Abstract
The prevalence of osteoporosis in recent years is rapidly increasing. For this reason, there is an urgent need to develop bone substitutes and composites able to enhance the regeneration of damaged tissues which meet the patients’ needs. In the case of osteoporosis, personalized, tailored materials should enhance the impaired healing process and restore the balance between osteoblast and osteoclast activity. In this study, we fabricated a novel hybrid material (Co0.5Mn0.5Fe2O4@PMMA) and investigated its properties and potential utility in the treatment of osteoporosis. The material structure was investigated with X-ray diffraction, Fourier-transform infrared spectroscopy with attenuated total reflectance, FTIR-ATR, transmission electron microscopy (TEM), scanning electron microscopy (SEM) and selected area (electron) diffraction (SAED). Then, the biological properties of the material were investigated with pre-osteoblast (MC3T3-E1) and pre-osteoclasts (4B12) and in the presence or absence of magnetic field, using RT-qPCR and RT-PCR. During the studies, we established that the impact of the new hybrids on the pre-osteoblasts and pre-osteoclasts could be modified by the presence of the magnetic field, which could influence on the PMMA covered by magnetic nanoparticles impact on the expression of genes related to the apoptosis, cells differentiation, adhesion, microRNAs or regulating the inflammatory processes in both murine cell lines. In summary, the Co0.5Mn0.5Fe2O4@PMMA hybrid may represent a novel approach for material optimization and may be a way forward in the fabrication of scaffolds with enhanced bioactivity that benefits osteoporotic patients.
Keywords: osteoporosis, pre-osteoblasts, osteoclasts, PMMA, Co0.5Mn0.5Fe2O4, magnetic field, apoptosis, integrins, microRNAs
1. Introduction
Osteoporosis (OP) is to the most frequent bone disease which can occur at any age stage, although women are more predisposed than men [1]. OP is common in societies all over the world due to progressive aging. The disease is characterized by reduced bone strength, mineral density and biomechanical properties, which together trigger bone fractures. According to the NIH Consensus Development Panel on Osteoporosis Prevention, osteoporosis is defined to “primarily reflect the integration of bone density and bone quality [2]. OP related bone fracture seriously affects patients’ quality of life, with increased mortality and disability. More than 50% of postmenopausal women suffer from bone fracture and less than 30% of them will recover to normal life after hip fracture. Although the incidents of OP among men are estimated to be around 20%, mortalities due to bone fractures including the hip are twice as frequent in women [3]. Together with progressing aging in society, it is easily predicted that OP-related bone fracture incidents will steadily grow in the future; therefore, further research searching for factors that could improve bone regeneration are strongly required and fully reasonable. The bone microstructure under OP condition is seriously deteriorated and decreased bone mineral density (BMD), causing abnormal bone remodeling in which the advantage of anabolic process over catabolic occurs. This process is mediated by two critical cell populations, i.e., osteoblasts that are responsible for the synthesis and secretion of multiplate factors leading to bone formation and osteoclasts that resorb the bone. Osteoblasts originate from multipotent stem progenitor cells that give them rise and are critical for the development of functionally active osteoblasts. These multipotent stem progenitor cells, apart from osteoblasts, give rise to other adult cell populations including chondrocytes, adipocytes or myocytes underlining their importance during tissue formation [4]. Within osteoblasts, runt-related transcription factor 2 (RUNX2), bone γ-carboxyglutamate protein 2 (Bglap2) and dentin matrix protein 1 (Dmp1) are recognized as a critical regulator of osteoblast differentiation and function. Osteoblast formation, function and proliferative activity are mediated by multiplate signaling pathways and proteins including TGF-β/BMP and WNT pathways and osteopontin (OPN), which modulates the expression of common transcripts involved in the bone formation process [5]. OPN plays an important role in bone metabolism and homeostasis, especially in endocrine-regulated bone mass, osteoblast proliferation, migration and adhesion. OPN has been proposed in several studies to be closely related to the development of many bone diseases including osteosarcoma and osteoporosis as its impact on the bone formation/resorption process seems to be critical [6]. In turn, bone resorption is critically regulated by osteoclasts which are cells differentiated from the myeloid precursors. The activity of osteoclasts is mediated by various factors including cell-to-cell contact (osteoblast-osteoclasts), secretion of a broad range of cytokines colony-stimulating factor 1 (CSF1), receptor activator of NF-κB ligand (RANKL) that activate signaling pathways in myeloid precursors leading to enhanced expression of transcription factors critical for osteoclasts differentiation including PU.1, cFOS and NF-κB [7]. Pu.1 seems to play a critical role since its depletion in mouse model directly leads to osteoporosis development. Pu.1 is the master regulator of hematopoietic precursors to the myeloid lineage and it has been shown to be a critical activator of osteoclast differentiation [8].
Interest in organic-inorganic hybrid materials is caused by the possibility of mixing different material properties into one single product to deliver smart and multifunctional platforms for broad biomedical applications. In the case of ferrite compounds, the tailoring of the elemental composition of magnetic spinal leads to the mixed character of magnetic properties, i.e., hard–soft behavior that enhances their response to the applied magnetic field and increases the efficacy of temperature generation in hyperthermia [9]. The addition of the polymeric outer layer to the inorganic core improves the biocompatibility by protecting particle surfaces from contact with biological objects. Among many accessible polymers, polymethacrylate (PMMA) deserves special attention since it is used as the main component of injectable medical cement and a popular filling material characterized at the same time with very low toxicity [10]. The role of hyamine 1622 (benzethonium chloride) is essential since except for being a cationic surfactant, it can deliver anticancer and antiviral activity to organic-inorganic hybrid materials [11]. Recently, it was shown that this type of organic-magnetic hybrid materials has great potential in the treatment of deep-seated cancer, as well as being important composites in a new class of bone replacement materials for temperature stimulation of regenerative processes [12,13].
For that reason, in the presented study, we developed Co0.5Mn0.5Fe2O4@PMMA nanohybrids that were cultured with both pre-osteoblasts and osteoclasts under normal and static magnetic field conditions. In this study, we have found that under normal and magnetic field condition, Co0.5Mn0.5Fe2O4@PMMA improves pre-osteoblast activity and induces the expression of OPN-BGLAP2-DMP1 axis by activation of integrins, while inhibiting osteoclastogenesis.
In summary, by utilizing Co0.5Mn0.5Fe2O4@PMMA nanohybrids modulating of osteoblasts/osteoclast activity might occur, therefore becoming an interesting approach for developing a strategy for future osteoporotic related fracture and bone regeneration.
2. Materials and Methods
2.1. In Situ Synthesis of the Binary Co0.5Mn0.5Fe2O4@PMMA Hybrids
The magnetic field responsive stock colloidal suspension of the Co0.5Mn0.5Fe2O4 nanoparticles was prepared via microwave driven non-hydrolytic approach described in detail elsewhere [14]. The magnetic characterization of Co0.5Mn0.5Fe2O4 was presented by our group previously interested reader is advised to follow that article [14]. In short, the following metal acetylacetonates were taken 1 mmol (257 mg) of Co(acac)2 (99.9%, Alfa Aesar, Kandel, Germany), 1 mmol (253 mg) of Mn(acac)2 (99.9% Alfa Aesar, Kandel Germany), 4 mmol (1413 mg) of Fe(acac)3 (99.99%, Alfa Aesar, Kandel Germany) and subsequently dissolved in 70 mL of acetophenone (99% Sigma Aldrich, Poznań, Poland, used without further purification). All handling with the metal complexes has been done under an inert atmosphere of N2 using an acrylic glove box (GS Glove Box Systemtechnik GMBH P10R250T2, Sömmerda, Germany). The reaction mixture was directly transferred into the Teflon vessel, secured and placed inside of the Ertec® Magnum V2 microwave reactor (Ertec, Wrocław, Poland). The process was carried out under autogenous pressure of 15 atm, at 200 °C for 60 min. Afterward, Co0.5Mn0.5Fe2O4 nanoparticles were separated from the solvent through washing, centrifuging cycles and re-suspended in 30 mL of de-ionized water. The final concentration of the Co0.5Mn0.5Fe2O4 particles was measured using the micro-scale technique. In the case of the binary Co0.5Mn0.5Fe2O4@PMMA hybrids, the in situ polymerization protocol was adopted without any changes as described previously [15]. The main chemicals were methyl methacrylate (MMA) monomer (99% Sigma Aldrich, Poznań, Poland), potassium peroxydisulfate as a polymerization initiator (≥99.0%, KPS, Sigma Aldrich, Poznań, Poland), benzethonium chloride (≥96.0%, Hyamine®1622, Sigma Aldrich, Poznań, Poland), as well as stock Co0.5Mn0.5Fe2O4 particles. The MMA monomer was purified from the hydroquinone (inhibitor) through washing with 10% water solution of NaOH (≥97.0% Sigma Aldrich, Poznań, Poland) and, finally, dried with MgSO4 (≥97.0% Sigma Aldrich, Poznań, Poland) prior usage. Briefly, 3 mL (4 mmol) of hyamine containing aqueous solution was added to the 6 mL of the Co0.5Mn0.5Fe2O4 (concentrated stock suspension containing 100 mg particles) and mixed with 13 mL of de-ionized water. An ultrasound bath was used to homogenize dispersion for 20 min. After that, the mixture was transferred to a four-neck flask equipped with a mechanical stirrer, gas inlet (N2), dropping funnels and Pt-100 sensor for temperature control. The MMA (in proportion of 80% to 20% of particles) was slowly injected and a KPS initiator was added. The reaction vessel was heated up to 80 °C for 3 h under constant stirring and nitrogen blanket. The binary hybrids were separated using a laboratory magnet and carefully dried under a vacuum.
2.2. Characterization of Basic Physicochemical Properties of PMMA@Co0.5Mn0.5Fe2O4 Hybrids
Structure identification of Co0.5Mn0.5Fe2O4 nanoparticles and Co0.5Mn0.5Fe2O4@PMMA hybrid materials was carried out employing X-ray powder diffraction technique using a PANalytical X’Pert PRO X-ray diffractometer (Cu-Kα1 = 1.54060 Å, nickel filtering, Malvern, UK) recording XRD patterns at the range of 2Q = 10–75° and their direct comparison with the reference standards from the ICDD database (International Centre for Diffraction Data). The morphology and particle size were discussed based on the transmission electron microscopy (TEM) in the case of the nanoparticles whereas hybrid materials were subjected to the scanning electron microscopy (SEM) technique to prevent hybrids from unwanted deterioration induced by the high energy electron beam. Therefore, a Philips CM-20 Super Twin microscope (Philips, Amsterdam, The Netherlands), operated at 200 kV was used for the TEM characterization while hybrids samples were imaged with a Nova Nano-SEM 230 microscope (FEI Company, ThermoFisher Scienitific, Waltham, MA, USA). The measurements of the FTIR-ATR spectra were performed on a Nicolet iZ10 spectrometer (Thermo Fischer Scientific, Waltham, MA, USA) equipped in diamond ATR accessory covering the spectral range between 4000–500 cm−1 at room temperature. Magnetic characterization of the Co1−xMnxFe2O4 colloids was presented by us previously [16]; thus, the interested reader is advised to follow that article for details. The hybrid particle mean size and distribution were estimated by using free-image processing software ImageJ v.1.46 [17] through analysis of SEM images by taking into consideration of 100 hybrid particles (longest diameter was measured due to elongated shape of objects).
2.3. Cell Lines
The mouse pre-osteoblast mouse cell line (MC3T3-E1-subclone 4) was obtained from the European Collection of Authenticated Cell Cultures (EACC, Sigma-Aldrich, Munich, Germany), while the mouse pre-osteoclast cell line (4B12) was a kind gift from the Department of Oral Biology and Tissue Engineering, Meikai University School of Dentistry (Professor S. Amano) [18]. The MC3T3-E1 cell line was maintained in Minimum Essential Medium Alpha (MEM-α, Gibco, Waltham, MA, USA) without ascorbic acid supplemented with 10% Fetal Bovine Serum (FBS, Merck, KGaA, Darmstadt, Germany) and 1% of standard antibiotics (Merck KGaA, Darmstadt, Germany). The 4B12 cell line was also cultured in MEM-α with the addition of 30% CSCM (calvaria-derived stromal cell conditioned media), 10% of FBS and 1% of antibiotics in standard conditions.
2.4. Magnetic Field (MF)
The cells were exposed to the magnetic field using the static magnetic field (SMF) stimulation system designed in Wroclaw University of Science and Technology. This system is appropriate to produce a uniform SMF through action of two parallel magnets with opposite polarity. Plates with samples were placed in the central core, as shown in Figure 1. In this place, the MF strength was equaled 0.3T. The daily exposure of cells on the MF was 15 min.
Figure 1.
Diagram of the magnetic field.
2.5. Cell Proliferation Assay
In order to determine the viability of cells after treating them with PMMA, Co0.5Mn0.5Fe2O4 and their combination (Co0.5Mn0.5Fe2O4@PMMA) in a ratio of 80/20 and at a final concentration of 90.8 μg/mL, after 24, 48 and 72 h in the magnetic field conditions, TOX-8 kit (Merck KGaA, Darmstadt, Germany) was performed according to the manufacturer’s protocol. The absorbance in the appropriate wells was evaluated by 96-well microplate reader (Epoch; Biotek Instruments, Winnoski, VT, USA) equipped with Gen5 software version 2.0 [19]. The measurements were taken at 600 nm and 690 nm as the reference lengths. Each experiment was performed at least three times independently.
2.6. Morphology and Mitochondria Status Analysis
The mitochondria, actin filaments and the nucleus of treated/untreated pre-osteoblasts and pre-osteoclasts were stained as described previously [20]. Briefly, mitochondria were stained using MitoRed dye, the F-actin filaments with Phalloidin-Atto 488 and the cells nuclei with 4′,6-diamidino-2-phenylindole DAPI (all from Life Technologies, Carslbad, CA, USA).
Briefly, the cells after incubation with the PMMA and its combination, were incubated for 30 min with MitoRed solution at concentration 1:1000 at 37 °C and fixed with 4% PFA (POCh, Gliwice, Poland). Then, the cells were stained with Phalloidin-Atto 488 for 45 min at RT and then stained with DAPI. Visualization was made by a confocal microscope (Leica TCS SPE, Leica Microsystems, Wetzlar, Germany) at 0.5 µm steps up to a final depth of 25 µm. Images were captured at magnification 630× and analyzed using Fiji New ImageJ with Colour Pixel Counter plugin version 1.52 developed by Wayne Rasband from NIH, USA. Each photograph was taken at least three times independently.
2.7. Gene Expression Analysis
The gene expression analysis was performed using qPCR technique. Briefly, cells seeded at the plastic plates at the density of 1 × 104/well in the appropriate medium with the addition of PMMA or its modification and placed in the magnetic field were collected after 24 h and suspended in the Extrazol (BLIRT DNA, Gdańsk, Poland). The total RNA was obtained using acid guanidinium thiocyanate-phenol-chloroform extraction method described by Chomczynski and Sacchi [21] using the reagents from Merck KGaA, Darmstadt, Germany. The total of RNA quality and quantity were determined using a spectrophotometer (Epoch, Biotek Instruments, Winnoski, VT, USA).
The process of digestion of gDNA and cDNA synthesis was performed using Takara PrimeScript™ RT Reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, Bio Europe, Goteborg, Sweden) according to the manufacturer protocol. Both processes were performed using a T100 Thermal Cycler (Bio-Rad, Hercules, CA, USA).
Each cDNA template was amplified by the quantitative reverse transcription polymerase chain reaction, using SensiFAST™ SYBR No-ROX Kit (Bioline, London, UK) in total volume of 10 µL (for a single reaction-1 μL of cDNA and 500 nM of each primer, according to the protocol). The sequences of the specific primers obtained from Merck KGaA, are listed in Table 1. The qRT-PCR reactions were performed using a CFX Connect Real-Time PCR Detection System (CFX Connect Optics Module, Bio-Rad, Hercules, CA, USA) equipped with the software BioRad CFX Maestro and the transcript levels were normalized to Gaph as a standard control (house-keeping gene).
Table 1.
Sequences of primers used in the actual studies.
| Gene | Forward (5′→3′) | Reverse (3′→5′) | Length of Amplicon |
|---|---|---|---|
| P21 | TGTTCCACACAGGAGCAAAG | AACACGCTCCCAGACGTAGT | 175 |
| P53 | AGTCACAGCACATGACGGAGG | GGAGTCTTCCAGTGTGATGATGG | 287 |
| CASP9 | CCGGTGGACATTGGTTCTGG | GCCATCTCCATCAAAGCCGT | 278 |
| BAD | ACATTCATCAGCAGGGACGG | ATCCCTTCATCCTCCTCGGT | 115 |
| BAX | AGGACGCATCCACCAAGAAGC | GGTTCTGATCAGCTCGGGCA | 251 |
| BCL2 | GGATCCAGGATAACGGAGGC | ATGCACCCAGAGTGATGCAG | 141 |
| RUNX2 | TCCGAAATGCCTCTGCTGTT | GCCACTTGGGGAGGATTTGT | 130 |
| RANKL | TTAAGCCAGTGCTTCACGGG | ACGTAGACCACGATGATGTCGC | 493 |
| OPG | TGGCACACAGTGATGAATGCG | GCTGGAAAGTTTGCTCTTGCG | 149 |
| ALP | TTCATAAGCAGGCGGGGGAG | TGAGATTCGTCCCTCGCTGG | 198 |
| COL1A1 | CCAGCCGCAAAGAGTCTACA | CAGGTTTCCACGTCTCACCA | 175 |
| OPN | AGACCATGCAGAGAGCGAG | GCCCTTTCCGTTGTTGTCCT | 340 |
| BGLAP2 | CTCCTGAGAGTCTGACAAAGCCTT | GCTGTGACATCCATTACTTGC | 320 |
| DMP1 | CCCAGAGGCACAGGCAAATA | TCCTCCCCAATGTCCTTCTT | 211 |
| MMP9 | TTGCCCCTACTGGAAGGTATTAT | GAGAATCTCTGAGCAATCCTTGA | 172 |
| PU.1 | GAGAAGCTGATGGCTTGGAG | TTGTGCTTGGACGAGAACTG | 175 |
| ITGAV | ACAATGTAAGCCCAGTTGTGTCT | TTTGTAAGGCCACTGGAGATTTA | 236 |
| C-FOS | CCAGTCAAGAGCATCAGCAA | TAAGTAGTGCAGCCCGGAGT | 248 |
| INTa1 | CACCTTTCAAACTGAGCCCGCCA | GCTGCCCAGCGATGTAGAGCACAT | 110 |
| INTa3 | TGGGCAAGTGCTATGTGCGTGGCA | TCTGGGTGAAGCCGCCGCTGGT | 147 |
| INTa6 | CTGGCTTCCTCGTTTGGCTATG | TGCCTTGCTGGTTAATGTAGACGT | 145 |
| INTb1 | TCTCACCAAAGTAGAAAGCAGGGA | ACGATAGCTTCATTGTTGCCATTC | 138 |
| IL6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA | 141 |
| TGFβ1 | GGAGAGCCCTGGATACCAAC | CAACCCAGGTCCTTCCTAAA | 171 |
| TNFα | GAACTGGCAGAAGAGGCACT | AGGGTCTGGGCCATAGAACT | 203 |
| miR-7a-5p | TGGAAGACTAGTGATTTTGTTGT | * | |
| miR-17-5p | CAAAGTGCTTACAGTGCAGGTAG | * | |
| miR-145-5p | GTCCAGTTTTCCCAGGAATCCCT | * | |
| miR-21-5p | TAGCTTATCAGACTGATGTTGA | * | |
| miR-124-3p | TAAGGCACGCGGTGAATGCCAA | * | |
| miR-203a | GUGAAAUGUUUAGGACCACUAG | * | |
| miR-223a | TGTCAGTTTGTCAAATACCCCA | * | |
| GAPDH | TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC | 177 |
p21: cyclin-dependent kinase inhibitor 1; p53: tumor suppressor factor; Casp9: caspase 9; Bad: Bcl-2 associated agonist of cell death; Bax: Bcl-2 associated X protein; Bcl-2: B-cell lymphoma 2; RUNX-2: Runt-related transcription factor 2; RANKL: Receptor Activator for Nucleat Factor κβ Ligand; Opg: osteoprotegerin; Alp: phosphatase alkaline; Col1A1: collagen alpha-1 chain precursor; Opn: osteoponin; Bglap2: bone-carboxyglutamic acid-containing protein; Dmp-1: dentin matrix protein 1; Mmp-9: matrix metalloproteinase 9; PU.1: protein in human encoded by the SPI1 gene; Itgav: Integrin Subunit Alpha V; c-fos: protoonkogene, cellular oncogene fos; INTa1: intergrin alpha 1; INTa3: integrin alpha 3; INTa6: integrin alpha 6; INTb1: integrin beta 1; Il-6: interleukin-6, Tgfβ1: tumor-growth factor beta 1; Tnfa; tumor-necrosis factor alpha, miR; microRNA, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.* there is only one sequence.
2.8. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 Software and the statistical significance was marked with asterisk (*). The p value less than 0.05 (p < 0.05) are marked with one asterisk (*), while p value less than 0.01 (p < 0.01) with two asterisks (**) and, finally, the p values less than 0.001 (p < 0.001) with three asterisks (***).
3. Results
3.1. Characterization of Physicochemical Properties of the Co0.5Mn0.5Fe2O4 Nanoparticles and PMMA@Co0.5Mn0.5Fe2O4
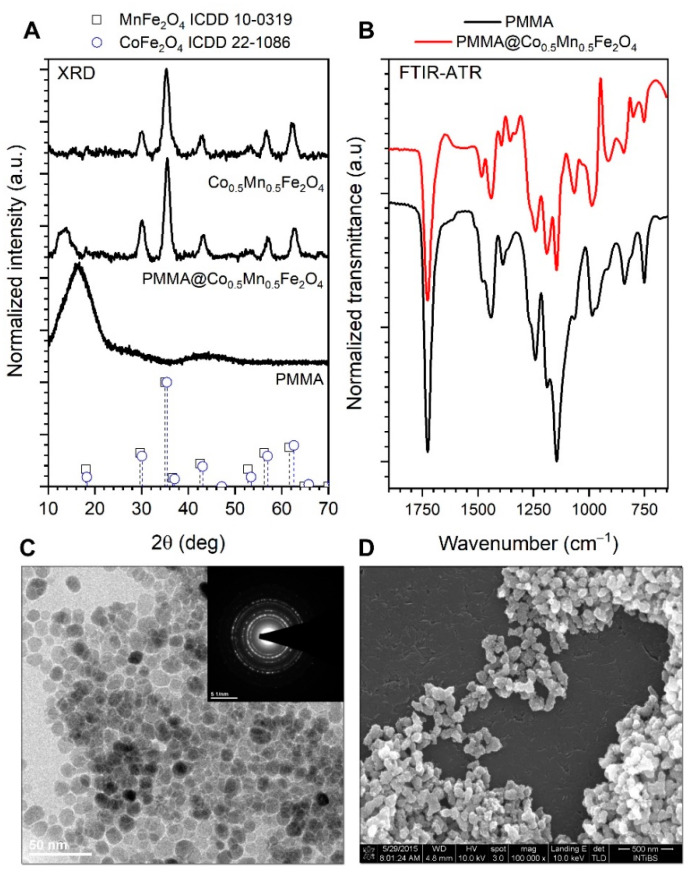
The analysis of the diffraction patterns (Figure 2A) leads to the conclusion that the structure of the Co0.5Mn0.5Fe2O4 can be ascribed to the spinel-type materials as supported by reference card no. ICDD 10–0319 as well as 22–1086. A detailed analysis of the structural properties of the same nanoparticles with a broader concentration range of Mn doping was a subject of our previous article [15] where it was proven that the final compound formed solid solution of respective elements in appropriate ratio. The results of the formation of the hybrid material were confirmed by the FTIR-ATR spectra analysis (Figure 2B). One can see a range of the PMMA characteristic vibration modes which are present either in the case of hybrid Co0.5Mn0.5Fe2O4@PMMA and reference polymer sample prepared in the same way as composite. The difference in peak positions and their structure reflects the interaction of the PMMA with the Co0.5Mn0.5Fe2O4 surface [22]. The average crystallite size was calculated from the peak broadening with help of Scherrer’s formula:
| (1) |
where k stands for constant value set at 0.9, λ is the wavelength of the Cu lamp (1.54060 Å), β0 means apparatus broadening; β is full width at half maximum (FWHM) and θ corresponds with the peak maximum taken for the calculations [22] and compared with the size extracted from the TEM image (Figure 2C). We noted that there is a very good match between both size estimation methods. The mean crystallite size calculated from the broadening of the (220) crystallographic plane reflection was around 7 nm, whereas the particle size obtained from the TEM imaging is 8 ± 2 nm. Analysis of the SEM micrographs leads to the observation that the hybrid materials have elongated shapes, sample morphology shows sufficient homogeneity, while the size of the objects has been estimated to range from 120 to 200 nm.
Figure 2.
X-ray diffraction patterns of the Co0.5Mn0.5Fe2O4 and Co0.5Mn0.5Fe2O4@PMMA (A,B) FTIR-ATR spectra of the reference PMMA and composite sample; (C) TEM and SAED images of the Co0.5Mn0.5Fe2O4 nanoparticles, as well as (D) SEM picture of the binary hybrid.
All reflections were indexed accordingly; the 14 2θ broad peak corresponds to the amorphous PMMA shell.
The Co0.5Mn0.5Fe2O4 sample was taken as a core material due to its best magnetic properties within the whole concentration range of Mn2+ studied in Ref. [14]. The sample and the sample after covering it with the PMMA shell (to improve biocompatibility) assure the best response upon action of static magnetic field.
3.2. Anti-Proliferative Effect of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
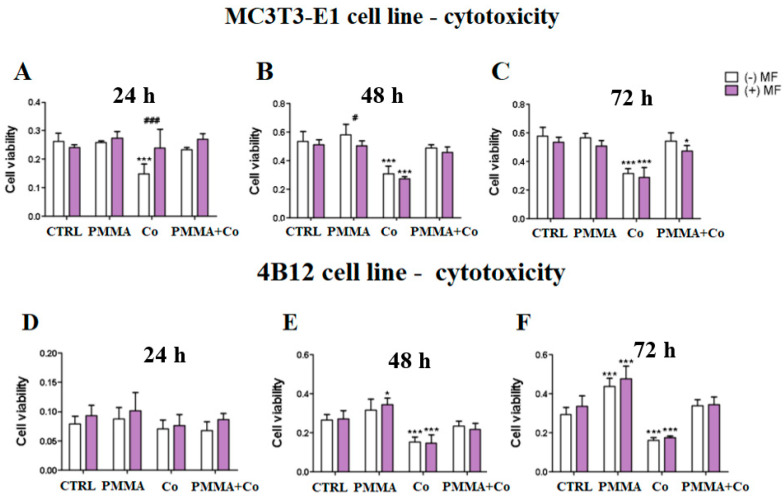
The effect of PMMA and Co0.5Mn0.5Fe2O4@PMMA modification was evaluated on the pre-osteoblasts in the presence of magnetic field and the results showed that applying of magnetic fields decrease the viability of MC3T3-E1 after 48 h (Figure 3B).
Figure 3.
The kinetics of anti-proliferative effects of PMMA and PMMA@Co0.5Mn0.5Fe2O4 towards mouse pre-osteoblasts (MC3T3-E1 cell line) after 24 h (A), 48 h (B) and 72 h (C) and towards mouse pre-osteoclasts (4B12 cell line) after 24 h (D), 48 h (E) and 72 h (F) in the presence of the magnetic field. Notes: CTRL—control, PMMA—poly(methyl (methylacrylate), Co—Co0.5Mn0.5Fe2O4, PMMA + Co—Co0.5Mn0.5Fe2O4@PMMA in ratio 80/20, MF—magnetic field. Statistical differences are indicated by * p < 0.005 and *** p < 0.0001 (in comparison to control) and by # p < 0.005; ### p < 0.0001 (comparison MF+/MF−).
In turn, Co0.5Mn0.5Fe2O4@PMMA increases the viability in the MF(+) condition in comparison to control cells (Figure 3C).
In the case of pre-osteoclasts, we observed statistically significant increase of viability of cells treated with the PMMA in the presence of MF(+) after 48 h (Figure 3E) and after 72 h, independently of the MF application (Figure 3F)
3.3. Morphology and Mitochondria Network Development Related to PMMA and PMMA@Co0.5Mn0.5Fe2O4 towards Osteoblasts and Osteoclasts in the Presence of Magnetic Field
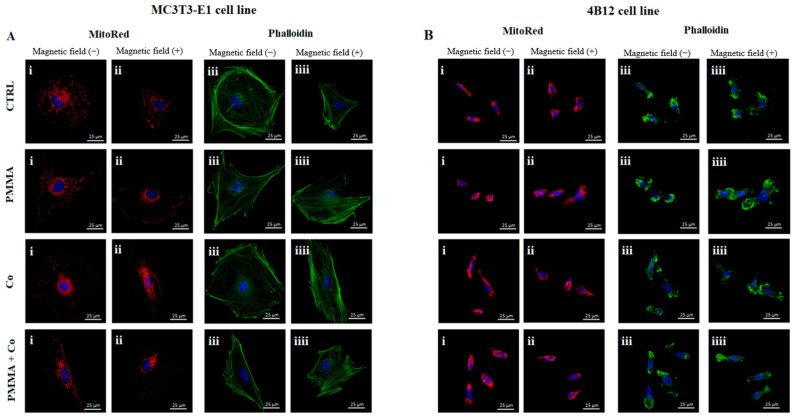
The impact of the PMMA and its modification on the morphology and mitochondria network rearrangement in the magnetic field on the pre-osteoblasts and pre-osteoclasts was determined using MitoRed and F-actin staining.
Our studies showed that PMMA alone Co0.5Mn0.5Fe2O4@PMMA limited the growth of cytoskeleton in the pre-osteoblasts in the MF(−) condition (Figure 4A(iii)). Moreover, the comparison between MF(+) and MF(−) revealed that pre-osteoblasts cultured in the MF(+) condition showed weak cytoskeleton development than these in the MF(−) (Figure 4A(iii,iiii)). From the other side, the mitochondrial network was less developed in case of PMMA combination in the MF(−) condition in comparison to control cells (Figure 4A(i)).
Figure 4.
The impact of the PMMA and its modification on the mitochondria status and cytoskeleton in the MC3T3-E1 (A) and 4B12 (B) cell line in the presence of the magnetic field. Notes: CTRL—control, PMMA—poly(methyl (methylacrylate)), Co—Co0.5Mn0.5Fe2O4, PMMA+ Co—Co0.5Mn0.5Fe2O4@PMMA in ratio 80/20.
The opposite effect was reported in the pre-osteoclasts treated by PMMA and its combination in both magnetic conditions, where the clearly visible mitochondrial network and cytoskeleton development were reported (Figure 4B(i–iiii)).
The photographs were captured at 60× magnification: scale bar, 25 μm.
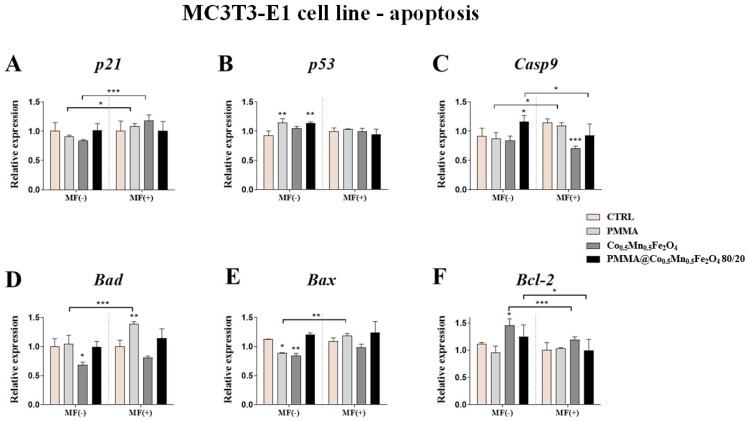
3.4. The Impact of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 on the Expression of Genes Related to the Apoptosis towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
The impact of the PMMA and its modification on the apoptosis of pre-osteoblasts and pre-osteoclasts was determined using qPCR. The expression of p21, p53, Casp9, Bad, Bax and Bcl-2 was analyzed after 24 h of incubation of the cells with the addition of PMMA and Co0.5Mn0.5Fe2O4@PMMA in ratio 80/20 in the MF condition.
Our studies showed that PMMA alone increasing the expression of p53 gene as compared to control in the MF(−) condition (Figure 5B). Moreover, the effect of the magnetic field application was observed after pre-osteoblasts culturing with PMMA. The addition of PMMA caused increase of the expression of the p21, Casp9, Bad and Bax in the MF(+) as compared to MF(−) (Figure 5A,C–E).
Figure 5.
The impact of PMMA and its modification on the expression of genes related to apoptosis: (p21 (A), p53 (B), Casp-9 (C), Bad (D), Bax (E) and Bcl-2 (F)) in the magnetic field condition towards mouse pre-osteoblasts (MC3T3-E1 cell line). Statistical differences are indicated by * p < 0.005; ** p < 0.001 and *** p < 0.0001.
In turn, the expression of the p53 gene after 24 h exposition to Co0.5Mn0.5Fe2O4@PMMA was increased in the MF(−) condition, compared to control (Figure 5B). In parallel, the expression of the Casp9 and Bcl-2 were increased after PMMA@Co0.5Mn0.5Fe2O4 incubation in the MF(−), in comparison to MF(+) (Figure 5C,F).
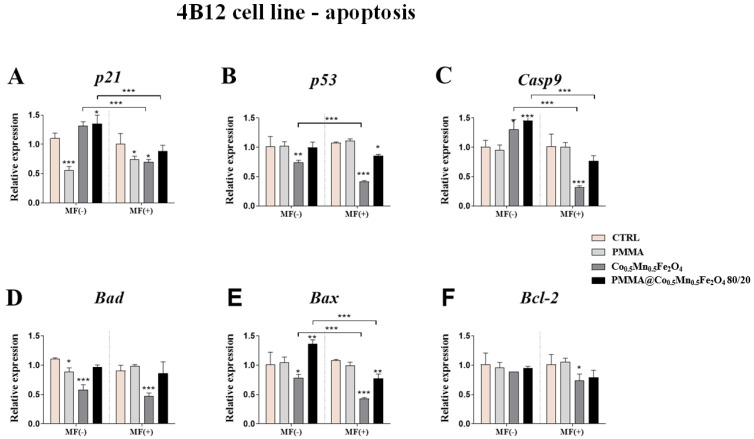
The effect of PMMA alone on the pre-osteoclasts expression of genes associated with apoptosis was similar in the MF(+) and MF(−) conditions. The significant decrease in the expression of p21 and Bad in the MF(−) condition, as well as the decrease of the expression of p21 in the MF(+) condition, was observed (Figure 6A,D).
Figure 6.
The impact of PMMA and its modification on the expression of genes related to apoptosis: (p21 (A), p53 (B), Casp-9 (C), Bad (D), Bax (E) and Bcl-2 (F)) in the magnetic field condition towards mouse pre-osteoclasts (4B12 cell line). Statistical differences are indicated by * p < 0.005; ** p < 0.001 and *** p < 0.0001.
The opposite effect was observed after pre-osteoclasts treating with Co0.5Mn0.5Fe2O4@PMMA in ratio 80/20 in the MF(−) condition, where we observed the increase of the expression of the p21, Casp9 and Bax (Figure 6A,C,E), while after the applied magnetic field, the significant decrease of p53 and Bax was reported (Figure 6B,E). Interestingly, applying of magnetic field decreased the expression of p21, Casp9 and Bax after treating pre-osteoclasts with modified PMMA (Figure 6A,C,E).
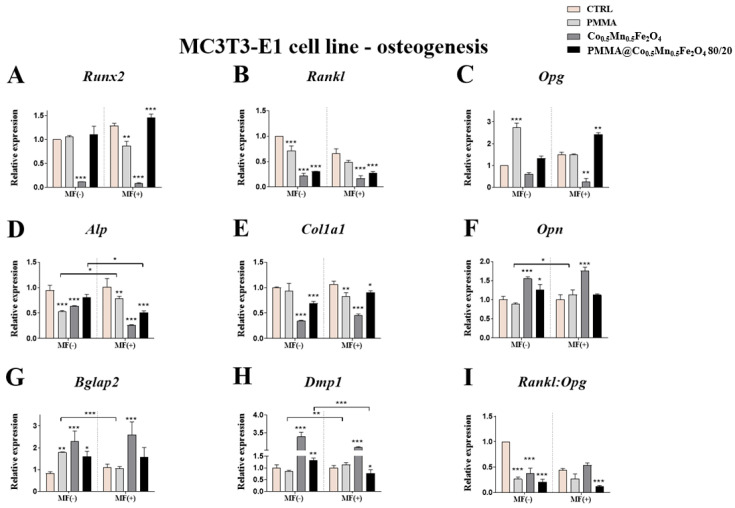
3.5. The Impact of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 on the Expression of Genes and Proteins Related to Osteogenesis/Osteoclastogenesiss towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
Our studies revealed the impact of the PMMA on the expression of genes related to process of osteogenesis and osteoclastogenesis in the case of the exposition of them to the magnetic field. Interesting observation was noticed in case of the expression of Alp gene after pre-osteoblasts incubation with PMMA. From one side, the expression of the Alp was decreased independently of MF application, but from the other side, its expression was lower in the MF(−) than in MF(+) condition (Figure 7B). Moreover, PMMA caused the statistical significant decrease of the Col1A1 expression in the MF(+) condition in comparison to control (Figure 7C).
Figure 7.
The impact of PMMA and its modification on the expression of genes related to osteogenesis: Runx2 (A), Rankl (B), Opg (C), Alp (D), Col1A1 (E), Opn (F), Bglap2 (G), Dmp1 (H), Rankl:Opg (I) in the magnetic field condition towards mouse pre-osteoblasts (MC3T3-E1 cell line).Statistical differences are indicated by * p < 0.005; ** p < 0.001 and *** p < 0.0001.
The impact of the magnetic field application was also observed in the case of Bglap 2 and Dmp1 expression after PMMA addition to culture. In the MF(−) condition, the expression of Bglap 2 was increased as compared to control, while after comparison Bglap2 expression between MF(−) and MF(+), we noticed the increase expression after PMMA in the MF(−) than in MF(+) (Figure 7E). Moreover, the expression of Dmp1 in the MF(+) was higher than in the MF(−) condition (Figure 7F).
In turn, the combination of the Co0.5Mn0.5Fe2O4@PMMA increased the expression of Alp in the pre-osteoblasts in MF(+) as compared to MF(−) condition (Figure 7B), while Opn and Bglap 2 was increased as compared to the control only in the case of the MF(−) condition (Figure 7D,E). Additionally, the combination of the Co0.5Mn0.5Fe2O4@PMMA in a ratio of 80/20 increases the expression of the Col1A1 as compared to control in both magnetic conditions (Figure 7C).
Interestingly, the expression of the Dmp1 was increased in the MF(−) condition as compared to control, while in the MF(+) was decreased. Moreover, the expression of the Dmp1 in the MF(−) condition was increased as compared to MF(+) (Figure 7F).
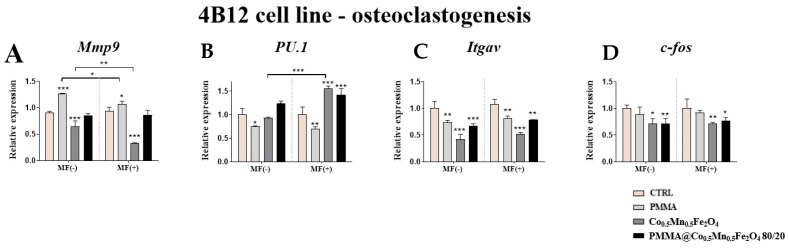
The impact of the PMMA and its combination in the magnetic field condition on the pre-osteoclasts was determined based on the expression of the Mmp9, PU.1, Itgav and c-fos.
Our studies revealed the enhanced expression of the Mmp9 after PMMA incubation of pre-osteoclasts as compared to control cells independently of the magnetic field conditions (Figure 8A). In addition, we reported that presence of the magnetic field is associated with decreasing expression of Mmp9 (Figure 8A). Additionally, it was noticed that in both magnetic conditions the expression of PU.1 and Itgav was decreased as compared to control (Figure 8B,C).
Figure 8.
The impact of PMMA and its modification on the expression of genes related to osteoclastogenesis; Mmp9 (A), PU.1 (B), Itgav (C), c-fos (D) in the magnetic field condition towards mouse pre-osteoclasts (4B12 cell line). Statistical differences are indicates by * p < 0.005; ** p < 0.001 and *** p < 0.0001.
Similar effect we observed after treating osteoclasts with the combination of Co0.5Mn0.5Fe2O4 and PMMA, where the expression of the PU.1 was increased, while Itgav and c-fos decreased as compared to control in both magnetic conditions (Figure 8B–D).
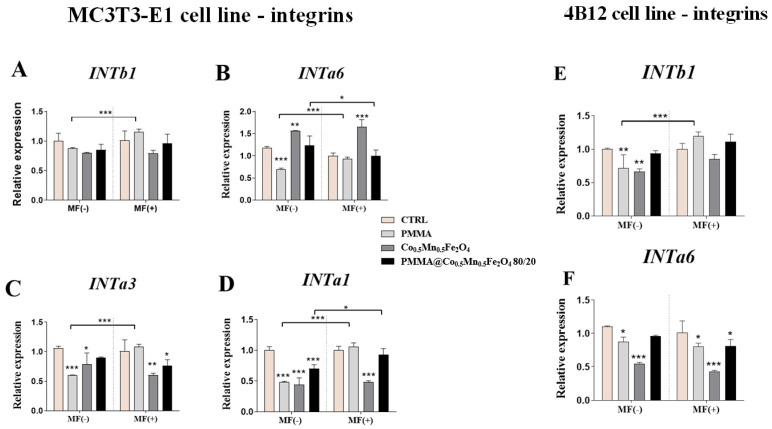
3.6. The Impact of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 on the Expression of Genes Related to Integrins towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
Finally, we established the impact of the PMMA and its modification on the integrins expression in mouse pre-osteoblasts and pre-osteoclasts cell lines. In the case of INTb1, INTa1, INTa3 and INTa6, the increase of expression was observed in the MF(+) condition as compared to MF(−) (Figure 9A–D). Moreover, in the absence of magnetic field, the decrease of the expression of the INTa6, INTa1 and INTaα3 after PMMA culturing of the pre-osteoblasts was observed in the MF(−) conditions (Figure 9B–D).
Figure 9.
The impact of PMMA and its modification on the integrins expression in the magnetic field condition towards mouse pre-osteoblasts (MC3T3-E1 cell line) Intb1 (A), Inta6 (B), Inta1 (C), Inta3 (D) and mouse pre-osteoclasts (4B12 cell line) Intb1 (E), Inta6 (F). Notes: CTRL—control, PMMA—poly(methyl (methylacrylate)), Co—Co0.5Mn0.5Fe2O4, PMMA+ Co—Co0.5Mn0.5Fe2O4@PMMA in ratio 80/20. Statistical differences are indicated by * p < 0.005; ** p < 0.001 and *** p < 0.0001.
In turn, the combination of the PMMA and Co0.5Mn0.5Fe2O4@PMMA decreased the expression of the INTa1 in the MF(−) condition as well as INTa3 in the MF(+) condition in pre-osteoblasts (Figure 9C,D). Additionally, the impact of the magnetic field on the expression of INTa6 and INTa3 after this combination was reported, although in case of the INTa6 the expression was decreased in the MF(+) condition as compared to MF(−), while in case of the INTa3, it was increased in the MF(+) condition (Figure 9B,D).
In the case of the pre-osteoclasts, PMMA alone decreased the expression of the INTb1 in the presence of the magnetic field and also INTa6 in both magnetic conditions (Figure 9E,F). Moreover, the combination of the PMMA and Co0.5Mn0.5Fe2O4 decrease the expression of the INTa6 in the magnetic field condition (Figure 9F). Interestingly, the expression of the INTb1 was increased in the pre-osteoclasts in the MF(+) condition after culturing them in the presence of the PMMA combination.
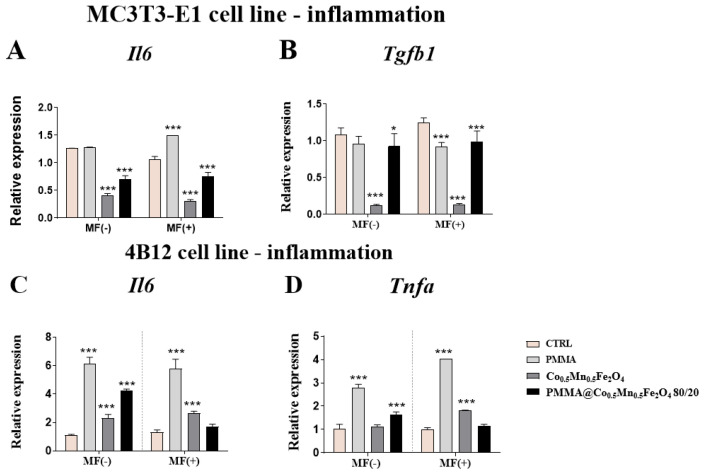
3.7. The Impact of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 on the Expression of Genes Related to Inflammation Process towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
Interestingly, the results associated with the inflammation profile showed that the new modification of PMMA could decrease the expression of Il-6 independently of the presence of magnetic field in the pre-osteoblasts, while increasing in the pre-osteoclasts (Figure 10A,C). The opposite, also independently of the presence of MF, Co0.5Mn0.5Fe2O4 @PMMA in a ratio of 80/20 caused the decrease of the Tgfb in MC3T3-E1 cell line and enhanced the expression of Tnfa (Figure 10B,D).
Figure 10.
The impact of PMMA and its modification on the expression of genes related to inflammation profile under the magnetic field condition towards mouse pre-osteoblasts (MC3T3-E1 cell line) Il-6 (A), Tgfb1 (B) and mouse pre-osteoclasts (4B12 cell line) Il-6 (C) and Tnfa (D). Statistical differences are indicated by * p < 0.005; and *** p < 0.0001.
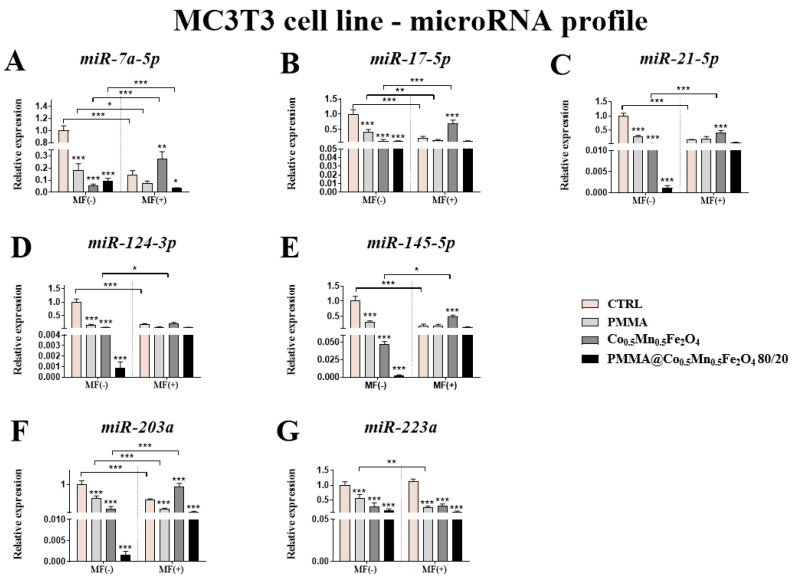
3.8. The Impact of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 on the Expression of Genes Related to microRNA Involved in the Process of Osteoblastogenesis and Osteoclatogenesis towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
Additional studies revealed statistically significant decrease of the expression of miR-17-5p, miR-21-5p, miR-124-3p and miR-145-5p in comparison to control only in case of MF absence, while the expression of miR-7a-5p, miR-203a and miR-223a was decreased independently of the magnetic field presence in the MC3T3-E1 cell line (Figure 11A–G).
Figure 11.
The impact of PMMA and its modification on the microRNA profile associated with osteoblastogenesis and osteoclastogenesis expression of miR-7a-5p (A), miR-17-5p (B), miR-21-5p (C), miR-124-3p (D), miR-145-5p (E), miR-203a (F) and miR-223a (G) in the magnetic field condition towards mouse pre-osteoblasts (MC3T3-E1 cell line. Statistical differences are indicated by * p < 0.005; ** p < 0.001 and *** p < 0.0001.
Comparing the results between the presence and absence of the magnetic field, only in the case of miR-7a-5p was the slightly decreased of expression of this miR observed under magnetic field condition (Figure 11A).
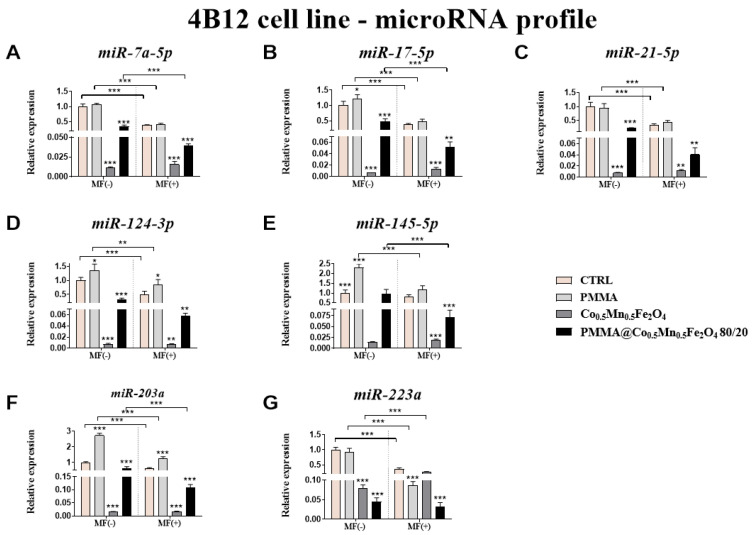
From the other side, in pre-osteoclasts (4B12 cell line) almost all tested microRNA (miR-7a-5p, miR-17-5p, miR-21-5p, miR124-3p, miR-203a and miR-223a) was decreased after incubation with Co0.5Mn0.5Fe2O4@PMMA in ratio 80/20 independently of the presence of the magnetic field (Figure 12A–D,F,G). However, the expression of miR-145-5p was diminished only in the presence of magnetic field (Figure 12E). It was interesting that MF caused strongest decrease of the expression of miR-7a-5p, miR-17-5p, miR-145-5p, miR-203a and miR-223a, comparing to the results obtained in case of MF absence (Figure 12A–C,E–G).
Figure 12.
The impact of PMMA and its modification on the microRNA profile associated with osteoblastogenesis and osteoclastogenesis expression of miR-7a-5p (A), miR-17-5p (B), miR-21-5p (C), miR-124-3p (D), miR-145-5p (E), miR-203a (F) and miR-223a (G) in the magnetic field condition towards mouse pre-osteoclasts (4B12 cell line). Statistical differences are indicated by * p < 0.005; ** p < 0.001 and *** p < 0.0001.
4. Discussion
The effectiveness of bone implants tailored to osteoporotic patients depends on both stimulation of osteoblast proliferation and differentiation, as well as inhibition of osteoclasts activity. Poly(methyl methacrylate) (PMMA) has become one of the attractive and frequently used polymers in the synthesis of bone cements since its first biomedical application in 1937. In order to enhance its bioactivity, PMMA can be combined with a wide range of chemicals in order to synthetize nanoparticles with improved osteoinductive properties. One of the potential candidates for small molecule additives are bioinorganic ions which represents an inexpensive and stable alternative to peptides, nucleic acids and growth factors. In the presented study, we doped PMMA with Co, Mn and Fe2O4 and analyzed in vitro the osteogenic and osteoclastogenic properties of composite. Furthermore, we have investigated whether exposition of fabricated biomaterials to magnetic field enhance their bioactive properties. Previous studies have confirmed that cobalt ions and MF are both able to enhance angiogenesis and bone tissue regeneration, which supports their application in the fabrication of nanomaterials for bone tissue engineering.
For that reason, in order to confirm the utility of bone-filling material, its impact on the cells surrounding the microenvironment of damaged tissue should be investigated carefully. Biocompatibility results revealed that pure Co decreased proliferation of MC3T3-E1 cell line in comparison to control group; however, that effect was ameliorated in PMMA + Co group. Previous studies revealed that a concentration of cobalt ions <10 ppm enhance proliferation of bone cells [23,24]. In the presented study, we employed the MC3T3-E1 cell line as it has behaviors similar to primary calvarial osteoblasts and for the evaluation of biocompatibility of implants is preferable to osteosarcoma cell line, since it better reflects physiological condition. A similar phenomenon was observed for 4B12 cells, in which addition of Co resulted in decreased growth kinetics.
In the presented study, we demonstrated that Co0.5Mn0.5Fe2O4@PMMA hybrid and Co0.5Mn0.5Fe2O4 alone exert a pro-ostegoenic and anit-osteoclastogenic effects. The biological activity of the materials has been demonstrated by the analysis of gene expression related to bone cells metabolism under normal and MF condition. Interestingly, hybrid material showed the better cell response for the expression of Runx2, Alp, Col1a1, while Co0.5Mn0.5Fe2O4 enhanced expression of Opn, Bglap2, Dmp1. Observed phenomenon can be explained at least by two different ways. Enhanced differentiation of MC3T3-E1 cells may by directly activated by each hybrid components. Modification of PMMA surface with different inorganic compounds was proved to enhance osteoblasts adhesion and response. Recently, it was shown by Phakatkar et al. [25] that novel PMMA bone cement nanocomposites containing magnesium phosphate nanosheets and hydroxyapatite nanofibers possess antibacterial attributes with enhanced cytocompatibility and mechanical properties. Another group showed that incorporation of hydroxyapatite into PMMA increases the biological response to the cement from tissue around the implant site [20]. The authors revealed that, after the transplantation of material in vivo, its surface is immediately covered by the cells with initiate the osteointegration. They proved that the incorporation of hydroxyapatite improves the attachment of extracellular matrix (ECM) protein in comparison to PMMA only. In the same analogy, enhanced osteogenesis on the PMMA hybrid observed in the presented study may results from its modification with inorganic ions. Fan et al. [23] have shown that cobalt chloride (CoCl2-treated bone progenitor cells induced higher degree of vascularization and enhanced osteogenesis. Furthermore, cobalt-substituted hydroxyapatite (COHA) effectively promotes bone cell growth, reduces the inflammatory response and is an antibacterial agent [26].
Another possible mechanism is related to the presence of iron oxide in fabricated materials. Recently, iron oxide nanoparticles (IONPs) have been widely studied in the areas of bone regenerative medicine. It was shown that IONPs incorporated to gelatin sponge scaffold enhance bone formation in vivo and is visible in MRI imaging without using of external magnetic field [27]. On the other hand, nanocomposites of iron oxide and hydroxyapatite were characterized by superparamagnetic and biocompatible properties [28]. Vlad et al. [27] have shown that incorporation of iron oxide nanoparticles into the powder phase of an alpha-tricalcium phosphate-based cement improved injectability of apatitic bone cement for vertebroplasty. The application of IONPs in bone tissue engineering was also investigated in our own studies. We have shown previously that α-Fe2O3/γ-Fe2O3 nanocomposite exerts dual action as they enhance osteogenic differentiation while reduce the activity of osteoclast [20]. We also found that polyurethane/poly(lactic) acid sponges doped with iron oxide nanoparticles under magnetic field enhance osteogenesis of adipose derived mesenchymal stem cells by enhanced expression of osteopontin and collagen type I.
An additional mechanism for better cell response may result from the application of external magnetic field (MF), which is therapeutic agent per se and further enhance the bioactive properties of materials doped with magnetic responsive particles. MF potential to affect osteoblast behavior on different biomaterials have been proved in multiple studies including our own. Based on the obtained data, MF represent a potential tool to improve the clinical outcome of selected regenerative therapies not only in orthopedics but also in dentistry. However, due to discrepancies between some research works, MF should be more thoroughly investigated by proper clinical trials. Herein, we have shown that application of MF enhances the cellular response of scaffold doped with Fe2O4. It stands with our and other previous research which showed that MF application enhance osteogenesis, modulates progenitor stem cells fate and diminish osteoclasts activity.
It also should be mentioned that fabricated, hybrid material, due to the incorporation of Co and iron oxides, represents a potent MRI contrasting agent. In diagnostics, MRI is applied to differ between healthy and tumor tissue and to visualize the location of lesions. However, metal implants can interfere with the MRI, causing misinterpretation of the obtained results. In previous experiments, it was confirmed that COHAC can be used as a T2 contrast localization agent and does not cause image interference [29,30].
In the next step, we investigated how fabricated nanocomposites affects the expression of the integrins as they are involved in the regulation of multiple cell functions, including their interactions with matrix, proliferation and differentiation. It was shown that cell movement on the material surface is possible through the formation of cytoskeletal projections called filopodia, which in turn stimulates the activation of integrins [18,31]. Transmembrane receptors in a great amount can be found in cells forming focal contacts (adhesion plaques), which are directly responsible for the adhesion of cells to material surface. Integrins bound to selected ECM components, e.g., collagen, fibronectin, osteopontin and through signal transduction modulates the fate of bone forming cells and, thus, are directly involved in the regeneration process. Their activation stimulates cells to migration, movement, adhesion and, finally, differentiation. The findings in this study show that both Co0.5Mn0.5Fe2O4@PMMA and Co0.5Mn0.5Fe2O4 in control and MF condition modulate the integrins expression. We found that the abovementioned materials enhanced the mRNA levels of INTa6, INTa1 and INTa3 in relation to pure PMMA.
Here, we also proved that the new biomaterials could influence on the action of miRNAs involved in the processes of bone remodeling and can modulate the pro-inflammatory response that is important in the case of the potential future use of such biomaterials in the OP treatments.
5. Conclusions
In the presented paper, we fabricated a Co0.5Mn0.5Fe2O4@PMMA hybrid and investigated its physicochemical and biological properties. The bioactivity of scaffolds was determined using in vitro osteoblast and osteoclasts system. The hybrid material showed better cellular response in comparison to pure PMMA under control and magnetic field condition. This can be explained by the presence of bioactive, inorganic ions- Fe2O4 and Co, which are known to enhance bone regeneration. Therefore, a newly developed Co0.5Mn0.5Fe2O4@PMMA might be useful for a bone substitute or filler.
The material for bone regeneration should be characterized by activation of preosteoblasts, which induce deposition of ECM proteins and leads to its mineralization. Yet, while taking into consideration its application in osteoporotic patients, novel, smart biomaterials should be incorporated with particles that not only stimulate bone forming cells, but at the same time inhibit the overactivity of osteoclasts. The enhanced activity of bone resorbing cells disrupts tissue homeostasis, contributing to bone mass loss and altered bone microstructures, which make it prone to fractures. Thus, in order to re-establish normal bone repair, bone grafts should be tailored to meet the patients’ need and restore the balance between cells in affected tissue. Here, we provide a proof of evidence that the modification of PMMA with Co0.5Mn0.5Fe2O may represent a novel approach for the material optimization and may be the way forward in the fabrication of scaffold with enhanced bioactivity that benefits osteoporotic patients.
Acknowledgments
The authors are grateful of Shigeru Amano from the Department of Oral Biology and Tissue Engineering, Meikai University School of Dentistry for sharing with the 4B12 cell line.
Abbreviations
| ALP | alkaline phosphatase |
| BAD | Bcl-2 associated agonist of cell death |
| BAX | Bcl-2 associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| BGLAP | bone gamma-carboxyglutamic acid-containing protein (OC) |
| BMD | bone mineral density |
| CASP | caspase |
| c-FOS | cellular protooncogene |
| COHA | cobalt-substitute hydroxyapatitie |
| COL1A1 | Collagen alpha-1 (I) chain precursor |
| CSF1 | Colony stimulating factor 1 |
| CSCM | 30% calvaria-derived stromal cell conditioned media |
| DEPC | sterile filter water treated with diethyl pyrocarbonate |
| DMP1 | dentin matrix acidic phosphoprotein 1 |
| ECM | extracellular matrix |
| FBS | Fetal Bovine Serum |
| FTIR-ATR | Fourier Transform Infrared-Attenuated Total Reflectance |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| IL | interleukin |
| IONs | iron oxide nanoparticles |
| ITGAV | Integrin Subunit AlphaV |
| iNOS | inducible nitric oxide synthase |
| INT | integrin |
| MEMα | Minimum Essential Medium α |
| miRNA | microRNA |
| MF | magnetic field |
| MMP | matrix metalloproteinase |
| MNPs | magnetic nanoparticles |
| OC | osteocalcin (BGLAP) |
| OPN | osteopontin |
| OS | osteoporosis |
| p21 | cyclin-dependent kinase inhibitor 1 |
| p53 | tumor suppressor factor |
| PMMA | polymethylcrylate |
| PU.1 | protein in human encoded by the SPI1 gene |
| RANKL | Receptor activator of NFκβ ligand |
| RUNX2 | Runt-related transcription factor 2 |
| SAED | selected area (electron) diffraction |
| SEM | scanning electron microscopy |
| SMF | static magnetic field |
| TEM | transmission electron microscopy |
| TNFα | tumor necrosis factor α |
| TGFβ | transforming growth factor β |
Author Contributions
R.P. and K.M. designed the research; E.T., E.Z., A.T., M.K.-G., R.P. and K.K.-G., performed the experiments and collected and analyzed the data; K.K.-G., E.T., R.P., A.T., E.Z. and M.K.-G. validated the results; K.K.-G., K.M., E.T. and R.P.; wrote the manuscript text and prepared the figures.; K.M., E.T., K.K.-G., E.Z., A.T., M.K.-G. and R.P. have read critically and edited the manuscript.; K.M. and R.P. acquired the funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Centre Poland grant no: 2017/26/M/NZ5/01184. The publication is financed under the Leading Research Groups support project from the subsidy increased for the period 2020–2025 in the amount of 2% of the subsidy referred to Art. 387 (3) of the Law of 20 July 2018 on Higher Education and Science, obtained in 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ongoing studies.
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akkawi I., Zmerly H. Osteoporosis: Current Concepts. Joints. 2018;6:122–127. doi: 10.1055/s-0038-1660790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redlich K., Smolen J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 3.Teng G.G., Curtis Effrey R., Saag K.G. Mortality and osteoporotic fractures: Is the link causal, and is it modifiable? Clin. Exp. Rheumatol. 2008;26:S125–S137. [PMC free article] [PubMed] [Google Scholar]
- 4.Knight M.N., Hankenson K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care. 2013;2:306–316. doi: 10.1089/wound.2012.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caetano-Lopes J., Canhão H., Fonseca J.E. Osteoblasts and bone formation. Acta Reumatol. Port. 2007;32:103–110. [PubMed] [Google Scholar]
- 6.Si J., Wang C., Zhang D., Wang B., Hou W., Zhou Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020;26:e919159-1. doi: 10.12659/MSM.919159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles J., Aliprantis A.O. Osteoclasts: More than ‘bone eaters’. Trends Mol. Med. 2014;20:449–459. doi: 10.1016/j.molmed.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nerlov C., Graf T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12:2403–2412. doi: 10.1101/gad.12.15.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giustini A.J., Petryk A.A., Cassim S.M., Tate J.A., Baker I., Hoopes P.J. MAGNETIC NANOPARTICLE HYPERTHERMIA IN CANCER TREATMENT. Nano Life. 2010;01:17–32. doi: 10.1142/S1793984410000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frazer R.Q., Byron R.T., Osborne P.B., West K.P. PMMA: An Essential Material in Medicine and Dentistry. J. Autom. Inf. Sci. 2005;15:629–639. doi: 10.1615/JLongTermEffMedImplants.v15.i6.60. [DOI] [PubMed] [Google Scholar]
- 11.Yip K.W., Mao X., Au P.B., Hedley D., Chow S., Dalili S., Mocanu J.D., Bastianutto C., Schimmer A., Liu F.-F. Benzethonium Chloride: A Novel Anticancer Agent Identified by Using a Cell-Based Small-Molecule Screen. Clin. Cancer Res. 2006;12:5557–5569. doi: 10.1158/1078-0432.CCR-06-0536. [DOI] [PubMed] [Google Scholar]
- 12.Shima H., Kawai T., Matsushima Y., Unuma H., Kawamura K., Li Z., Kawashita M. Magnetite fine particles highly loaded PMMA microspheres for hyperthermia of deep-seated cancer. J. Ceram. Soc. Jpn. 2013;121:802–806. doi: 10.2109/jcersj2.121.802. [DOI] [Google Scholar]
- 13.Kulpa-Greszta M., Pązik R., Kłoda P., Tomaszewska A., Zachanowicz E., Pałka K., Ginalska G., Belcarz A. Efficient non-contact heat generation on flexible, ternary hydroxyapatite/curdlan/nanomagnetite hybrids for temperature controlled processes. Mater. Sci. Eng. C. 2021;118:111360. doi: 10.1016/j.msec.2020.111360. [DOI] [PubMed] [Google Scholar]
- 14.Zachanowicz E., Zięcina A., Mikołajczyk P.A., Rogacki K., Małecka M., Marycz K., Marędziak M., Poźniak B., Nowakowska M., Tikhomirov M., et al. Cytotoxic Effects of Co1−xMnxFe2O4 Ferrite Nanoparticles Synthesized under Non-Hydrolytic Conditions (Bradley’s Reaction)—In Vitro. Eur. J. Inorg. Chem. 2016;2016:5315–5323. doi: 10.1002/ejic.201600720. [DOI] [Google Scholar]
- 15.Zachanowicz E., Pigłowski J., Grzymajło M., Poźniak B., Tikhomirov M., Pierunek N., Śniadecki Z., Idzikowski B., Marycz K., Maredziak M., et al. Efficient synthesis of PMMA@Co0.5Ni0.5Fe2O4 organic-inorganic hybrids containing hyamine 1622—Physicochemical properties, cytotoxic assessment and antimicrobial activity. Mater. Sci. Eng. C. 2018;90:248–256. doi: 10.1016/j.msec.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Zachanowicz E., Kulpa-Greszta M., Tomaszewska A., Gazińska M., Marędziak M., Marycz K., Pązik R. Multifunctional Properties of Binary Polyrhodanine Manganese Ferrite Nanohybrids—from the Energy Converters to Biological Activity. Polymer. 2020;12:2934. doi: 10.3390/polym12122934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ImageJ, v.1.46. [(accessed on 1 September 2021)]; Available online: https://imagej.nih.gov/ij/
- 18.Amano S., Sekine K., Bonewald L.F., Ohmori Y. A novel osteoclast precursor cell line, 4B12, recapitulates the features of primary osteoclast differentiation and function: Enhanced transfection efficiency before and after differentiation. J. Cell. Physiol. 2009;221:40–53. doi: 10.1002/jcp.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gen5, Version 2.0, Data Analysis Software. [(accessed on 1 September 2021)]; Available online: https://www.labbulletin.com/articles/gen5-version-2-0-data-analysis-software-now-available-from-biotek.
- 20.Marycz K., Sobierajska P., Roecken M., Kornicka-Garbowska K., Kępska M., Idczak R., Nedelec J.-M., Wiglusz R.J. Iron oxides nanoparticles (IOs) exposed to magnetic field promote expression of osteogenic markers in osteoblasts through integrin alpha-3 (INTa-3) activation, inhibits osteoclasts activity and exerts anti-inflammatory action. J. Nanobiotechnol. 2020;18:1–24. doi: 10.1186/s12951-020-00590-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chomczynski P. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 22.Klug H.P., Alexander L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials. 2nd ed. Wiley-VCH; Weinheim, Germany: 1974. [(accessed on 1 September 2021)]. p. 992. Available online: https://ui.adsabs.harvard.edu/abs/1974xdpf.book.....K/abstract. [Google Scholar]
- 23.Fan W., Crawford R., Xiao Y. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials. 2010;31:3580–3589. doi: 10.1016/j.biomaterials.2010.01.083. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y., Yang Y., Deng Y. Dual therapeutic cobalt-incorporated bioceramics accelerate bone tissue regeneration. Mater. Sci. Eng. C. 2019;99:770–782. doi: 10.1016/j.msec.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Phakatkar A.H., Shirdar M.R., Qi M.-L., Taheri M.M., Narayanan S., Foroozan T., Sharifi-Asl S., Huang Z., Agrawal M., Lu Y.-P., et al. Novel PMMA bone cement nanocomposites containing magnesium phosphate nanosheets and hydroxyapatite nanofibers. Mater. Sci. Eng. C. 2020;109:110497. doi: 10.1016/j.msec.2019.110497. [DOI] [PubMed] [Google Scholar]
- 26.Sundaram N.M., Murugesan S. Preparation and characterization of an iron oxide-hydroxyapatite nanocomposite for potential bone cancer therapy. Int. J. Nanomed. 2015;10:99–106. doi: 10.2147/IJN.S79985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vlad M.D., del Valle L.J., Barracó M., Torres R., López J., Fernández E. Iron Oxide Nanoparticles Significantly Enhances the Injectability of Apatitic Bone Cement for Vertebroplasty. Spine. 2008;33:2290–2298. doi: 10.1097/BRS.0b013e31817eccab. [DOI] [PubMed] [Google Scholar]
- 28.Hu S., Zhou Y., Zhao Y., Xu Y., Zhang F., Gu N., Ma J., Reynolds M.A., Xia Y., Xu H.H. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018;12:e2085–e2098. doi: 10.1002/term.2641. [DOI] [PubMed] [Google Scholar]
- 29.Dalby M., Di Silvio L., Harper E., Bonfield W. Increasing hydroxyapatite incorporation into poly(methylmethacrylate) cement increases osteoblast adhesion and response. Biomaterials. 2002;23:569–576. doi: 10.1016/S0142-9612(01)00139-9. [DOI] [PubMed] [Google Scholar]
- 30.Pazik R., Piasecka E., Małecka M., Kessler V., Idzikowski B., Sniadecki Z., Wiglusz R.J. Facile non-hydrolytic synthesis of highly water dispersible, surfactant free nanoparticles of synthetic MFe2O4 (M–Mn2+, Fe2+, Co2+, Ni2+) ferrite spinel by a modified Bradley reaction. RSC Adv. 2013;3:12230–12243. doi: 10.1039/c3ra40763b. [DOI] [Google Scholar]
- 31.Alicka M., Major P., Wysocki M., Marycz K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. J. Clin. Med. 2019;8:765. doi: 10.3390/jcm8060765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ongoing studies.