Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a pandemic fatal infection with no known treatment. The severity of the disease and the fast viral mutations forced the scientific community to search for potential solution. Here in the present manuscript, some benzofused1,2,3triazolesulfonamide hybrids were synthesized and evaluated for their anti- SARS-CoV-2 activity using in silico prediction then the most potent compounds were assessed using in-Vitro analysis. The in-Silico study was assessed against RNA dependent RNA polymerase, Spike protein S1, Main protease (3CLpro) and 2′-O-methyltransferase (nsp16). It was found that 4b and 4c showed high binding scores against RNA dependent RNA polymerase reached −8.40 and −8.75 kcal/mol, respectively compared to the approved antiviral (remdesivir −6.77 kcal/mol). Upon testing the binding score with SARS-CoV-2 Spike protein it was revealed that 4c exhibited the highest score (−7.22 kcal/mol) compared to the reference antibacterial drug Ceftazidime (−6.36 kcal/mol). Surprisingly, the two compounds 4b and 4c showed the highest binding scores against SARS-CoV-2 3CLpro (−8.75, −8.48 kcal/mol, respectively) and nsp16 (- 8.84 and – 8.89 kcal/mol, respectively) displaying many types of interaction with all the enzymes binding sites. The derivatives 4b and 4c were examined in vitro for their potential anti-SARS-CoV-2 and it was revealed that 4c was the most promising compound with IC50 reached 758.8108 mM and complete (100%) inhibition of the binding of SARS-CoV-2 virus to human ACE2 can be accomplished by using 0.01 mg.
Keywords: Anti-COVID-19, Docking study, Remdesivir, Lopinavir, N3, Sinefungin, In vitro
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a recently human threatening infectious virus causing COVID-19 infection that has been reported as a pandemic disease at 11th March 2020. SARS-CoV-2 is an RNA virus (singlestrand) containing a spike protein gene (S), membrane protein gene (M), nonstructural proteins (nsps), envelope protein gene (E), nucleocapsid protein gene (N), and many unidentified nonstructural open reading frames [1]. Recently, the urged need has been increased to discover an efficient drug that can be subjected to clinical investigations as anti-COVID-19 drugs. Remdesivir inhibits the RNA-dependent RNA polymerase (RdRp) (responsible for the viral replication) while umifenovir can block the viral entry by inhibiting the membrane fusion [2]. SARS-CoV-2 can be inhibited by several routes including the inhibition of viral and host protease, host-directed treatment, and interferons (IFNs) [3]. Spike protein (S protein) has a critical role in the viral fusion with the human cell, so it became a vital therapeutic target [4]. While the main protease enzyme (Mpro or 3CLpro) plays a vital rule in viral replication and maturation [5]. This protein is the most exposed part of COVID-19 because it belongs to the class of non-structural proteins of the viral genome [6]. Lately, another target was explored it's known as 2′-O-methyltransferase (nsp16) which is crucial enzyme in the viral survival. 2′-O-methyltransferase protects the viral RNA from the human innate immunity by forming a special array (Cap) at the RNA 5′ end consisting of N-methylated guanosine triphosphate and C2′-O-methyl-ribosyladenine. The formed RNA cap is like the native mRNA of the human cells which helps in the RNA stabilization and guarantees its effective translation [7].
Heterocyclic compounds specially the five membered (thiazoles, thiadiazoles, triazoles, 1,3,4-oxadiazoles and benzimidazole ring) have shown a very interesting biological activity [8]. These compounds also showed very promising antiviral activity against different viruses [9]. Triazole based derivatives has been attracting the researcher's attention due to its effectiveness as therapeutic drugs in the field of antibacterial, antiviral, antifungal, antitumor and antihypertensive agents. 1,2,3triazole ring acts as a linker to enhance the biological activity through hybrids formation [10]. Ribavirin is a triazole nucleoside antiviral drug which magnetizes the researches into modifying the antiviral molecules containing the triazole moiety [11]. For example, 1-benzyl-1H-1,2,3-triazoles coupled with different carbohydrate molecules were examined as anti HIV activities and one the tested compounds showed a higher cytotoxic effect than azidothymidine (the reference drug) with a higher selective index (SI) than zalcitabine and didadosine [11]. Their potential theoretical profile, low cytotoxicity and high activity made these compounds potent leading molecules for further investigations.
Based on these observation, we have anticipated to investigate the anti-COVID-19 activity of some benzofused1,2,3triazolesulfonamide hybrids through viral enzymes inhibition against RNA dependent RNA polymerase, Spike protein S1, Main protease and 2′-O-methyltransferase (nsp16) using docking study. While the most potent molecules were further investigated using SARS-COV-2 inhibitor screening kit.
2. Materials and methods
2.1. Chemical synthesis
In the present study, we have reproduced the synthesis of some novel 1,2,3-triazole-sulfa drug hybrids carrying different heterocyclic entities, including benzothiazole, isatin and/or benzimidazole core according to our reported procedure [12,13] (Scheme 1 ). The synthetic methodology adopted required Cu(I)-catalyzed click 1,3-dipolar cycloaddition reaction between the appropriate heterocyclic akynes 1a-c with a variety of sulfa drug azides 2a-e in the presence of CuSO4 and sodium ascorbate as catalysts. The click reactions were carried out in an aqueous solution of DMSO, at room temperature and/or under heating, according to the nature of the alkyne.
Scheme 1.
Click synthesis of some novel 1,2,3-triazole-sulfadrug hybrids carrying benzofused heterocycles 3a-i.
The study includes also the synthesis of bis-(1,2,3-triazole-sulfa-drug) benzimidazole hybrids 4a-c, through the coupling of the bis-propargylated benzimidazole with the appropriate sulfa drug azides in 1:2 ratio, under the same optimized Cu(I)-click conditions (Scheme 2 ). Full characterization of the newly designed 1,2,3-triazole hybrids has been reported in detail in our previously reported works (Table 1 ) [12,13].
Scheme 2.
Structures of some novel bis-(1,2,3-triazole-sulfadrug hybrids) carrying benzimidazole moiety 4a-c.
Table 1.
Structures of the synthesized benzofused1,2,3triazolesulfonamide hybrids 3a-i and 4a-c.
| Compound code | Structure |
|---|---|
| 3a |  |
| 3b |  |
| 3c |  |
| 3d |  |
| 3e |  |
| 3f |  |
| 3g |  |
| 3h |  |
| 3i |  |
| 4a |  |
| 4b |  |
| 4c |  |
2.2. Molecular modeling
In order to fight the global pandemic of COVID-19 several essential targets have been utilized in anti-COVID-19 drug discovery. Most of these enzyme targets have a potential role in replication of viral genome, viral entry into the host cell, viral replication process, and viral survival. Examples of these enzymes are RNA dependent RNA polymerase (RdRp) [14], spike protein S1 [15], main protease (3CLpro) [16], and 2′-O-methyltransferase (nsp16) [17]. Thus, docking studies were performed to predict the binding affinity and the binding features of 12 investigated compounds toward SARS-CoV-2 RdRp, SARS-CoV-2 Spike protein S1, SARS-CoV-2 3CLpro, and SARS-CoV-2 nsp16 to develop novel potential inhibitors for these four main targets.
2.2.1. Viral enzymes preparation
For molecular docking calculations, the MOE 2019 was utilized, the X-ray crystal structures of the docking targets; SARS-CoV-2 RdRp (PDB ID: 7BTF) [18], SARS-CoV-2 Spike protein S1 (PDB ID: 6VW1) [19], SARS-CoV-2 3CLpro (PDB ID: 6Y2G) [20], and SARS-CoV-2 nsp16 (PDB ID: 6wkq) [21] were downloaded from the Protein Data Bank (PDB) website and were taken as templates, followed by addition of all missing hydrogen atoms, then the target protein was prepared employing the default “Structure preparation” module settings. ‘Site Finder’ feature of MOE was employed in search for the receptor binding site.
2.2.2. Database generation and optimization
Twelve test compounds were drawn using ChemDraw program and collected in database in MOE 2019. This database was subjected to displaying hydrogens, default energy minimization and computation of partial charges. Triangular matcher algorithm was applied to set the ligand placement. The default scoring function was alpha HB which generated the top 5 non-redundant poses of the lowest binding energy conformers of the tested compounds. Docking was conducted for the prepared database with induced fitting protocol to record the best possible molecular interactions. The consensus scoring in Kcal-mol was calculated using two scoring functions, alpha hydrogen bonding and London dG forces. Results were listed based on the S-scores with RMSD value < 2 Å. Molecular docking results are often validated using a training set of experimental ligand–protein complexes, and the accuracy of this docking software is mainly dependent on the used training set [22]. Due to the lack of known COVID-19 active ligands, it is important to ensure that the used program can replicate the binding mode of a known experimental inhibitor for the studied enzyme. Although no effective antiviral drug against COVID-19 infection is currently available, several reports have indicated that RdRp inhibitor (Remdesivir) [23], spike protein inhibitor (Ceftazidime) [24], protease inhibitor (N3) [25], in addition to methyltransferase inhibitor (Sinefungin) [18] have the potential for designing active SARS-CoV-2 inhibitors. In the attempt to have reference values (positive control) the anti-viral drug Remdesivir, antibacterial drug Ceftazidime, Michael acceptor inhibitor N3 and Sinefungin were considered as comparative standards for the molecular docking. Finally, conformers that give best docking scores and ligand-enzyme interactions were selected and analyzed. Graphical representations of the interactions were then generated and inspected.
2.3. In vitro antiviral activity
SARS-COV-2 inhibitor screening kit (COVID 19 Coronavirus Assay Kit, Biosource, USA) was used to evaluate the anti-COVID-19 activity of the promising compounds in vitro. The proposed inhibitor screening assay is based on a colorimetric ELISA kit, which measures the binding of the RBD of the Spike S protein from SARS-CoV-2 to its human receptor ACE2. Thus, this assay allows identifying and characterizing the effect of different inhibitory compounds on the inhibition of the binding of SARS-CoV-2 virus to human ACE2. According to the sample manual different concentration of the compounds were prepared and incubated with Spike S protein for 1 h at 37 °C then the OD was measured at 450 nm using ELISA reader [26].
2.4. In silico prediction of physicochemical properties and drug likeness score
In silico prediction of physicochemical properties and drugability evaluation are nowadays used to select the most promising drug like candidates and reduce the risk of late-stage drug attrition [[27], [28], [29]]. In the present work, compounds 4b and 4c were subjected to prediction of the physical and molecular properties using various tools, such as Molinspiration and Mol-Soft software [30,31]. Molinspiration chemoinformatic server was used to calculate topological polar surface area (PSA) and Lipinski's rule violation. This rule concluded the required parameters for drug likeness: molecular weight should be less than 500, logP ≤5, hydrogen bond donors (HBD) ≤ 5, and hydrogen bond acceptors (HBA) ≤ 10. Compounds which comply with this rule are considered as drug-like candidates [[32], [33], [34], [35]]. Drug likeness score and solubility parameters were calculated using Mol-Soft software.
3. Results and discussion
Several repurposed drugs alone or in combinations have been subjected to clinical trials to treat COVID-19 infection [8]. The mechanism of action of most of these drugs is to target the viral replication process or block viral entry into the host cell.
3.1. Molecular docking calculations
The predicted binding affinities and features of the investigated compounds towards the 4 main enzyme targets (SARS-CoV-2 RdRp, SARS-CoV-2 Spike protein S1, SARS-CoV-2 3CLpro, and SARS-CoV-2 nsp16) are listed in Table 2, Table 3, Table 4, Table 5 . The 2D and 3D representations of interactions of the inspected compounds with the key amino acid residues of the target enzymes are illustrated in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5 .
Table 2.
Calculated docking scores (in Kcal/mol) and binding features for the COVID-19 drug candidates against SARS-CoV-2RdRp.
| Compound Code | Docking score (Kcal/mol) | Binding Features (Hydrogen bond) |
|---|---|---|
| Remdesivir | −6.77 | GLU167, ASP618, LYS621& LYS798 |
| 3a | −6.39 | LYS621 |
| 3b | −6.43 | LYS621, TYR619 |
| 3c | −6.32 | ARG553, ARG555, LYS621 |
| 3d | −7.04 | ARG555, THR556 |
| 3e | −6.54 | ARG555 |
| 3f | −6.84 | ARG555, ASP618 |
| 3g | −7.12 | ARG555, ASP618 |
| 3h | −7.00 | ARG553, ARG555, ASP623 |
| 3i | −6.95 | ARG555 |
| 4a | −7.99 | GLN444, ASN552, LYS798, |
| 4b | −8.40 | LYS545, ARG555, LYS621 |
| 4c | −8.75 | ARG553, TYR619, ASP623, ARG624 |
Table 3.
Calculated docking scores (in Kcal/mol) and binding features for the COVID-19 drug candidates against SARS-CoV-2 Spike protein S1.
| Compound Code | Docking score (Kcal/mol) | Binding Features (Hydrogen bond) |
|---|---|---|
| Ceftazidime | −6.36 | LYS403, GLY504, TYR505 |
| 3a | −5.23 | ARG408 |
| 3b | −5.58 | ASP406, GLN409, VAL417, TYR453, GLY496 |
| 3c | −5.71 | LYS403, ARG408, VAL417 |
| 3d | −6.07 | LYS403, ARG408 |
| 3e | −5.72 | LYS403, ASP406, GLY496 |
| 3f | −5.66 | ASP406, GLY496 |
| 3g | −5.78 | LYS403, GLN409 |
| 3h | −6.03 | ARG408, THR415 |
| 3i | −5.52 | LYS403, THR415 |
| 4a | −6.44 | ARG408, THR415, GLY496 |
| 4b | −7.15 | LYS403, ASP406, GLN409 |
| 4c | −7.22 | LYS403, ARG408, GLY496 |
Table 4.
Calculated docking scores (in Kcal/mol) and binding features for the COVID-19 drug candidates against SARS-CoV-23CLpro.
| Compound Code | Docking score (Kcal/mol) | Binding Features (Hydrogen bond) |
|---|---|---|
| N3 | −8.45 | GLY143, HIS163, HIS164, MET165, GLU166, GLN189, THR190 |
| 3a | −6.31 | CYS145 |
| 3b | −6.43 | CYS145, MET165, GLU166, GLN192 |
| 3c | −6.09 | ASN142, GLY143, THR190, GLN192 |
| 3d | −6.90 | ASN142, HIS163, GLN192 |
| 3e | −6.80 | CYS145, GLN192 |
| 3f | −6.95 | MET49, MET165, GLN192 |
| 3g | −6.61 | CYS145 |
| 3h | −7.44 | MET165, GLN192 |
| 3i | −7.09 | ASN142, HIS163, GLN192 |
| 4a | −8.19 | MET49, MET165, GLU166, GLN192 |
| 4b | −8.75 | CYS145, HIS163, GLN192 |
| 4c | −8.48 | MET49, HIS164, MET165, GLN192 |
Table 5.
Calculated docking scores (in Kcal/mol) and binding features for the COVID-19 drug candidates against SARS-CoV-2nsp16.
| Compound Code | Docking score (Kcal/mol) | Binding Features (Hydrogen bond) |
|---|---|---|
| Sinefungin | −7.35 | ASN6841, ASP6897, ASP6912, CYS6913 |
| 3a | −7.09 | ASN6841, LEU6898, CYS6913, MET6929 |
| 3b | −6.67 | LYS6844, ASP6897 |
| 3c | −7.16 | ASP6912, ASP6897 |
| 3d | −7.46 | LYS6844, ASP6897 |
| 3e | −7.26 | ASN6841, ASP6897 |
| 3f | −7.32 | ASP6897, ASP6912, LYS6935 |
| 3g | −7.40 | ASP6897, LYS6935 |
| 3h | −7.47 | ASN6841, ASP6897, MET6929, TYR6930 |
| 3i | −7.09 | ASN6841, GLY6871, CYS6913, ASP6928, |
| 4a | −8.65 | ASP6912, TYR6930, LYS6968, ASN6996 |
| 4b | −8.84 | ASN6841, GLY6869, ASP6897, ASN6996 |
| 4c | −8.89 | ASP6873, ASP6912, ASP6928 |
Fig. 1.
(A) 3D binding mode of Remdesivir, (B) 2D binding mode of Remdesivir, (C) 3D binding mode of compound 4b, (D) 2D binding mode of compound 4b, (E) 3D binding mode of compound 4c, (F) 2D binding mode of compound 4c inside SARS-CoV-2 RdRp active site.
Fig. 2.
Mode of binding of (A) 3D binding mode of Ceftazidime, (B) 2D binding mode of Ceftazidime, (C) 3D binding mode of compound 4b, (D) 2D binding mode of compound 4b, (E) 3D binding mode of compound 4c, (F) 2D binding mode of compound 4c inside SARS-CoV-2 Spike protein S1 active site.
Fig. 3.
3D Binding mode of compound 4c (blue) in the binding interface of SARS-CoV-2 Spike protein S1 in complex with hACE2. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Mode of binding of (A) 3D binding mode of N3, (B) 2D binding mode of N3, (C) 3D binding mode of compound 4b, (D) 2D binding mode of compound 4b, (E) 3D binding mode of compound 4c, (F) 2D binding mode of compound 4c inside SARS-CoV-2 3CLpro active site.
Fig. 5.
Mode of binding of (A) 3D binding mode of Sinefungin, (B) 2D binding mode of Sinefungin, (C) 3D binding mode of compound 4b, (D) 2D binding mode of compound 4b, (E) 3D binding mode of compound 4c, (F) 2D binding mode of compound 4c inside SARS-CoV-2 nsp16 active site.
3.1.1. SARS-CoV-2 RdRp
As can be observed from data in Table 2, the binding scores of the investigated compounds with SARS-CoV-2 RdRp ranged from −6.32 to −8.75 kcal/mol, with 8 Compounds among the 12 potential leads, were able to achieve better binding scores than the reference drug Remedisivir (−6.77 kcal/mol). Moreover, compounds 4b and 4c showed a strong binding affinity with many types of interactions with the enzyme binding site (Fig. 1). Inspecting the compound-RdRp interactions indicated that most of the compounds possess a similar binding mode inside the RdRp active site, exhibiting fundamental hydrogen bonds with ARG553, ARG555 and LYS621. Further interactions, including hydrogen bonds, hydrophobic interactions, and pi-based interactions, were also seen between the compounds and the proximal amino acids inside the SARS-CoV-2 RdRp active site.
3.1.2. SARS-CoV-2 spike protein S1
As can be seen from data in Table 3, all the investigated compounds demonstrated promising docking scores toward SARS-CoV-2 Spike ranging from −5.23 to −7.22 kcal/mol which were comparable to the reference antibacterial drug Ceftazidime (−6.36 kcal/mol). In particular, compounds 4b and 4c (Fig. 2) exhibited the best scoring results (−7.15 and −7.22 kcal/mol) among the 12 test compounds. Most of the investigated compounds were able to accommodate into the receptor-binding domain (RBD) of the spike protein exhibiting similar binding features and interact with the key amino acids LYS403, ASP406 and ARG408 through hydrogen bonds which may destabilize or even prevent spike protein–ACE2 interaction.
Compound 4c was selected for further analysis to examine the binding affinity between 4c and S-RBD or ACE2-ECD. Docking of the compound 4c at the interface of SARS-CoV-2 Spike protein S1 in complex with hACE2 (PDB ID: 6VW1) (Fig. 3) indicated a strong and stable interactions between the compound 4c and S-RBD. By contrast, compound 4c and ACE2-ECD showed no binding interactions.
3.1.3. SARS-CoV-2 3CLpro
As can be noticed from data in Table 4, compounds 4b and 4c (Fig. 4) among all test compounds, exhibited higher docking scores against SARS-CoV-2 3CLpro than the reference N3 (−8.45 kcal/mol), while the other compounds demonstrated promising binding affinities with docking scores ranging from −6.09 to −7.44 kcal/mol. Structural insights into the binding modes of the investigate compounds with 3CLpro demonstrated that most of the compounds achieve similar binding interactions inside the 3CLpro active site which is comparable to that exhibited by the reference N3. Most of the compounds exhibit essential hydrogen bonds with ASN142, MET165 and GLN192 in addition to hydrophobic interactions, and pi-based interactions with the proximal amino acids inside the SARS-CoV-2 3CLpro active site.
3.1.4. SARS-CoV-2 nsp16
As can be observed from data in Table 5, the docking scores of the inspected compounds with SARS-CoV-2 nsp16 ranged from −5.68 to −10.61 kcal/mol, with 6 Compounds among the 12 potential leads, demonstrated higher binding score than the reference drug Sinefungin (−7.35 kcal/mol). Surprisingly, the two compounds 4b and 4c (Fig. 5) achieved the highest docking score (- 8.84 and – 8.89 kcal/mol) displaying many types of interaction with the enzyme binding site. Examining the compound-nsp16 binding features indicated that most of the compounds possess a close binding mode inside the nsp16 active site, which is similar to that exhibited by the reference drug Sinefungin. It is also worth noting that most of the compounds displaying a key hydrogen bond with ASP6897, as well as, hydrophobic interactions, and pi-based interactions, were also seen between the compounds and the proximal amino acids.
3.2. In vitro antiviral activity
The most potent compounds 4b and 4c that proved to have the best scoring docking results with many types of interaction with COVID-19 enzymes were tested for the in vitro anit-COVID-19 activity. Results were in accordance with the docking results. Data in Fig. 6 proved that 4c was the most promising compound with IC50 reached 758.8108 mM. In the present research, the complete (100%) inhibition of the binding of SARS-CoV-2 virus to human ACE2 can be accomplished by using 0.01 mg of the potent compound 4c.
Fig. 6.
The remaining viral binding activity upon using different concentrations of the most potent compounds.
Sulfonamides hybrids attracted so many researchers attention to develop non-nucleoside antivirals due to the increased cross-resistance to nucleoside inhibitors. Hybridization of bioactive pharmacophores with sulfonamides has been used as a strategy to develop sulfonamide antivirals [36]. In the present research different hybrids of benzofused1,2,3triazolesulfonamide were tested for their anti-COVID-19 activity. Compounds 4b and 4c showed the most promising scoring results with many types of interaction with viral enzymes. However, Compounds 4c exhibited the highest antiviral activity in vitro with IC50 was 758.8108 mM. Our results were in accordance with Shin et al. [37] who proved that cyclic sulfonamide derivatives will be the savior against SARS-CoV-2 because of their IC50 inhibition reached 0.88 μM with no proved cytotoxic effect (CC50 > 25 μM), and high oral bioavailability equaled 77% with good metabolic stability. He et al. [38] tested the antiviral activity of some purine nucleoside derivatives containing a sulfonamide moiety and it was proved that the antiviral activity was related to the immune induction effect. Azzam et al. [39] proved the antiviral effect of some (3-benzo- [d]thiazole-2-yl)-2-pyridonesagainst both DNA viruses (herpes simplex virus type 1 (HSV-1)) and adenovirus type 7 (HAdV7) in addition to RNA viruses (coxsackie virus B4 (CBV4), hepatitis A virus (HAV) HM175, and HCVcc genotype). The in silico toxicity of the compounds 4b and 4c were reported in our previous procedure [12,13] which predicted that both compounds were non-carcinogenic. Further acute toxicity study using in vivo analysis was performed [12,13] and LD50 in rat was proved to be 2.29–2.41 mol/kg.
3.3. In silico prediction of physicochemical properties and drug likeness score
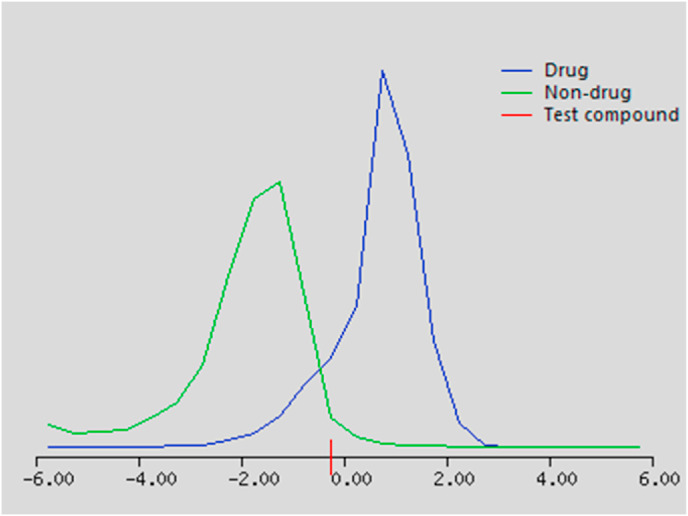
The predicted physicochemical properties and drug likeness scores of the investigated compounds 4b and 4c are listed in Table 6, Table 7 . According to Molinspiration and Molsoft software calculations, the two compounds 4b and 4c showed logP value less than 5, number of H-bond donors less than 5. While the violations of Lipinski's rule were in two parameters, molecular weight above 500 Da and number of hydrogen bond acceptors more than 10. It is worth mentioning that number of rotatable bonds is significant for prediction of conformational changes and binding to receptors. All compounds fulfilled this criterion and displayed high conformational flexibility (13 rotatable bond). On the other hand, solubility of drugs is a crucial issue for reasonable absorption. Both compounds 4b and 4c were considered as good absorbable drugs as their solubility values were more than 0.0001 mg/L [31,40]. Bioactivity prediction results revealed that these compounds showed acceptable drug-likeness scores as being enzyme inhibitors (Fig. 7, Fig. 8 ).
Table 6.
In silico physicochemical properties data of compounds 4b and 4c using Molinspiration software.
| Comp No. | LogPa | M.Wtb | HBAc | HBDd | Lipiniski's violation | NROTBe | TPSAf A2 |
|---|---|---|---|---|---|---|---|
| 4b | 2.35 | 778.14 | 14 | 2 | 2 | 13 | 223 |
| 4c | 4.11 | 834.20 | 14 | 2 | 2 | 13 | 223 |
g%ABS: percentage of absorption.
LogP: logarithm of compound partition coefficient between n-octanol and water.
M.Wt: molecular weight.
HBA: number of hydrogen bond acceptors.
HBD: number of hydrogen bond donors.
NROTB: number of rotatable bonds.
TPSA: topological polar surface area.
Table 7.
In silico prediction of drug likeness score of compounds 4b and 4c using Molsoft software.
| Comp No. | Sa (mg/L) | Drug likeness model score |
|---|---|---|
| 4b | 871.32 | −0.24 |
| 4c | 81.17 | −0.27 |
S: solubility.
Fig. 7.
Drug-likeness plot of compound 4b.
Fig. 8.
Drug-likeness plot of compound 4c.
4. Conclusion
Nowadays several computational studies are done to predict some structural information about repurposed drugs to treat COVID-19. These studies can predict physicochemical properties, type of interactions with target macromolecules, bioactivity score, pharmacokinetic profile, and alignment with reference drugs. In the current study, molecular docking was performed in order to predict the binding affinity and the binding features of the 24 drug candidates toward the enzyme targets (SARS-CoV-2 RdRp, SARS-CoV-2 Spike protein S1, SARS-CoV-2 3CLpro, and SARS-CoV-2 nsp16). Results of the docking studies revealed that most of the investigated compounds demonstrated promising binding scores, in addition to excellent binding manner with the active site of target enzymes in comparison to the reference (Remdesivir, Ceftazidime, N3 and Sinefungin). In particular, Compounds 4b, 4c, and 4a exhibited the best scoring results with many types of interaction with the target enzymes. In the present research, the complete (100%) inhibition of the binding of SARS-CoV-2 virus to human ACE2 can be accomplished by using 0.01 mg of the potent compounds 4c. In addition, both compounds 4b and 4c showed reasonable drug-likeness scores which advocated these compounds to be drug-like candidates.
CRediT authorship contribution statement
Abdullah Y. Alzahrani: Funding acquisition, Project administration, Resources, Supervision, Formal analysis. Marwa M. Shaaban: Software, Validation, Methodology, Writing – original draft, Formal analysis, Writing – review & editing. Bassma H. Elwakil: Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Moaaz T. Hamed: Conceptualization, Data curation, Formal analysis, Investigation, Methodology. Nadjet Rezki: Conceptualization, Data curation, Formal analysis, Investigation, Methodology. Mohamed R. Aouad: Conceptualization, Data curation, Formal analysis, Investigation, Methodology. Mohamed A. Zakaria: Conceptualization, Data curation, Formal analysis, Investigation, Methodology. Mohamed Hagar: Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Research Project under grant number (RGP.2/83/42).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemolab.2021.104421.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Fig. S1.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H.…Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. 10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.H Elwakil B., Shaaban M.M., Bekhit A.A., El-Naggar M.Y., Olama Z.A. Potential anti-COVID-19 activity of Egyptian propolis using computational modeling. Future Virol. 2021;16(2):107–116. . [Google Scholar]
- 3.Andersen P.I., Ianevski A., Lysvand H., et al. Discovery and development of safe-in-man broad-spectrum antiviral agents. Int. J. Infect. Dis. 2020;93:268–276. doi: 10.1016/j.ijid.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robson B. Bioinformatics studies on a function of the SARS-CoV-2 spike glycoprotein as the binding of host sialic acid glycans. Comput. Biol. Med. 2020;122:103849. doi: 10.1016/j.compbiomed.2020.103849. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi R.S., Jagdale S.S., Bansode S.B., Shankar S.S., Tellis M.B., Pandya V.K., Chugh A., Giri A.P., Kulkarni M.J. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1760137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in-silico approach. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krafcikova P., Silhan J., Nencka R., et al. Structural analysis of the SARS-CoV-2 methyltransferase complex involved in RNA cap creation bound to sinefungin. Nat. Commun. 2020;11:3717. doi: 10.1038/s41467-020-17495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saghyan A.S., Simonyan H.M., Petrosyan S.G., Geolchanyan A.V., Roviello G.N., Musumeci D., Roviello V. Thiophenyl-substituted triazolyl-thione L-alanine: asymmetric synthesis, aggregation and biological properties. Amino Acids. 2014;46(10):2325–2332. doi: 10.1007/s00726-014-1782-3. . [DOI] [PubMed] [Google Scholar]
- 9.Tawfik S.S., Liu M., Farahat A.A. Antiviral activity of thiadiazoles, oxadiazoles, triazoles and thiazoles. Org. Chem. 2020:180–218. (part i) [Google Scholar]
- 10.H Zhou C., Wang Y. Recent researches in triazole compounds as medicinal drugs. Curr. Med. Chem. 2012;19(2):239–280. doi: 10.2174/092986712803414213. . [DOI] [PubMed] [Google Scholar]
- 11.Da Silva F.D.C., De Souza M.C.B.V., Frugulhetti I.I.P., Castro H.C., Souza S.L.D.O., De Souza T.M.L., Rodrigues D.Q., Souza A.M.T., Abreu P.A., Passamani F., Rodrigues C.R., Ferreira V.F. Synthesis, HIVRT inhibitory activity and SAR of 1-benzyl-1H-1,2,3-triazole derivatives of carbohydrates. Eur. J. Med. Chem. 2009;44:373–383. doi: 10.1016/j.ejmech.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 12.Rezki N., Almehmadi M.A., Ihmaid S., Shehata A.M., Omar A.M., Ahmed H.E., Aouad M.R. Novel scaffold hopping of potent benzothiazole and isatin analogues linked to 1, 2, 3-triazole fragment that mimic quinazoline epidermal growth factor receptor inhibitors: synthesis, antitumor and mechanistic analyses. Bioorg. Chem. 2020;103:104133. doi: 10.1016/j.bioorg.2020.104133. . [DOI] [PubMed] [Google Scholar]
- 13.Al-blewi F.F., Almehmadi M.A., Aouad M.R., et al. Design, synthesis, ADME prediction and pharmacological evaluation of novel benzimidazole-1,2,3-triazole-sulfonamide hybrids as antimicrobial and antiproliferative agents. Chem. Cent. J. 2018;12:110. doi: 10.1186/s13065-018-0479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Fu X., Duan D., Liu X., Xu J., Gao X. Extraction and identification of phlorotannins from the brown alga, sargassumfusiforme (Harvey) setchell. Mar. Drugs. 2017;15(2):49. doi: 10.3390/md15020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borbone N., et al. Nucleoside analogs and nucleoside precursors as drugs in the fight against SARS-CoV-2 and other coronaviruses. Molecules. 2021;26(4):986. doi: 10.3390/molecules26040986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Hawary S.S., et al. In silico identification of SARS-CoV-2 spike (S) protein–ACE2 complex inhibitors from eight Tecoma species and cultivars analyzed by LC-MS. RSC Adv. 2020;10(70):43103–43108. doi: 10.1039/d0ra08997d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vicidomini C., Roviello V., Roviello G.N. In silico investigation on the interaction of chiral phytochemicals from opuntia ficus-indica with SARS-CoV-2 mpro. Symmetry. 2021;13(6):1041. [Google Scholar]
- 18.Krafcikova P., et al. Structural analysis of the SARS-CoV-2 methyltransferase complex involved in RNA cap creation bound to sinefungin. Nat. Commun. 2020;11(1):3717. doi: 10.1038/s41467-020-17495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Y., et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368(6492):779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang J., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L., et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosas-Lemus M., et al. High-resolution structures of the SARS-CoV-2 2'-O-methyltransferase reveal strategies for structure-based inhibitor design. Sci. Signal. 2020;13(651) doi: 10.1126/scisignal.abe1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin C., et al. Ceftazidime is a potential drug to inhibit SARS-CoV-2 infection in vitro by blocking spike protein–ACE2 interaction. Signal Transduction and Targeted Therapy. 2021;6(1):198. doi: 10.1038/s41392-021-00619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Z., et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 26.Chua M.H., Cheng W., Goh S.S., Kong J., Li B., Lim J.Y.…Loh X.J. 2020. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van De Waterbeemd H., Gifford E. ADMET in silico modelling: towards prediction paradise? Nat. Rev. Drug Discov. 2003;2(3):192–204. doi: 10.1038/nrd1032. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita F., Hashida M. In silico approaches for predicting ADME properties of drugs. Drug Metabol. Pharmacokinet. 2004;19(5):327–338. doi: 10.2133/dmpk.19.327. [DOI] [PubMed] [Google Scholar]
- 29.Mälkiä A., et al. Drug permeation in biomembranes: in vitro and in silico prediction and influence of physicochemical properties. Eur. J. Pharmaceut. Sci. 2004;23(1):13–47. doi: 10.1016/j.ejps.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 30.http://molsoft.com/mprop/
- 31.http://www.molinspiration.com/
- 32.Grime K.H., Barton P., McGinnity D.F. Application of in silico, in vitro and preclinical pharmacokinetic data for the effective and efficient prediction of human pharmacokinetics. Mol. Pharm. 2013;10(4):1191–1206. doi: 10.1021/mp300476z. [DOI] [PubMed] [Google Scholar]
- 33.Onguéné P.A., et al. The potential of anti-malarial compounds derived from African medicinal plants, part III: an in silico evaluation of drug metabolism and pharmacokinetics profiling. Organic and medicinal chemistry letters. 2014;4(6):1–9. doi: 10.1186/s13588-014-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butina D., Segall M.D., Frankcombe K. Predicting ADME properties in silico: methods and models. Drug Discov. Today. 2002;7(11):S83–S88. doi: 10.1016/s1359-6446(02)02288-2. [DOI] [PubMed] [Google Scholar]
- 35.Abad-Zapatero C. Ligand Efficiency Indices for Drug Discovery. Academic Press; San Diego: 2013. Chapter 5 - analysis of the content of SAR databases; pp. 67–79. [Google Scholar]
- 36.Dash R.N., Moharana A.K., Subudhi B.B. Sulfonamides: antiviral strategy for neglected tropical disease virus. Curr. Org. Chem. 2020;24(9):1018–1041. . [Google Scholar]
- 37.Shin Y.S., Lee J.Y., Noh S., Kwak Y., Jeon S., Kwon S.…Park C.M. Discovery of cyclic sulfonamide derivatives as potent inhibitors of SARS-CoV-2. Bioorg. Med. Chem. Lett. 2021;31:127667. doi: 10.1016/j.bmcl.2020.127667. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He F., Shi J., Wang Y., Wang S., Chen J., Gan X.…Hu D. Synthesis, antiviral activity, and mechanisms of purine nucleoside derivatives containing a sulfonamide moiety. J. Agric. Food Chem. 2019;67(31):8459–8467. doi: 10.1021/acs.jafc.9b02681. . [DOI] [PubMed] [Google Scholar]
- 39.Azzam R.A., Elboshi H.A., Elgemeie G.H. Novel synthesis and antiviral evaluation of new benzothiazole-bearing N-sulfonamide 2-pyridone derivatives as USP7 enzyme inhibitors. ACS Omega. 2020;5(46):30023–30036. doi: 10.1021/acsomega.0c04424. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y.H., et al. Rate-limited steps of human oral absorption and QSAR studies. Pharmaceut. Res. 2002;19(10):1446–1457. doi: 10.1023/a:1020444330011. [DOI] [PubMed] [Google Scholar]