Abstract
Siglec-8, an immune-inhibitory sialoglycan binding lectin (S8), is expressed on the surface of eosinophils and mast cells, which are potent mediators of allergic inflammation. When S8 engages endogenous sialoglycan ligands, eosinophils undergo apoptosis and mast cell mediator release is inhibited. In the human airway, Siglec-8 ligands (S8L) are sialylated keratan sulfate chains carried on isoforms of the protein Deleted in Malignant Brain Tumors-1 (DMBT1), an immunoregulatory protein that we recently identified as the endogenous ligand for S8, DMBT1S8. We herein report that S8L is overexpressed in chronic rhinosinusitis with nasal polyposis (CRSwNP), a prevalent eosinophilic laden airway disease. Quantification and comparison of the degree to which DMBT1 carries the S8L by immunoblot analysis and lectin blot overlay, respectively, from nasal lavage showed that the S8L/DMBT1 ratio was significantly increased in CRSwNP vs. control or CRS patients. We identified the histological sites of S8L and DMBT1 expression in fresh surgically resected human nasal polyps. Histochemistry of diseased polyps and adjacent nondiseased middle turbinate (MT) tissue from CRSwNP demonstrated colocalization of S8L and DMBT1 with highest staining in submucosal glands >> epithelium > stoma. S8L expression was specifically elevated in the submucosal glands and epithelium of polyp tissue compared to MT. We hypothesize that expression of the isoform of DMBT1 carrying the Siglec-8 binding sialoglycan, DMBT1S8, is induced in polyps of CRSwNP specifically at the site of disease, is produced in the submucosal glands of polyps and secreted into the lumen of the sinonasal cavity as a host response to mitigate eosinophil-mediated inflammation.
Keywords: chronic rhinosinusitis with nasal polyposis, deleted in malignant brain tumors-1, Siglec-8 ligand, eosinophilic nasal polyps, Type 2 inflammation
Introduction
Chronic rhinosinusitis (CRS) is the second most common chronic condition in the United States (Chaaban et al. 2013). CRS with nasal polyposis (CRSwNP), the most severe subset form of CRS characterized by tissue and peripheral eosinophilia, with 4% prevalence or 13 million individuals in the United States, incurs the majority of the health care cost. Despite the significant morbidity of disease, central mechanisms regarding the pathogenesis of eosinophilic CRSwNP remain unclear. Eosinophilic CRSwNP is characterized by edematous outpouchings of sinus and nasal wall mucosa into the nasal cavity which display expression of Type 2 inflammatory mediators IL-4, IL-5, IL-13 (Tan et al. 2013; Shah et al. 2016). These responses are thought to recruit eosinophils, promote local IgE production and goblet hyperplasia. Thus inflammatory cascade with resultant intense eosinophilic infiltration has become a hallmark of the Type 2 inflammation seen in polyp tissue. Presently, there is no effective lasting medical or surgical treatment (Wynn and Har-El 2004). A new era of monoclonal antibodies directed against Type 2 mediators IL5, IL5 receptor, IL4/IL13 receptor and IgE now offer new treatment options for CRSwNP with purportedly low side effect profiles (Casale 2017; Rivero and Liang 2017; Tsetsos et al. 2018; Kartush et al. 2019). However, in each of these clinical trials, there still remains a significant group of CRSwNP subjects that did not respond, suggesting endotype variability. Thus, even with demonstration of efficacy in control of cytokine driven inflammation, the variability in response in CRSwNP suggests that additional mechanisms of eosinophil survival and function are involved that require further investigation. We herein examine the role of S8L as an immunoinhibitory proteoglycan that is induced as a response to unrelenting eosinophilic inflammation that is the hallmark of CRS with nasal polyp disease.
In the first genetic array analysis of human nasal polyp tissue reported, we previously showed that expression of deleted in malignant brain tumors-1 (DMBT1, also known as SALSA, GP340 or SAG) was the third most highly expressed gene. DMBT1 was induced by 30-fold and expressed predominantly in the submucosal glands of polyps (Liu et al. 2004). The significance of this finding and its relation to the eosinophilic infiltration observed in polyp tissue was unclear. Eosinophils are known to express an eosinophil-specific death cell receptor, Siglec-8 (S8) (Floyd et al. 2000; Bochner 2009; Kiwamoto et al. 2012). S8 crosslinking with specific antibodies rapidly induces caspase 3-like activity and induction of eosinophil apoptosis. (Nutku et al. 2003). S8 is a member of the family of sialic acid-binding immunoglobulin-like lectins, many of which mediate immune inhibition upon engaging their extracellular sialoglycan ligands. Gonzalez-Gil et al. demonstrated that sialylated keratan sulfate chains are necessary structural determinants for binding the S8, and that these were expressed in human post mortem trachea and submucosal glands of inferior turbinates (Jia et al. 2015b; Gonzalez-Gil et al. 2018). However, the identity of the endogenous natural human airway ligand for S8 remained unidentified. In our recent studies, we found that the endogenous human airway natural ligand (S8L) is a high molecular weight O-linked sialoglycoprotein (Gonzalez-Gil et al. 2020). This structure was identified as a heavily O-glycosylated keratan sulfate proteoglycan with a Neu5Acα2-3 (6-sulfo)-Gal terminus that was recovered from human nasal secretions. Purification and mass spectrometric proteomic analysis of nasal lavage aspirates revealed that DMBT1 is the predominant protein carrier of S8L in human airways (Gonzalez-Gil et al. 2020). Given that our independent line of investigation demonstrated that DMBT1 was elevated in CRSwNP polyp tissue (Liu et al. 2004), we sought in determine the clinical significance of S8L in CRSwNP by: (1) determining whether the eosinophilic inflammation observed in CRSwNP results in induction of expression of the endogenous ligand for the S8 eosinophil- (and mast cell-) specific death cell receptor; (2) examining whether sinonasal tissues express DMBT1 in conjunction with the endogenous S8L and (3) comparing the cellular distribution and expression pattern of S8L and DMBT1 in human nasal polyp and nonpolypoid middle turbinate (MT) mucosal tissue.
Results
Patient demographics
Table I shows the demographic and clinical characteristics of the 55 study subject cohorts who underwent functional endoscopic sinus surgery for medically indicated reasons. Patients were designated phenotype: CRSwNP (n = 30), CRS (n = 9) and control (n = 16) according to guidelines (Benninger 2007; Bhattacharyya and Lee 2010; Fokkens et al. 2012c; Steinke and Borish 2016; Khan et al. 2019). The most common comorbidities were asthma (40% overall, 57% of CRSwNP group), allergic fungal sinusitis (9% overall, 13% of CRSwNP group), allergic bronchopulmonary aspergillosis (9% overall, 13% of CRSwNP group) and AERD (4% overall, 7% of CRSwNP). Patients with CRSwNP had significantly elevated peripheral blood absolute eosinophil count, with CRSwNP > CRS > Control (P < 0.01 between groups) and elevated serum IgE levels, with CRSwNP ~ CRS > Control (P < 0.01 vs. Control group). Of the entire cohort, all but 17 patients (n = 33) were exposed to topical nasal, inhaled or oral steroids for CRSwNP, asthma or COPD (fluticasone, budesonide or beclomethasone). Nineteen of 30 CRSwNP patients were exposed to systemic steroids in the form of oral steroid courses of methylprednisolone (ranging from 5 to 10 mg) (n = 7) or inhaled steroids (n = 13) at the time of surgery. One control subject with COPD (2% overall, 6% of control group) was being treated with inhaled glucocorticoid. Three patients were on immunomodulatory drugs at the time of surgery (see Table I).
Table I.
Patient subject demographics, clinical laboratory characteristics, glucocorticoid and immunomodulatory biologic medication exposure of CRSwNP, CRS and control subjects
| CRSwNP | CRS | Control | Total | |
|---|---|---|---|---|
| Number of patients (% of total) | 30 (51) | 9 (18.2) | 16 (31) | 55 (100) |
| Female (% of phenotype) | 17 (57) | 4 (50) | 7 (41) | 28 (51) |
| Male (% of phenotype) | 13 (43) | 5 (50) | 9 (59) | 27 (49) |
| Median age, years (Interquartile range) | 46 (34–55) | 55 (39–64) | 41 (34–48) | 46 (33–57) |
| Comorbidities (% of phenotype) | ||||
| Asthma | 17 (57) | 2 (22) | 3 (19) | 22 (40) |
| ABPA | 4 (13) | 1 (11) | 0 | 5 (9) |
| AERD | 2 (7) | 0 | 0 | 2 (4) |
| AFS | 4 (13) | 1 (11) | 0 | 5 (9) |
| COPD | 0 | 0 | 2 (13) | 2 (4) |
| Absolute blood eosinophil per mm3 +/– SEM | 450 + 60 | 295 + 53 | 184 + 49 | |
| Serum IgE IU/mL +/– SEM | 474 + 121 | 496 + 216 | 44 + 15 | |
| Medication (% of phenotype) | ||||
| No steroid | 4 (13) | 4 (44) | 9 (56) | 17 (31) |
| Intranasal steroid only | 7 (23) | 5 (56) | 6 (38) | 18 (33) |
| Systemic steroids: inhaled or oral +/– intranasal | 19 (63) | 0 | 1 (6) | 20 (36) |
| Inhaled only | 6 (20) | 0 | 1 (6) | 7 (13) |
| Inhaled + intranasal | 6 (20) | 0 | 0 | 6 (11) |
| Inhaled + oral | 3 (10) | 0 | 0 | 3 (5) |
| Intranasal + oral | 1 (3) | 0 | 0 | 1 (2) |
| Intranasal + inhaled + oral | 3 (10) | 0 | 0 | 3 (5) |
| Systemic immunomodulators mepolizumab mycophenolate mofetil infliximab | 2 (7) 1 (4) 1 (4) | 0 | 1 (6) 1 (6) | 3 (5) 1 (2) 1 (2) 1 (2) |
Expression of S8L and DMBT1 in human nasal lavage aspirates from CRSwNP, CRS and control subjects
To determine the clinical significance of S8L in upper airways as it relates to CRSwNP, we compared expression of S8L and DMBT1 from nasal lavage from patients with CRSwNP, CRS and control patients using immunofluorescent analysis. The method of nasal secretion sampling by analysis of nasal lavage aspirates was chosen over nasal absorption sampling using synthetic sponges due to concerns of difficulty and inconsistency in elution of the large size of the high molecular weight O-linked sialoglycoprotein structure that was confirmed for the natural ligand for S8 (Gonzalez-Gil et al. 2020). In addition, the method of nasal absorption sampling using sponges has not been validated for large molecular weight structures in the range of 1 million Daltons. Western immunoblot shown in Figure 1A using previously validated antibodies, shows that S8L migrates at a high molecular weight of ~1 million Daltons, consistent with the structure of a large proteoglycan as predicted by previous work of Gonzalez-Gil et al. (2020). Nasal lavage expressed a single major band at approximately 1 million Daltons (arrow shows molecular weight marker 900 kDa) in all 55 samples screened with variability in levels of expression between samples. Staining for DMBT1 (Figure 1A panel 1) shows that the pattern of the staining of that major band mimics that of S8L staining (Figure 1A panel 2). The green signal for DMBT1 and red signal for S8L comigrate to identical fluorescent bands at ~1 MDa, consistent with the conclusion that S8L is carried on the protein backbone of DMBT1, as reported in Gonzalez-Gil et al. (2020).
Fig. 1.
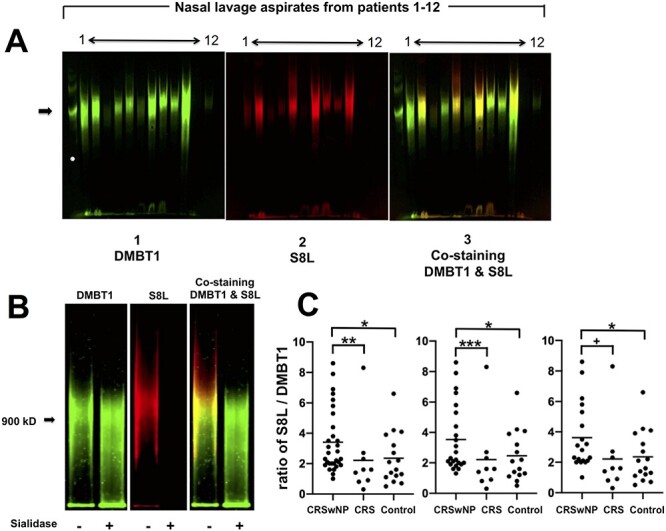
(A) DMBT1 and S8L expression in human nasal lavage aspirates from CRSwNP, CRS and control subjects. Representative immunoblot of nasal lavage samples from 12 patient subjects was double labeled for DMBT1 (left: IRDye 680RD donkey anti-rabbit IgG) and S8L (center: IRDye 800CW donkey anti-goat IgG) and signals overlaid (right). Arrow shows migration of molecular weight marker at 900,000 Daltons. (B) Sialidase sensitivity of S8L, but not DMBT1, in human nasal lavage from CRSwNP subject. Immunoblot was double labeled for DMBT1 (left) and S8L (center) and signals overlaid (right) in the absence (–) and presence (+) of V. cholera sialidase (100 mU/mL as described in Methods). Arrow = 900 kDa. (C) Induction of S8L expression in CRSwNP. Right panel shows staining intensity of bands from immunoblot analysis of nasal lavage aspirates compared from CRSwNP (n = 30), CRS (n = 9) and control (n = 16). *P < 0.05 CRSwNP vs. control, **P < 0.02 CRSwNP vs. CRS by Kruskall–Wallis test. Middle panel shows subgroup analysis after removal of subjects with oral steroid or immunomodulatory drugs from CRSwNP (n = 23), CRS (n = 9) and control (n = 15) subjects. *P < 0.05 CRSwNP vs. control, ***P < 0.03 CRSwNP vs. CRS by Kruskall–Wallis test. Left panel shows subgroup analysis of CRSwNP subjects specifically exposed to oral and/or inhaled steroids (n = 19), CRS (n = 9) and control (n = 16) subjects. *P < 0.05 CRSwNP vs. control, +P < 0.01 CRSwNP vs. CRS by Kruskall–Wallis test.
To further validate these findings, we enzymatically characterized S8L in nasal lavage by testing the sialidase sensitivity of S8L. By definition, S8L is a glycosylated sialic acid containing moiety and thus is known to be sialidase sensitive (Moustafa et al. 2004). Since the sialic acid residue is located specifically on the S8 binding determinant of S8L, and not on the DMBT1 carrier protein of the natural ligand, we predicted that sialidase pretreatment of nasal lavage aspirates would abolish S8L staining, but have no effect on DMBT1 staining. Figure 1B shows nasal lavage aspirate from a CRSwNP subject express DMBT1 (left panel (green (-)) and S8L (center panel, red (-)), which comigrates with significant signal overlay (right panel, yellow (-)), at ~1 million Da, consistent with the structure of a large proteoglycan as predicted by our recent publication (Gonzalez-Gil et al. 2020). Pretreatment with sialidase results in preservation of DMBT1 (left panel, green (+), elimination of S8L (center panel, red (+)) and lack of overlay and remaining DMBT1 staining (right panel, green (+)), confirming sialidase sensitivity of the natural ligand that is present in human nasal lavage aspirates. As detailed here and in our recent publication (Gonzalez-Gil et al. 2020), DMBT1 protein, per se, is not the endogenous bioactive S8 ligand. Instead, an isoform of DMBT1 that is post-translationally decorated with an array of specific sialylated keratan sulfate chains will convert the DMBT1 protein carrier to a functional bioactive S8 ligand (DMBT1S8). The degree to which DMBT1 is converted to DMBT1S8 during biosynthesis is expected to enhance its anti-inflammatory activity.
Total levels of S8L and DMBT1 in nasal lavage are subject to variation based on airway accessibility and lavage logistics. Due to inherent variability between patients’ sinonasal anatomy, quantitative comparison of S8L and DMBT1 expressed as a concentration per volume recovered does not take into consideration the variability of total surface area of each individual patient’s sinonasal cavity that is sampled by lavage technique. However, the ratio of S8L to DMBT1 is an indication of its anti-inflammatory potency. That is, the conversion of the precursor molecule DMBT1 to its biologically active endogenous ligand for S8, DMBT1S8 is a reflection of the expression of the anti-inflammatory activity within the tissues. To examine whether the relative levels of S8L on DMBT1 vary according to clinical phenotype we compared the levels of their expression in nasal lavage aspirates from three of our patient cohorts: CRSwNP, CRS and control. DMBT and S8L staining intensity was independently quantitated. Figure 1C shows that CRSwNP subjects express significantly increased ratio of S8L / DMBT1 (3.4 +/– 0.4), as compared to CRS (2.2 +/– 0.8, P < 0.02) or control subjects (2.4 +/– 0.4, P < 0.05), suggesting induction of S8L expression in CRSwNP patients. Not surprisingly, comparison of absolute concentration values of S8L and DMBT1 showed no difference between groups (data not shown),
To address potential confounding variables, subgroup analysis was performed after removing the subjects who had concurrent systemic oral steroid or immunomodulatory medication exposure. Analysis shows that the pattern of induction was unchanged (Table I and Figure 1C center panel, CRSwNP vs. CRS or (P < 0.03) CRSwNP vs. control (P < 0.05)). Inhaled glucocorticoids, similar to oral but unlike topical nasal steroids, are known to have broad systemic anti-inflammatory effects which may confound results (Barnes 1998, 2011; Dahl 2006; Oakley and Cidlowski 2013). Therefore, further subgroup analysis of CRSwNP subjects who were specifically exposed to inhaled and/or oral steroids was performed. As shown in Figure 1C right panel, comparison of systemic steroid exposed CRSwNP subjects to CRS (P < 0.01) or CRSwNP to control subjects (P < 0.05) showed that there was no apparent effect of systemic inhaled or oral steroid exposure on the ratio of S8L to DMBT1 in our limited cohort of steroid exposed CRSwNP patients.
To examine whether the finding of elevated ratio of S8L to DMBT1 in nasal lavage may serve as a biomarker for eosinophilic nasal polyp disease, we analyzed the correlation between ratio of S8L to DMBT1 in nasal lavage and peripheral blood eosinophil levels the in our cohort of patient subjects. Analysis of spearman rank correlation coefficients demonstrated lack of correlation for both CRSwNP subjects [r = 0.16, P = 0.42, n = 28] and for entire study cohort [r = 0.048, P = 0.84, n = 55], indicating that the inflammation observed at the precise site of disease by this specific mediator (ratio of S8L to DMBT1) is not reflected by a peripheral blood marker of Type 2 inflammatory disease.
Distribution of DMBT1 and S8L in nasal polyp and middle turbinate tissue from CRSwNP subjects
To determine which cellular compartments of polyp tissue were expressing the natural ligand for Siglec 8, we performed immunohistochemical analysis of DMBT1 and S8L. Consistent with our previously published findings (Liu et al. 2004), Figure 2A, panel 1 shows that DMBT1 is strongly expressed in the submucosal glands, as compared to the epithelium or stroma. Background staining without anti-DMBT1 primary antibody shows absence of nonspecific staining (see inset Figure 2A panel 1). Notably, as shown by this representative specimen, the distribution of the submucosal glands is not equally dispersed throughout the whole polyp. The submucosal glands appeared to aggregate at the proximal segment and near skull base attachment of the polyps and at right side surface (see arrows). We observed consistent lack of architectural homogeneity in the distribution of the submucosal glands, microvasculature and edematous stroma in whole polyp specimens (n = 6), representing the distortion of these structures by the tissue edema characteristic of nasal polyps. To examine whether DMBT-1 and S8L colocalize to the same cellular structures in nasal polyps, we performed double label immunofluorochemical staining for DMBT1 and S8L on adjacent whole polyp tissue section. Figure 2A panels 2, 3 and 4 show that DMBT1 (panel 2) and S8L (panel 3) colocalize to the submucosal glands (panel 4), with less staining of the polyp epithelium.
Fig. 2.
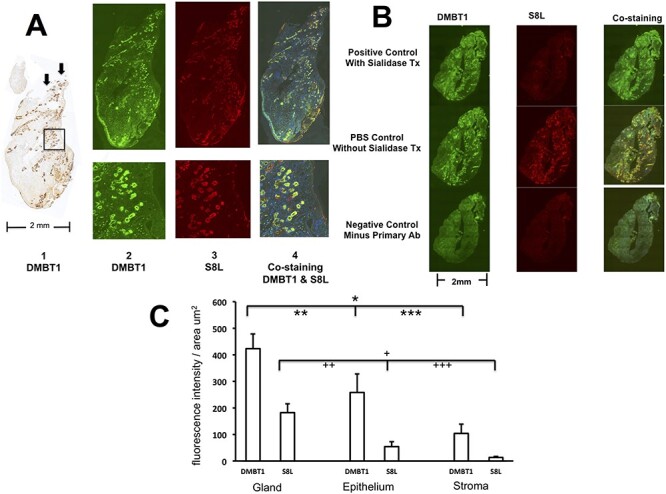
(A) Colocalization of DMBT1 and S8L in nasal polyp tissue. Tissue was surgically excised en bloc and stained for DMBT1 by immunohistochemistry and DMBT1 and S8L double label immunofluorescence using adjacent serial tissue sections as described in methods. Panel 1 left shows DMBT1 staining using DAB as substrate. Upper left inset shows negative control background control staining without anti-DMBT1 primary ab. Arrows indicate the cut edge proximal attachment to the skull base. Panels 2–4 were double labeled for DMBT1 using Alexa fluor green (488 nm) and S8L using Alexa fluor red (598 nm) tagged secondary ab, and visualized showing overlay of both 488 and 598 nm filters, respectively. Bottom panels show detailed views of area illustrated by square with 10 X magnification inset (200 microns width of inset). (B) S8L in nasal polyp tissue is sialidase sensitive. Double label immunofluoroscopy of DMBT1 (left) and S8L (center) from CRSwNP subject was performed using Alexa fluor green (488 nm filter) and Alexa fluor red (598 nm filter) tagged secondary abs, respectively, and visualized showing overlay (right, both filters) in the presence of sialidase, 100 mU/mL using primary antibody anti-DMBT1 and S8-Fc (upper panels positive control), in the absence of sialidase (center panels PBS control) and in the absence of anti-DMBT1 and S8-Fc (lower panels) as described in Methods. (C) Distribution of DMBT1 and S8L in gland, epithelium and stromal compartments of nasal polyp tissue (n = 6). *P < 0.001 DMBT1 in glands vs. stroma, **P < 0.005 DMBT1 in glands vs. epithelium, ***P < 0.01 DMBT1 in epithelium vs. stroma. +P < 0.02 S8L in glands vs. stroma, ++P < 0.04 S8L in glands vs. epithelium, +++P = NS S8L in epithelium vs. stroma by ANOVA with posthoc Bonferroni test.
To further validate these findings, we tested the sialidase sensitivity of S8L of S8-Fc binding on whole polyp tissue. Because the sialic acid residue is located specifically on the S8 binding determinant of S8L, and not on the DMBT1 backbone of the natural ligand, we reasoned that S8L staining should be specifically abolished by sialidase exposure. Figure 2B upper panel shows that pretreatment of adjacent tissue sections with sialidase results in preservation of DMBT1 (green signal, left), significant reduction of S8L (center) and only DMBT1 staining on the overlay (green signal, right), confirming sialidase sensitivity of the S8L. PBS control without sialidase confirms findings seen in Figure 1A: DMBT1 and S8L colocalize to the submucosal glands. Figure 2B lower panel shows negative control staining in the absence of primary immunogens, anti-DMBT1 antibody and S8-Fc, demonstrating absence of nonspecific binding. These results are consistent with previous findings demonstrating that the binding of S8-Fc to human tissue by immunohistochemical analysis in both lung and inferior turbinate is sialidase sensitive (Jia et al. 2015a; Yu et al. 2017).
To identify which cellular compartment of polyp tissue may be responsible for expression of the natural ligand, we performed quantitative analysis using Nikon Eclipse 90i high-resolution microscopy. Figure 2C shows quantitation of the cellular compartment regions of interest (ROI): gland, epithelium and stroma. Both DMBT1 and S8L display parallel patterns of expression within these cellular compartments. Polyp tissue displays the highest staining of DMBT1 in submucosal glands, followed by epithelium, then stroma. Similarly polyps display highest staining of S8L in submucosal glands, followed by epithelium, with negligible staining of stroma.
As a tissue control, we used MT mucosa obtained from the same CRSwNP subject as a matched pair internal control, since there is no analogous structural analog of polyps in control patients. Similar to polyp tissue, the MT tissue shown in Figure 3A panel 1 displays DMBT1 that is strongly expressed in the submucosal glands, as compared to the epithelium or stroma. Double label staining of DMBT1 (Figure 3A panel 2) and S8L (panel 3) show that both markers colocalize to the same cellular compartments (panel 4). In addition, MT tissue displayed the same pattern of sialidase sensitivity as polyp tissue. Figure 3B upper panel shows that pretreatment with sialidase results in preservation of DMBT1 (green signal, left), abolishment of S8L staining (center) and persistence of DMBT1 staining on the overlay (green signal, right), confirming sialidase sensitivity specifically for S8L that is present in the PBS treated, sialidase absent control (Figure 3B center panels). Figure 3B lower panel shows negative control staining in the absence of primary immunogens, anti-DMBT1 antibody and S8-Fc, demonstrating lack of nonspecific binding. Quantitative analysis of staining shown in Figure 3C shows that MT, like polyps, displays highest staining of DMBT1 in submucosal glands, followed by epithelium, then stroma. However, in contrast to matching polyp tissue, S8L expression is decreased in all three cellular compartments in MT, without any preferential cellular compartment distribution. In contrast to diseased nasal polyp tissue, the MT submucosal glands are widely and homogeneously dispersed throughout the entire body of MT, with no preference for proximal skull base versus distal luminal distribution (Figure 3A).
Fig. 3.
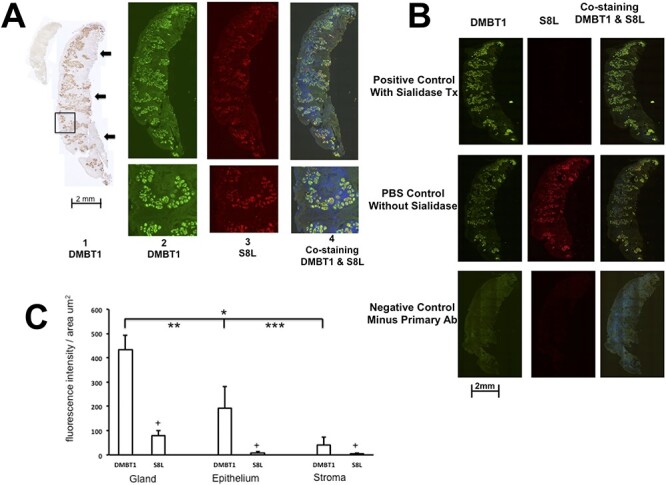
(A) Colocalization of DMBT1 and S8L in MT tissue. Tissue was surgically excised en bloc and stained for DMBT1 by immunohistochemistry and DMBT1 and S8L double label immunofluorescence using adjacent serial tissue sections as described in methods. Panel A1 shows DMBT1 staining using DAB as substrate. Upper left inset shows negative control background control staining without anti-DMBT1 primary ab. Arrows indicate the cut edge proximal attachment to the skull base. Panels 2–4 were double labeled for DMBT1 using Alexa fluor green (488 nm) and S8L using Alexa fluor red (598 nm) tagged secondary ab, and visualized showing overlay using both 488 and 598 nm filters, respectively. Bottom panels show detailed views of area illustrated by square (inset width is 200 microns). (B) S8L in nasal MT tissue is sialidase sensitive. Double label immunofluoroscopy of DMBT1 (left) and S8L (center) from CRSwNP subject was performed using Alexa fluor green (488 nm filter) and Alexa fluor red (598 nm filter) tagged secondary abs, respectively, and visualized showing overlay (right, both filters) in the presence of sialidase,100 mU/mL using primary antibody anti-DMBT1 and S8-Fc (upper panels positive control), in the absence of sialidase (center panel PBS) and in the absence of anti-DMBT1 and S8-Fc (lower panels) as described in Methods. (C) Distribution of DMBT1 and S8L in gland, epithelium and stromal compartments of MT tissue (n = 6). *P < 0.0001 DMBT1 in glands vs. stroma, **P < 0.0001 DMBT1 in glands vs. epithelium, ***P = 0.15 NS DMBT1 in epithelium vs. stroma. +P = NS comparison of S8L in glands vs. stroma, glands vs. epithelium, epithelium vs. stroma by ANOVA with posthoc Bonferroni test.
Next, to examine whether DMBT1 or S8L was induced specifically in the site of disease, the whole polyp tissue, as compared to its nondiseased adjacent nasal mucosa, we compared expression of DMBT1 and S8L in polyp tissue against its matched MT as internal control tissue samples. Figure 4 shows that both polyp and MTs display identical pattern of DMBT1 expression in each of the gland, epithelium or stromal tissue compartments. However, polyps display a significant increase in S8L expression in each of the submucosal gland, epithelium or stromal tissue compartments, as compared to matching MT. This suggests that polyps are the source of elevated S8L expression observed in CRSwNP, leading to increase secretion of S8L detected in nasal lavage aspirates of CRSwNP patients.
Fig. 4.
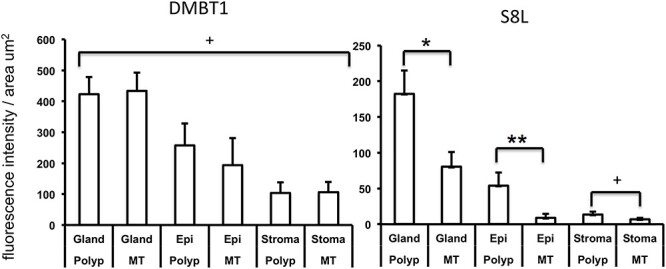
Comparison of expression of DMBT1 (left) and S8L (right) in nasal polyp vs. MT tissue (n = 6). Each CRSwNP subject (n = 6) contributed polyp tissue and matching MT from the same nasal cavity. *P < 0.0001 S8L in polyp vs. MT in glands, **P < 0.05 S8L in polyp vs. MT in epithelium (Epi), +P = NS polyp vs. MT in gland, epithelium, or stroma by ANOVA with posthoc Bonferroni test.
Discussion
Previously, our laboratory identified DMBT1 as one of the top genes to be up regulated in CRSwNP. But the significance of this finding was unclear (Liu et al. 2004). We recently reported purification and biochemical characterization of the endogenous human airway ligand for S8, the eosinophil- (and mast cell-) specific death cell receptor from human nasal lavage aspirates (Gonzalez-Gil et al. 2020). Structural analysis confirmed DMBT1 as the protein carrier for the sialoside ligand that is characterized by terminal epitope containing both sialic acid and sulfate attached to the same galactose residue, designated as S8L. We have extended these findings to examine the significance of S8L in the context of human disease. Our results show that both S8L and DMBT1 comigrate on western immunoblot analysis of nasal lavage aspirate. Both S8L and DMBT1 colocalize to identical histologic compartments in both disease polyp tissue and nondiseased nasal MT tissue. Our results confirm that the S8L detected in both nasal lavage and diseased polyp and nonpolyp nasal tissues demonstrate the key biochemical structure containing the sialic acid residue that confers bioactivity. Detailed quantitative analysis of tissue compartments shows that polyp tissue expresses elevated S8L relative to its nondiseased adjacent MT counterpart from the same patient. Thus as disease transforms nonpolypoid to polypoid mucosa in the sinonasal cavity, expression of the bioactive glycan terminus of the endogenous ligand is increased from the major cellular site of production, the submucosal glands. Thus, S8L appears to be synthesized mostly by the submucosal glands, and subsequently released into the airway lumen, as observed in nasal lavage aspirates. This process appears to be induced in patients with CRSwNP.
In our studies, histologic analysis of nasal polyps consistently showed that the submucosal glands were located near the cut edge, origin along the skull base. The distal tip where the edematous out pouching of polyp tissue occurs was devoid of submucosal gland structural elements. Additionally, the extracellular tissue edema displaces these structures to result in heterogeneity of tissue histology. We noticed that unless the entire polyp from the origin along the skull base to the distal tip was excised, the submucosal glands that were critical to our analysis would have been inconsistently omitted from our studies. This emphasizes the importance of en bloc dissection and orientation of the tissue for analysis, so that the results can be appropriately interpreted in the context of the entire diseased tissue specimen.
The CRSwNP phenotype is characterized by intense eosinophilic infiltration. In this phenotype, we found that both DMBT1 and now report that S8L per DMBT1 is induced in polyp tissue. Thus our findings suggest that S8L, like DMBT1, may serve as a tissue biomarker for eosinophilic inflammation observed polyposis. Subgroup analysis was performed on oral glucocorticoid and biologic naïve patients, and on CRSwNP subjects who were specifically exposed to systemic (oral and inhaled) steroids in order to determine whether these medications served as potential confounding variables. Subgroup analysis shows that the induction of DMBT1 and S8L observed in CRSwNP subjects was unchanged, irrespective of glucocorticoid or immunomodulatory medication exposure. This preliminary subgroup analysis suggests that the local polyp tissue production of the bioactive endogenous ligand for S8 is insensitive to exogenous glucocorticoids. Thus, induction of S8L expression may reflect a host response that is attempting to mitigate and contain the eosinophilic driven inflammation. As to whether this immune response is independent of the effect of glucocorticoids or immunomodulatory medication remains to be determined with further studies that specifically address this question. In addition, the lack of correlation of the bioactive endogenous ligand DMBT1S8 (expressed as the ratio of S8L to DMBT1) to peripheral blood eosinophil levels indicate that the inflammation observed in the precise site of disease is not reflected by a phenotypically relevant peripheral blood laboratory marker in the systemic circulation. This implies that there are multiple complex mechanistic steps between expression of peripheral blood eosinophilia, homing of eosinophils to site of disease and expression of eosinophils in polyp tissue that require further detailed studies of the human polyp tissue itself. This also highlights the complexities and challenges in the identification of clinically feasible biomarkers for nasal polyposis.
DMBT1, itself, is a glycoprotein that has multiple O-glycosylation sites that confer potential for an extensive array of possible protein–protein ligand binding, and thereby potential for vast diversity of functional roles (Mollenhauer et al. 2000; Madsen et al. 2010). DMBT1 is thought to function in mucosal immunity as a pattern recognition receptor that can bind lactoferrin, IgA, and activate the complement system (Madsen et al. 2010). DMBT1 can also act in an anti-inflammatory manner by inhibiting both NOD2- and TLR4-mediated NF-kB-activation (Rosenstiel et al. 2007). In particular, DMBT1 carries the specific epitope (sialyl Lex) that can potentially bind bacteria, including Streptococcus mutans and Helicobacter pylori (Prakobphol et al. 2000). In addition, DMBT1 is thought to participate in epithelial cell proliferation during inflammation (Madsen et al. 2010). Thus, DMBT1 may have multiple functions relevant to CRSwNP, independent of S8L. The endogenous ligand for S8 on human airway, DMBT1S8, is a large glycoprotein in which the polypeptide DMBT1 is post-translationally modified by a suite of glycosyltransferases and carbohydrate sulfotransferases to create the specific sialoside receptor for S8 which confers function (Gonzalez-Gil et al. 2020). Of the family of Siglec receptors, S8 has one of the most stringent specificity requirements for ligand binding (Kiwamoto et al. 2012). S8 is expressed only in humans and selectively on eosinophils and mast cells (and less so on basophils). S8 has the highest affinity to sialosides that have in common a terminal epitope with both sialic acid and sulfate attached to the same galactose residue (NeuAcα2-3(6S) Galβ1-4(±Fucα1-3)GlcNAc-) (Kiwamoto et al. 2012). This glycosylation pattern dictates its three-dimensional structure and thus determines the specificity of binding (Mollenhauer et al. 2000; Robbe et al. 2005; Purushotham and Deivanayagam 2013; Pröpster et al. 2016). This suggests that expression of the natural ligand for S8 is tightly controlled.
Genetic polymorphisms in S8 receptor are associated with susceptibility to allergic asthma (Gao et al. 2010; Sajay-asbaghi et al. 2020). Although the genetic bases for changes in S8L biosynthesis have not been established, the findings reported here indicate a role for DMBT1S8, the endogenous ligand for S8, in a human disease, CRSwNP. S8 has roles in other eosinophilic and mast cell disorders, including asthma, eosinophilic esophagitis, Churg-Strauss syndrome and hematologic malignancies involving eosinophils and mast cells. Future studies are needed to understand the mechanism of DMBT1S8 and S8 receptor expression in CRSwNP and other eosinophilic diseases. Encouraging results of a phase 2 clinical trial using anti-Siglec 8 antibody therapy in eosinophilic esophagitis and duodenitis has shown efficacy in reducing both gastrointestinal symptoms and tissue eosinophil infiltration (AK002, Allakos Inc.) (Dellon et al. 2020). Given the inherent specificity of DMBT1S8 for its receptor and target tissues, DMBT1S8 and S8 may serve as tissue biomarkers and plausible drug targets in CRSwNP and other eosinophilic and mast cell diseases.
The pathophysiological consequences of increased production of DMBT1S8 at the site of eosinophilic inflammation in polyposis have yet to be determined. We hypothesize that expression of DMBT1S8 is induced in submucosal gland cells of polyps in CRSwNP and secreted into the lumen of the sinonasal cavity as a host response to mitigate eosinophil-mediated inflammation. Whether the disease-associated upregulation we report generates enhanced (although incomplete) control of eosinophils, is overmatched by eosinophil infiltration, or is compromised by fine structure changes in the secreted ligand are questions for future investigations.
Materials and methods
Human subjects and inclusion/exclusion criteria
This prospective cross-sectional study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. The study population was comprised of 55 patients who underwent functional endoscopic sinus surgery between December 2016 and March 2019. The decision to undergo surgery and all clinical interventions including medical treatment, nasal endoscopy, clinical laboratory blood work and sinus CT scan were obtain during the course of clinical care and not part of the study protocol. Demographic data including age, comorbidities, surgical history, medication history, peripheral blood eosinophil and serum IgE laboratory values, were extracted from medical records. Medication exposures including topical, inhaled, systemic steroids or biologic immunomodulatory medications were assessed.
Patients were identified as CRSwNP or CRS without polyps on the basis of historical, endoscopic and radiographic criteria as defined by the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) Chronic Rhinosinusitis Task Force (Benninger et al. 2003b) and the European Rhinologic Society/European Academy of Allergy and Clinical Immunology guidelines (Fokkens et al. 2012a, 2012b). For CRSwNP, additional inclusion criteria included: (1) clinical diagnosis of CRSwNP; and (2) findings of chronic inflammation and eosinophilic infiltration of sinus surgical tissues on pathology report. Normal controls were identified as patients failing to meet criteria for CRS as previously defined (Benninger et al. 2003a; Fokkens et al. 2012a, 2012b), lacking history CRS, nasal endoscopy with no evidence of mucosal inflammation, sinus CT scan Lund Mackay score of zero, who were undergoing endoscopic nasal surgery for nasal obstruction, septal deviation, inferior turbinate hypertrophy or other anatomic reasons. Patients who underwent surgery for sinonasal benign tumor or malignancy were excluded.
Collection of nasal lavage aspirates and surgical sinonasal tissue
Tissue excised and studied using JHU IRB approved protocols included: (1) Polyps from the nasal and sinus cavities; (2) nasal lavage that is routinely performed at the start of surgery to clean the nasal cavity of inspissated mucous, mucopurulence and debride in preparation for the surgical dissection and (3) inferior edge of MT partially excised. In subset of CRSwNP patients, MT tissue was reduced by partial surgical excision for clinically indicated purpose. In those patients, MT reduction provided improved and safer access to the deeper sinus cavities and skull base for complete polyp removal. Nasal lavage was collected according to previously described methods (Lee et al. 2009). With the patient’s head in the recumbent position, 10 mL of saline was instilled into each nasal cavity with a syringe while simultaneously suctioning the irrigation fluid using a straight 8-0 suction and mucous collection trap. The lavage aspirates were spun at 3600 RPM for 15 min and aliquots were frozen at –80°C until analysis. Human nasal and sinus tissue from CRSwNP subjects were obtained during functional endoscopic sinus surgery. Multiple representative sinonasal polyps were removed from the nasal and/or sinus cavities by identifying the most superior skull base origin of the polyp and dissecting the entire polyp en bloc from its proximal origin near the skull base. MT specimens were obtained by excising en bloc the anterior and inferior border. From each CRSwNP subject who donated surgical tissue for study, both a polyp specimen and a matching MT specimen was surgically excised, immediately placed in 4% paraformaldehyde and processed for analysis.
DMBT1 immunoblotting and Siglec-8 lectin blotting of nasal lavage aspirates
The nasal lavage samples from CRSwNP, CRS alone and control patients who underwent functional endoscopic sinus surgery were analyzed for DMBT1 and S8L by immunoblot for DMBT1 or lectin overlay with S8, respectively. Nasal lavage aspirates were immediately spun at 20,000g and the clear supernatant was prepared for loading by mixing equal amount of LDS loading buffer (Nupage) containing 100 mM dithiothreitol and boiling for 10 min at 95°C. Large glycoprotein molecules in each sample were resolved by SDS gel electrophoresis on composite agarose–acrylamide gels (2%: 1.5%) then blotted to polyvinylidene fluoride membranes as previously described (Jia et al. 2015b; Yu et al. 2017). Membranes were blocked with 5% nonfat dry milk dissolved in PBS supplemented with 0.1% Tween-20 (PBST), then incubated with 1% nonfat dry milk in PBST containing 1:1000 of rabbit polyclonal anti-DMBT1 antibody (Aviva Systems Biology, San Diego, CA).
The antibody reagents used in binding to high molecular weight glycoproteins were validated as follows. For detection of DMBT1, we used the Aviva rabbit anti-DMBT1 polypclonal antibody that was previously validated in our studies (Gonzalez-Gil et al. 2020). Our recent publication demonstrates that this antibody binds to a protein with DMBT1 primary sequence that was confirmed by mass spectrometry, demonstrated a molecular weight of 1 million Daltons, and comigrates at the same molecular weight by western immunoblot staining. In addition, this Aviva antibody has been previously shown to bind only to human cells expressing DMBT1 (Bathum Nexoe et al. 2020) and the data product sheet demonstrates specific binding to a single high molecular weight protein in human lung extracts and in tetracycline inducible human cancer cell line that is specifically and only expressed by tetracycline exposure (https://www.avivasysbio.com/sd/tds/html_datasheet.php?sku=ARP42352_P050). For S8L detection, a chimera of the extracellular domain of S8 fused to human Fc (S8-Fc) fragment was expressed by recombinant methods and purified as previously described (Yu et al. 2017). The same S8-Fc fragment was used as the reagent to specifically detect S8L in these studies. S8-Fc (1 μg) and unconjugated goat anti-human IgG, Fc-specific (Millipore Sigma, 0.7 μg) were preincubated for 30 min on ice. After the blot had incubated with anti-DMBT1 antibody for 2 h at ambient temperature, precomplexed S8-Fc was added and blots were incubated an additional 16 h at 4°C. After incubation, the blot was washed three times with PBST, 5 min each, then overlaid with PBST containing both a 1:2000 dilution of IRDye 680RD donkey anti-Rabbit IgG (for DMBT1) and a 1:4000 dilution of IRDye 800CW donkey anti-Goat IgG (for S8L). IRDye secondary antibodies were from LI-COR Biosciences (Lincoln, NE). After 1 h at room temperature blots were washed with PBST three times, 10 min each, then scanned using an Odyssey CLx infrared imager (LI-COR). A large molecular weight marker was custom prepared by crosslinking 1 mg/mL IgM in PBS containing 2.5 mM bis-(sulfosuccinimidyl) suberate and stained with Visio real time stain (Advansta, San Jose, CA). The dyed crosslinked IgM band is visible at 900 kDa. S8L/DMBT1 ratio was quantitated by measuring the intensity signal of ~1 MDa molecular weight band on the 680 channel (DMBT1) and 800 channel (S8L) using Image Studio Lite Version 5.2 (LI-COR Biosciences). The ratio was computed by taking the signal intensity of S8L (800-channel) over the signal intensity of DMBT1 (680-channel).
Enzymatic characterization of S8L immunoblot analysis of nasal lavage was performed by examination of sialidase sensitivity. Aliquots of nasal lavage supernatant were incubated either with PBS (incubated with no enzyme) or with sialidase glycolytic enzyme in PBS containing 100 mU/mL Vibrio cholerae sialidase isolated from Escherichia coli as previously described (Mountney et al. 2010) (Sigma Aldrich). Equal amounts from each reaction were resolved by electrophoresis and blotted, and Siglec-8–Fc binding was determined by lectin overlay as previously described and noted above (Gonzalez-Gil et al. 2020).
Assay of S8L and DMBT1 from nasal lavage was performed as single measurements to obtain the ratio of S8L/DMBT1 for our cohort of patient subjects due to the limited availability of human sample. However, a few samples were run in triplicate by loading different volumes onto the gel. For example, one nasal lavage sample demonstrated S8L/DMBT1 average ratio of 14.09 +/– 1.05 (mean +/– SD of values of 15.37, 14.10, 12.80). Another sample demonstrated S8L/DMBT1 average ratio of 4.82 +/– 1.67 (mean +/– SD of values of 5.47, 5.09, 3.91). These results confirm that the ratio of S8/DMBT1 remained unchanged. This data further validate the analysis and approach since the ratio is independent of volume loaded.
Immunohistochemistry and immunofluorochemistry
Expression of DMBT1 and S8L in human nasal polyp surgical tissue was analyzed by immunohistochemistry and double-label immunofluorochemistry using monoclonal antibody to DMBT1 (HYB 213-06-02, Invitrogen, Carlsbad, CA) and the chimera of extracellular domain of S8 fused to human Fc fragment (Yu et al. 2017). Paraformaldehyde-fixed sinonasal tissue was dehydrated, paraffin embedded and sectioned at 3 μm onto glass slides with a Leica RM2245 Cryostat (Leica Microsystems, Buffalo Grove, Ill). After xylene deparaffinization and rehydration, slides were immersed in Antigen Unmasking Solution (Vector Lab, Burlingame, CA), pH 6.0, at 95°C to 100°C , then blocked with Dako dual enzyme block (Dako, Carpinteria, Calif) and Fc receptor blocker (Innovex, Richmond, Calif). For single label immunohistochemistry, DMBT1 was detected using monoclonal mouse anti-human DMBT1 ab (Invitrogen, Carlsbad, CA) at 1:300 and biotinylated goat anti-mouse secondary ab conjugated to peroxidase (Invitrogen, Carlsbad, CA) with diaminobenzidine substrate (Vector Lab, Burlingame, CA), as previously described (Liu et al. 2004). Adjacent tissue section was identically treated except without primary antibody as negative control. For single label immunohistochemistry of S8L, Siglec-8-Fc (20 μg/mL) was preconjugated to secondary antibody (2 μg/mL of AP-conjugated goat anti-human IgG Fc-specific, 109-055-008, Jackson Immunoresearch, West Grove, PA) in PBS containing 0.1% Triton X-100 (PBST) supplement with 10 mg/mL bovine serum albumin (BSA) for 30 min as previously described (Gonzalez-Gil et al. 2020). Blocked slides were washed in PBST and overlaid with the precomplexed Siglec-8-Fc solution for 2 h. Slides were washed with PBST and equilibrated in 100 mM Tris-HCl pH 8.3 containing 0.1% Tween-20 before detection of bound lectin with Vector Red AP substrate (Vector Laboratories, Burlingame, CA). Slides were dehydrated, mounted in Krystalon (MilliporeSigma, Burlington, MA) and imaged using a Nikon Eclipse 90i microscope (Nikon Instruments, Melville, NY). As indicated, sialidase sensitivity of S8L was performed on adjacent tissue sections as a positive control. Prior to Siglec-8-Fc histochemistry, adjacent tissue sections were incubated for 2.5 h at 37°C with PBS (control) or PBS containing 100 mU/mL V. cholerae sialidase (Moustafa et al. 2004; Gonzalez-Gil et al. 2020).
For double label immunofluorescence staining with anti-DMBT1 and S8-Fc, the slide was overlaid with anti-DMBT1 antibody at 1:300 in PBS containing 0.1% Triton X-100 (TX100PBS) supplemented with 10 mg/mL BSA. The slide was incubated for 1.5 h at room temperature. During the incubation, S8-Fc was preconjugated with Alexa 594 labeled goat anti-human Fc (Jackson Immuno Research, West Grove, PA) as follows. To S8-Fc (15 μg/mL) in TX100PBS/BSA was added secondary goat anti-human Ig at 7 μg/mL. After 30 min on ice anti-DMBT1 (1:300) was added. The solution on the slide was removed and replaced with the precomplexed S8-Fc/anti-DMBT1 mixture. After additional incubation overnight at 4°C, the slide was washed with TX100PBS, the slide was overlaid with donkey anti-mouse Ig conjugated to Alexa Fluor 488 (Invitrogen, 1:200) for DMBT1 and donkey anti-goat Ig conjugated to Alexa Fluor 598 (Invitrogen, 1:200) for S8L detection in the same buffer for 1 h and washed in TX100PBS. Tissue was exposed to DAPI stain (Vector Laboratories) at 1:1000 dilution, then washed, dried and mounted prior to microscopy.
Microscopy and quantification of immunofluorescent tissue staining
Images of stained sections were captured with a Nikon Eclipse 90i automated research microscope calibrated to established threshold windows (Nikon Instruments, Melville, NY). Immunofluorescent signal was captured using green fluorescent filters for DMBT1 (488 nm) and red (598 nm) for S8L. Using the matching immunohistochemical staining of DMBT1, the following ROIs were delineated in each polyp or MT tissue specimen: (1) submucosal glands, (2) epithelium and (3) stroma. Signal was captured and scanned using Nikon NIS Elements Advanced Research software. For each specimen, five separate samplings of ROIs were measured and signals normalized to area in microns. Thus, each specimen’s ROI value represents the average of five independent area samplings of submucosal glands, epithelium or stroma, as shown in Figures 2 and 3.
Statistical methods
The Kruskal–Wallis nonparametric analysis of variance was used to compare expression levels of peripheral blood absolute eosinophil counts, serum IgE, S8L/DMBT1 between CRSwNP, CRS and controls using Prism8 (Graphpad; San Diego, USA). Significance was determined at P < 0.05. ANOVA with posthoc Bonferroni analysis was used to compare expression levels of S8L and DMBT1 in sinus polyps and patient matched MT tissue specimens from cellular compartments: submucosal glands, epithelium and stroma. Spearman’s rank correlation coefficients (nonparametric) was used to analyze the relationships between peripheral blood eosinophil counts and ratio of S8L to DMBT1. Significance was determined at P < 0.05.
Acknowledgments
None.
Contributor Information
Hyun Sil Lee, Department of Medicine, Division of Allergy and Clinical Immunology, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA; Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
Anabel Gonzalez-Gil, Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Virginia Drake, Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
T August Li, Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Ronald L Schnaar, Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Jean Kim, Department of Medicine, Division of Allergy and Clinical Immunology, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA; Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
Funding
National Institutes of Health (grant nos K12 HL141952 to A.G.G., J.K., T32 GM0087623 to T.A.L., U19 AI136443 to R.L.S.); the Flight Attendent Medical Research Institute (to R.S); research gift from the Carl and Kara Pittinger Family (to H.L., J.K.).
Conflict of interest disclosures
Jean Kim has consulted for ALK, GSK and Genentech. Other authors declare no conflicts of interest.
References
- Barnes PJ. 1998. Efficacy of inhaled corticosteroids in asthma. J Allergy Clin Immunol. 102:531–538. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. 2011. Glucocorticosteroids: Current and future directions. Brit J Pharmacol. 163:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathum Nexoe A, Pedersen AA, von Huth S, Detlefsen S, Hansen PL, Holmskov U. 2020. Immunohistochemical localization of deleted in malignant brain tumors 1 in normal human tissues. J Histochem Cytochem. 68:377–387. [DOI] [PubMed] [Google Scholar]
- Benninger M. 2007. Guidelines on the treatment of ABRS in adults. Int J Clin Pract. 61:873–876. [DOI] [PubMed] [Google Scholar]
- Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, Lanza DC, Marple BF, Osguthorpe JD, Stankiewicz JA et al. 2003a. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 129:S1–S32. [DOI] [PubMed] [Google Scholar]
- Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, Lanza DC, Marple BF, Osguthorpe JD, Stankiewicz JA et al. 2003b. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngology–Head and Neck Surgery. 129(3_suppl):S1–S32. [DOI] [PubMed]
- Bhattacharyya N, Lee LN. 2010. Evaluating the diagnosis of chronic rhinosinusitis based on clinical guidelines and endoscopy. Otolaryngol–Head Neck Surg. 143:147–151. [DOI] [PubMed] [Google Scholar]
- Bochner BS. 2009. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 39:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale TB. 2017. Biologics and biomarkers for asthma, urticaria, and nasal polyposis. J Allergy Clin Immunol. 139:1411–1421. [DOI] [PubMed] [Google Scholar]
- Chaaban MR, Walsh EM, Woodworth BA. 2013. Epidemiology and differential diagnosis of nasal polyps. Am J Rhinol Allergy. 27:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R. 2006. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 100:1307–1317. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Peterson KA, Murray JA, Falk GW, Gonsalves N, Chehade M, Genta RM, Leung J, Khoury P, Klion AD et al. 2020. Anti–Siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med. 383:1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, Steel J, Crocker PR. 2000. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 275:861–866. [DOI] [PubMed] [Google Scholar]
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P et al. 2012a. European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 50:1–12. [DOI] [PubMed] [Google Scholar]
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P et al. 2012b. European position paper on rhinosinusitis and nasal polyps 2012. Rhinology. 50(1):1–298. [DOI] [PubMed] [Google Scholar]
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P et al. 2012c. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 50:1–12. [DOI] [PubMed] [Google Scholar]
- Gao P-S, Shimizu K, Grant AV, Rafaels N, Zhou L-F, Hudson SA, Konno S, Zimmermann N, Araujo MI, Ponte EV et al. 2010. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Eur J Hum Genet. 18:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gil A, Li TA, Porell RN, Fernandes SM, Tarbox HE, Lee HS, Aoki K, Tiemeyer M, Kim J, Schnaar RL. 2020. Isolation, identification and characterization of the human airway ligand for the eosinophil and MAST cell immunoinhibitory receptor SIGLEC-8. J Allergy Clin Immunol. 10.1016/j.jaci.2020.08.001. PMCID 32791164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gil A, Porell RN, Fernandes SM, Wei Y, Yu H, Carroll DJ, McBride R, Paulson JC, Tiemeyer M, Aoki K et al. 2018. Sialylated keratan sulfate proteoglycans are Siglec-8 ligands in human airways. Glycobiology. 28:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, Vajn K, Stevens WW, Peters AT, Bochner BS et al. 2015a. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allerg Clin Immunol. 135:799–810.e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, Vajn K, Stevens WW, Peters AT, Bochner BS et al. 2015b. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allergy Clin Immunol. 135:799, e797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartush AG, Schumacher JK, Shah R, Patadia MO. 2019. Biologic agents for the treatment of chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 33:203–211. [DOI] [PubMed] [Google Scholar]
- Khan A, Vandeplas G, Huynh TMT, Joish VN, Mannent L, Tomassen P, Van Zele T, Cardell LO, Arebro J, Olze H et al. 2019. The global allergy and asthma European network GALEN rhinosinusitis cohort: A large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 57:32–42. [DOI] [PubMed] [Google Scholar]
- Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. 2012. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Therapeutics. 135:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Myers A, Kim J. 2009. Vascular endothelial growth factor drives autocrine epithelial cell proliferation and survival in chronic rhinosinusitis with nasal polyposis. Am J Respir Crit Care Med. 180:1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Kim J, Sypek JP, Wang IM, Horton H, Oppenheim FG, Bochner BS. 2004. Gene expression profiles in human nasal polyp tissues studied by means of DNA microarray. J Allergy Clin Immunol. 114:783–790. [DOI] [PubMed] [Google Scholar]
- Madsen J, Mollenhauer J, Holmskov U. 2010. Review: Gp-340/DMBT1 in mucosal innate immunity. Innate Immunity. 16:160–167. [DOI] [PubMed] [Google Scholar]
- Mollenhauer J, Herbertz S, Holmskov U, Tolnay M, Krebs I, Merlo A, Daa Schrøder H, Maier D, Breitling F, Wiemann S et al. 2000. DMBT1 encodes a protein involved in the immune defense and in epithelial differentiation and is highly unstable in cancer. Cancer Res. 60(6):1704–1710. [PubMed] [Google Scholar]
- Mountney A, Zahner MR, Lorenzini I, Oudega M, Schramm LP, Schnaar RL. 2010. Sialidase enhances recovery from spinal cord contusion injury. Proc Natl Acad Sci. 107:11561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa I, Connaris H, Taylor M, Zaitsev V, Wilson JC, Kiefel MJ, von Itzstein M, Taylor G. 2004. Sialic acid recognition by vibrio cholerae neuraminidase. J Biol Chem. 279:40819–40826. [DOI] [PubMed] [Google Scholar]
- Nutku E, Aizawa H, Hudson SA, Bochner BS. 2003. Ligation of Siglec-8: A selective mechanism for induction of human eosinophil apoptosis. Blood. 101(12):5014–5020. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski JA. 2013. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J Aller Clin Immunol. 132:1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakobphol A, Xu F, Hoang VM, Larsson T, Bergstrom J, Johansson I, Frangsmyr L, Holmskov U, Leffler H, Nilsson C et al. 2000. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J Biol Chem. 275:39860–39866. [DOI] [PubMed] [Google Scholar]
- Pröpster JM, Yang F, Rabbani S, Ernst B, Allain FH-T, Schubert M. 2016. Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc Natl Acad Sci. 113:E4170–E4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham S, Deivanayagam C. 2013. Cloning, expression and purification of the SRCR domains of glycoprotein 340. Prot Express Purif. 90(2):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero A, Liang J. 2017. Anti-IgE and anti-IL5 biologic therapy in the treatment of nasal polyposis: A systematic review and meta-analysis. The Annals of otology, rhinology, and laryngology. 126:739–747. [DOI] [PubMed] [Google Scholar]
- Robbe C, Paraskeva C, Mollenhauer J, Michalski JC, Sergi C, Corfield A. 2005. DMBT1 expression and glycosylation during the adenoma-carcinoma sequence in colorectal cancer. Biochem Soc Trans. 33:730–732. [DOI] [PubMed] [Google Scholar]
- Rosenstiel P, Sina C, End C, Renner M, Lyer S, Till A, Hellmig S, Nikolaus S, Fölsch UR, Helmke B et al. 2007. Regulation of DMBT1 via NOD2 and TLR4 in intestinal epithelial cells modulates bacterial recognition and invasion. J Immunol. 178(12):8203–8211. [DOI] [PubMed] [Google Scholar]
- Sajay-asbaghi M, Sadeghi-shabestrai M, Monfaredan A, Seyfizadeh N, Razavi A, Kazemi T. 2020. Promoter region single nucleotide polymorphism of siglec-8 gene associates with susceptibility to allergic asthma. Personalized Med. 17(3):195–201. [DOI] [PubMed] [Google Scholar]
- Shah SA, Ishinaga H, Takeuchi K. 2016. Pathogenesis of eosinophilic chronic rhinosinusitis. J Inflamm. 13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke JW, Borish L. 2016. Chronic rhinosinusitis phenotypes. Ann Allerg Asthma Immunol. 117:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BK, Kern RC, Schleimer RP, Schwartz BS. 2013. Chronic rhinosinusitis: The unrecognized epidemic. Am J Respir Crit Care Med. 188:1275–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsos N, Goudakos JK, Daskalakis D, Konstantinidis I, Markou K. 2018. Monoclonal antibodies for the treatment of chronic rhinosinusitis with nasal polyposis: A systematic review. Rhinology. 56:11–21. [DOI] [PubMed] [Google Scholar]
- Wynn R, Har-El G. 2004. Recurrence rates after endoscopic sinus surgery for massive sinus polyposis. Laryngoscope. 114:811–813. [DOI] [PubMed] [Google Scholar]
- Yu H, Gonzalez-Gil A, Wei Y, Fernandes SM, Porell RN, Vajn K, Paulson JC, Nycholat CM, Schnaar RL. 2017. Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology. 27:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]


