Abstract
Direct repeat spoligotyping of 85 paraffin-embedded lung biopsies was used to investigated the occurrence around Beijing of the Beijing family of Mycobacterium tuberculosis. Samples ranged in time from 1956 to 1990. Hybridization patterns were found with 49 (58%) samples, and 45 (92%) produced typical Beijing family patterns extending over the 34-year period.
Within the past few years, new molecular fingerprinting techniques have made it possible to study mycobacteria and the epidemiology of tuberculosis with greater sensitivity and precision than previously possible. Strains can now be typed according to the polymorphism of DNA fragments. Their spread between and within communities can be monitored (5, 11), and common source outbreaks can be identified and investigated (1–3, 7, 10).
Our previous discovery of a predominant clone of Mycobacterium tuberculosis isolates in China, the Beijing family, which has radiated to neighbor countries (8, 11), raises the question of how long this family of strains was present in the Beijing area. In cultures collected from 1994 to 1996 from patients living in the Beijing area, we found more than 80% of isolates were members of the Beijing family. Not knowing if this represented a recent spread of the Beijing family, we looked at stored samples collected from individuals residing in different districts around the Beijing area. Since there was no access to old cultures from the past, we obtained paraffin-embedded lung tissues of tuberculosis patients, which had been stored at the Beijing Tuberculosis Research Institute (The National Tuberculosis Control Center). The dates of these samples ranged from 1956 to 1990. PCR-based spoligotyping (6) was chosen because of its sensitivity, specificity, and easy recognition of the nine-spacer spoligotyping pattern of Beijing family isolates (11).
Eighty-five formalin-fixed paraffin-embedded lung biopsy specimens were kindly provided by the Department of Pathology, Beijing Tuberculosis Research Institute, People’s Republic of China. Samples were from patients, who lived in at least 17 different localities around Beijing city and counties or the surrounding counties of Hebei province, had a clinical diagnosis of pulmonary tuberculosis, and were undergoing pneumonectomy from 1956 to 1990. Ten of the samples with positive spologotyping patterns were from Hebei province, and 17 from Beijing had no district or counties recorded. In addition, specimens were selected based on the pathological confirmation of pulmonary tuberculosis. Samples were grouped in 10-year intervals according to the time when preparation was performed: 1956–1960 (group 1, n = 25), 1969–1970 (group 2, n = 18), 1979–1980 (group 3, n = 23), and 1989–1990 (group 4, n = 19). The age and sex of the patients were chosen randomly.
Each tissue sample was prepared with the following procedures. Once the microtome had been well cleaned using 10% freshly diluted bleach, the first section was taken to expose a “PCR-clean” surface by sectioning a companion negative control tissue (14 μm), a healthy lung tissue. Two 3-μm specimen tissues were then sectioned. One of them was subjected to acid-fast staining and one to conventional hematoxylin and eosin (H&E) staining. This procedure allowed confirmation of the original histological and clinical diagnosis. Six 14-μm sections were then sectioned successively; four of them were placed separately in 1.5-ml screw-cap microcentrifuge tubes, and the remaining two were placed in one tube for the purpose of doubling the concentration of the M. tuberculosis DNA for better detection. The blade and gloves were discarded after each sample was handled, and a new blade was replaced for sectioning the next sample. To avoid several steps of centrifugation and washes and multiple tube transfers, a one-step DNA extraction method was developed and applied based on the Chelex 100 method for extraction of DNA (9). Briefly, one tissue section (14 μm thick) was placed into a 1.5-ml microcentrifuge tube. A total of 300 μl of extraction solution, including 5% Chelex 100 (Bio-Rad), 0.1% (wt/vol) lauryl sulfate (Sigma), 1% (vol/vol) Nonidet P-40 (Sigma), and 1% (vol/vol) Tween 20 (Sigma), was added. The mixture was then mixed and heated at 100°C for 30 min. The paraffin then appeared floating on the surface of the solution. After centrifugation at 13,000 rpm for 10 minutes (Eppendorf centrifuge model 5415C), the supernatant under the solidified paraffin layer was transferred to a new tube carefully to avoid taking Chelex particles. The supernatant then was centrifuged for 10 s at 13,000 rpm. Two volumes, 10 and 2 μl of the suspension, were used as DNA templates for PCR.
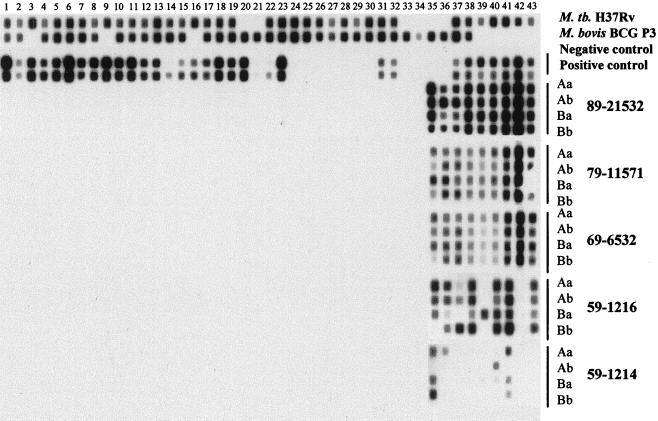
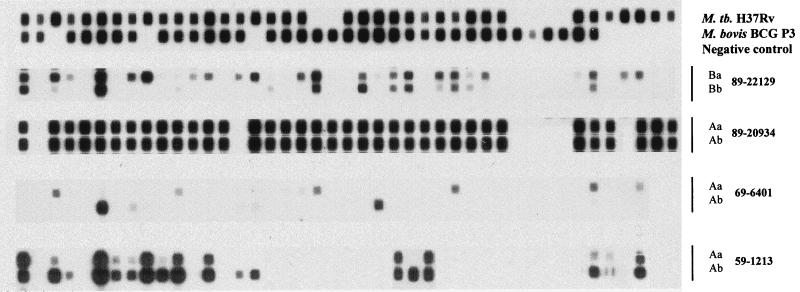
Spoligotyping (spacer oligotyping) exploits the DNA polymorphism at a unique chromosomal locus, the direct repeat (DR) region, of M. tuberculosis complex bacteria (5). Details of the method were described by Kamerbeek et al. (6). As described previously (11), a typical Beijing family strain contained only 9 out of the 43 spacers, located near the 3′ end of the DR region of strain H37Rv. The samples with all nine spacers were counted as having the complete Beijing pattern (Fig. 1, samples: 89-21532, 79-11571 Aa and Ab, and 69-6532), and those with one to eight spacers within the nine-spacer region were counted as having the incomplete pattern (Fig. 1, samples 79-11571 Ba, 59-1216, and 59-1214). Among the 49 samples giving positive spoligotyping patterns, 45 (92%) showed the Beijing patterns (Table 1). However, there were four positive samples demonstrated the non-Beijing patterns (Fig. 2). Although one sample (89-20934) exhibited the complete spoligotyping pattern, the other three (89-22129, 69-6401, and 59-1213) showed truncated patterns. Spoligotyping patterns of M. tuberculosis strains were managed and analyzed using Microsoft Excel for Windows 95, version 7.0a. Statistical analysis of data was performed using EPIINFO 6.0.
FIG. 1.
Hybridization patterns (spoligotypes) of five M. tuberculosis strains from preserved lung biopsy samples (89-21532, 79-11571, 69-6532, 59-1216, and 59-1214). Numbers on the top correspond to the 43 applied spacers (6). The numbers: 89-, 79-, 69-, and 59- represent the year when the tissues were preserved. For each sample, duplicated sections (A and B) were run simultaneously for spoligotyping PCR. For each section, two volumes (10 μl [a] and 2 μl [b]) of DNA templates were used as templates for PCR. A black dot represents the presence of the particular spacer within amplified DNA of the corresponding sample. Between each sample, there is a negative tissue control. Typical complete Beijing family patterns are shown as 89-21532 A and B. The truncated Beijing patterns are demonstrated as 59-1216 and 59-1214. M. tuberculosis H37Rv and Mycobacterium bovis BCG P3 DNAs were used as the hybridization pattern controls. One normal lung tissue specimen without acid-fast bacilli was used as the negative tissue control. Another lung tissue specimen with confirmed acid-fast bacilli was used as the positive lung tissue control (10 μl [upper lane] and 2 μl [lower lane]).
TABLE 1.
Distribution of spoligotyping positive reactivity of M. tuberculosis strains in preserved lung tissues
| Sample collect-ing perioda | No. of samples | No. (%) with positive reactions | No. (%) with Beijing patterns |
|---|---|---|---|
| 1 | 25 | 10 (40) | 9 (90) |
| 2 | 18 | 9 (50) | 8 (89) |
| 3 | 23 | 18 (78) | 18 (100) |
| 4 | 19 | 12 (63) | 10 (83) |
| Total | 85 | 49 (58) | 45 (92) |
1, 1956–1960; 2, 1969–1970; 3, 1979–1980; 4, 1989–1990.
FIG. 2.
Spoligotypes of four M. tuberculosis strains from paraffin-embedded lung samples (89-22129, 89-20934, 69-6401, and 59-1213). Compared with the Fig. 1, these four samples demonstrate the Non-Beijing patterns. For each sample, either section A (89-20934, 69-6401, and 59-1213) or B (89-22129) is shown. For each section, two volumes (10 μl [a] and 2 μl [b]) of DNA templates were used respectively as templates for PCR. M. tuberculosis H37Rv and Mycobacterium bovis BCG P3 DNAs were used as the hybridization pattern controls. One normal lung tissue specimen without acid-fast bacilli was used as the negative tissue control.
As shown in Table 1, we found that the Beijing family M. tuberculosis strains have been dominant since the mid-1950s (90%) and have remained so to the 1990s, with an average of 92% of the positive biopsies being positive for the Beijing family pattern over the 34-year time period. Statistically, there are no difference between the percentages of the Beijing family strains in four time groups (χ2 = 2.1, P = 0.55). However, older samples had a lower positive rate of spoligotyping reactions (Table 1). In the first time period (1956–60) only 40% (10 of 25) of the samples were spoligotyped positive. Samples from the second time period (1969–70) were 50% (9 of 18) positive, and samples from 1979–80 were 78% (18 of 23) positive. Samples from the most recent time period (1989–90) were positive 63% (12 of 19) of the time. These findings demonstrated an increased detection rate for DR spacer DNA with the more recent samples. Comparison of the positive reactivity or detection rate of groups 1 and 2, with a mean of 44.2%, and that of groups 3 and 4, with a mean of 71.4%, revealed that specimens in the latter groups gave a significantly higher positive rate than the former groups (χ2 = 6.46, P = 0.011). The truncated/incomplete spoligotyping patterns (Fig. 1 and Fig. 2) frequently found in older samples indicated that the old DNA templates might have become damaged and fragmented during the course of storage. Humidity, pH, temperature, and nuclease activity, etc. could be the possible factors associated with DNA damage. We considered restoration of intact DNA templates, such as using DNA ligation method(s), which might have been helpful for restoration of original spoligotyping patterns. However, artifact(s) created after the DNA ligation might have resulted in false-positive reactions. Therefore, we did not use this technique. For the negative samples, we thought that using a smaller volume of DNA template in the PCR might give better detection of M. tuberculosis DNA, based on the postulate that the dilution of the DNA template will also cause the dilution of inhibitor(s) of DNA polymerase(s) and therefore increase the PCR sensitivity. The DNA templates were tested at two volumes (10 and 2 ml) to limit the possible presence of an inhibitor. We found no significant difference between the two volumes in the development of the spoligotyping patterns (labeled a and b in Fig. 1 and Fig. 2).
The Beijing family was highly prevalent in at least 17 different areas around Beijing, as far back as 1956 to 1960. Although randomly chosen, most of the subjects from this period were under the age of 30 (65%) and males (81%). These data suggested a relatively recent tuberculosis infection among this group. The more recent groupings (1970, 1980, and 1990) were gradually skewed toward older individuals and an increasing proportion of females. This might suggest a shift in the mixture of recent infections and reactivated cases during the past 20 years toward more reactivated cases, indicating an improved living standard and tuberculosis control activities. The dominance of the Beijing family genotype was well established based on the fact that 92% of the total and a similar percentage of positive samples for each 10-year time period were of this type. If we factor in the possibility of reactivation in older patients found in the more recent groupings, we still come up with a limit in our study to the 1950s.
In summary, we demonstrated that the Beijing family has been dominant in the Beijing area since the middle of 1950s. Our efforts to improve sensitivity by increasing the dilution factor of the DNA templates did not enhance detection. Finally, we demonstrated that the spoligotyping method is a valuable and sensitive tool for providing typing information on stored specimens. Further studies of older biopsies or older patients from Beijing or other areas where the Beijing family strains are currently dominant, such as Taipei, Taiwan (our unpublished data), may allow us to determine the time when the Beijing family became dominant in corresponding areas. Also, studies using these methods would give us some insights into the dynamics of tuberculosis strains within and between populations in the past.
Acknowledgments
We are indebted to the personnel of the Laboratory of Medical Microbiology, Deventer, The Netherlands, and the Department of Pathology, Beijing Tuberculosis Research Institute and National Tuberculosis Control Center, Beijing, People’s Republic of China, whose assistance have made this work possible. We thank Annelies Bunschoten and Ria Hoentjen for technical assistance in spoligotyping. Norbert Tiemessen is acknowledged for help in sectioning the lung biopsy. Haiqing Zhang is acknowledged for assistance with the selection and organization of the lung biopsies.
Lishi Qian was financially supported by the Leahi Trust, Hawaii Community Foundation, Pacific Health Institute, Honolulu, Hawaii, and the Art and Science Advisory Council Award, University of Hawaii.
REFERENCES
- 1.Daley C L, Small P M, Schecter G F, Schoolnik G K, McAdam R A, Jacobs W R, Jr, Hopewell P C. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: an analysis using restriction fragment length polymorphism. N Engl J Med. 1992;326:231–235. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 2.Dwyer B, Jackson K, Raios K, Sievers A, Wilshire E, Ross B. DNA restriction fragment analysis to define an extended cluster of tuberculosis in homeless men and their associates. J Infect Dis. 1993;167:490–494. doi: 10.1093/infdis/167.2.490. [DOI] [PubMed] [Google Scholar]
- 3.Edlin B R, Tokars J I, Grieco M H, Crawford J T, Williams J, Sordillo E M, Ong K R, Kilburn J O, Dooley S W, Castro K G, Jarvis W R, Holmberg S D. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–1521. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 4.Greenfield L, White T J. Sample preparation methods. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular biology: principles and applications. Washington, D.C: American Society for Microbiology; 1993. pp. 122–137. [Google Scholar]
- 5.Hermans P W M, van Soolingen D, Bik E M, de Haas P E W, Dale J W, van Embden J D A. Insertion element IS987 from Mycobacterium bovis BCG is located in a hot-spot integration region for insertion elements in Mycobacterium tuberculosis complex strains. Infect Immun. 1991;59:2695–2705. doi: 10.1128/iai.59.8.2695-2705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J D A. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazurek G H, Cave M D, Eisenach K D, Wallace R J, Jr, Bates J H, Crawford J T. Chromosomal DNA fingerprint patterns produced with IS6110 as strain-specific markers for epidemiologic study of tuberculosis. J Clin Microbiol. 1991;29:2030–2033. doi: 10.1128/jcm.29.9.2030-2033.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian L, Traore H, van Soolingen D, van Embden J D A, Huang Z, Portaels F, Douglas J T. Abstracts of the 95th General Meeting of American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. IS6110-based RFLP Analysis of M. tuberculosis strains from P. R. China. [Google Scholar]
- 9.van der Zanden A G M, Hoentjen A H, Heilmann F G C, Weltevreden E F, Schouls L M, van Embden J D A. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis complex in paraffin wax embedded tissues and in stained microscopic preparations. Mol Pathol. 1998;51:209–214. doi: 10.1136/mp.51.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P W M, Martin C, Mcadam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Soolingen D, Qian L, de Haas P E W, Douglas J T, Enkhasaikan D, van Embden J D A. Predominance of a single clone of Mycobacterium tuberculosis in countries of East Asia. J Clin Microbiol. 1995;33:3234–3238. doi: 10.1128/jcm.33.12.3234-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Soolingen D, Hermans P W M, de Haas P E W, Soll D R, van Embden J D A. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]