Abstract
Purpose
Oxidative stress is a major factor underlying many neurodegenerative diseases. However, antioxidant therapy has had mixed results, possibly because of its indiscriminate activity. The purpose of our study was to determine if the human OXR1 (hOXR1) antioxidant regulatory gene could protect neurons from oxidative stress and delay photoreceptor cell death.
Methods
The cone-like 661W cell line was transfected to stably express the hOXR1 gene. Oxidative stress was induced by the addition of hydrogen peroxide (H2O2). Intracellular levels of reactive oxygen species (ROS), caspase cleavage, and cellular resistance to oxidative stress were determined and compared between the control and hOXR1 cells. For in vivo analysis, AAV8-hOXR1 was injected subretinally into the rd1 mouse model of retinal degeneration. Functional and structural integrity of the photoreceptors were assessed using electroretinography (ERG), histology, and immunofluorescence analysis.
Results
Expression of hOXR1 increased cellular resistance and reduced ROS levels and caspase cleavage in the 661W cell line after H2O2-induced oxidative stress. Subretinal injection of AAV8-hOXR1 in the rd1 mice improved their photoreceptor light response, expression and localization of photoreceptor-specific proteins, and delayed retinal degeneration.
Conclusions
Our results suggest that OXR1 is a potential therapy candidate for retinal degeneration. Because OXR1 targets oxidative stress, a common feature of many retinal degenerative diseases, it should be of therapeutic value to multiple retinal degenerative diseases.
Keywords: oxidative stress, gene therapy, retinal degeneration
Photoreceptors (rods and cones) are the light-sensing neurons of the retina, a multilayered neuronal tissue located in the back of the eye. Degeneration of photoreceptors due to genetic or environmental insults underlies the pathogenesis of the majority of retinal degenerative diseases, such as retinitis pigmentosa (RP).1
RP is characterized by the loss of peripheral vision and night blindness due to the loss of rod photoreceptors followed by progressive loss of the central vision due to cone photoreceptor dysfunction.2 Mutations in genes that are expressed specifically in rod photoreceptors have been found to cause RP (https://sph.uth.edu/retnet/disease.htm). These gene mutations lead to rod cell death, which is accompanied or followed by cone cell death, although the causative gene is not expressed in cones.3 Such bystander effects on cone survival have been attributed to several mechanisms, including oxidative stress.4–9 Among these, oxidative stress due to hyperoxic conditions in the absence of rod photoreceptors is a major contributor to the secondary cone death. Such conditions arise because photoreceptors are highly metabolically active neurons and consume significantly high levels of oxygen.4,10–13
The oxidation resistance 1 (OXR1) gene is induced by oxidative stress and is required for the expression of a number of genes that contribute to oxidative stress resistance, either by directly controlling their expression, or by controlling the transcription factors that regulate genes involved in oxidative stress resistance.14–17 OXR1 is required for the expression of several genes required for repair of oxidative DNA damage and the cell cycle arrest genes required for repair to be efficient. OXR1 controls genes required for detoxification of reactive oxygen species (ROS) and genes involved in ROS production as well as pro and anti-apoptotic genes contributing to increased oxidative stress resistance upon exposure to an oxidizing agent.15,17
In mouse models of diabetic retinopathy and oxygen induced retinopathy, OXR1 mRNA and protein levels decline shortly before the onset of retinal degeneration.18,19 A reduction in OXR1 mRNA and protein prior to the onset of neurodegeneration has also been found to occur in several neurodegenerative disease models, including Parkinson's disease,20–23 neuronal cell death due to ischemia,24 and a cellular model of sevoflurane-induced neurotoxicity.25 Further evidence that OXR1 functions to prevent neurodegeneration is that a mouse carrying an OXR1 deletion mutation incurs high levels of oxidative damage in cerebellar granular layer neurons, which are involved in motion control. This mouse develops a rapidly progressing ataxia beginning at 2 weeks of age and death ensues by 4 weeks of age. This phenotype can be reversed by reintroduction of OXR1.26 These results clearly suggest that expression of OXR1 plays a critical role in a number of retinal degenerative and neurodegenerative diseases (for review see Ref. 27).
We hypothesized that OXR1 is an excellent candidate to reduce oxidative stress in retinal degeneration. To test this hypothesis, we delivered the human OXR1 gene packaged into the adeno-associated virus serotype 8 (AAV8-hOXR1) subretinally in the rd1 mutant mouse of early onset aggressive retinal degeneration in order to test if the human gene can function in this regard. Human and mouse OXR1 proteins are 83% identical and 88% similar indicating they are highly conserved. Retinal degeneration in the rd1 mouse is much more rapid than that seen in human RP. However, it follows a similar pattern of rod cell death followed by secondary cone cell death as is seen in patients with RP and the rd10 and Rho-KO mouse models.28,29 AAV8 has a high affinity for photoreceptors, but also enters the RPE.30 This was deemed beneficial, as it has been shown that reducing oxidative stress in both cell types can reduce retinal degeneration in the rd10 mouse model.5,6 In the rd1 mouse, retinal degeneration is due to a mutation in rod-specific gene Pdeβ (beta-subunit of phosphodiesterase).31,32 We found that OXR1 overexpression leads to significantly improved photoreceptor survival and light response kinetics. Our results suggest that OXR1 gene therapy is a viable approach to the treatment of retinal degenerative diseases and possibly other neurodegenerative diseases.
Methods
Construction of 661W Cells Overexpressing OXR1B2
The 661W cone-like cells were obtained from Dr. Muayyad R. Al-Ubaidi (University of Houston). The human OXR1B2 (hOXR1B2) isoform (accession no. 851999.2) was cloned into the pcDNA3.1 vector plasmid to produce pMV1632 for construction of stably transfected 661W mouse photoreceptor-like cells overexpressing this gene. After G418 selection, 10 independent clones were combined to produce a stock of cells containing a random pool of insertions of the cytomegalovirus (CMV) promoter driven OXR1 expression plasmid, pMV1632, to produce the cell line MVL120. Control cells were produced in an identical manner using the pcDNA3.1 vector plasmid for stable transfection.
RNA Extraction and Quantitative Reverse Transcriptase-Polymerase Chain Reaction Analysis
Total RNAs were isolated from 661w cells with QIAzol Lysis Reagent (Qiagen) and precipitated according to the manufacturer's instructions. Reverse transcription was performed with 1 µg of total RNAs with the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). The resulting cDNA was used to perform gene expression analysis using the BioRad CFX96 qPCR instrument and Power SYBR Green PCR Master Mix (Thermo Fisher Scientific). The following quantitative PCR (qPCR) primers were used to quantify OXR1 expression: OXR1_Fwd1: 5′-GCAAACTTTGGAAAACCCATACTA-3′, OXR1_Rev1: 5′-GATGCCTTTGCTTTTCTTTTAAAAG-3′, OXR1_Fwd2: 5′-CAAACTTTGGAAAACCCATAC-3′, OXR1_Rev2: 5′-AACTTATGTAATCGATGCC-3′, β-Actin_Fwd: 5′-GGCTGTATTCCCCTCCATCG-3′, and β-Actin_Rev: 5′-CCAGTTGGTAACAATGCCATG-3′.
Immunoblotting
The cells were lysed on ice in Pierce RIPA Buffer (Thermo Scientific) containing Halt Protease Inhibitor Cocktail (100X; Thermo Fisher). Lysates were sonicated and centrifugated at 12,000 g for 15 minutes. Supernatants were quantified and 20 µg of total protein lysate was incubated for 5 minutes at 95°C with Laemmli sample buffer. Proteins were separated on a 4 to 20% Mini-PROTEAN TGX Precast Protein Gel (BioRad) and transferred to a nitrocellulose membrane (BioRad). The blots were processed using LI-COR (per manufacturer's instructions) and protein expression was detected using LI-COR Odyssey Fc detection system and quantified with Image Studio Lite quantification software.33
Intracellular ROS Levels
The levels of ROS were measured by growing cells to 70 to 80% confluence. The 2′,7′-dicholorfluorescin diacetate (DCFDA; Millipore Sigma) was added at a final concentration of 1 mM to the cells and incubated for 1 hour. Cells were washed three times with DPBS, then fresh medium containing H2O2 was added at the indicated concentrations and cells were incubated for 20 minutes. ROS levels were measured immediately with a fluorescence plate reader using 480 nm excitation and measuring fluorescence at 530 nm.
Caspase Activity
Cells were seeded to reach 70 to 80% confluence, treated with H2O2 at the indicated concentrations and incubated for 16 hours in the presence of H2O2. Caspase 3/7 cleavage was determined using the Caspase-Glo 3/7 assay kit (Promega) according to manufacturer's instructions. Briefly, cells were lysed in Pierce RIPA Buffer (Thermo Scientific) and the protein concentration was determined using the Pierce BCA Protein Assay kit (Thermo Fisher Scientific). Cell lysates (5 µg of protein) were incubated with the caspase substrates and luminescence was quantified after 30 minutes using Cytalion 5 cell imaging multi model reader from Biotek. Caspase activity was normalized to the protein concentration.
Cell Viability
To measure cellular resistance to oxidative stress, cells were grown and treated with H2O2 as indicated for caspase assays. After 16 hours of incubation in the presence of H2O2, viability was measured using the Cell Titer-Glo kit (Promega) according to the manufacturer's protocols.
Virus Vector Construction
For AAV production, human OXR1 variant hOXR1-B2 (accession no. 851999.2) cDNA was cloned in the pAAV2 viral cassette to produce the plasmid pMV1622 (Supplementary Fig. S1). Digestion of this plasmid with the restriction enzyme SmaI releases four fragments. Two fragments of 11 bp each, the vector backbone, and the region to be packaged, which lies between the two inverted terminal repeat (ITR) sequences. Contained within the packaged region are the following sequences: the first ITR sequence, the chicken beta actin 6 (CB6) promoter, the CMV enhancer, a short hybrid intron comprised of parts of the beta-actin and the immunoglobulin heavy chain gene to activate splicing and mRNA processing. This was followed by the hOXR1B2 coding sequence, an IRES sequence to drive transcription of the downstream EGFP gene, and a polyadenylation signal sequence, followed by the second ITR sequence. The hOXR1B2 sequence is comprised of OXR1 exons 4 to 12 and 15 to 19.27 This cassette was packaged into the AAV8 capsid by the UMass Medical School, Horae Gene Therapy Center's Viral Vector Core Facility, using the HEK293-triple transfection method and purification by CsCl gradient centrifugation, as described previously.34 The purified AAV8 carrying the hOXR1B2 gene (AAV8-hOXR1) was delivered in the subretinal space to rd1 mouse eyes as described below.
Antibodies
The following antibodies and dilutions were used: OXR1 antibody (1:500) was described previously,35 β-Tubulin (Thermo Fisher; #MA5-16308, 1:2000), Goat Anti-Mouse secondary Ab 680RD (LiCor 926-68070, 1:5000), and Goat Anti-Rabbit Secondary Ab 800CW (Licor 926-32211, 1:5000). For immunostaining studies, the following antibodies and dilutions were used: rhodopsin antibody (Mouse Anti-Rhodopsin monoclonal; MAB5356, 1:500, EMD Millipore), red-green opsin antibody (Rabbit Red/Green opsin; AB5405, 1:500, EMD Millipore), OXR1 antibody, described previously35 (1:200) were used. Secondary antibodies: (Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 546 goat anti-mouse IgG; 1:500; Life Technology Corporation).
Animals
All animal experiments were performed in accordance with institutional guidelines and all animal procedures were approved by the Institutional Animal Care and Use Committee, UMASS Medical School, and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Mice were housed in 12 hours light and 12 hours dark cycle rooms. The Pde6βrd1/rd1 (referred as rd1) mice were provided Dr. Claudio Punzo (Department of Ophthalmology, UMass Medical School). All mice were genotyped to exclude the rd8 allele.
For anesthesia, ketamine and xylazine cocktail (Ketamine, 10 mg/mL and Xylazine, 1 mg/mL) with volume 10 uL/gm body weight was used to anesthetize the mice. For euthanasia, mice in cages were placed in a CO2 chamber. The chamber was slowly filled with CO2 replacing 30 to 70% of chamber volume/min. Gas flow was slowly stopped after all animal movement had ceased. Secondary physical method of cervical dislocation was used to confirm the death.
Subretinal Injection
AAV8-hOXR1 was injected subretinally in 3 different locations in the eye with 0.5 µL per injection for a total of 1.5 uL virus (1 × 1012 genome copies/mL) per eye.36,37 Control eyes were injected with balanced salt solution (BSS) at either day P0/1 or P10 following identical procedures. Subretinal injection of either virus or mock-BSS control was performed on 8 to 10 mice in each group and both eyes of each animal were treated with either virus or BSS control. Injections were performed in three different regions with either virus or BSS solution. Two injection sites were around the temporal and nasal regions. The third injection site was centered between these two locations. Multiple injections were performed to increase the area of the retina that was treated. A small incision along the closed lid fissure was made. The eyes were proptosed and the eyelids were pinched slightly to hold the eye in position for injection. A glass needle filled with virus was inserted in the sclera in the subretinal space by holding the needle parallel to outer wall of the eye. Although no substantial ocular injuries were noted in this study, cataract formation is the usual exclusion criteria for subretinal injection.
Electroretinography
Scotopic and photopic electroretinography (ERG) were performed as described earlier,36,38,39 using the CELERIS Next Generation rodent ERG testing (ERG Diagnosys LLC). The flash intensities for scotopic ERG were 0.01, 0.1, and 1 cd.s/m2 and for photopic ERG were 3 cd.s/m2 and 10 cd.s/m2. Light adaptation was performed with a background illumination of 30 cd/m2 for 5 minutes.
Immunofluorescence Microscopy
Staining of the retinal cryosections was performed essentially as described previously.36,38 The sections were stained with primary antibodies overnight followed by washing and incubation with appropriate secondary antibodies and DAPI (stock 300 µm/mL, diluted 1:1000 for treatment) for 1 hour at room temperature. The sections were then washed in 0.1 M phosphate buffer 3 times for 5 minutes each, air dried, and mounted with fluoro-gel (Electron Microscopy Sciences) with a cover slip. The images were produced using a Leica upright microscope (Leica Microsystems).
Statistical Analysis
All data are presented as mean ± standard error of the mean. We have used 1-way ANOVA for multiple comparisons. The groups are statistically significant if P < 0.05. Statistically significant P values were indicated by asterisks (∗ P < 0.01–0.05; ∗∗ P < 0.001–0.01; ∗∗∗ P < 0.0001–0.001; and ∗∗∗∗ P < 0.00001).
Results
OXR1 Expression Vectors
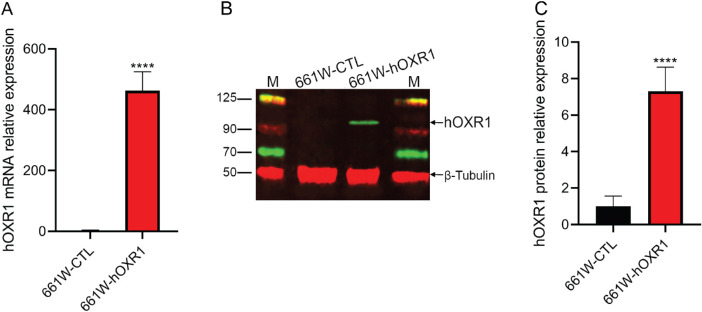
To confirm that the 661W cells overexpressed hOXR1, qRT-PCR using the human OXR1-specific primers was performed. Figure 1A shows that these primers do not detect mouse Oxr1 mRNA in the untransfected MVL118 control (CTL) cells. However, the human OXR1 mRNA is highly expressed in the stably transfected MVL120 cell line. Protein expression, measured by Western blot analysis using an antibody that recognizes both human and mouse OXR1, revealed approximately 7-fold higher OXR1 protein levels in the MVL120 cells compared to the MVL118 control cells (Figs. 1B, 1C).
Figure 1.
In vitro validation of hOXR1 overexpression in 661W cone derived cell lines. (A) Expression of hOXR1 in 661W cells was analyzed by qRT-PCR. ****: P < 0.0001. (B) Protein extracts (40 µg) of the indicated 661W cells were analyzed by SDS-PAGE and immunoblotting using OXR1 specific (green) or β-tubulin (red; loading control) antibodies. CTL, control cells. (C) Total OXR1 protein expression was calculated relative to the β-tubulin levels, which were uniform among all samples. Data are mean ± SD from two independent experiments.
Elevated Expression of OXR1 Reduces Cellular Levels of ROS
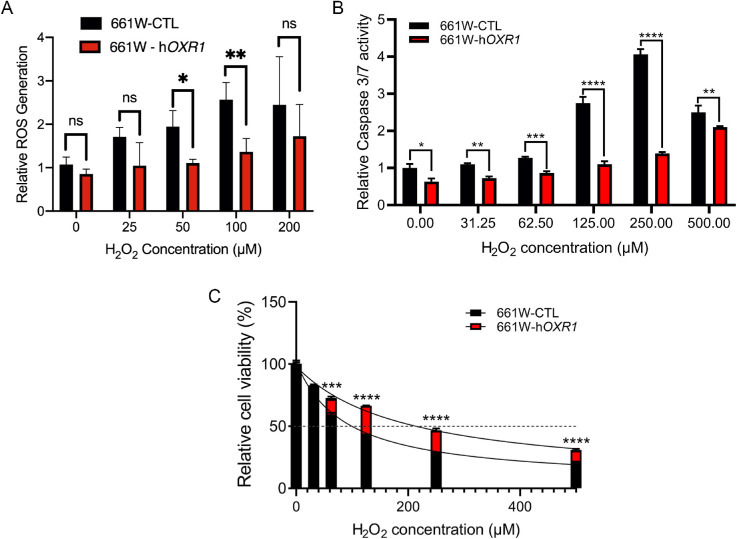
Because OXR1 is known to affect multiple enzymes involved in H2O2 processing and ROS production and detoxification,16,17 we tested if elevating OXR1 protein levels could reduce intracellular levels of these toxic compounds in 661W cells. The DCFDA, which functions only after intracellular deacetylation, was used to measure intracellular H2O2 and ROS levels.40 Figure 2A shows that MVL120 cells overexpressing OXR1 have lower intracellular levels of H2O2 and ROS prior to treatment and after exposure to exogenous to H2O2 than the MVL118 control cells. This demonstrates that increasing OXR1 expression levels increases H2O2 and ROS detoxification, production of these reactants, or both.
Figure 2.
Protective effects of hOXR1 in the 661W cone-derived photoreceptor cell line after H2O2 treatment. (A) ROS levels in 661W cells overexpressing hOXR1 normalized to control cells was determined using DCF-DA. Fluorescence was measured 20 minutes after treatment with the indicated concentrations of H2O2. (B) Caspase activity of 661W cells overexpressing hOXR1 (MVL120 cells) normalized to control (CTL) cells was determined with the Caspase 3/7-Gloassay 16 hours following the addition of the indicated concentrations of H2O2. (C) Cell titer-Gloassay was used to determine percent cell viability 16 hours after the addition of the indicated concentrations of H2O2. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, ns = nonsignificant, 2-way ANOVA test. Data are mean ± SD from two independent experiments, with three sets of samples per experiment.
Elevated OXR1 Expression Decreases Caspase Activation
In HeLa cells, repression of OXR1 levels by an inhibitory RNA causes an increase in caspase 9 expression and expression of multiple genes involved in activation of the caspase cascade.15 To determine if OXR1 overexpression causes a general inhibition of caspase activation, we measured cleavage of caspase 3 and 7. Cells were treated with peroxide and caspase 3 and 7 cleavage was measured using the Caspase-Glo 3/7 cleavage assay. Figure 2B shows that caspase cleavage is reduced in the MVL120 - 661W OXR1 overexpressing cells compared to the MVL118 controls at all doses tested. In the control cells, caspase cleavage increases up to a peroxide concentration of 250 µM. At 500 µM, the level of caspase cleavage is reduced in the control cells compared to 250 µM. This may be because cell death, cell lysis, and/or excessive damage to remaining cells, which impairs their ability to respond and produce caspases resulting in lower levels cleaved caspase. By contrast, in the OXR1 overexpressing cells caspase cleavage is still increasing at 500 µM compared to the level seen at 250 µM, but still remains lower than the control cells. Our results demonstrate that OXR1 overexpression reduces caspase 3 and 7 activation and therefore reduces the level of apoptosis, which leads to cell death and degeneration of retinal photoreceptors.
Elevated OXR1 Expression Increases Oxidative Stress Resistance
The decrease in ROS levels and activation of apoptosis seen when OXR1 is overexpressed suggests that cells should be more resistant to oxidative stress. To test this, we treated 661w cells overexpressing OXR1 (MVL120) and compared their resistance to H2O2 with 661w vector control cells (MVL118). The cells were treated with increasing concentrations of H2O2 and assessed for viability using the Cell-Glo cell viability assay (Promega). Figure 2C shows that elevating the level of OXR1 in 661w cells markedly increases the cellular resistance to oxidative stress.
AAV8-hOXR1 Improves Photoreceptor Light Response in the rd1 Mouse
Because hOXR1 is effective at protecting cells from oxidative stress, we constructed a plasmid, pMV1622, that expresses this isoform and contains a region suitable for packaging into AAV8 capsids (see Supplementary Fig. S1).
In the rd1 mouse model of aggressive and early onset retinal degeneration, rod photoreceptors die by 3 to 4 weeks of age with secondary cone death initiating only after approximately 50% rod photoreceptor loss.4 In the initial proof of concept experiments, we delivered AAV8-hOXR1 subretinally at day P0/P1 into the rd1 mice. The mice were evaluated by scotopic (rod-mediated) and photopic (cone-mediated) ERG starting at 4, 6, 9, and 13-weeks post-injection (WPI), which also corresponds to their chronological age. Mock (BSS) injected mice showed no detectable scotopic or photopic ERG activity at 4 weeks of age, the time at which rd1 mutant mice typically do not exhibit detectable light responses.41 The lack of measurable ERG activity is also seen at all later time points for the mock treated eyes. By contrast, mice injected with AAV8-hOXR1 showed detectable levels of both scotopic (Fig. 3A) and photopic (Fig. 3B) ERG activity, indicating they were still capable of responding to light and retained partial photoreceptor activity (Supplementary Fig. S2). The improved ERG activity in the AAV8-hOXR1 treated mice peaked at 6 weeks PI. By 9 and 13 weeks of age, visual activity was undetectable in the AAV8-hOXR1 treated eyes. Thus, early intervention and injection of AAV8-hOXR1 at P0/P1 resulted in an increase in the retention of partial visual activity in the rd1 mutant mouse that remained well beyond the time the eyes of control mice lost visual activity.
Figure 3.
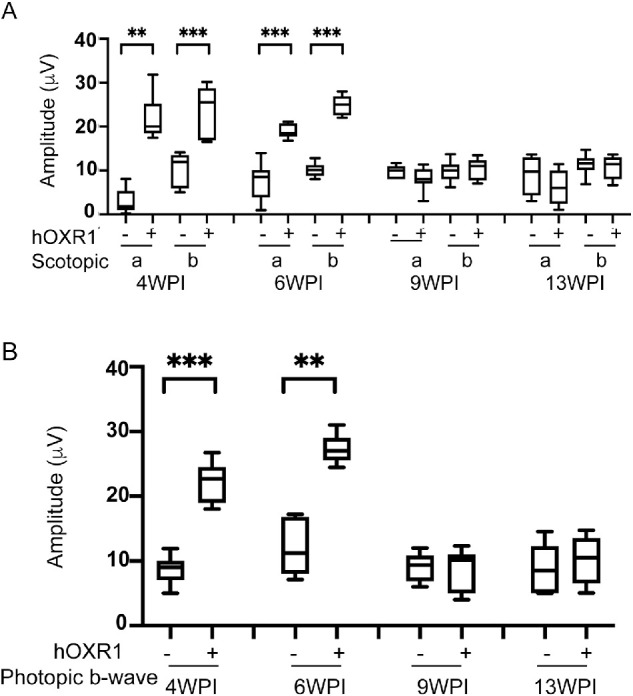
Protection of retinal function in rd1 mice after AAV8-hOXR1 injection at P1. Eyes of rd1 mice were injected with AAV8-hOXR1 vector and BSS at P1 via the subretinal route. ERG recordings were performed at 4, 6, 9, and 13 weeks post-injection (A). Quantification of scotopic a-wave and b-wave amplitudes are presented as box-whisker plots (n = 10–14 eyes for BSS injection, and n = 8–15 eyes for AAV8-hOXR1 injection). (B) Photopic b-wave ERG responses are presented as box-whisker plots (n = 9–12 eyes for BSS injection, and n = 9–13 eyes for AAV8-hOXR1 injection). ANOVA was used to determine the statistical significance. Data are represented as mean ± SEM. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001.
Later-Stage Injection of AAV8-hOXR1 Results in Greater Photoreceptor Survival
To demonstrate translatability of our approach, we next tested if injection at a later time (P10) also provided retention of visual activity. In these experiments, ERG activity was monitored at 4, 6, 8, and 10 WPI. Figures 4A and 4B and Supplementary Figures S3 show that rd1 mice treated with AAV8-hOXR1 exhibited both scotopic and photopic ERG activity at 4 WPI. At this time, the mock treated eyes showed no detectable photopic or scotopic ERG activity. ERG activity was retained up to 8 WPI. By 10 WPI, ERG activity was no longer significantly above that of the mock treated mice. The increased age at which measurable ERG activity could be detected when later injection was performed suggests this is the more effective procedure. These results reinforce the role of OXR1 in delaying the loss of visual activity in the rd1 mutant mouse.
Figure 4.
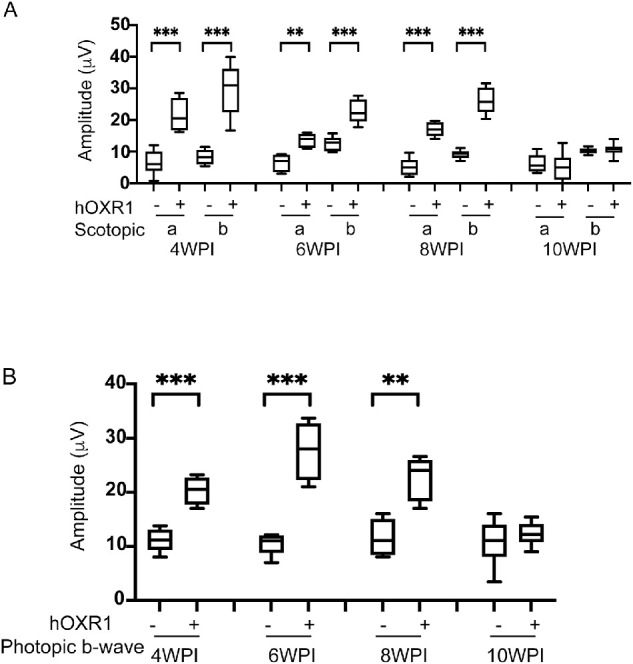
Rescue of retinal function in rd1 mice after AAV8-hOXR1 injection at P10. Eyes of rd1 mice were injected with AAV8-hOXR1 vector and BSS at P10 via subretinal route. Scotopic and photopic ERGs were recorded at 4, 6, 8, and 10 weeks post-injection (A) Scotopic a-wave and b-wave ERG responses are presented in box-whisker plots (n = 8–15 eyes BSS injection and n = 8–11 eyes AAV8-hOXR1 injection). (B) Photopic b-wave amplitudes are shown in box-whisker plots (n = 8–11 eyes for BSS injection and n = 8–14 eyes for AAV8-hOXR1 injection). ANOVA was used to determine the statistical significance. Data are represented as mean ± SE. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001.
Morphological Improvement of Retina Following AAV8-hOXR1 Gene Delivery
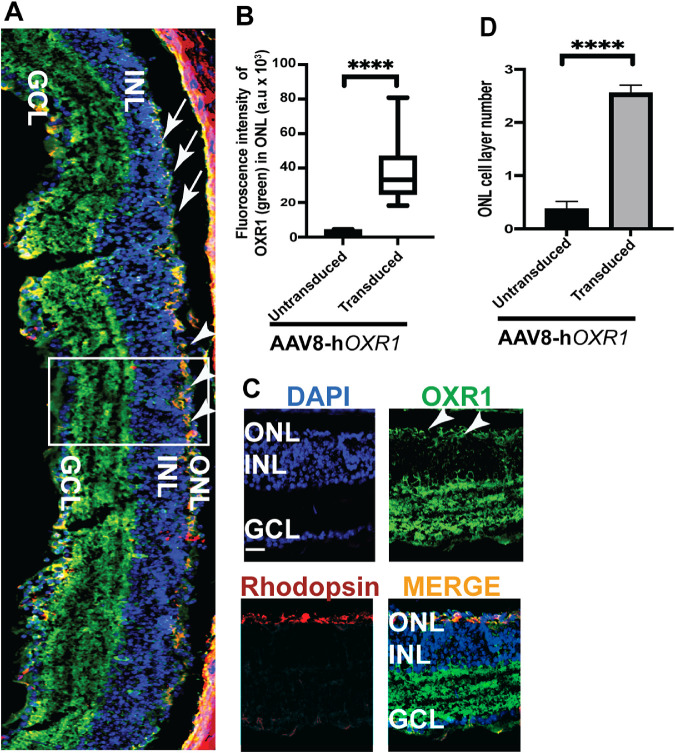
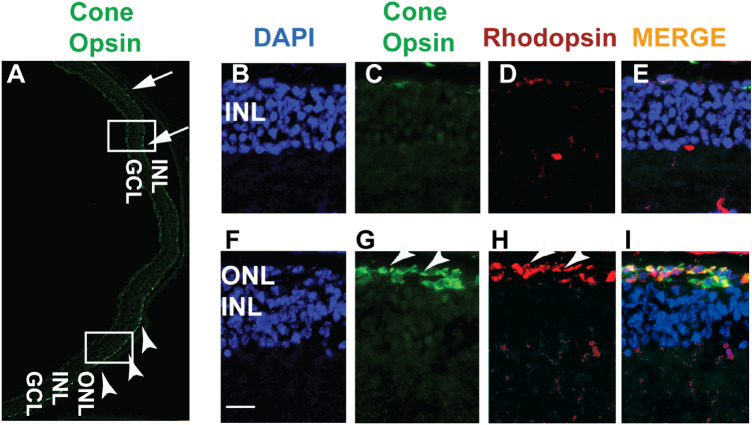
The rd1 mice exhibit undetectable outer segment protein expression and massive loss of the rod and cone photoreceptors by 2 weeks of age. We therefore investigated whether OXR1 protein expression also results in improvement of the photoreceptor outer segment protein expression. Figures 5A and 5B indicate transduced and untransduced regions of the retina. The AAV8-hOXR1 transduced region was identified by the presence of higher levels of OXR1 (green) and rhodopsin (red) staining (see arrowheads in Figs. 5A–C) and 2 to 3 layers of the photoreceptor nuclei (see Figs. 5A, 5D). The transduced region also revealed higher levels of red/green cone opsin expression (arrowheads in Figs. 6A, 6G), indicating preservation of cones as compared to barely detectable cone-opsin staining in the untransduced region (see Figs. 6A–E; arrows). As both OXR1 and cone-opsin antibodies are raised in rabbits, we used the rhodopsin antibody to mark the transduced region. Subretinal injection is unlikely to transduce the inner retinal layers. The intense OXR1 staining of this region is presumably because the OXR1 antibody reacts well with both mouse and human OXR1 protein, suggesting these regions either have high constitutive levels of mouse OXR1 expression or are also experiencing oxidative stress and have induced the endogenous mouse OXR1 to high levels.
Figure 5.
HOXR1 expression and rod preservation in AAV8-hOXR1 injected retinas in rd1 mice. (A) Retinal cryosections of rd1 mice were immunostained with OXR1 (green) and rhodopsin (red) antibodies. Nuclei were stained with DAPI (blue). Arrowheads indicate expression of OXR1 and rhodopsin (orange = transduced area) in the preserved outer nuclear layer (ONL). Arrows indicate untransduced region and undetectable ONL. (B) Quantification of OXR1 intensity was performed in the transduced area as compared to the untransduced area. Panel (C) depicts the transduced area marked by box in A showing OXR1 and rhodopsin expression. (D) Quantification of the nuclear layers in the ONL indicates a significant improvement in the preservation of the nuclei in the transduced area. INL, inner nuclear layer; GCL, ganglion cell layer. Scale 200 µm. **** P < 0.0001.
Figure 6.
Cone survival in rd1 mouse retina treated with AAV8-hOXR1. The retinal cryosections were immunostained with red/green cone opsin (green) and rhodopsin (red) antibodies. Nuclei were stained with DAPI (blue). (A) Lower magnification image shows the expression of cone opsin (green; arrowheads) marking the transduced area. The corresponding untransduced area showing reduced to no cone-opsin staining is marked by arrows. The untransduced (B–E) and transduced (F–I) areas (marked by white rectangles in A) were further examined under higher magnification. Arrowheads in F indicate the presence of the outer nuclear layer (ONL). **** P < 0.0001. INL, inner nuclear layer; GCL, ganglion cell layer. Scale 200 µm.
Discussion
OXR1 is a regulatory gene that functions upstream of a number of transcription factors.15,17 It also interacts and stimulates the protein methyltransferases (PRMT5 and PRMT1), two proteins involved in chromatin remodeling and protein methylation.16 It has been suggested that OXR1 may function as a sensor of oxidative stress that signals downstream regulatory elements that control expression of protective genes.15 Among the many genes that are differentially expressed when OXR1 is inhibited are genes that protect cells from oxidative stress, caspases, and caspase activation factors, DNA repair genes, and the p53 response to oxidative stress.15,17 OXR1 overexpression also alleviates inflammation,42 however, it is not known if this is a direct or indirect effect due to reduced oxidative stress. OXR1 is highly expressed in neurons and is induced when cells encounter oxidative stress (for review see Ref. 27). Based on our results, increasing OXR1 levels above the normal background expression in the 661w cell line by adding a highly expressed copy of hOXR1, increases cellular resistance to peroxide induced oxidative stress, and reduces caspase activation and cellular ROS levels. These results suggest that elevating OXR1 protein levels in degenerating neurons protects them from oxidative stress induced death.
We tested the hypothesis that increasing OXR1 expression can prevent or delay retinal degeneration by treating the rd1 mutant mouse model of retinal degeneration with AAV8-hOXR1 to see if this would retard the rate of neurodegeneration of the photoreceptor cells. We demonstrate that subretinal injection of this viral vector is effective at delaying the loss of retinal activity and that the retinal structure is maintained at a time when untreated regions of the same retina show signs of severe degradation. Untreated or mock treated eyes of rd1 mice lose all retinal activity, as measured by ERG, by 4 weeks of age (see Figs. 3 4). However, increasing OXR1 expression by hOXR1 addition reduces the rate of degeneration and ERG activity remains detectable up to 9.5 weeks of age (8 WPI) when injected on day P10. Although we presume that much of this is due to the reduction in ROS levels and the reduction in the activation of caspase, other factors, such as effects on inflammation, which are also modulated by OXR1 may also play a role.43 Thus, OXR1 gene therapy in the rd1 mouse model results in a significant delay in retinal degeneration.
In rd1 mice, the rod photoreceptors are completely lost by postnatal day 21 and this leads to the eventual degeneration of cone photoreceptors.44 We investigated the hypothesis of structural preservation of rod and cone cells in the injected area. At first, we revealed 2 to 3 layers of outer nuclear layer (ONL) in the injected area by DAPI staining when compared to uninjected area at 4-weeks post-injection (5.5 week age). ONL layer protection improves ERG activity in the rd1 mice at 4 weeks post injection. This protection is not uniform but very regional, which may also reflect the less than full improvement of scotopic and photopic ERG activity in rd1 mice after AAV8-hOXR1 treatment. The rd1 mice treated with AAV8-hOXR1 displayed 25% to 30% of wild-type scotopic ERG activity, which is also similar to the regional restoration of rod photoreceptor function in the retina. A significant expression of hOXR1 in this area was revealed by OXR1 antibody treatment and was accompanied by retention of rod photoreceptors as indicated by staining with rhodopsin antibody. The conclusion of the effects of OXR1 on the retinal structure is based on the analysis of the transduced regions of the retina. Future studies using slower degenerating mouse models will be useful for validation of the results from the rd1 mouse study.
We hypothesize that hOXR1 alleviates oxidative stress and reduces ROS levels in photoreceptors, as was seen in cells in culture, and delays photoreceptor degeneration. AAV expressing SOD2 and catalase seems to reduce superoxide radicals (O2−) generated by oxidative stress in rd1 mice and, like OXR1, increases photoreceptor survival.6 We visualized maintenance of significant photopic function in rd1 mice after AAV8-hOXR1 injection. Therefore, we also examined structural maintenance of cones in the injected area. As the majority of cones in mice are of the M/L cone cell type, the green/red opsin antibody was used to check the cone preservation in the retina.45 The green/red opsin antibody usually stains the outer segment and cell body in cone cells. We observed a significant number of M/L cone cells in the treated area. Although the complete structural features of the cone cell types are lost, presumably because of degeneration of the structurally supportive rod cells, partial preservation of cone cells was seen and is sufficiently sensitive to light to allow improved photopic ERG activity.
AAV8-hOXR1 is not gene or disease-specific, because it treats oxidative stress, which is a common factor in most retinal degenerative diseases. Therefore, modulation of OXR1 levels could be therapeutic in many neurodegenerative diseases. In other mouse models, such as the rhodopsin mutant in which retinal degeneration proceeds more slowly, resulting in blindness at about 16 weeks of age,4 it is possible that OXR1 may be more beneficial. This is based on the hypothesis that the slower retinal degeneration may correlate with lower levels of oxidative stress. If this predicted correlation is correct, then providing the same level of protection as was achieved in the rd1 mutant mouse should result in a higher ratio of OXR1-dependent protective activities to oxidative stress levels.
Many gene therapies are directed at curing a disease by adding a wild type copy of the defective disease-causing gene. OXR1 gene therapy differs as it is not curative of the underlying cause of the disease, instead it treats oxidative stress which triggers apoptosis and leads to death of photoreceptor cells.46 Inhibiting oxidative stress has the potential to treat many different degenerative retinopathies by slowing down oxidative stress-induced apoptosis of retinal cells. Previous attempts to limit oxidative stress to prevent retinal degeneration used Nrf2 gene therapy. Like OXR1, Nrf2 is a regulatory factor that controls a number of oxidative stress resistance genes, including glutathione peroxidase 2 (GPX2) and Heme Oxygenase 1 (HMOX1).47,48 Nrf2 gene therapy, either by targeting the photoreceptor and retinal pigmented epithelial (RPE) cells or by targeting only the RPE, increases the retention of ERG activity in the rd10 mouse model of retinal degeneration and extended the preservation of the integrity of the cone structure in several mouse models of retinal degeneration.5,6 The rd10 mutation, like rd1, affects the Pde6β gene, however, retinal degeneration proceeds much more slowly than in the rd1 mouse resulting in blindness at about 8 weeks of age. The result that both Nrf2 and OXR1 can delay retinal degeneration demonstrates that controlling oxidative stress by genetic means can provide a substantial benefit to the prevention of retinal degeneration and retention of visual activity.
Like Nrf2, OXR1 is required for the expression of the GPX2 and HMOX1 genes, possibly through its interaction with KEAP1, which controls Nrf2's regulatory activity.16 However, OXR1 controls many additional pathways that contribute to resistance to oxidative stress. It controls the p53 response to oxidative stress, delaying the cell cycle to allow more time for DNA repair and increases the expression of several DNA repair factors. OXR1 also regulates genes involved in apoptosis and genes that modulate the levels of reactive oxygen in the cell.15,17 It acts upstream of several transcription factors that control oxidative stress resistance genes, as well as modulating the activity of oxidant and antioxidant proteins to reduce ROS levels and increase resistance to oxidative stress. Thus, OXR1 appears to have a comprehensive effect on cellular resistance to oxidative stress.
Long-term overexpression of OXR1 in brain neurons in transgenic mice expressing OXR1 from the neuron-specific Prnp prion promoter or the ubiquitously expressed chicken ß-actin promoter showed no deleterious consequences. This suggests that OXR1 is not associated with detectable toxicity and is likely to be relatively safe.14,26
In this study, we demonstrated that AAV8-hOXR1 gene therapy can delay the onset of neurodegeneration in the retina of the rd1 mutant mouse and serves as a proof of concept that OXR1 gene therapy may be beneficial as a therapeutic for many retinal degenerative diseases. Because OXR1 functions in the brain as well as retinal neurons and its repression has been implicated in several mouse models of neurodegeneration, it has the potential to be therapeutically effective at retarding neurodegeneration more generally if delivery problems can be conquered. In wild type mice that overexpress OXR1 in brain neurons, no pathology was found,14,26 suggesting it is an ideal candidate for gene therapy of oxidative stress induced neurodegenerative diseases. The extent to which it will be effective in such therapies remains to be determined.6,49
Supplementary Material
Acknowledgments
Funded by Grants from the International Retinal Research Foundation, The Dan and Diane Riccio Fund for Neuroscience, the UMASS Medical School Bridge Fund to MRV, and the National Institutes of Health grant EY022372 to HK. L.M.L. is supported by a postdoctoral fellowship from the Horae Gene Therapy Center, University of Massachusetts Medical School.
Disclosure: B. Sahu, None; L.M. Leon, None; W. Zhang, None; N. Puranik, None; R. Periasamy, None; H. Khanna, None; M. Volkert, None
References
- 1.Beshares JC.Photosensitive Membrane Turnover: Differentiated Membrane Domains and Cell-Cell Interaction. New York, NY: Academic Press; 1983: 363. [Google Scholar]
- 2.Bird AC.Clinical investigation of retinitis pigmentosa. Prog Clin Biol Res. 1987; 247: 3–20. [PubMed] [Google Scholar]
- 3.Sahel JA, Leveillard T.. Maintaining cone function in rod-cone dystrophies. Adv Exp Med Biol. 2018; 1074: 499–509. [DOI] [PubMed] [Google Scholar]
- 4.Punzo C, Xiong W, Cepko CL.. Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame? J Biol Chem. 2012; 287: 1642–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu DM, Ji X, Ivanchenko MV, et al.. Nrf2 overexpression rescues the RPE in mouse models of retinitis pigmentosa. JCI Insight. 2021; 6: e145029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015; 125: 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cronin T, Raffelsberger W, Lee-Rivera I, et al.. The disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress. Cell Death Differ. 2010; 17: 1199–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei X, Chaffiol A, Kole C, et al.. The thioredoxin encoded by the rod-derived cone viability factor gene protects cone photoreceptors against oxidative stress. Antioxid Redox Signal. 2016; 24: 909–923. [DOI] [PubMed] [Google Scholar]
- 9.Komeima K, Rogers BS, Lu L, Campochiaro PA.. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2006; 103: 11300–11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calderon GD, Juarez OH, Hernandez GE, Punzo SM, De la Cruz ZD.. Oxidative stress and diabetic retinopathy: development and treatment. Eye (Lond). 2017; 31: 1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami Y, Nakabeppu Y, Sonoda KH.. Oxidative stress and microglial response in retinitis pigmentosa. Int J Mol Sci. 2020; 21: 7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roehlecke C, Schumann U, Ader M, et al.. Stress reaction in outer segments of photoreceptors after blue light irradiation. PLoS One. 2013; 8: e71570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campochiaro PA, Mir TA.. The mechanism of cone cell death in retinitis pigmentosa. Prog Retin Eye Res. 2018; 62: 24–37. [DOI] [PubMed] [Google Scholar]
- 14.Finelli MJ, Liu KX, Wu Y, Oliver PL, Davies KE.. Oxr1 improves pathogenic cellular features of ALS-associated FUS and TDP-43 mutations. Hum Mol Genet. 2015; 24: 3529–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M, Lin X, Rowe A, Rognes T, Eide L, Bjoras M.. Transcriptome analysis of human OXR1 depleted cells reveals its role in regulating the p53 signaling pathway. Sci Rep. 2015; 5: 17409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M, Lin X, Segers F, et al.. OXR1A, a coactivator of PRMT5 regulating histone arginine methylation. Cell Rep. 2020; 30: 4165–4178 e4167. [DOI] [PubMed] [Google Scholar]
- 17.Yang M, Luna L, Sorbo JG, et al.. Human OXR1 maintains mitochondrial DNA integrity and counteracts hydrogen peroxide-induced oxidative stress by regulating antioxidant pathways involving p21. Free Radic Biol Med. 2014; 77: 41–48. [DOI] [PubMed] [Google Scholar]
- 18.Murray AR, Chen Q, Takahashi Y, Zhou KK, Park K, Ma JX.. MicroRNA-200b downregulates oxidation resistance 1 (Oxr1) expression in the retina of type 1 diabetes model. Invest Ophthalmol Vis Sci. 2013; 54: 1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natoli R, Provis J, Valter K, Stone J.. Expression and role of the early-response gene Oxr1 in the hyperoxia-challenged mouse retina. Invest Ophthalmol Vis Sci. 2008; 49: 4561–4567. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Liu J, Chen L, et al.. Serum secreted miR-137-containing exosomes affects oxidative stress of neurons by regulating OXR1 in Parkinson's disease. Brain Res. 2019; 1722: 146331. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Pan X, Zhang J, et al.. Plasma levels of miR-137 and miR-124 are associated with Parkinson's disease but not with Parkinson's disease with depression. Neurol Sci. 2017; 38: 761–767. [DOI] [PubMed] [Google Scholar]
- 22.Puspita L, Chung SY, Shim JW.. Oxidative stress and cellular pathologies in Parkinson's disease. Mol Brain. 2017; 10: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamper C, Siegel A, Liang WS, et al.. Neuronal gene expression correlates of Parkinson's disease with dementia. Mov Disord. 2008; 23: 1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mo JL, Pan ZG, Chen X, et al.. MicroRNA-365 knockdown prevents ischemic neuronal injury by activating oxidation resistance 1-mediated antioxidant signals. Neurosci Bull. 2019; 35: 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Shen Q, Xia Y, Lei X, Peng J.. Sevoflurane induced neurotoxicity is driven by OXR1 posttranscriptional downregulation involving hsamiR302e. Mol Med Rep. 2018; 18: 4657–4665. [DOI] [PubMed] [Google Scholar]
- 26.Oliver PL, Finelli MJ, Edwards B, et al.. Oxr1 is essential for protection against oxidative stress-induced neurodegeneration. PLoS Genet. 2011; 7: e1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volkert MR, Crowley DJ.. Preventing Neurodegeneration by controlling oxidative stress: the role of OXR1. Front Neurosci. 2020; 14: 611904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalloniatis M, Nivison-Smith L, Chua J, Acosta ML, Fletcher EL.. Using the rd1 mouse to understand functional and anatomical retinal remodelling and treatment implications in retinitis pigmentosa: A review. Exp Eye Res. 2016; 150: 106–121. [DOI] [PubMed] [Google Scholar]
- 29.Petit L, Khanna H, Punzo C.. Advances in gene therapy for diseases of the eye. Hum Gene Ther. 2016; 27: 563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allocca M, Mussolino C, Garcia-Hoyos M, et al.. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol. 2007; 81: 11372–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowes Rickman C, Farsiu S, Toth CA, Klingeborn M.. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci. 2013; 54: ORSF68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pittler SJ, Baehr W.. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the RD mouse. Proc Natl Acad Sci USA. 1991; 88: 8322–8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno-Leon L, West EL, O'Hara-Wright M, et al.. RPGR isoform imbalance causes ciliary defects due to exon ORF15 mutations in X-linked retinitis pigmentosa (XLRP). Hum Mol Genet. 2021; 29: 3706–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sena-Esteves M, Gao G.. Introducing genes into mammalian cells: viral vectors. Cold Spring Harb Protoc. 2020; 2020: 095513. [DOI] [PubMed] [Google Scholar]
- 35.Elliott NA, Volkert MR.. Stress induction and mitochondrial localization of Oxr1 proteins in yeast and humans. Mol Cell Biol. 2004; 24: 3180–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Li L, Su Q, Gao G, Khanna H.. Gene therapy using a mini CEP290 fragment delays photoreceptor degeneration in a mouse model of Leber congenital amaurosis. Hum Gene Ther. 2018; 29: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkatesh A, Ma S, Langellotto F, Gao G, Punzo C.. Retinal gene delivery by rAAV and DNA electroporation. Curr Protoc Microbiol. 2013; Chapter 14:Unit 14D 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao KN, Zhang W, Li L, Ronquillo C, Baehr W, Khanna H.. Ciliopathy-associated protein CEP290 modifies the severity of retinal degeneration due to loss of RPGR. Hum Mol Genet. 2016; 25: 2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahu B, Sun W, Perusek L, et al.. Conditional ablation of retinol dehydrogenase 10 in the retinal pigmented epithelium causes delayed dark adaption in mice. J Biol Chem. 2015; 290: 27239–27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crow JP.Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide. 1997; 1: 145–157. [DOI] [PubMed] [Google Scholar]
- 41.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR.. Retinal degeneration mutants in the mouse. Vision Res. 2002; 42: 517–525. [DOI] [PubMed] [Google Scholar]
- 42.Finelli MJ, Sanchez-Pulido L, Liu KX, Davies KE, Oliver PL.. The evolutionarily conserved Tre2/Bub2/Cdc16 (TBC), lysin motif (LysM), domain catalytic (TLDc) domain is neuroprotective against oxidative stress. J Biol Chem. 2016; 291: 2751–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu KX, Edwards B, Lee S, et al.. Neuron-specific antioxidant OXR1 extends survival of a mouse model of amyotrophic lateral sclerosis. Brain. 2015; 138: 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sancho-Pelluz J, Arango-Gonzalez B, Kustermann S, et al.. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol Neurobiol. 2008; 38: 253–269. [DOI] [PubMed] [Google Scholar]
- 45.Ortin-Martinez A, Nadal-Nicolas FM, Jimenez-Lopez M, et al.. Number and distribution of mouse retinal cone photoreceptors: differences between an albino (Swiss) and a pigmented (C57/BL6) strain. PLoS One. 2014; 9: e102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunchithapautham K, Rohrer B.. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy. 2007; 3: 433–441. [DOI] [PubMed] [Google Scholar]
- 47.Tonelli C, Chio IIC, Tuveson DA.. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018; 29: 1727–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He F, Ru X, Wen T.. NRF2, a transcription factor for stress response and beyond. Int J Mol Sci. 2020; 21: 4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komeima K, Rogers BS, Campochiaro PA.. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007; 213: 809–815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.