Abstract
Objective:
The basic objective of this systematic review was to identify potential biomarkers for chronic stress.
Methods:
A systematic review of studies linking biomarkers in people with chronic stress was conducted using PRISMA guidelines. The last 40 years’ studies were included in the systematic review with no age restrictions; animal studies were excluded from the study. Electronic databases including PubMed, Embase, and Google Scholar were searched for the study purpose. The studies were searched using the combinations of search terms that comprised chronic stress together with the keywords hypothalamic-pituitary-adrenal axis (HPA axis), autonomic nervous system (ANS), immune system, metabolic biomarkers, cortisol, hair cortisol, salivary cortisol, urinary cortisol, epinephrine, norepinephrine, adrenocorticotropic hormone (ACTH), brain-derived neurotropic factor (BDNF), metabolic biomarkers, antioxidants, glucose, hemoglobin, C-reactive protein (CRP), cytokines, pro-inflammatory cytokines, anti-inflammatory cytokines, and tumor necrosis factor (TNF).
Results:
A total of 37 studies out of 671 studies met the eligibility criteria and were included in this review. Potential diagnostic biomarkers of chronic stress included cortisol, ACTH, BDNF, catecholamines, glucose, HbA1c, triglycerides, cholesterol, prolactin, oxytocin, dehydroepiandrosterone sulfate (DHEA-S), CRP, and interleukin - 6 and 8. While the others including antioxidants and natural killer (NK) cells require further validation. Taken together, addition, these stress biomarkers have critical prognostic capacities for stress-associated diseases and therapeutic guidance.
Conclusion:
This systematic review provides an update to the literature by highlighting the role of physiological biomarkers in chronic stress and describing their prognostic and therapeutic values.
Keywords: Chronic stress, physiological biomarkers, stress, diagnosis, prognosis
Introduction
Stress is a usual psychophysiological response generated by the body due to the undesirable, challenging, and difficult circumstances or stressors.[1-3] The stress effects on the nervous system are known for more than 50 years;[4] and literature confirms the adverse effects of stress on the human brain.[5] The structural changes, like that in brain atrophy due to chronic stress,[6] lead to differential response and its impact on cognition and memory is also reported.[5] The intensity of effects varies as per the duration of stress, and it causes long-term structural defects to the brain, leading to psychological alterations.[5,7] Any traumatic event can serve as the trigger, for instance, being part of a road accident and getting a lifetime disability after it. Sometimes even witnessing any horrible event can cast a lifelong impact on mental health and well-being.[6] Furthermore, maternal separation is also determined as a powerful stressor that affects the individual in the postnatal period, ultimately extending till adulthood.[5]
Acute stress refers to a short-term and adaptive state. In contrast, chronic stress is a long-lasting condition known to be related to maladaptive response, implying harmful effects on bodily mechanisms.[3,8] Persistent or prolonged stress results in the secretion of certain hormones or chemicals from the body, which indicates the body’s constant stressful condition and affects the vital organs such as the brain, heart, or liver, in various aspects that might not favor the subject’s health. There are many systems in the body that either individually or collectively regulate the level of stress,[9,10] including the hypothalamic–pituitary–adrenal axis (HPA-Axis), Autonomic Nervous System (ANS), and immune system.[11-14] HPA axis reacts to stressors by secreting cortisol, a chemical that prepares the body for fight and flight conditions and is a significant biomarker for stress.[9] Cortisol is mostly concerned with psychological stress making HPA axis a system that mainly responds to psychosocial stress and interacts with ANS and the immune system.[9,10] Due to this interaction, the HPA axis is considered the mediating system and used to determine the effects of stress on disease processes, playing an essential role in cognition, metabolism, behavior, and immune reactivity.[9,15] Although usually, the cortisol level remains high in the body at specific times of the day, for instance, in the morning, if it is persistent throughout the day, it is an alarming situation. Cortisol levels are detectable through various biological media such as saliva, blood, urine, and hair sample.[9]
As mentioned, ANS also contributes to acute and chronic stress generation and regulation. It regulates bodily functions by autonomic reflexes in response to external (environmental, vision, smell, touch, etc.) and internal stimuli (maintenance of body homeostasis including temperature, blood sugar, removal of excess water, obesity etc.).[12,16-19] Epinephrine, norepinephrine, and acetylcholine (ACh) are the prime neurotransmitters released by the sympathetic and parasympathetic ANS. During a stressful condition, catecholamines level increases while acetylcholine decreases, regulating the fight, and flight response.[13,20] The HPA-axis and ANS regulate a series of non-immunological biomarkers, for example, arginine vasopressin (AVP) and dehydroepiandrosterone that are not involved in an immune response. Still, a few of the immunological biomarkers regulated by these are accountable for the immune system’s response to stress. The body’s immune system has fought against these stressors; initiation of such stressful events, for instance, any injury or infection, promotes immune cells’ release into the bloodstream, which then induce fight and flight response.[21] Increase inflammatory responses due to the immune system dysregulation among acute and chronic stress subjects, as the level of pro-inflammatory cytokines rapidly excels under both conditions increasing the likelihood of chronic diseases and associated frailty.[22-24] Moreover, chronic stress’s health consequences are severe compared to acute and lead to latent viral activation, ultimately affecting the immune system.[25] These responses and their intensity may vary from person to person, as an exaggerated immune response to stress might be generated among some individuals.[26,27]
These immunological biomarkers are mainly cytokines, that is, interleukin 6 (IL-6),[9,28,29] IL1- beta,[9] C-Reactive Protein (CRP),[9,30] tumor necrosis factor-alpha (TNF-alpha)[9,28] also termed as pro-inflammatory cytokines, and natural killer cells (NK).[31-33] These immunological indicators and mediators, in particular cytokines and CRP, not only prepare the body against pathogens but also play an essential role in stimulating either acute or chronic stress concerning psychological stimuli or social interactions.[34]
Along with these specified mediators, there are also other metabolic biomarkers such as Fasting glucose, glucose tolerance,[35,36] glycosylated hemoglobin (HbA1c),[37] triglycerides, and cholesterol levels,[38,39] for examining the association of chronic stress with prolonged diseases. Other than these stress hormones, endocrine hormones such as prolactin,[40,41] estradiol,[42] oxytocin,[40] Growth Factor (GF), and Dehydroepiandrosterone Sulfate (DHEA-S)[43,44] are also studied to evaluate the level of chronic stress through blood samples. Hence, these neural endocrine-immune system interactions play an essential role in shaping an individual’s reactivity to persistent stress. In addition to these hormones, the human body also has a complex array of enzymatic and non-enzymatic antioxidants such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GTPx), malondialdehyde (MDA) and Ascorbic Acid, which are involved in antioxidant defense system, that is one of the natural defense systems against the harmful effects of reactive oxygen species (ROS) inducing oxidative stress.[45-47]
Therefore, it provides a useful link to understand the association between stress and diseases also specifies the significance of testing these biomarkers among stressed subjects in routine clinical practices.
Methodology
Study design and search strategy
A systematic search of articles was conducted using PubMed, Embase, and Google scholar, using the combinations of search terms such as chronic stress together with the keywords HPA-Axis, ANS, immune system, metabolic biomarkers, cortisol, hair cortisol, salivary cortisol, urinary cortisol, epinephrine, norepinephrine, Adrenocorticotropic hormone (ACTH), BDNF, metabolic biomarkers, antioxidants, glucose, hemoglobin, CRP, cytokines, Pro-inflammatory cytokines, anti-inflammatory cytokines, and TNF.
Participants
The study population was human subjects. Since the aim of the study is to identify the potential biomarkers for chronic stress. Hence, all similar studies with the selected biomarkers, without restricting age and gender of the participants, were studied.
Data sources, studies selection, and data extraction
Studies of the last 40 years were included in the systematic review. All the articles fulfilling the eligibility criteria were read thoroughly for relevancy. The data were extracted, screened, and scrutinized for the robustness of the article and errors in the inclusion, to confirm the authenticity of the review. The primary information sources were the published research articles, while the reference lists of eligible articles were also searched and scrutinized to obtain all potential data.
Quality of studies
The quality of the included studies was assessed on the basis of selection bias, study design, confounders, and data collection methods.
Inclusion and exclusion criteria
Eligible studies reporting empirical findings on the association between physiological biomarkers and chronic stress were included in the study. Studies of the last 40 years were included in the systematic review with no age restrictions, only human studies published in English were included in the study. While all studies on non-human subjects, outside specified time duration, review articles, and studies with biomarkers other than those studied were excluded from this review.
Data analysis
A systematic summary was established through data synthesis including extraction, and management of the studies. The results were then screened, and combined according to the PRISMA guidelines.
Results
The systematic search and review yielded 628 studies from electronic database searching and 43 additional studies identified through other sources. A total of 616 non-human subject studies were excluded and remaining 55 full-text experimental studies were assessed for eligibility. Finally, 37 studies were included in the quantitative synthesis [Figure 1].
Figure 1.
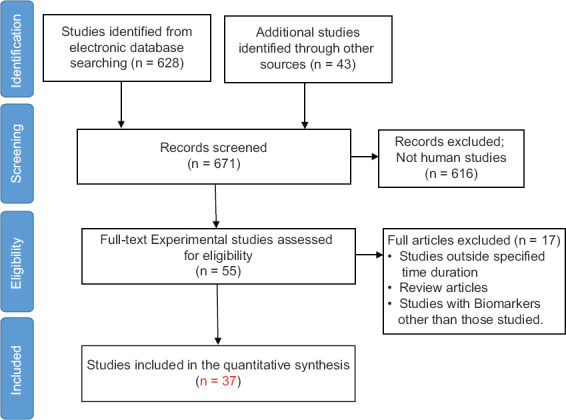
Flow diagram for study selection in this systematic review
Biomarkers of HPA-axis
Cortisol
Among the biomarkers of HPA-Axis in relation to chronic stress, cortisol, ACTH, and BDNF were studied. A total of nine studies supported cortisol measurement as a chronic stress biomarker through hair samples. HPA-axis starts from the hypothalamus by secreting the corticotropin-releasing hormone (CRH) is secreted that further induces the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary stimulating the secretion of cortisol.[9] Cortisol is mainly synthesized from cholesterol as the main glucocorticoid in zona fasciculata of the human adrenal cortex secreted by the influence of biochemical stress.[15,48,49] Cortisol is present as bound and unbound form; the unbound cortisol is of low molecular weight and is lipophilic. It enters the cell through passive diffusion, thus, easily determined in body fluids.[50] The blood cortisol measurement timings have significant importance as it is increased in blood cortisol level in the early morning and decreases in the evening time and initial phase of sleep.[50] Herein, we focused on those studies that helped us identify cortisol as a biomarker of chronic stress and cortisol quantification methods through hair samples, saliva, and urine. Rauel et al. were the first who examined cortisol through hair samples.[51]
Hair cortisol
According to Table 1, hair cortisol is demonstrated as a potential clinical biomarker for infants to address neonatal chronic stress better using a baseline measure comparison in which hair cortisol is influenced by day of ventilation in the neonatal intensive care unit (NICU).[52] Moreover, another study assessed stress among mothers and their infants, using intra stability of hair, cortisol suggests its potential biomarker in detecting chronic stress experiences during the first year of life and the postpartum period.[53] Furthermore, hair cortisol can be a good source for measuring chronic stress in pregnant women.[54] This long-term biomarker provides a vital implication in clinical practices and research.[54] Moreover, another study examined the role of cortisol in pregnant women’s hair. This study suggested that hair cortisol is a potential biomarker of chronic stress. Hence, this long-term biological marker has a key association in clinical research practices.[54-57]
Table 1.
Studies on hair cortisol
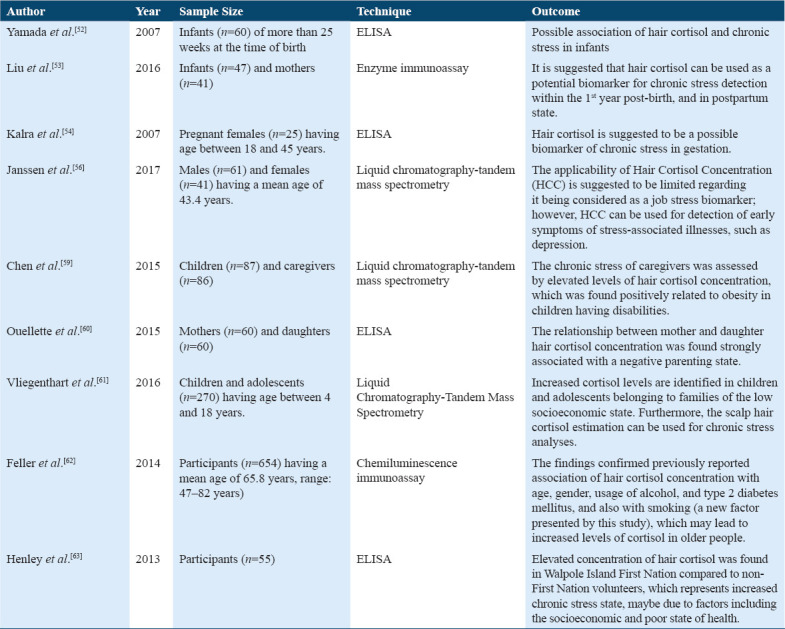
The quantification of scalp hair cortisol in children seems to be a biomarker for long-term cortisol measurement and thereby a biomarker of stress. A study examined HCC in children in the early phase of elementary school. It proposed a high-stress hormone level in fearful children at that stage.[58] Furthermore, another study demonstrated that the chronic stress evaluated by increased cortisol levels in hair were positively associated with obesity measures among children with disabilities.[59] Besides, Quellete et al. examined the validity of hair cortisol concentration (HCC) in mother and daughter characterized by higher and lower maternal chronic stress, and overall findings indicated HCC as a useful biomarker of cortisol in response to chronic stress.[60]
Moreover, chronic stress is associated with low socioeconomic status (SES) in children of young age and adolescent population by activating HPA-axis and releasing a high level of the stress hormone cortisol. According to this study, chronically high hair cortisol levels were found in children of young age and adolescents in low SES.[61] However, older people with increasing age have more chances for a stress-related disease that correlates with chronically elevated cortisol secretion.[62] HCC showed a positive relation with age, female sex, alcohol consumption, and other factors such as smoking, contributing to significantly elevated cortisol levels in older people.[62] Moreover, increased hair cortisol suggested a high level of chronic stress among the Walpole island first nation volunteers compared to volunteers of the non-first nation.[63] Thus, the most reliable biomarker of chronic stress and the most readily used validation exhibited hair cortisol measurements.
Salivary cortisol
There were a total of seven studies for salivary cortisol as a biomarker of chronic stress (Table 2). The use of salivary cortisol as a stress biomarker increases and provides useful results when estimating cortisol through saliva samples.[64-70] However, when investigated the role of the physiological stress response and basal physiological values in health and stress (burnout), individual study suggested that stress (burnout) patients showed higher heart rate and high cortisol level during the 1st h after awakening from the sleep when compared with healthy subjects.[64] Furthermore, this study also suggested the association between chronic stress and cortisol changes during the 1st h of post-sleep in the morning. The salivary cortisol was found to be elevated in chronically stressed subjects compared to unstressed subjects.[65] According to Soderstrom et al., 2006, burnout (stress), patients showed a high awakening cortisol response during the workday than during the weakened.[66] However, when examined the role of free cortisol response after awakening among both genders, the study suggested high cortisol response in females with high (stress) burnout than to females with low burnout (stress) at awakening and +15 min, +30 min, and +60 min after awakening whereas, male participants with moderate burnout (stress) showed high cortisol level at +60 min, after awakening than to low burnout (stress) male participants.[67] Therefore, the measurement of free cortisol in response to awakening should be considered a possible biomarker for chronic stress.[64] Moreover, this study examined whether chronic stress (burnout) is associated with somatic and physiological arousal, intensified somatic arousals and elevated salivary cortisol levels.[68] In addition, the study for determining the physiological biomarker of chronic stress through a salivary sample on middle-aged women revealed that stressed women have high evening (9 pm) salivary cortisol level and they had high urinary testosterone from the first-morning void whereas no association found between overnight urinary cortisol and catecholamines Table 6.[69]
Table 2.
Studies on salivary cortisol
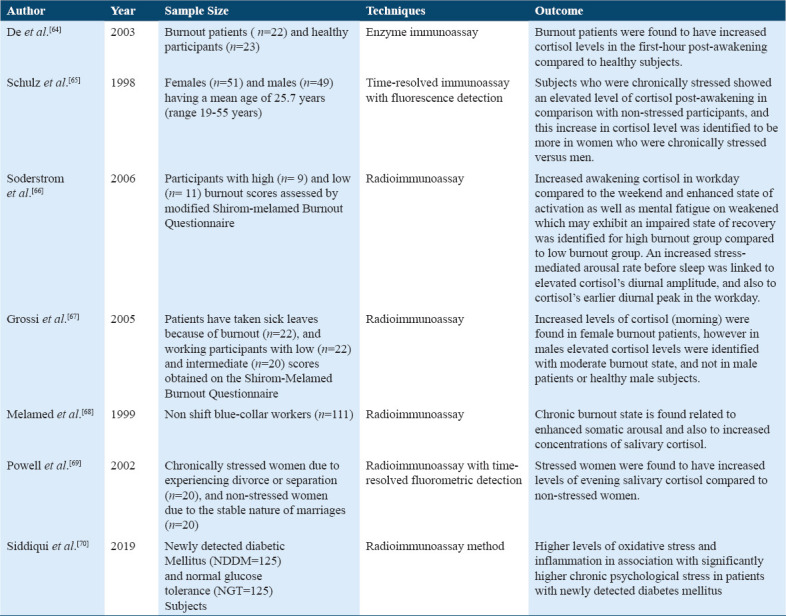
Table 6.
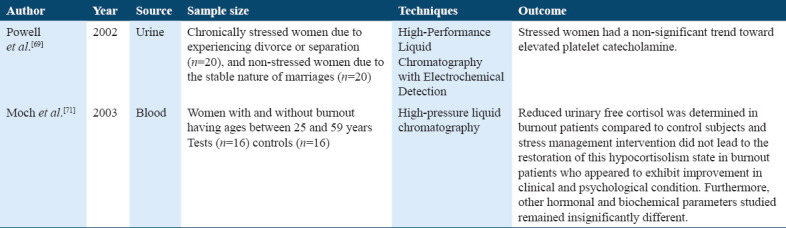
Studies on catecholamine
Urinary cortisol
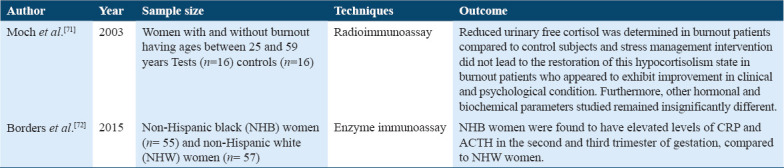
Free cortisol was found in chronically stressed (burnout) patients when compared to control healthy subjects. Hormonal and biochemical changes remained indifferent in these patients versus control subjects. These results indicated hypocortisolism in the stressed patient Tables 3 and 6.[71]
Table 3.
Study on urinary cortisol

Adrenocorticotropic hormone (ACTH)
Corticotrophin releasing hormone (CRH) is a crucial mediator of endocrine, autonomic, and immune responses to stress. There are two studies in which ACTH was used as a measure to evaluate chronic stress (Table 4).[71,72]
Table 4.
Studies on adrenocorticotropic hormone
Brain-derived neurotropic factor (BDNF)
BDNF is considered necessary in the regulation, development, and survival of neurons and the maintenance of their physiological activity.[35] However, the studies suggested that the stress (burnout) subjects have significantly lowered the level of BDNF and symptoms such as altered mood and cognition factor when compared with healthy control subjects (Table 5).[36]
Table 5.
Study on brain-derived neurotropic factor

Biomarkers of Autonomic Nervous System (ANS)
Two physiological systems commonly; HPA-axis and sympathetic adrenergic medullary-axis (SAM) are involved in biomarkers of the autonomic nervous system. HPA-axis has a slow response toward a stressor whereas the SAM axis activates instantaneously and shows a very adaptive response to a stressor.[9,10] Moreover, SAM deals with immediate sympathetic activation. Hence, it stimulates the ability of an individual to cope with the conditions such as increased heart rate, blood pressure, and the release of catecholamines which include epinephrine and norepinephrine.[65]
Catecholamines
Catecholamine is one of the major biomarkers for the ANS activity. Epinephrine and norepinephrine are mainly synthesized by the central noradrenergic neurons and are released mainly from the adrenal medulla in the bloodstream.[20]
Biomarkers of Metabolic Processes
Like HPA-axis and SAM-axis, several metabolic biomarkers play a major role in the physiology of stress, entailing a group of biomarkers such as glucose, glycosylated hemoglobin (HbA1c), cholesterols, and insulin. Thus, categorizing this group as metabolic biomarkers can be an effective tool for a biological measure of chronic stress level in the body.
Glucose, HbA1c, triglycerides, and cholesterol
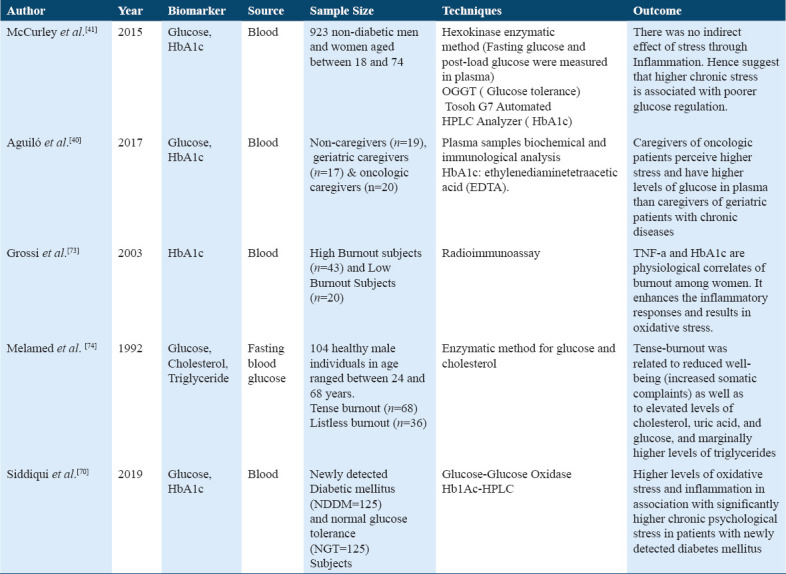
The first study in Table 7 shows that the association of chronic stress with fasting glucose, glucose tolerance, HbA1c also involves the indirect effect of stress through inflammatory marker CRP.[73,74] However, it was found that high chronic stress leads to elevated levels of fasting glucose, post loaded glucose, and HbA1c level that there was no indirect effect of stress with CRP, this result suggested that high chronic stress is associated with poor glucose regulation in Hispanics subjects before onset of clinical diabetes diagnosis.[42]
Table 7.
Studies on glucose, HbA1c, cholesterol, and triglycerides
Endocrine Hormones
Prolactin and oxytocin
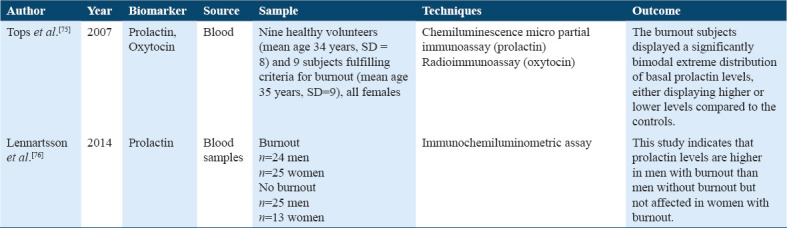
Many other hormones such as prolactin, oxytocin, Dehydroepiandrosterone Sulfate (DHEA-S), and growth hormone are studied in various stress groups to determine the effects of these endocrine hormones as a physiological biomarker (Table 8).[75,76]
Table 8.
Studies on prolactin and oxytocin
Dehydroepiandrosterone sulfate (DHEA-S)
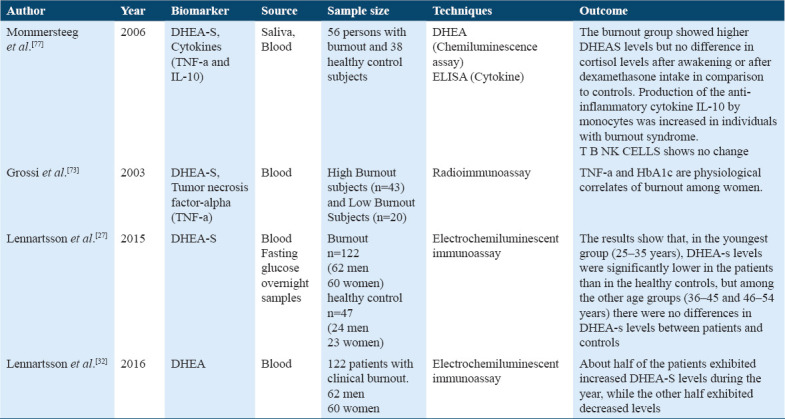
It is a steroid hormone with a capability of immunomodulatory function opposite to cortisol, and it is produced by the zona - reticularis in the adrenal cortex in response to ACTH Table 9.[43,45,77] It has a significant role as a regeneration and protection function that’s leads to maintenance of health status,[46] whereas increased and decreased levels of DHEA-S are associated with the diagnosis of health issues and outcomes.
Table 9.
Studies on Dehydroepiandrosterone sulfate and Cytokines
Antioxidants
Several enzymes such as SOD, catalase, and MOD are involved in the body’s natural defense system against reactive oxygen species (ROS), contributing to aging, and disease-related problems.[78]
SOD and catalase
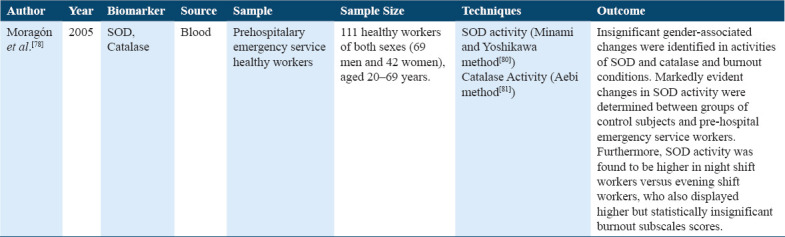
The role of two antioxidant enzymes, SOD and catalase, in stress in healthy workers has been studied,[78,79] it was shown that SOD was higher in workers of evening and night shift Table 10.
Table 10.
Studies on superoxide dismutase and catalase
Malondialdehyde (MDA)
The stressful condition leads to free radicals along with MDA as an end product of lipid peroxidation. The relationship between MDA and burnout levels in workers, when identified at night and evening show increased level of MDA and high-stress level. In contrast, MDA increases with age. No changes in MDA levels are found for sex; however, MDA is considered a biomarker of lipid peroxidation, occupational, and oxidative stress rather than chronic stress biomarker Table 11.[29,82]
Table 11.
Study on Malondialdehyde

Biomarkers of Immune System
The immunological biomarkers are an effective tool in creating a link between pathways between the immune and neuroendocrine systems in chronic stress-related outcomes.[84] However, few studies determine the role of immunological biomarkers and the association of different cytokines measures in plasma in the blood samples and it involves individual cytokine anti-inflammatory and pro-inflammatory or it showed the ratio of anti-inflammatory and pro-inflammatory in several studies to detect the role of cytokines as a biomarker of chronic stress.
Cytokines
There are certain groups of cytokines such as growth factors, interleukins, and interferon. There are certain immune biomarkers such as pro-inflammatory (IL-6, IL-1b) cytokines, anti-inflammatory (IL-10), TNF-alpha, T, B cell, and NK cells and some of them are discussed below. All of these play a major role in immune responses by promoting the cell to cell communication and thus stimulates its role at the side of inflammation, infection, and trauma.[30]
Anti-inflammatory and pro-inflammatory cytokines
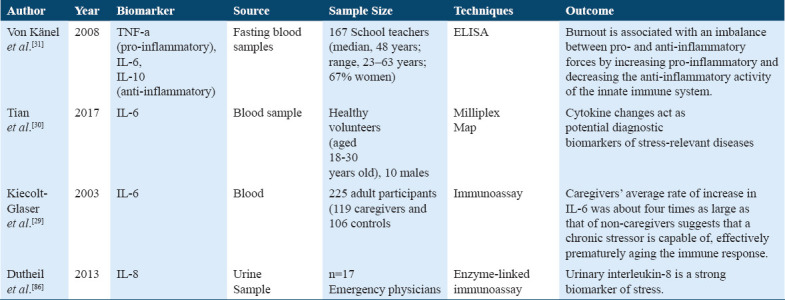
As discussed earlier (Table 12), few studies demonstrated cytokines’ role in stress physiology.[32,85-88] A previous study has presented the association of stress (burnout) with circulating anti-inflammatory (IL-4, IL-10) and pro-inflammatory (TNF-alpha) cytokines, where burnout stress teachers possessed higher levels of TNF-alpha/IL-4 ratio,[32] suggests burnout stress subjects are related to increased systemic inflammation.[32]
Table 12.
Studies on anti-inflammatory and pro-inflammatory cytokines
Natural killer cells (NK) and leukocytes
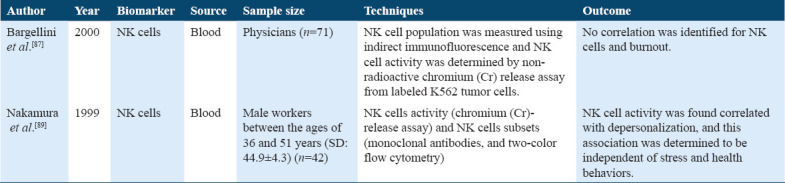
Few studies have found the association between different types of white blood cells (leukocytes) and the levels of stress (burnout) state (Table 13).[28,89] The present study demonstrated the relationship between burnout (stress) state and immune variables indicating that high scores for stress lead toward an increased leukocyte level and several T cell counts above the normal range.[90] Several studies do not involve the increased number of lymphocytes subsets due to burnout stress state.[28,90] Hence, to summarize, burnout (stress) does not usually associate with a large number of leukocyte changes in the blood.[28]
Table 13.
Studies on Natural Killer cells and leukocytes
C-reactive protein (CRP) and fibrinogen
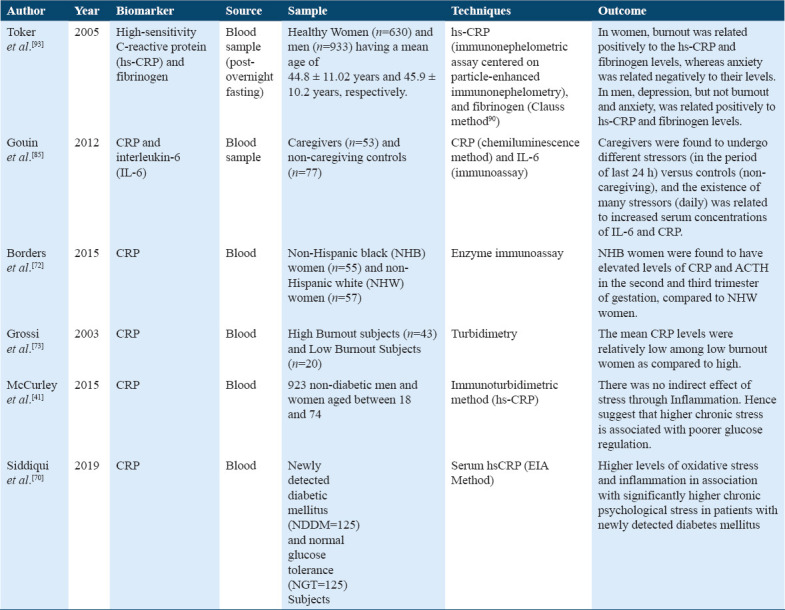
A study in support showed a significant relationship between chronic stress, inflammatory markers, and daily stressors (Table 14). It suggested that among family dementia caregivers, the increased daily stressors experience contributes to an elevation in the IL-6 and CRP levels. However, CRP showed more contrasting results with such variations, that either showing no relationship between burnout stress state and non-burnout state,[42,74] while in some studies it showed increased levels of CRP for burnout (stress) state.[91]
Table 14.
Studies on C-reactive protein and fibrinogen
Discussion
All of the included 37 studies were original researches from the past 40 years and included any of the specific biomarkers that we have studied in this review. Among the biomarkers of HPA-Axis in relation to chronic stress, cortisol, ACTH, and BDNF were studied. A total of nine studies supported cortisol measurement as a chronic stress biomarker through hair samples. The useful cortisol parameters are urine, saliva, and blood; however, these parameters have some limitations.[33] The best and recent innovation for cortisol estimation is from hair. According to Kimberly et al, potential biomarker during maternal stress is cortisol; they proposed that hair cortisol as a valid biomarker rather than samples from urine or salivary samples. Hair cortisol concentration (HCC) is a useful tool in evaluating long-term cortisol levels. It provides a reliable measurement of HPA-axis activity throughout pregnancy and the reflecting total cortisol release.[56] However, the working population depicted the association between chronic stress and hair cortisol concentration and suggested that hair cortisol concentration’s limited applicability is an essential biomarker in work-related stress. Besides, it may be useful for the early detection of depression.[57] Compared to salivary cortisol, hair cortisol appears to be a significant biomarker for evaluating stress and is a better indicator of stress system.[58] Furthermore, the role of ACTH as a biological measure of chronic stress in the study of non-Hispanic black and white pregnant women suggested that black Hispanic pregnant women had elevated mean levels of ACTH and C - reactive protein (CRP) in the 2nd and 3rd trimester of pregnancy.[72] However, another study did not show any ACTH association with biological measures of chronic stress in blood samples.[72] Similarly, for BDNF studies suggest that the stress subjects have significantly low BDNF levels.[35,36]
Among the ANS biomarkers, Catecholamines were studied while among those for the metabolic processes were glucose, HbA1c, triglycerides, and cholesterol. The included study suggested that there is no association found between ANS mediated hormones and chronic stress. The previous studies did not identify changes in stressed subjects versus healthy subjects,[70,72] thus, it cannot be used as a valid biomarker for chronic stress detection. A study determined the role of the stressor on the individual, through metabolic endocrine, and immunological biomarker without any significant changes found through endocrine and immunological markers; however, statistical changes were found in glucose and HbA1c in two different caregivers group.[41] However, another study determined metabolic, endocrine, and immune correlates of burnout (stress) state among women, and high HbA1c and TNF-alpha levels in women with burnout and suggested these women had enhanced inflammatory responses and oxidative stress.[74] Moreover, the association of tensed burnout with cardiovascular disease (CVD) risk factors results showed reduced health state and elevated levels of glucose, cholesterols, triglycerides, and uric acid.[75]
Prolactin, oxytocin, and Dehydroepiandrosterone Sulfate (DHEA-S) were the endocrine hormones studied. Three articles compared DHEA-S levels through blood samples and showed the association of DHEA-S levels between stress (burnout) subjects and controlled healthy subjects. The low dose dexamethasone and DHEA-S levels were identified; however, higher DHEA-S levels were found in burnout, and no difference was found in cortisol level. Furthermore, Pro-inflammatory cytokine IL-10 was found to be increased in stress subjects.[78] When DHEA-S levels were compared between stress subjects and healthy subjects within each three 10 years interval age group, the youngest age group (25-34 years) showed lower DHEA-S levels more in females than in males of stress subjects. In comparison, no change in stress subjects for DHEA-S levels was found in the age group (35–44 years) and (45–54 years) when compared with control subjects.[28] Moreover, changes in DHEA-S levels in stress patients during the 1st year of their treatment leads led to the development in health, hence changes in DHEA-S sulfate associated with the prognosis of outcome in stress patients.[33] Furthermore, we studied SOD and catalase among the antioxidants, only one study analyzed the role of two antioxidant enzymes, SOD, MDA, and catalase, in stress in healthy workers, showed activity SOD was higher in workers of evening and night shift.[80]
Biomarkers of immune system included cytokines, anti-inflammatory, pro-inflammatory cytokines, NK cells, leukocytes, CRP, and fibrinogen. A reviewed study showed high levels of IL-6 due to chronic stress that explains the changes due to chronic stress that produces adaptations in the immunological system reflected in the certain number of cytokines and immune cells, which do not return to normal baseline even after removal of stressors. That justify decreased immune function in people with chronic stress provides a possibility to identify a personal vulnerability with specific biomarkers.[31,86] Furthermore, the presented study that shows showed the association of IL-6 and chronic stress; hence, it was found that IL-6 was four times greater in caregivers than non-caregivers, and this provides an evidenced mechanism through which chronic stressors may be related to age-associated disease and ageing of the immune system.[30] However, this present study indicates that chronic stress and its responses are widely associated with oxidative stress and inflammation, which may further lead to the risk of type 2 diabetes.[71] The anti-inflammatory cytokines (IL-10) found to be increased in patients with burnout (stress) state.[80] However, another study shows that interleukin-8 is a robust biomarker of chronic stress, and elevated levels of IL-8 are associated with cardiovascular disease and negative psychological consequences.[87]
While, changes in NK cell activity and its number due to chronic stress were found in a few studies. One study shows that due to high burnout, depersonalization scores indicate decreased NK cell activity in male office workers and no changes in NK cell activity due to other burnout scores were found to in these male workers.[89] The above studies summarized that the exact role of NK cell activity and variation in leukocyte number and activity due to chronic stress response is not clear. Hence, NK cells and leukocytes cannot be such valid biomarkers as compared to that of others. Furthermore, CRP is one of the significant biomarkers of inflammation,[80] and studies have presented its enhanced levels in chronic stress conditions.[92,93] A previous study has reported a positive relation of burnout with hs-CRP and fibrinogen levels in women, but not in men.[94]
Summary of Main Findings and Study Limitations
Each biomarker plays an important role in stress, and each portray different outcomes. Moreover, this review helps to identify the relevance for biomarkers in chronic stress that can be helpful as the biological predecessor of disease, prognosticator of disease progression, and a potential target for behavioral interferences in chronic stress. Among the major limitation of this review was a few of the studies had a fragmented outcomes; however, conclusions are drawn based on this descriptive systematic analysis. However, we recommend a more extensive and yet specific literature search for each of these included biomarkers.
Conclusion
This review highlights the physiological biomarkers of HPA axis, ANS, immune, endocrine, metabolic, and defense system for how each of these play a major role in chronic stress. Hair and salivary cortisol, HPA axis biomarkers are considered as a major source of evaluating chronic stress levels in the targeted individuals. As the salivary and urinary biomarkers provide convenient measurement methods.
References
- 1.Selye H. Psychopathology of Human Adaptation. Berlin, Germany: Springer; 1976. Stress without Distress; pp. 137–46. [Google Scholar]
- 2.Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function:A review. EXCLI J. 2017;16:1057. doi: 10.17179/excli2017-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneiderman N, Ironson G, Siegel SD. Stress and health:Psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–28. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thierry AM, Javoy F, Glowinski J, Kety SS. Effects of stress on the metabolism of norepinephrine, dopamine and serotonin in the central nervous system of the rat I. Modifications of norepinephrine turnover. J Pharmacol Exp Ther. 1968;163:163–71. [PubMed] [Google Scholar]
- 5.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 6.Sarahian N, Sahraei H, Zardooz H, Alibeik H, Sadeghi B. Effect of memantine administration within the nucleus accumbens on changes in weight and volume of the brain and adrenal gland during chronic stress in female mice. Modares J Med Sci. 2014;17:71–82. [Google Scholar]
- 7.Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala:Differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109–14. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- 8.Selye H. The Stress of Life. New York: McGraw-Hill; 1956. [Google Scholar]
- 9.Nater UM, Skoluda N, Strahler J. Biomarkers of stress in behavioural medicine. Curr Opin Psychiatry. 2013;26:440–5. doi: 10.1097/YCO.0b013e328363b4ed. [DOI] [PubMed] [Google Scholar]
- 10.Kudielka BM, Wüst S. Human models in acute and chronic stress:Assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- 11.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neuro. 2006;8:383–95. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morey JN, Boggero IA, Scott AB, Segerstrom SC. Current directions in stress and human immune function. Curr Opin Psychol. 2015;5:13–7. doi: 10.1016/j.copsyc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Won E, Kim YK. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr Neuropharmacol. 2016;14:665–73. doi: 10.2174/1570159X14666151208113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology. 2006;31:1583–91. doi: 10.1038/sj.npp.1301012. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DS. Clinical assessment of sympathetic responses to stress. Ann N Y Acad Sci. 1995;771:570–93. doi: 10.1111/j.1749-6632.1995.tb44711.x. [DOI] [PubMed] [Google Scholar]
- 16.Rasheed N. Prolonged stress leads to serious health problems:Preventive approaches. Int J Health Sci (Qassim) 2016;10:5–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Sedky NA. Perceived sources of stress among junior and mid-senior Egyptian dental students. Int J Health Sci. 2012;6:141–57. doi: 10.12816/0005990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasheed N. Stress-associated eating leads to obesity. Int J Health Sci. 2017;11:1–2. [PMC free article] [PubMed] [Google Scholar]
- 19.Farooq A, Patel M, Ahmed S, Noushad S. Obesity as a noticeable cause of physical stress;a study on relationship of physical exertion and cardiovascular parameters. Int J Endors Health Sci Res. 2016;4:39–4. [Google Scholar]
- 20.Segerstrom SC, Miller GE. Psychological stress and the human immune system:A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlsson E, Frostell A, Ludvigsson J, Faresjo M. Psychological stress in children may alter the immune response. J Immunol. 2014;192:2071–81. doi: 10.4049/jimmunol.1301713. [DOI] [PubMed] [Google Scholar]
- 22.Fagundes CP, Way B. Early-life stress and adult inflammation. Curr Dir Psychol. 2014;23:277–83. [Google Scholar]
- 23.Ershler WB. Interleukin-6:A cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–81. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 24.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence:Is it infectious? Immunol Rev. 2005;205:257–68. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 25.McEwen BS. Brain on stress:How the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17180–5. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 responses to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–23. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lennartsson AK, Theorell T, Kushnir MM, Jonsdottir IH. Low levels of dehydroepiandrosterone sulfate in younger burnout patients. PLoS One. 2015;10:e0140054. doi: 10.1371/journal.pone.0140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casado Á, de Lucas N, López-Fernández E, Sánchez A, Jimenez JA. Lipid peroxidation, occupational stress and aging in workers of a prehospital emergency service. Eur J Emerg Med. 2006;13:165–71. doi: 10.1097/01.mej.0000194404.61076.88. [DOI] [PubMed] [Google Scholar]
- 29.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian R, Hou G, Song L, Zhang J, Yuan TF. Chronic grouped social restriction triggers long-lasting immune system adaptations. Oncotarget. 2017;8:33652. doi: 10.18632/oncotarget.16856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Känel R, Bellingrath S, Kudielka BM. Association between burnout and circulating levels of pro-and anti-inflammatory cytokines in schoolteachers. J Psychosom Res. 2008;65:51–9. doi: 10.1016/j.jpsychores.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Lennartsson AK, Theorell T, Kushnir MM, Jonsdottir IH. Changes in DHEA-s levels during the first year of treatment in patients with clinical burnout are related to health development. Biol Psychol. 2016;120:28–34. doi: 10.1016/j.biopsycho.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans:A review and meta-analysis. Brain Behav Immun. 2007;21:901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Panek M, Jonakowski M, Zioło J, Pietras T, Wieteska Ł, Małachowska B, et al. Identification of relationships between interleukin 15 mRNA and brain-derived neurotrophic factor II mRNA levels with formal components of temperament in asthmatic patients. Mol Neurobiol. 2017;54:1733–44. doi: 10.1007/s12035-016-9768-7. [DOI] [PubMed] [Google Scholar]
- 35.Sertoz OO, Binbay IT, Koylu E, Noyan A, Yıldırım E, Mete HE. The role of BDNF and HPA axis in the neurobiology of burnout syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1459–65. doi: 10.1016/j.pnpbp.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Steptoe A, Cropley M, Joekes K. Job strain, blood pressure and response to uncontrollable stress. J Hypertens. 1999;17:193–200. doi: 10.1097/00004872-199917020-00003. [DOI] [PubMed] [Google Scholar]
- 37.Toker S, Melamed S, Berliner S, Zeltser D, Shapira I. Burnout and risk of coronary heart disease:A prospective study of 8838 employees. Psychosom Med. 2012;74:840–7. doi: 10.1097/PSY.0b013e31826c3174. [DOI] [PubMed] [Google Scholar]
- 38.Appels A, Schouten E. Burnout as a risk factor for coronary heart disease. Behav Med. 1991;17:53–9. doi: 10.1080/08964289.1991.9935158. [DOI] [PubMed] [Google Scholar]
- 39.Michels N, Sioen I, Clays E, de Buyzere M, Ahrens W, Huybrechts I, et al. Children's heart rate variability as stress indicator:Association with reported stress and cortisol. Biol Psychol. 2013;94:433–40. doi: 10.1016/j.biopsycho.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Aguiló S, García E, Arza A, Garzón-Rey JM, Aguiló J. Evaluation of chronic stress indicators in geriatric and oncologic caregivers:A cross-sectional study. Stress. 2018;21:36–42. doi: 10.1080/10253890.2017.1391211. [DOI] [PubMed] [Google Scholar]
- 41.McCurley JL, Mills PJ, Roesch SC, Carnethon M, Giacinto RE, Isasi CR, et al. Chronic stress, inflammation, and glucose regulation in US Hispanics from the HCHS/SOL sociocultural ancillary study. Psychophysiology. 2015;52:1071–9. doi: 10.1111/psyp.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danhof-Pont MB, van Veen T, Zitman FG. Biomarkers in burnout:A systematic review. J Psychosom Res. 2011;70:505–24. doi: 10.1016/j.jpsychores.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Johansson GG, Karonen SL, Laakso ML. Reversal of an elevated plasma level of prolactin during prolonged psychological stress. Acta Physiol Scand. 1983;119:463–4. doi: 10.1111/j.1748-1716.1983.tb07364.x. [DOI] [PubMed] [Google Scholar]
- 44.Nieschlag E, Loriaux DL, Ruder H, Zucker I, Kirschner M, Lipsett M. The secretion of dehydroepiandrosterone and dehydroepiandrosterone sulphate in man. J Endocrinol. 1973;57:123–34. doi: 10.1677/joe.0.0570123. [DOI] [PubMed] [Google Scholar]
- 45.Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone (DHEA)-a precursor steroid or an active hormone in human physiology (CME) J Sex Med. 2011;8:2960–82. doi: 10.1111/j.1743-6109.2011.02523.x. [DOI] [PubMed] [Google Scholar]
- 46.Staab CA, Maser E. 11b-Hydroxysteroid dehydrogenase Type 1 is an important regulator at the interface of obesity and inflammation. J Steroid Biochem Mol Biol. 2010;119:56–72. doi: 10.1016/j.jsbmb.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Noushad S, Sajid U, Ahmed S, Saleem Y. Oxidative stress mediated neurodegeneration;a cellular perspective. Int J Endors Health Sci Res. 2019;7:192–212. [Google Scholar]
- 48.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Miller WL. Disorders of the Human Adrenal Cortex. Vol. 13. Basel, Switzerland: Karger Publishers; 2008. Steroidogenic enzymes; pp. 1–18. [Google Scholar]
- 50.Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 51.Raul JS, Cirimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 2004;37:1105–11. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, et al. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 2007;92:42–9. doi: 10.1159/000100085. [DOI] [PubMed] [Google Scholar]
- 53.Liu CH, Snidman N, Leonard A, Meyer J, Tronick E. Intra-individual stability and developmental change in hair cortisol among postpartum mothers and infants:Implications for understanding chronic stress. Dev Psychobiol. 2016;58:509–18. doi: 10.1002/dev.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalra S, Einarson A, Karaskov T, van Uum S, Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med. 2007;30:E103–7. doi: 10.25011/cim.v30i2.986. [DOI] [PubMed] [Google Scholar]
- 55.D'Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy:Comparison to salivary cortisol. Physiol Behav. 2011;104:348–53. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janssens H, Clays E, Fiers T, Verstraete A, de Bacquer D, Braeckman L. Hair cortisol in relation to job stress and depressive symptoms. Occup Med (Lond) 2017;67:114–20. doi: 10.1093/occmed/kqw114. [DOI] [PubMed] [Google Scholar]
- 57.Iglesias S, Jacobsen D, Gonzalez D, Azzara S, Repetto EM, Jamardo J, et al. Hair cortisol:A new tool for evaluating stress in programs of stress management. Life Sci. 2015;141:188–92. doi: 10.1016/j.lfs.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Groeneveld MG, Vermeer HJ, Linting M, Noppe G, van Rossum EF, van IJzendoorn MH. Children's hair cortisol as a biomarker of stress at school entry. Stress. 2013;16:711–5. doi: 10.3109/10253890.2013.817553. [DOI] [PubMed] [Google Scholar]
- 59.Chen X, Gelaye B, Velez JC, Barbosa C, Pepper M, Andrade A, et al. Caregivers'hair cortisol:A possible biomarker of chronic stress is associated with obesity measures among children with disabilities. BMC Pediatr. 2015;15:9. doi: 10.1186/s12887-015-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ouellette SJ, Russell E, Kryski KR, Sheikh HI, Singh SM, Koren G, et al. Hair cortisol concentrations in higher-and lower-stress mother-daughter dyads:A pilot study of associations and moderators. Dev Psychobiol. 2015;57:519–34. doi: 10.1002/dev.21302. [DOI] [PubMed] [Google Scholar]
- 61.Vliegenthart J, Noppe G, van Rossum E, Koper J, Raat H, van den Akker E. Socioeconomic status in children is associated with hair cortisol levels as a biological measure of chronic stress. Psychoneuroendocrinology. 2016;65:9–14. doi: 10.1016/j.psyneuen.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 62.Feller S, Vigl M, Bergmann MM, Boeing H, Kirschbaum C, Stalder T. Predictors of hair cortisol concentrations in older adults. Psychoneuroendocrinology. 2014;39:132–40. doi: 10.1016/j.psyneuen.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Henley P, Jahedmotlagh Z, Thomson S, Hill J, Darnell R, Jacobs D, et al. Hair cortisol as a biomarker of stress among a first nation in Canada. Ther Drug Monit. 2013;35:595–9. doi: 10.1097/FTD.0b013e318292eb84. [DOI] [PubMed] [Google Scholar]
- 64.de Vente W, Olff M, van Amsterdam J, Kamphuis J, Emmelkamp P. Physiological differences between burnout patients and healthy controls:Blood pressure, heart rate, and cortisol responses. Occup Environ Med. 2003;60(Suppl 1):i54–61. doi: 10.1136/oem.60.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schulz P, Kirschbaum C, Prüßner J, Hellhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med. 1998;14:91–7. [Google Scholar]
- 66.Soderstrom M, Ekstedt M, Akerstedt T. Weekday and weekend patterns of diurnal cortisol, activation and fatigue among people scoring high for burnout. Scand J Work Environ Health. 2006;32:35–40. doi: 10.5271/sjweh.987. [DOI] [PubMed] [Google Scholar]
- 67.Grossi G, Perski A, Ekstedt M, Johansson T, Lindström M, Holm K. The morning salivary cortisol response in burnout. J Psychosom Res. 2005;59:103–11. doi: 10.1016/j.jpsychores.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Melamed S, Ugarten U, Shirom A, Kahana L, Lerman Y, Froom P. Chronic burnout, somatic arousal and elevated salivary cortisol levels. J Psychosom Res. 1999;46:591–8. doi: 10.1016/s0022-3999(99)00007-0. [DOI] [PubMed] [Google Scholar]
- 69.Powell LH, Lovallo WR, Matthews KA, Meyer P, Midgley AR, Baum A, et al. Physiologic markers of chronic stress in premenopausal, middle-aged women. Psychosom Med. 2002;64:502–9. doi: 10.1097/00006842-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 70.Siddiqui A, Desai NG, Sharma SB, Aslam M, Sinha UK, Madhu SV. Association of oxidative stress and inflammatory markers with chronic stress in patients with newly diagnosed Type 2 diabetes. Diabetes Metab Res Rev. 2019;35:e3147. doi: 10.1002/dmrr.3147. [DOI] [PubMed] [Google Scholar]
- 71.Moch SL, Panz VR, Joffe BI, Havlik I, Moch JD. Longitudinal changes in pituitary-adrenal hormones in South African women with burnout. Endocrine. 2003;21:267–72. doi: 10.1385/ENDO:21:3:267. [DOI] [PubMed] [Google Scholar]
- 72.Borders AE, Wolfe K, Qadir S, Kim KY, Holl J, Grobman W. Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. J Perinatol. 2015;35:580–4. doi: 10.1038/jp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grossi G, Perski A, Evengård B, Blomkvist V, Orth-Gomér K. Physiological correlates of burnout among women. J Psychosom Res. 2003;55:309–16. doi: 10.1016/s0022-3999(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 74.Melamed S, Kushnir T, Shirom A. Burnout and risk factors for cardiovascular diseases. Behav Med. 1992;18:53–60. doi: 10.1080/08964289.1992.9935172. [DOI] [PubMed] [Google Scholar]
- 75.Tops M, Boksem MA, Wijers AA, van Duinen H, den Boer JA, Meijman TF, et al. The psychobiology of burnout:Are there two different syndromes? Neuropsychobiology. 2007;55:143–50. doi: 10.1159/000106056. [DOI] [PubMed] [Google Scholar]
- 76.Lennartsson AK, Billig H, Jonsdottir IH. Burnout is associated with elevated prolactin levels in men but not in women. J Psychosom Res. 2014;76:380–3. doi: 10.1016/j.jpsychores.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 77.Mommersteeg PM, Heijnen CJ, Kavelaars A, van Doornen LJ. Immune and endocrine function in burnout syndrome. Psychosom Med. 2006;68:879–86. doi: 10.1097/01.psy.0000239247.47581.0c. [DOI] [PubMed] [Google Scholar]
- 78.Moragón AC, García ND, Fernandez ME, Rodriguez-Manzaneque AS, Fraile JA. Antioxidant enzymes, occupational stress and burnout in workers of a prehospitalary emergency service. Eur J Emerg Med. 2005;12:111–5. doi: 10.1097/00063110-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Ismail MK, Samera MY, Abid SK. Oxidative stress markers and antioxidant activity in patients admitted to intensive care unit with acute myocardial infarction. Int J Health Sci (Qassim) 2018;12:14–9. [PMC free article] [PubMed] [Google Scholar]
- 80.Minami M, Yoshikawa H. A simplified assay method of superoxide dismutase activity for clinical use. Clin Chem Acta. 1979;92:337–42. doi: 10.1016/0009-8981(79)90211-0. [DOI] [PubMed] [Google Scholar]
- 81.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 673–6. [Google Scholar]
- 82.Shahzad A, Ali SA, Fatima U, Erum H, Munawar R. Level of occupational stress and its associated factors among house officers of Dow University of health sciences. Int J Endors Health Sci Res. 2019;7:33–8. [Google Scholar]
- 83.Bull AW, Marnett LJ. Determination of malondialdehyde by ion-pairing highperformance liquid chromatography. Anal Biochem. 1985;149:284–90. doi: 10.1016/0003-2697(85)90506-8. [DOI] [PubMed] [Google Scholar]
- 84.Hänsel A, Hong S, Camara RJ, von Kaenel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35:115–21. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 85.Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser J. Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 2012;31:264–8. doi: 10.1037/a0025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dutheil F, Trousselard M, Perrier C, Lac G, Chamoux A, Duclos M, et al. Urinary interleukin-8 is a biomarker of stress in emergency physicians, especially with advancing age-the JOBSTRESS*randomized trial. PLoS One. 2013;8:e71658. doi: 10.1371/journal.pone.0071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bargellini A, Barbieri A, Rovesti S, Vivoli R, Roncaglia R, Borella P. Relation between immune variables and burnout in a sample of physicians. Occup Environ Med. 2000;57:453–7. doi: 10.1136/oem.57.7.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shamoon N, Ahmed S. Stress:A public health concern for progression of neurodegeneration and cognitive decline in Pakistani population. Int J Med Res Health Sci. 2019;8:167–74. [Google Scholar]
- 89.Nakamura H, Nagase H, Yoshida M, Ogino K. Natural killer (NK) cell activity and NK cell subsets in workers with a tendency of burnout. J Psychosom Res. 1999;46:569–78. doi: 10.1016/s0022-3999(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 90.Sonawane MD, Nimse SB. C-reactive protein:A major inflammatory biomarker. Anal Methods. 2017;9:3400–13. [Google Scholar]
- 91.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17:237–46. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 92.Johnson TV, Abbasi A, Master VA. Systematic review of the evidence of a relationship between chronic psychosocial stress and C-reactive protein. Mol Diagn Ther. 2013;17:147–64. doi: 10.1007/s40291-013-0026-7. [DOI] [PubMed] [Google Scholar]
- 93.Toker S, Shirom A, Shapira I, Berliner S, Melamed S. The association between burnout, depression, anxiety, and inflammation biomarkers:C-reactive protein and fibrinogen in men and women. J Occup Health Psychol. 2005;10:344–62. doi: 10.1037/1076-8998.10.4.344. [DOI] [PubMed] [Google Scholar]
- 94.Metlaine A, Sauvet F, Gomez-Merino D, Boucher T, Elbaz M, Delafosse JY, et al. Sleep and biological parameters in professional burnout:A psychophysiological characterization. PLoS One. 2018;13:e019060. doi: 10.1371/journal.pone.0190607. [DOI] [PMC free article] [PubMed] [Google Scholar]


