Abstract
It is well-known that the P-acids including phosphonic acids resist undergoing direct esterification. However, it was found that a series of alkylphoshonic acids could be involved in monoesterification with C2–C4 alcohols under microwave (MW) irradiation in the presence of [bmim][BF4] as an additive. The selectivity amounted to 80–98%, while the isolated yields fell in the range of 61–79%. The method developed is a green method for P-acid esterification. DFT calculations at the M062X/6–311+G (d,p) level of theory (performed considering the solvent effect of the corresponding alcohol) explored the three-step mechanism, and justified a higher enthalpy of activation (160.6–194.1 kJ·mol−1) that may be overcome only by MW irradiation. The major role of the [bmim][BF4] additive is to increase the absorption of MW energy. The specific chemical role of the [BF4] anion of the ionic liquid in an alternative mechanism was also raised by the computations.
Keywords: alkylphosphonic acid, monoesterification, selectivity, microwave, ionic liquid, mechanism, energetics, theoretical calculations
1. Introduction
Dialkyl phosphonates and their derivatives may be important intermediates or starting materials in different reactions [1], they have importance in the pharmaceutical industry [2] and in biochemistry [3]. Phosphonates find application also in environmental chemistry [4] and as flame retardants [5]. The typical preparation of phosphonates (III) involves the reaction of aryl- or alkylphosphonic dichlorides (I) with alcohols or phenols, or the Arbuzov reaction of trialkyl phosphites (IV) with alkyl- or aryl halides (Scheme 1) [1,2].
Scheme 1.
Conventional synthesis of dialkyl phosphonates.
According to a newer approach, the syntheses were based on the direct esterification of the P(O)OH moiety of the P-acids. Among a series of phosphinic acids, phenyl-H-phosphinic acid (V) was also subjected to direct esterification under microwave (MW) conditions using [bmim][PF6] ionic liquid (IL) as the catalyst [6]. In the absence of an IL, the esterification was not so efficient [7]. The alkyl phenyl-H-phosphinates (VI) so obtained were oxidized by m-chloro-perbenzoic acid to the corresponding phosphonic ester-acids (VII) (Scheme 2) [7]. The MW-assisted direct esterification of ester-acids VII furnished the dialkyl phenylphosphonates (VIII) in lower yields of 25–62% [7]. A more appropriate protocol was, when phenylphosphonic acid (IX) was converted to the mono ester VII under MW irradiation and IL catalysis, and the ester-acid (VII) so obtained was converted to the diester (VIII) by alkylation (Scheme 2) [8].
Scheme 2.
Novel approaches to dialkyl phenylphosphonates.
The ionic liquids comprising a cation and an anion are regarded “green” solvents due to their inflammability, negligible vapor pressure (low volatility), and solvation power [9,10,11]. Ionic liquids are especially good solvents for metal complexes [12]. Although the primary function of ionic liquids is to serve as solvents, they find more and more applications as catalysts/additives [13].
In this article, we describe the MW-assisted monoesterification of alkylphosphonic acids. Beyond the preparative work, we also aimed at the investigation of the theoretical background of the target reaction.
2. Results and Discussion
2.1. Preparative Experiments on the Microwave-Assited Monoesterification of Alkylphosphonic Acids
The model reaction studied experimentally and theoretically is shown in Scheme 3. The monoesterification of the alkylphosphonic acids (1A–D) was performed using 15 equivalents of the alcohol and 10% of [bmim][BF4] under MW conditions. [Bmim][BF4] was selected on the basis of our earlier studies [8]. Formation of some of the diester (3A–D) was inevitable. The pure monoesters 2A–D/a–d could be obtained by converting them to the corresponding sodium salts by treatment with 10% aqueous NaOH, removing the diester (3A–D/a–d) by extraction with dichloromethane, and liberating the free acid by acidification with hydrochloric acid.
Scheme 3.
Monoesterification of alkylphosphonic acids (1A–D).
The results obtained with methylphosphonic acid (1A) are summarized in Table 1. Due to the volatility and taking into account the pressure limit of the MW reactor, the esterification with EtOH had to be carried out at 160 °C. The almost complete conversion was attained after an irradiation of 5 h. The ratio of the mono and the diester (2Aa and 3Aa) was 94–2 (Table 1/Entry 1). The reactivity of PrOH and iPrOH was significantly different, after a reaction at 180 °C for 3 h, the conversion was 97% and 77%, respectively, while the proportion of the mono and diesters (2Ab/2Ac and 3Ab/3Ac) was 78–19 and 74–3, respectively (Table 1/Entries 2 and 3). Using iPrOH, the conversion was complete after 5 h (Table 1/Entry 4). Employing BuOH, the appropriate reaction conditions were either 180 °C/3 h or 200 °C/1.25 h. In these cases, the ratio of 2Ad and 3Ad was ca. 81:19 (Table 1/Entries 5 and 6). On the effect of prolonged heating, the ratio of the diester 3Ad increased to 55% (Table 1/Entry 7). It is noteworthy that a control experiment carried out in the lack of [bmim][BF4] took place in a conversion of 19% (Table 1/Entry 6, footnote “b”). Hence, the role of IL is unambiguous. In another comparative experiment performed on conventional heating without IL, the conversion was 10% (Table 1/Entry 6, footnote “c”). This proves that both MW and IL are needed for efficient esterification.
Table 1.
Direct esterification of methylphosphonic acid (1A) in the presence of [bmim][BF4] under MW conditions.

| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Temperature (°C) |
Time (h) |
Conversion a (%) |
Monoester a (2A) (%) |
Diester a (3A) (%) |
Yield (%) |
| 1 | Et (a) | 160 | 5 | 96 | 94 | 2 | 78 |
| 2 | Pr (b) | 180 | 3 | 97 | 78 | 19 | 62 |
| 3 | iPr (c) | 180 | 3 | 77 | 74 | 3 | – |
| 4 | iPr (c) | 180 | 5 | 100 | 98 | 2 | 75 |
| 5 | Bu (d) | 180 | 3 | 100 | 81 | 19 | 66 |
| 6 | Bu (d) | 200 b,c | 1.25 | 99 | 81 | 18 | 65 |
| 7 | Bu (d) | 200 | 2 | 100 | 45 | 55 | – |
a Conversion and product composition was determined on the basis of relative 31P NMR intensities; b The control experiment carried out in the absence of [bmim][BF4] took place in a conversion of 19% affording only the monoester 2Ad (19%); c The comparative thermal experiment performed in the lack of the IL proceeded to a conversion of 10%.
The results of the direct esterification of ethylphosphonic acid 1B can be found in Table 2. Using EtOH at 160 °C, completion required > 6 h. After an irradiation of 6 h, the conversion was 95%, and the ratio of the mono and the diester (2Ba and 3Ba) was 87:8 (Table 2/Entry 1). The esterification with PrOH and iPrOH at 180 °C was almost complete after 3.5 h and 7 h, respectively, leading to ester mixtures (2Bb–3Bb and 2Bc–3Bc) 79:16 and 91:7, respectively (Table 2/Entries 2 and 3). Using BuOH the completion took >4 h at 180 °C. After an irradiation of 3.5 h, the ratio of the mono and the diester (2Bd and 3Bd) was 89:7 (Table 2/Entry 4). Performing the esterification at a somewhat higher temperature of 200 °C, the ratio of 2Bd and 3Bd was 79:20 (Table 2/Entry 5).
Table 2.
Direct esterification of ethylphosphonic acid (1B) in the presence of [bmim][BF4] on MW irradiation.

| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Temperature (°C) |
Time (h) |
Conversion a (%) |
Monoester a (2B) (%) |
Diester a (3B) (%) |
Yield (%) |
| 1 | Et (a) | 160 | 6 | 95 | 87 | 8 | 71 |
| 2 | Pr (b) | 180 | 3.5 | 95 | 79 | 16 | 63 |
| 3 | iPr (c) | 180 | 7 | 98 | 91 | 7 | 72 |
| 4 | Bu (d) | 180 | 4 | 96 | 89 | 7 | 69 |
| 5 | Bu (d) | 200 | 2 | 99 | 79 | 20 | 61 |
a Conversion and product composition was determined on the basis of relative 31P NMR intensities.
The results obtained during the esterification of propylphosphonic acid (1C) are listed in Table 3. One can see that the data on the reaction times, conversions, and monoester—diester ratios are rather similar to those obtained for the esterification of ethylphosphonic acid (1B). This means that the reactivity of the ethyl- and propylphosphonic acid is rather similar in the monoesterification under discussion.
Table 3.
Reaction of propylphosphonic acid (1C) in the presence of [bmim][BF4] on MW irradiation.

| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Temperature (°C) |
Time (h) |
Conversion a (%) |
Monoester a (2C) (%) |
Diester a (3C) (%) |
Yield (%) |
| 1 | Et (a) | 160 | 6 | 99 | 90 | 9 | 79 |
| 2 | Pr (b) | 180 | 3.5 | 94 | 82 | 12 | 65 |
| 3 | iPr (c) | 180 | 7 | 98 | 94 | 4 | 77 |
| 4 | Bu (d) | 180 | 5 | 98 | 83 | 15 | 67 |
| 5 | Bu (d) | 200 | 2 | 97 | 83 | 14 | 65 |
a Conversion and product composition was determined on the basis of relative 31P NMR intensities.
As can be seen from Table 4, butylphosphonic acid (1D) was the less reactive in the series. The esterification with EtOH at 165 °C was not even complete after 7 h (Table 4/Entry 1). Applying PrOH and iPrOH at 200 °C, the reaction time was 4 h, and, as extrapolated, 10 h, respectively (Table 4/Entries 3 and 4). With BuOH, the esterification required 200 °C/3 h.
Table 4.
Direct esterification of butylphosphonic acid (1D) in the presence of [bmim][BF4] on MW irradiation.

| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Temperature (°C) |
Time (h) |
Conversion a (%) |
Monoester a (2D) (%) |
Diester a (3D) (%) |
Yield (%) |
| 1 | Et (a) | 165 | 7 | 70 | 67 | 3 | 51 |
| 2 | Pr (b) | 200 | 4 | 99 | 82 | 17 | 66 |
| 3 | iPr (c) | 200 | 7 b | 77 | 71 | 6 | 60 |
| 4 | Bu (d) | 200 | 3 | 100 | 87 | 13 | 70 |
a Conversion and product composition was determined on the basis of relative 31P NMR intensities; b Extrapolated reaction time: 10 h.
The ester-acid species 2A–D/a–d were isolated from the best mixtures by treatment with NaOH/H2O, extraction with dichloromethane, and liberating the ester-acid (2A–D/a–d) with hydrochloric acid. A final extraction with dichloromethane furnished the pure monoesters that were identified by 31P NMR chemical shifts and HRMS (See Experimental, Table 7). From among the 16 ester-acids, 9 were described earlier, and were identified by us by 31P NMR shifts [14,15,16,17]. The remaining 7 ester-acids (2Bb, 2Bd, 2Cb, 2Da, 2Db, 2Dc, and 2Dd) were also characterized by us by 1H and 13C NMR spectral data (see Supplementary Materials). The minor components, dialkyl alkylphosphonates 3A–D/a–d were identified by 31P NMR and HRMS (See Experimental, Table 8).
The synthetic method developed for the selective monoesterification of phosphonic acids (1) is green, as it avoids the application of P-chlorides.
2.2. Theoretical Calculations on the Direct Esterification of Alkylphosphonic Acids
We have analyzed the energetics of the direct esterification of phosphonic acids (1, R = Me, Et, Bu) with alcohols (MeOH, BuOH) using DFT computations at the M062X/6–311+G (d,p) level of theory considering the solvent effect of the corresponding alcohol (Scheme 4, Table 5). Based on our previous model [18], we proposed a reaction complex containing three alcohol reagents and two phosphonic acids, where one alcohol molecule acts as the reagent in the monoesterification of one of the phosphonic acid units. The other P- and ROH species in the reaction complex are responsible for the proton transfer chain that promotes the formation of the new P-O bond and the departure of an H2O molecule. The formation of the reaction complex (4) is highly exothermic marked by a ΔH of (−123)–(−146.6) kJ·mol−1, but the significant decrease in entropy (ΔS) by the assessment of five molecules results in an increased Gibbs free energy value (ΔG = 46.0–66.0 kJ·mol−1). The first transition state (TS1) belongs to the addition of the reacting alcohol onto the P atom of the P=O function resulting in intermediate 5. This reaction is moderately endothermic with an activation enthalpy requirement of 70.7–94.5 kJ·mol−1. The second step is the removal of a hydroxy group from species 5 that is realized via dehydration taking a proton from a nearby OH unit. Notably, the second phosphonic acid unit remains intact and acts only as a proton transfer additive. Interestingly, although the intermediate following TS1 has a higher enthalpy, a similar or slightly lower Gibbs free energy value may suggest a slight stabilization after the transition. The enthalpy of activation requirement for TS2 belonging to the dehydration is, as compared to the starting level [19], 160.6–194.1 kJ·mol−1. The Gibbs free energy values are also over 150 kJ·mol−1. The higher barrier may be overcome at a higher temperature of 180–200 °C utilizing MW assistance [20,21]. Finally, the product complex (6) breaks up, and the liberation of the product (2) is slightly exothermic as compared to the enthalpy level of the starting reactants (meaning an enthalpy gain of 8.4–15.8 kJ·mol−1).
Scheme 4.
Mechanism for the direct monoesterification of alkylposphonic acids (1) with alcohols obtained by DFT computations at the M062X/6–311+G (d,p) level of theory considering the solvent effect of the corresponding alcohol.
Table 5.
Energetics for the monoesterification of alkylphosphonic acids (1) obtained by DFT computations at the M062X/6–311+G (d,p) level of theory considering the solvent effect of the corresponding alcohol.
| 1 | 4 | TS1 | 5 | TS2 | 6 | 2 | ||
|---|---|---|---|---|---|---|---|---|
| R1 = Me, R2 = Me |
ΔH (kJ·mol−1) | 0.0 | −123.0 | −52.3 | −47.2 | 49.1 | −116.8 | −10.6 |
| ΔG (kJ·mol−1) | 0.0 | 66.0 | 154.3 | 151.6 | 245.2 | 63.2 | −5.5 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 68.6 | 54.9 | 61.1 | 63.3 | 76.0 | 49.7 | |
| R1 = Me, R2 = Bu |
ΔH (kJ·mol−1) | 0.0 | −146.6 | −52.1 | −48.6 | 47.5 | −120.3 | −15.8 |
| ΔG (kJ·mol−1) | 0.0 | 49.8 | 156.5 | 156.4 | 250.5 | 68.4 | −5.1 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 70.9 | 61.2 | 63.8 | 65.6 | 76.9 | 50.4 | |
| R1 = Et, R2 = Bu |
ΔH (kJ·mol−1) | 0.0 | −141. 7 | −47.9 | −42.1 | 18.9 | −122.3 | −13.2 |
| ΔG (kJ·mol−1) | 0.0 | 62.3 | 169.4 | 168.4 | 223.8 | 74.1 | −9.1 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 67.1 | 56.6 | 62.1 | 66.6 | 73.4 | 56.6 | |
| R1 = Bu, R2 = Bu |
ΔH (kJ·mol−1) | 0.0 | −138.5 | −42.4 | −39.1 | 36.7 | −117.1 | −8.4 |
| ΔG (kJ·mol−1) | 0.0 | 48.2 | 163.8 | 158.3 | 230.7 | 63.3 | −5.7 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 85.3 | 70.0 | 76.9 | 79.6 | 90.4 | 58.6 | |
| R1 = Ph, R2 = Me |
ΔH (kJ·mol−1) | 0.0 | −144.1 | −64.5 | −58.4 | 47.7 | −120.6 | −12.8 |
| ΔG (kJ·mol−1) | 0.0 | 46.0 | 141.8 | 138.4 | 256.6 | 58.4 | −10.2 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 75.2 | 62.3 | 69.9 | 50.2 | 84.0 | 52.4 |
The question is raised, what the role of the IL may be? Well, it is assumed that similar to earlier cases, the role of [bmim][BF4] is to increase the MW absorbing ability of the medium [22,23].
It is worth noting that according to the energetics, the esterification of phenylphosphonic acid that was studied earlier [8] goes with similar enthalpy, energy, and entropy changes (see the last row of Table 5) as the alkylphosphonic acids (1), meaning that the reactivity of the phosphonic acids in monoesterification is not influenced much by the nature of the substituent.
The enthalpy diagram for the monoesterification of ethylphosphonic acid (1B) with butanol is shown in Figure 1. The gross enthalpy of activation is 160.6 kJ·mol−1.
Figure 1.
Enthalpy diagram for the direct monoesterification of ethylphosphonic acid (1B) with BuOH.
Next, the [bmim][BF4]-promoted esterification was studied for a few alkylphosphonic acid–alcohol combinations (Scheme 5, Table 6). Similar to the additive-free way, the reaction goes through a multicomponent complex (7) containing the phosphonic acid, the BF4− anion, and two alcohol molecules. The formation of this complex (7) is exothermic regarding enthalpy [the gain is (−56.8)–(−63.4) kJ·mol−1], but considering the Gibbs free energy values, again an increase may be observed. The esterification, in this case, is a single-step process involving a rate-determining transition state (TS3) with a somewhat lower ΔH value (162.4–171.0 kJ·mol−1) as compared to the other pathway shown in Scheme 4. This leads to a product complex (8) that results in the formation of the monoesterified product (2) after decomplexation.
Scheme 5.
Mechanism for the [bmim][BF4]–promoted monoesterification of alkylphosphonic acids (1) with alcohols obtained by DFT computations at the M062X/6–311+G (d,p) level of theory considering the solvent effect of the corresponding alcohol.
Table 6.
Energetics for the [bmim][BF4]-promoted esterification of alkylphosphonic acids (1) with alcohols obtained by DFT computations at the M062X/6–311+G (d,p) level of theory considering the solvent effect of the corresponding alcohol.
| 1 | 7 | TS3 | 8 | 2 | ||
|---|---|---|---|---|---|---|
| R1 = Me, R2 = Me |
ΔH (kJ·mol−1) | 0.0 | −63.4 | 105.8 | −65.7 | −10.6 |
| ΔG (kJ·mol−1) | 0.0 | 69.2 | 248.7 | 58.9 | −5.5 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 59.3 | 51.0 | 65.9 | 49.7 | |
| R1 = Me, R2 = Bu |
ΔH (kJ·mol−1) | 0.0 | −58.5 | 103.9 | −66.6 | −15.8 |
| ΔG (kJ·mol−1) | 0.0 | 80.2 | 253.5 | 63.2 | −5.1 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 60.2 | 50.4 | 70.2 | 56.6 | |
| R1 = Et, R2 = Bu |
ΔH (kJ·mol−1) | 0.0 | −56.8 | 114.2 | −45.8 | −13.2 |
| ΔG (kJ·mol−1) | 0.0 | 85.6 | 268.9 | 84.0 | −9.1 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 76.2 | 67.3 | 82.9 | 50.4 |
The enthalpy diagram for the [bmim][BF4]-promoted monoesterification of ethylphosphonic acid (1B) with butanol is shown in Figure 2. The enthalpy of activation is 171.0 kJ·mol−1 that is comparable with that of the gross value (160.6 kJ·mol−1) of the other mechanism (Figure 1).
Figure 2.
Enthalpy diagram for the monoesterification of ethylphosphonic acid (1B) with BuOH in the presence of [bmim][BF4].
Analyzing the datasets computed for the direct esterification and BF4-promoted version, one might conclude that according to the calculations, at least at this level, there is no significant discrimination among the P-substituents or among the alcohols. However, when comparing the two mechanistic pathways, one can see that while the direct way (A) is a two-step process, the ionic-liquid-promoted version (B) involves just one step. Another difference is that the formation of the primary reaction complexes (4 in “A” and 7 in “B”) needs the association of five and four molecules, respectively. So, route B seems to be simpler. However, the decisive factor may be that the enthalpy of activation values are comparable for the two kinds of mechanisms (A and B): for the selected examples represented in Figure 1 and Figure 2, the enthalpy of activation value is 160.6 kJ·mol−1 and 171.0 kJ·mol−1, respectively. These high values may be overcome by MWs. The final message is that in the cases studied, both mechanisms (A and B) may be operative. The role of ionic liquid may be merely to increase MW absorption (as in earlier cases [20,21]), but it is also possible that the BF4 anion of the ionic liquid participates chemically, and hence promotes the esterification.
In summary, an MW-assisted, IL-promoted method was elaborated for the selective monoesterification of alkylphosphonic acids with simple alcohols. This is a green method, as avoids the use of P-chlorides. The mechanism explored by high-level theoretical calculations suggested the formation of a ring associate comprising two phosphonic acid molecules and three alcohol units, the nucleophilic attack of the alcohol on the P atom of the P=O moiety, and dehydration exhibiting a gross enthalpy of activation of 160.6–194.1 kJ·mol−1. The high barrier could be overcome by the beneficial effect of MWs. A [bmim][BF4] additive ensured the efficient absorption of MWs. An alternative mechanism involving the participation of the BF4 anion of the ionic liquid was also substantiated, but the activation energy requirement of this option was also high (162.4–171.0 kJ·mol−1).
3. Experimental
3.1. General
The 31P, 13C, and 1H-NMR spectra were taken on a Bruker DRX-500 spectrometer operating at 202.4, 125.7, and 500 MHz, respectively. The couplings are given in Hz. LC-MS measurements were performed with an Agilent 1200 liquid chromatography system coupled with a 6130 quadrupole mass spectrometer equipped with an ESI ion source (Agilent Technologies, Palo Alto, CA, USA).
3.2. Use of the 31P NMR Spectra in Quantitative Analysis
The composition of the reaction mixture was determined by the integration of the areas under the corresponding peaks of the starting material and product in the 31P NMR spectra.
3.3. General Procedure for the Direct Esterification of Akylphosphonic Acids in the Presence of Ionic Liquids
A mixture of 1.44 mmol of alkylphosphonic acid (methylphosphonic acid: 0.14 g, ethylphosphonic acid: 0.16 g, propylphosphonic acid: 0.18 g, butylphosphonic acid: 0.20 g), 21.72 mmol of alcohol (1.28 mL of ethanol, 1.62 mL of propanol, 1.68 mL of i-propanol, 2.0 mL of butanol) and 0.144 mmol (27 µL) [bmim][BF4] was stirred under MW conditions (max 100 W). After evaporation and flash column chromatography (silica gel, DCM–MeOH 97:3), the reaction mixture was analyzed by 31P NMR spectroscopy. The crude products of the best experiments were purified by extraction. The residue obtained after evaporation was taken up in 5 mL of CH2Cl2, and the solution was stirred with a mixture of 0.60 mL of 10% NaOH/H2O. The phases were separated, the water phase was acidified with 0.14 mL of 37% hydrochloric acid, and stirred with 5 mL of CH2Cl2. The organic phase was dried (Na2SO4) and its concentration afforded the corresponding ester-acids (2A–D) as colorless oils.
Identification data of ester acids 2A–D/a–d and diesters 3A–D/a–d are listed in Table 7 and Table 8, respectively.
Table 7.
Identification of the ester-acids (2A–D/a–d).
| Compound | δP[found] | δP[lit] | HRMS | |||
|---|---|---|---|---|---|---|
| [M + Na]+found | Formula | [M + Na]+calculated | ||||

|
(2Aa) | 32.8 (CDCl3) | 32.5 [14] (CDCl3) | 147.0190 | C3H9O3PNa | 147.0187 |

|
(2Ab) | 32.6 (CDCl3) | 32.8 [14] (CDCl3) | 161.0341 | C4H11O3PNa | 161.0344 |

|
(2Ac) | 32.5 (CDCl3) | 33.8 [14] (CDCl3) | 161.0343 | C4H11O3PNa | 161.0344 |

|
(2Ad) | 32.6 (CDCl3) | 33.9 [14] (CDCl3) | 175.0504 | C5H13O3PNa | 175.0500 |

|
(2Ba) | 36.3 (CDCl3) | 37.5 [15] (CDCl3) | 161.0341 | C4H11O3PNa | 161.0344 |

|
(2Bb) | 36.1 (CDCl3) | – | 175.0500 | C5H13O3PNa | 175.0500 |
|
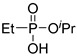
(2Bc) | 35.4 (CDCl3) | 33.6 [16] (CD3OD) | 175.0505 | C5H13O3PNa | 175.0500 |

|
(2Bd) | 35.1 (CDCl3) | – | 163.0835 a | C6H16O3P | 163.0837 |

|
(2Ca) | 35.0 (CDCl3) | 34.1 [16] (CD3OD) | 175.0501 | C5H13O3PNa | 175.0500 |

|
(2Cb) | 34.9 (CDCl3) | – | 189.0655 | C6H15O3PNar | 189.0657 |

|
(2Cc) | 34.4 (CDCl3) | 34.2 b [17] | 189.0658 | C6H15O3PNa | 189.0657 |

|
(2Cd) | 35.2 (CDCl3) | 33.8 [16] (CD3OD) | 203.0815 | C7H17O3PNa | 203.0813 |

|
(2Da) | 35.2 (CDCl3) | – | 189.0662 | C6H15O3PNa | 189.0657 |
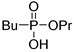
|
(2Db) | 35.4 (CDCl3) | – | 203.0815 | C7H17O3PNa | 203.0813 |

|
(2Dc) | 34.4 (CDCl3) | – | 203.0815 | C7H17O3PNa | 203.0813 |

|
(2Dd) | 35.6 (CDCl3) | – | 217.0971 | C8H19O3PNa | 217.0970 |
a Identified as [M + H]+; b No solvent was provided.
Table 8.
Identification of the diesters (3A–d/a–d).
| Compound | δP[found] | δP[lit] | HRMS | |||
|---|---|---|---|---|---|---|
| [M + Na]+found | Formula | [M + Na]+calculated | ||||

|
(3Aa) | 30.1 (DMSO) | 31.1 a [24] | 175.0499 | C5H13O3PNa | 175.0500 |

|
(3Ab) | 30.2 (DMSO) | 30.8 a [24] | 203.0811 | C7H17O3PNa | 203.0813 |

|
(3Ac) | 28.2 (DMSO) | 30.9 a [24] | 181 b | C7H18O3P | 181 |

|
(3Ad) | 30.2 (DMSO) | 30.1 a [24] | 231.1132 | C9H21O3PNa | 231.1126 |
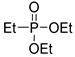
|
(3Ba) | 33.1 (DMSO) | 34.3 a [24] | 189.0655 | C6H15O3PNa | 189.0657 |

|
(3Bb) | 33.1 (DMSO) | 33.0 a [24] | 217.0966 | C8H19O3PNa | 217.0970 |

|
(3Bc) | 31.2 (DMSO) | 32.9 a [24] | 195 b | C8H20O3P | 195 |

|
(3Bd) | 33.1 (DMSO) | 31.5 a [24] | 245.1279 | C10H23O3PNa | 245.1283 |

|
(3Ca) | 31.7 (DMSO) | 31.8 a [24] | 203.0815 | C7H17O3PNa | 203.0813 |
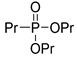
|
(3Cb) | 31.1 (DMSO) | 31.1 a [24] | 231.1126 | C9H21O3PNa | 231.1126 |

|
(3Cc) | 29.8 (DMSO) | 30.9 a [24] | 209 b | C9H22O3P | 209 |

|
(3Cd) | 31.7 (DMSO) | 30.7 a [24] | 259.1440 | C11H25O3PNa | 259.1439 |

|
(3Da) | 32.1 (DMSO) | 33.5 [25] (CDCl3) | 217.0963 | C8H19O3PNa | 217.0970 |
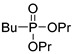
|
(3Db) | 32.0 (DMSO) | – | 245.1282 | C10H23O3PNa | 245.1283 |

|
(3Dc) | 30.1 (DMSO) | – | 245.1283 | C10H23O3PNa | 245.1283 |

|
(3Dd) | 32.1 (DMSO) | 33.1 [26] (CDCl3) | 273.1598 | C12H27O3PNa | 273.1596 |
a No solvent was provided; b Identified as [M + H]+.
3.4. Additional Spectral Data for the New Ester-Acids
3.4.1. Monopropyl Ethylphosphonate (2Bb)
13C NMR (CDCl3) Δ: 6.3 (d, 2JP,C = 6.7, PCH2CH3), 10.0 (s, OCH2CH2CH3), 19.0 (d, 1JP,C = 145.1, PCH2), 23.7 (d, 3JP,C = 6.1, OCH2CH2), 66.5 (d, 2JP,C = 6.9, OCH2); 1H NMR (CDCl3) Δ: 0.96 (t, J = 7.4, 3H, CH3), 1.14–1.21 (m, 3H, CH3), 1.66–1.80 (m, 4H, PCH2, CH2), 3.98 (q, J = 6.8, 2H, OCH2), 9.62 (s, 1H, OH).
3.4.2. Monobutyl Ethylphosphonate (2Bd)
13C NMR (CDCl3) Δ: 6.4 (d, 2JP,C = 6.7, PCH2CH3), 13.6 (s, OCH2CH2CH2CH3), 18.7 (s, OCH2CH2CH2), 19.0 (d, 1JP,C = 145.0, PCH2), 32.5 (d, 3JP,C = 6.0, OCH2CH2), 64.7 (d, 2JP,C = 6.8, OCH2); 1H NMR (CDCl3) Δ: 0.94 (t, J = 7.3, 3H, CH3), 1.13–1.21 (m, 3H, CH3), 1.37–1.45 (m, 2H, CH2), 1.63–1.79 (m, 4H, PCH2, CH2), 4.02 (q, J = 6.7, 2H, OCH2), 8.32 (s, 1H, OH).
3.4.3. Monopropyl Propylphosphonate (2Cb)
13C NMR (CDCl3) Δ: 10.0 (s, OCH2CH2CH3), 15.2 (d, 3JP,C = 17.3, PCH2CH2CH3), 16.0 (d, 2JP,C = 4.8, PCH2CH2), 23.8 (d, 3JP,C = 6.0, OCH2CH2), 27.9 (d, 1JP,C = 142.7, PCH2), 66.4 (d, 2JP,C = 6.8, OCH2); 1H NMR (CDCl3) Δ: 0.96 (t, J = 7.3, 3H, CH3), 1.03 (t, J = 7.1, 3H, CH3), 1.64–1.77 (m, 6H, PCH2, 2 CH2), 3.97 (q, J = 6.6, 2H, OCH2), 7.96 (s, 1H, OH).
3.4.4. Monoethyl Butylphosphonate (2Da)
13C NMR (CDCl3) Δ: 13.5 (s, PCH2CH2CH2CH3) 16.3 (s, OCH2CH3), 23.6 (d, 2JP,C = 16.8, PCH2CH2CH2), 24.3 (s, PCH2CH2), 25.6 (d, 1JP,C = 143.0, PCH2), 61.0 (d, 2JP,C = 5.1, OCH2); 1H NMR (CDCl3) Δ: 0.92 (t, J = 7.3, 3H, CH3), 1.33 (t, J = 6.7, 3H, CH3), 1.38–1.45 (m, 2H, CH2), 1.57–1.64 (m, 2H, CH2), 1.71–1.78 (m, 2H, PCH2), 4.06–4.12 (m, J = 7.3, 2H, OCH2), 10.08 (s, 1H, OH).
3.4.5. Monopropyl Butylphosphonate (2Db)
13C NMR (CDCl3) Δ: 10.0 (s, OCH2CH2CH3), 13.6 (s, PCH2CH2CH2CH3) 23.6 (d, 3JP,C = 17.6, PCH2CH2CH2), 23.8 (d, 2JP,C = 5.9, OCH2CH2), 24.2 (d, 3JP,C = 4.8, PCH2CH2), 25.5 (d, 1JP,C = 143.8, PCH2), 66.4 (d, 2JP,C = 6.8, OCH2); 1H NMR (CDCl3) Δ: 0.92 (t, J = 7.3, 3H, CH3), 0.96 (t, J = 7.4, 3H, CH3), 1.38–1.45 (m, 2H, CH2), 1.56–1.64 (m, 2H, CH2), 1.66–1.78 (m, 4H, PCH2, CH2), 3.97 (q, J = 6.8, 2H, OCH2), 9.62 (s, 1H, OH).
3.4.6. Monoisopropyl Butylphosphonate (2Dc)
13C NMR (CDCl3) Δ: 13.6 (s, PCH2CH2CH2CH3) 23.7 (d, 3JP,C = 17.4, PCH2CH2CH2), 24.0 (d, 2JP,C = 3.1, OCH(CH3)2), 24.4 (d, 3JP,C = 2.9, PCH2CH2), 26.1 (d, 1JP,C = 143.8, PCH2), 70.0 (d, 2JP,C = 6.0, OCH2); 1H NMR (CDCl3) Δ: 0.91 (t, J = 7.3, 3H, CH3), 1.33 (d, J = 6.0, 6H, 2 CH3), 1.37–1.45 (m, 2H, CH2), 1.56–1.62 (m, 2H, CH2), 1.69–1.76 (m, 2H, PCH2), 4.65–4.72 (m, J = 6.5, 1H, OCH), 10.46 (s, 1H, OH).
3.4.7. Monobutyl Butylphosphonate (2Dd)
13C NMR (CDCl3) Δ: 13.6 (s, OCH2CH2CH2CH3), 13.6 (s, PCH2CH2CH2CH3), 18.8 (s, OCH2CH2CH2) 23.7 (d, 3JP,C = 17.5, PCH2CH2CH2), 24.3 (d, 2JP,C = 4.5, PCH2CH2), 25.6 (d, 1JP,C = 143.2, PCH2), 32.5 (d, 3JP,C = 5.9, OCH2CH2), 64.7 (d, 2JP,C = 6.7, OCH2); 1H NMR (CDCl3) Δ: 0.93 (dt, J = 9.9, 7.3, 6H, 2 CH3), 1.37–1.45 (m, 4H, 2 CH2) 1.57–1.77 (m, 6H, PCH2, 2 CH2), 4.01 (q, J = 6.7, 2H, OCH2), 9.73 (s, 1H, OH).
3.5. Theoretical Calculations
DFT computations at the M062X/6–311+G (d,p) level of theory were performed considering the solvent effect of the corresponding alcohol using the SMD solvent model with the Gaussian 09 program package [27,28,29]. The geometries of the molecules were optimized in all cases, and frequency calculations were also performed to assure that the structures are in a local minimum or in a saddle point. The conformations of the reported structures have been determined by conformational analysis. The solution-phase Gibbs free energies were obtained by frequency calculations as well. The G values obtained were given under standard conditions, the corrected total energies of the molecules were taken into account. Entropic and thermal corrections were evaluated for isolated molecules using standard rigid rotor harmonic oscillator approximations. That is, the Gibbs free energy was taken as the “sum of electronic and thermal free energies” printed in a Gaussian 09 vibrational frequency calculation. Standard state correction was taken into account. The transition states were optimized with the QST3 or the TS (Berny) method. Transition states were identified by having one imaginary frequency in the Hessian matrix, and IRC calculations were performed in order to prove that the transition states connect two corresponding minima.
Supplementary Materials
The following are available online, 31P, 1H and 13C NMR spectra of the new ester acids, energetics and geometrical data belonging to the theoretical calculations.
Author Contributions
G.K. fund raising, planning, coordinating, and supervising the work, drawing the conclusions, writing the paper; N.H. critical survey of the literature, carrying out the preparative work, and interpretation of the results; P.Á.-B. and R.H. planning and performing the theoretical calculations, and interpreting the results of the calculations; L.D. obtaining and processing the mass spectral data. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Research, Development and Innovation Office (K134318).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Quin L.D. A Guide to Organophosphorus Chemistry. Wiley; New York, NY, USA: 2000. [Google Scholar]
- 2.Horsman G.P., Zechel D.L. Phosphonate Biochemistry. Chem. Rev. 2017;117:5704–5783. doi: 10.1021/acs.chemrev.6b00536. [DOI] [PubMed] [Google Scholar]
- 3.Pradere U., Garnier-Amblard E.C., Coats S.J., Amblard F., Schinazi R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014;114:9154–9218. doi: 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nowack B. Environmental chemistry of phosphonates. Water Res. 2003;37:2533–2546. doi: 10.1016/S0043-1354(03)00079-4. [DOI] [PubMed] [Google Scholar]
- 5.Xiang H.F., Xu H.Y., Wang Z.Z., Chen C.H. Dimethyl methylphosphonate (DMMP) as an efficient flame retardant additive for the lithium-ion battery electrolytes. J. Power Sources. 2007;173:562–564. doi: 10.1016/j.jpowsour.2007.05.001. [DOI] [Google Scholar]
- 6.Kiss N.Z., Keglevich G. Direct esterification of phosphinic and phosphonic acids enhanced by ionic liquid additives. Pure Appl. Chem. 2019;91:59–65. doi: 10.1515/pac-2018-1008. [DOI] [Google Scholar]
- 7.Kiss N.Z., Mucsi Z., Böttger É., Drahos L., Keglevich G. A three-step conversion of phenyl-1H-phosphinic acid to dialkyl phenylphosphonates including two microwave-assisted direct esterification steps. Curr. Org. Synth. 2014;11:767–772. doi: 10.2174/1570179410666131212231130. [DOI] [Google Scholar]
- 8.Henyecz R., Kiss A., Mórocz V., Kiss N.Z., Keglevich G. Synthesis of phosphonates from phenylphosphonic acid and its monoesters. Synth. Commun. 2019;49:2642–2650. doi: 10.1080/00397911.2019.1637894. [DOI] [Google Scholar]
- 9.Welton T. Ionic liquids: A brief history. Biophys. Rev. 2018;10:691–706. doi: 10.1007/s12551-018-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S.K., Savoy A. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020;297:112038. doi: 10.1016/j.molliq.2019.112038. [DOI] [Google Scholar]
- 11.Karmakar A., Mukundan R., Yang P., Batista E.R. Solubility model of metal complex in ionic liquids from first principle calculations. RSC Adv. 2019;9:18506–18526. doi: 10.1039/C9RA04042K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keaveney S.T., Haines R.S., Harper J.B. Ionic liquid solvents: The importance of microscopic interactions in predicting organic reaction outcomes. Pure App. Chem. 2017;89:745–757. doi: 10.1515/pac-2016-1008. [DOI] [Google Scholar]
- 13.Rádai Z., Kiss N.Z., Keglevich G. An Overview of the Applications of Ionic Liquids as Catalyst and Additives in Organic Chemical Reactions. Curr. Org. Chem. 2018;22:533–556. doi: 10.2174/1385272822666171227152013. [DOI] [Google Scholar]
- 14.Barucki H., Black R.M., Kinnear K.I., Holden I., Read R.W., Timperley C.M. Solid-phase synthesis of some alkyl hydrogen methylphosphonates. Phosphorus Sulfur Silicon. 2003;178:2279–2286. doi: 10.1080/713744564. [DOI] [Google Scholar]
- 15.Raghuraman K., Pillarsetty N., Prabhu K.R., Katti K.K., Katti K.V. Unprecedented rhodium-mediated catalytic transfer hydrogenation of a phosphonate functionalized olefin in ecofriendly media. Inorg. Chim. Acta. 2004;357:2933–2938. doi: 10.1016/j.ica.2004.01.005. [DOI] [Google Scholar]
- 16.Gupta A.K., Kumar R., Gupta H.K., Tak V., Dubey D.K. DCC-Celite hybrid immobilized solid support as a new, highly efficient reagent for the synthesis of O-alkyl hydrogen alkylphosphonates under solvent-free conditions. Tetrahedron Lett. 2008;49:1656–1659. doi: 10.1016/j.tetlet.2008.01.016. [DOI] [Google Scholar]
- 17.Pienaar A., Erasmus C.M., Wentzel M., Cowley E.H. Synthesis of alkyl hydrogen alkylphosphonates. Phosphorus Sulfur Silicon. 1999;148:149–159. doi: 10.1080/10426509908037007. [DOI] [Google Scholar]
- 18.Mucsi Z., Kiss N.Z., Keglevich G. A quantum chemical study on the mechanism and energetics of the direct esterification, thioesterification and amidation of 1-hydroxy-3-methyl-3-phospholene 1-oxide. RSC Adv. 2014;4:11948–11954. doi: 10.1039/c3ra47456a. [DOI] [Google Scholar]
- 19.Ansly E.V., Dougherty D.A. Modern Physical Organic Chemistry. University Science Books; Mill Valley, CA, USA: 2006. pp. 361–362. [Google Scholar]
- 20.Keglevich G., Kiss N.Z., Mucsi Z., Körtvélyesi T. Insights into a surprising reaction: The microwave-assisted direct esterification of phosphinic acids. Org. Biomol. Chem. 2012;10:2011–2018. doi: 10.1039/c2ob06972e. [DOI] [PubMed] [Google Scholar]
- 21.Keglevich G., Mucsi Z. 4. Interpretation of the rate enhancing effect of microwaves. In: Giancarlo C., Diego C., editors. Microwave Chemistry. De Gruyter; Berlin, Germany: Boston, MA, USA: 2017. pp. 53–64. [DOI] [Google Scholar]
- 22.Hohmann E., Keglevich G., Greiner I. The effect of onium salt additives on the Diels Alder reactions of a 1-phenyl-1,2-dihydrophosphinine oxide under microwave conditions. Phosphorus Sulfur Silicon. 2007;182:2351–2357. doi: 10.1080/10426500701441473. [DOI] [Google Scholar]
- 23.Harsági N., Kiss N.Z., Drahos L., Keglevich G. Synthesis of Cyclic Phosphinates by Microwave-Assisted Ionic Liquid-Promoted Alcoholysis. Synthesis. 2021 doi: 10.1055/a-1504-8924. in press. [DOI] [Google Scholar]
- 24.Acharya J., Shakya P.D., Pardasani D., Palit M., Dubey D.K., Gupta A.K. Surface-mediated solid phase reactions: A simple, efficient and base-free synthesis of phosphonates and phosphates on Al2O3. J. Chem. Res. 2005:194–196. doi: 10.3184/0308234054213591. [DOI] [Google Scholar]
- 25.Lai C., Xi C., Chen W., Hua R. Metallo-phosphorylation of alkenes: A highly regioselective reaction of zirconocene-alkene complexes with chlorophosphate. Tetrahedron. 2006;62:6295–6302. doi: 10.1016/j.tet.2006.04.048. [DOI] [Google Scholar]
- 26.Li C., Wang Q., Zhang J.Q., Ye J., Xie J., Xu Q., Han L.B. Water determines the products: An unexpected Bronsted acid-catalyzed PO-R cleavage of P(III) esters selectively producing P(O)-H and P(O)-R compounds. Green Chem. 2019;21:2916–2922. doi: 10.1039/C9GC01254K. [DOI] [Google Scholar]
- 27.Gaussian 09, Revision D.01. Gaussian, Inc.; Wallingford, CT, USA: 2009. [Google Scholar]
- 28.Zhao Y., Truhlar D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008;120:215–241. doi: 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- 29.Petersson G.A., Bennett A., Tensfeldt T.G., Al-Laham M.A., Shirley W.A. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988;89:2193–2218. doi: 10.1063/1.455064. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.









