Abstract
Background
The aim of this study was to evaluate the efficacy and safety of Sofosbuvir/Velpatasvir prophylaxis in hepatitis C virus (HCV)-negative recipients who received a transplant kidney from HCV-infected donors.
Material/Methods
This retrospective cohort study enrolled consecutive HCV-negative recipients between January 2019 and February 2021. All the recipients were treated with Sofosbuvir/Velpatasvir (400 mg/100 mg) once daily for 12 weeks after receiving a transplant kidney from HCV-infected donors. We collected data on renal function and liver function and HCV RNA were collected during the study. We also compared the rates of adverse events.
Results
A total of 26 patients were included in the cohort. All the recipients (100%) completed 12 weeks of treatment and the entire follow-up. All recipients (100%) had negative HCV RNA, but 4 recipients (15.4%) were HCV antibody (Ab)-positive after transplantation. Fifteen adverse events (57.7%) occurred during the study. Three recipients (11.5%) experienced graft rejection, 6 recipients (23.1%) had delayed graft function, and 3 recipients (11.5%) had bleeding. However, none of them were related to study medication. Renal function was stable in all patients.
Conclusions
Sofosbuvir/Velpatasvir pre- and post-transplantation treatment was effective and safe in HCV-uninfected recipients who received a transplant kidney from HCV-infected donors.
Keywords: Hepatitis C Protein F, Hepatitis C Virus; Kidney Transplantation; Treatment Outcome
Background
Renal transplantation is the best therapy for patients with end-stage renal disease (ESRD). More than 300 000 patients with ESRD in China are waiting for kidney transplantation [1]. Donation after citizen death and donation from a living relative are currently the 2 main organ sources [2]. However, the number of donor kidneys is far from meeting the increasing needs for kidney transplantation. Therefore, it is imperative to increase access to donor kidneys. Efforts to expand the criteria for optimal donor organs have never stopped.
Chronic HCV infection is a global public health problem that affects approximately 170 million people worldwide [3] and there are an estimated 8.9 million of these patients living in China. How to properly utilize HCV-positive donor organs needs to be further explored. In the past few years, the use of expanded standard donor kidneys was increased, and hepatitis C donors were also considered [4–8]. This brings the hope of allowing more patients with end-stage renal disease to survive, but also brings concerns about transmission of HCV from donors to recipients [9,10]. Hepatitis C infection can cause liver failure in kidney transplant patients, so it is necessary to carefully select the kidneys from hepatitis C-positive donors and promptly treat them.
A new drug for chronic hepatitis C, Sofosbuvir/Velpatasvir has been officially launched in China for the treatment of adult patients with chronic hepatitis C virus (HCV) genotype 1–6 infection.
Introduction of direct acting antiviral (DAA) therapy has made HCV a curable infection, which allows HCV-infected patients to achieve extremely high sustained virologic response (SVR) with good safety performance [11,12]. In recent years, DAA therapy was also reported in a few studies focused on kidney transplantation from HCV-infected donors to HCV-uninfected recipients [4,5,7,13]. In these studies, data showed that HCV-negative recipients could also achieve SVR12 under DAA therapy after receiving an allograft from HCV-positive donors, and without experiencing drug-related severe adverse events. These results showed clinicians that using kidneys from HCV-infected donors could be an efficient way to expand the current donor pool.
Therefore, there is a growing need to assess the feasibility of use of HCV-positive donor kidneys for transplantation into HCV-negative recipients. In this single-center retrospective study, we evaluated the efficacy and safety of Sofosbuvir/Velpatasvir prophylaxis for 12 weeks, with the first dose given 2 h before renal transplantation, in HCV-negative recipients who received kidney transplants from HCV-infected donors.
Material and Methods
Patients
In this retrospective study, 26 consecutive HCV-negative recipients who underwent kidney transplant surgery from HCV-infected donors during the period of January 2019 to February 2021 were enrolled. Patients who were co-infected with HIV or with decompensated liver disease, or who had a serious alcohol abuse problem were excluded. Data retrieval was approved by the Institutional Review Board of Affiliated Renji Hospital, School of Medicine, Shanghai Jiao Tong University. Informed consent was obtained from all recipients (Figure 1).
Figure 1.
Flow chart. A total of 837 people received kidney transplants during this period, of which 26 received HCV RNA(+) kidney transplants, and all recipients completed the follow-up. At the end of follow-up, HCV RNA was negative.
Immunosuppressive Therapy
Patients received an immunosuppressive regimen comprising intravenous rabbit-anti-human-thymocyte-immunoglobulin (RATG) or basiliximab in combination with methylprednisolone for induction immunosuppressive therapy (except No. 9), followed by oral calcineurin inhibitors, prednisolone, and mycophenolate mofetil (MMF) for maintenance immunosuppression therapy.
Antiviral Therapy and Follow-up Schedule
All patients received 1 Sofosbuvir/Velpatasvir (400 mg/100 mg) tablet once daily for 12 weeks. The first dose was given 2 h before renal transplant surgery.
Patients were followed up at weeks 2, 4, 8, and 12 after renal transplant surgery and weeks 4, 8, and 12 after the end of antiviral treatment.
Outcomes
The primary endpoint was the proportion of patients with negative HCV RNA at week 12 after discontinuation of antiviral treatment. HCV RNA was determined using the Roche COBAS AmpliPrep/COBAS TaqMan HCV Quantitative Test, v2.0. The lower limit of quantitation (LLOQ) of the assay was 15 IU/mL. Secondary outcomes included liver and renal function, transplant rejection, and graft survival rate. The safety endpoints included any adverse events occurring during the study.
Statistical Analysis
A percentage form was used for qualitative data. Descriptive analysis was used for quantitative data by SAS, version 9.2. Data are expressed as mean±SD.
Results
Patient Characteristics
We enrolled 26 patients in the final analysis. All patients had completed 12 weeks of antiviral treatment and 12 weeks of follow-up. Baseline characteristics were collected at the time the patient entered the waitlist. Among them, 19 (73.1%) were male, the median age was 42 (range 20–73) years old, and none had compensated cirrhosis. The median time on the waitlist prior to transplantation was 198 days. The median time on dialysis prior to transplantation was 330 days.
There were 15 donors for 26 recipients. All donors were HCV RNA-positive, and all the donors were HCV Ab-positive. The median donor age was 47 (range 32–53) years old. The median donor terminal serum creatine was 83 (range 24–189) umol/L.
Twenty-six patients were receiving dialysis at baseline, including 5 on peritoneal dialysis and 21 on hemodialysis. All of them had negative HCV RNA at baseline (Table 1).
Table 1.
Demographic and clinical characteristics of recipient and donor.
| Recipient characteristics (n=26) | |
|---|---|
| Median age (IQR), years | 42 (20~73) |
| Male, n (%) | 19 (73.1%) |
| Median body mass index, kg/m2 | 23.295 (17.03~33.66) |
| Cirrhosis, n (%) | 0 |
| ALT level (U/L) | 10.5 (3~70) |
| Serum creatinine level (μmol/L) | 894 (373~1705) |
| eGFR (mL/min) | 7.715 (4~20.77) |
| Primary cause of renal failure (n=26) | |
| IgA nephropathy | 4 |
| PKD | 3 |
| FSGS | 3 |
| DN | 0 |
| HN | 1 |
| Unknown cause | 15 |
| History of diabetes (n) | 2 |
| HBsAg-positive (n) | 2 |
| HCV Ab-positive (n) | 0 |
| Median time on dialysis before transplantation (d) | 330 (0~4340) |
| Median time on waitlist before transplantation (d) | 198 (29~865) |
| Induction immunosuppression, (n=26) | |
| rATG+prednisone | 23 |
| Basiliximab+prednisone | 2 |
| Prednisone | 1 |
| Maintenance immunosuppression, (n=26) | |
| FK+MPA+Pred | 26 |
| Donor Characteristics (n=15) | |
| Male, n (%) | 14 (93.3%) |
| Cause of death, (n) | |
| DBD | 13 |
| DCD | 2 |
| Median terminal serum creatine (μmol/L) | 84.5 (24~194.5) |
ALT – alanine aminotransferase; eGFR – estimated glomerular filtration rate; PKD – polycystic kidney disease; FSGS – focal segmental glomerulosclerosis; DN – diabetic nephropathy; HN – hypertensive nephrosclerosis; rATG – rabbit anti-human thymocyte; FK – tacrolimus; MPA – mycophenolic acid; DBD – donation after brain death; DCD – donation after cardiac death.
Efficacy
Outcomes
Among the 26 recipients, 22 patients remained HCV Ab-negative after transplantation. No patients had detectable HCV RNA at the end of follow-up (Table 2).
Table 2.
Viremic parameter of donor and recipient before and after transplantation.
| Recipient | Donor | Paired recipient | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| HCV RNA IU/mL | HCV Ab | Genotype | HCV RNA IU/mL | |||
| Week 12 ot * | Week 12 fu # | Week 4 ot * | Week 2 ot * | 3a | 583 | 1 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.37/0.11 | 1b | 369000 | 2 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.04/0.2 | 2a | 727000 | 3 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.06/0.04 | 1b | 168000 | 4 |
|
|
|
|
||||
| 0 | 0 | 0 | 2.07/1.68 | 3b | 147000 | 5 |
|
|
|
|
||||
| 0 | 0 | 0 | 1.72/1.29 | 3a | 654000 | 6 |
|
| ||||||
| 0 | 0 | 0 | 0.11 | |||
|
|
|
|
||||
| 0 | 0 | 0 | 0.02 | 2a | 342000 | 7 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.08/0.09 | 3b | 113000 | 8 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.05 | 1b | 217000 | 9 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.04/0.07 | 3a | 668000 | 10 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.42 | 2a | 438000 | 11 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.09/0.08 | 3a | 935000 | 12 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.03/0.07 | 3b | 477000 | 13 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.2/0.08 | 1b | 537000 | 14 |
|
|
|
|
||||
| 0 | 0 | 0 | 0.08/0.05 | 3b | 1100000 | 15 |
ot – on treatment of antiviral therapy;
fu – follow-up after antiviral treatment.
Renal function parameters remained stable among all patients. Serum creatine level was monitored at days 14 and 90 after renal transplant surgery (Figure 2). All patients completed the entire course of treatment and follow-up. The median serum creatine level at week 12 off treatment was 101.5 (IQR 61–213) umol/L. Median eGFR at week 12 after transplantation was 67.22 (IQR 32.17–93.1) ml/min/1.73 m2 and 74.09 (IQR 36.96–102.4) ml/min/1.73 m2 at last visit.
Figure 2.
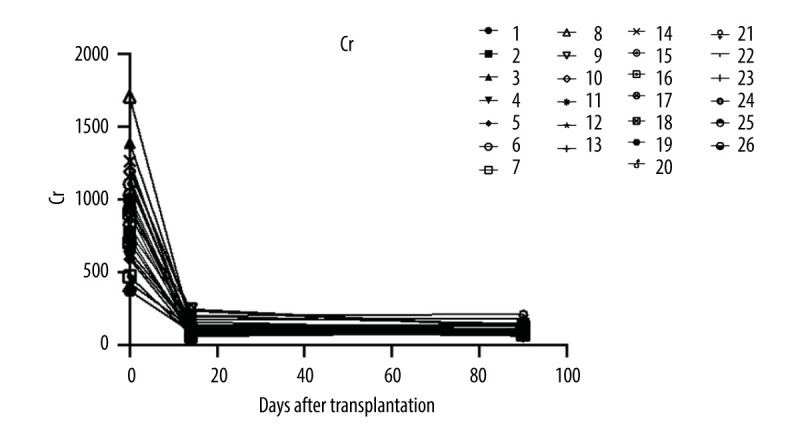
The serum creatine levels of patients were followed up at days 14 and 90 after renal transplant surgery (26 patients were enrolled and 24 patients completed the follow-up).
Liver function maintained stable during the whole study in all patients (Figure 3).
Figure 3.
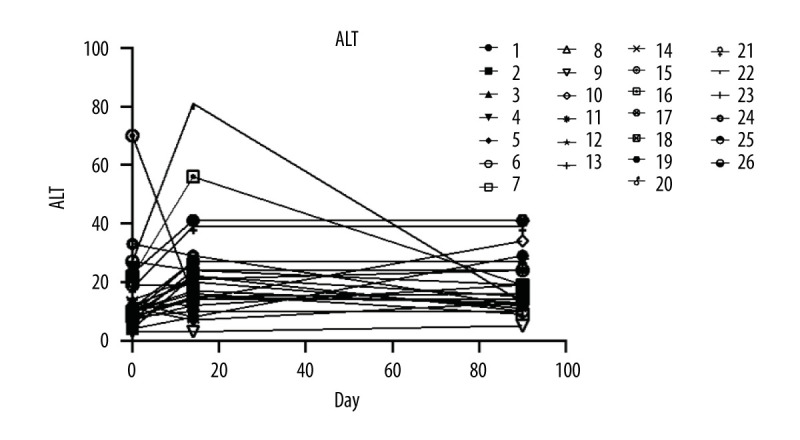
The level of alanine aminotransferase.
Safety
The majority of adverse events reported in the study were mild and consistent with those in previous Sofosbuvir/Velpatasvir studies. Adverse events were reported in 15 patients (Table 3), but none was considered related to the drugs.
Table 3.
Safety.
| Sofosbuvir/Velpatasvir 12 weeks (n=26) | |
|---|---|
| Any adverse event | 15 |
| 3/4 treatment-emergent adverse events | 0 |
| Severe adverse event | 0 |
| Deaths | 0 |
| Adverse events occurring in ≥10% of patients | |
| DGF | 6 |
| Rejection | 3 |
| Heart disease | 3 |
| Bleeding | 3 |
DGF – delayed graft function.
Three (11.5%) patients experienced transplant rejection. Recipient No. 9, who did not receive RATG or basiliximab for induction immunosuppressive therapy, had antibody-mediated rejection (AMR) on day 13 after renal transplantation. This patient was treated with Rituximab and intravenous immunoglobulin, and the renal function was stable.
Recipients No. 18 and No. 25 experienced acute cellular rejection (ACR) on day 16 and day 11 after surgery, respectively. None of them were considered related to Sofosbuvir/Velpatasvir treatment. No graft failure occurred in this study.
Discussion
In this retrospective study, 26 HCV-negative recipients received 12 weeks of Sofosbuvir/Velpatasvir treatment before and after renal transplant surgery from HCV-positive donors, and none had detectable HCV RNA to date. Three patients experienced graft rejection at days 11, 13, and 16 after transplantation, respectively. None of them were considered to be related to study medication. No graft failure happened in this study. Renal function remained stable during both during treatment and in follow-up. Adverse events were common, but most of them were mild and consistent with those reported in previous Sofosbuvir/Velpatasvir studies. Severe adverse events (SAEs) were reported in 6 patients, but none were thought to be related to HCV infection or antiviral treatment. To the best of our knowledge, this is the first study to investigate the efficacy and safety of Sofosbuvir/Velpatasvir prophylaxis in HCV-uninfected recipients who received transplant kidneys from HCV-infected donors in China.
In China, the shortage of qualified donor kidneys is continuing to grow, and there is an urgent demand to increase access to donor kidneys. In the past few years, several studies have been published on kidney transplantation from HCV-infected donors to HCV-uninfected recipients. Meghan et al reported on 30 HCV-negative recipients, who received HCV viremic kidneys and were initiated with glecaprevir-pibrentasvir treatment 3 days after transplantation [7]. All patients achieved SVR. Three out of 7 recipients who underwent kidney biopsy at the end of follow-up showed acute cellular rejection. Sixteen recipients experienced a total of 21 SAEs during glecaprevir-pibrentasvir treatment, but none of them were considered related to study medication. Christine et al showed that an immediate dose of grazoprevir-elbasvir with or without sofosbuvir prior to transplantation and continued for 12 weeks was effective [3]. All 10 recipients had undetectable HCV RNA 12 weeks after treatment. No recipient developed acute rejection, but 1 recipient experienced severe transaminase elevation greater than 5 times the upper limit of normal. The efficacy and tolerance of Sofosbuvir/Velpatasvir was recently evaluated in the PROACT study [4]. HCV-uninfected recipients were treated with 12 weeks of Sofosbuvir/Velpatasvir once they were confirmed HCV-positive after transplantation, and all of them achieved SVR12 (n=9). None of them experienced ACR.
Sofosbuvir/Velpatasvir has been proved to be effective and safe in the general HCV-infected population regardless of genotype and degree of fibrosis in many previous clinical trials and real-world studies [9,12,14]. A single-tablet regimen and fixed treatment duration allows the possibility to simplify anti-HCV therapy [15]. Sofosbuvir/Velpatasvir was approved to be used in severe renal impairment patients, including those with ESRD and on dialysis, by the FDA in 2019. It was based on a real-world, open-label study that evaluated the safety and efficacy of 12 weeks of Sofosbuvir/Velpatasvir in treatment-naïve and treatment-experienced patients with ESRD and on dialysis [16]. The total SVR12 was 95% (56/59). Sofosbuvir/Velpatasvir was safe and well tolerated in that study and there were no treatment-related discontinuation or serious adverse events during the whole treatment course. However, the effectiveness and safety of Sofosbuvir/Velpatasvir prophylaxis has not been evaluated in HCV-negative recipients receiving HCV-positive renal transplantation. Our study results add treatment experience in this special population.
Three of 26 recipients were treated for acute rejection after transplant in this study. Although some studies reported that DAA therapy might affect immunosuppression levels, and HCV treatment after transplant might be associated with rejection due to declined immunosuppression levels or changes in the immune profile, the rate of rejection in our study was comparable with those in the general kidney transplant population. However, this should be further comprehensively evaluated in a large population in the future [17–19].
The limitations of our study are obvious. First, this was a retrospective, single-center study with small sample size and limited follow-up duration. Second, data on viremic parameters in the first 3 days after transplantation were unavailable in this study, so we could not tell the viral load immediately after the surgery, which may have an association with liver function fluctuation. We expect to conduct a prospective controlled study to further demonstrate the virologic kinetics, as well as the effectiveness and tolerance, of Sofosbuvir/Velpatasvir in this specific population.
Conclusions
In summary, this study showed that initiation of Sofosbuvir/Velpatasvir prophylaxis at the day of renal transplantation surgery could effectively block HCV transmission or cure HCV infection in HCV-negative recipients receiving allografts from HCV-positive donors. Most patients remained negative for HCV Ab and no patient had detectable HCV RNA at the end of the study. Sofosbuvir/Velpatasvir was also well tolerated. No SAEs were considered to be related to study medication. These results may contribute to enlarging the number of qualified donor kidneys in China.
Footnotes
Conflict of interest: Consensus on all papers was reached through discussion by the authors and there was no identified conflict of interest
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: National Natural Science Fund of China (ID 81800657)
References
- 1.GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J, Millis JM, Mao Y, et al. A pilot programme of organ donation after cardiac death in China. Lancet. 2012;379(9818):862–65. doi: 10.1016/S0140-6736(11)61086-6. [DOI] [PubMed] [Google Scholar]
- 3.Petruzziello A, Marigliano S, Loquercio G, et al. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22(34):7824–40. doi: 10.3748/wjg.v22.i34.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sise ME, Strohbehn IA, Chute DF, et al. Preemptive treatment with elbasvir and grazoprevir for hepatitis C-viremic donor to uninfected recipient kidney transplantation. Kidney Int Rep. 2020;5(4):459–67. doi: 10.1016/j.ekir.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sise ME, Goldberg DS, Kort JJ, et al. Multicenter Study to Transplant Hepatitis C-Infected Kidneys (MYTHIC): An open-label study of combined glecaprevir and pibrentasvir to treat recipients of transplanted kidneys from deceased donors with hepatitis C virus infection. J Am Soc Nephrol. 2020;31(11):2678–87. doi: 10.1681/ASN.2020050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrault NA, Burton J, Ghobrial M, et al. Prospective multicenter study of early antiviral therapy in liver and kidney transplant recipients of HCV-viremic donors. Hepatology. 2021;73(6):2110–23. doi: 10.1002/hep.31551. [DOI] [PubMed] [Google Scholar]
- 7.Kapila N, Menon KVN, Al-Khalloufi K, et al. Hepatitis C virus NAT-positive solid organ allografts transplanted into hepatitis C virus-negative recipients: A real-world experience. Hepatology. 2020;72(1):32–41. doi: 10.1002/hep.31011. [DOI] [PubMed] [Google Scholar]
- 8.Durand CM, Bowring MG, Brown DM, et al. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients: An open-label nonrandomized trial. Ann Intern Med. 2018;168(8):533–40. doi: 10.7326/M17-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flohr TR, Bonatti H, Hranjec T, et al. Elderly recipients of hepatitis C positive renal allografts can quickly develop liver disease. J Surg Res. 2012;176(2):629–38. doi: 10.1016/j.jss.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckman MH, Woodle ES, Thakar CV, et al. Cost-effectiveness of using kidneys from HCV-viremic donors for transplantation into HCV-uninfected recipients. Am J Kidney Dis. 2020;75(6):857–67. doi: 10.1053/j.ajkd.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Foster GR, Afdhal N, Roberts SK, et al. ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–17. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 12.Feld JJ, Jacobson IM, Hézode C, et al. ASTRAL-1 Investigators. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 13.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangia A, Milligan S, Khalili M, et al. Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts. Liver Int. 2020;40(8):1841–52. doi: 10.1111/liv.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson WE, Everson GT. Sofosbuvir and velpatasvir for the treatment of hepatitis C. Expert Rev Gastroenterol Hepatol. 2017;11(6):501–5. doi: 10.1080/17474124.2017.1326817. [DOI] [PubMed] [Google Scholar]
- 16.Borgia SM, Dearden J, Yoshida EM, et al. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J Hepatol. 2019;71(4):660–65. doi: 10.1016/j.jhep.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Terrault NA, Berenguer M, Strasser SI, et al. International Liver Transplantation Society consensus statement on hepatitis C management in liver transplant recipients. Transplantation. 2017;101(5):956–67. doi: 10.1097/TP.0000000000001704. [DOI] [PubMed] [Google Scholar]
- 18.Gentil MA, González-Corvillo C, Perelló M, et al. Hepatitis C treatment with direct-acting antivirals in kidney transplant: Preliminary results from a multicenter study. Transplant Proc. 2016;48(9):2944–46. doi: 10.1016/j.transproceed.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Chan C, Schiano T, Agudelo E, et al. Immune-mediated graft dysfunction in liver transplant recipients with hepatitis C virus treated with direct-acting antiviral therapy. Am J Transplant. 2018;18(10):2506–12. doi: 10.1111/ajt.15053. [DOI] [PubMed] [Google Scholar]


