Abstract
Protein kinase B (PKB or Akt), a downstream effector of phosphoinositide 3-kinase (PI 3-kinase), has been implicated in insulin signaling and cell survival. PKB is regulated by phosphorylation on Thr308 by 3-phosphoinositide-dependent protein kinase 1 (PDK1) and on Ser473 by an unidentified kinase. We have used chimeric molecules of PKB to define different steps in the activation mechanism. A chimera which allows inducible membrane translocation by lipid second messengers that activate in vivo protein kinase C and not PKB was created. Following membrane attachment, the PKB fusion protein was rapidly activated and phosphorylated at the two key regulatory sites, Ser473 and Thr308, in the absence of further cell stimulation. This finding indicated that both PDK1 and the Ser473 kinase may be localized at the membrane of unstimulated cells, which was confirmed for PDK1 by immunofluorescence studies. Significantly, PI 3-kinase inhibitors prevent the phosphorylation of both regulatory sites of the membrane-targeted PKB chimera. Furthermore, we show that PKB activated at the membrane was rapidly dephosphorylated following inhibition of PI 3-kinase, with Ser473 being a better substrate for protein phosphatase. Overall, the results demonstrate that PKB is stringently regulated by signaling pathways that control both phosphorylation/activation and dephosphorylation/inactivation of this pivotal protein kinase.
The lipid kinase phosphoinositide 3-kinase (PI 3-kinase) is activated following stimulation of cells by various growth and survival factors (reviewed in reference 52) and has been implicated in a number of cellular responses, including cell adhesion and motility, vesicular trafficking, gluconeogenesis, protein synthesis, and cell survival and transformation (52). The activation of PI 3-kinase results in the production of the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate (PI-3,4,5P3), whose major targets are pleckstrin homology (PH) domain-containing proteins (24, 31, 52). Protein kinase B (PKB) is a member of the second-messenger subfamily of serine/threonine kinases (3, 16, 37) and is a major target of PI 3-kinase (12, 25). Three mammalian isoforms, termed PKBα, -β, and -γ, have been identified so far (10, 37, 42); all contain an N-terminal PH domain (29) capable of binding PI-3,4,5P3 and phosphatidylinositol 3,4-bisphosphate (PI-3,4P2) (26, 35). PKB mediates many PI 3-kinase-regulated biological responses, including glucose uptake, glycogen and protein synthesis (17, 40, 54), and promotion of cell survival through the inhibition of apoptosis (21, 38, 39; reviewed in references 20 and 32). The antiapoptotic role of PKB could account for its transforming potential (9, 55). In addition, overexpression of the α and β isoforms of PKB has been detected in certain human carcinomas (reviewed in reference 27).
The main mechanism for regulation of PKB activity is phosphorylation, which occurs on Thr308/309 in the activation loop of the catalytic domain and Ser473/474 in the C-terminal region of the human α and β isoforms, respectively, following stimulation of the cells by insulin or insulin-like growth factor 1 (IGF-1) (1, 45). The kinase that phosphorylates Thr308 of PKBα has been isolated, cloned (2, 3, 50, 51), and termed 3-phosphoinositide-dependent protein kinase 1 (PDK1). It also has a PH domain at the C terminus which is likely to be responsible for high-affinity binding of PDK1 to PI-3,4,5P3 and PI-3,4P2 (50). PDK1 phosphorylates all three isoforms of PKB in vitro, in the presence of 3-phosphorylated phospholipids (2, 11, 51, 56). However, PDK1 is able to phosphorylate and activate truncated forms of PKB lacking the PH domain in the absence of 3-phosphoinositides (3, 51), implying that the lipids are required to induce an activating conformational change in PKB by binding to its PH domain, thus allowing phosphorylation in the activation loop. The current model for PKB regulation envisages the following steps (33): (i) activated PI 3-kinase through 3-phosphoinositide production recruits PKB to the membrane; (ii) phospholipid binding to the PH domain reverses its inhibitory effect on the activation loop site; (iii) PKB is phosphorylated on Thr308 and then on Ser473 by upstream kinases; and (iv) activated PKB detaches from the membrane, which allows phosphorylation of substrates in the cytosol and nucleus.
The model has been supported by findings from several laboratories. First, PKB is found to associate with the plasma membrane following stimulation by growth factors, which is then accompanied by the translocation to the nucleus (6). Second, artificial membrane targeting activates PKB due to the phosphorylation of the same set of regulatory sites (6, 45). These reports indicate that both PDK1 and the Ser473 kinase are constitutively active and able to phosphorylate their substrate, as soon as it becomes available in the same cellular compartment. However, in these membrane targeting experiments PKB accumulated in the fully active state over a 2-day transfection period, which impaired analysis of the sequence and kinetics of events leading to its activation (and inactivation).
To overcome this limitation, we created a series of constructs which allow rapid, inducible translocation of PKB to the membrane. The results revealed that following translocation PKB is rapidly and efficiently phosphorylated by PDK1 and Ser473 kinase. Furthermore, we show that PKB is rapidly dephosphorylated following the inhibition of PI 3-kinase, implying that there are also signaling pathways responsible for kinase inactivation.
MATERIALS AND METHODS
Construction of expression vectors.
The pCMV5 construct encoding hemagglutinin (HA) epitope-tagged PKBα has been described elsewhere (1). HA epitope-tagged PKB-ΔPH was created by PCR, using a 5′ oligonucleotide encoding the HA epitope, followed by the sequence Glu-Arg-Pro-Gln, containing a NotI restriction site, and amino acids 119 to 125 of human PKBα, and a 3′ oligonucleotide encoding amino acids 468 to 480. The resulting product was subcloned as a SalI/XbaI fragment into the pECE vector (23). The C1 domain of bovine protein kinase Cα (PKCα) (amino acids 26 to 162) was amplified by PCR using a 5′ oligonucleotide encoding the HA epitope, followed by amino acids 26 to 34 of bovine PKCα, and a 3′ oligonucleotide encoding amino acids 154 to 162 of bovine PKCα, followed by the sequence Glu-Arg-Pro-Gln containing a NotI restriction site. To create C1-PKB-ΔPH, the PCR product was subcloned as a SalI/NotI fragment in frame into the above-described vector pECE.PKB-ΔPH. Both PKB-ΔPH and C1-PKB-ΔPH were subcloned from pECE into the pCMV5 vector (4) as BglII/XbaI fragments. pCMV5.C1-PKB-ΔPH-S473A was created by subcloning a CelII/XbaI fragment from pECE.PKB-S473A (1) into this vector. pCMV5.C1-PKB-ΔPH-T308A was prepared by Quickchange (Stratagene) according to the manufacturer’s instructions, using mutating oligonucleotides and pCMV5.C1-PKB-ΔPH as the template. Glycogen synthase kinase 3β (GSK-3β) was tagged with the Myc epitope at the C terminus by PCR and subcloned into pRK5 mammalian expression vector (22). The pCMV5 construct encoding Myc epitope-tagged PDK1 lacking the N-terminal 51 amino acids and pcDNA3 constructs encoding the active and kinase-dead versions of the membrane-targeted catalytic subunit of PI 3-kinase (HA-p110.CAAX) have already been described (19, 48). The constructs were confirmed by restriction analysis and sequencing.
Cell culture.
Human embryonic kidney (HEK) 293 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (Life Technologies, Inc.) at 37°C in an atmosphere containing 5% CO2. Cells seeded at 0.5 × 106 per 6-cm-diameter dish were transfected the following day, by a modified calcium phosphate method (13), with plasmid DNA (0.5 μg/ml). The transfection mixture was removed after a 16-h incubation, and cells were serum starved for 24 h before stimulation with 12-O-tetradecanoylphorbol 13-acetate (TPA; 100 ng/ml; Life Technologies), 1,2-dioctanoyl-sn-glycerol (DAG; 200 μM; Sigma), mezerein (500 ng/ml; Biomol Research Laboratories), 4α-phorbol (200 ng/ml; LC Laboratories), IGF-1 (100 ng/ml; Life Technologies), or 0.2 mM pervanadate prepared as described previously (5). In some cases cells were pretreated with LY 294002 (50 to 100 μM; Calbiochem), 17-hydroxywortmannin (wortmannin; 200 nM; gift from M. Thelen, Theodor Kocher-Institut, Bern, Switzerland), staurosporine (200 ng/ml; Biomol Research Laboratories), bisindolylmaleimide I (1 μg/ml; LC Laboratories), or calphostin C (1 μM; LC Laboratories). Transfection and starvation times were reduced in immunofluorescence studies in order to avoid strong overexpression which could impair analysis.
Cell fractionation.
HEK 293 cells were washed and collected in ice-cold hypotonic buffer containing 10 mM Tris (pH 7.5), 10 mM NaF, 1 mM EDTA, 1 μM mycrocystin LR (LC Laboratories), 0.1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM benzamidine and lysed by 30 strokes in a Dounce homogenizer. Nuclei were removed by centrifugation for 10 min at 1,000 × g at 4°C. The P100 and S100 fractions were obtained by additional centrifugation at 100,000 × g for 30 min at 4°C. P100 was resuspended in lysis buffer.
Immunoprecipitation and in vitro kinase assays.
Cells were extracted on plates in lysis buffer containing 50 mM Tris-HCl (pH 7.5), 1% (wt/vol) Nonidet P-40, 120 mM NaCl, 25 mM NaF, 40 mM β-glycerol phosphate, 0.1 mM sodium orthovanadate, 1 μM mycrocystin LR (LC Laboratories), 1 mM PMSF, and 1 mM benzamidine. Lysates were centrifuged for 15 min at 12,000 × g. The HA epitope-tagged PKB protein was immunoprecipitated from 100 to 200 μg of cell extracts, with the anti-HA epitope monoclonal antibody 12CA5 coupled to protein A-Sepharose or the anti-Myc epitope antibody 9E10 coupled to protein G-Sepharose. The immune complexes on beads were washed once with lysis buffer containing 0.5 M NaCl followed by lysis buffer and finally with 50 mM Tris-HCl (pH 7.5)–1 mM PMSF–1 mM benzamidine. In vitro kinase assays were performed for 30 min at 30°C in a 50-μl reaction volume containing 50 mM Tris-HCl (pH 7.5), 1 mM PMSF, 1 mM benzamidine, 10 nM okadaic acid (LC Laboratories), 0.1% (vol/vol) 2-mercaptoethanol, 10 mM MgCl2, 1 μM protein kinase A inhibitor peptide (Bachem), 50 μM [γ-32P]ATP (1,000 to 2,000 cpm/pmol; Amersham), and 30 μM peptide GRPRTSSAEG as PKB substrate (17) or 30 μM phospho-glycogen synthase (GS) peptide 2 (Upstate Biotechnology) as substrate for GSK-3β, and activity was determined as described previously (1).
Immunoblot analysis.
Cell extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% gel) and transferred to Immobilon P membranes (Millipore). The filters were blocked for 30 min with 5% skim milk in 1× Tris-buffered saline–1% Triton X-100–0.5% Tween 20, followed by a 2-h incubation with the 1,000-fold-diluted rabbit polyclonal antibodies raised against phosphorylated Ser473 (phosphoSer473) phosphopeptide or phosphoThr308 phosphopeptide of PKBα (New England Biolabs) or with the anti-HA epitope 12CA5 or anti-Myc epitope 9E10 monoclonal antibody diluted 100-fold in the same blocking solution. The secondary antibodies were 5,000-fold-diluted alkaline phosphatase-conjugated anti-rabbit immunoglobulin G (IgG) (Sigma) and 1,000-fold-diluted anti-mouse Ig (Southern Biotechnology Associates). Detection was performed with alkaline phosphatase color development reagents from Bio-Rad. To normalize expression levels of PKB, blots were scanned with Umax MagicScan 3.11 supported by AdobePhotoshop 4.1 and quantified with ImageQuant software (Molecular Dynamics).
Immunofluorescence.
293 cells were plated and transfected on sterile coverslips. Fixation of cells with formaldehyde and permeabilization with 0.2% Triton X-100 were performed as described elsewhere (28). The mixture of the 12CA5 monoclonal and rabbit polyclonal anti-PKB antibodies (Abα469/480 [36]) diluted 50- and 5-fold, respectively, in phosphate-buffered saline (PBS) or of the 9E10 monoclonal and rabbit polyclonal anti-phosphoSer473 antibodies diluted 5- and 500-fold, respectively, in PBS was applied for 1 h at 37°C. The cells were subsequently washed twice with PBS and incubated with 50-fold-diluted fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (Sigma) together with 100-fold-diluted biotinylated anti-mouse IgG (Sigma), followed by 100-fold-diluted streptavidin coupled to Texas red (Amersham). DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI). The coverslips were washed twice with PBS and once with H2O and then mounted on glass slides, using Gelvatol. Confocal images were collected on a Leica TCS 4D microscope.
RESULTS
Activation of PKB containing the C1 domain by inducible membrane translocation.
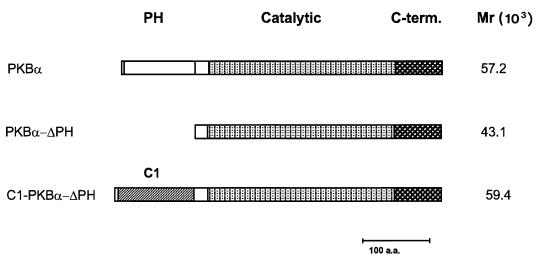
To analyze the role of membrane localization and lipid signaling in regulation of PKB activity, we constructed a variety of chimeric molecules that allow inducible membrane translocation of the kinase. In these constructs, the PH domain of PKB was replaced with other signaling modules such as the phosphotyrosine-binding (PTB) domain of insulin receptor substrate 1 (IRS-1), Src homology 2 domains of the regulatory p85 subunit of PI 3-kinase, and the C1 domain of PKC. From all the chimeras described above, exchange of the PH domain of the α isoform by the membrane-targeting C1 domain of bovine PKCα turned out to be the most efficient. The C1 domain is a cysteine-rich region which has the ability to bind DAG and its functional analogs, tumor-promoting phorbol esters (reviewed in reference 47). Most of the PKC isoforms contain two C1 domains, but only one of them appears to be responsible for phorbol ester binding in vivo (reference 47 and references therein). The main advantage of the C1 domain is that phorbol ester binding induces tight membrane association by increasing the hydrophobic surface without a significant conformational change within the region (57). The PKBα construct containing the C1 domain at the N terminus instead of the PH domain was termed C1-PKB-ΔPH. Properties of this chimeric protein were compared with those of the wild-type enzyme and PKB lacking the PH domain (PKB-ΔPH). All of the proteins were tagged with the HA epitope at the N terminus. The constructs used in this study are presented in Fig. 1.
FIG. 1.
Schematic representation of PKBα constructs used to study kinase regulation by inducible membrane translocation.
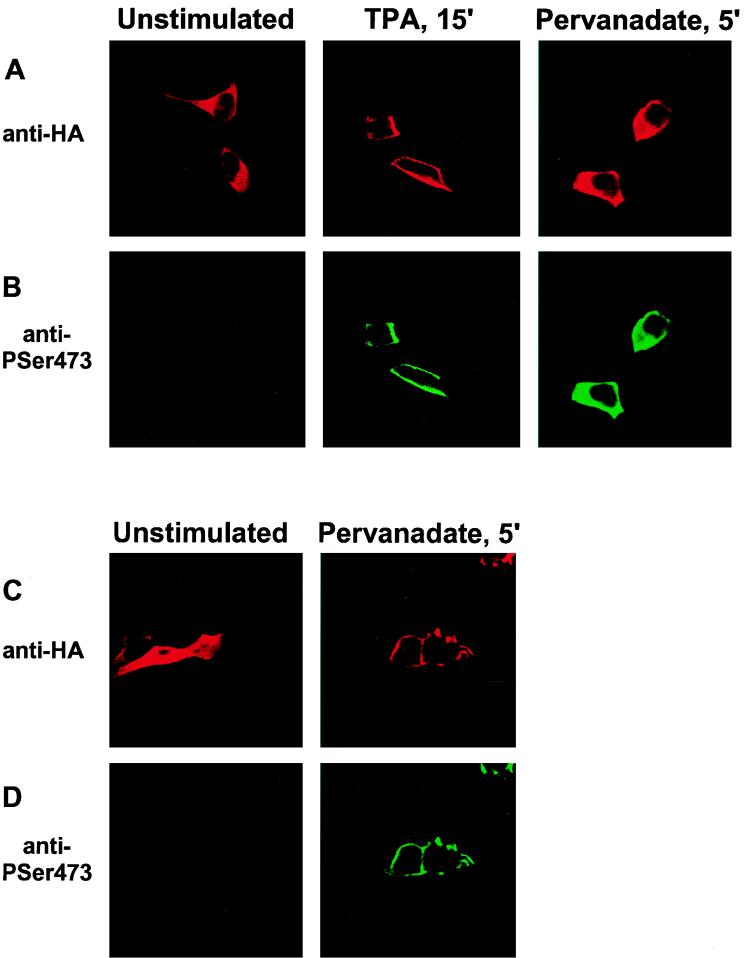
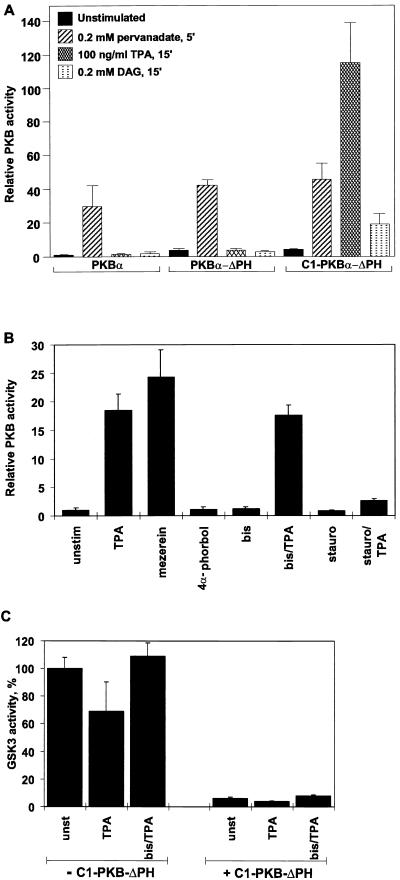
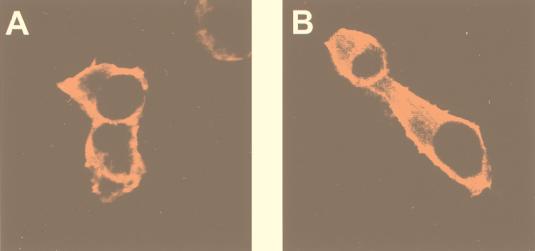
To confirm that the C1 domain-containing PKB is capable of translocating to the plasma membrane upon phorbol ester treatment, the subcellular localization of the fusion protein was monitored before and after stimulation of transiently transfected HEK 293 cells (Fig. 2A, top row). The protein was found to be mainly cytosolic in serum-starved, unstimulated cells (Fig. 2A, left), whereas a 15-min treatment with the phorbol ester TPA led to membrane association of C1-PKB-ΔPH (Fig. 2A, middle). Similarly, DAG treatment of cells resulted in membrane localization of the fusion protein (data not shown). TPA-induced membrane association of C1-PKB-ΔPH was accompanied by 20- to 40-fold stimulation of the activity of the chimera (Fig. 3 and 4A), whereas DAG stimulation of cells led to a modest 5-fold activation (Fig. 3A), consistent with a 2-orders-of-magnitude-lower affinity of the C1 domain for the latter lipid (47). Wild-type PKB and the protein lacking the PH domain could not be activated by either agonist (Fig. 3A), in accordance with previous reports (12, 45).
FIG. 2.
Activation of C1-PKB-ΔPH by TPA is due to membrane translocation. HEK 293 cells plated on coverslips were transfected with C1-PKB-ΔPH (A and B) or wild-type PKB (C and D) and serum starved for 16 h prior to stimulation with TPA or pervanadate. Fixed and permeabilized cells were incubated with the anti-HA epitope monoclonal antibody 12CA5 (A and C) and a rabbit polyclonal antibody raised against the phosphoSer473 phosphopeptide (B and D), followed by a biotinylated anti-mouse antibody/streptavidin-conjugated Texas red and FITC-conjugated anti-rabbit antibody, and analyzed by confocal microscopy.
FIG. 3.
Activation of C1-PKB-ΔPH and effects on GSK-3β activity. (A) Activation of wild-type PKB, PKB-ΔPH, and C1-PKB-ΔPH by pervanadate, TPA, or DAG. The PKB constructs were expressed in HEK 293 cells. Transfected cells were starved 24 h prior to stimulation with pervanadate, TPA, or DAG, as indicated. (B) Effects of PKC inhibitors on C1-PKB-ΔPH activation. Transfected cells were serum starved for 24 h before 15 min of stimulation with TPA, mezerein, or 4α-phorbol. Pretreatment with bisindolylmaleimide I (bis) and staurosporine (stauro) was done for 30 min before the addition of vehicle or TPA. PKB activity is the average (±standard deviation) of two experiments with duplicate immunoprecipitates and was corrected for different expression levels for each construct. The activity of wild-type PKB (A) and C1-PKB-ΔPH (B) from unstimulated cells was taken as 1. (C) Inactivation of GSK-3β by C1-PKB-ΔPH. Myc epitope-tagged GSK-3β was expressed in HEK 293 cells either alone or together with C1-PKB-ΔPH. Transfected cells were serum starved for 24 h before 15 min of stimulation with TPA. Pretreatment with bisindolylmaleimide I was as described for panel B. GSK-3β activity is the average (±standard deviation) of two experiments with duplicate immunoprecipitates and was corrected for different expression levels in coexpression experiments. Kinase activity determined from unstimulated cells expressing GSK-3β was taken as 100%.
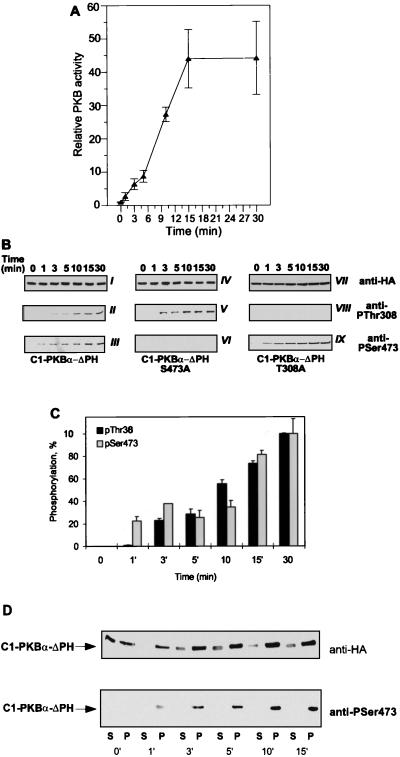
FIG. 4.
Time course of C1-PKB-ΔPH activation, phosphorylation, and translocation to the particulate fraction. (A) C1-PKB-ΔPH was expressed in HEK 293 cells that were serum starved 24 h prior to stimulation with TPA (100 ng/ml) for the indicated time periods. C1-PKB-ΔPH activity was determined following immunoprecipitation with the anti-HA epitope antibody. PKB activity is the average (±standard deviation) of two experiments with duplicate immunoprecipitates. The activity of C1-PKB-ΔPH from unstimulated cells was taken as 1. (B) Phosphorylation state of C1-PKB-ΔPH, C1-PKB-ΔPH-S473A, and C1-PKB-ΔPH-T308A was determined by immunoblot analysis using the antibody specific for phosphoThr308 phosphopeptide (gels II, V, and VIII) and phosphoSer473 phosphopeptide (gels III, VI, and IX). Expression levels for each C1-PKB-ΔPH construct were monitored by the anti-HA epitope antibody (gels I, IV, and VII). (C) Quantification of phosphorylation depicts the average (±standard deviation) of two representative experiments out of four. Phosphorylation levels of Thr308 and Ser473 at the 30-min time point were taken as 100%. (D) Transfected HEK 293 treated with TPA (100 ng/ml) for the indicated time periods were subjected to hypotonic lysis, and cytosolic (S100) and particulate (P100) fractions were prepared as described in Materials and Methods. Changes in the distribution of C1-PKB-ΔPH protein and phosphorylation were revealed by immunoblotting with the anti-HA epitope antibody (upper gel) and anti-phosphoSer473 antibody (lower gel), respectively.
To determine whether the C1 domain can fully substitute for PH domain functions, we compared the activation of C1-PKB-ΔPH by pervanadate with that of wild-type PKB. This phosphatase inhibitor was shown previously to be a potent activator of PKB in several cell lines (5, 6). C1-PKB-ΔPH responded to pervanadate stimulation, reaching a specific activity similar to those of the wild-type kinase and PKB-ΔPH (Fig. 3A). The stimulation of C1-PKB-ΔPH and PKB-ΔPH activity induced by pervanadate was 3-fold lower than that of the wild-type kinase (10-fold versus 30-fold), due to a higher basal activity of the constructs lacking the PH domain. In addition, stimulation with pervanadate for 5 min did not lead to significant changes in the subcellular distribution of C1-PKB-ΔPH (Fig. 2A, right). In contrast, the same treatment induced membrane translocation of wild-type PKB from the cytosol to the plasma membrane (Fig. 2C; compare images on the left and right). This finding agrees with previous reports concerning IGF-1-induced translocation of PKB to the plasma membrane, which also requires the PH domain (6). Pervanadate treatment appears to overcome the requirement for plasma membrane association because C1-PKB-ΔPH was enriched in the particulate fraction of HEK 293 cells prior to any cell stimulation (Fig. 4D; see Fig. 7C). Therefore, the C1 domain serves exclusively as a plasma membrane targeting module in the presence of a specific class of lipid second messengers.
FIG. 7.
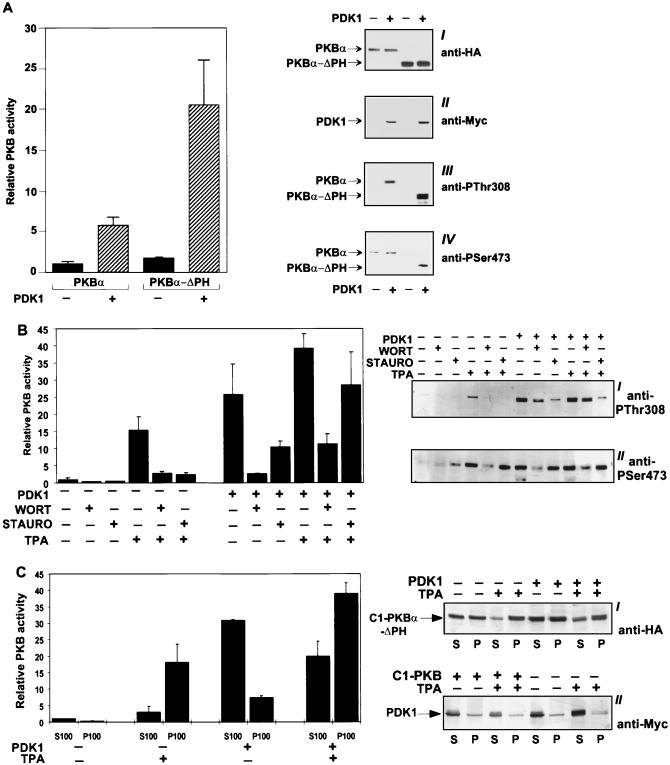
Expression of PDK1 activates PKB, PKB-ΔPH, and C1-PKB-ΔPH in vivo. (A) HEK 293 cells were transfected with PKB and PKB-ΔPH alone or with an equal amount of Myc epitope-tagged PDK1. PKB was immunoprecipitated from cells serum starved for 24 h with the anti-HA epitope antibody. Kinase activity is the average (±standard deviation) of two experiments with duplicate immunoprecipitates and was corrected for different expression levels of the constructs. The activity of wild-type PKB from transfected cells was taken as 1. PKB and PDK1 expression was confirmed by immunoblot analysis using the anti-HA epitope antibody (gel I) and anti-Myc epitope antibody (gel II), respectively. Phosphorylation state was determined by immunoblot analysis using the antibody specific for phosphoThr308 phosphopeptide (gel III) and phosphoSer473 phosphopeptide (gel IV). (B) C1-PKB-ΔPH was transfected into 293 cells alone or together with an equal amount of Myc epitope-tagged PDK1. Cells were either treated with vehicle following 24 h of serum starvation or subjected to one of the following treatments before lysis: 15-min TPA stimulation (100 ng/ml), 30-min incubation with wortmannin (WORT; 200 nM), 30-min incubation with staurosporine (STAURO; 200 ng/ml), 30-min wortmannin treatment before TPA addition, or 30-min staurosporine treatment prior to TPA stimulation. C1-PKB-ΔPH was immunoprecipitated with the anti-HA epitope antibody. PKB activity is the average (±standard deviation) of two experiments with duplicate immunoprecipitates. The activity of C1-PKB-ΔPH from transfected, unstimulated cells was taken as 1. PKB phosphorylation state was determined by immunoblot analysis using the antibody specific for phosphoThr308 phosphopeptide (gel I) and phosphoSer473 phosphopeptide (gel II). (C) Subcellular distribution of C1-PKB-ΔPH activity and protein in the presence of PDK1 and TPA. HEK 293 cells cotransfected and serum starved as described for panel B were treated with either vehicle or TPA for 15 min. The cytosolic (S100) and particulate (P100) fractions were prepared as described in Materials and Methods. C1-PKB-ΔPH was immunoprecipitated from S100 and P100 with the anti-HA epitope antibody. PKB activity is the average (±standard deviation) of two experiments with duplicate immunoprecipitates. The activity of C1-PKB-ΔPH from the S100 fraction of transfected, unstimulated cells was taken as 1. Changes in C1-PKB-ΔPH and PDK1 distribution were detected by immunoblotting with the anti-HA epitope antibody (gel I) and anti-Myc antibody (gel II), respectively.
To establish that the mechanism of C1-PKB-ΔPH activation by TPA relies exclusively on the presence of the C1 domain and is not influenced by other phorbol ester receptors in the cells, we used a number of compounds that bind to this domain and/or affect PKC activity. Treatment of the cells with the non-phorbol ester mezerein, which binds to phorbol ester receptors in the cells (34), led to C1-PKB-ΔPH activation comparable to that induced by TPA (Fig. 3B). Addition of a structurally related negative control compound, 4α-phorbol, which does not bind to the C1 domain, did not have any influence on C1-PKB-ΔPH activity (Fig. 3B). To rule out any involvement of PKC in TPA-mediated C1-PKB-ΔPH activation, we carried out activation experiments in the presence of PKC inhibitors. Pretreatment of HEK 293 cells with bisindolylmaleimide I at concentrations which inhibit TPA-responsive PKC activity (53) did not prevent TPA-induced C1-PKB-ΔPH activation. In contrast, a less specific protein kinase inhibitor, staurosporine (49), had an inhibitory effect (Fig. 3B), due to the inhibition of Thr308 phosphorylation (see Fig. 7B).
Furthermore, we analyzed the ability of C1-PKB-ΔPH to mediate downstream signaling. Overexpression of C1-PKB-ΔPH was sufficient to inhibit GSK-3β kinase activity by 90%, similar to overexpression of wild-type PKB (22). C1-PKB-ΔPH-induced inactivation of GSK-3β was neither enhanced by TPA nor affected by bisindolylmaleimide I pretreatment (Fig. 3C). Therefore, conditional activation of PKB by membrane targeting and downstream signaling occurs independent of the activity of the TPA-responsive PKC isoforms. This apparent signaling without TPA stimulation is due to the higher levels of PKB basal activity in the overexpressing transfected cells.
Activation of C1-PKB-ΔPH by phorbol ester is due to rapid phosphorylation of Thr308 and Ser473.
To learn more about the mechanism underlying conditional activation of the kinase, C1-PKB-ΔPH activation and phosphorylation were monitored during the course of TPA treatment. Activation of C1-PKB-ΔPH by TPA occurred within 1 min, reaching a plateau after 15 min, and remained constant for at least 30 min (Fig. 4A). The activation was accompanied by phosphorylation on both Thr308 and Ser473 (Fig. 4B, gels II and III), as determined using phospho-specific antibodies for these phosphorylation sites. The increase in phosphorylation levels of both sites was observed within minutes following phorbol ester addition, which was in agreement with the activity changes during TPA treatment (Fig. 4B and C). Mutation of either regulatory site to Ala did not affect the kinetics of phosphorylation of the remaining site (Fig. 4B, gels V and IX). Consistent with the lack of the regulatory site, phosphorylation of Ser473 and Thr308 was not observed in the respective Ala mutants (Fig. 4B, gels VI and VIII).
The data presented above suggest that TPA-induced membrane association of C1-PKB-ΔPH leads to rapid activation and phosphorylation by upstream kinases already present at the membrane of unstimulated cells. To explore this possibility, we expressed Myc epitope-tagged PDK1 in HEK 293 cells and monitored its localization by indirect immunofluorescence before and after TPA stimulation. The kinase was found to be both at the membrane and cytosolic in unstimulated cells. This distribution was not affected by TPA treatment (Fig. 5). This finding was confirmed by cell fractionation experiments (see Fig. 7C). We investigated the localization of Ser473-phosphorylated protein by using the specific antiphosphopeptide antibody. As expected, Ser473 phosphorylation could not be detected in unstimulated cells expressing either C1-PKB-ΔPH or wild-type PKB (Fig. 2B and D, left). In the case of TPA- and pervanadate-stimulated cells, anti-phosphoSer473 staining colocalized with C1-PKB-ΔPH, being at the membrane and cytosolic, respectively (Fig. 2B, middle and right). Membrane-associated wild-type PKB in pervanadate-stimulated cells was also found to be phosphorylated on Ser473 (Fig. 2D, right). The data demonstrated membrane localization of phosphorylated C1-PKB-ΔPH 15 min after TPA stimulation which did not reflect where the initial phosphorylation event occurred. To monitor dynamic changes of C1-PKB-ΔPH localization and activity, distribution and phosphorylation of the protein between the cytosolic and particulate fractions were analyzed during the course of cell stimulation. An increase in PKB content as well as the appearance of phosphoSer473 was observed in the particulate fraction already 1 min after TPA treatment (Fig. 4D), in good agreement with the C1-PKB-ΔPH activation and phosphorylation presented in Fig. 4A to C. Therefore, it is likely that kinase activity responsible for phosphorylation of Ser473 is present at the membrane of unstimulated and stimulated HEK 293 cells.
FIG. 5.
Subcellular localization of PDK1. HEK 293 cells transfected on coverslips with PDK1 were either left unstimulated following 16 h of serum starvation (A) or stimulated with TPA (100 ng/ml) for 15 min (B). Cells were immunostained with the anti-Myc epitope monoclonal antibody 9E10, followed by biotinylated anti-mouse antibody/streptavidin-conjugated Texas red, and analyzed by confocal microscopy.
C1-PKB-ΔPH activation by phorbol ester, but not membrane association, requires PI 3-kinase activity.
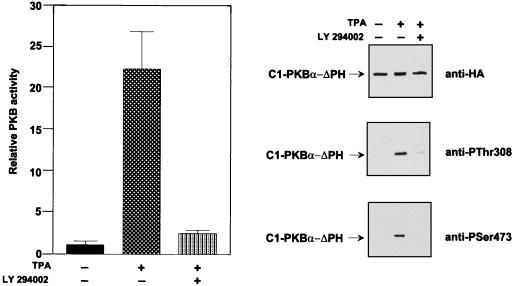
Inducible membrane recruitment of PKB provided a means to study the role of PI 3-kinase in the part of the activation process involving phosphorylation of Thr308 and Ser473. TPA-induced activation of C1-PKB-ΔPH was found to be sensitive to pretreatment of cells with the PI 3-kinase inhibitors LY 294002 and wortmannin (Fig. 6 and 7B). Analysis of the phosphorylation state of the chimeric PKB from LY 294002-treated cells revealed a significant reduction of both phosphoThr308 and phosphoSer473 (Fig. 6, middle and bottom gels). The PI 3-kinase inhibitors did not prevent TPA-induced membrane association of C1-PKB-ΔPH (data not shown). The data indicated that basal PI 3-kinase activity in HEK 293 cells was required to support membrane localization and activity of upstream kinases. Alternatively, the results suggest that PI 3-kinase regulates the activity of phosphatase specific for Thr308 and Ser473 (see below).
FIG. 6.
Activation of C1-PKB-ΔPH by TPA is sensitive to the PI 3-kinase inhibitor. HEK 293 cells expressing C1-PKB-ΔPH were subjected to a 24-h serum starvation before stimulation with TPA (100 ng/ml), with or without a 15-min pretreatment with LY 294402. C1-PKB-ΔPH was immunoprecipitated with the anti-HA epitope antibody. Kinase activity is the average (±standard deviation) of two experiments with duplicate immunoprecipitates. The activity of C1-PKB-ΔPH from unstimulated cells was taken as 1. Phosphorylation state was determined by immunoblot analysis using the antibody specific for the HA epitope (top gel), phosphoThr308 phosphopeptide (middle gel), or phosphoSer473 phosphopeptide (bottom gel).
PDK1 activates PKB-ΔPH and C1-PKB-ΔPH in vivo due to phosphorylation on both Thr308 and Ser473.
In vitro phosphorylation of PKB by PDK1 depends on the presence of 3-phosphoinositides and occurs only on Thr308 (3). The removal of the PH domain of PKB apparently reduces the requirements for phospholipids, resulting in phosphorylation by PDK1 that is insensitive to 3-phosphoinositides (3). Consistent with these data, C1-PKB-ΔPH was activated in vitro by PDK1 to a level similar to that of PKB-ΔPH (seven- and eightfold, respectively; data not shown), without the addition of any phospholipids or phorbol ester, under conditions when wild-type PKB is not phosphorylated and activated (3, 51). It was reported that coexpression of PDK1 activates PKB in vivo due to phosphorylation exclusively on Thr308. Consistent with these data, we found a modest, sixfold activation of wild-type PKB upon cotransfection with PDK1 (Fig. 7A), concomitant with the phosphorylation of Thr308 (Fig. 7A, gel III). We have consistently detected significant phosphorylation of Ser473 in HEK 293 cells prior to stimulation (Fig. 7A, gel IV) probably reflecting the partially transformed phenotype of these cells. This basal phosphorylation of Ser473 could not be increased by coexpression of PDK1 (Fig. 7A, gel IV). Removal of the PH domain of PKB enhanced the in vivo effect of PDK1, in terms of both fold activation (10-fold) and specific activity (Fig. 7A). Immunoblot analysis revealed a dramatic increase of both phosphoThr308 and phosphoSer473 in cells coexpressing PDK1 and PKB-ΔPH (Fig. 7A, gels III and IV). The lower Ser473 phosphorylation levels of PKB-ΔPH from unstimulated cells (Fig. 7A, gel IV) could be a consequence of a higher turnover of the phosphate on this regulatory site in the mutant protein, as a result of its predominantly cytosolic localization (see below). Expression of PDK1 did not affect PKB protein levels in either case (Fig. 7A, gels I and II).
Coexpression of C1-PKB-ΔPH with PDK1 in HEK 293 cells resulted in an ∼20- to 25-fold increase in kinase activity (Fig. 7B), coinciding with phosphorylation of both Thr308 and Ser473 (Fig. 7B, gels I and II). PDK1 coexpression resulted in a more robust activation than TPA stimulation of the cells, apparently due to a more efficient Thr308 phosphorylation, whereas phorbol ester treatment led to a greater increase in phosphoSer473 content (Fig. 7B, gels I and II). TPA stimulation of cells coexpressing PDK1 led to a further 30% augmentation of C1-PKB-ΔPH activity, mainly due to an increase in phosphoSer473 (Fig. 7B, gel II), which occurred in the particulate fraction (data not shown).
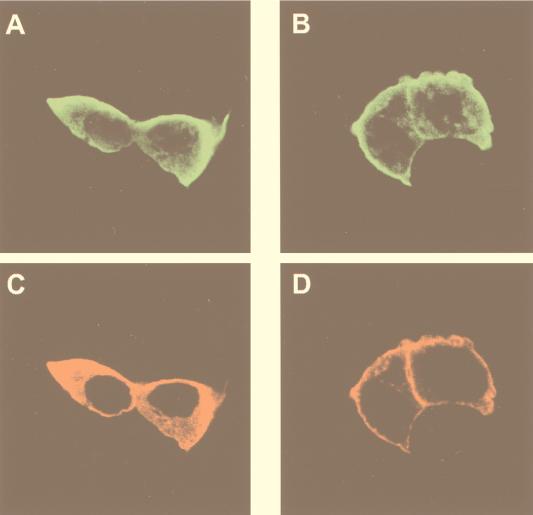
In parallel, we analyzed the subcellular localization of both kinases and the distribution of C1-PKB-ΔPH activity in unstimulated and stimulated cells. The fusion PKB protein was found in both cytosolic and particulate fractions of unstimulated cells, independently of coexpressed PDK1 (Fig. 7C, gel I). Similar results were obtained by indirect immunofluorescence experiments, in which the presence of PDK1 did not alter the cytosolic localization of C1-PDK1-ΔPH as previously observed in unstimulated cells (compare Fig. 2A and 8A). In the same cells, PDK1 was found predominantly in the cytosol and to a lesser extent in the particulate fraction (Fig. 7C, gel II). Consistent with these data, activated C1-PKB-ΔPH was detected predominantly in the cytosolic fraction of coexpressing cells (Fig. 7C). TPA treatment led to an increase in C1-PKB-ΔPH protein and activity in the particulate fraction independently of PDK1 coexpression (Fig. 7C), observed as plasma membrane association of the fusion protein (Fig. 8B). In the case of coexpressing cells, this was accompanied by anti-Myc-PDK1 membrane staining (Fig. 8D). Hence, translocation of C1-PKB-ΔPH from the cytosol to the membrane appeared to affect the localization of coexpressed PDK1. However, no significant increase of the PDK1 protein in the particulate fraction was observed upon TPA stimulation of coexpressing cells (Fig. 7C). The difference between this result and the immunofluorescence data can be explained by different sensitivities of the two assays.
FIG. 8.
Activation of C1-PKB-ΔPH with TPA induces translocation of PDK1. HEK 293 cells cotransfected on coverslips with PDK1 and C1-PKB-ΔPH were left unstimulated following 16 h of serum starvation (A and C) or stimulated with TPA (100 ng/ml) for 15 min (B and D). Cells were immunostained with a polyclonal anti-PKB antibody (Abα469/480) (A and B) and the anti-Myc epitope 9E10 monoclonal antibody (C and D), followed by an FITC-conjugated anti-rabbit antibody and biotinylated anti-mouse antibody/streptavidin-conjugated Texas red, and analyzed by confocal microscopy.
The phosphorylation of Ser473 on C1-PKB-ΔPH (Fig. 7B, gel IV) could be possibly due to PH domain removal resulting in a conformation which favors phosphorylation of the C-terminal regulatory site by PDK1. Alternatively, it could be a side effect of overexpression of PDK1 and subsequent activation of distinct downstream targets such as p70S6K and PKC (43, 48), which may result in the activation of other signaling pathways. We therefore analyzed if PDK1 coexpression is sufficient to confer on C1-PKB-ΔPH resistance to dephosphorylation of Ser473 in the presence of PI 3-kinase or PKC inhibitors. Wortmannin treatment of coexpressing cells completely abolished Ser473 phosphorylation and decreased phosphoThr308 levels, resulting in C1-PKB-ΔPH inactivation (Fig. 7B). TPA addition partially suppressed wortmannin inhibition of PDK1-mediated C1-PKB-ΔPH activation (Fig. 7B), due to protection of Thr308 and, to a lesser extent, of Ser473 phosphorylation (Fig. 7B, gels I and II). Staurosporine treatment of coexpressing cells led to a loss of phosphoThr308 immunoreactivity without any effect on Ser473 phosphorylation, resulting in a partial inhibition of C1-PKB-ΔPH activity. Addition of TPA to staurosporine-treated cells led to a modest increase of Ser473 phosphorylation, without protection of phosphoThr308. From these experiments, we cannot conclude that staurosporine inhibits PDK1 activity, only that it leads to the inhibition of Thr308 phosphorylation. Our staurosporine data appear to rule out the involvement of TPA-responsive PKCs in the phosphorylation of Ser473. Obviously, the kinase responsible for Ser473 phosphorylation must be identified to fully resolve these issues. However, these data, together with the mutant analysis presented in Fig. 4B, clearly show that phosphorylation of Thr308 and that of Ser473 can be uncoupled.
Differential control of Thr308 and Ser473 dephosphorylation by PI 3-kinase.
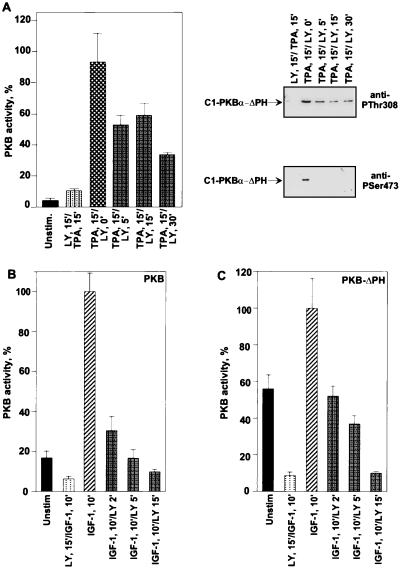
To assess the regulation of Thr308 and Ser473 by the endogenous phosphatases, we determined the influence of the PI 3-kinase inhibitors on dephosphorylation and inactivation of activated C1-PKB-ΔPH at the membrane. Treatment of membrane-associated C1-PKB-ΔPH with LY 294002 led to an ∼65% reduction of TPA-stimulated activity within 30 min (Fig. 9A). Decrease of activity was accompanied by complete dephosphorylation of Ser473 after a 5-min treatment with the PI 3-kinase inhibitor, whereas Thr308 phosphorylation appeared to be more resistant, accounting for the residual PKB activity (Fig. 9A, top and bottom gels).
FIG. 9.
Effects of LY 294003 posttreatment on TPA-induced activity of C1-PKB-ΔPH and IGF-1-induced activity of PKB and PKB-ΔPH. HEK 293 cells expressing C1-PKB-ΔPH (A), wild-type PKB (B), or PKB-ΔPH (C) were serum starved for 24 h before 15 min of stimulation with TPA (100 ng/ml) (A) or IGF-1 (100 ng/ml) (B and C), followed by treatment with 50 μM LY 294002 (LY) or vehicle for the indicated time periods, or pretreated with the same concentration of LY 294002 for 15 min before addition of agonist. Protein was precipitated with the anti-HA epitope antibody. PKB activity is the average (±standard deviation) of two experiments with duplicate immunoprecipitates. Kinase activity from stimulated cells was taken as 100%. PKB phosphorylation state in panel A was determined by immunoblot analysis using the antibodies specific for phosphoThr308 (top gel) and phosphoSer473 (bottom gel).
In a similar experiment, LY 294002 treatment of IGF-1-stimulated cells expressing either PKB or PKB-ΔPH was also evaluated. This agonist is able to stimulate activity of both PKB forms in HEK 293 cells (1, 3), accompanied by transient membrane association in the case of the wild-type kinase (6). Treatment with the PI 3-kinase inhibitor promoted rapid and complete loss of PKB activity within 15 min (Fig. 9B and C). The results indicate that PI 3-kinase may not only regulate the upstream signaling components but also control a signal transduction pathway that rapidly promotes dephosphorylation and inactivation of PKB. Furthermore, it appears that membrane localization apparently partially protects PKB from inactivation.
DISCUSSION
Stimulation of growth factor receptors leads to the recruitment of adapter proteins and activation of mitogenic signaling pathways (30). Molecules that are recruited into signaling complexes include mitogen-activated PI 3-kinase and phospholipase Cγ. Activation of both enzymes results in the production of second messengers, 3-phosphoinositides by the former and DAG by the latter, which operate on distinct signaling pathways. PI 3-kinase has been implicated in the activation of PKB and p70S6K, whereas DAG production leads to the activation of conventional and novel PKC isoforms. Although both PKB and PKC are highly related in their catalytic domains (16, 36) and are regulated by phosphorylation at the homologous sites (1, 47), different signaling domains are responsible for their selective activation by lipid second messengers. Conventional and novel PKC isoforms possess the C1 domain, which binds DAG and acts in cooperation with the phosphatidylserine- as well as Ca2+-binding C2 domain to provide the high-affinity interaction with cell membranes and subsequent activation of the enzyme (47).
PKB possesses a different class of membrane-targeting module, the PH domain, which binds 3-phosphoinositides (26, 35). It has been largely accepted that the physiological role of PI 3-kinase in the regulation of PKB activity is to induce the translocation of the kinase to the membrane, thereby promoting a conformational change in PKB and thus allowing phosphorylation by upstream kinases on Thr308 and Ser473 (reviewed in reference 33). The replacement of the PH domain of PKB with another lipid-binding signaling module represents a useful strategy for creating a functional molecule able to respond to a different second-messenger system. In the case of C1-PKB-ΔPH, this produced a kinase which can be activated in vivo by a second messenger other than 3-phosphorylated phospholipids, namely, DAG and its functional analogs. Significantly, the C1-PKB fusion protein uses the upstream signaling components from the PKB signaling and is completely independent of PKC signaling, as confirmed by the use of inhibitors specific for either pathway. Therefore, such a chimeric molecule can be used to mediate physiological downstream responses, as it is able to overcome defects upstream of PKB in the signaling pathway (in the case of lack of PI 3-kinase activation). Significantly, C1-PKB-ΔPH was able to inactivate GSK-3β in the absence of PKC activity, confirming that there is no interference of the two signaling pathways, although they are using the same second messenger as activator.
The ability of C1-PKB-ΔPH to be activated by pervanadate or coexpressed PDK1, similar to PKB-ΔPH, demonstrates that the C1 domain in the chimera exists as an independent module which does not affect the conformation of the rest of the protein and, unlike the PH domain, does not occlude the regulatory site in the activation loop. The PH domain has an inhibitory effect on PKB activity, because removal of this domain facilitates the activation by phosphorylation not only on Thr308 but also on Ser473, as observed by coexpressing PDK1 with the PKB constructs lacking this domain. Therefore, while the PH domain plays targeting and regulatory roles in PKB regulation, the C1 domain in the fusion protein serves solely as a membrane-targeting module. It is also worthwhile to note that the exchange of the PH domain with the PTB domain of IRS-1 did not have an inhibitory effect on PKB activity (data not shown). Although the three-dimensional structures of the PH and PTB domains reveal that they have the same fold, they appear to have acquired different functions subsequently (reviewed in reference 44).
A recently described (41) version of a conditionally active allele of PKB was created by fusing a hormone-binding domain to constitutively active PKB lacking the PH domain, thereby rendering membrane localization of the kinase responsive to synthetic steroids. Such a construct was also able to mediate downstream signaling responses including p70S6K, 4E-binding protein 1 phosphorylation, and glucose uptake (41). However, the advantage of the C1-containing PKB fusion construct is that it can be activated by a second messenger (DAG) that is produced in vivo.
Inducible membrane translocation of PKB also provided a means for studying the regulation of the kinase. The rapid kinetics of phosphorylation of the regulatory sites strongly suggest that the upstream kinases are associated with the plasma membrane of unstimulated cells. Regulation of the phosphorylation sites appears to be independent, which may reflect individual regulation of the upstream kinases. In addition, the increase in Ser473 occurs in the particulate fraction and coincides with C1-PKB-ΔPH translocation to the plasma membrane. It has to be borne in mind that C1-PKB-ΔPH stimulation by vanadate and phosphorylation on Ser473 occur without any apparent plasma membrane association. However, this does not exclude the possibility that the activation takes place in the particulate fraction of the cytoplasm, as the protein was found to be present in this fraction of unstimulated cells (Fig. 7C). The identification of the Ser473 kinase and its localization should resolve these apparent contradictions (see below).
PDK1 activity is not apparently regulated by growth factors or PI 3-kinase products (3, 51), but its localization may be controlled by 3-phosphoinositides, based on the fact that the kinase binds 3-phosphorylated phospholipids, with an affinity that is substantially higher than that of PKB (50). This may be sufficient to promote membrane association of PDK1 at basal 3-phosphoinositide levels. This was confirmed by immunofluorescence localization studies of PDK1 expressed in 293 cells. Immunolocalization studies of coexpressed C1-PKB-ΔPH and PDK1, in contrast to biochemical fractionation experiments, suggested that the kinases may interact in vivo and form a complex which translocates to the plasma membrane following TPA stimulation, allowing phosphorylation to take place. However, there is no evidence that phosphorylation of Thr308 in coexpressing cells occurs at the plasma membrane; on the contrary, C1-PKB-ΔPH activated in vivo by PDK1 was found in both the cytosolic and particulate fractions. Further phosphorylation on Ser473 induced by TPA treatment of coexpressing cells apparently takes place in the particulate fraction, and this suggests that the kinase responsible may be regulated by lipid second messengers. DAG and TPA are not likely to stimulate Ser473 kinase activity, as they do not lead to PKB or PKB-ΔPH activation. In addition, Ser473 phosphorylation is not affected by specific and nonspecific PKC inhibitors. PI 3-kinase-generated phospholipids may be involved in the regulation of Ser473 kinase, which would make it similar to PDK1 and PKB. Regulation of Ser473 by PI 3-kinase may involve two distinct but not exclusive modes: 3-phosphoinositide production may be required only to provide membrane localization without affecting its activity, as is the case for PDK1, or it may also influence kinase activity, directly or indirectly, which is more similar to the case for PKB. A recent report showed that the integrin-linked kinase mediates activation of PKB and Ser473 phosphorylation in vivo, in a PI 3-kinase-dependent manner (18). However, in our system we were not able to see an effect on C1-PKB-ΔPH phosphorylation or activation upon coexpression of integrase-linked kinase (data not shown). We have recently identified and partially characterized a protein kinase activity capable of phosphorylating Ser473 (58). It was initially identified based on its ability to phosphorylate a homologous site in PKCα and PKCδ. Further characterization of this activity will provide insights into the regulation of serine/threonine kinases of the second-messenger subfamily.
Dephosphorylation of Thr308 and Ser473 display differential regulation, based on their sensitivity to inhibition of PI 3-kinase. This may be a consequence of Ser473 being a better substrate than Thr308 for protein phosphatase 2A (5). Significantly, the inactivation of PKB from stimulated cells by osmotic stress is also initiated by Ser473 dephosphorylation (14, 46), confirming that this site is a major target of phosphatase. Also, PKB localization seems to be an important determinant in regulating its phosphorylation state, as the kinase was found to be more resistant to dephosphorylation at the plasma membrane than in the cytosol. Experiments with the PKC inhibitor staurosporine confirmed independent regulation of Thr308 and Ser473 dephosphorylation and demonstrated that phosphoThr308 can be partially protected when C1-PKB-ΔPH is at the membrane (Fig. 7C).
The role of PI 3-kinase in maintaining the phosphorylation state of activated PKB may be twofold: (i) to promote membrane localization and stimulated activity of upstream kinases, which should overcome the effect of a constitutively active phosphatase, and (ii) through additional inhibition of PKB phosphatases. Significantly, the activation of C1-PKB-ΔPH by the phosphatase inhibitor calyculin A is partly sensitive to PI 3-kinase inhibitor pretreatment (data not shown), which implies that stimulation of PKB through the inhibition of phosphatase still requires PI 3-kinase. It has been reported that PI 3-kinase mediates insulin-induced downregulation of protein phosphatase 2A (8). Furthermore, there is evidence for the existence of a novel signaling pathway activated by osmotic stress, which leads to dephosphorylation of activated PKB and is sensitive to calyculin A (14, 46).
In summary, we have developed a system for inducible membrane translocation of PKB which allows a temporal and spatial dissection of the regulatory events leading to PKB activation and inactivation. Creation of inducible PKB alleles not only represents a useful tool for studying regulation of the kinase but also should provide valuable insights into the downstream targets of the PKB family.
ACKNOWLEDGMENTS
We thank Michael Comb (New England Biolabs) for providing anti-phosphoThr308 antibody, A. Dufner for the Myc-tagged GSK-3β construct, P. Müller for oligonucleotide synthesis, and D. Brodbeck and D. Evans for critical comments on the manuscript.
This work was partially supported by a grant from the Schweizerische Krebsliga (to B.A.H.).
REFERENCES
- 1.Alessi D R, Andjelković M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R J, Reese C, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDoucall C N, Harbison D, Ashwarth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 4.Andersson S, Davie D N, Dahlbäck H, Jörnvall H, Russell D W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile-acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- 5.Andjelković M, Jakubowicz T, Cron P, Ming X-F, Han J H, Hemmings B A. Activation and phosphorylation of a pleckstrin homology domain-containing protein-kinase (RAC-PK/PKB) promoted by serum and protein-phosphatase inhibitors. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andjelković M, Alessi D R, Meier R, Fernandez A, Lamb N J C, Frech M, Cron P, Lucocq J M, Hemmings B A. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 7.Andjelković N. Structure, regulation and targeting of protein phosphatase 2A. Basel, Switzerland: University of Basel; 1997. [Google Scholar]
- 8.Begum N, Ragolia L. cAMP counter-regulates insulin-mediated protein phosphatase 2A inactivation in rat skeletal muscle cells. J Biol Chem. 1996;271:31166–31171. doi: 10.1074/jbc.271.49.31166. [DOI] [PubMed] [Google Scholar]
- 9.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. A retroviral oncogene, akt, encoding a serine threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 10.Brodbeck D, Cron P, Hemmings B A. A human protein kinase Bγ with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J Biol Chem. 1999;274:9133–9136. doi: 10.1074/jbc.274.14.9133. [DOI] [PubMed] [Google Scholar]
- 11.Brodbeck, D., and B. A. Hemmings. Unpublished data.
- 12.Burgering B M T, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Okayama H. Calcium phosphate mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. BioTechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- 14.Chen D, Fucini R V, Olson A L, Hemmings B A, Pessin J E. Osmotic shock inhibits insulin signaling by maintaining Akt/protein kinase B in an inactive dephosphorylated state. Mol Cell Biol. 1999;19:4684–4694. doi: 10.1128/mcb.19.7.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng X, Yuliang M, Moore M, Hemmings B A, Tailor S S. Phosphorylation and activation of cAMP-dependent protein kinase by phosphoinositide-dependent protein kinase. Proc Natl Acad Sci USA. 1998;95:9849–9854. doi: 10.1073/pnas.95.17.9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffer P J, Woodgett J R. Molecular-cloning and characterization of a novel putative protein-serine kinase related to the cAMP-dependent and protein-kinase-C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 17.Cross D A E, Alessi D R, Cohen P, Andjelković M, Hemmings B A. Inhibition of glycogen-synthase kinase-3 by insulin mediated by protein-kinase-B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 18.Delcommene M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-2-OH-kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Didichenko S A, Tilton B, Hemmings B A, Ballmer-Hofer K, Thelen M. Constitutive activation of protein kinase B and phosphorylation of p47phox by a membrane-targeted phosphoinositide 3-kinase. Curr Biol. 1996;10:1271–1278. doi: 10.1016/s0960-9822(02)70713-6. [DOI] [PubMed] [Google Scholar]
- 20.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 21.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 22.Dufner, A., M. Andjelković, B. M. T. Burgering, B. A. Hemmings, and G. Thomas. Protein kinase B localization and activation differentially affect S6 kinase activity and eukaryotic transcription initiation factor 4E-binding protein 1 phosphorylation. Mol. Cell. Biol. 19:4525–4534. [DOI] [PMC free article] [PubMed]
- 23.Ellis L, Clauser E, Morgan D O, Edery M, Roth R A, Rutter W J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986;45:721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- 24.Falasca M, Logan S K, Lehto V P, Baccante G, Lemmon M A, Schlessinger J. Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke T F, Yang S-I, Chan T O, Datta K, Kazlauskas A, Morrison D, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 26.Frech M, Andjelković M, Ingley E, Reddy K K, Falck J R, Hemmings B A. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 27.Galetić, I., M. Andjelković, R. Meier, D. Brodbeck, J. Park, and B. A. Hemmings. Mechanism of protein kinase B activation by insulin/IGF1 revealed by specific inhibitors of phosphoinositide 3-kinase—significance for diabetes and cancer. Pharmacol. Ther., in press. [DOI] [PubMed]
- 28.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 29.Haslam R J, Koide H B, Hemmings B A. Pleckstrin domain homology. Nature. 1993;363:309–310. doi: 10.1038/363309b0. [DOI] [PubMed] [Google Scholar]
- 30.Heldin C H. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 31.Hemmings B A. PH domains—a universal membrane adaptor. Science. 1997;275:1889. doi: 10.1126/science.275.5308.1899. [DOI] [PubMed] [Google Scholar]
- 32.Hemmings B A. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 33.Hemmings B A. PtdIns(3,4,5)P3 gets its message across. Science. 1997;277:534. doi: 10.1126/science.277.5325.534. [DOI] [PubMed] [Google Scholar]
- 34.Jaken S, Shupnik M A, Blumberg P M, Tashjian A H., Jr Relationship between mezerein-mediated biological responses and phorbol ester receptor occupancy. Cancer Res. 1983;43:11–14. [PubMed] [Google Scholar]
- 35.James S R, Downes C P, Gigg R, Grove S J A, Holmes A B, Alessi D R. Specific binding of the AKT-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J. 1996;315:709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones P F, Jakubowicz T, Pitossi F J, Maurer F, Hemmings B A. Molecular cloning and identification of a serine threonine protein-kinase of the second messenger subfamily. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones P F, Jakubowicz T, Hemmings B A. Molecular cloning of a second form of rac protein kinase. Cell Regul. 1991;2:1001–1009. doi: 10.1091/mbc.2.12.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 40.Kohn A D, Summers S A, Birnbaum M, Roth R A. Expression of a constitutive active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 41.Kohn A D, Barthel A, Kovacina K S, Boge A, Wallach B, Summers S, Birnbaum M, Scott P H, Lawrence J C, Roth R A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 42.Konishi H, Kuroda S, Tanaka M, Matsuzaki H, Ono Y, Kameyama K, Haga T, Kikkawa U. Molecular-cloning and characterization of a new member of the RAC protein-kinase family—association of the pleckstrin homology domain of 3 types of RAC protein-kinase with protein-kinase-C subspecies and beta-gamma-subunits of G-proteins. Biochem Biophys Res Commun. 1995;216:526–534. doi: 10.1006/bbrc.1995.2654. [DOI] [PubMed] [Google Scholar]
- 43.Le Good A J, Ziegler W H, Alessi D, Parker P J. Characterization of a PKC activation loop kinase. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 44.Lemmon M A, Ferguson K M, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 45.Meier R, Alessi D R, Cron P, Andjelković M, Hemmings B A. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bβ. J Biol Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 46.Meier R, Thelen M, Hemmings B A. Inactivation and dephosphorylation of protein kinase Bα (PKBα) promoted by hyperosmotic stress. EMBO J. 1998;17:7294–7303. doi: 10.1093/emboj/17.24.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newton A. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 48.Pullen N, Dennis P B, Andjelković M, Dufner A, Kozma S C, Hemmings B A, Thomas G. Phosphorylation and activation of p70S6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 49.Seynaeve C M, Kazanietz M G, Blumberg P M, Sausville E A, Worland P J. Differential inhibition of protein kinase C izozymes by UCN-01, a staurosporine analogue. Mol Pharmacol. 1994;45:1207–1214. [PubMed] [Google Scholar]
- 50.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter G F, Holmes A, Gaffney P R J, Reese C, McCormick F, Tempst P, Coadwell J, Hawkins P T. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 51.Stokoe D, Stephens L R, Copeland T, Gaffney P R J, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 52.Toker A, Cantley L. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 53.Toullec D, Pianetti P, Coste H, Belleverrgue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 54.Ueki K, Yammamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering B M, Coffer P J, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 55.Wada H, Matsuo M, Uenaka A, Shimbara N, Shimizu K, Nakayama E. Rejection antigen peptides on BALB/c RL♂1 leukemia recognized by cytotoxic T lymphocytes: derivation from the normally untranslated 5′ region of the c-Akt proto-oncogene activated by log terminal repeat. Cancer Res. 1995;55:4780–4783. [PubMed] [Google Scholar]
- 56.Walker K S, Deak M, Paterson A, Hudson K, Cohen P, Alessi D R. Activation of protein kinase Bβ and γ isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase Bα. Biochem J. 1998;331:299–308. doi: 10.1042/bj3310299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang G, Kazanietz M G, Blumberg P M, Hurley J H. Crystal structure of the Cys2 activator-binding domain of protein kinase Cδ in complex with phorbol ester. Cell. 1995;81:917–924. doi: 10.1016/0092-8674(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 58.Ziegler, W. H., D. B. Parekh, J. A. Le Good, R. D. H. Whelan, J. J. Kelly, M. Frech, B. A. Hemmings, and P. J. Parker. Rapamycin-sensitive phosphorylation of PKC on a carboxy-terminal site by an atypical PKC complex. Curr. Biol., in press. [DOI] [PubMed]