The OGTT will be performed after an overnight fast of 10-12 hours. An oral glucose load with 75 g of anhydrous glucose dissolved in 250-350 mL of water will be administered over 3-5 minutes immediately after the basal sample. The blood samples for LH, FSH, testosterone, AMH, SHBG, and DHEAS will be collected on days 3-7 (early follicular phase) of spontaneous or medroxyprogesterone-induced (in amenorrhea patients) menstrual cycle to confirm PCOS. Hematological parameters (hemoglobin, red blood cells, white blood cells, etc), biochemical tests like lipid profile, liver function, kidney function, blood glucose (OGTT), and hormonal assays like T4, thyrotropin, LH, FSH, PRL, E2, P4, cortisol, testosterone, AMH, DHEAS, 17OHP, and insulin at 0 hours, 30 minutes, and 2 hours (optional) will be carried out at the respective centers, and special hormones like testosterone and SHBG will be done in the central facility to be created at the coordinating center (SKIMS, Srinagar). On the day of testing, women will be called after 10-12 hours of fast, and blood samples (15 ml) will be drawn. Blood will be also collected for glucose and insulin estimation after 30 minutes and 120 minutes of 75 g oral glucose challenge. Insulin samples will be immediately put in a refrigerator, separated in cold centrifuge, and stored at −20 °C until the assay. Plasma will be separated in a refrigerated centrifuge at 1560g for 10 minutes, and sera will be stored at −80 °C until tested.
Plasma glucose will be measured using the glucose oxidase–peroxidase method, and hormonal tests (LH, FSH, T4, thyrotropin, PRL, E2, cortisol, testosterone, AMH, hsCRP, cytokines, and 25OHD) will use ECLIA on Cobas e411 (Roche Diagnostics USA).
Methodology: Protocol for Substudy 3
The study will be conducted only at 2 centers: AIIMS, New Delhi, and SKIMS, Srinagar. Women (aged 18-40 years) residing in the communities (rural and urban) identified in the prevalence study and those attending outpatient clinics in the departments of either endocrinology, gynecology, or dermatology of the selected institutes who are willing to participate will be recruited in the interventional part of the study. All these women will undergo clinical, biochemical, and hormonal assessment at baseline and at 3 and 6 months (Figure 4).
Figure 4.
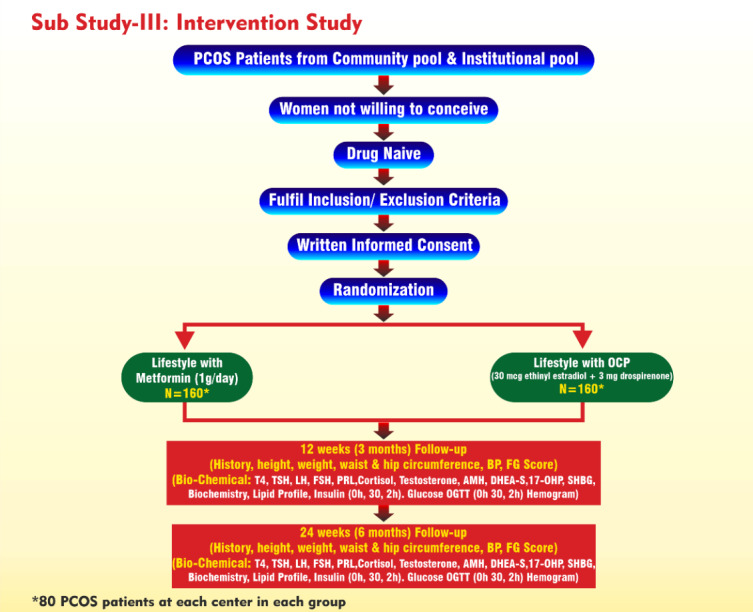
Study design and workflow chart of substudy 3. 17-OHP: 17-hydroxyprogesterone; AMH: anti-Müllerian hormone; BP: blood pressure; DHEA-S: dehydroepiandrosterone sulfate; FG: Ferriman-Gallwey; FSH: follicle-stimulating hormone; LH: luteinizing hormone; OCP: oral contraceptive pill; OGTT: oral glucose tolerance test; PCOS: polycystic ovary syndrome; PRL: prolactin; SHBG: sex hormone–binding globulin; T4: thyroxine; TSH: thyrotropin.
Sample Size Calculation for Substudy 3
On the basis of one study, metformin could improve the Homeostatic Model Assessment of IR from 2.5 (SD 0.39) to 2.79 (SD 0.39) [38]. Assuming that the baseline was similar, at an α error of 0.05 with power of 90%, and with a projected attrition rate of 20%, we need to recruit 153 women per group. To round off, we will recruit and randomize 160 women with PCOS per group (ie, a total of 320 women). A total of 160 subjects will be recruited and randomized in each of the 2 groups, with 80 subjects in each arm.
Randomization (Drug Intervention)
Eligible consenting subjects will be randomized in a 1:1 ratio into 2 arms through a blinding procedure.
Arm A compares lifestyle (32 kcal/kg diet per day with 55% carbohydrates, 20% protein, 20% fat, and 20 g of fiber with 30 minutes of brisk walking daily) and metformin (1 g /day) versus lifestyle alone (n=160; 80 per center).
Arm B compares lifestyle (32 kcal/kg diet/day with 55% carbohydrates, 20% protein, 20% fat, and 20 g of fiber with 30 minutes of brisk walking daily) and OCP (30 µg ethinyl estradiol plus 2 mg drospirenone) versus lifestyle alone (n=160; 80 per center).
Subject Selection
The subjects will be recruited from the community pool of substudy 1 and the institutional pool. The eligibility based on inclusion and the investigators will assess exclusion criteria. The institutional pool, which comprises the women attending endocrinology, gynecology, or other clinics for various complaints suggestive of PCOS, will be taken for purposes of recruitment in the intervention part of phase 1 or for further assessment in forming a long-term cohort. Potentially eligible patients will be recruited from the outpatient department services of the participating centers. If the subjects qualify on the inclusion and exclusion criteria, they will be informed about the details of the study and hence will be approached for written informed consent. All potentially eligible patients will undergo clinical, biochemical, and imaging (US) assessment to confirm PCOS. A logbook will be maintained for all patients undergo screening, randomized, eligible but not randomized along with reasons for non-randomization. For the intervention study (substudy 3), the subjects will be recruited only from AIIMS, New Delhi, and SKIMS, Srinagar. The sleep study and carotid Doppler at SKIMS and AIIMS, New Delhi, will be conducted for 100 subjects at each site.
The drug-naïve women who qualify on the 1990 NIH criteria (from both the institutional pool and the community pool) will be invited for the study. This will be an open-label randomized intervention trial and will follow a common protocol at each participating center. Women who do not desire fertility and who furnish written informed consent (second time) will undergo a detailed clinical assessment including details of menstrual, drug, and other relevant history followed by clinical examination involving anthropometry (height, weight, waist circumference); Ferriman-Gallwey scoring for hirsutism; grading of acanthosis nigricans, androgenic alopecia, and acne vulgaris (grades 1-3); blood pressure measurement; and a brief relevant systemic examination. Biochemical and hormone estimation will be done at baseline from the second day to the seventh day of the menstruation cycle and at 3 and 6 months. The subjects will be followed up at 6 months, and all these investigations will be repeated at the follow-up. A total of 320 women for the intervention phase (ie, 80 subjects per arm and per center) will be recruited. The study will be done only in 2 participating centers (SKIMS, Srinagar, and AIIMS, New Delhi). The intervention phase will be initially done for a period of 24 weeks.
Baseline Assessment
Standardized study tools will be used to record patient name, contact details, history, family history, personal information (age of menarche, menstrual regularity information, acne severity), food habits, lifestyle habits, previous disease information, drug intake history information, and clinical details including anthropometry (height, skinfold, waist circumference) by using anthropometric tools (SECA 213 height scale, SECA 813 weight scale, SECA 203 nonstretchable tape, and calipers measuring skinfold thickness), blood pressure (using Omron blood pressure instruments), Ferriman-Gallwey score, androgenic alopecia, acanthosis nigricans, acne vulgaris, and systemic examination. Measurement of biochemical parameters (blood glucose, OGTT, lipid profile, liver function, kidney function), protein:creatinine ratio, and 25OHD (in selected cases); hormonal assays (like T4, thyrotropin, LH, FSH, PRL, E2, P4—21st day optional, cortisol, testosterone, AMH, DHEAS, 17OHP, SHBG, and insulin); and radiological assessment will be done at baseline and after 6 months of treatment.
Follow-Up and Safety Evaluation
Patients will be evaluated for any adverse events and efficacy at 90 days (−7 days to +7 days) and 180 days (−7 days to +7 days) through follow-up. All clinical and biochemical investigation will be performed in a similar fashion as done at baseline. If the patient is not available for assessment at 180 days (−7 days to +7 days) follow-up, this will be considered a protocol deviation. The safety of different interventions will be assessed by recording the development of immediate or delayed complications recorded in a form with a prespecified list of safety parameters. Immediate reactions will include allergic reactions, diarrhea, vomiting, deep vein thrombosis, etc in a prespecified checklist.
Field Activities
Field activities will be conducted for the purposes of screening. It will include rapport building among locals and the selection of the study subjects (1 woman per household). It will be the duty of the research staff to approach the selected individuals and to make them understand their role in the study. Consenting individuals will be screened for PCOS phenotype using the predesigned structured questionnaire. The screening questionnaire will be used to obtain information on personal identification details, demographics, socioeconomic status, menstrual history and clinical information, history of any systemic disease, and QOL. The women who have hyperandrogenism or oligomenorrhea with or without obesity and acanthosis nigricans (IR) will be taken as probable cases of PCOS. Screen-positive and selected screen-negative (regular menstrual cycles, no features of hyperandrogenism or insulin resistance) individuals will be informed to visit the hospital for further investigations.
Statistical Analysis
Data will be collected on predesigned proformas. The data obtained will then be entered using pilot-tested CSPro software (version 6.2; United States Census Bureau). After assessing for approximate normal distribution, all continuous variables will be summarized as mean (SD) or median (IQR). Categorical variables will be expressed as n (%). Student t test and analysis of variance will be used to compare the groups. Statistical analysis will be performed using reliable SPSS statistical software (version 26; IBM Corp).
Results
The study was ethically approved and funded in November 2017 by ICMR, New Delhi, India. The subject recruitment from the community pool and implementation of the intervention for evaluating the treatment responses in a subset of PCOS subjects started in 2018 and is expected to be completed in October 2021. The study results will be compiled and statistically analyzed by April 2022 and will be published in a peer-reviewed scientific journal.
Discussion
Polycystic ovary syndrome is a multifactorial disorder with unknown etiology and numerous clinical manifestations in reproductive-aged women around the world [4-7]. The diagnostic criteria used to define PCOS include menstrual irregularity, hyperandrogenism, and polycystic ovary morphology. To date, PCOS has remained a major clinical challenge due to its poor prognosis, limited treatment options, and late diagnosis of the disease.
The prevalence of PCOS is increasing the world over and is showing a galloping increase in India. To date, there have been no systematic studies available either globally or in India. In India, there are undersized, convenience-based studies reported, which might not reflect the true status of PCOS prevalence in the community [32,33]. It is difficult to draw a clear conclusion from this limited number of studies conducted across the country. These studies hint at the potential for large-scale epidemiological studies on PCOS across India. This ongoing study will be the first of its kind in the country to generate the actual prevalence and picture of the disease in the community.
It is the first nationwide initiative by ICMR, New Delhi, to comprehensively study the prevalence, comorbidities (obesity, AGT, IR, NAFLD, sleep apnea, stroke, CVD, neuropsychiatric comorbidities), and phenotypes of PCOS in India using robust diagnostic methodology. On its completion, the study will generate an unprecedented amount of data about PCOS and the efficacy of interventions, which will facilitate the health care professionals, policy makers, and government of India to formulate new strategies that may shift the existing treatment paradigm in the near future.
Acknowledgments
We thank the ICMR, Monitoring Committee, and PCOS study group for their valuable suggestions and scientific inputs.
The ICMR–PCOS study group consists of MAG, SC, V Suri, NM, PKB, SA, BJ, RS, RR, PKJ, V Sreenivas, MS, IAW, SS, RSS, Abilash Nair, Zafar Amin Shah, Shariq Masoodi, Parvaiz Koul, Tabassum Parvez, Ghulam Nabi Yatoo, Naseer Ahmed, Tariq Gojwari, Muzafar Wani, Seema Qayoom, Wahid Khan, Rama Walia, Dipankar De, Tulika Singh, Neena Khanna, Nitish Naik, Shyam Prakash, Nandita Gupta, Rajesh Sagar, Jai Bhagwan Sharma, Devasenathipathy Kandasamy, Narendra Kuber Bodhey, Eli Mohapatra, Sabah Siddiqui, Gourisankar Kamilya, Archana Singh, Pradip Mukhopadhyay, Mousumi Mukhopadhyay, Nehar Ranjan Sarkar, Alice Abraham Ruram, Donboklang Lynser, Manika Agrawal, Ashok Anand, Shahina Begum, Anushree Patil, Shrabani Mukherjee, Hemangini Thakkar, Aruna Ramaiah, Lakshman Rao, Sudha Bindu, Malathi Ponnuru, Nadeem Ahmad, Sujatha Rani, Aleem Ahmed Khan, Sultan Rizwan Ahmed, Prabhakar Rao, Nirmala C, Anuja Elizabeth George, Jayasree Leelamma, Jayakumari, Aafia Rashid, Aarifa Mehraj, Rahul Harish, Mudasir Makhdoomi, Nowsheen Hamid, Shadab Wani, Paramjit Kaur, Neha Ravi, Ajay Kumar, Garima Rana, Siffali Chandrakar, Nithlesh Kumar, Sudipta Banerjee, Gazala Hasan, Nitin Deshpande, Himali Vadhan, Rita Parab, Balaji Bhasker, Shaik Iqubal Ahmed, Ram Babu, and Arya Suresh.
Abbreviations
- 17OHP
17-hydroxyprogesterone
- 25OHD
25-hydroxyvitamin D
- AE-PCOS
Androgen Excess and Polycystic Ovary Syndrome
- AGT
abnormal glucose tolerance
- AIIMS
All India Institute of Medical Sciences
- AMH
anti-Müllerian hormone
- BMI
body mass index
- CVD
cardiovascular diseases
- DHEAS
dehydroepiandrosterone sulfate
- E2
estradiol
- ECLIA
electrochemiluminescence assay
- FSH
follicle-stimulating hormone
- hsCRP
high-sensitivity C-reactive protein
- ICMR
Indian Council of Medical Research
- IR
insulin resistance
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NIH
National Institutes of Health
- OCP
oral contraceptive pill
- OGTT
oral glucose tolerance test
- P4
progesterone
- PCOS
polycystic ovary syndrome
- PRL
prolactin
- QOL
quality of life
- SHBG
sex hormone–binding globulin
- T4
thyroxine
- US
ultrasonography; ultrasound
ICMR-PCOS Study-Expert committee recommendations.
Footnotes
Authors' Contributions: MAG, national chief coordinator of the study, is the first author of the study protocol. MAG and RSS conceived the proposal. Coauthors SC, V Suri, BJ, PKB, SA, NM, RS, RR, PKJ, MS, IAW, and SS provided their expertise in the design of and contributed to finalize the study protocol and are involved in data capturing. V Sreenivas provided statistical assistance and sample size calculation. All authors revised the paper and approved the final version of the protocol.
Conflicts of Interest: None declared.
References
- 1.Vos T, Flaxman A, Naghavi M, Lozano Rafael, Michaud Catherine, Ezzati Majid, Shibuya Kenji, Salomon Joshua A, Abdalla Safa, Aboyans Victor, Abraham Jerry, Ackerman Ilana, Aggarwal Rakesh, Ahn Stephanie Y, Ali Mohammed K, Alvarado Miriam, Anderson H Ross, Anderson Laurie M, Andrews Kathryn G, Atkinson Charles, Baddour Larry M, Bahalim Adil N, Barker-Collo Suzanne, Barrero Lope H, Bartels David H, Basáñez Maria-Gloria, Baxter Amanda, Bell Michelle L, Benjamin Emelia J, Bennett Derrick, Bernabé Eduardo, Bhalla Kavi, Bhandari Bishal, Bikbov Boris, Bin Abdulhak Aref, Birbeck Gretchen, Black James A, Blencowe Hannah, Blore Jed D, Blyth Fiona, Bolliger Ian, Bonaventure Audrey, Boufous Soufiane, Bourne Rupert, Boussinesq Michel, Braithwaite Tasanee, Brayne Carol, Bridgett Lisa, Brooker Simon, Brooks Peter, Brugha Traolach S, Bryan-Hancock Claire, Bucello Chiara, Buchbinder Rachelle, Buckle Geoffrey, Budke Christine M, Burch Michael, Burney Peter, Burstein Roy, Calabria Bianca, Campbell Benjamin, Canter Charles E, Carabin Hélène, Carapetis Jonathan, Carmona Loreto, Cella Claudia, Charlson Fiona, Chen Honglei, Cheng Andrew Tai-Ann, Chou David, Chugh Sumeet S, Coffeng Luc E, Colan Steven D, Colquhoun Samantha, Colson K Ellicott, Condon John, Connor Myles D, Cooper Leslie T, Corriere Matthew, Cortinovis Monica, de Vaccaro Karen Courville, Couser William, Cowie Benjamin C, Criqui Michael H, Cross Marita, Dabhadkar Kaustubh C, Dahiya Manu, Dahodwala Nabila, Damsere-Derry James, Danaei Goodarz, Davis Adrian, De Leo Diego, Degenhardt Louisa, Dellavalle Robert, Delossantos Allyne, Denenberg Julie, Derrett Sarah, Des Jarlais Don C, Dharmaratne Samath D, Dherani Mukesh, Diaz-Torne Cesar, Dolk Helen, Dorsey E Ray, Driscoll Tim, Duber Herbert, Ebel Beth, Edmond Karen, Elbaz Alexis, Ali Suad Eltahir, Erskine Holly, Erwin Patricia J, Espindola Patricia, Ewoigbokhan Stalin E, Farzadfar Farshad, Feigin Valery, Felson David T, Ferrari Alize, Ferri Cleusa P, Fèvre Eric M, Finucane Mariel M, Flaxman Seth, Flood Louise, Foreman Kyle, Forouzanfar Mohammad H, Fowkes Francis Gerry R, Franklin Richard, Fransen Marlene, Freeman Michael K, Gabbe Belinda J, Gabriel Sherine E, Gakidou Emmanuela, Ganatra Hammad A, Garcia Bianca, Gaspari Flavio, Gillum Richard F, Gmel Gerhard, Gosselin Richard, Grainger Rebecca, Groeger Justina, Guillemin Francis, Gunnell David, Gupta Ramyani, Haagsma Juanita, Hagan Holly, Halasa Yara A, Hall Wayne, Haring Diana, Haro Josep Maria, Harrison James E, Havmoeller Rasmus, Hay Roderick J, Higashi Hideki, Hill Catherine, Hoen Bruno, Hoffman Howard, Hotez Peter J, Hoy Damian, Huang John J, Ibeanusi Sydney E, Jacobsen Kathryn H, James Spencer L, Jarvis Deborah, Jasrasaria Rashmi, Jayaraman Sudha, Johns Nicole, Jonas Jost B, Karthikeyan Ganesan, Kassebaum Nicholas, Kawakami Norito, Keren Andre, Khoo Jon-Paul, King Charles H, Knowlton Lisa Marie, Kobusingye Olive, Koranteng Adofo, Krishnamurthi Rita, Lalloo Ratilal, Laslett Laura L, Lathlean Tim, Leasher Janet L, Lee Yong Yi, Leigh James, Lim Stephen S, Limb Elizabeth, Lin John Kent, Lipnick Michael, Lipshultz Steven E, Liu Wei, Loane Maria, Ohno Summer Lockett, Lyons Ronan, Ma Jixiang, Mabweijano Jacqueline, MacIntyre Michael F, Malekzadeh Reza, Mallinger Leslie, Manivannan Sivabalan, Marcenes Wagner, March Lyn, Margolis David J, Marks Guy B, Marks Robin, Matsumori Akira, Matzopoulos Richard, Mayosi Bongani M, McAnulty John H, McDermott Mary M, McGill Neil, McGrath John, Medina-Mora Maria Elena, Meltzer Michele, Mensah George A, Merriman Tony R, Meyer Ana-Claire, Miglioli Valeria, Miller Matthew, Miller Ted R, Mitchell Philip B, Mocumbi Ana Olga, Moffitt Terrie E, Mokdad Ali A, Monasta Lorenzo, Montico Marcella, Moradi-Lakeh Maziar, Moran Andrew, Morawska Lidia, Mori Rintaro, Murdoch Michele E, Mwaniki Michael K, Naidoo Kovin, Nair M Nathan, Naldi Luigi, Narayan K M Venkat, Nelson Paul K, Nelson Robert G, Nevitt Michael C, Newton Charles R, Nolte Sandra, Norman Paul, Norman Rosana, O'Donnell Martin, O'Hanlon Simon, Olives Casey, Omer Saad B, Ortblad Katrina, Osborne Richard, Ozgediz Doruk, Page Andrew, Pahari Bishnu, Pandian Jeyaraj Durai, Rivero Andrea Panozo, Patten Scott B, Pearce Neil, Padilla Rogelio Perez, Perez-Ruiz Fernando, Perico Norberto, Pesudovs Konrad, Phillips David, Phillips Michael R, Pierce Kelsey, Pion Sébastien, Polanczyk Guilherme V, Polinder Suzanne, Pope C Arden, Popova Svetlana, Porrini Esteban, Pourmalek Farshad, Prince Martin, Pullan Rachel L, Ramaiah Kapa D, Ranganathan Dharani, Razavi Homie, Regan Mathilda, Rehm Jürgen T, Rein David B, Remuzzi Guiseppe, Richardson Kathryn, Rivara Frederick P, Roberts Thomas, Robinson Carolyn, De Leòn Felipe Rodriguez, Ronfani Luca, Room Robin, Rosenfeld Lisa C, Rushton Lesley, Sacco Ralph L, Saha Sukanta, Sampson Uchechukwu, Sanchez-Riera Lidia, Sanman Ella, Schwebel David C, Scott James Graham, Segui-Gomez Maria, Shahraz Saeid, Shepard Donald S, Shin Hwashin, Shivakoti Rupak, Singh David, Singh Gitanjali M, Singh Jasvinder A, Singleton Jessica, Sleet David A, Sliwa Karen, Smith Emma, Smith Jennifer L, Stapelberg Nicolas J C, Steer Andrew, Steiner Timothy, Stolk Wilma A, Stovner Lars Jacob, Sudfeld Christopher, Syed Sana, Tamburlini Giorgio, Tavakkoli Mohammad, Taylor Hugh R, Taylor Jennifer A, Taylor William J, Thomas Bernadette, Thomson W Murray, Thurston George D, Tleyjeh Imad M, Tonelli Marcello, Towbin Jeffrey A, Truelsen Thomas, Tsilimbaris Miltiadis K, Ubeda Clotilde, Undurraga Eduardo A, van der Werf Marieke J, van Os Jim, Vavilala Monica S, Venketasubramanian N, Wang Mengru, Wang Wenzhi, Watt Kerrianne, Weatherall David J, Weinstock Martin A, Weintraub Robert, Weisskopf Marc G, Weissman Myrna M, White Richard A, Whiteford Harvey, Wiersma Steven T, Wilkinson James D, Williams Hywel C, Williams Sean R M, Witt Emma, Wolfe Frederick, Woolf Anthony D, Wulf Sarah, Yeh Pon-Hsiu, Zaidi Anita K M, Zheng Zhi-Jie, Zonies David, Lopez Alan D, Murray Christopher J L, AlMazroa Mohammad A, Memish Ziad A. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2163–96. doi: 10.1016/S0140-6736(12)61729-2. http://europepmc.org/abstract/MED/23245607 .S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vidya Bharathi R, Swetha S, Neerajaa J, Varsha Madhavica J, Janani DM, Rekha S, S. R, B. U. An epidemiological survey: Effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertility Society Journal. 2017 Jun 07;22(4):313–316. doi: 10.1016/j.mefs.2017.05.007. [DOI] [Google Scholar]
- 3.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. American Journal of Obstetrics and Gynecology. 1935 Jan 01;29(2):181–191. doi: 10.1016/s0002-9378(15)30642-6. [DOI] [Google Scholar]
- 4.Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes. 2002 Jun 25;26(7):883–896. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 5.Legro RS, Driscoll D, Strauss JF, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998 Dec 08;95(25):14956–60. doi: 10.1073/pnas.95.25.14956. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=9843997 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman RJ. Obesity, polycystic ovary syndrome and anovulation--how are they interrelated? Curr Opin Obstet Gynecol. 2001 Jun;13(3):323–7. doi: 10.1097/00001703-200106000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz B, Yarali H, Oguz H, Bayraktar Miyase. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003 May;88(5):2031–6. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- 8.Westerveld H, Hoogendoorn M, de Jong A, Goverde A, Fauser B, Dallinga-Thie G. Cardiometabolic abnormalities in the polycystic ovary syndrome: pharmacotherapeutic insights. Pharmacol Ther. 2008 Sep;119(3):223–41. doi: 10.1016/j.pharmthera.2008.04.009.S0163-7258(08)00099-5 [DOI] [PubMed] [Google Scholar]
- 9.McGowan MP. Polycystic ovary syndrome: a common endocrine disorder and risk factor for vascular disease. Curr Treat Options Cardiovasc Med. 2011 Aug;13(4):289–301. doi: 10.1007/s11936-011-0130-0. [DOI] [PubMed] [Google Scholar]
- 10.Xita N, Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab. 2006 May;91(5):1660–6. doi: 10.1210/jc.2005-2757.jc.2005-2757 [DOI] [PubMed] [Google Scholar]
- 11.Gill H, Tiwari P, Dabadghao P. Prevalence of polycystic ovary syndrome in young women from North India: A Community-based study. Indian J Endocrinol Metab. 2012 Dec;16(Suppl 2):S389–92. doi: 10.4103/2230-8210.104104. http://europepmc.org/abstract/MED/23565440 .IJEM-16-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi B, Mukherjee S, Patil A, Purandare A, Chauhan S, Vaidya R. A cross-sectional study of polycystic ovarian syndrome among adolescent and young girls in Mumbai, India. Indian J Endocrinol Metab. 2014 May;18(3):317–24. doi: 10.4103/2230-8210.131162. http://www.ijem.in/article.asp?issn=2230-8210;year=2014;volume=18;issue=3;spage=317;epage=324;aulast=Joshi .IJEM-18-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005 Aug;90(8):4650–8. doi: 10.1210/jc.2005-0628.jc.2005-0628 [DOI] [PubMed] [Google Scholar]
- 14.Franks S, Gharani N, Waterworth D, Batty S, White D, Williamson R, McCarthy M. The genetic basis of polycystic ovary syndrome. Hum Reprod. 1997 Dec;12(12):2641–8. doi: 10.1093/humrep/12.12.2641. [DOI] [PubMed] [Google Scholar]
- 15.Roldán Belén, San Millán José L, Escobar-Morreale HF. Genetic basis of metabolic abnormalities in polycystic ovary syndrome: implications for therapy. Am J Pharmacogenomics. 2004;4(2):93–107. doi: 10.2165/00129785-200404020-00004.424 [DOI] [PubMed] [Google Scholar]
- 16.Escobar-Morreale Héctor F, Luque-Ramírez Manuel, San Millán José L. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev. 2005 Apr;26(2):251–82. doi: 10.1210/er.2004-0004.er.2004-0004 [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee S, Shaikh N, Khavale S, Shinde Gayatri, Meherji Pervin, Shah Nalini, Maitra Anurupa. Genetic variation in exon 17 of INSR is associated with insulin resistance and hyperandrogenemia among lean Indian women with polycystic ovary syndrome. Eur J Endocrinol. 2009 May;160(5):855–62. doi: 10.1530/EJE-08-0932.EJE-08-0932 [DOI] [PubMed] [Google Scholar]
- 18.Ganie MA, Marwaha RK, Dhingra A, Nisar S, Mani K, Masoodi S, Chakraborty S, Rashid A. Observation of phenotypic variation among Indian women with polycystic ovary syndrome (PCOS) from Delhi and Srinagar. Gynecol Endocrinol. 2016 Jul;32(7):566–70. doi: 10.3109/09513590.2016.1141879. [DOI] [PubMed] [Google Scholar]
- 19.Ganie MA, Marwaha RK, Nisar S, Farooqi KJ, Jan RA, Wani SA, Gojwari T, Shah ZA. Impact of hypovitaminosis D on clinical, hormonal and insulin sensitivity parameters in normal body mass index polycystic ovary syndrome women. J Obstet Gynaecol. 2016 May;36(4):508–12. doi: 10.3109/01443615.2015.1103715. [DOI] [PubMed] [Google Scholar]
- 20.Ganie M, Marwaha R, Aggarwal R, Singh Satveer. High prevalence of polycystic ovary syndrome characteristics in girls with euthyroid chronic lymphocytic thyroiditis: a case-control study. Eur J Endocrinol. 2010 Jun;162(6):1117–22. doi: 10.1530/EJE-09-1012.EJE-09-1012 [DOI] [PubMed] [Google Scholar]
- 21.Hussain A, Chandel RK, Ganie MA, Dar MA, Rather YH, Wani ZA, Shiekh JA, Shah MS. Prevalence of psychiatric disorders in patients with a diagnosis of polycystic ovary syndrome in kashmir. Indian J Psychol Med. 2015;37(1):66–70. doi: 10.4103/0253-7176.150822. http://europepmc.org/abstract/MED/25722515 .IJPsyM-37-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulshreshtha B, Ganie MA, Praveen EP, Gupta N, Lal Khurana M, Seith A, Dwivedi SN, Kumar G, Ammini AC. Insulin response to oral glucose in healthy, lean young women and patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008 Nov;24(11):637–43. doi: 10.1080/09513590802342858.905851238 [DOI] [PubMed] [Google Scholar]
- 23.Kulshreshtha B, Gupta N, Ganie MA, Ammini AC. Effect of metformin and spironolactone therapy on OGTT in patients with polycystic ovarian syndrome - a retrospective analysis. Gynecol Endocrinol. 2012 Oct;28(10):823–6. doi: 10.3109/09513590.2012.671394. [DOI] [PubMed] [Google Scholar]
- 24.Ganie MA, Nisar S, Shah P, Kuchay M, Bhat M, Rashid A, Ahmed S. Association of polycystic ovary syndrome and Graves′ disease: Is autoimmunity the link between the two diseases. Indian J Endocr Metab. 2012 Nov 31;16(6):982–986. doi: 10.4103/2230-8210.103006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shabir I, Ganie MA, Praveen EP, Khurana ML, John J, Gupta N, Kumar G, Ammini AC. Morning plasma cortisol is low among obese women with polycystic ovary syndrome. Gynecol Endocrinol. 2013 Dec;29(12):1045–7. doi: 10.3109/09513590.2013.829449. [DOI] [PubMed] [Google Scholar]
- 26.Shabir Iram, Ganie Mohd Ashraf, Zargar Mohd Afzal, Bhat Dilafroz, Mir Mohd Muzzafar, Jan Aleem, Shah Zaffar Amin, Jan Vicar, Rasool Riyaz, Naqati Andleeb. Prevalence of metabolic syndrome in the family members of women with polycystic ovary syndrome from North India. Indian J Endocrinol Metab. 2014 May;18(3):364–9. doi: 10.4103/2230-8210.131186. http://www.ijem.in/article.asp?issn=2230-8210;year=2014;volume=18;issue=3;spage=364;epage=369;aulast=Shabir .IJEM-18-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yousuf S, Ganie M, Zargar M, Amin Shajr Ul, Bhat Gulzar A, Masood Akbar, Rashid Fouzia. The Lys469glu/K469E Polymorphism of the Inflammatory Gene Intercellular Adhesion Molecule-1 Lacks any Apparent Role in the Polycystic Ovary Syndrome in Kashmiri Women: A Case Control Study. Asian Pac J Cancer Prev. 2017 Nov 26;18(11):2925–2930. doi: 10.22034/APJCP.2017.18.11.2925. http://journal.waocp.org/?sid=Entrez:PubMed&id=pmid:29172260&key=2017.18.11.2925 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yousuf SD, Ganie MA, Zargar MA, Parvez T, Rashid F. The ICAM-1 Gly241Arg Polymorphism is Not Associated With Polycystic Ovary Syndrome - Results from a Case Control study in Kashmir, India. Asian Pacific Journal of Cancer Prevention. 2016 Apr 11;17(3):1583–1588. doi: 10.7314/apjcp.2016.17.3.1583. [DOI] [PubMed] [Google Scholar]
- 29.Yousuf SD, Rashid F, Mattoo T, Shekhar C, Mudassar S, Zargar MA, Ganie MA. Does the Oral Contraceptive Pill Increase Plasma Intercellular Adhesion Molecule-1, Monocyte Chemoattractant Protein-1, and Tumor Necrosis Factor-α Levels in Women with Polycystic Ovary Syndrome: A Pilot Study. J Pediatr Adolesc Gynecol. 2017 Feb;30(1):58–62. doi: 10.1016/j.jpag.2016.06.010.S1083-3188(16)30091-2 [DOI] [PubMed] [Google Scholar]
- 30.Zargar AH, Gupta VK, Wani AI, Masoodi SR, Bashir MI, Laway BA, Ganie MA, Salahuddin M. Prevalence of ultrasonography proved polycystic ovaries in North Indian women with type 2 diabetes mellitus. Reprod Biol Endocrinol. 2005 Aug 11;3:35–42. doi: 10.1186/1477-7827-3-35. https://rbej.biomedcentral.com/articles/10.1186/1477-7827-3-35 .1477-7827-3-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattacharya S, Basu A, Biswas B. Obesity and selected inflammatory markers in polycystic ovary syndrome (PCOS): a BMI (body mass index) -matched case-control study. Fertility and Sterility. 2017 Sep 01;108(3):e249–e249. doi: 10.1016/j.fertnstert.2017.07.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganie M, Vasudevan V, Wani I, Baba M, Arif T, Rashid A. Indian J Med Res. 2019 Oct;150(4):333–344. doi: 10.4103/ijmr.IJMR_1937_17. http://www.ijmr.org.in/article.asp?issn=0971-5916;year=2019;volume=150;issue=4;spage=333;epage=344;aulast=Ganie .IndianJMedRes_2019_150_4_333_272092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinde KS, Patil SS. Incidence and risk factors of polycystic ovary syndrome among women in reproductive age group attending a tertiary health care hospital in Western Maharashtra. Int J Reprod Contracept Obstet Gynecol. 2019 Jun 29;8(7):2804–2809. doi: 10.18203/2320-1770.ijrcog20193046. [DOI] [Google Scholar]
- 34.Ganie Mohd Ashraf, Rashid Aafia, Sahu Danendra, Nisar Sobia, Wani Ishfaq A, Khan Junaida. Prevalence of polycystic ovary syndrome (PCOS) among reproductive age women from Kashmir valley: A cross-sectional study. Int J Gynaecol Obstet. 2020 May;149(2):231–236. doi: 10.1002/ijgo.13125. [DOI] [PubMed] [Google Scholar]
- 35.Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva: 2008. Dec, [2021-01-01]. https://www.who.int/publications/i/item/9789241501491 . [Google Scholar]
- 36.Misra A, Chowbey P, Makkar B M, Vikram N K, Wasir J S, Chadha D, Joshi Shashank R, Sadikot S, Gupta R, Gulati Seema, Munjal Y P, Concensus Group Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009 Feb;57:163–70. [PubMed] [Google Scholar]
- 37.Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981 Aug 01;140(7):815–30. doi: 10.1016/0002-9378(81)90746-8.0002-9378(81)90746-8 [DOI] [PubMed] [Google Scholar]
- 38.Palomba Stefano, Falbo Angela, Zullo Fulvio, Orio Francesco. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. 2009 Feb;30(1):1–50. doi: 10.1210/er.2008-0030.er.2008-0030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICMR-PCOS Study-Expert committee recommendations.


