Dear Editors:
The core-binding factor (CBF) acute myeloid leukemias (AML) are considered a distinct subgroup of AML due to similar molecular pathogenesis and clinical features.[1] The two forms of CBF AML have rearrangements t(8;21)(q22;q22.1) [t(8;21)] and inv(16)(p13.1q22) or t(16;16)(p13.1;q22) [inv(16)] which result in the fusion genes RUNX1/RUNXT1 and CBFB/MYH11, respectively. Although survival of CBF-AML is superior than other subtypes of AML, relapse occurs in approximately 30-40% of patients.[1-4] Finding risk factors for treatment failure (e.g., relapse or death) may be useful to manage patients individually. However, the relative rarity of CBF-AML (approximately 15-20% of AML cases) and its relatively good prognosis has confounded these efforts.[2, 3] Moreover, recent studies clearly indicate each CBF AML is a distinct disease regarding patient and disease characteristics [2-5], and should be evaluated separately. We previously published a scoring system (I-CBFit) for treatment failure for t(8;21) [6], now here we present our analysis for inv(16).
Eleven US and Europe centers collected data on 550 patients with CBF AML; 290 patients had inv(16). Inclusion criteria were: a) AML patients with inv(16), t(16;16) or CBFB/MYH11 by fluorescence in situ hybridization confirmed by the reporting institutions; b) cases diagnosed between July 1996 and January 2017, c) diagnostic or at least postinduction bone marrow sample was available for review in the participating institution’s pathology department. Data were uniformly collected using a predesigned data spread sheet. The data forms included the following: patient characteristics (age, sex, race); disease-characteristics [date of diagnosis, white blood cell count (WBC) at diagnosis (x 109/L), cytogenetics, KIT D816V mutational status, primary or secondary AML]; hematopoietic cell transplantation (HCT) (autologous HCT or allogeneic HCT (alloHCT), donor type, remission status at HCT); and events (relapse, death, or alive at last contact). Deidentified patient data were transferred to the University of Minnesota where the main database was created and managed. This study was approved by the Institutional Review Board: Human Subjects Committee at the University of Minnesota.
Secondary AML was assigned if a patient had a history of chemotherapy/radiation therapy for a malignancy and/or had a history of preleukemic neoplastic bone marrow disease. The presence of 46 chromosomes in one clone or each clone was defined as pseudodiploidy (due to inversion or t(16;16) it was not named diploidy), and if the chromosome number was higher or lower than 46 chromosomes in any clone (in patients with more than one clone) it was defined as hyperdiploidy and hypodiploidy, respectively. Event-free survival (EFS) was defined as the time from diagnosis to relapse, death from any cause, or failure to achieve complete remission. Disease-free survival (DFS) was defined as the time from complete remission to relapse or death from any cause. Overall survival (OS) was defined as the time from diagnosis to last follow-up alive or date of death of any cause. We described patients’ characteristics using the median and range for continuous variables, and frequency and percentage for categorical variables. All patients were included EFS and OS analyses, but subjects who did not achieve complete remission (CR) were excluded from DFS analysis, Subjects that were censored before 2 years of follow-up were considered to be missing for the primary outcome. Covariates considered for inclusion were sex, age, race, primary or secondary AML, WBC at diagnosis, + 22, +8, and diploid status. Other chromosome abnormalities (4, 5, 7, 9, X, Y) were not considered for the prognostic model due to their rarity in our sample. KIT mutations were not included due to too many with missing data. Missing data were imputed with multivariate Imputation by Chained Equations in R. Twenty-five imputed datasets were generated. Stepwise model selection based on Akaike Information Criterion was performed on each imputed dataset. Covariates that were selected in at least half of the imputed datasets were included in the final model. Parameter estimates and standard errors were computed according to Rubin’s rules. A Cox proportional hazards model was initially used to investigate the effects of age, sex, WBC at diagnosis, +8, +22, and hyperdiploidy on EFS. An event was defined as either relapse, death, or treatment failure (defined as not achieving CR at 30 days after diagnosis. Patients were considered to be censored if they were lost to follow-up or received alloHCT in CR1. When it was found that age and WBC were the only significant predictors, cut-off values were determined to group patients into high and low risk categories. Models for DFS and OS were also computed. Separately for age and WBC, a ROC curve was generated for EFS for generated at 2 years after diagnosis using the survivalROC package in R. Cutoff points were chosen to maximize the Youden Index (Specificity + Sensitivity − 1). Kaplan-Meier curves and log-rank test were used for survival analyses in patients who had known diagnostic WBC and age (n=282).
Of the 290 patients with inv16 (median age of 49 years), 264 achieved CR1 (Table 1). The most common additional cytogenetic abnormalities were +8 and +22. Hyperdiploidy (23.5%) was more frequent. KIT D816V mutation was present in 17.7% of tested patients.
Table 1.
Characteristics of patients with inv(16)
| Variable | Total |
|---|---|
| n | 264 |
| Age (median [range]) | 49 [5-78] |
| Sex, n (%) | |
| Female | 129 (48.9) |
| Male | 117 (44.3) |
| Missing | 18 (6.8) |
| WBC at Diagnosis (109/L) (median [range]) | 21.30 [1-373] |
| Missing n (%) | 5 (1.9) |
| AML type, n (%) | |
| Primary | 219 (83.0) |
| Secondary | 32 (12.1) |
| Missing | 13 (4.9) |
| Chromosome 4 abnormalities, n (%) | |
| No | 243 (92.0) |
| Yes | 5 (1.9) |
| Missing | 16 (6.1) |
| Chromosome 5 or 7 abnormalities, n (%) | |
| No | 233 (88.3) |
| Yes | 15 (5.7) |
| Missing | 16 (6.1) |
| +8, n (%) | |
| No | 212 (80.3) |
| Yes | 36 (13.6) |
| Missing | 16 (6.1) |
| Chromosome 9 abnormalities, n (%) | |
| No | 243 (92.0) |
| Yes | 5 (1.9) |
| Missing | 16 (6.1) |
| + 22, n (%) | |
| No | 208 (78.8) |
| Yes | 40 (15.2) |
| Missing | 16 (6.1) |
| −X ,n (%) | |
| No | 245 (92.8) |
| Yes | 3 (1.1) |
| Missing | 16 (6.1) |
| −Y,n (%) | |
| No | 239 (90.5) |
| Yes | 9 (3.4) |
| Missing | 16 (6.1) |
| Number of Chromosomes, n (%) | |
| <46 | 6 (2.3) |
| =46 | 180 (68.2) |
| >46 | 62 (23.5) |
| Missing | 16 (61) |
| KIT D816V mutation, n (%) | |
| Negative | 135 (51.1) |
| Positive | 29 (11.0) |
| Missing | 100 (37.9) |
| AlloHCT, n (%) | 92 (34.8) |
| Disease status at alloHCT, n (%) | |
| No CR | 7 (7.7) |
| CR1 | 28 (30.8) |
| CR2 | 54 (59.3) |
| >CR2 | 1 (1) |
| Missing | 1 (1) |
| Relapsed within 2 years (%) in CR1 | 91 (34.5) |
| Non-relapse mortality within 2 years in CR1, n (%) | 15 (5.7) |
| EFS duration, months (median) | 25.5 |
| DFS duration, months (median) | 29.5 |
| OS duration, months (median) | Not reached |
Abbreviations: AlloHCT, allogeneic hematopoietic cell transplantation; CR, complete remission, DFS, disease-free survival; EFS, event-free survival; OS, overall survival; WBC, white blood cell count.
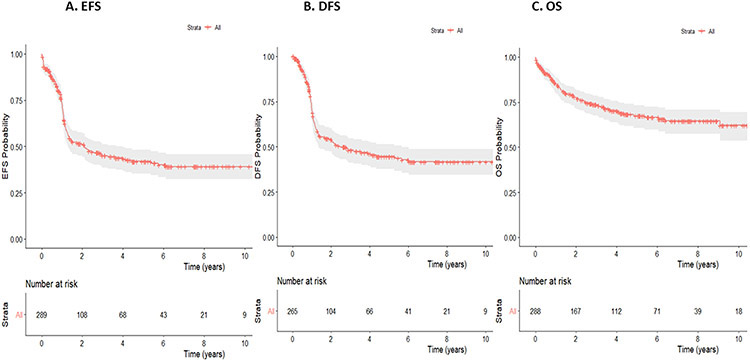
Relapse or death occurred in 106 patients (40.2%) within 2 years after CR1 at a median of 10.7 months (range 0.8 to 22.8 months). The median EFS and DFS were 25.5 and 29.5 months, respectively while OS was not reached. (Table 1 and Figure 1). In multivariate analysis (MVA) for survival at 2-year, older age (≥43 years) was associated with inferior EFS [(Hazard ratio (HR): 1.017, 95% Confidence Interval (CI): (1.007-1.028), p=0.0014], DFS [HR: 1.014, 95% CI (1.003-1.026), p=0.0123], and OS [HR: 1.034, 95% CI (1.018-1.049), p=0.00002]. Higher white blood cell counts (WBC≥98 x 109/L) were associated with a poorer EFS [HR: 1.005, 95% CI (1.002-1.008), p=0.0003] and DFS [HR: 1.0006, 95% CI (1.004-1.009), p=0.0001], but not with OS [HR: 1,000 95% CI (0.995-1.005), p=0.958].
Figure 1.
EFS (A), DFS (B), and OS (C) in patients with inv(16).
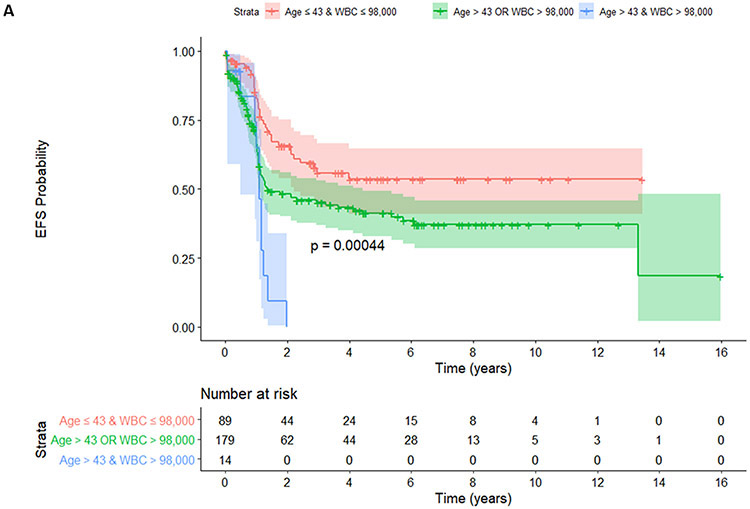
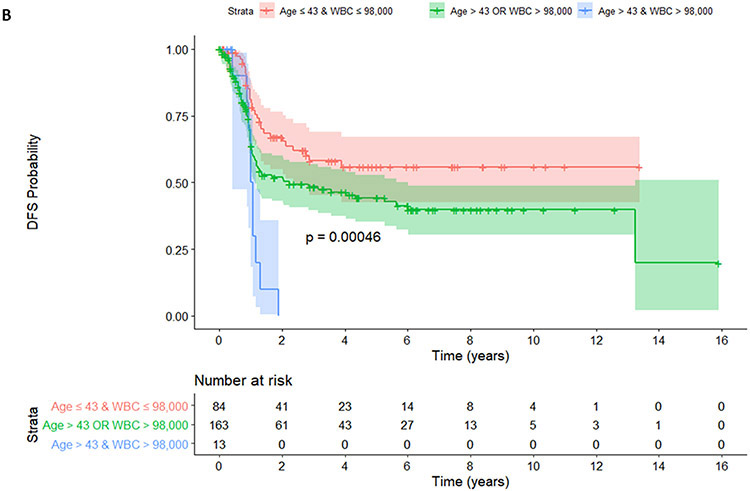
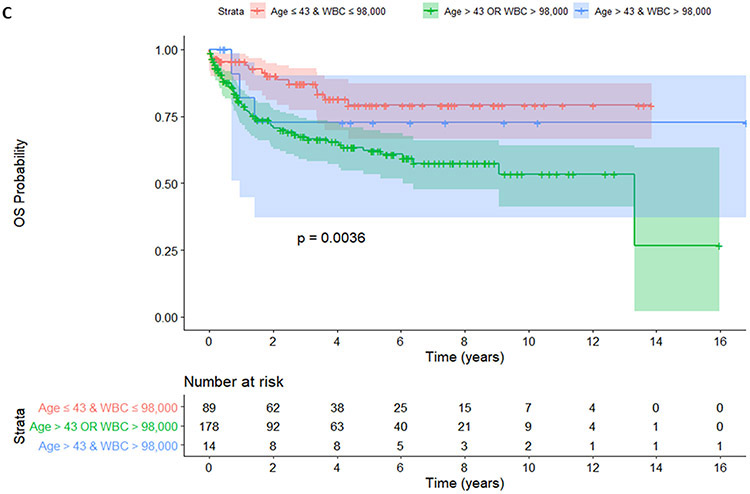
Patients stratified by age and WBC into 3 groups (age ≤43 years and WBC ≤ 98 x 109/L vs. age ≤43 years or WBC ≤ 98 x 109/L vs. age >43 years and WBC > 98 x 109/L). Regarding EFS, younger patients with a lower WBC (no risk group, age ≤43 years and WBC ≤ 98 x 109/L) did not reach median survival whereas those with 1 risk (age ≤43 years or WBC ≤ 98 x 109/L) and patients with 2 risks (age >43 years and WBC > 98 x 109/L) had a median of EFS of 16.3 months and 13.1 months, respectively, p=0.00044 (Figure 2A). Regarding DFS, patients with no risk did not reach median survival whereas those with 1 risk and those 2 risks (age >43 years and WBC > 98 x 109/L) had a median DFS of 26.8 months and 12.5 months, respectively, p=0.00046 (Figure 2B). Regarding OS, patients with no risk or with both risks did not reach median survival whereas those with 1 risk had a median OS of 160 months, p=0.0036 (Figure 2C).
Figure 2.
EFS (A), DFS (B), and OS (C) were shown among patients with no (age ≤43 years and WBC ≤ 98 x 109/L), 1 (age ≤43 years or WBC ≤ 98 x 109/L) and 2 risk factors (age >43 years and WBC > 98 x 109/L).
Although this multicenter study had limitations (due to retrospective in nature and thus missing detailed molecular data and no data on minimal residual disease), the data from a large number of patients with long-term follow-up allowed us to identify clear risk factors for treatment failure; older patients and higher WBC counts. Hoyos et al also showed that higher WBC count (> 20×109/L) had higher relapse at 5-year (40% vs. 16%, p = 0.001) in 150 patients with CBF AML (76 with CBFB-MYH11).[7]. When patients were stratified by age and WBC (age >50 years and WBC > 20×109/L), similar to our results, patients who had no risk factor had a superior 5-year OS (80%). Other studies have also supported that WBC (one defined it similar to ours, 100 x 109/L) is associated with poor DFS, mainly due to increased relapse.[8, 9]. Pashka et al reported that patients with higher WBC had decreased DFS (HR=1.33, p=0.02) and more mutated KIT and FLT3. [8] De Jonge et al showed low WBC (< 20×109/L) was associated with a higher CR rate (p=0.024), improved EFS (median 77.2 vs.9.7 months) and improved OS (median 85.5 vs. 28.9 months, p=0.001) in favorable prognostic AML.[10] Delauney et al found that older aged (>36 years), +22, and severe thrombocytopenia (<30 x 109/L) were associated with poor DFS in 110 patients with inv(16).[11] In MVA, age was the only risk factor for poor DFS. The impact of +22 on survival is conflicting [2, 3, 8, 11] and might be more prominent in patients with lower WBC.[3]
A KIT mutation (exons 8-13 & 17), present in up to 37% of patients, was associated with poor prognosis for survival in CBF AML.[4, 8] KIT mutations seem to have an impact in patients with t(8;21), but not in those with inv16.[6, 12] However, in the current study of patients with inv16, KIT mutations could not be tested due to excessive missing data. Similar to our findings, complex karyotype [2, 5, 11] and secondary AML did not have effect in treatment failure.[13]
Prospective studies are warranted to improve survival in older inv(16) patients with a higher WBC at diagnosis.
Acknowledgements:
Dr. Bloomfield unexpectedly, unfortunately passed away during the preparation of the final manuscript
Footnotes
Conflict of Interest
The authors have no conflict of interest relevant to the study to disclose.
References
- 1.Ustun C, Marcucci G. Emerging diagnostic and therapeutic approaches in core binding factor acute myeloid leukaemia. Curr Opin Hematol 2015;22:85–91. [DOI] [PubMed] [Google Scholar]
- 2.Marcucci G, Mrozek K, Ruppert AS, et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): a Cancer and Leukemia Group B study. J Clin Oncol 2005;23:5705–5717. [DOI] [PubMed] [Google Scholar]
- 3.Schlenk RF, Benner A, Krauter J, et al. Individual patient data-based meta-analysis of patients aged 16 to 60 years with core binding factor acute myeloid leukemia: a survey of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol 2004;22:3741–3750. [DOI] [PubMed] [Google Scholar]
- 4.Duployez N, Marceau-Renaut A, Boissel N, et al. Comprehensive mutational profiling of core binding factor acute myeloid leukemia. Blood 2016;127:2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosna F, Papayannidis C, Martinelli G, et al. Complex karyotype, older age, and reduced first-line dose intensity determine poor survival in core binding factor acute myeloid leukemia patients with long-term follow-up. Am J Hematol 2015;90:515–523. [DOI] [PubMed] [Google Scholar]
- 6.Ustun C, Morgan E, Moodie EEM, et al. Core-binding factor acute myeloid leukemia with t(8;21): Risk factors and a novel scoring system (I-CBFit). Cancer Med 2018;7:4447–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoyos M, Nomdedeu JF, Esteve J, et al. Core binding factor acute myeloid leukemia: the impact of age, leukocyte count, molecular findings, and minimal residual disease. Eur J Haematol 2013;91:209–218. [DOI] [PubMed] [Google Scholar]
- 8.Paschka P, Du J, Schlenk RF, et al. Secondary genetic lesions in acute myeloid leukemia with inv(16) or t(16;16): a study of the German-Austrian AML Study Group (AMLSG). Blood 2013;121:170–177. [DOI] [PubMed] [Google Scholar]
- 9.Martin G, Barragan E, Bolufer P, et al. Relevance of presenting white blood cell count and kinetics of molecular remission in the prognosis of acute myeloid leukemia with CBFbeta/MYH11 rearrangement. Haematologica 2000;85:699–703. [PubMed] [Google Scholar]
- 10.de Jonge HJ, Valk PJ, de Bont ES, et al. Prognostic impact of white blood cell count in intermediate risk acute myeloid leukemia: relevance of mutated NPM1 and FLT3-ITD. Haematologica 2011;96:1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaunay J, Vey N, Leblanc T, et al. Prognosis of inv(16)/t(16;16) acute myeloid leukemia (AML): a survey of 110 cases from the French AML Intergroup. Blood 2003;102:462–469. [DOI] [PubMed] [Google Scholar]
- 12.Kawashima N, Ishikawa S, Atsuta Y, et al. Prospective Evaluation of Prognostic Relevance of KIT Mutations in Core-Binding Factor Acute Myeloid Leukemia: Results from the JALSG CBF-AML209-KIT Study. Blood; 2018. p 438. [Google Scholar]
- 13.Seymour JF, Juneja SK, Campbell LJ, et al. Secondary acute myeloid leukemia with inv(16): report of two cases following paclitaxel-containing chemotherapy and review of the role of intensified ara-C therapy. Leukemia 1999;13:1735–1740. [DOI] [PubMed] [Google Scholar]