Abstract
We report the first case of fungemia due to Candida catenulata, a contaminant of dairy products. C. catenulata was isolated from three blood cultures of a patient with gastric cancer. The patient failed to respond to fluconazole but recovered after treatment was switched to amphotericin B. In vitro, C. catenulata was susceptible to amphotericin B and itraconazole and was also susceptible to fluconazole in dose-dependent manner. The likely portal of entry was the digestive tract, as the patient often ate cheese and had multiple gastric ulcerations.
CASE REPORT
Candida catenulata (formerly also named C. ravautii and C. brumptii) is considered a natural contaminant of dairy products and has not yet been associated with invasive infection in humans (13, 14). We describe a case of C. catenulata fungemia in a patient with metastatic gastric carcinoma and discuss the origin of the infection.
A 42-year-old woman was referred to Hôpital de Hautepierre, Strasbourg, France, on 6 May 1997 with fatigue, weight loss, back and abdominal pain, melena, and mild fever (38 to 38.5°C). The first symptoms had appeared 4 weeks earlier. Results of a physical examination were normal apart from showing hepatomegaly. Laboratory investigations showed severe pancytopenia (hemoglobin, 3.3 g/dl; platelets, 46,000/μl; leukocytes, 3,470/μl; and neutrophils, 940/μl) and mildly increased liver enzyme activities (glutamyl transferase, alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase). Renal function was normal, but levels of the C-reactive protein were markedly increased (111 mg/liter). Gastroscopy showed superficial gastric ulceration and diffuse infiltration of the fundus. Gastric biopsy confirmed the diagnosis of linitis plastica. Staging showed massive bone marrow involvement and vertebral metastasis, but no evidence of hepatic metastasis was found on the computerized tomography scan. Combination chemotherapy with doxorubicin, 5-fluorouracil, and cisplatin was started.
On 12 May 1997, in spite of broad-spectrum antibiotic therapy combining piperacillin-tazobactam and netilmicin given for 5 days, the patient developed a high fever (up to 40°C) without chills. Blood drawn for fungal and bacterial culture, on the same day and on the following two days, still yielded negative results. Three separate blood samples cultured on 15 May 1997 yielded a yeast later identified as C. catenulata. The patient was not neutropenic (neutrophil count, 1,990/μl) when the latter samples were drawn, and she had only a peripheral venous line. She stated that she often ate various cheeses.
Treatment with intravenous fluconazole (400 mg/day) was started on 17 May 1997. Further blood cultures were negative, but the patient remained febrile. Fluconazole was replaced with amphotericin B (1.1 mg/kg of body weight/day) on day 6 of therapy. A central venous line was inserted for amphotericin B administration. The patient’s temperature slowly normalized, and she was discharged after 10 days of amphotericin B therapy. She continued to take fluconazole orally (100 mg/day). Culture of the catheter gave negative results.
The patient was again admitted 4 days later with a new episode of fever. Fluconazole was again replaced with amphotericin B, and broad-spectrum antibiotic therapy including vancomycin was added. Attempts to microbiologically document this new episode were unfruitful. In particular, a search for a deep-seated fungal infection (including funduscopy and echocardiography) was unsuccessful. Apyrexia was obtained after 9 days, and the antimicrobial regimen was continued unchanged for a total of 20 days. A second course of chemotherapy was given during the same hospital stay. The patient was discharged in late June and readmitted in July with rapidly fatal brain metastasis.
Mycological findings.
Three blood samples cultured on the same day yielded a yeast after 48 h of incubation at 36°C in the Bactec NR860 system (Becton Dickinson, New York, N.Y.). The organism was identified as C. catenulata by using the ID 32C strip (BioMerieux, Marcy-l’Etoile, France). Each isolate was subcultured on Sabouraud’s glucose agar at 27°C for 48 h to further study the morphology of the colonies, which were soft, slightly wrinkled, and whitish to creamy.
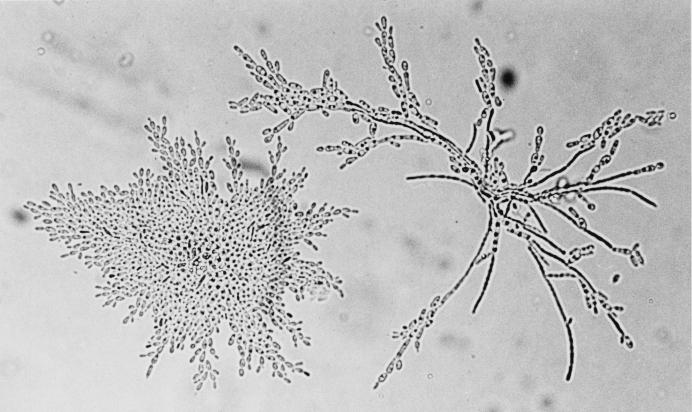
Microscopic examination of subcultures on PCB medium (potato, carrot, and bile) after incubation at 27°C for 48 h showed a well-developed pseudomycelium with ramified chains of often-curved pseudohyphae and elongated or cylindrical cells measuring 1 to 2 by 5 to 7 μm (Fig. 1). The yeast assimilated galactose and trehalose but not cellobiose, raffinose, or inositol. It was resistant to 0.01% cycloheximide and did not grow at 40°C. All these characteristics were consistent with its identification as C. catenulata (16).
FIG. 1.
C. catenulata from a subculture on PCB medium. Magnification, ×600. Well-developed curved pseudohyphae and elongated cylindrical cells are shown.
Susceptibility and MICs were determined by the standard Fungitotal micromethod (International Mycoplasma, Signes, France). The isolate was susceptible to amphotericin B (MIC < 1 μg/ml), flucytosine (MIC < 8 μg/ml), and miconazole, econazole, tioconazole, and ketoconazole (MIC < 1 μg/ml). Susceptibilities to fluconazole and itraconazole were evaluated by using the E-test method (BMD Diagnostics, Marne la Vallée, France). The strain was susceptible to itraconazole (MIC = 0.25 μg/ml) and susceptible in a dose-dependent manner (intermediate) to fluconazole (MIC = 16 μg/ml).
Discussion.
Fungal infections are increasingly frequent in immunocompromised patients and have become a common cause of death in hospital oncology and hematology departments. Candida albicans is still the most frequently encountered fungal pathogen in cancer patients, but the range of causative agents is expanding (3). C. catenulata was first isolated from human feces in Puerto Rico (16). DNA recombination studies subsequently showed that C. catenulata, C. brumptii, and C. ravautii were the same species (10).
Most C. catenulata isolates have been recovered from bovine mammary secretions, milk, Camembert and blue-veined cheeses, and the vaginas of cows, sheep, and pigs (2, 5, 9–11, 13, 14).
Together with Debaryomyces hansenii, Candida lipolytica, Candida kefyr and Candida famata, C. catenulata is a predominant yeast in Australian Camembert cheese (13). These yeasts are natural contaminants in the cheese-making process; more than 106 C. catenulata CFU per g were found in half the samples of a brand of Australian Camembert. C. catenulata has also been isolated in similar amounts from Australian blue-veined cheeses, with the highest counts found in the outer layers. C. catenulata has not been found in European Camembert but has been isolated from other European cheeses (12, 13, 17).
Since the organism lacks the ability to ferment lactose, C. catenulata growth in milk may be explained by its strong proteolytic and lipolytic activities (14). At 25°C, the maximum density in milk (107 to 108 CFU/ml) is reached after 2 to 3 days. The growth rate and proteolytic and lipolytic activities are reduced but not abrogated by lowering the temperature to 10°C and by adding NaCl.
C. catenulata has been linked to bovine mastitis (1, 6, 11). Richard et al. collected 91 yeast strains from infected bovine mammary glands (11) and found that Candida tropicalis, Candida rugosa, and Candida krusei together accounted for more than 60% of the isolates. C. catenulata and C. brumptii, which the authors considered different species, ranked fourth, together representing 4% of isolates.
Intravenous injection of C. catenulata isolates from bovine mastitis secretions into steroid-immunosuppressed mice has been found to produce no visceral lesions at autopsy, even with an inoculum as high as 107 CFU per animal (6).
C. catenulata can occasionally cause superficial infections in humans. In a review of skin diseases in 68 renal transplant patients, Kopsa et al. found 16 cases of dermatomycosis (8). C. catenulata was isolated from one of these patients and was considered responsible for the skin lesions. No details of the aspect of the lesion or treatment were given.
Crozier and Coats reported a case of onychomycosis due to C. ravautii in a 50-year-old Australian man (4). The yeast was isolated from both big toenails on three separate occasions. The only clinical manifestation was darkish grey discoloration of the distal portion of the nails. The patient said he had had the condition for about 5 years and had no predisposing conditions. The isolate did not possess keratinolytic properties. The authors did not state whether treatment was given.
C. catenulata has also been linked to fungal vaginitis and gastric colonization after surgery (7, 15). Investigating the epidemiology of fungal vaginitis, Knippenberger et al. found that 3% of fungal isolates were C. catenulata (7). Ström and coworkers reported 17 cases of massive fungal overgrowth in the stomach after gastric surgery (15) and isolated 20 different strains of Candida (13 C. albicans, 5 C. glabrata, 1 C. pseudotropicalis, and 1 C. catenulata strain). While most patients with C. albicans or C. glabrata overgrowth complained of bloating, epigastric pain, nausea, vomiting, or weight loss, the patient from whom C. catenulata was isolated was asymptomatic. No details of therapy or outcome were given by the authors.
In conclusion, we describe the first case of invasive C. catenulata infection. The patient had several potentially predisposing factors, including gastric carcinoma, immunosuppressive therapy, and broad-spectrum antibiotic therapy. The likely source of infection was cheese, and the likely portal of entry was the digestive tract. Treatment with intravenous amphotericin B was successful after fluconazole had failed.
REFERENCES
- 1.Aalbaek B, Stenderup J, Jensen H E, Valbak J, Nylin B, Huda A. Mycotic and algal bovine mastitis in Denmark. Acta Pathol Microbiol Immunol Scand. 1994;102:451–456. doi: 10.1111/j.1699-0463.1994.tb04898.x. [DOI] [PubMed] [Google Scholar]
- 2.Aller Gandeco J M. Incidence of yeasts in the vagina of cows and sheep, their experimental pathogenicity to different antimycotics. An Fac Vet Leon. 1976;22:375–428. [Google Scholar]
- 3.Chabasse D. New opportunistic fungi appearing in medical pathology. J Mycol Med. 1994;4:9–28. [Google Scholar]
- 4.Crozier W J, Coats H. A case of onychomycosis due to Candida ravautii. Aust J Dermatol. 1977;18:139–140. doi: 10.1111/j.1440-0960.1977.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 5.Hajsig M, Nalevski M, Herak M. Yeasts in the vagina of healthy swine. Vet Arh. 1983;53:51–58. [Google Scholar]
- 6.Jensen H E, Aalbaeck B. Pathogenicity of yeasts and algae isolated from bovine mastitis secretions in a murine model. Mycoses. 1994;37:101–107. doi: 10.1111/j.1439-0507.1994.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 7.Knippenberger H, Vanselow H, Barth H. Untersuchung zur Sprosspilzepidemiologie an 1000 Patientinnen. Geburtsh Frauenheilkd. 1979;39:676–681. [PubMed] [Google Scholar]
- 8.Kopsa H, Zazgornik J, Schmidt P, Thurner J. Long-term observation of fungal and other skin disorders after renal transplantation. Med Klin. 1977;72:1447–1450. [PubMed] [Google Scholar]
- 9.McDonald J L, Richard J L, Anderson A J, Fichtner R E. In vitro antimycotic sensitivity of yeasts isolated from infected bovine mammary glands. Am J Vet Res. 1980;41:1987–1990. [PubMed] [Google Scholar]
- 10.Meyer S A, Ahearn D G, Yarrow D. Candida catenulata Diddens et Lodder. In: Kreger-van Rij N J W, editor. The yeasts. A taxonomic study. 3rd ed. Amsterdam, The Netherlands: Elsevier Science Publishers; 1984. pp. 648–650. [Google Scholar]
- 11.Richard J L, McDonald J S, Fichtner R E, Anderson A J. Identification of yeasts from infected bovine mammary glands and their experimental infectivity in cattle. Am J Vet Res. 1980;41:1991–1994. [PubMed] [Google Scholar]
- 12.Rohm H, Eliskases-Lechner F, Bräuer M. Diversity of yeasts in selected dairy products. J Appl Bacteriol. 1992;72:370–376. [Google Scholar]
- 13.Roostita R, Fleet G H. The occurrence and growth of yeasts in Camembert and blue-veined cheeses. Int J Food Microbiol. 1996;28:393–404. doi: 10.1016/0168-1605(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 14.Roostita R, Fleet G H. Growth of yeasts in milk and associated changes to milk composition. Int J Food Microbiol. 1996;31:205–219. doi: 10.1016/0168-1605(96)00999-3. [DOI] [PubMed] [Google Scholar]
- 15.Ström B G, Beaudry R, Morin F. Yeast overgrowth in operated stomachs. J Can Assoc Radiol. 1978;29:161–164. [PubMed] [Google Scholar]
- 16.Van Uden N, Buckley H. Candida catenulata Diddens et Lodder. In: Lodder J, editor. The yeasts. A taxonomic study. 2nd ed. Amsterdam, The Netherlands: North-Holland Publishing Company; 1971. pp. 937–939. [Google Scholar]
- 17.Welthagen J J, Viljoen B C. Yeast profile in Gouda cheese during processing and ripening. Int J Food Microbiol. 1998;41:185–194. doi: 10.1016/s0168-1605(98)00042-7. [DOI] [PubMed] [Google Scholar]