Abstract
Aggregation of RNA-binding proteins (RBPs) is a pathological hallmark of neurodegenerative disorders like amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). In these diseases, TDP-43 and FUS RBPs are both depleted from the nuclear compartment, where they are normally localized, and found within cytoplasmic inclusions in degenerating regions of patient postmortem tissue. The mechanisms responsible for the aggregation of these proteins has remained elusive but recent studies suggest that liquid-liquid phase separation (LLPS) might serve as a critical nucleation step in the formation of pathological inclusions. The process of phase separation also underlies the formation and maintenance of several functional membraneless organelles (MLOs) throughout the cell, some of which contain TDP-43, FUS, and other disease-linked RBPs. One common ligand of disease-linked RBPs, RNA, is a major component of MLOs containing RBPs and has been demonstrated to be a strong modulator of RBP phase transitions. While early evidence suggested a largely synergistic effect of RNA on RBP phase separation and MLO assembly, recent work indicates that RNA can also antagonize RBP phase behavior in certain physiological and pathological conditions. In this review, we describe the mechanisms underlying RNA-mediated phase transitions of RBPs and examine the molecular properties of these interactions, such as RNA length, sequence, and secondary structure, that mediate physiological or pathological LLPS.
RNA binding protein aggregation in neurodegenerative disease
The intracellular deposition of misfolded proteins is a well characterized pathological feature of a number of neurodegenerative disorders, such as Alzheimer’s Disease (AD), Parkinson’s Disease (PD), Huntington’s Disease (HD), chronic traumatic encephalopathy (CTE), amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) (Kumar et al., 2016; McKee et al., 2015; Ross and Poirier, 2004). While typically associated with divergent clinical presentations, pathway perturbations and genetic/environmental factors, the unifying observation of abnormal protein inclusions in postmortem tissue of these patients may suggest some common downstream mechanisms driving neurodegeneration in these diseases.
Though the specific protein components of neuropathological inclusions vary, both across disorders and between subtypes of disease, spanning a wide range of biological functions and subcellular distributions, one class of proteins that is increasingly overrepresented are RNA-binding proteins (Maziuk et al., 2017). For example, aggregation of the DNA/RNA binding protein TDP-43 is estimated to occur in up to ~97% of ALS, ~60% of AD, ~50% of FTD, and ~85% of CTE patients (McKee et al., 2010; Prasad et al., 2019; Tremblay et al., 2011). Aberrant aggregation of different RNA-binding proteins (RBPs) is also documented in neurodegenerative disorders, including: FUS, TAF15, EWSR1, hnRNPA1, hnRNPA2B1, TIA-1, hnRNPA3 and hnRNPD/AUF1 (Table 1). Furthermore, other well-recognized disease-linked proteins like tau and α-synuclein not typically considered “traditional” RNA binding proteins were recently predicted and/or demonstrated to interact with RNA and other nucleic acids (Schaser et al., 2019; Zanzoni et al., 2013; Zhang et al., 2017), suggesting that alterations in protein:RNA interactions or global RNA homeostasis may participate in the pathological deposition of protein inclusions across many different disorders.
Table 1:
Disease-linked RBPs with PrLDs that undergo LLPS.
| RNA-binding protein (RBP) | Genetic disease-association | Pathology disease-association | Identified PrLD (1,2) | In vitro LLPS? | Example MLO-association |
|---|---|---|---|---|---|
| TDP-43 | ALS (3), FTD (4) | ALS (5,6), FTLD (5,6), LATE (7), CTE (8), AD (9,10), CBD (10), PD (11) | a.a. 277–414 | Full-length protein (12–14) C-terminal PrLD (15–18) |
mRNP transport granules (19,20), Nuclear gems (21,22), Nuclear stress bodies (23), Stress granules (24,25), Paraspeckles (26) |
| FUS | ALS (27,28), FTD (29,30) | ALS (27,28), FTLD-FUS (31), HD (32), SCA1/2/3 (33) | a.a. 1–237 | Full-length protein (34–36) N-terminal PrLD/LCD (34,36,37) |
Paraspeckles (38), Nuclear gems (22), Stress granules (39–41), mRNP transport granules (42,43) |
| EWSR1 | ALS (44) | FTLD-FUS (45) | a.a. 1–280 | Full-length protein (46,47) | DNA damage response foci (48), Stress granules (49), Paraspeckles (26) |
| TAF15 | ALS (50,51) | FTLD-FUS (45) | a.a. 1–152 | Full-length protein (46,47) | DNA damage response foci (48), Stress granules (49,52), Paraspeckles (26) |
| hnRNPA1 | MSP (53), ALS (53) | MSP* (53), IBM* (53), FTLD-FUS (54) | a.a. 186–372 | Full-length protein (12,47,55) C-terminal PrLD/LCD (12,55) |
Stress granules (12,56), mRNP transport granules (42), Paraspeckles (26) |
| hnRNPA2B1 | MSP (53), ALS (53) | MSP* (53), IBM* (53) | a.a. 197–353 | Full-length protein (46,57,58) C-terminal PrLD/LCD (58,59) |
Stress granules (53,60), mRNP transport granules (42) |
| TIA-1 | ALS (61,62), WDM* (63,64) | AD (65) | a.a. 292–386 | Full-length protein (46,61,66) C-terminal PrLD/IDR (55) |
Stress granules (67) |
| hnRNPA3 | Unknown | C9orf72-ALS (68,69) | a.a. 207–378 | Full-length protein (46) | mRNP transport granules (70), Stress granules (71) |
| hnRNPD/AUF1 | Unknown | FTLD-FUS (54) | a.a. 262–365 | Full-length protein (46) | mRNP transport granules (42) |
Muscular diseases/pathology
Abbreviations: AD = Alzheimer’s disease; ALS = amyotrophic lateral sclerosis; CBD = corticobasal degeneration; CTE = chronic traumatic encephalopathy; FTD = frontotemporal dementia; FTLD = frontotemporal lobar degeneration; HD = Huntington’s disease; IBM = Inclusion body myopathy; IDR = Intrinsically-disordered region; LATE = Limbic-predominant age-related TDP-43 encephalopathy; LCD = Low-complexity domain; LLPS = Liquid-liquid phase separation; MLO = Membraneless organelle; PD = Parkinson’s disease; MSP = Multisystem proteinopathy; PrLD = Prion-like domain; SCA1/2/3 = Spinocerebellar ataxia types 1, 2 & 3; VCPDM = vocal cord and pharyngeal weakness with distal myopathy; WDM = Welander distal myopathy
While these observations from postmortem patient tissue suggest some participation of RNA-binding protein aggregation in the pathogenesis of neurodegenerative disease, a critical question remains: how do RBP inclusions arise and what upstream mechanisms initiate this event? One major hypothesis that has recently emerged from the fields of polymer chemistry and biophysics revolves around the concept of phase separation. In addition to interactions with nucleic acids, another commonality across many RNA-binding proteins and proteins misfolded in neurodegenerative diseases, is their ability to undergo a particular type of phase transition, called liquid-liquid phase separation (LLPS) and this is proposed to serve as a critical nucleation event for the deposition of pathological protein aggregates (Babinchak and Surewicz, 2020).
Liquid-liquid phase separation in biological systems
In biological terms, phase separation refers to a process by which certain organic molecules dynamically transition from an initially mixed population into separate compartments, or phases. In the same manner that non-biological molecules can transition between different states (i.e. gas, liquid, and solid) based upon variables like temperature, it is becoming increasingly recognized that biological molecules, such as proteins, are found in different biophysical states within a cell (i.e. soluble proteins or solid-like aggregates) based upon changes in the intra- and extracellular environment such as heat (Wallace et al., 2015), nutrient starvation (Narayanaswamy et al., 2009) or proteostatic stress (Sui et al., 2020).
Phase transitions are also utilized to dynamically compartmentalize and organize the cell in the absence of lipid membranes. Complementing more canonical lipid-bound organelles like mitochondria and the endoplasmic reticulum, these “membraneless organelles” (MLOs) can be found all over the cell and include such structures as the nucleolus, RNA transport granules, processing bodies (P-bodies), and paraspeckles (reviewed by Gomes and Shorter, 2019). Following pioneering biophysical investigations into the C. elegans germline P granule (Brangwynne et al., 2009) and the X. laevis oocyte nucleoli (Brangwynne et al., 2011), it is now established that many MLOs arise through the process of liquid-liquid phase separation (LLPS). Commonly presented as analogous to the behavior of oil in water, LLPS involves the selective condensation of molecules (i.e. proteins and nucleic acids) into distinct droplets, or a condensed phase, within a surrounding liquid environment. These condensed droplets exhibit many properties of “classical” liquids, for example: spherical shape, fission/fusion, dripping, surface wetting, and dynamic molecular exchange both within droplets and with the surrounding environment (reviewed by Hyman et al., 2014; Shin and Brangwynne, 2017).
The resulting biophysical characteristics of these condensed assemblies provide a unique advantage over membrane-bound organelles. For example, recent work investigating LLPS at the neuronal synapse suggests that condensed phases function to rapidly concentrate and release specific pre- and post-synaptic components in response to neuronal activity without the need for active transporters typically utilized by membrane-bound compartments (Milovanovic et al., 2018; Zeng et al., 2016). Liquid-like assemblies at the plasma membrane of non-neuronal cells also play a role in T-cell receptor signaling, where downstream signaling proteins condense and organize specific adapter/effector proteins, such as ZAP70 and Nck, while excluding phosphatases that function to dissolve condensates via protein dephosphorylation (Su et al., 2016). These localized condensates promote actin filament assembly at rates that exceeded those resulting from simple membrane targeting of the same molecules (Su et al., 2016). Additional examples of functional biomolecular condensates are being increasingly discovered and associated with novel cellular pathways, thus highlighting that the ability of proteins and other molecules to undergo phase transitions is a fundamental principle of cellular physiology.
Intrinsically-disordered regions (IDRs) as key players in the formation and material properties of phase-separated protein assemblies
As the number and diversity of proteins reported to utilize phase separation across the proteome has grown, so too has our understanding of the molecular determinants governing the formation and biophysical properties of different membraneless assemblies. While the precise domain composition and molecular interactions vary, recent efforts in the purification and proteomic analysis of various MLOs, such as the nucleolus (Andersen et al., 2002, 2005; Scherl et al., 2002), stress granules (Jain et al., 2016; Kuechler et al., 2020) P-bodies (Hubstenberger et al., 2017), and other RNA granules (Han et al., 2012; Kato et al., 2012), suggest that a common feature allowing proteins to undergo phase separation is multivalency. This multivalency can be achieved in a number of ways, but generally involves weak, transient contacts, often through repeated interaction motifs or modules, within and between molecules (reviewed by Posey et al., 2018; Shin and Brangwynne, 2017). Initial investigations utilizing synthetic linear peptides comprised of repeated interaction modules, such as the SH3/PRM (Li et al., 2012) and SUMO/SIM (Banani et al., 2016) domains, in heterotypic reactions have highlighted the importance of valency (i.e. the number of interaction modules) in the ability of proteins to undergo phase separation, but later studies also point to a distinct role for the interspersing of these motifs by flexible linker sequences as a critical determinant of phase behavior in these systems (Harmon et al., 2017). Interestingly, another key feature of proteins found within MLOs by the studies mentioned above, particularly in stress and other RNA granules, was an enrichment of intrinsically disordered domains or intrinsically-disordered regions (IDRs) (Han et al., 2012; Jain et al., 2016; Kato et al., 2012; Kuechler et al., 2020).
IDRs are typically defined as regions of a protein sequence that lack a defined, or ordered, secondary structure and thus are capable of adopting a range of three-dimensional conformations (Dunker et al., 2013). The structural ambiguity of intrinsically-disordered regions are conferred by amino acid compositions of limited sequence complexity, and regions conforming to these characteristics are commonly referred to as low-complexity domains (LCDs) or low-complexity IDRs (van der Lee et al., 2014). IDRs are also typically enriched in charged (i.e. arginine, lysine, glutamic acid), polar (i.e. serine, glutamine, asparagine), and/or aromatic (i.e. phenylalanine, tyrosine, tryptophan) amino acids and contain amino acids like glycine and proline that may convey some structural information (Romero et al., 2001; Vucetic et al., 2003). Based on the specific composition and clustering of certain subsets of these enriched amino acids, IDRs can be further classified into distinct subtypes, such as RG/RGG domains (Elbaum-Garfinkle et al., 2015; Wang et al., 2018b), FG domains (Hülsmann et al., 2012; Schmidt and Görlich, 2015) and prion-like domains (PrLDs, discussed below), that engage in a variety of non-covalent interactions responsible for driving phase transitions. While interaction types can vary both across and within IDRs, they generally involve one or more of the following: i) charge-based interactions (charge-charge or cation-π), ii) dipole-dipole interactions, and iii) π-π stacking of aromatic residues.
IDR amino acid composition dictates biomolecular condensate material properties and function
Recent simulations and mutational studies predict that the heterogeneity of both structural conformation and interaction type (via amino acid composition) in IDRs have profound effects on the formation and biophysical properties of higher-order assemblies (Tüű-Szabó et al., 2018; Wang et al., 2018b). The material properties, in turn, reflect specific functions that are encoded within the sequence of IDRs and other interaction domains within protein components of various membraneless structures. One example of this notion is found within the central channel of the nuclear pore complex, where condensation of intrinsically-disordered FG-nucleoporins form a selectivity barrier of the nuclear pore complex through multivalent interactions between repetitive aromatic patches (i.e. FxFG, GLFG, xxFG) contained within FG domains of these proteins (Li et al., 2016) (Figure 1a). In vitro, the FG domains of several nucleoporins, such as Nup62 (Labokha et al., 2013), Nup98 (Hülsmann et al., 2012), and Nup153 (Milles and Lemke, 2011), undergo hydrogel formation. Despite divergence of exact amino acid sequence, overall amino acid composition and structural disorder is a well-conserved feature of FG domains spanning from yeast to human nucleoporins (Denning and Rexach, 2007). Yeast FG-nucleoporins also form hydrogels when sufficiently saturated in vitro and function as selective permeability barriers in these systems, allowing the rapid translocation of importin-β-bound cargo via successive high-affinity interactions with FG-nucleoporins while blocking the diffusion of non-specific molecules (Celetti et al., 2020; Frey and Görlich, 2007, 2009). Importantly, deletion of FG domains, or mutation of phenylalanine residues to allow liquid-liquid phase separation but prevent hydrogelation impairs the barrier function of nucleoporins like Nup98 (Hülsmann et al., 2012) and Nup100 (Patel et al., 2007), highlighting the functional benefit of hydrogel formation driven by conserved FG-repeat interactions.
Figure 1. Examples of biophysical state dictating structure function in vivo.
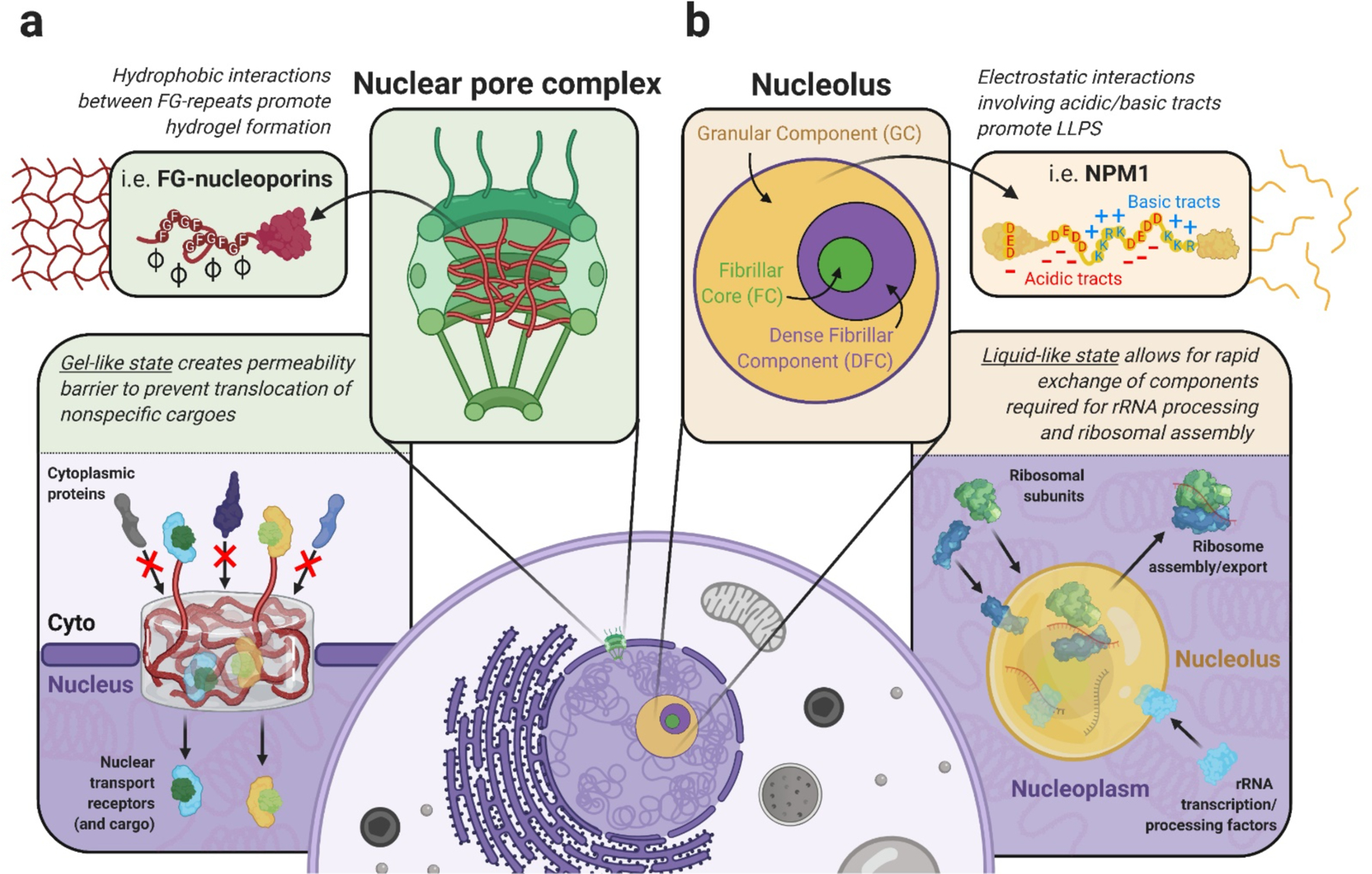
(a) Within the central channel of the nuclear pore complex (NPC), hydrophobic interactions between FG-repeat regions of the FG-nucleoporins (i.e. Nup98, Nup62) contribute to the formation of a hydrogel-like assembly. This gel-like state forms a permeability barrier to block the translocation of nonspecific cargo in and out of the nucleus, while allowing for specific interactions between nuclear transport receptors and nucleoporins that drive cargo tranport. (b) The nucleolar protein NPM1 utilizes electrostatic interactions provided by acidic and basic tracts within its central IDR to undergo LLPS as part of the the granular component (GC) of the nucleolus. The liquid-like state of the GC allows for rapid exchange of newly-transcribed and processed rRNA, from the dense fibrillar component (DFC) and the fibrillar core (FC) subcompartments of the nucleolus, and ribosomal subunits, from the surrounding nucleoplasm, that is required for proper ribosomal assembly and export.
Another example is found in the phase behavior of the nucleolar protein, nucleophosmin 1 (NPM1). NPM1 utilizes charge-based interactions driven by acidic and basic tracts within its central IDR to achieve homotypic LLPS in vitro (Mitrea et al., 2018) and heterotypic LLPS with arginine-rich peptides and rRNA. This results in the formation of the liquid-like granular component (GC) of the nucleolus (Feric et al., 2016; Mitrea et al., 2016) (Figure 1b). In addition to compartmentalization of the nucleolar machinery within the surrounding nucleoplasm, the liquid-like state of NPM1 within the granular component is essential for rRNA processing, as solidification of the GC phase by optogenetic oligomerization (Zhu et al., 2019) or the infiltration of toxic arginine-rich dipeptide-repeat proteins (Lee et al., 2016) leads to the accumulation of rRNA precursors and reduction of mature rRNA. The liquid-like material properties of the GC also play an important role in the cellular stress response, facilitating the entry and dynamic storage of misfolded proteins to allow for Hsp70-mediated refolding and prevent irreversible aggregate formation prior to stress recovery (Frottin et al., 2019). Disruption of the liquid-like state of NPM1 through prolonged stress or accumulation of dipeptide-repeat proteins, severely compromises the protein quality control function of the GC phase. Thus, each distinct interaction type (i.e. aromatic or charge-based) encoded within these regions were likely evolutionarily tuned to allow for the formation of assemblies with specific, functionally-beneficial material properties.
Prion-like domains and their relevance to aberrant RNA-binding protein aggregation in neurodegenerative disease
Another subtype of IDR that has been identified to mediate phase separation of various proteins into assemblies with unique biophysical properties are prion-like domains (PrLDs). First identified in the yeast proteins Ure2 and Sup35, PrLDs were shown to drive the assembly of self-propagating, amyloid-like aggregates that were capable of templating their aggregated state to soluble versions of the proteins (Patino et al., 1996; Ter-Avanesyan et al., 1994; Wickner, 1994). Subsequent analysis of these domains revealed that PrLDs were intrinsically-disordered and contained very limited sequence complexity, skewing towards amino acid compositions of glycine and largely uncharged, polar amino acids like glutamine, asparagine, serine, and tyrosine (Verdile et al., 2019).
When the human proteome was later scanned for proteins containing PrLD-like amino acid compositions, a heavy enrichment of proteins identified as containing PrLDs was found in RNA-binding proteins (Alberti et al., 2009; Couthouis et al., 2011; King et al., 2012). Furthermore, a number of these PrLD-containing RBPs overlap with those reported to form pathological inclusions in postmortem patient tissue mentioned above (Table 1), suggesting a role for prion-like domains in the aberrant aggregation of proteins observed in disease (Couthouis et al., 2011). Purified preparations of these disease-linked RBPs also undergo LLPS in vitro and are components of RNA-containing MLOs within the cell, including RNA transport granules, paraspeckles, and nuclear gems (Table 1). The PrLDs of many RBPs are necessary and sufficient for phase separation in these systems (Babinchak et al., 2019; Conicella et al., 2016, 2020; Molliex et al., 2015; Patel et al., 2015; Ryan et al., 2018; Schmidt and Rohatgi, 2016), highlighting the importance of prion-like domains in regulating physiological self-assembly of disease-linked RNA-binding proteins under normal conditions.
While initial assemblies formed by PrLD-containing RBPs in vitro, as well as their PrLDs in isolation, often display liquid-like biophysical properties, extended incubation of certain RBPs (such as FUS, hnRNPA1, and the TDP-43 LCD) promote the maturation or hardening of droplets into more gel-like structures or solid-state aggregates that resemble the pathological state of these proteins observed in patient tissue (Babinchak et al., 2019; Conicella et al., 2016; Lin et al., 2015; Patel et al., 2015; Zhuo et al., 2020). Similar droplet-like states precede amyloid formation of Sup35 in yeast (Patel et al., 2015; Serio et al., 2000), suggesting that PrLDs harbor evolutionarily-conserved elements that allow for the formation of liquid-like compartments in response to physiological stimuli but also confer a propensity for solid-state aggregation under pathological conditions.
Supporting this potential duality, liquid-to-solid state transitions of certain RBPs, also called aberrant phase transitions, are promoted by disease-linked familial mutations within their PrLDs. An ALS-linked mutation in the FUS PrLD (G156E) was recently shown to gradually increase the viscosity of initially liquid-like FUS droplets in vitro and promote emergent fibrillization at incubation times in which wildtype droplets remained dynamic (Patel et al., 2015). Similar results were observed when examining the effect of a multisystem proteinopathy (MSP)-linked PrLD mutation on the in vitro phase behavior of hnRNPA1, which underwent LLPS indistinguishable from wildtype protein at initial timepoints and were readily partitioned into liquid-like droplets with wildtype protein when mixed (Molliex et al., 2015). However, over time, the mutant protein began to fibrillize and emerged from wildtype droplets, eventually seeding wildtype protein into similar fibril-like assemblies. These effects were similarly observed for other PrLD-containing RBPs like TDP-43 and TIA-1 in cellular systems, where disease-linked mutations within these regions corresponded with increased protein droplet viscosity and/or delayed dissolution of membraneless structures containing mutant proteins (Gopal et al., 2017; Mackenzie et al., 2017; Mann et al., 2019; Schmidt and Rohatgi, 2016). Though the maturation of initial protein assemblies has only been directly interrogated for a subset of disease-linked RBPs, it is hypothesized that LLPS may serve as a general initiation event that results in the deposition of these proteins within insoluble inclusions in neurodegenerative diseases.
RNA as a scaffold for promoting physiological phase transitions and MLO formation
In addition to prion-like IDRs, many protein components of MLOs also contain multiple RNA-binding domains, either in the form of canonical RNA-recognition motifs (RRMs) or RGG/ZnF regions (reviewed by Gomes and Shorter, 2019). RNA is major component of a number of MLOs within the cell, including paraspeckles (Bond and Fox, 2009; Clemson et al., 2009), processing bodies (Sheth and Parker, 2003; Teixeira et al., 2005), and neuronal transport granules (Krichevsky and Kosik, 2001). Taken together, this suggests that RBP:RNA interctions may regulate phase separation. Recent in vitro studies uncovered some of the basic mechanisms underlying the effects of RNA on RBP phase behavior. One of the first direct investigations of RNA’s effect on RBP phase separation reported a strong enhancement of LLPS observed of artificial fusion proteins containing RNA-binding elements from the polypyrimidine tract-binding protein (PTB) along with intrinsically disordered regions from various other RBPs (including FUS, hnRNPA1, TIA-1, and others) upon addition of RNA (Lin et al., 2015). The enhancement of PTB assembly by RNA was dependent upon the presence of IDRs, whereas phase separation of PTB alone with RNA only occurred at high protein concentrations (50 μM alone versus 1.25–2.5 μM with IDRs). The addition of RNA did not promote the LLPS of intrinsically disordered regions alone (without PTB RNA-binding regions), suggesting a synergistic effect of PTB:RNA and IDR:IDR interactions in the driving of phase separation. Similar findings were reported for full-length RBPs, including FUS (Burke et al., 2015; Han et al., 2012) and hnRNPA1 (Lin et al., 2015; Molliex et al., 2015), which led to the hypothesis that binding of RNA promotes a high local concentration of IDR-containing RBPs that contribute additional multivalent interactions required to drive phase separation of RNP structures.
The coaction of RNA:RBP and IDR:IDR interactions is particularly important for the assembly of multicomponent MLOs, such as the nucleolus, and was recently conceptually described in terms of client-scaffold relationships (Banani et al., 2016; Ditlev et al., 2018). In brief, the client-scaffold framework describes the formation and composition of phase-separated compartments being dictated by interactions between multivalent “scaffold molecules” (i.e. RNA) and “client molecules” (i.e. RBPs). The enhanced multivalency afforded by interactions between RNA-binding regions within RBPs and specific binding motifs encoded within RNA nucleotide sequence drive heterotypic phase separation (i.e. through lowering the concentration threshold required for a protein to undergo LLPS), and provide some specificity in the recruitment of particular RBPs to ribonucleoprotein (RNP) granules (reviewed by Langdon and Gladfelter, 2018). This concept is well-exemplified in the granular component (GC) subcompartment of the nucleolus by interaction between rRNA and NPM1, serving as scaffold and client respectively. In vitro, rRNA is capable of driving phase separation of NPM1 into co-assemblies that mirror the liquid-like state of the GC phase of the nucleolus in vivo (Feric et al., 2016; Mitrea et al., 2018). Co-assembly is mediated by specific interactions between the C-terminal RNA binding domain of NBM1 and rRNA. Poly-U50 RNA cannot induce NPM1 LLPS and deletion of the RNA binding domain disrupts NPM1 phase separation with rRNA and localization to the GC phase of nucleoli (Feric et al., 2016; Mitrea et al., 2016, 2018). Interestingly, NPM1 localization within the GC requires the N-terminal oligomerization domain (Feric et al., 2016; Mitrea et al., 2016), demonstrating the cooperative effects of homotypic and heterotypic protein:RNA multivalent interactions often underlying the assembly of multicomponent MLOs.
Other factors such as relative scaffold/client concentrations, client affinity/valency, and additional multivalent interactions between different scaffold and client molecules also play a critical role in determining the effect of RNA in promoting MLO assembly and RBP incorporation into these structures (reviewed by Boeynaems et al., 2018). Other work investigating the formation of other naturally-occurring MLOs within intracellular systems support the general framework of a client-scaffold relationship between RBPs and RNA, as the presence of certain RNAs within a cell is required for the assembly of many different cellular RNP compartments including P-bodies (Teixeira et al., 2005), bacterial RNP (BR) bodies (Al-Husini et al., 2018), paraspeckles (Clemson et al., 2009), nuclear amyloid bodies (Audas et al., 2016), and the nucleolus (Berry et al., 2015; Falahati et al., 2016). Furthermore, MLOs, such as nuclear speckles, paraspeckles, Cajal bodies, and nuclear stress bodies, can be formed de novo by the introduction of excess RNA substrates into the cell (Bounedjah et al., 2014; Kaiser et al., 2008; Shevtsov and Dundr, 2011). These observations suggest that RNA is not merely a passive component of many MLOs within the cell but is a necessary and active participant in their assembly and maintenance.
Stress granules as an intersection between physiological and pathological RBP phase transitions
One of the best-characterized multi-component MLOs in the cell are stress granules. First described in chicken embryo fibroblasts (Collier and Schlesinger, 1986; Collier et al., 1988), stress granules are cytoplasmic RNP granules that form in response to cellular stressors or pharmacological/genetic intervention and contain an abundance of RBPs, translation initiation factors, and mRNAs (reviewed by Buchan and Parker, 2009; Protter and Parker, 2016). These granules exhibit liquid-like properties in cells and are thus presumed to arise through the process of LLPS. Stress granules have been proposed to function in translational regulation and mRNA homeostasis, given the presence of stalled translation initiation complexes and the global inhibition of protein synthesis observed in conditions triggering their formation (Kedersha et al., 2000, 2002, 1999; Kimball et al., 2003; Mazroui et al., 2006; Mokas et al., 2009). Stress granules also sequester a number of other non-RNA binding proteins involved in various signaling pathways (Jain et al., 2016) and thus may participate in additional or alternative functions.
Recent in-depth investigations into the molecular mechanisms of stress granule formation show that RNA is a critical factor mediating their initial assembly and maturation in conditions of translational arrest. Specifically, the release of non-translating mRNAs from polysomes upon translational inhibition triggers phase separation of stress granule core proteins like G3BP1/2 upon binding to newly available RNA (Guillén-Boixet et al., 2020; Sanders et al., 2020; Yang et al., 2020). Initial core assemblies of G3BP1/2 and non-translating mRNA then serve as multivalent network nodes that provide a physical platform for the subsequent recruitment of client molecules required for stress granule condensation through crosslinking (Guillén-Boixet et al., 2020; Sanders et al., 2020; Yang et al., 2020). Early studies indicate that recruitment of additional client RBPs is indeed dependent upon their binding to RNA, as deletion or mutation of RNA binding domains of RBPs, such as TDP-43 and FUS, antagonizes their co-localization with stress granules (Bentmann et al., 2012; Chen and Cohen, 2019; Colombrita et al., 2009; Daigle et al., 2013; Guillén-Boixet et al., 2020; Mann et al., 2019), and RNaseI-mediated degradation of single-stranded RNA significantly reduces their enrichment within stress granule fractions upon the induction of cellular stress (Fang et al., 2019).
Stress granules are a major focus in the field of neurodegeneration following the discoveries that disease-linked RBPs like TDP-43, FUS, TIA-1, hnRNPA1 and hnRNPA2/B1 localize to this MLO (Bosco et al., 2010; Colombrita et al., 2009; Dormann et al., 2010; Gal et al., 2011; Guil et al., 2006; Kim et al., 2013; Liu-Yesucevitz et al., 2010; McDonald et al., 2011; Molliex et al., 2015) (see also Table 1). Many of the above-mentioned RBPs exert some regulatory capacity over stress granule formation and disease-linked mutations often enhance recruitment to stress granules (Baron et al., 2013; Bosco et al., 2010; Gilks et al., 2004; Kim et al., 2013; McDonald et al., 2011). Additionally, familial disease-linked mutations in some stress granule components, such as FUS and TIA-1, alter stress granule assembly and/or dynamics (Baron et al., 2013; Mackenzie et al., 2017).
These observations led to the emerging hypothesis linking stress granule dysfunction to the protein depositions in neurodegenerative diseases. Specifically, during the physiological stress response, proteins like TDP-43 and FUS shuttle out to the cytoplasm and are incorporated into stress granules. In normal conditions, stress granules are then disassembled and/or cleared by autophagy during stress recovery, allowing for translational restart of stalled mRNPs and degradation or return to normal function of other RBP components (reviewed by Protter and Parker, 2016). Conversely, during pathological conditions in which stress granule clearance or disassembly is delayed, these liquid-like structures could serve as reaction crucibles to promote the aggregation of proteins like TDP-43 and FUS in a similar mechanism to that observed in vitro (reviewed by Wolozin and Ivanov, 2019). In this sense, the prolonged incubation of aggregate-prone RBPs present at high local concentrations within stress granules could promote a maturation of these assemblies and result in their deposition into insoluble inclusions capable of driving disease. However, while one might expect other stress granule components to co-aggregate with TDP-43 or FUS in patient inclusions as a result of stress granule maturation, postmortem analyses of TDP-43 or FUS proteinopathy patient tissue has yielded largely negative results (Chen and Cohen, 2019; Hirsch-Reinshagen et al., 2017; Mackenzie et al., 2017; Mann et al., 2019). ALS patients harboring TIA-1 mutations shown to delay stress granule disassembly in cellular models, there are TIA-1-positive inclusions nor co-localization of TIA-1 with TDP-43 pathology was observed in postmortem tissue (Hirsch-Reinshagen et al., 2017; Mackenzie et al., 2017). Still, the possibility does exist that initial TDP-43 or FUS aggregate formation may occur within stress granules, which may persist following disassembly of other stress granule components. However, it is still unclear whether seeding of RBP aggregation within stress granules, based on observations in cellular models, recapitulates the pathogenic processes occurring in human disease and remains the focus of continued study.
The role of RNA in the maturation of stress granules also remains an important question in the field. Recent transcriptomic studies have begun to uncover some common elements of SG-enriched RNAs (discussed below) and potential mechanisms driving their partitioning into stress granules (Khong et al., 2017; Matheny et al., 2019; Van Treeck et al., 2018). Additionally, some shifts in the protein and RNA composition of stress granules induced by acute or chronic stress have been reported (Reineke et al., 2018), but future studies aimed at directly modulating the RNA content of these assemblies are needed to determine the contribution of RNA to the maintenance of SG fluidity and prevention of protein aggregation under chronic stress conditions.
RNA antagonizes the phase transitions of RBPs at physiological concentrations
In addition to influencing the formation and composition of membraneless assemblies, studies show that RNA can promote RBP phase separation and incorporation into MLOs both in vitro and in cellular environments. The effects of RNA on RBP LLPS are often recognized as heavily dependent upon RNA:protein molar ratios, with lower RNA concentrations promoting phase separation of excess protein concentrations in most in vitro experiments (Burke et al., 2015; Lin et al., 2015; Molliex et al., 2015; Schwartz et al., 2013; Zhang et al., 2015) (Figure 2a). However, in a cellular environment under physiological conditions, many RBPs reside in the nuclear compartment where these molar ratios are generally reversed (Maharana et al., 2018) (Figure 2b). FUS for example, which has been predicted to be endogenously expressed at ~7 μM within the nucleus, readily undergoes LLPS at the same concentration in vitro (Burke et al., 2015; Monahan et al., 2017), but remains largely soluble within the nuclear compartment in cells (Maharana et al., 2018). While addition of low concentrations of total RNA (~0.4:1 RNA:FUS ratio) enhances in vitro LLPS, multiple studies noted that increasing RNA concentrations to excess, thus mimicking the native intracellular environment, reverses the enhancement of, and prevents, FUS phase separation in vitro (Burke et al., 2015; Maharana et al., 2018; Schwartz et al., 2013). In fact, total RNA was shown to effectively solubilize FUS droplets in vitro at concentrations over 10-fold lower than that predicted to be present within the nucleus under normal conditions (Maharana et al., 2018). A similar biphasic effect of RNA on phase separation in vitro was demonstrated for other RBPs like Whi3 (Zhang et al., 2015), G3BP1 (Yang et al., 2020), and artificial arginine-rich peptides (RP3/SR8) (Banerjee et al., 2017), suggesting a complex relationship between RNA and RBP phase behavior at work within the cell (Figure 2b).
Figure 2. RNA concentration is an important determinant of RBP phase behavior in vitro and in vivo.

(a) In vitro LLPS of RBPs can be stimulated by the addition of low concentrations of RNA, wherein excess concentrations of RBPs are allowed to multimerize on single RNA molecules and undergo phase transitions. In contrast, in vitro phase separation of RBPs resulting from molecular crowding or high RBP concentrations can be buffered by the addition of RNA at high/excess concentrations. The increased availability of RNA binding partners can prevent RBP multimerization on single RNAs and thus may prevent/reverse LLPS. (b) The effects of these molar ratios of RBP:RNA can be observed in the cell, where high RNA:RBP ratios in the nucleus maintain RBP solubility and low RNA:RBP ratios in the cytoplasm can promote physiological/pathological phase transitions.
In support of an oppositional role for RNA in physiological contexts, mutation of the RNA-binding domains of RBPs like TDP-43, FUS, Matrin-3, and Whi3 to mitigate RNA binding efficiency promotes LLPS and/or aggregation when localized to their native compartments within the cell (Daigle et al., 2013; Flores et al., 2019; Malik et al., 2018; Mann et al., 2019; Yu et al., 2021; Zhang et al., 2015). Degradation of endogenous RNA substrates exerts a similar effect as artificial RNA-binding domain mutations, resulting in the rapid phase separation of wildtype RBPs, such as FUS, TDP-43, hnRNPA1, EWSR1, and TAF15, upon RNase microinjection in the nucleus (Maharana et al., 2018). Interestingly, phase separation of nuclear RBPs is not observed upon DNase microinjection, suggesting a specific role for RNA in the buffering of these proteins in the cell. In support of this notion, novel ALS/FTD-linked mutations adjacent to the RNA-recognition motifs (RRMs) of TDP-43 were recently identified (Chen et al., 2019). Two of these mutations (K181E/K263E) disrupt RNA binding and promote the intranuclear aggregation of TDP-43, while the D169G mutation neither affected RNA binding or intracellular aggregation of the protein. Additional studies indicate that nuclear aggregation of TDP-43 with compromised RNA-binding capacity are initiated through LLPS, as TDP-43 containing ALS/FTD-linked (P112H/K181E/K263E) or acetylation-mimicking mutations within RNA-recognition motifs was observed to form liquid-like anisosomal structures capable of conversion to more gel- or solid-like structures following Hsp70 inhibition or ATP depletion (Yu et al., 2021).
Recent proteomic analysis of cell and tissue lysates subjected to RNase treatment additionally demonstrated that this phenomenon is not restricted to a small subset of disease-linked RBPs, as over 1300 individual proteins become insoluble following cellular RNA degradation (Aarum et al., 2020). Other disease-associated proteins not typically considered RBPs, such as Tau and Huntingtin, also became aggregated upon RNase digestion, suggesting that RNA:protein interactions contribute to aberrant phase transitions and resulting protein depositions found in a variety of neurodegenerative disorders. Another key observation of this study was the over-representation of proteins associated with the Gene Ontology (GO) terms of “RNA binding” and “translation initiation” that became aggregated upon RNase treatment, which mirrors the major protein composition of stress granules determined by a similar proteomic analysis (Aarum et al., 2020; Markmiller et al., 2018). These results are especially interesting in light of recent work suggesting a protective role for stress granules in the maintenance of TDP-43 and FUS solubility during periods of cellular stress (Gasset-Rosa et al., 2019; Hans et al., 2020; Mann et al., 2019; McGurk et al., 2018; Shelkovnikova et al., 2013). Here, acute RNA-dependent sequestration of proteins like TDP-43, FUS, and others within RNA-rich stress granules may actually prevent their aggregation during environmental conditions that trigger protein misfolding or damage in a similar manner to that proposed for the yeast prion protein Sup35 during pH stress (Franzmann et al., 2018). Osmotic stress conditions that are typically used to initiate stress granule assembly directly trigger TDP-43 insolubility and ubiquitination in the presence of pharmacological inhibitors of stress granule formation (Hans et al., 2020), further supporting a possible stress granule-independent pathway to RBP aggregation during periods of cellular stress. Together, these results suggest that intracellular RNA is an endogenous buffering system evolved to maintain the solubility of RBPs under physiological conditions. RNA likely acts in concert with other molecular chaperones previously shown to prevent aberrant RBP aggregation, including various heat-shock proteins (HSPs) and nuclear import receptors (Carlomagno et al., 2014; Chen et al., 2016; Guo et al., 2018). Breakdown of any of these systems in pathological states, such as the disrupted interaction between ALS-linked mutant FUS and its nuclear import receptor Kapβ2 (Hofweber et al., 2018), may promote the deposition of insoluble inclusions of these proteins observed in disease.
RNA properties differentially regulate RBP phase behavior
The intracellular and in vitro findings described above have shed new light on the complex regulatory role of RNA on the phase behavior of various RBPs. However, considering the variety of RNA species present within the intracellular environment and total RNA preparations used for many in vitro assays, it is challenging to identify RNA species responsible for promoting or inhibiting RBP phase separation in current model systems or the mechanisms by which they may be exerting their effects. Recent investigations into RBP phase behavior with individual RNA species revealed that the properties of RNA molecules exert differential effects on the formation, material properties, and composition of RNP assemblies.
Long RNAs as scaffolds for RBP phase transitions and MLO assembly
Though direct comparisons are limited, RNA length is one variable that mediates phase behavior of RBPs. Longer RNAs exhibit an enhanced ability to drive in vitro protein phase separation, as has been observed with the RBP, PGL-3 (Saha et al., 2016). Recent smFRET studies of LAF-1 and FUS interactions with synthetic oligonucleotide sequences of varying lengths support these studies, where longer oligonucleotides promote multimerization of RBPs with RNA and dynamic protein:RNA interactions that represent the initiation of phase separation (Kim and Myong, 2016; Niaki et al., 2020). Longer RNAs preferentially drive LLPS of the SG-nucleating protein, G3BP1, with a minimum length requirement about 10-fold higher than that required for G3BP1 binding (~250 nt), further demonstrating the importance of protein multimerization upon single RNA molecules to drive LLPS and MLO assembly (Guillén-Boixet et al., 2020; Yang et al., 2020). Interestingly, ultra-course-grained models generated to simulate G3BP1/RNA interactions revealed that longer RNAs promote the formation of an interconnected meshwork of G3BP1 and RNA, resulting in higher-order assembly not observed in simulations involving shorter RNA species (Guillén-Boixet et al., 2020). These phase-separated networks of G3BP1/RNA, achieved via crosslinking mediated by G3BP1 clustering at distinct foci along long RNAs, serve as assembly platforms in the nucleation of stress granules through the recruitment of other protein/RNA components to unoccupied RNA binding sites and additional protein/RNA interconnectivity (Guillén-Boixet et al., 2020; Sanders et al., 2020; Yang et al., 2020). Recent transcriptomic studies highlight the importance of RNA length in the scaffolding of MLO assembly and revealed an enrichment in long RNAs contained within stress granules across various stressors (Khong et al., 2017; Namkoong et al., 2018).
Other anecdotal observations regarding the RNA content of other MLOs, such as paraspeckles and nuclear stress bodies, similarly support a positive relationship between RNA length and scaffolding of MLO assembly. For example, alternative isoforms of the long noncoding RNA NEAT1 have differing effects in nucleating paraspeckle assembly, with replacement of only the longer of the two isoforms (NEAT1_2) able to recover paraspeckle assembly in NEAT1 knockout MEF cells (Fox et al., 2018; Naganuma et al., 2012). The unique ability of the longer NEAT1_2 isoform drives the recruitment and resulting phase separation of PrLD-containing RBPs, like FUS and RBM14, through functionally-redundant elements not present in the shorter NEAT1_1 isoform (Chujo and Hirose, 2017; Yamazaki et al., 2018). A similar mechanism is proposed to underlie the formation of nuclear stress bodies, where heat-induced transcription of highly-repetitive satellite III (HSATIII) long noncoding RNA is linked to the recruitment of various IDR-containing RBPs and nucleation of these droplet-like structures (Biamonti and Vourc’h, 2010; Metz et al., 2004; Ninomiya et al., 2020).
Short RNAs prevent RBP multimerization and higher-order assembly
RNA length might also be a strong determinant to inhibit RBP phase separation. While direct comparisons between RNAs of differing length but similar valence and affinity are lacking, recent investigations of the buffering capacity of different types of RNAs (tRNA, NEAT1 lncRNA, and rRNA) revealed a near-linear relationship between RNA length and the antagonization of FUS LLPS (Maharana et al., 2018) (Figure 3a). Specifically, shorter RNA species (tRNA) were the most efficient solubilizers of FUS droplets, with the longer NEAT1 and rRNAs requiring much higher concentrations in solution for effective inhibition of phase separation (Maharana et al., 2018). Short tRNA species were also shown to outcompete other, presumably longer, RNAs to bind and prevent FUS assembly within stress granules. The above-mentioned smFRET studies of FUS multimerization with polyU RNA of different lengths demonstrates a length-dependence in the buffering of protein phase transitions, with longer polyU species allowing for FUS multimerization and dynamic FRET fluctuations which are representative of condensate formation (Niaki et al., 2020). Conversely, shorter polyU tracts (U10-U30) only allowed binding of single FUS monomers and did not promote FUS multimerization or dynamic RNA interactions in this study.
Figure 3. RNA length contributes to regulation of RBP LLPS and MLO dynamics.
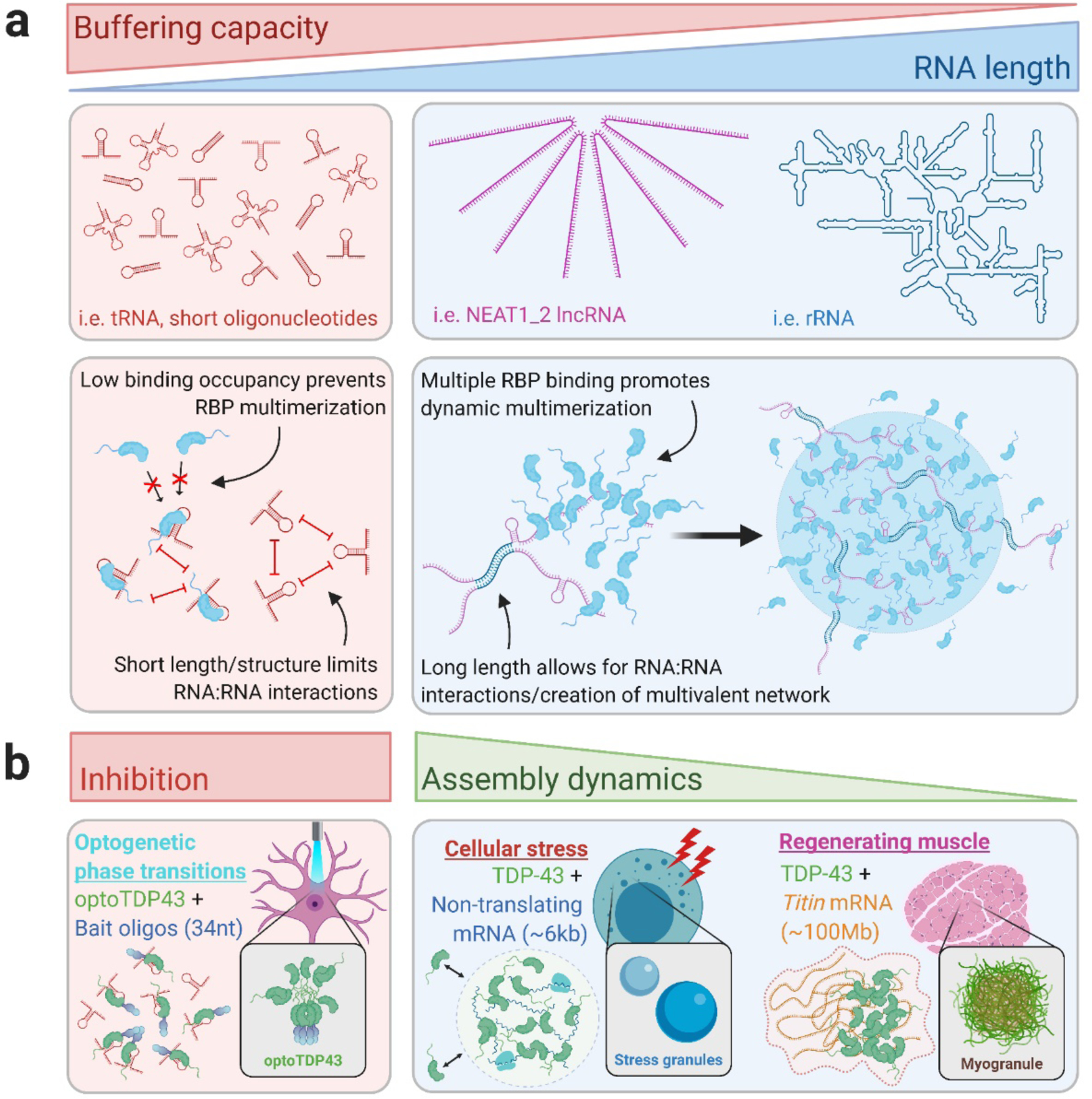
(a) RNA length seemingly inversely correlates with its capacity to buffer phase transitions of RBPs (i.e. FUS). The low binding occupancy of short oligonucleotides and/or tRNA molecules may prevent multiple RBP binding, while longer RNAs (i.e. NEAT1_2 lncRNA, rRNA) may contain many binding sites that enable RNA scaffolding of RBP LLPS. Longer RNA length also may promote RNA:RNA interactions that contribute to LLPS and MLO formation, while shorter RNAs have less capacity to form these multivalent RNA/RBP networks. (b) Examples of different intracellular condensates/MLOs that contain RNAs of different lengths. While short RNA oligonucleotides are capable of effectively buffering RBP phase transitions in the cell (i.e. bait RNA oligonucleotides), longer RNAs tend to promote the formation of MLOs and tune their characteristics. MLOs with longer RNA components may exhibit a less dynamic biophysical state (i.e. myogranules, Titin mRNA) than those containing shorter RNA components (i.e. stress granules, non-translating mRNA) due to an increased propensity for RNA self-interaction and reduced molecular exchange.
Similarly, TDP-43 self-assembly is antagonized by short, TDP-43 targeting RNA oligonucleotides (French et al., 2019; Mann et al., 2019), but conversely can be promoted by long RNAs like NEAT1 lncRNA and titin mRNA that contain multiple binding sites for RBPs on single transcripts (Tollervey et al., 2011; Vogler et al., 2018) (Figure 3b). Thus, the ability of shorter oligonucleotides to inhibit higher-order assembly of these RBPs may be intrinsically linked to the low-occupancy binding capacity of these molecules, in contrast to longer multivalent RNA molecules that act as scaffolds for RBP multimerization and subsequent phase separation (Figure 3a).
RNA secondary structure influences RBP phase transitions
In addition to specific sequence motifs, many RBPs display some structural biases in their interaction with RNA substrates (Dominguez et al., 2018). The RNA secondary structures that are preferred by different RBPs tends to vary, likely as a result of the type and combination of RNA-binding domain(s) utilized for RNA interaction, but structure-specific recognition is often used along with cooperative sequence-based binding to achieve target specificity across RBPs, especially in those utilizing redundant binding motifs (Dominguez et al., 2018). The structural content of RNA molecules positively correlates with the number of individual protein interactors for a given RNA, suggesting that RNA secondary structure may enhance specific RBP binding at distinct transcript loci (Sanchez de Groot et al., 2019). Given these observations, one might expect RNA secondary structure to contribute to the coordinated nucleation of various MLOs. However, recent studies indicate that long, single-stranded, unstructured RNAs are preferred for initiation of stress granule assembly, and were more efficient drivers of G3BP1 LLPS in vitro (Guillén-Boixet et al., 2020; Yang et al., 2020). Transcriptomic analyses of RNAs found within stress granules support a possible lack of structural dependence for RNA scaffolding or incorporation within SGs, revealing weak associations between SG-enriched transcripts and either mRNA folding energy or GC-content (Namkoong et al., 2018). Conversely, RNAs enriched with linear sequence motifs like AU-rich elements (AREs) were more likely to be found within SGs in this study. Similar observations have arisen from investigations of NEAT1_2 lncRNA required for paraspeckle formation (Lin et al., 2018). Structural analysis showed a lack of conservation in secondary structure of the NEAT_2 lncRNA across evolution, despite the maintenance of its essential function in coordinating paraspeckle assembly (Lin et al., 2018). Additional studies identified a set of redundant subdomains within the middle domain of NEAT1_2 that contain multiple binding sites for essential paraspeckle proteins like NONO, SFPQ, FUS, and RBM14. These are proposed to initiate paraspeckle assembly via multimerization and phase separation upon cooperative binding to NEAT1_2 (Yamazaki et al., 2018).
Linear motifs commonly bound by RBPs are predicted to have a lower propensity to form defined secondary structures (Dominguez et al., 2018). This suggests that a higher overall structure of RNAs may diminish their ability to scaffold MLOs assembly by limiting RBP binding accessibility. However, local structural elements within or adjacent to binding motifs are likely important in determining interaction specificity in interactions with certain RBPs and may aid in the nucleation of MLOs (Dominguez et al., 2018). In a similar manner, overall or local structural flexibility of RNA molecules may be a major determinant of their regulation of RBP phase behavior. Multiple investigations show that structural rigidity of nucleic acids, for example due to complementary base-pairing or nucleotide composition, suppress LLPS or favor the assembly of less dynamic assemblies (Boeynaems et al., 2019; Shakya and King, 2018). Modulation of RBP phase behavior by RNA structural flexibility was also observed with endogenous RNA targets (CLN3/BNI1) for the RBP Whi3, found within functionally distinct assemblies in Ashbya gossypii (Langdon et al., 2018; Zhang et al., 2015). Here, Whi3 condensates formed along with CLN3 mRNA, which showed a more-compacted baseline conformation by smFRET, exhibit significantly increased viscosity when compared with the more-extended BNI1 mRNA that exhibited more dynamic interactions with Whi3 and incorporated more Whi3 protein into droplets than CLN3 (Langdon et al., 2018; Zhang et al., 2015). Thus, the structural flexibility of RNA molecules may allow for dynamic conformational remodeling of RNA upon RBP binding, as observed for RBPs like FUS, LAF-1, DDX3X, and Whi3 (Kim and Myong, 2016; Langdon et al., 2018; Loughlin et al., 2019), that promotes RBP multimerization and interactions across RBP/RNA complexes responsible for subsequent phase separation. Conversely, rigid RNA structures, may result in more static or tight monomeric binding that would oppose LLPS.
The influence of secondary structure is highlighted by the capacity of short synthetic oligonucleotides that solubilize protein aggregation induced by RNA digestion in whole cell lysate. The most effective solubilizing molecules containing single-stranded motifs including loops or bulges interspersed by double stranded regions (Aarum et al., 2020). Similar results were demonstrated following RBP precipitation via biotinylated isoxazole (b-isox) treatment in HeLa cell lysate, where mass spectrometry revealed a preferential antagonization of protein aggregation following incubation with highly-structured RNAs, whereas low-structure RNAs produced results similar to background controls (Sanchez de Groot et al., 2019).
Secondary structure drives RNA partitioning into condensates
In addition to directly affecting protein binding and phase behavior, RNA complementarity and secondary structure can also influence the incorporation of RNA into pre-existing membraneless assemblies. For example, secondary structures within nascent pre-rRNA are required for proper sorting in the nucleolus where a stem-loop structure within the 5’ end of the 47S pre-rRNA is necessary and sufficient for incorporation into pre-formed fibrillarin (FBL) droplets in vitro (Yao et al., 2019). Similar results were observed for oligonucleotide incorporation into pre-formed Ddx4 droplets, where ssDNA/ssRNA hairpins were preferentially absorbed over their unstructured ssDNA/ssRNA counterparts (Nott et al., 2016). Both single-stranded oligonucleotides exhibited enhanced partitioning when compared to dsDNA/dsRNA, and those double-stranded molecules that were absorbed were unwound and stabilized in single-stranded conformation within droplets. The favoring of compact, single-stranded nucleic acid structures within Ddx4 droplets was due to a reduced disruption of condensate organization arising from extended and/or rigid conformations that alter the transient interactions required to maintain the dynamic nature of these assemblies (Nott et al., 2016).
Beyond recruitment by RBP components of biomolecular condensates, secondary structure also mediates RNA incorporation into MLOs via RNA:RNA interactions. In the Whi3 studies mentioned above for example, it was shown that in addition to modulating interactions with Whi3 protein alone, RNA structure determined heterotypic RNA:RNA interactions and resulting RNA partitioning into Whi3 droplets formed with other RNA species (Langdon et al., 2018). Exposed regions of RNA complementarity were proposed to drive the differential incorporation of RNAs into Whi3/RNA assemblies, either contained within native structures of mRNAs found co-localized within Ashbya gossypii polarity granules in vivo or artificially induced in normally-segregated mRNAs by thermal unfolding or point mutations. Distinct mRNA partitioning was also observed in the absence of Whi3 protein, highlighting the role for RNA:RNA interactions in the sorting of functionally-related RNAs into specific RNP assemblies.
RNA:RNA interactions and membraneless organelle assembly
RNA self-assembly also contributes to the formation of physiological RNA-containing MLOs like stress granules. It is well known that RNA is capable of self-assembly into higher-order structures (reviewed by Grabow and Jaeger, 2014). However, RNA homopolymers, as well as total yeast RNA, also self-assemble into liquid-like droplets that recruit RBPs like hnRNPA1 (Van Treeck et al., 2018). Sequencing of RNAs enriched within total RNA assemblies created in vitro revealed a striking similarity to the stress granule-enriched transcriptome mentioned above, indicating overlaps in both RNA characteristics (longer RNAs enriched) and specific RNA transcripts contained within these assemblies (Namkoong et al., 2018). In addition to the above observations, alterations in osmotic conditions that favor RNA:RNA interactions (i.e. hyperosmotic stress) (Bounedjah et al., 2012) or the introduction of G-quadruplex-forming RNAs initiate intracellular stress granule assembly (Fay et al., 2017). Together, this raised the hypothesis that intermolecular interactions between RNAs play an active role in physiological RNP assembly (Van Treeck and Parker, 2018).
Homotypic RNA phase separation was recently shown to participate in the formation of pathological RNA foci in repeat expansion disorders like Huntington’s Disease (HD) and C9ORF72-ALS/FTD (Fay et al., 2017; Jain and Vale, 2017). Disease-associated repeat RNA (CAGn for HD/GGGGCCn for C9ORF72-ALS/FTD) can phase separate in vitro and form intracellular liquid- or gel-like RNA assemblies in an RNA length-dependent manner (Fay et al., 2017; Jain and Vale, 2017). Phase transitions of repeat RNA arose from the long GC-rich tracts contained within these repeats that allow for multivalent base-pairing and the formation of structural elements, such as hairpins and/or G-quadruplexes, since foci formation was reversed by interrupting complementary base-pairing interactions with blocking antisense oligonucleotides or the nucleic acid intercalator doxorubicin (Jain and Vale, 2017). Another similar repeat-expansion (CGGn) in the FMR1 5’UTR linked to Fragile-X associated tremor ataxia syndrome (FXTAS) can also drive the formation of nuclear RNA foci (Cid-Samper et al., 2018). In an analogous manner to CAG/GGGGCC repeats described above, these CGGn repeats recruit RNA-binding proteins in cellular models that are often observed to be sequestered within foci in patient tissue (Cid-Samper et al., 2018; Jain and Vale, 2017). Disruption of distinct hairpin structures formed by these repeats prevented both nuclear foci formation and RBP recruitment, again highlighting the role of RNA structure in mediating homo- and heterotypic RNA/RBP phase separation. Structure-dependent phase transitions of nucleic acids are also observed in non-disease contexts as other G-quadruplex-forming nucleic acids, such as regions of the SHR mRNA and ssDNA from the c-myc promoter region, similarly undergo LLPS that is highly sensitive to structural perturbations and can recruit proteins like linker histone H1 to condensates (Mimura et al., 2020; Zhang et al., 2019).
Taken together, these observations provide insight into the dynamic regulation of RBP phase behavior and MLO formation by distinct RNA species. Similarly, the above studies highlight the potential differences between RNA properties important for initial condensate nucleation and subsequent maintenance/recruitment of additional components to membraneless assemblies. Returning to the conceptual framework of client-scaffold relationships as it relates to MLO dynamics, it is possible that scaffold RNAs exist as a distinct class of components from client RNAs and tend to exhibit divergent characteristics that mirror their function (Ditlev et al., 2018). In this sense, longer, flexible, generally-unstructured RNAs that contain a multitude of different RBP binding motifs likely serve as the building blocks of MLOs through the scaffolding of important multivalent RBP:RNA, RBP:RBP, and RNA:RNA interactions responsible for phase separation. Following initial assembly, functionally-related client RNA molecules may then be partitioned into condensates through specific complementary interactions with scaffold RNAs or interactions with RBP components mediated by specific sequence and/or structural elements, which are then unwound/stabilized upon entry to maintain the biophysical state of the condensate and provide additional multivalency (Ditlev et al., 2018). Conversely, shorter RNAs suppress LLPS and MLOs assembly by limiting RBP multimerization due to the inherently lower binding occupancy of these molecules. High RNA concentrations, such as those observed in the nucleus of cells, result in a similar buffering effect, as the enhanced binding availability of different RNAs likely support monomeric RBP:RNA interactions.
Conclusions and future directions
The emerging field of biomolecular condensates indicates that the assembly and maintenance of MLOs is a crucial component of normal cellular physiology. However, as is the case with many other functional pathways, disruption of this dynamic process can have devastating consequences. The pathological deposition of RBPs in neurodegenerative disease provides an interesting hypothesis that highlights how alterations in the regulation of protein phase transitions drive disease via protein LLPS and MLO dysfunction. One common element of disease-linked RBPs, prion-like domains, may lie at the nexus of the dichotomy between physiological and pathological phase separation. PrLDs allow for functional phase transitions during normal cell physiology but also confer a propensity for aberrant phase transitions in pathological contexts, such as the presence of genetic mutations within these regions (Harrison and Shorter, 2017), diminished proteostasis during aging (Hipp et al., 2019), and/or chronic cellular stress (Baradaran-Heravi et al., 2020). However, recent studies demonstrated that another commonality between disease-linked RBPs, interaction with RNA ligands, has a profound modulatory influence over the assembly and dynamics of membraneless assemblies in vitro and within the cell.
On one hand, RNA exists as a frequent and often critical component of many MLOs in the cell and promotes phase separation of multiple RBPs through a scaffolding mechanism as described above (Banani et al., 2016; Ditlev et al., 2018). In contrast, RNA seems to oppose phase transitions of disease-linked RBPs, both in vitro and within the cell, and prevents toxicity associated with their aberrant assembly (Maharana et al., 2018; Mann et al., 2019). One potential explanation for the contrasting effects of RNA on RBP phase behavior is the relative subcellular concentrations or molar ratios of RBPs and RNAs, such as the nucleus, or in vitro reactions (Burke et al., 2015; Maharana et al., 2018; Zhang et al., 2015), where excess RNA inhibits RBP phase separation (Figure 2a–b). However, recent work indicates that the distinct properties of different RNA species play a critical role in the assembly and composition of membraneless assemblies. Some of the few examples point to a positive correlation between RNA length and the ability to scaffold RBP phase transitions (Niaki et al., 2020; Wang et al., 2020) (Figure 3), but direct evidence for the effects of different RNA secondary structures on MLO assembly and function remains limited to draw precise and generalizable conclusions. Nonetheless, these studies demonstrate a key concept outlined in this review: that RNA is not a uniform entity in its participation in biological phase transitions and should thus be carefully considered in the same detail as diverse protein components of membraneless assemblies. Of course, future studies aimed at exploring the effects of diverse RNA species on the phase behavior of single RBPs and modulating the RNA contents of various MLOs will be needed to truly decipher the intricacies of RNA’s role in MLO form and function. This knowledge, in turn, will hopefully deepen our understanding of biomolecular condensates in cellular physiology and inform future efforts in the development of RNA-based therapeutics to counter pathological RBP phase transitions in disease.
Acknowledgements:
C.J.D. laboratory is funded by grants from the National Institutes of Health, a grant from Target ALS Foundation and the Association for Frontotemporal Degeneration, and the LiveLikeLou Center for ALS Research at the University of Pittsburgh Brain Institute. C.J.D. is a Co-Founder and Scientific Advisory Board Chair of Vivid Sciences, LLC. Figures were created with BioRender.com.
Abbreviations:
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- CBD
corticobasal degeneration
- CTE
chronic traumatic encephalopathy
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- HD
Huntington’s disease
- IBM
Inclusion body myopathy
- IDR
Intrinsically-disordered region
- LATE
Limbic-predominant age-related TDP-43 encephalopathy
- LCD
Low-complexity domain
- LLPS
Liquid-liquid phase separation
- MLO
Membraneless organelle
- PD
Parkinson’s disease
- MSP
Multisystem proteinopathy
- PrLD
Prion-like domain
- SCA1/2/3
Spinocerebellar ataxia types 1, 2 & 3
- VCPDM
vocal cord and pharyngeal weakness with distal myopathy
- WDM
Welander distal myopathy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarum J, Cabrera CP, Jones TA, Rajendran S, Adiutori R, Giovannoni G, Barnes MR, Malaspina A, and Sheer D (2020). Enzymatic degradation of RNA causes widespread protein aggregation in cell and tissue lysates. EMBO Rep. 21, e49585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Husini N, Tomares DT, Bitar O, Childers WS, and Schrader JM (2018). α-Proteobacterial RNA Degradosomes Assemble Liquid-Liquid Phase-Separated RNP Bodies. Mol. Cell 71, 1027–1039.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alami NH, Smith RB, Carrasco MA, Williams LA, Winborn CS, Han SSW, Kiskinis E, Winborn B, Freibaum BD, Kanagaraj A, et al. (2014). Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron 81, 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S, Halfmann R, King O, Kapila A, and Lindquist S (2009). A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 137, 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmeyer M, Neelsen KJ, Teloni F, Pozdnyakova I, Pellegrino S, Grøfte M, Rask M-BD, Streicher W, Jungmichel S, Nielsen ML, et al. (2015). Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat. Commun 6, 8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Ortiz C, Lin W-L, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, and Dickson DW (2007). TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann. Neurol 61, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JS, Lyon CE, Fox AH, Leung AKL, Lam YW, Steen H, Mann M, and Lamond AI (2002). Directed proteomic analysis of the human nucleolus. Curr. Biol 12, 1–11. [DOI] [PubMed] [Google Scholar]
- Andersen JS, Lam YW, Leung AKL, Ong S-E, Lyon CE, Lamond AI, and Mann M (2005). Nucleolar proteome dynamics. Nature 433, 77–83. [DOI] [PubMed] [Google Scholar]
- Andersson MK, Ståhlberg A, Arvidsson Y, Olofsson A, Semb H, Stenman G, Nilsson O, and Aman P (2008). The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. (2006). TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun 351, 602–611. [DOI] [PubMed] [Google Scholar]
- Audas TE, Audas DE, Jacob MD, Ho JJD, Khacho M, Wang M, Perera JK, Gardiner C, Bennett CA, Head T, et al. (2016). Adaptation to stressors by systemic protein amyloidogenesis. Dev. Cell 39, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulas A, Fay MM, Lyons SM, Achorn CA, Kedersha N, Anderson P, and Ivanov P (2017). Stressspecific differences in assembly and composition of stress granules and related foci. J. Cell Sci 130, 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinchak WM, and Surewicz WK (2020). Liquid-Liquid Phase Separation and Its Mechanistic Role in Pathological Protein Aggregation. J. Mol. Biol 432, 1910–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinchak WM, Haider R, Dumm BK, Sarkar P, Surewicz K, Choi J-K, and Surewicz WK (2019). The role of liquid-liquid phase separation in aggregation of the TDP-43 low-complexity domain. J. Biol. Chem 294, 6306–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani SF, Rice AM, Peeples WB, Lin Y, Jain S, Parker R, and Rosen MK (2016). Compositional Control of Phase-Separated Cellular Bodies. Cell 166, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PR, Milin AN, Moosa MM, Onuchic PL, and Deniz AA (2017). Reentrant phase transition drives dynamic substructure formation in ribonucleoprotein droplets. Angew. Chem. Int. Ed. Engl 56, 11354–11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran-Heravi Y, Van Broeckhoven C, and van der Zee J (2020). Stress granule mediated protein aggregation and underlying gene defects in the FTD-ALS spectrum. Neurobiol. Dis 134, 104639. [DOI] [PubMed] [Google Scholar]
- Baron DM, Kaushansky LJ, Ward CL, Sama RRK, Chian R-J, Boggio KJ, Quaresma AJC, Nickerson JA, and Bosco DA (2013). Amyotrophic lateral sclerosis-linked FUS/TLS alters stress granule assembly and dynamics. Mol. Neurodegener 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentmann E, Neumann M, Tahirovic S, Rodde R, Dormann D, and Haass C (2012). Requirements for stress granule recruitment of fused in sarcoma (FUS) and TAR DNA-binding protein of 43 kDa (TDP-43). J. Biol. Chem 287, 23079–23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J, Weber SC, Vaidya N, Haataja M, and Brangwynne CP (2015). RNA transcription modulates phase transition-driven nuclear body assembly. Proc. Natl. Acad. Sci. USA 112, E5237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biamonti G, and Vourc’h C (2010). Nuclear stress bodies. Cold Spring Harb. Perspect. Biol 2, a000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechingberg J, Luo Y, Bolund L, Damgaard CK, and Nielsen AL (2012). Gene expression responses to FUS, EWS, and TAF15 reduction and stress granule sequestration analyses identifies FET-protein non-redundant functions. PLoS One 7, e46251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, et al. (2018). Protein phase separation: A new phase in cell biology. Trends Cell Biol. 28, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S, Holehouse AS, Weinhardt V, Kovacs D, Van Lindt J, Larabell C, Van Den Bosch L, Das R, Tompa PS, Pappu RV, et al. (2019). Spontaneous driving forces give rise to protein-RNA condensates with coexisting phases and complex material properties. Proc. Natl. Acad. Sci. USA 116, 7889–7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CS, and Fox AH (2009). Paraspeckles: nuclear bodies built on long noncoding RNA. J. Cell Biol 186, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ, Sapp P, McKenna-Yasek D, Brown RH, and Hayward LJ (2010). Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum. Mol. Genet 19, 4160–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounedjah O, Hamon L, Savarin P, Desforges B, Curmi PA, and Pastré D (2012). Macromolecular crowding regulates assembly of mRNA stress granules after osmotic stress: new role for compatible osmolytes. J. Biol. Chem 287, 2446–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounedjah O, Desforges B, Wu T-D, Pioche-Durieu C, Marco S, Hamon L, Curmi PA, Guerquin-Kern J-L, Piétrement O, and Pastré D (2014). Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules. Nucleic Acids Res. 42, 8678–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, and Hyman AA (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Mitchison TJ, and Hyman AA (2011). Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA 108, 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, and Parker R (2009). Eukaryotic stress granules: the ins and outs of translation. Mol. Cell 36, 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KA, Janke AM, Rhine CL, and Fawzi NL (2015). Residue-by-Residue View of In Vitro FUS Granules that Bind the C-Terminal Domain of RNA Polymerase II. Mol. Cell 60, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlomagno Y, Zhang Y, Davis M, Lin W-L, Cook C, Dunmore J, Tay W, Menkosky K, Cao X, Petrucelli L, et al. (2014). Casein kinase II induced polymerization of soluble TDP-43 into filaments is inhibited by heat shock proteins. PLoS One 9, e90452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo P, Camuzat A, Guillot-Noel L, Thomas-Antérion C, Couratier P, Wong TH, Teichmann M, Golfier V, Auriacombe S, Belliard S, et al. (2016). Defining the spectrum of frontotemporal dementias associated with TARDBP mutations. Neurol. Genet 2, e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celetti G, Paci G, Caria J, VanDelinder V, Bachand G, and Lemke EA (2020). The liquid state of FG-nucleoporins mimics permeability barrier properties of nuclear pore complexes. J. Cell Biol 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, and Cohen TJ (2019). Aggregation of the nucleic acid-binding protein TDP-43 occurs via distinct routes that are coordinated with stress granule formation. J. Biol. Chem 294, 3696–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-J, Mitchell JC, Novoselov S, Miller J, Nishimura AL, Scotter EL, Vance CA, Cheetham ME, and Shaw CE (2016). The heat shock response plays an important role in TDP-43 clearance: evidence for dysfunction in amyotrophic lateral sclerosis. Brain 139, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-J, Topp SD, Hui HS, Zacco E, Katarya M, McLoughlin C, King A, Smith BN, Troakes C, Pastore A, et al. (2019). RRM adjacent TARDBP mutations disrupt RNA binding and enhance TDP-43 proteinopathy. Brain 142, 3753–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chujo T, and Hirose T (2017). Nuclear bodies built on architectural long noncoding rnas: unifying principles of their construction and function. Mol. Cells 40, 889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid-Samper F, Gelabert-Baldrich M, Lang B, Lorenzo-Gotor N, Ponti RD, Severijnen L-AWFM, Bolognesi B, Gelpi E, Hukema RK, Botta-Orfila T, et al. (2018). An Integrative Study of Protein-RNA Condensates Identifies Scaffolding RNAs and Reveals Players in Fragile X-Associated Tremor/Ataxia Syndrome. Cell Rep. 25, 3422–3434.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A, and Lawrence JB (2009). An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol. Cell 33, 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier NC, and Schlesinger MJ (1986). The dynamic state of heat shock proteins in chicken embryo fibroblasts. J. Cell Biol 103, 1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier NC, Heuser J, Levy MA, and Schlesinger MJ (1988). Ultrastructural and biochemical analysis of the stress granule in chicken embryo fibroblasts. J. Cell Biol 106, 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, Silani V, and Ratti A (2009). TDP-43 is recruited to stress granules in conditions of oxidative insult. J. Neurochem 111, 1051–1061. [DOI] [PubMed] [Google Scholar]
- Conicella AE, Zerze GH, Mittal J, and Fawzi NL (2016). ALS Mutations Disrupt Phase Separation Mediated by α-Helical Structure in the TDP-43 Low-Complexity C-Terminal Domain. Structure 24, 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conicella AE, Dignon GL, Zerze GH, Schmidt HB, D’Ordine AM, Kim YC, Rohatgi R, Ayala YM, Mittal J, and Fawzi NL (2020). TDP-43 α-helical structure tunes liquid-liquid phase separation and function. Proc. Natl. Acad. Sci. USA 117, 5883–5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couthouis J, Hart MP, Shorter J, DeJesus-Hernandez M, Erion R, Oristano R, Liu AX, Ramos D, Jethava N, Hosangadi D, et al. (2011). A yeast functional screen predicts new candidate ALS disease genes. Proc. Natl. Acad. Sci. USA 108, 20881–20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couthouis J, Hart MP, Erion R, King OD, Diaz Z, Nakaya T, Ibrahim F, Kim H-J, Mojsilovic-Petrovic J, Panossian S, et al. (2012). Evaluating the role of the FUS/TLS-related gene EWSR1 in amyotrophic lateral sclerosis. Hum. Mol. Genet 21, 2899–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle JG, Lanson NA, Smith RB, Casci I, Maltare A, Monaghan J, Nichols CD, Kryndushkin D, Shewmaker F, and Pandey UB (2013). RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Hum. Mol. Genet 22, 1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson YS, Flood L, Robinson AC, Nihei Y, Mori K, Rollinson S, Richardson A, Benson BC, Jones M, Snowden JS, et al. (2017). Heterogeneous ribonuclear protein A3 (hnRNP A3) is present in dipeptide repeat protein containing inclusions in Frontotemporal Lobar Degeneration and Motor Neurone disease associated with expansions in C9orf72 gene. Acta Neuropathol. Commun 5, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning DP, and Rexach MF (2007). Rapid evolution exposes the boundaries of domain structure and function in natively unfolded FG nucleoporins. Mol. Cell Proteomics 6, 272–282. [DOI] [PubMed] [Google Scholar]
- Ditlev JA, Case LB, and Rosen MK (2018). Who’s In and Who’s Out-Compositional Control of Biomolecular Condensates. J. Mol. Biol 430, 4666–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi H, Koyano S, Suzuki Y, Nukina N, and Kuroiwa Y (2010). The RNA-binding protein FUS/TLS is a common aggregate-interacting protein in polyglutamine diseases. Neurosci. Res 66, 131–133. [DOI] [PubMed] [Google Scholar]
- Dominguez D, Freese P, Alexis MS, Su A, Hochman M, Palden T, Bazile C, Lambert NJ, Van Nostrand EL, Pratt GA, et al. (2018). Sequence, structure, and context preferences of human RNA binding proteins. Mol. Cell 70, 854–867.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IRA, Capell A, Schmid B, et al. (2010). ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 29, 2841–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker AK, Babu MM, Barbar E, Blackledge M, Bondos SE, Dosztányi Z, Dyson HJ, Forman-Kay J, Fuxreiter M, Gsponer J, et al. (2013). What’s in a name? Why these proteins are intrinsically disordered. Intrinsically Disord Proteins 1, e24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CC-H, Eckmann CR, Myong S, and Brangwynne CP (2015). The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA 112, 7189–7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falahati H, Pelham-Webb B, Blythe S, and Wieschaus E (2016). Nucleation by rRNA Dictates the Precision of Nucleolus Assembly. Curr. Biol 26, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang MY, Markmiller S, Vu AQ, Javaherian A, Dowdle WE, Jolivet P, Bushway PJ, Castello NA, Baral A, Chan MY, et al. (2019). Small-Molecule Modulation of TDP-43 Recruitment to Stress Granules Prevents Persistent TDP-43 Accumulation in ALS/FTD. Neuron 103, 802–819.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay MM, Anderson PJ, and Ivanov P (2017). ALS/FTD-Associated C9ORF72 Repeat RNA Promotes Phase Transitions In Vitro and in Cells. Cell Rep. 21, 3573–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, and Brangwynne CP (2016). Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores BN, Li X, Malik AM, Martinez J, Beg AA, and Barmada SJ (2019). An Intramolecular Salt Bridge Linking TDP43 RNA Binding, Protein Stability, and TDP43-Dependent Neurodegeneration. Cell Rep. 27, 1133–1150.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AH, Nakagawa S, Hirose T, and Bond CS (2018). Paraspeckles: where long noncoding RNA meets phase separation. Trends Biochem. Sci 43, 124–135. [DOI] [PubMed] [Google Scholar]
- Franzmann TM, Jahnel M, Pozniakovsky A, Mahamid J, Holehouse AS, Nüske E, Richter D, Baumeister W, Grill SW, Pappu RV, et al. (2018). Phase separation of a yeast prion protein promotes cellular fitness. Science 359. [DOI] [PubMed] [Google Scholar]
- French RL, Grese ZR, Aligireddy H, Dhavale DD, Reeb AN, Kedia N, Kotzbauer PT, Bieschke J, and Ayala YM (2019). Detection of TAR DNA-binding protein 43 (TDP-43) oligomers as initial intermediate species during aggregate formation. J. Biol. Chem 294, 6696–6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, and Görlich D (2007). A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 130, 512–523. [DOI] [PubMed] [Google Scholar]
- Frey S, and Görlich D (2009). FG/FxFG as well as GLFG repeats form a selective permeability barrier with self-healing properties. EMBO J. 28, 2554–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frottin F, Schueder F, Tiwary S, Gupta R, Körner R, Schlichthaerle T, Cox J, Jungmann R, Hartl FU, and Hipp MS (2019). The nucleolus functions as a phase-separated protein quality control compartment. Science 365, 342–347. [DOI] [PubMed] [Google Scholar]
- Gal J, Zhang J, Kwinter DM, Zhai J, Jia H, Jia J, and Zhu H (2011). Nuclear localization sequence of FUS and induction of stress granules by ALS mutants. Neurobiol. Aging 32, 2323.e27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gami-Patel P, Bandopadhyay R, Brelstaff J, Revesz T, and Lashley T (2016). The presence of heterogeneous nuclear ribonucleoproteins in frontotemporal lobar degeneration with FUS-positive inclusions. Neurobiol. Aging 46, 192–203. [DOI] [PubMed] [Google Scholar]
- Gasset-Rosa F, Lu S, Yu H, Chen C, Melamed Z, Guo L, Shorter J, Da Cruz S, and Cleveland DW (2019). Cytoplasmic TDP-43 De-mixing Independent of Stress Granules Drives Inhibition of Nuclear Import, Loss of Nuclear TDP-43, and Cell Death. Neuron 102, 339–357.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, and Anderson P (2004). Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15, 5383–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E, and Shorter J (2019). The molecular language of membraneless organelles. J. Biol. Chem 294, 7115–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal PP, Nirschl JJ, Klinman E, and Holzbaur ELF (2017). Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons. Proc. Natl. Acad. Sci. USA 114, E2466–E2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabow WW, and Jaeger L (2014). RNA self-assembly and RNA nanotechnology. Acc. Chem. Res 47, 1871–1880. [DOI] [PubMed] [Google Scholar]
- Guil S, Long JC, and Cáceres JF (2006). hnRNP A1 relocalization to the stress granules reflects a role in the stress response. Mol. Cell. Biol 26, 5744–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén-Boixet J, Kopach A, Holehouse AS, Wittmann S, Jahnel M, Schlüßler R, Kim K, Trussina IREA, Wang J, Mateju D, et al. (2020). RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell 181, 346–361.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Kim HJ, Wang H, Monaghan J, Freyermuth F, Sung JC, O’Donovan K, Fare CM, Diaz Z, Singh N, et al. (2018). Nuclear-Import Receptors Reverse Aberrant Phase Transitions of RNA-Binding Proteins with Prion-like Domains. Cell 173, 677–692.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman P, Sarparanta J, Lehtinen S, Vihola A, Evilä A, Jonson PH, Luque H, Kere J, Screen M, Chinnery PF, et al. (2013). Welander distal myopathy is caused by a mutation in the RNA-binding protein TIA1. Ann. Neurol 73, 500–509. [DOI] [PubMed] [Google Scholar]
- Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, et al. (2012). Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell 149, 768–779. [DOI] [PubMed] [Google Scholar]
- Hans F, Glasebach H, and Kahle PJ (2020). Multiple distinct pathways lead to hyperubiquitylated insoluble TDP-43 protein independent of its translocation into stress granules. J. Biol. Chem 295, 673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon TS, Holehouse AS, Rosen MK, and Pappu RV (2017). Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AF, and Shorter J (2017). RNA-binding proteins with prion-like domains in health and disease. Biochem. J 474, 1417–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp MS, Kasturi P, and Hartl FU (2019). The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol 20, 421–435. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Pottier C, Nicholson AM, Baker M, Hsiung G-YR, Krieger C, Sengdy P, Boylan KB, Dickson DW, Mesulam M, et al. (2017). Clinical and neuropathological features of ALS/FTD with TIA1 mutations. Acta Neuropathol. Commun 5, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofweber M, Hutten S, Bourgeois B, Spreitzer E, Niedner-Boblenz A, Schifferer M, Ruepp M-D, Simons M, Niessing D, Madl T, et al. (2018). Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation. Cell 173, 706–719.e13. [DOI] [PubMed] [Google Scholar]
- Hubstenberger A, Courel M, Bénard M, Souquere S, Ernoult-Lange M, Chouaib R, Yi Z, Morlot J-B, Munier A, Fradet M, et al. (2017). P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Mol. Cell 68, 144–157.e5. [DOI] [PubMed] [Google Scholar]
- Huey ED, Ferrari R, Moreno JH, Jensen C, Morris CM, Potocnik F, Kalaria RN, Tierney M, Wassermann EM, Hardy J, et al. (2012). FUS and TDP43 genetic variability in FTD and CBS. Neurobiol. Aging 33, 1016.e9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsmann BB, Labokha AA, and Görlich D (2012). The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell 150, 738–751. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, and Jülicher F (2014). Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol 30, 39–58. [DOI] [PubMed] [Google Scholar]
- Jain A, and Vale RD (2017). RNA phase transitions in repeat expansion disorders. Nature 546, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, and Parker R (2016). ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 164, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard J-P, Lacomblez L, Pochigaeva K, Salachas F, et al. (2008). TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet 40, 572–574. [DOI] [PubMed] [Google Scholar]
- Kaiser TE, Intine RV, and Dundr M (2008). De novo formation of a subnuclear body. Science 322, 1713–1717. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, and Hirokawa N (2004). Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron 43, 513–525. [DOI] [PubMed] [Google Scholar]
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, et al. (2012). Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, and Anderson P (2000). Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol 151, 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, and Anderson P (2002). Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 13, 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha NL, Gupta M, Li W, Miller I, and Anderson P (1999). RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol 147, 1431–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, and Parker R (2017). The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Mol. Cell 68, 808–820.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, and Myong S (2016). RNA remodeling activity of DEAD box proteins tuned by protein concentration, RNA length, and ATP. Mol. Cell 63, 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim NC, Wang Y-D, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, et al. (2013). Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Horetsky RL, Ron D, Jefferson LS, and Harding HP (2003). Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am. J. Physiol. Cell Physiol 284, C273–84. [DOI] [PubMed] [Google Scholar]
- King OD, Gitler AD, and Shorter J (2012). The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 1462, 61–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar J, Sobol M, Melberg A, Mäbert K, Ameur A, Johansson ACV, Feuk L, Entesarian M, Orlén H, Casar-Borota O, et al. (2013). Welander distal myopathy caused by an ancient founder mutation in TIA1 associated with perturbed splicing. Hum. Mutat 34, 572–577. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, and Kosik KS (2001). Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32, 683–696. [DOI] [PubMed] [Google Scholar]
- Kuechler ER, Budzyńska PM, Bernardini JP, Gsponer J, and Mayor T (2020). Distinct features of stress granule proteins predict localization in membraneless organelles. J. Mol. Biol 432, 2349–2368. [DOI] [PubMed] [Google Scholar]
- Kumar V, Sami N, Kashav T, Islam A, Ahmad F, and Hassan MI (2016). Protein aggregation and neurodegenerative diseases: From theory to therapy. Eur. J. Med. Chem 124, 1105–1120. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. (2009). Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208. [DOI] [PubMed] [Google Scholar]
- Labokha AA, Gradmann S, Frey S, Hülsmann BB, Urlaub H, Baldus M, and Görlich D (2013). Systematic analysis of barrier-forming FG hydrogels from Xenopus nuclear pore complexes. EMBO J. 32, 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon EM, and Gladfelter AS (2018). A New Lens for RNA Localization: Liquid-Liquid Phase Separation. Annu. Rev. Microbiol 72, 255–271. [DOI] [PubMed] [Google Scholar]
- Langdon EM, Qiu Y, Ghanbari Niaki A, McLaughlin GA, Weidmann CA, Gerbich TM, Smith JA, Crutchley JM, Termini CM, Weeks KM, et al. (2018). mRNA structure determines specificity of a polyQ-driven phase separation. Science 360, 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Langenhove T, van der Zee J, Sleegers K, Engelborghs S, Vandenberghe R, Gijselinck I, Van den Broeck M, Mattheijssens M, Peeters K, De Deyn PP, et al. (2010). Genetic contribution of FUS to frontotemporal lobar degeneration. Neurology 74, 366–371. [DOI] [PubMed] [Google Scholar]
- Lee K-H, Zhang P, Kim HJ, Mitrea DM, Sarkar M, Freibaum BD, Cika J, Coughlin M, Messing J, Molliex A, et al. (2016). C9orf72 Dipeptide Repeats Impair the Assembly, Dynamics, and Function of Membrane-Less Organelles. Cell 167, 774–788.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Goryaynov A, and Yang W (2016). The selective permeability barrier in the nuclear pore complex. Nucleus 7, 430–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H-R, Chen T-C, Hsiao C-L, Shi L, Chou C-Y, and Huang J-R (2018a). The physical forces mediating self-association and phase-separation in the C-terminal domain of TDP-43. Biochim. Biophys. Acta Proteins Proteom 1866, 214–223. [DOI] [PubMed] [Google Scholar]
- Li H-R, Chiang W-C, Chou P-C, Wang W-J, and Huang J-R (2018b). TAR DNA-binding protein 43 (TDP-43) liquid-liquid phase separation is mediated by just a few aromatic residues. J. Biol. Chem 293, 6090–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Banjade S, Cheng H-C, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, et al. (2012). Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Protter DSW, Rosen MK, and Parker R (2015). Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol. Cell 60, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Schmidt BF, Bruchez MP, and McManus CJ (2018). Structural analyses of NEAT1 lncRNAs suggest long-range RNA interactions that may contribute to paraspeckle architecture. Nucleic Acids Res. 46, 3742–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bilgutay A, Zhang Y-J, Vanderweyde T, Citro A, Mehta T, Zaarur N, McKee A, Bowser R, Sherman M, et al. (2010). Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One 5, e13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin FE, Lukavsky PJ, Kazeeva T, Reber S, Hock E-M, Colombo M, Von Schroetter C, Pauli P, Cléry A, Mühlemann O, et al. (2019). The Solution Structure of FUS Bound to RNA Reveals a Bipartite Mode of RNA Recognition with Both Sequence and Shape Specificity. Mol. Cell 73, 490–504.e6. [DOI] [PubMed] [Google Scholar]
- Ma ASW, Moran-Jones K, Shan J, Munro TP, Snee MJ, Hoek KS, and Smith R (2002). Heterogeneous nuclear ribonucleoprotein A3, a novel RNA trafficking response element-binding protein. J. Biol. Chem 277, 18010–18020. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, Pottier C, Annu K, Baker M, Perkerson RB, Kurti A, et al. (2017). TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95, 808–816.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharana S, Wang J, Papadopoulos DK, Richter D, Pozniakovsky A, Poser I, Bickle M, Rizk S, Guillén-Boixet J, Franzmann TM, et al. (2018). RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 360, 918–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AM, Miguez RA, Li X, Ho Y-S, Feldman EL, and Barmada SJ (2018). Matrin 3-dependent neurotoxicity is modified by nucleic acid binding and nucleocytoplasmic localization. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JR, Gleixner AM, Mauna JC, Gomes E, DeChellis-Marks MR, Needham PG, Copley KE, Hurtle B, Portz B, Pyles NJ, et al. (2019). RNA Binding Antagonizes Neurotoxic Phase Transitions of TDP-43. Neuron 102, 321–338.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markmiller S, Soltanieh S, Server KL, Mak R, Jin W, Fang MY, Luo E-C, Krach F, Yang D, Sen A, et al. (2018). Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules. Cell 172, 590–604.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny T, Rao BS, and Parker R (2019). Transcriptome-Wide Comparison of Stress Granules and P-Bodies Reveals that Translation Plays a Major Role in RNA Partitioning. Mol. Cell. Biol 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny T, Van Treeck B, Huynh TN, and Parker R RNA partitioning into stress granules is based on the summation of multiple interactions. RNA 27, 174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziuk B, Ballance HI, and Wolozin B (2017). Dysregulation of RNA binding protein aggregation in neurodegenerative disorders. Front. Mol. Neurosci 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazroui R, Sukarieh R, Bordeleau M-E, Kaufman RJ, Northcote P, Tanaka J, Gallouzi I, and Pelletier J (2006). Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2alpha phosphorylation. Mol. Biol. Cell 17, 4212–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KK, Aulas A, Destroismaisons L, Pickles S, Beleac E, Camu W, Rouleau GA, and Vande Velde C (2011). TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum. Mol. Genet 20, 1400–1410. [DOI] [PubMed] [Google Scholar]
- McGurk L, Gomes E, Guo L, Mojsilovic-Petrovic J, Tran V, Kalb RG, Shorter J, and Bonini NM (2018). Poly(ADP-Ribose) Prevents Pathological Phase Separation of TDP-43 by Promoting Liquid Demixing and Stress Granule Localization. Mol. Cell 71, 703–717.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, et al. (2010). TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J. Neuropathol. Exp. Neurol 69, 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Kiernan PT, and Alvarez VE (2015). The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 25, 350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz A, Soret J, Vourc’h C, Tazi J, and Jolly C (2004). A key role for stress-induced satellite III transcripts in the relocalization of splicing factors into nuclear stress granules. J. Cell Sci 117, 4551–4558. [DOI] [PubMed] [Google Scholar]
- Milles S, and Lemke EA (2011). Single molecule study of the intrinsically disordered FG-repeat nucleoporin 153. Biophys. J 101, 1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic D, Wu Y, Bian X, and De Camilli P (2018). A liquid phase of synapsin and lipid vesicles. Science 361, 604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura M, Tomita S, Shinkai Y, Shiraki K, and Kurita R (2020). Quadruplex Folding of DNA Promotes the Condensation of Linker Histones via Liquid-Liquid Phase Separation. ChemRxiv. [DOI] [PubMed] [Google Scholar]
- Mitrea DM, Cika JA, Guy CS, Ban D, Banerjee PR, Stanley CB, Nourse A, Deniz AA, and Kriwacki RW (2016). Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrea DM, Cika JA, Stanley CB, Nourse A, Onuchic PL, Banerjee PR, Phillips AH, Park C-G, Deniz AA, and Kriwacki RW (2018). Self-interaction of NPM1 modulates multiple mechanisms of liquid-liquid phase separation. Nat. Commun 9, 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokas S, Mills JR, Garreau C, Fournier M-J, Robert F, Arya P, Kaufman RJ, Pelletier J, and Mazroui R (2009). Uncoupling stress granule assembly and translation initiation inhibition. Mol. Biol. Cell 20, 2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, and Taylor JP (2015). Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan Z, Ryan VH, Janke AM, Burke KA, Rhoads SN, Zerze GH, O’Meally R, Dignon GL, Conicella AE, Zheng W, et al. (2017). Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 36, 2951–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Lammich S, Mackenzie IRA, Forné I, Zilow S, Kretzschmar H, Edbauer D, Janssens J, Kleinberger G, Cruts M, et al. (2013). hnRNP A3 binds to GGGGCC repeats and is a constituent of p62-positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol. 125, 413–423. [DOI] [PubMed] [Google Scholar]
- Mori S, Honda H, Ishii T, Yoshimura M, Sasagasako N, Suzuki SO, Taniwaki T, and Iwaki T (2019). Expanded polyglutamine impairs normal nuclear distribution of fused in sarcoma and poly (rC)-binding protein 1 in Huntington’s disease. Neuropathology 39, 358–367. [DOI] [PubMed] [Google Scholar]
- Murakami T, Qamar S, Lin JQ, Schierle GSK, Rees E, Miyashita A, Costa AR, Dodd RB, Chan FTS, Michel CH, et al. (2015). ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron 88, 678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma T, Nakagawa S, Tanigawa A, Sasaki YF, Goshima N, and Hirose T (2012). Alternative 3’-end processing of long noncoding RNA initiates construction of nuclear paraspeckles. EMBO J. 31, 4020–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, et al. (2007). Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 114, 221–229. [DOI] [PubMed] [Google Scholar]
- Namkoong S, Ho A, Woo YM, Kwak H, and Lee JH (2018). Systematic Characterization of Stress-Induced RNA Granulation. Mol. Cell 70, 175–187.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswamy R, Levy M, Tsechansky M, Stovall GM, O’Connell JD, Mirrielees J, Ellington AD, and Marcotte EM (2009). Widespread reorganization of metabolic enzymes into reversible assemblies upon nutrient starvation. Proc. Natl. Acad. Sci. USA 106, 10147–10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C, et al. (2019). Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142, 1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. (2006). Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133. [DOI] [PubMed] [Google Scholar]
- Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, and Mackenzie IRA (2009). A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 132, 2922–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Bentmann E, Dormann D, Jawaid A, DeJesus-Hernandez M, Ansorge O, Roeber S, Kretzschmar HA, Munoz DG, Kusaka H, et al. (2011). FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations. Brain 134, 2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaki AG, Sarkar J, Cai X, Rhine K, Vidaurre V, Guy B, Hurst M, Lee JC, Koh HR, Guo L, et al. (2020). Loss of Dynamic RNA Interaction and Aberrant Phase Separation Induced by Two Distinct Types of ALS/FTD-Linked FUS Mutations. Mol. Cell 77, 82–94.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya K, Adachi S, Natsume T, Iwakiri J, Terai G, Asai K, and Hirose T (2020). LncRNA-dependent nuclear stress bodies promote intron retention through SR protein phosphorylation. EMBO J. 39, e102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott TJ, Craggs TD, and Baldwin AJ (2016). Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. Nat. Chem 8, 569–575. [DOI] [PubMed] [Google Scholar]
- Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et al. (2015). A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 162, 1066–1077. [DOI] [PubMed] [Google Scholar]
- Patel SS, Belmont BJ, Sante JM, and Rexach MF (2007). Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell 129, 83–96. [DOI] [PubMed] [Google Scholar]
- Patino MM, Liu JJ, Glover JR, and Lindquist S (1996). Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science 273, 622–626. [DOI] [PubMed] [Google Scholar]
- Posey AE, Holehouse AS, and Pappu RV (2018). Phase separation of intrinsically disordered proteins. Meth. Enzymol 611, 1–30. [DOI] [PubMed] [Google Scholar]
- Prasad A, Bharathi V, Sivalingam V, Girdhar A, and Patel BK (2019). Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci 12, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protter DSW, and Parker R (2016). Principles and properties of stress granules. Trends Cell Biol. 26, 668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman JB, Karl KA, and Kandel ER (2018). TIA-1 Self-Multimerization, Phase Separation, and Recruitment into Stress Granules Are Dynamically Regulated by Zn2. Cell Rep. 22, 59–71. [DOI] [PubMed] [Google Scholar]
- Reineke LC, Cheema SA, Dubrulle J, and Neilson JR (2018). Chronic starvation induces noncanonical pro-death stress granules. J. Cell Sci 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, and Dunker AK (2001). Sequence complexity of disordered protein. Proteins 42, 38–48. [DOI] [PubMed] [Google Scholar]
- Ross CA, and Poirier MA (2004). Protein aggregation and neurodegenerative disease. Nat. Med 10 Suppl, S10–7. [DOI] [PubMed] [Google Scholar]
- Ryan VH, Dignon GL, Zerze GH, Chabata CV, Silva R, Conicella AE, Amaya J, Burke KA, Mittal J, and Fawzi NL (2018). Mechanistic View of hnRNPA2 Low-Complexity Domain Structure, Interactions, and Phase Separation Altered by Mutation and Arginine Methylation. Mol. Cell 69, 465–479.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan VH, Watters S, Amaya J, Khatiwada B, Venditti V, Naik MT, and Fawzi NL (2020). Weak binding to the A2RE RNA rigidifies hnRNPA2 RRMs and reduces liquid-liquid phase separation and aggregation. Nucleic Acids Res. 48, 10542–10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan VH, Perdikari TM, Naik MT, Saueressig CF, Lins J, Dignon GL, Mittal J, Hart AC, and Fawzi NL (2021). Tyrosine phosphorylation regulates hnRNPA2 granule protein partitioning and reduces neurodegeneration. EMBO J. 40, e105001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Weber CA, Nousch M, Adame-Arana O, Hoege C, Hein MY, Osborne-Nishimura E, Mahamid J, Jahnel M, Jawerth L, et al. (2016). Polar Positioning of Phase-Separated Liquid Compartments in Cells Regulated by an mRNA Competition Mechanism. Cell 166, 1572–1584.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez de Groot N, Armaos A, Graña-Montes R, Alriquet M, Calloni G, Vabulas RM, and Tartaglia GG (2019). RNA structure drives interaction with proteins. Nat. Commun 10, 3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DW, Kedersha N, Lee DSW, Strom AR, Drake V, Riback JA, Bracha D, Eeftens JM, Iwanicki A, Wang A, et al. (2020). Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell 181, 306–324.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaser AJ, Osterberg VR, Dent SE, Stackhouse TL, Wakeham CM, Boutros SW, Weston LJ, Owen N, Weissman TA, Luna E, et al. (2019). Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders. Sci. Rep 9, 10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherl A, Couté Y, Déon C, Callé A, Kindbeiter K, Sanchez J-C, Greco A, Hochstrasser D, and Diaz J-J (2002). Functional proteomic analysis of human nucleolus. Mol. Biol. Cell 13, 4100–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HB, and Görlich D (2015). Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore-like permselectivity. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HB, and Rohatgi R (2016). In Vivo Formation of Vacuolated Multi-phase Compartments Lacking Membranes. Cell Rep. 16, 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JC, Wang X, Podell ER, and Cech TR (2013). RNA seeds higher-order assembly of FUS protein. Cell Rep. 5, 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio TR, Cashikar AG, Kowal AS, Sawicki GJ, Moslehi JJ, Serpell L, Arnsdorf MF, and Lindquist SL (2000). Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science 289, 1317–1321. [DOI] [PubMed] [Google Scholar]
- Shakya A, and King JT (2018). DNA Local-Flexibility-Dependent Assembly of Phase-Separated Liquid Droplets. Biophys. J 115, 1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelkovnikova TA, Robinson HK, Connor-Robson N, and Buchman VL (2013). Recruitment into stress granules prevents irreversible aggregation of FUS protein mislocalized to the cytoplasm. Cell Cycle 12, 3194–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelkovnikova TA, Robinson HK, Troakes C, Ninkina N, and Buchman VL (2014). Compromised paraspeckle formation as a pathogenic factor in FUSopathies. Hum. Mol. Genet 23, 2298–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U, and Parker R (2003). Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300, 805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsov SP, and Dundr M (2011). Nucleation of nuclear bodies by RNA. Nat. Cell Biol 13, 167–173. [DOI] [PubMed] [Google Scholar]
- Shin Y, and Brangwynne CP (2017). Liquid phase condensation in cell physiology and disease. Science 357. [DOI] [PubMed] [Google Scholar]
- Su X, Ditlev JA, Hui E, Xing W, Banjade S, Okrut J, King DS, Taunton J, Rosen MK, and Vale RD (2016). Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Pires DEV, Ormsby AR, Cox D, Nie S, Vecchi G, Vendruscolo M, Ascher DB, Reid GE, and Hatters DM (2020). Widespread remodeling of proteome solubility in response to different protein homeostasis stresses. Proc. Natl. Acad. Sci. USA 117, 2422–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, and Parker R (2005). Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 11, 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Avanesyan MD, Dagkesamanskaya AR, Kushnirov VV, and Smirnov VN (1994). The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [psi+] in the yeast Saccharomyces cerevisiae. Genetics 137, 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticozzi N, Vance C, Leclerc AL, Keagle P, Glass JD, McKenna-Yasek D, Sapp PC, Silani V, Bosco DA, Shaw CE, et al. (2011). Mutational analysis reveals the FUS homolog TAF15 as a candidate gene for familial amyotrophic lateral sclerosis. Am. J. Med. Genet. B, Neuropsychiatr. Genet 156B, 285–290. [DOI] [PubMed] [Google Scholar]
- Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, König J, Hortobágyi T, Nishimura AL, Zupunski V, et al. (2011). Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat. Neurosci 14, 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Treeck B, and Parker R (2018). Emerging Roles for Intermolecular RNA-RNA Interactions in RNP Assemblies. Cell 174, 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Treeck B, Protter DSW, Matheny T, Khong A, Link CD, and Parker R (2018). RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc. Natl. Acad. Sci. USA 115, 2734–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay C, St-Amour I, Schneider J, Bennett DA, and Calon F (2011). Accumulation of transactive response DNA binding protein 43 in mild cognitive impairment and Alzheimer disease. J. Neuropathol. Exp. Neurol 70, 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuiji H, Iguchi Y, Furuya A, Kataoka A, Hatsuta H, Atsuta N, Tanaka F, Hashizume Y, Akatsu H, Murayama S, et al. (2013). Spliceosome integrity is defective in the motor neuron diseases ALS and SMA. EMBO Mol. Med 5, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tüű-Szabó B, Kóczy LT, and Fuxreiter M (2018). Simulations of Higher-Order Protein Organizations Using a Fuzzy Framework. Complexity 2018, 1–10. [Google Scholar]
- Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM-Y, Trojanowski JQ, et al. (2008). Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J. Neuropathol. Exp. Neurol 67, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, et al. (2014). Classification of intrinsically disordered regions and proteins. Chem. Rev 114, 6589–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, et al. (2009). Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderweyde T, Yu H, Varnum M, Liu-Yesucevitz L, Citro A, Ikezu T, Duff K, and Wolozin B (2012). Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies. J. Neurosci 32, 8270–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdile V, De Paola E, and Paronetto MP (2019). Aberrant phase transitions: side effects and novel therapeutic strategies in human disease. Front. Genet 10, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler TO, Wheeler JR, Nguyen ED, Hughes MP, Britson KA, Lester E, Rao B, Betta ND, Whitney ON, Ewachiw TE, et al. (2018). TDP-43 and RNA form amyloid-like myo-granules in regenerating muscle. Nature 563, 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic S, Brown CJ, Dunker AK, and Obradovic Z (2003). Flavors of protein disorder. Proteins 52, 573–584. [DOI] [PubMed] [Google Scholar]
- Wallace EWJ, Kear-Scott JL, Pilipenko EV, Schwartz MH, Laskowski PR, Rojek AE, Katanski CD, Riback JA, Dion MF, Franks AM, et al. (2015). Reversible, Specific, Active Aggregates of Endogenous Proteins Assemble upon Heat Stress. Cell 162, 1286–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Conicella AE, Schmidt HB, Martin EW, Rhoads SN, Reeb AN, Nourse A, Ramirez Montero D, Ryan VH, Rohatgi R, et al. (2018a). A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing. EMBO J 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Duan Y, Duan G, Wang Q, Zhang K, Deng X, Qian B, Gu J, Ma Z, Zhang S, et al. (2020). Stress Induces Dynamic, Cytotoxicity-Antagonizing TDP-43 Nuclear Bodies via Paraspeckle LncRNA NEAT1-Mediated Liquid-Liquid Phase Separation. Mol. Cell 79, 443–458.e7. [DOI] [PubMed] [Google Scholar]
- Wang I-F, Reddy NM, and Shen C-KJ (2002). Higher order arrangement of the eukaryotic nuclear bodies. Proc. Natl. Acad. Sci. USA 99, 13583–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Choi J-M, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, Pozniakovsky A, Drechsel D, et al. (2018b). A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 174, 688–699.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB (1994). [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science 264, 566–569. [DOI] [PubMed] [Google Scholar]
- Wolozin B, and Ivanov P (2019). Stress granules and neurodegeneration. Nat. Rev. Neurosci 20, 649–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Souquere S, Chujo T, Kobelke S, Chong YS, Fox AH, Bond CS, Nakagawa S, Pierron G, and Hirose T (2018). Functional Domains of NEAT1 Architectural lncRNA Induce Paraspeckle Assembly through Phase Separation. Mol. Cell 70, 1038–1053.e7. [DOI] [PubMed] [Google Scholar]
- Yang P, Mathieu C, Kolaitis R-M, Zhang P, Messing J, Yurtsever U, Yang Z, Wu J, Li Y, Pan Q, et al. (2020). G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 181, 325–345.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R-W, Xu G, Wang Y, Shan L, Luan P-F, Wang Y, Wu M, Yang L-Z, Xing Y-H, Yang L, et al. (2019). Nascent Pre-rRNA Sorting via Phase Separation Drives the Assembly of Dense Fibrillar Components in the Human Nucleolus. Mol. Cell 76, 767–783.e11. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Zhang H, Loiselle D, Haystead T, Macara IG, and Mili S (2013). The RNA-binding protein Fus directs translation of localized mRNAs in APC-RNP granules. J. Cell Biol 203, 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H, Triandafillou C, and Drummond DA (2019). Cellular sensing by phase separation: Using the process, not just the products. J. Biol. Chem 294, 7151–7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Lu S, Gasior K, Singh D, Vazquez-Sanchez S, Tapia O, Toprani D, Beccari MS, Yates JR, Da Cruz S, et al. (2021). HSP70 chaperones RNA-free TDP-43 into anisotropic intranuclear liquid spherical shells. Science 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Jiao B, Hou L, Xiao T, Liu X, Wang J, Xu J, Zhou L, Yan X, Tang B, et al. (2018). Mutation analysis of the TIA1 gene in Chinese patients with amyotrophic lateral sclerosis and frontotemporal dementia. Neurobiol. Aging 64, 160.e9–160.e12. [DOI] [PubMed] [Google Scholar]
- Zanzoni A, Marchese D, Agostini F, Bolognesi B, Cirillo D, Botta-Orfila M, Livi CM, Rodriguez-Mulero S, and Tartaglia GG (2013). Principles of self-organization in biological pathways: a hypothesis on the autogenous association of alpha-synuclein. Nucleic Acids Res. 41, 9987–9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M, Shang Y, Araki Y, Guo T, Huganir RL, and Zhang M (2016). Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. Cell 166, 1163–1175.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, Brangwynne CP, and Gladfelter AS (2015). RNA controls polyq protein phase transitions. Mol. Cell 60, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lin Y, Eschmann NA, Zhou H, Rauch JN, Hernandez I, Guzman E, Kosik KS, and Han S (2017). RNA stores tau reversibly in complex coacervates. PLoS Biol. 15, e2002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang M, Duncan S, Yang X, Abdelhamid MAS, Huang L, Zhang H, Benfey PN, Waller ZAE, and Ding Y (2019). G-quadruplex structures trigger RNA phase separation. Nucleic Acids Res. 47, 11746–11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Richardson TM, Wacheul L, Wei M-T, Feric M, Whitney G, Lafontaine DLJ, and Brangwynne CP (2019). Controlling the material properties and rRNA processing function of the nucleolus using light. Proc. Natl. Acad. Sci. USA 116, 17330–17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo X-F, Wang J, Zhang J, Jiang L-L, Hu H-Y, and Lu J-X (2020). Solid-State NMR Reveals the Structural Transformation of the TDP-43 Amyloidogenic Region upon Fibrillation. J. Am. Chem. Soc 142, 3412–3421. [DOI] [PubMed] [Google Scholar]
Numbers correspond to references listed below:
- 1.King OD, Gitler AD, Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012June26;1462:61–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009April3;137(1):146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008May;40(5):572–574. [DOI] [PubMed] [Google Scholar]
- 4.Caroppo P, Camuzat A, Guillot-Noel L, Thomas-Antérion C, Couratier P, Wong TH, et al. Defining the spectrum of frontotemporal dementias associated with TARDBP mutations. Neurol Genet. 2016June;2(3):e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006October6;314(5796):130–133. [DOI] [PubMed] [Google Scholar]
- 6.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006December22;351(3):602–611. [DOI] [PubMed] [Google Scholar]
- 7.Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019June1;142(6):1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010September;69(9):918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amador-Ortiz C, Lin W-L, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007May;61(5):435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008June;67(6):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007September;114(3):221–229. [DOI] [PubMed] [Google Scholar]
- 12.Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015September24;163(1):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGurk L, Gomes E, Guo L, Mojsilovic-Petrovic J, Tran V, Kalb RG, et al. Poly(ADP-Ribose) Prevents Pathological Phase Separation of TDP-43 by Promoting Liquid Demixing and Stress Granule Localization. Mol Cell. 2018September6;71(5):703–717.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang A, Conicella AE, Schmidt HB, Martin EW, Rhoads SN, Reeb AN, et al. A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing. EMBO J 2018March1;37(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conicella AE, Zerze GH, Mittal J, Fawzi NL. ALS Mutations Disrupt Phase Separation Mediated by α-Helical Structure in the TDP-43 Low-Complexity C-Terminal Domain. Structure. 2016September6;24(9):1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H-R, Chen T-C, Hsiao C-L, Shi L, Chou C-Y, Huang J-R. The physical forces mediating self-association and phase-separation in the C-terminal domain of TDP-43. Biochim Biophys Acta Proteins Proteom. 2018February;1866(2):214–223. [DOI] [PubMed] [Google Scholar]
- 17.Conicella AE, Dignon GL, Zerze GH, Schmidt HB, D’Ordine AM, Kim YC, et al. TDP-43 α-helical structure tunes liquid-liquid phase separation and function. Proc Natl Acad Sci USA. 2020March17;117(11):5883–5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H-R, Chiang W-C, Chou P-C, Wang W-J, Huang J-R. TAR DNA-binding protein 43 (TDP-43) liquid-liquid phase separation is mediated by just a few aromatic residues. J Biol Chem. 2018April20;293(16):6090–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alami NH, Smith RB, Carrasco MA, Williams LA, Winborn CS, Han SSW, et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014February5;81(3):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gopal PP, Nirschl JJ, Klinman E, Holzbaur ELF. Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons. Proc Natl Acad Sci USA. 2017March21;114(12):E2466–E2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang I-F, Reddy NM, Shen C-KJ. Higher order arrangement of the eukaryotic nuclear bodies. Proc Natl Acad Sci USA. 2002October15;99(21):13583–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuiji H, Iguchi Y, Furuya A, Kataoka A, Hatsuta H, Atsuta N, et al. Spliceosome integrity is defective in the motor neuron diseases ALS and SMA. EMBO Mol Med. 2013February;5(2):221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Duan Y, Duan G, Wang Q, Zhang K, Deng X, et al. Stress Induces Dynamic, Cytotoxicity-Antagonizing TDP-43 Nuclear Bodies via Paraspeckle LncRNA NEAT1-Mediated Liquid-Liquid Phase Separation. Mol Cell. 2020August6;79(3):443–458.e7. [DOI] [PubMed] [Google Scholar]
- 24.Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, et al. TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem. 2009November;111(4):1051–1061. [DOI] [PubMed] [Google Scholar]
- 25.Liu-Yesucevitz L, Bilgutay A, Zhang Y-J, Vanderweyde T, Citro A, Mehta T, et al. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One. 2010October11;5(10):e13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naganuma T, Nakagawa S, Tanigawa A, Sasaki YF, Goshima N, Hirose T. Alternative 3’-end processing of long noncoding RNA initiates construction of nuclear paraspeckles. EMBO J. 2012October17;31(20):4020–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009February27;323(5918):1208–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwiatkowski TJ, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009February27;323(5918):1205–1208. [DOI] [PubMed] [Google Scholar]
- 29.Huey ED, Ferrari R, Moreno JH, Jensen C, Morris CM, Potocnik F, et al. FUS and TDP43 genetic variability in FTD and CBS. Neurobiol Aging. 2012May;33(5):1016.e9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Langenhove T, van der Zee J, Sleegers K, Engelborghs S, Vandenberghe R, Gijselinck I, et al. Genetic contribution of FUS to frontotemporal lobar degeneration. Neurology. 2010February2;74(5):366–371. [DOI] [PubMed] [Google Scholar]
- 31.Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IRA. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009November;132(Pt 11):2922–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori S, Honda H, Ishii T, Yoshimura M, Sasagasako N, Suzuki SO, et al. Expanded polyglutamine impairs normal nuclear distribution of fused in sarcoma and poly (rC)-binding protein 1 in Huntington’s disease. Neuropathology. 2019October9;39(5):358–367. [DOI] [PubMed] [Google Scholar]
- 33.Doi H, Koyano S, Suzuki Y, Nukina N, Kuroiwa Y. The RNA-binding protein FUS/TLS is a common aggregate-interacting protein in polyglutamine diseases. Neurosci Res. 2010January;66(1):131–133. [DOI] [PubMed] [Google Scholar]
- 34.Murakami T, Qamar S, Lin JQ, Schierle GSK, Rees E, Miyashita A, et al. ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron. 2015November18;88(4):678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell. 2015August27;162(5):1066–1077. [DOI] [PubMed] [Google Scholar]
- 36.Monahan Z, Ryan VH, Janke AM, Burke KA, Rhoads SN, Zerze GH, et al. Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 2017October16;36(20):2951–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke KA, Janke AM, Rhine CL, Fawzi NL. Residue-by-Residue View of In Vitro FUS Granules that Bind the C-Terminal Domain of RNA Polymerase II. Mol Cell. 2015October15;60(2):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shelkovnikova TA, Robinson HK, Troakes C, Ninkina N, Buchman VL. Compromised paraspeckle formation as a pathogenic factor in FUSopathies. Hum Mol Genet. 2014May1;23(9):2298–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ, et al. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet. 2010November1;19(21):4160–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 2010August18;29(16):2841–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gal J, Zhang J, Kwinter DM, Zhai J, Jia H, Jia J, et al. Nuclear localization sequence of FUS and induction of stress granules by ALS mutants. Neurobiol Aging. 2011December;32(12):2323.e27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004August19;43(4):513–525. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda K, Zhang H, Loiselle D, Haystead T, Macara IG, Mili S. The RNA-binding protein Fus directs translation of localized mRNAs in APC-RNP granules. J Cell Biol. 2013December9;203(5):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Couthouis J, Hart MP, Erion R, King OD, Diaz Z, Nakaya T, et al. Evaluating the role of the FUS/TLS-related gene EWSR1 in amyotrophic lateral sclerosis. Hum Mol Genet. 2012July1;21(13):2899–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neumann M, Bentmann E, Dormann D, Jawaid A, DeJesus-Hernandez M, Ansorge O, et al. FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations. Brain. 2011September;134(Pt 9):2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Choi J-M, Holehouse AS, Lee HO, Zhang X, Jahnel M, et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell. 2018July26;174(3):688–699.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maharana S, Wang J, Papadopoulos DK, Richter D, Pozniakovsky A, Poser I, et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science. 2018May25;360(6391):918–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altmeyer M, Neelsen KJ, Teloni F, Pozdnyakova I, Pellegrino S, Grøfte M, et al. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat Commun. 2015August19;6:8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersson MK, Ståhlberg A, Arvidsson Y, Olofsson A, Semb H, Stenman G, et al. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008July11;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Couthouis J, Hart MP, Shorter J, DeJesus-Hernandez M, Erion R, Oristano R, et al. A yeast functional screen predicts new candidate ALS disease genes. Proc Natl Acad Sci USA. 2011December27;108(52):20881–20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ticozzi N, Vance C, Leclerc AL, Keagle P, Glass JD, McKenna-Yasek D, et al. Mutational analysis reveals the FUS homolog TAF15 as a candidate gene for familial amyotrophic lateral sclerosis. Am J Med Genet B, Neuropsychiatr Genet. 2011April;156B(3):285–290. [DOI] [PubMed] [Google Scholar]
- 52.Blechingberg J, Luo Y, Bolund L, Damgaard CK, Nielsen AL. Gene expression responses to FUS, EWS, and TAF15 reduction and stress granule sequestration analyses identifies FET-protein non-redundant functions. PLoS One. 2012September25;7(9):e46251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HJ, Kim NC, Wang Y-D, Scarborough EA, Moore J, Diaz Z, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013March28;495(7442):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gami-Patel P, Bandopadhyay R, Brelstaff J, Revesz T, Lashley T. The presence of heterogeneous nuclear ribonucleoproteins in frontotemporal lobar degeneration with FUS-positive inclusions. Neurobiol Aging. 2016July15;46:192–203. [DOI] [PubMed] [Google Scholar]
- 55.Lin Y, Protter DSW, Rosen MK, Parker R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol Cell. 2015October15;60(2):208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guil S, Long JC, Cáceres JF. hnRNP A1 relocalization to the stress granules reflects a role in the stress response. Mol Cell Biol. 2006August;26(15):5744–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan VH, Watters S, Amaya J, Khatiwada B, Venditti V, Naik MT, et al. Weak binding to the A2RE RNA rigidifies hnRNPA2 RRMs and reduces liquid-liquid phase separation and aggregation. Nucleic Acids Res. 2020October9;48(18):10542–10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ryan VH, Dignon GL, Zerze GH, Chabata CV, Silva R, Conicella AE, et al. Mechanistic View of hnRNPA2 Low-Complexity Domain Structure, Interactions, and Phase Separation Altered by Mutation and Arginine Methylation. Mol Cell. 2018February1;69(3):465–479.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryan VH, Perdikari TM, Naik MT, Saueressig CF, Lins J, Dignon GL, et al. Tyrosine phosphorylation regulates hnRNPA2 granule protein partitioning and reduces neurodegeneration. EMBO J. 2021February1;40(3):e105001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDonald KK, Aulas A, Destroismaisons L, Pickles S, Beleac E, Camu W, et al. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet. 2011April1;20(7):1400–1410. [DOI] [PubMed] [Google Scholar]
- 61.Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, Pottier C, et al. TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron. 2017August16;95(4):808–816.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan Z, Jiao B, Hou L, Xiao T, Liu X, Wang J, et al. Mutation analysis of the TIA1 gene in Chinese patients with amyotrophic lateral sclerosis and frontotemporal dementia. Neurobiol Aging. 2018;64:160.e9–160.e12. [DOI] [PubMed] [Google Scholar]
- 63.Hackman P, Sarparanta J, Lehtinen S, Vihola A, Evilä A, Jonson PH, et al. Welander distal myopathy is caused by a mutation in the RNA-binding protein TIA1. Ann Neurol. 2013April;73(4):500–509. [DOI] [PubMed] [Google Scholar]
- 64.Klar J, Sobol M, Melberg A, Mäbert K, Ameur A, Johansson ACV, et al. Welander distal myopathy caused by an ancient founder mutation in TIA1 associated with perturbed splicing. Hum Mutat. 2013April;34(4):572–577. [DOI] [PubMed] [Google Scholar]
- 65.Vanderweyde T, Yu H, Varnum M, Liu-Yesucevitz L, Citro A, Ikezu T, et al. Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies. J Neurosci. 2012June13;32(24):8270–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rayman JB, Karl KA, Kandel ER. TIA-1 Self-Multimerization, Phase Separation, and Recruitment into Stress Granules Are Dynamically Regulated by Zn2. Cell Rep. 2018January2;22(1):59–71. [DOI] [PubMed] [Google Scholar]
- 67.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, et al. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004December;15(12):5383–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davidson YS, Flood L, Robinson AC, Nihei Y, Mori K, Rollinson S, et al. Heterogeneous ribonuclear protein A3 (hnRNP A3) is present in dipeptide repeat protein containing inclusions in Frontotemporal Lobar Degeneration and Motor Neurone disease associated with expansions in C9orf72 gene. Acta Neuropathol Commun. 2017April21;5(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mori K, Lammich S, Mackenzie IRA, Forné I, Zilow S, Kretzschmar H, et al. hnRNP A3 binds to GGGGCC repeats and is a constituent of p62-positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol. 2013March;125(3):413–423. [DOI] [PubMed] [Google Scholar]
- 70.Ma ASW, Moran-Jones K, Shan J, Munro TP, Snee MJ, Hoek KS, et al. Heterogeneous nuclear ribonucleoprotein A3, a novel RNA trafficking response element-binding protein. J Biol Chem. 2002May17;277(20):18010–18020. [DOI] [PubMed] [Google Scholar]
- 71.Markmiller S, Soltanieh S, Server KL, Mak R, Jin W, Fang MY, et al. Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules. Cell. 2018January25;172(3):590–604.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]


