Abstract
Phytochelatins (PyCs) are metal-binding compounds produced by plants. PyCs may reduce bioavailability of dietary toxic metals such as cadmium. However, the PyC concentrations in foods are unknown. The objective of this study was to analyze PyC contents in a subset of commonly consumed plant foods. Foods (20) across five groups were analyzed and PyCs quantified using liquid chromatography-mass spectrometry (LC-MS/MS). The impact of factors such as food processing were also explored. PyCs were in all 20 foods. Five PyC types were detected with PyC2-Gly, PyC3-Gly and PyC2-Ala at quantifiable concentrations. PyC2-Gly was found at the highest concentrations and most widely distributed. PyC2-Gly concentrations were highest in fruits and root vegetables. Foods with increased processing tended to have reduced PyC concentrations. This survey of commonly consumed plant foods in the United States demonstrates PyCs are widely distributed and provides a foundation for understanding their concentrations and impact in the human diet.
Keywords: toxic metals, nutritional metals, metal bioavailability, functional foods, plant-based nutrition, phytochemicals
1. Introduction
Dietary patterns emphasizing plant foods are consistently associated with reduced risks of cardiovascular disease, cancer, Type 2 diabetes, and other chronic diseases (Kahleova, Levin, & Barnard, 2017; WCRF, 2007). Plant foods contain health-promoting phytochemicals including flavonoids, carotenoids, and phenolic acids (Liu, 2013). Phytochemicals contribute to a range of protective mechanisms including regulation of inflammatory pathways, antioxidant functions, and cancer-preventing pathways via alterations in gene expression impacting cell proliferation and apoptosis (Liu, 2013). Through eating plant foods, individuals consume a diverse array of phytochemicals which can work synergistically or alone to promote internal biological environments with antioxidant, anticancer, and anti-inflammatory activities (Liu, 2013). However, a major barrier to studying the health impacts of consuming phytochemicals is understanding the distribution of specific compounds across the wide range of plant foods (Liu, 2013).
Phytochelatins (PyCs), are glutathione-(GSH) derived metal-binding polymers produced by plants, and are one class of phytochemicals not characterized in the human diet (Seregin & Kozhevnikova, 2020). PyCs consist of 2–11 repeating γ-glutamyl-cysteine (γ-Glu-Cys) peptides and are enzymatically formed by phytochelatin synthase (Seregin et al., 2020). Plants produce PyCs at basal levels and at increased levels as a protective response to metal exposure (Pal & Rai, 2010). The peptide repeats typically end with a terminal Gly but can also terminate with Ser, Glu, Gln, Ala, β-Ala, or no amino acid (Mou et al., 2016; Pal et al., 2010). Common names for PyCs include a subscript for the number of repeating γ-Glu-Cys units and the terminal amino acid (Dennis et al., 2019).
A distinctive feature of PyCs is their high Cys content. Metals bind with high affinity to the thiol (-SH) group of Cys at biological pH (close to neutral or slightly basic), resulting in significant capacity to bind and sequester metals in plants (Mehra, Kodati, & Abdullah, 1995). For example, PyC2-Gly [(γ-Glu-Cys)2-Gly] can bind a metal with an oxidation state of +2. With longer chain lengths, PyCs have increased metal-binding capacity (Mehra et al., 1995). As longer PyCs are formed through the addition of γ-Glu-Cys peptides in response to metal exposure, the most abundant PyCs tend to be the shorter chain lengths (Seregin et al., 2020).
Due to their metal-binding properties, PyCs may have an important role in the absorption of dietary metals. For example, average dietary cadmium (Cd) levels in the U.S. population are linked with poor health outcomes across multiple organ systems due to the long half-life of Cd and its accumulation in the body (Satarug, Vesey, & Gobe, 2017). Dietary factors which reduce Cd bioavailability could have an important role in reducing Cd-related health risks such as kidney dysfunction and cancer (Satarug et al., 2017). Studies of PyCs in mammalian systems have demonstrated PyCs protect against uptake of Cd. Researchers investigating the impact of PyC3-Gly and PyC5-Gly on Cd accumulation in rats observed reduced Cd in the liver and kidneys, common target organs for Cd accumulation, compared to rats exposed to only Cd (Fujita et al., 1993). Investigations in gastrointestinal cell models also demonstrated reduced cellular Cd accumulation when cells were exposed to PyC3-Ser in combination with Cd (Jumarie, Fortin, Houde, Campbell, & Denizeau, 2001). This evidence suggests consumption of PyCs in the diet may protect against Cd uptake (Fujita et al., 1993; Jumarie et al., 2001).
PyCs have been studied extensively in agricultural and bioremediation in efforts to improve soil and plant health. From this body of research, PyCs are known to occur in agricultural plants grown for human consumption, such as soybeans, corn, and rice (Mou et al., 2016; Seregin et al., 2020). However, these studies have focused on roots, stems, and leaves from plant seedlings exposed to heavy metals. There is little known about the amount of PyCs in the edible components of harvested mature plants. Studies show PyCs tend to concentrate in roots as they are produced in response to metals taken up from the soil (Bardarov, Naydenov, & Djingova, 2015; Mou et al., 2016). PyCs are also produced in or transferred to leaves and stems but typically at lower concentrations than roots (Gong, Lee, & Schroeder, 2003; Mendoza-Cozatl et al., 2008). The PyC types, distributions, and concentrations ultimately depend on a variety of factors including the plant species and growing conditions. Some plant families predominantly have PyCs with terminal amino acids other than Gly, such as Ala in Fabaceae (legumes) or Ser in Poaceae (cereal grasses/grains), and evidence demonstrates plants can produce multiple PyC types (Batista et al., 2014; Mou et al., 2016; Oven, Page, Zenk, & Kutchan, 2002). The PyC length and terminal amino acid influence the molecular environment of the metal-binding thiol group and consequently, the affinity with which metals bind to PyCs (Dennis et al., 2019). These could be important factors for understanding the impact of dietary PyCs on metal absorption in humans. For plants, soil heavy metal concentrations have the greatest influence on PyC production. In a study of plant communities along soil pollution gradients, plants had greater PyC content with increasing soil heavy metals (Dazy, Beraud, Cotelle, Grevilliot, Ferard, & Masfaraud, 2009). However, plants do not require heavy metal exposures to produce PyCs and will produce PyCs in response to essential metals (e.g. zinc, copper) (Oven et al., 2002).
The human diet is a mixture of exposures, including beneficial components such as fiber and phytochemicals, but also potentially harmful components such as pesticides, herbicides, and toxic metals. As with other phytochemicals, the health impact of PyCs will depend on the characteristics and concentrations of PyCs, other dietary components consumed, and nutritional status of the individual (Liu, 2013). Additionally, food processing impacts phytochemical concentrations and will likely influence PyCs as well (Palermo, Pellegrini, & Fogliano, 2014). The present study was designed to establish an understanding of PyC concentrations and types in commonly consumed foods in the U.S. diet for the first time. We tested the hypothesis that PyCs most directly related to the precursor GSH, i.e., PyC2-Gly and PyC3-Gly, are widespread among plant-derived foods, while other PyCs (PyC4-Gly, PyC5-Gly, PyC6-Gly, PyC2-Glu, PyC2-Ala) have more restricted distributions. Additionally, we examined how factors like food group, growing conditions, and food processing may impact PyC concentrations. Here we provide characterization of PyCs in twenty commonly consumed foods across five plant food groups. Through this work, we provide evidence of important food characteristics that impact PyC concentrations, demonstrate the widespread distribution of PyCs in plant foods, and establish relevant dietary PyC concentrations and types for understanding the impact of this relatively unexplored class of phytochemicals on human health.
2. Material and Methods
2.1. Materials
Acetonitrile (HPLC grade), formic acid (LC-MS grade), and water (HPLC grade) were purchased from Sigma Aldrich (St. Louis, MO, USA). HPLC grade water was prepared with 2% formic acid as a solvent for LC-MS/MS. Analytical standards of PyC2-Gly, PyC3-Gly, PyC4-Gly, PyC5-Gly, PyC6-Gly, PyC2-Glu, and PyC2-Ala at 95% purity were obtained from CPC Scientific Inc (San Jose, CA, USA). Individual stock solutions of PyCs were prepared in HPLC-grade water.
2.2. Selection of representative foods
Five plant food groups were selected based on expected distribution of PyCs due to known plant physiology as well as colloquial terms for common vegetable types (i.e. leafy greens, root vegetables, fruits, legumes, and grains). From each of the five plant food groups, four food types were selected based on commonly consumed foods in the U.S. in each group (USDA & ERS, 2014). Tomatoes were included with the fruit group due their designation in plant anatomy. Corn was considered in the grain group even though ground corn products and fresh corn are often categorized into different food groups.
For each food type, ten individual items were purchased from local grocery stores in Atlanta, Georgia (May 2019 to August 2019), totaling 40 samples per group. Food types included corn, brown rice, whole wheat flour, oats, oranges, apples, bananas, tomatoes, carrots, potatoes, onions, sweet potatoes, kale, romaine lettuce, head lettuce, spinach, peanuts, beans, tofu, and peas. Twelve individual items were purchased for apples to increase diversity for subset comparisons as described below. For each food, items were purchased with at least three different brand names or stores for analysis. To address PyC variability due to storage conditions and processing, a selection of fresh, frozen, and canned options was included when available (Supplemental Table 1). To explore other factors that could impact PyC concentrations, foods were selected with different varieties, growing locations, and growing conditions (e.g. conventional and organic). Fresh foods were kept refrigerated at 4°C and frozen foods were kept frozen at −20°C. Shelf stable items such as dry and canned foods were kept at room temperature until prepared for analysis.
2.3. Sample preparation
Samples were prepared from portions of the food item generally considered edible in the United States. For foods usually consumed cooked (i.e. rice, sweet potatoes, potatoes), foods were prepared using typical cooking directions. In brief, sweet potatoes and potatoes were baked in foil at 350ºF for 40–50 minutes until softened. Brown rice was cooked in 2:1 ratio of filtered water to rice by bringing to a boil and then reducing to a simmer for 40 minutes. If necessary, foods were cut before grinding with a mortar and pestle. Liquid nitrogen was used to freeze fruits, leafy greens, and root vegetables prior to grinding to facilitate breakdown of fibrous materials and transfer to extraction vials.
Ground food samples were loaded into 2 mL microcentrifuge tubes, weighed, and stored at – 80°C until extracted for LC-MS/MS analysis. A 2:1 acetonitrile to water solvent mixture was added to ground food samples at a ratio of 2 μL solvent to 1 mg food. Samples were vortexed, sonicated (10 seconds at 30% amp), incubated on ice for 30 min, and centrifuged for 10 min (14,000 rpm) at 4°C. The supernatant was removed and stored at −80°C until LC- MS/MS analysis. Sample extraction following reduction with dithiothreitol was tested and not included in the final method as it did not improve PyC recovery.
2.4. LC-MS/MS analysis
Food sample supernatants were thawed, vortexed, and loaded into autosampler vials. Pooled samples for each food type were mixed from the supernatant for use as a quality control reference, and seven PyC standards were added at 1 μM for use during quantification. Samples were randomized within each food type and analyzed on a high resolution LTQ-Velos Orbitrap mass spectrometer (Thermo Fisher). Pooled food samples with and without added PyC standards were analyzed at the end of each food type analysis for estimating PyC concentrations and validating PyCs with chromatographic characteristics and ion dissociation mass spectrometry (MS/MS). Each sample was analyzed in triplicate with a 10 μl injection volume on a HILIC column in positive electrospray ionization mode. The mass spectrometer was operated at 60,000 resolution and mass-to-charge (m/z) range of 500–1,250 following optimization for PyC detection and quantification.
2.5. Phytochelatin quantification
The intensity of each PyC (M+H adduct) was determined using the area under the curve (Thermo Scientific Xcalibur software, Genesis peak integration algorithm) (Supplemental Table 2) (Dennis et al., 2019). For some samples, reduced and oxidized PyC forms of the M+H adduct were detected. Tests with dithiothreitol treatment did not improve reproducible quantification and introduced an additional step in sample preparation, so the approach used for these samples was to sum the intensities for the multiple forms [e.g. PyC2-Gly: m/z 538.1272 (oxidized) and m/z 540.1429 (reduced)]. Comparisons of intensities for commercial standards treated in this manner showed this to be reliable, i.e., disulfide bonds form internally within PyC and the signal of the oxidized form is not decreased due to larger aggregate formation. Technical replicates were averaged. Quantification used a method of additions. To account for food specific matrix effects for each food item, intensity values of 1 μM PyC authentic standards were determined by subtracting the intensity of the pooled sample from intensity of the pooled sample with PyC standards. Estimates of PyC concentrations in each food item were then determined relative to the intensity of the added 1 μM PyC standard. Samples with MS/MS patterns matching the characteristic fragmentation pattern of the respective PyC standard but within an unreliable range for quantification were designated as “detected but not quantified”.
PyC quantities are reported as μg/g edible weight (EW), which refers to the weight of the food item as purchased or prepared. For example, oats and whole wheat flour are reported using the dry weights, potatoes are reported using the prepared weight (i.e. canned or baked), and apples are reported using the fresh weight. PyC quantities per serving size were calculated using the Food Pyramid Equivalent Database (FPED) serving equivalents converted to grams. FPED equivalents for fruits and vegetables are in 1 cup equivalents and for protein and whole grains are in 1 ounce equivalents (Bowman, Clemens, Friday, Thoerig, & Moshfegh, 2014).
2.6. Statistical analysis
One-way ANOVA with Tukey’s post-hoc test was used to compare PyC2-Gly concentrations across food groups (e.g. fruits, leafy greens, root vegetables, grains, legumes) and within food groups (e.g. apples, oranges, bananas, tomatoes), PyC3-Gly and PyC2-Ala concentrations across food types, and any food types with multiple varieties or processing levels. Unpaired t-tests were used to compare PyC concentrations between two groups (e.g. red kale vs. curly kale; fresh potatoes vs. canned potatoes). Pearson’s correlation was used to assess the association between concentrations of multiple PyCs within one food type. All statistical analyses were performed using the software GraphPad Prism version 8.3.1 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com. Significant differences were defined as p-values <0.05.
3. Results and Discussion
3.1. Overall distribution of PyCs in commonly consumed foods
Our results establish the novel finding that PyCs are widely distributed in plant foods. Foods from five plant food groups were analyzed with four food types per group and ten food items per type, totaling 202 individual food items (Table 1). PyCs were found in all five food groups and twenty food types analyzed, demonstrating PyCs are consumed via a variety of foods. Five PyC types were detectable and/or quantifiable in these foods. PyC2-Gly was the predominant PyC type with quantifiable concentrations in 18 of 20 food types and 182 of 202 food items. In alignment with previous studies in plant seedlings, the four other PyC types measured occurred much less frequently (Seregin et al., 2020). Quantifiable levels of PyC3-Gly were in six food types and PyC3-Gly was detectable but not quantifiable in one additional food. PyC2-Ala was quantifiable in four food types and detectable but not quantifiable in an additional four types (Supplemental Figure 1, Supplemental Table 3). PyC4-Gly and PyC2-Glu were detectable but not quantifiable in five and three food types, respectively (Supplemental Table 3). Long-chain PyCs, PyC5-Gly and PyC6-Gly, were not detected in any foods. Other studies have not observed long-chain PyCs in plant seedlings grown under control conditions and only trace quantities of long-chain PyCs when plants are grown with exposure to heavy metals (Seregin et al., 2020; Seregin, Vooijs, Kozhevnikova, Ivanov, & Schat, 2007).
Table 1.
Plant food groups and food types analyzed per group
| Food group | Whole grains | Fruits | Root vegetables | Leafy greens | Legumes |
|---|---|---|---|---|---|
| corn | orange | carrot | kale | peanuts | |
| brown rice | apple | potato | romaine lettuce | beans | |
| whole wheat flour | banana | onion | head lettuce | tofu | |
| oats | tomato | sweet potato | spinach | peas |
3.2. PyC2-Gly quantification in 18 of 20 food types
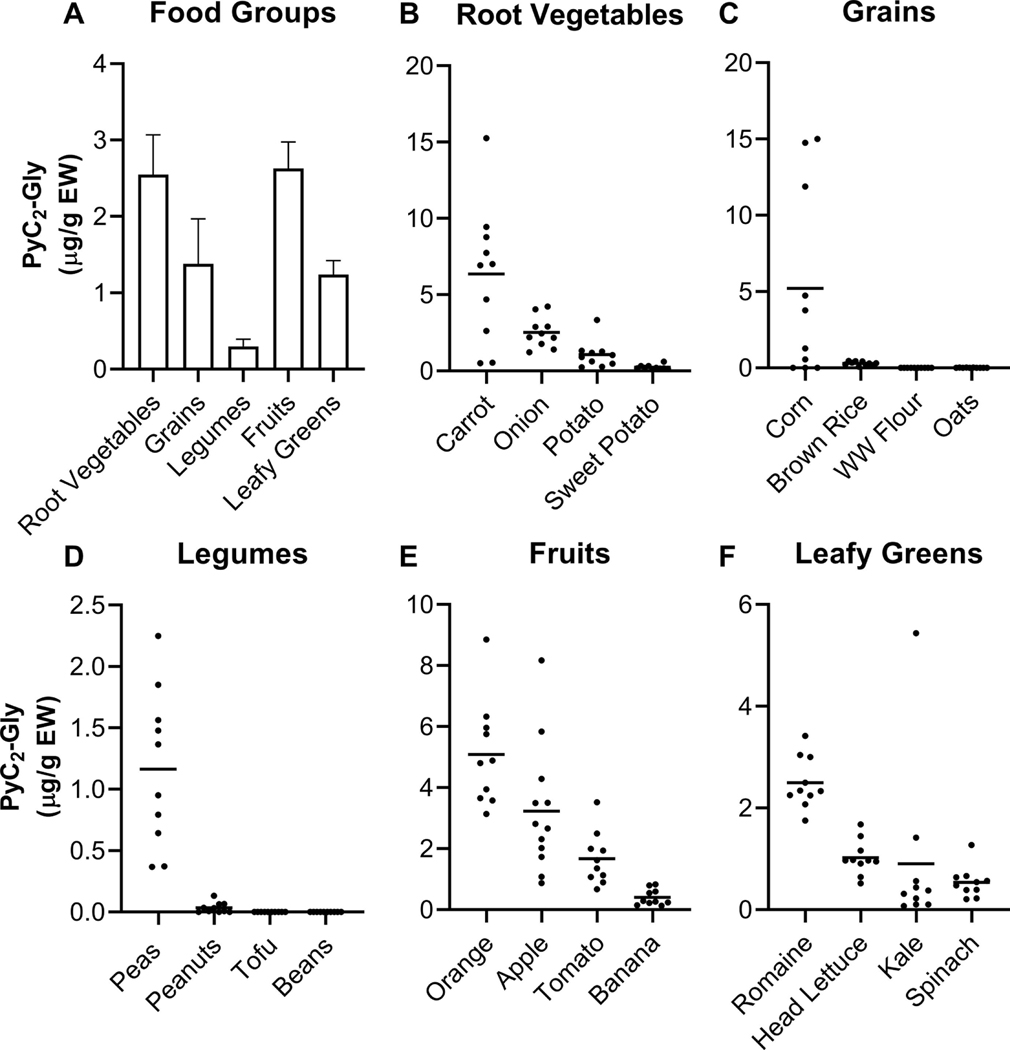
As PyC2-Gly is one of the most common PyCs in the plant kingdom, we focused our analysis on PyC2-Gly concentrations to determine if the distribution in foods was similar. PyC2-Gly concentrations were highly variable across the five food groups (one-way ANOVA, p<0.05) with fruits and root vegetables having the highest PyC2-Gly concentrations followed by grains, leafy greens, and legumes (Figure 1A). In root vegetables and fruits, average PyC2-Gly concentrations were 2.55 μg/g EW (range 0.09–15.26) and 2.63 μg/g EW (range 0.13–8.85), respectively. Root vegetables and fruits had significantly higher PyC2-Gly concentrations than average legume concentrations (0.30 μg/g EW, range 0.00–2.25, Tukey’s, p<0.05). Average PyC2-Gly concentrations in leafy greens and grains were similar (1.24 μg/g EW, range 0.07–5.44; 1.38 μg/g EW, range 0.00–15.01). The highest PyC2-Gly concentrations in individual food items were distributed across fruit and vegetable food groups; in descending order of PyC2-Gly content were carrots, corn, oranges, apples, onions, and romaine lettuce (Figure 1B, C, E, F). The lowest PyC2-Gly concentrations were in grains and legumes with no detection of PyC2-Gly in two legume food types, tofu and beans.
Figure 1.
PyC2-Gly concentration of 20 food types across five food groups. A, Bar graphs (mean ± SEM) show differences in PyC2-Gly concentrations across food groups (p<0.05, one- ay ANOVA). B-F, Scatter plots of each food group show variability in PyC2-Gly concentrations within food groups (p<0.05, one-way ANOVA) and some foods. Horizontal lines indicate mean values. Points represent individual PyC2-Gly levels in each food sample which are the average of three technical replicates. Carrots (B), corn (C), oranges (E), and apples (E) have the highest concentrations across all 20 food types. Tofu (D) and beans (D) contain no detectable PyC2-Gly.
3.2.1. Root vegetables
PyCs are produced by plants in response to soil metals. Previous studies demonstrate PyCs frequently concentrate in or near roots relative to stems and leaves (Bardarov et al., 2015; Mou et al., 2016). Therefore, we investigated whether edible components of plants which grow closer to the roots would have higher concentrations of PyCs. The four types of root vegetables varied significantly in their PyC2-Gly concentrations (Figure 1B). Of the two true roots in our study, carrots had high PyC2-Gly concentrations with an average of 6.36 μg/g EW. Sweet potatoes, the only other true root of the root vegetables studied, had the lowest average PyC2-Gly concentrations of the root vegetables at 0.24 μg/g EW with consistently low concentrations across all samples (range 0.09–0.61). Onion and potato samples had similar concentrations to fruits and leafy greens with similar average concentrations and variability across individual samples (2.53 μg/g EW, range 1.22–4.22 and 1.07 μg/g EW, range 0.24–3.35, respectively). These findings suggest that PyC types and concentrations in plant foods are specific to each food and more systematic and complete assessment should be conducted before generalizing to categories of plant food groups.
3.2.2. Grains and legumes
Grains are an important source of nutrition globally, accounting for 50% of calories consumed worldwide and therefore, may serve as an important source of PyCs in the diet. Legumes are a major source of protein in many low- and middle-income countries and have been identified in the U.S. dietary guidelines as an important component within a healthy dietary pattern. Although anatomically distal plant components like grains and legumes are farther from metal exposures at the roots, phytochelatin synthase, the enzyme producing PyCs, is expressed constitutively throughout the plant (Clemens & Ma, 2016). Among the grains measured, PyC2-Gly concentrations were very low except in fresh and canned/frozen corn samples (Figure 1C). Within corn samples, average PyC2-Gly concentrations were 5.20 μg/g EW and there was high variability in concentrations driven by the very low PyC2-Gly levels in cornmeal/flour and high levels in fresh corn (range 0.01–15.01). Legumes had low concentrations of PyC2-Gly (Figure 1D). Peas had an average PyC2-Gly concentration of 1.16 μg/g EW (range 0.37–2.25) and peanuts had an average of 0.03 μg/g EW (range 0.00–0.13). The other two legumes studied, tofu and beans, had no detectable PyC2-Gly. Other studies in beans and soybeans, from which tofu is made, found PyC-Ala but not PyC-Gly (Piechalak, Tomaszewska, Baralkiewicz, & Malecka, 2002; Vazquez, Goldsbrough, & Carpena, 2009).
3.2.3. Fruits
Among fruits, oranges had the highest average PyC2-Gly concentration at 5.09 μg/g EW (Tukey’s, p<0.05) (Figure 1E), followed by apples at 3.23 μg/g EW. Oranges and apples had similar variability in concentrations across samples (range 3.14–8.85 and 0.87–8.17, respectively). Tomatoes had an average PyC2-Gly concentration of 1.67 μg/g EW (range 0.67–3.52) and bananas had the lowest PyC2-Gly concentrations of all fruits at 0.40 μg/g EW (range 0.13–0.83). Although no previous studies have examined the PyC concentrations in apple, orange, or banana plants or their fruits, the phytochelatin synthase gene ortholog has been documented in each species (Yazdi, Kolahi, Mohajel Kazemi, & Goldson Barnaby, 2019). Additionally, PyCs have been identified in the roots of 1-year old citrus plants grown with and without Cd exposures (López-Climent, Arbona, Pérez-Clemente, Zandalinas, & Gómez-Cadenas, 2014). Similar studies in tomato plants have identified PyCs in leaves and roots with and without Cd exposures (Ben Ammar, Mediouni, Tray, Ghorbel, & Jemal, 2008; Hasan et al., 2015). In a study of tomato seedlings, PyC2-Gly concentrations were about 5 μg/g FW in leaves and roots with no Cd exposure (Hasan et al., 2015). The slightly higher concentrations observed in the tomato seedlings study are in alignment with previous studies demonstrating roots and young leaves have higher concentrations relative to old leaves and stems (Maier, Matthews, McDowell, Walden, & Ahner, 2003; Vazquez et al., 2009).
3.2.4. Leafy greens
Compared to fruits and grains, leafy greens are anatomically at an intermediate location between roots and the distal fruiting bodies of plants. Additionally, leafy greens are known to have higher concentrations of Cd than other plant foods. Therefore, we hypothesized leafy greens may have intermediate to high PyC concentrations based on plant anatomy and metal accumulation. Leafy greens actually had lower PyC2-Gly concentrations relative to root vegetable and fruit groups (Figure 1A). Romaine lettuce had the highest average PyC2-Gly concentration of 2.50 μg/g EW followed by head lettuce at 1.02 μg/g EW. Spinach had the lowest average PyC2-Gly concentrations of the leafy greens studied (0.54 μg/g EW). The average kale concentration was 0.90 μg/g with one kale sample having a 5-fold higher PyC2-Gly concentration (5.44 μg/g EW) (Figure 1F). High exposure to soil heavy metals while growing could explain the high concentrations in this kale plant. In a study of lettuce, increasing Cd exposures led to 2 to 10-fold higher PyC content relative to unexposed plants (Maier et al., 2003). Other studies of lettuce have found zero to very low levels of PyCs in leaves unexposed to heavy metals with total PyC concentrations around 0.1 μg/g FW (Maier et al., 2003). When plants are exposed to Cd in hydroponic growing systems, the concentrations increase to about 1.3 μg/g FW in new leaves (Maier et al., 2003). The low PyC levels may be due to the experimental growing conditions in these studies. In contrast to our findings, a study which grew both spinach and lettuce in a hydroponic system with the same exposures observed the highest PyC concentrations in Cd-exposed shoots and roots of spinach, not lettuce (Meng, Zhang, Wang, Zhou, Shangguan, & Yang, 2019). Nutrients in hydroponic systems differ from soil. As other soil metals like copper and zinc induce PyC generation, the difference in our results may reflect the impact of growing leafy greens in soil versus hydroponic conditions.
3.3. Difference in PyC types and concentrations across foods
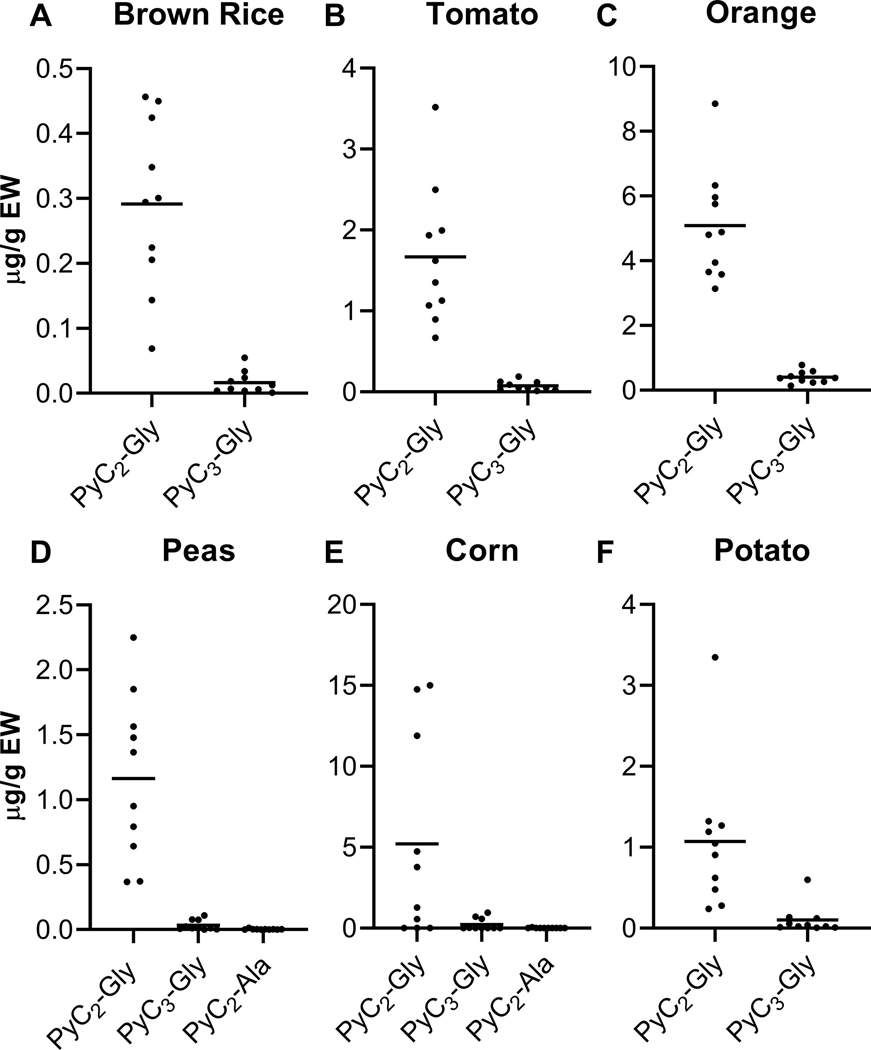
As hypothesized, short chain PyCs (PyC2-Gly and PyC3-Gly) are commonly distributed and present at the highest concentrations across all foods studied. Of the subset of six food types with multiple quantifiable PyCs, PyC2-Gly was at significantly higher concentrations than other PyCs in all foods (Figure 2). PyC2-Gly levels were 10 to 34-fold higher than PyC3-Gly in brown rice, tomatoes, oranges, peas, corn, and potatoes. Of the six foods with quantifiable PyC3-Gly concentrations, the highest concentrations were in oranges followed by corn and potatoes (Supplemental Figure 1A). In alignment with other studies, the PyC-Ala type predominated in legumes, occurring as the only detectable PyC in tofu and beans (Oven et al., 2002; Piechalak et al., 2002; Vazquez et al., 2009). In a study including beans and peas, peas contained PyC-Gly and PyC-Ala but beans only contained PyC-Ala (Piechalak et al., 2002). Of the four foods with PyC2-Ala, three of the food types were legumes, with tofu containing the highest concentrations (Supplemental Figure 1B). No PyC2-Ala was detected in peanuts but was detectable in other non-legume foods including corn, brown rice, and apples. PyCs other than PyC-Gly have been previously detected in corn and brown rice, including PyC-Ser and PyC-Glu, but to our knowledge, PyC-Ala has not (Mou et al., 2016; Seregin et al., 2007). In foods with multiple quantifiable PyCs, PyC concentrations were positively correlated (Supplemental Figure 2). In study in arsenic-exposed rice varieties, PyC types and chain lengths were also positively correlated (Batista et al., 2014).
Figure 2.
PyC2-Gly concentrations were higher in all foods with multiple quantifiable PyCs (A-F). PyC3-Gly and PyC2-Ala concentrations were quantifiable in only this subset of analyzed foods. Points represent individual PyC levels in each food sample and are the average of three technical replicates. Horizontal lines represent the mean value of each PyC in the respective food.
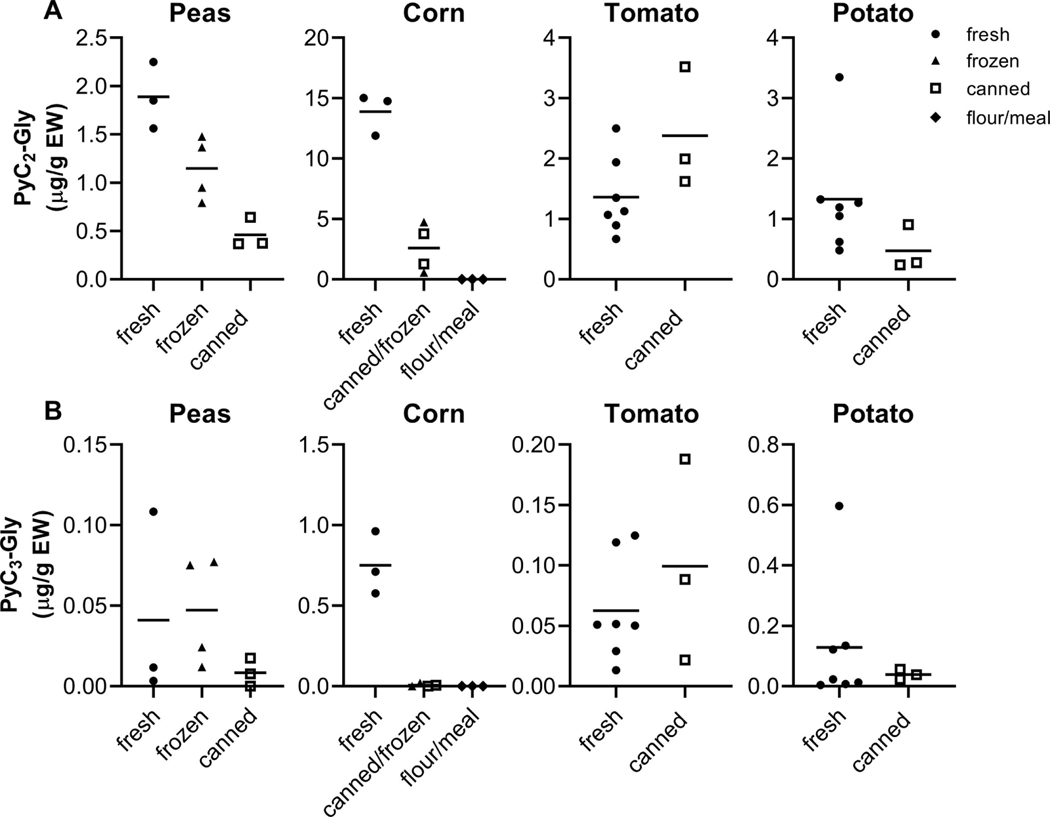
3.4. Difference in PyC content by level of food processing
The impact of food processing on PyC content has not been described. However, food processing is known to decrease GSH content (Jones et al., 1992), the precursor from which PyCs are formed. In alignment with previous research on GSH, foods with increased levels of food processing tended to have lower PyC2-Gly and PyC3-Gly content (Figure 3) (Jones et al., 1992). This trend was particularly pronounced in corn. Fresh corn had some of the highest PyC2-Gly concentrations while dried and milled corn had some of the lowest (Figure 3A). The milling technique could influence PyC concentrations as more or less of the corn kernel will be included in the resulting cornmeal or flour. Also, different corn varieties are used for fresh and canned corn versus milled corn which may contribute to this difference. With higher levels of processing, PyC2-Gly concentrations were consistently lower in peas and potatoes (Figure 3A). A significant trend was observed in peas across step-wise increases in food processing. This may be explained by thermal degradation, a process which frequently reduces phytochemical concentrations (Palermo et al., 2014). Dry grain products in our study had low PyC concentrations which may be due PyC degradation as these products are stored at room temperature. In our study, fresh peas, corn, and potatoes had the highest concentrations with decreasing concentrations in frozen and canned foods, respectively. Lower PyC concentrations in frozen foods relative to fresh may be due to blanching prior to freezing and packaging (Palermo et al., 2014). Cooking and canning can lead to greater phytochemical reductions due to increased thermal degradation with higher temperatures and longer cooking times (Ng & Rosman, 2019; Palermo et al., 2014). In a study of GSH in foods, raw spinach had the highest total GSH concentration (394 nmol/g) followed by cooked (234 nmol/g) and canned (71 nmol/g) (He, Openo, McCullough, & Jones, 2004). This study observed a similar pattern of total GSH concentrations in carrots (raw, 255 nmol/g; cooked 188 nmol/g; canned, 0 nmol/g) (He et al., 2004). Studies of other compounds have found similar patterns, with reductions in phytochemical content with increased food processing (Jones et al., 1992; Nayak, Liu, & Tang, 2015). Thermal processing could also disrupt the enzymatic activity of phytochelatin synthase, disrupting additional PyC synthesis which could otherwise occur in fresh foods. In some cases, thermal degradation can increase compound extraction through disrupting the food matrix and improving bioaccessibility (Palermo et al., 2014). In our study, tomatoes had slightly higher PyC2-Gly concentrations in canned as compared to fresh (canned: 2.38 μg/g EW, range 1.62–3.52; fresh: 1.36 μg/g EW, range 0.67–2.50; p>0.05). This may be due to increased extractability of PyCs from the canning process. Additionally, canning may concentrate PyCs in tomatoes more than other canned plant foods like potatoes and peas due to their high water content. Similar patterns were observed with PyC3-Gly concentrations for these foods (Figure 3B). Given the known influence and specificity of food processing on phytochemical concentrations, food processing will likely impact PyC concentrations in other plant foods.
Figure 3.
Food processing reduces PyC2-Gly and PyC3-Gly content in some foods. A, PyC2-Gly concentrations were higher in corn and pea food samples with less food processing. No difference was detected in concentrations in potato and tomato samples with different processing levels. B, PyC3-Gly concentrations were higher in corn samples with less food processing and there was with no difference in peas, potatoes, and tomatoes. Points indicate PyC concentrations of individual food samples and are the average of three technical replicates. Horizontal lines indicate mean values. EW, edible weight.
3.5. Comparison of PyC concentration by growing conditions and food variety
As PyCs are enzymatically produced by plants in response to their environment, we considered how growing conditions or food variety may impact PyC concentrations. Soil metal concentrations are known to impact PyC concentrations and vary geographically. When we examined how growing locations and organic versus conventional farming may impact PyC concentrations, no differences were found (Supplemental Figure 3, 4A). Agricultural growing practices do impact soil characteristics and metal exposures but conventional and organic labelling may not capture meaningful differences (ATSDR et al., 2012). Additionally, the data available about growing locations were limited to state or country-level which may not be granular enough to observe differences. For example, specific geographic regions may have increased heavy metals due to nearby manufacturing processes emitting heavy metals that deposit in nearby soils (ATSDR et al., 2012).
As indicated by the trends we observed, plant variety may impact PyC content in some foods. Many plant foods have multiple varieties from the same species widely available in grocery stores. For example, apple varieties include gala, fuji, red delicious, and many others. Our data indicate there may be some difference by food variety in apples, kale, and potatoes but these differences were not significant (Supplemental Figure 4B). In a study examining the response of six rice varieties to the same Cd exposures, PyC concentrations varied up to 10- fold (Batista et al., 2014). More broadly, phytochemical content is known to differ across common plant food varieties (Boyer & Liu, 2004). Our data indicate plant variety will be important to consider when studying PyCs in human foods.
3.6. PyC2-Gly content by serving size
To understand the relative proportion of PyC2-Gly obtained in the diet, we calculated PyC2-Gly for a typical serving using the mean PyC2-Gly concentrations in foods and the serving size from USDA Food Pyramid Equivalents Database. Fruits and vegetables had the highest quantity of PyC2-Gly (Table 2). The whole grain and protein foods had very low PyC2-Gly amounts per serving with the highest quantity coming from brown rice (Table 2). Raw corn and raw carrots had the highest quantity of PyC2-Gly with over 1000 μg/serving. Oranges, canned tomatoes, frozen or canned corn, and onions followed in descending order with over 400 μg/serving. As previously discussed, we observed higher quantities of PyCs in less processed foods. Raw corn, carrots, and peas had four times the PyC2-Gly per serving than the canned version, and baked potatoes had two times the quantity relative to canned. In tomatoes, the opposite relationship was observed with canned tomatoes containing two times the PyC2-Gly per serving than fresh tomatoes.
Table 2.
PyC2-Gly content per typical serving.
| Food | PyC2-Gly (μg/g EW)a | Serving in gramsb | PyC2-Gly (μg/serving) |
|---|---|---|---|
| Fruits & Vegetables | |||
| corn (raw) | 13.89 | 150 | 2080 |
| carrot (raw) | 8.48 | 125 | 1060 |
| orange (raw) | 5.09 | 185 | 942 |
| tomato (canned) | 2.38 | 245 | 583 |
| corn (frozen) | 2.59 | 165 | 437 |
| corn (canned) | 2.52 | 165 | 416 |
| onion (raw) | 2.53 | 160 | 405 |
| apple (raw) | 3.23 | 110 | 355 |
| carrot (canned) | 1.92 | 145 | 278 |
| peas (raw) | 1.89 | 145 | 274 |
| romaine (raw) | 2.50 | 95 | 238 |
| tomato (raw) | 1.36 | 170 | 231 |
| peas (frozen) | 1.15 | 145 | 167 |
| potato (baked) | 1.33 | 120 | 160 |
| head lettuce (raw) | 1.00 | 110 | 110 |
| spinach (canned) | 0.60 | 170 | 102 |
| peas (canned) | 0.46 | 160 | 73.6 |
| potato (canned) | 0.47 | 155 | 72.9 |
| kale (raw) | 0.90 | 70 | 63.0 |
| banana (raw) | 0.40 | 150 | 60.0 |
| sweet potato (baked) | 0.26 | 200 | 52.2 |
| spinach (raw) | 0.70 | 70 | 49.0 |
| Whole Grains | |||
| brown rice (cooked) | 0.29 | 98 | 28.6 |
| oats | 0.01 | 28 | 0.32 |
| corn (cornmeal) | 0.01 | 28 | 0.24 |
| whole wheat flour | 0.00 | 28 | 0.07 |
| Protein | |||
| peanuts | 0.04 | 14 | 0.50 |
| tofu | 0.00 | 31 | 0.00 |
| beans (canned) | 0.00 | 44 | 0.00 |
Values listed are mean edible weights (EW).
Servings are listed as Food Pyramid Equivalents Database (FPED) equivalents based on the USDA dietary guidelines for Americans. Fruits and vegetables are listed as gram weights for 1 cup equivalents. Proteins and whole grains are listed as gram weights for 1 ounce equivalents.
The values obtained indicate that the average amounts of PyC2-Gly in the human diet could range from 0.38 mg to 3.75 mg for individuals consuming less than 3 servings of fruits and vegetables per day to 0.88 mg to 8.75 mg for individuals consuming 7 or more servings per day. This may be highly impacted, however, by specific food selections. Of importance for mechanistic studies, the measured amounts suggest that relevant PyC concentration ranges in the intestinal lumen are 0.35 μM to 8.11 μM if one assumes dilution in 2 L from dietary intake and 0.08 μM to 1.80 μM if one assumes dilution in 9 L from endogenous and exogenous sources (Sullivan, Alpers, & Klein, 2012).
This study establishes PyC types and amounts coming from specific plant foods in the diet. Open questions remain about how the metal-binding properties of PyCs influence metal bioavailability. From previous research on Cd and PyCs, we know PyC exposure can lead to reduced absorption of Cd (Fujita et al., 1993; Jumarie et al., 2001). However, how PyCs impact bioavailability of other toxic metals or essential metals is unknown. Studies of iron bioavailability indicate compounds structurally similar to PyCs, GSH and Cys, can enhance iron absorption (Layrisse, Martinez-Torres, Leets, Taylor, & Ramirez, 1984). Although structurally different, phytic acid is another compound produced by grains, legumes, nuts, and some vegetables with metal-binding properties, which provides context and considerations for studying PyC health effects. Mineral bioavailability studies indicate the impact of phytic acid is highly dependent on dietary context. For example, absorption of minerals may not be negatively impacted in individuals who are well-nourished and eating a diverse diet (Silva & Bracarense, 2016). The affinity of binding will depend on the metal, ratio of phytic acid to metal, and other factors such as pH. Similar factors including gut microbiome activity are likely to influence the availability and function of PyCs in the gastrointestinal tract (Dennis et al., 2019). Other health effects of PyCs could include a role in nutritional immunity wherein the host controls access to micronutrients to protect from bacterial infections. Additionally, the structural similarity of PyCs to GSH, a major antioxidant, suggests PyCs may impact cellular redox mechanisms and availability of redox metals.
4. Conclusion
We determined PyCs are widely distributed across plant food groups, and high concentrations occur in specific food types and not necessarily specific food groups. PyC2-Gly was the predominant PyC in 18 of 20 food types, indicating focused study of PyC2-Gly may be warranted in future studies examining health effects. Although more extensive surveys of foods are required, our data provide a foundation for estimating PyC concentrations consumed in the population and examining these biologically relevant concentrations in model systems for their potential health effects. Additional research is needed to evaluate the extent to which PyC content contributes to health benefits of plant-based diets.
Supplementary Material
Metal-binding compounds, phytochelatins (PyCs), were characterized in plant foods.
PyCs may impact metal bioavailability but quantities in the human diet are unknown.
Five PyC types were detected across 20 commonly consumed plant foods.
PyC2-Glycine was at the highest concentrations and found in 18 of 20 foods.
PyC3-Glycine, PyC4-Glycine, PyC2-Alanine, and PyC2-Glutamate were also found.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases [T32 DK007734]; National Institute of Environmental Health Sciences [F31 ES030980, R01 ES023485, U2C ES030163]; and National Institutes of Health, Office of the Director [S10 OD018006].
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online.
CRediT author statement
Kristine Dennis: Conceptualization (lead), Methodology, Funding acquisition, Formal Analysis, Visualization, Writing – Original Draft (lead) and Review and Editing. Young-Mi Go: Funding acquisition, Methodology, Writing – Review and Editing. Ken Liu: Methodology, Validation, Writing – Review and Editing. Dean Jones: Conceptualization, Funding acquisition, Methodology, Writing – Review and Editing. Karan Uppal: Methodology, Writing Review and Editing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR, Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, … Rudisill C. (2012). Toxicological Profile for Cadmium. In A. f. T. S. a. D. Registry; (Ed.). Atlanta, GA. [PubMed] [Google Scholar]
- Bardarov K, Naydenov M, & Djingova R. (2015). HPLC-HRMS method for fast phytochelatins determination in plants. Application to analysis of Clinopodium vulgare L. Talanta, 142, 20–27. 10.1016/j.talanta.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Batista BL, Nigar M, Mestrot A, Rocha BA, Barbosa Junior F, Price AH, … Feldmann J. (2014). Identification and quantification of phytochelatins in roots of rice to long-term exposure: evidence of individual role on arsenic accumulation and translocation. J Exp Bot, 65(6), 1467–1479. 10.1093/jxb/eru018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ammar W, Mediouni C, Tray B, Ghorbel MH, & Jemal F. (2008). Glutathione and phytochelatin contents in tomato plants exposed to cadmium. Biologia Plantarum, 52(2), 314. 10.1007/s10535-008-0065-9. [DOI] [Google Scholar]
- Bowman SA, Clemens JC, Friday JE, Thoerig RC, & Moshfegh AJ (2014). Food Patterns Equivalents Database 2005–06: Methodology and User Guide. In B. H. N. R. C. Food Surveys Research Group, Agricultural Research Service, U.S. Department of Agriculture; (Ed.). Beltsville, Maryland. [Google Scholar]
- Boyer J, & Liu RH (2004). Apple phytochemicals and their health benefits. Nutr J, 3, 5. 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, & Ma JF (2016). Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu Rev Plant Biol, 67, 489–512. 10.1146/annurev-arplant-043015-112301. [DOI] [PubMed] [Google Scholar]
- Dazy M, Beraud E, Cotelle S, Grevilliot F, Ferard JF, & Masfaraud JF (2009). Changes in plant communities along soil pollution gradients: responses of leaf antioxidant enzyme activities and phytochelatin contents. Chemosphere, 77(3), 376–383. 10.1016/j.chemosphere.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Dennis KK, Uppal K, Liu KH, Ma C, Liang B, Go YM, & Jones DP (2019). Phytochelatin database: a resource for phytochelatin complexes of nutritional and environmental metals. Database (Oxford), 2019. 10.1093/database/baz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, el Belbasi HI, Min KS, Onosaka S, Okada Y, Matsumoto Y, … Tanaka K. (1993). Fate of cadmium bound to phytochelatin in rats. Res Commun Chem Pathol Pharmacol, 82(3), 357–365. https://www.ncbi.nlm.nih.gov/pubmed/8122036. [PubMed] [Google Scholar]
- Gong JM, Lee DA, & Schroeder JI (2003). Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc Natl Acad Sci U S A, 100(17), 10118–10123. 10.1073/pnas.1734072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan MK, Ahammed GJ, Yin L, Shi K, Xia X, Zhou Y, … Zhou J. (2015). Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front Plant Sci, 6, 601. 10.3389/fpls.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Openo K, McCullough M, & Jones DP (2004). Total equivalent of reactive chemicals in 142 human food items is highly variable within and between major food groups. J Nutr, 134(5), 1114–1119. 10.1093/jn/134.5.1114. [DOI] [PubMed] [Google Scholar]
- Jones DP, Coates RJ, Flagg EW, Eley JW, Block G, Greenberg RS, …Jackson B. (1992). Glutathione in foods listed in the National Cancer Institute’s Health Habits and History Food Frequency Questionnaire. Nutr Cancer, 17(1), 57–75. 10.1080/01635589209514173. [DOI] [PubMed] [Google Scholar]
- Jumarie C, Fortin C, Houde M, Campbell PG, & Denizeau F. (2001). Cadmium uptake by Caco-2 cells: effects of Cd complexation by chloride, glutathione, and phytochelatins. Toxicol Appl Pharmacol, 170(1), 29–38. 10.1006/taap.2000.9075. [DOI] [PubMed] [Google Scholar]
- Kahleova H, Levin S, & Barnard N. (2017). Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients, 9(8). 10.3390/nu9080848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layrisse M, Martinez-Torres C, Leets I, Taylor P, & Ramirez J. (1984). Effect of histidine, cysteine, glutathione or beef on iron absorption in humans. J Nutr, 114(1), 217–223. 10.1093/jn/114.1.217. [DOI] [PubMed] [Google Scholar]
- Liu RH (2013). Dietary bioactive compounds and their health implications. J Food Sci, 78 Suppl 1, A18–25. 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- López-Climent MF, Arbona V, Pérez-Clemente RM, Zandalinas SI, & Gómez-Cadenas A. (2014). Effect of cadmium and calcium treatments on phytochelatin and glutathione levels in citrus plants. Plant Biol (Stuttg), 16(1), 79–87. 10.1111/plb.12006. [DOI] [PubMed] [Google Scholar]
- Maier EA, Matthews RD, McDowell JA, Walden RR, & Ahner BA (2003). Environmental cadmium levels increase phytochelatin and glutathione in lettuce grown in a chelator-buffered nutrient solution. J Environ Qual, 32(4), 1356–1364. 10.2134/jeq2003.1356. [DOI] [PubMed] [Google Scholar]
- Mehra RK, Kodati VR, & Abdullah R. (1995). Chain length-dependent Pb(II)-coordination in phytochelatins. Biochem Biophys Res Commun, 215(2), 730–736. 10.1006/bbrc.1995.2524. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cozatl DG, Butko E, Springer F, Torpey JW, Komives EA, Kehr J, & Schroeder JI (2008). Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant Journal, 54(2), 249–259. 10.1111/j.1365-313X.2008.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Zhang L, Wang L, Zhou C, Shangguan Y, & Yang Y. (2019). Antioxidative enzymes activity and thiol metabolism in three leafy vegetables under Cd stress. Ecotoxicol Environ Saf, 173, 214–224. 10.1016/j.ecoenv.2019.02.026. [DOI] [PubMed] [Google Scholar]
- Mou RX, Cao ZY, Lin XY, Wu L, Cao ZZ, Zhu ZW, & Chen MX (2016). Characterization of the phytochelatins and their derivatives in rice exposed to cadmium based on high-performance liquid chromatography coupled with data-dependent hybrid linear ion trap orbitrap mass spectrometry. Rapid Commun Mass Spectrom, 30(16), 1891–1900. 10.1002/rcm.7669. [DOI] [PubMed] [Google Scholar]
- Nayak B, Liu RH, & Tang J. (2015). Effect of processing on phenolic antioxidants of fruits, vegetables, and grains--a review. Crit Rev Food Sci Nutr, 55(7), 887–919. 10.1080/10408398.2011.654142. [DOI] [PubMed] [Google Scholar]
- Ng ZX, & Rosman NF (2019). In vitro digestion and domestic cooking improved the total antioxidant activity and carbohydrate-digestive enzymes inhibitory potential of selected edible mushrooms. J Food Sci Technol, 56(2), 865–877. 10.1007/s13197-018-3547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oven M, Page JE, Zenk MH, & Kutchan TM (2002). Molecular characterization of the homo-phytochelatin synthase of soybean Glycine max: relation to phytochelatin synthase. J Biol Chem, 277(7), 4747–4754. 10.1074/jbc.M108254200. [DOI] [PubMed] [Google Scholar]
- Pal R, & Rai JP (2010). Phytochelatins: peptides involved in heavy metal detoxification. Appl Biochem Biotechnol, 160(3), 945–963. 10.1007/s12010-009-8565-4. [DOI] [PubMed] [Google Scholar]
- Palermo M, Pellegrini N, & Fogliano V. (2014). The effect of cooking on the phytochemical content of vegetables. J Sci Food Agric, 94(6), 1057–1070. 10.1002/jsfa.6478. [DOI] [PubMed] [Google Scholar]
- Piechalak A, Tomaszewska B, Baralkiewicz D, & Malecka A. (2002). Accumulation and detoxification of lead ions in legumes. Phytochemistry, 60(2), 153–162. 10.1016/s0031-9422(02)00067-5. [DOI] [PubMed] [Google Scholar]
- Satarug S, Vesey DA, & Gobe GC (2017). Health Risk Assessment of Dietary Cadmium Intake: Do Current Guidelines Indicate How Much is Safe? Environ Health Perspect, 125(3), 284–288. 10.1289/EHP108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seregin IV, & Kozhevnikova AD (2020). Low-molecular-weight ligands in plants: role in metal homeostasis and hyperaccumulation. Photosynth Res. 10.1007/s11120-020-00768-1. [DOI] [PubMed] [Google Scholar]
- Seregin IV, Vooijs R, Kozhevnikova AD, Ivanov VB, & Schat H. (2007). Effects of cadmium and lead on phytochelatin accumulation in maize shoots and different root parts. Dokl Biol Sci, 415, 304–306. 10.1134/s0012496607040163. [DOI] [PubMed] [Google Scholar]
- Silva EO, & Bracarense AP (2016). Phytic Acid: From Antinutritional to Multiple Protection Factor of Organic Systems. J Food Sci, 81(6), R1357–1362. 10.1111/1750-3841.13320. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Alpers D, & Klein S. (2012). Nutritional Physiology of the Alimentary Tract. In Ross AC, Caballero B. & Cousins RJ (Eds.), Modern Nutrition in Health and Disease (pp. 540–573). Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- USDA, & ERS. (2014). Food Consumption and Nutrient Intakes. Retrieved from: https://www.ers.usda.gov/data-products/food-consumption-and-nutrient-intakes/Accessed October 2016. [Google Scholar]
- Vazquez S, Goldsbrough P, & Carpena RO (2009). Comparative analysis of the contribution of phytochelatins to cadmium and arsenic tolerance in soybean and white lupin. Plant Physiol Biochem, 47(1), 63–67. 10.1016/j.plaphy.2008.09.010. [DOI] [PubMed] [Google Scholar]
- WCRF. (2007). Food, Nutrition, and Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR: World Cancer Research Fund/American Institute for Cancer Research. [Google Scholar]
- Yazdi M, Kolahi M, Mohajel Kazemi E, & Goldson Barnaby A. (2019). Study of the contamination rate and change in growth features of lettuce (Lactuca sativa Linn.) in response to cadmium and a survey of its phytochelatin synthase gene. Ecotoxicol Environ Saf, 180, 295–308. 10.1016/j.ecoenv.2019.04.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.