Abstract
Purpose
In clinical practice, we found some of the patients who received transarterial chemoembolization (TACE) with molecular targeted agents (MTGs) plus immune checkpoint inhibitors (ICIs) for hepatocellular carcinoma (HCC) had obvious liquefactive necrosis formation within the tumor and some even progressed to a liver abscess, which seems more frequent than patients who received other treatments. Thus, we aim to identify this condition and analyze the potential risk factors.
Patients and Methods
Medical records of 72 consecutive patients with intermediate (BCLC B) and advanced (BCLC C) HCC who received TACE plus MTGs combined with (n=30) or without (n=42) ICIs were reviewed. Liquefactive necrosis formation was defined as the presence of obvious liquefactive necrosis within the tumor that required intervention.
Results
The liquefactive necrosis rate was higher in the TACE+MTGs+ICIs group than in the TACE+MTGs group (30% vs 4.8%, P=0.006). Moreover, 18.2% (2/11) of the patients with liquefactive necrosis within the tumor had a bacterial infection. We then take the binary logistic regression analysis model to identify the predictors of liquefactive necrosis formation, and which showed the tumor size (P=0.006, OR=1.355, 95% CI: 1.090–1.684), alpha-fetoprotein level (P=0.036, OR=6.745, 95% CI: 1.130–40.262) and treatment modality (P=0.015, OR=11.717, 95% CI: 1.617–84.887) were the independent risk factor for liquefactive necrosis formation within the tumor.
Conclusion
Patients with HCC who received TACE combined with MTGs plus ICIs have increased liquefactive necrosis formation, and the larger tumor size and higher alpha-fetoprotein level were associated with more liquefactive necrosis formation within the tumor.
Keywords: liver cancer, transarterial chemoembolization, molecular targeted agents, immune checkpoint inhibitors, liquefactive necrosis
Introduction
Liver cancer is a serious health event worldwide, which is estimated to be the sixth most common malignant tumor and ranked the fourth common cause of cancer-related deaths.1 Despite the development of diagnostic methods for early detection of liver cancer, a large number of patients are diagnosed at an intermediate or advanced stage, which is not eligible for curative treatment.2–4 The Barcelona Clinic Liver Cancer (BCLC) staging system has been widely used to guide treatment decisions and prognostic prediction, and the system identifies patients at intermediate (BCLC B) or advanced stage (BCLC C) who may benefit from intra-arterial or systemic therapy respectively.5,6
Transarterial chemoembolization (TACE) for treatment of hepatocellular carcinoma (HCC) has shown promising outcomes according to the reported studies’ results and it has been recommended as the first-line treatment for HCC patients at the intermediate (BCLC B) stage, and even the Chinese guidelines extend the indication for selected advanced HCC cases.5,7–9 Drug-eluting bead (DEB)-TACE, which aims to increase the local concentration of chemotherapeutic agents, has been applied in clinical practice, but the published studies reported that it has a similar therapeutic effect as conventional TACE (cTACE).10,11 Systemic therapy, including molecular targeted agents (MTGs) and immune checkpoint inhibitors (ICIs), has shown satisfactory results in clinical trials, and many new agents are emerging.12–14
More recently, many combination treatment modalities, such as TACE+ MTGs, have been used and the outcomes were better than single treatment and without increased toxicity.12,15,16 MTGs play the role of anti-angiogenesis by blocking one or several complicated pathways; ICIs, targeting cytotoxic T lymphocyte protein 4 (CTLA-4), programmed cell death protein-1 (PD-1), and its ligand (PD-L1), were mainly used to enhance the activation of T cell; TACE contributes to the angiogenesis by inducing the hypoxic response and could lead to higher neoantigen presentation and immunogenic cell death.12,17,18 Thus, the combination therapy may be a potential therapy for advanced HCC with promising results in theory.
It has been reported that HCC patients with liquefactive necrosis experienced tumor recurrence and metastasis earlier and had a worse prognosis than patients with coagulative necrosis.19 In clinical practice, we found some of the patients who received TACE with MTGs plus ICIs for HCC had obvious liquefactive necrosis formation within the tumor and some even progressed to a liver abscess, which seems more frequent than patients who received other treatment. Thus, we aim to identify this condition and analyze the potential risk factors based on our cases in the present study.
Materials and Methods
Study Populations
The study was approved by the institutional ethics review board of Wuhan Union Hospital and written informed consent was waived because of the retrospective nature. We confirmed that all the data was anonymized or maintained with confidentiality and the study was performed in accordance with the ethical standards of the Declaration of Helsinki. From August 2018 to September 2020, medical records of consecutive patients with intermediate (BCLC B) and advanced (BCLC C) HCC who received TACE plus MTGs combined with or without ICIs were reviewed. Patients with incomplete medical records and patients who developed liquefactive necrosis before the administration of MTGs and ICIs were excluded.
We reviewed the medical records of each patient. The clinical data included age, gender, Child–Pugh score, BCLC stage, and number of TACE including cTACE and DEB-TACE. The laboratory data included total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alpha-fetoprotein (AFP), and hepatitis B surface antigen (HBsAg) status. The imaging data including the tumor size (defined as the largest diameter) and the condition of liquefactive necrosis within the tumor (defined as the presence of obvious liquefactive necrosis within the tumor which required intervention) were evaluated on abdominal contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI). Moreover, patients with obvious liquefactive necrosis within the tumor recorded the following: clinical manifestations, laboratory data including white cell count and the neutrophils ratio, and the properties of the liquefactive necrosis including color and the bacterial culture results of the liquefactive necrosis.
TACE Procedure
TACE was performed by doctors with more than 10 years of experience in the procedure under local anesthesia. First, both the superior mesenteric artery and common hepatic artery angiography were performed with a nonionic contrast agent through a 5- or 4-F catheter (COOK) to assess the patency of the portal vein, anatomy, and tumor burden. Second, chemoembolization was performed by selective catheterization of the feeding arteries with a 3-F coaxial microcatheter (Terumo, Tokyo, Japan). For cTACE, a mixture of 50 mg epirubicin was manually emulsified with 5–10 mL lipiodol followed by embolization with absorbable gelatin sponge particles until the blood flow of the feeding arteries was stagnated. For DEB-TACE, 30 mg of epirubicin was dissolved in 2 mL saline and loaded into 100–300 µm DEB (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) and then mixed with nonionic contrast medium. After catheterization into the feeding arteries, the suspended DEBs were administered slowly. Finally, angiography was performed to confirm the complete embolism of the feeding arteries. Repeated TACE would be performed once the CT or MRI imaging showed residual lesions.
MTGs and ICIs Administration
The administration of MTGs and ICIs was initiated within 2 weeks post-TACE therapy based on the proper liver function (AST <40 U/L and Child–Pugh A). Oral MTGs used in the TACE+MTGs group included sorafenib at a dose of 400 mg bid or lenvatinib at a dose of 8 mg qd or apatinib at a dose of 500 mg qd or regorafenib at a dose of 120 mg qd, and it was suspended 3 days before and after the following TACE procedure. The selection of the MTGs was dependent on the doctors’ experience and mostly be influenced by the patients’ economic condition. Patients in the TACE+MTGs+ICIs group received MTGs at the same dose and in combination with intravenous administration of Camrelizumab (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) at a dose of 200mg, which was injected every 3 weeks If patients could not tolerate the side effects, dose reduction was recommended. Once serious adverse events occur, drug administration should be stopped.
Follow-Up Protocol
All patients were prescribed to have a follow-up protocol including assessment of clinical symptoms, survival, and enhanced CT or MRI images. The first follow-up was recommended within 1 month and then was recommended every 1–3 months, and 3–6 months if the lesion kept stable.
Statistical Analysis
All statistical analyses were performed using SPSS 19.0 software (IBM Corporation, Armonk, NY USA). The categorical variables were reported as numbers (percentages) and all the continuous variables as means ± standard error or median (minimum, maximum), depending on the variable distribution. Numeric variables were compared using the Student's t-test or Wilcoxon rank-sum test, and chi-squared or Fisher’s exact test for categorical variables. The variables with a P-value of <0.1 were considered statistically significant in the univariate analysis, and then these significant variables were included in the multivariate model, which was refined using binary logistic regression, and a two-sided P-value of <0.05 was considered statistically significant.
Results
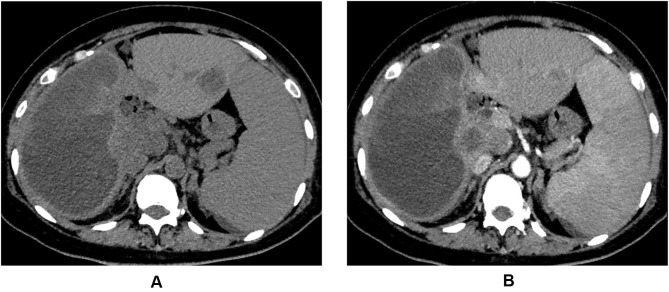
A total of 72 consecutive patients were included in the present study, and 42 patients who received TACE plus MTGs treatment belonged to the TACE+MTGs group and 30 patients who received TACE plus MTGs combined with ICIs treatment were classified as TACE+ MTGs +ICIs group. The comparison of clinical features between the two groups is shown in Table 1. During a median follow-up of 8.2 (range 1.3–45.3) months, there were 9 cases, and 2 cases had obvious liquefactive necrosis within the tumor in the TACE+ MTGs +ICIs group and TACE+MTGs group, respectively, and the difference was statistically significant (30% vs 4.8%, P=0.006). The other baseline characteristics were not significantly different between the two groups. Moreover, 18.2% (2/11) of the patients with liquefactive necrosis within the tumor had a bacterial infection confirmed by the bacterial culture of the liquefactive necrosis content (Figure 1A and B), and both of the patients had a fever with an increased white cell count and the neutrophils ratio, and other patients with liquefactive necrosis within the tumor presented with abdominal pain and had a normal white cell count and the neutrophils ratio, and none of them showed positive in bacterial culture.
Table 1.
Clinical Characteristics Comparison Between the TACE+ MTGs +icis Group and TACE+ MTGs Group
| Variables | TACE+MTGs+ICIs Group (n=30) | TACE+MTGs Group (n=42) | P value |
|---|---|---|---|
| Age (years) | 52.1±9.4 | 54.7±11 | 0.378 |
| Gender | 0.302 | ||
| Male | 24 (80.0) | 38 (90.5) | |
| Female | 6(20.0) | 4 (9.5) | |
| Child–Pugh classification | 0.659 | ||
| A | 7 (23.3) | 8 (19.0) | |
| B | 23 (76.7) | 34 (81.0) | |
| BCLC stage | 0.261 | ||
| B | 7 (23.3) | 15 (35.7) | |
| C | 23 (76.7) | 27 (64.3) | |
| AFP (ng/mL) | 0.561 | ||
| <400 | 18 (60) | 28 (66.7) | |
| >400 | 12 (40) | 14 (33.3) | |
| Hepatitis B | 1.000 | ||
| Yes | 28 (93.3) | 40 (95.2) | |
| No | 2 (6.7) | 2 (4.8) | |
| Liquefactive necrosis | 0.006 | ||
| Yes | 9 (30.0) | 2 (4.8) | |
| No | 21 (70.0) | 40 (95.2) | |
| Tumor size (cm) | 8.8±4.4 | 7.5±4.4 | 0.218 |
| Number of TACE | 3.9±2.4 | 3.9±1.7 | 0.114 |
| Number of cTACE | 2 (0.10) | 2 (0.9) | 0.875 |
| Number of DEB-TACE | 1.7±1.4 | 1.6±1.5 | 0.786 |
| TBIL (µmol/L) | 16.6 (8.2,45.2) | 20.9 (7.0,44.5) | 0.059 |
| AST (U/L) | 57.0 (20.0,134.0) | 54.0 (18.0,130.0) | 0.991 |
| ALT (U/L) | 51.0 (3.0,127.0) | 59.0 (4123.0) | 0.417 |
Notes: The categorical variables were reported as numbers (percentages) and all the continuous variables as means ± standard error or median (minimum, maximum), depending on variable distribution.
Abbreviations: MTGs, molecular targeted agents; ICIs, immune checkpoint inhibitors; TACE, transarterial chemoembolization; DEB-TACE, drug-eluting bead (DEB)-TACE; cTACE, conventional TACE; TBIL, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha-fetoprotein.
Figure 1.
A 43-year-old patient who received transarterial chemoembolization combined with molecular targeted agents plus immune checkpoint inhibitors for hepatocellular carcinoma presented with abdominal pain and fever. The computed tomography plain scan (A) and enhanced scan (B) images of a patient demonstrated obvious liquefactive necrosis within the tumor (the right lobe of the liver). Catheter drainage was performed under the guidance of ultrasound and the bacterial culture result of the liquefactive necrosis content was positive.
We then take the binary logistic regression analysis model to identify the predictors of liquefactive necrosis formation within the tumor. Univariate analysis was firstly used to evaluate all the potential factors affecting the liquefactive necrosis formation within the tumor. Value of P<0.1 was obtained for the AFP level (P=0.013), the tumor size (P<0.001), the treatment modality (P=0.006), and the ALT level (P=0.007). Then, the above statistically significant variables combined with age and gender were included in the binary logistic regression analysis model, and the result revealed that tumor size (P=0.006, OR=1.355, 95% CI: 1.090–1.684), AFP level (P=0.036, OR=6.745, 95% CI: 1.130–40.262) and treatment modality (P=0.015, OR=11.717, 95% CI: 1.617–84.887) were the independent risk factors for liquefactive necrosis formation within the tumor (Table 2).
Table 2.
Univariate and Binary Logistic Regression Analysis Model to Identify Predictors of Liquefactive Necrosis Formation Within the Tumor
| Variables | LN Group (n=11) | Non-LN Group (n=61) | Univariate Analysis P value | Binary Logistic Regression P value, OR (95% CI) |
|---|---|---|---|---|
| Age (years) | 50.5±8.6 | 54.2±10.5 | 0.274 | |
| Gender | 1.000 | |||
| Male | 10 (90.9) | 52 (85.2) | ||
| Female | 1 (9.1) | 9 (14.8) | ||
| Child–Pugh | 0.105 | |||
| A | 0 (0) | 15 (24.6) | ||
| B | 11 (100.0) | 46 (75.4) | ||
| BCLC stage | 0.485 | |||
| B | 2 (18.2) | 20 (32.8) | ||
| C | 9 (81.8) | 41 (67.2) | ||
| AFP (ng/mL) | 0.013 | 0.036,6.745 (1.130–40.262) | ||
| <400 | 3 (27.3) | 43 (70.5) | ||
| >400 | 8 (72.7) | 18 (29.5) | ||
| Hepatitis B | 0.493 | |||
| Yes | 10 (90.9) | 58 (95.1) | ||
| No | 1 (9.1) | 3 (4.9) | ||
| Treatment modality | 0.006 | 0.015,11.717 (1.617–84.887) | ||
| TACE+MTGs+ICIs | 9 (81.8) | 21 (34.4) | ||
| TACE+MTGs | 2 (18.2) | 40 (65.6) | ||
| Tumor size (cm) | 12.4±3.4 | 7.2±4.1 | <0.001 | 0.006, 1.355 (1.090–1.684) |
| No. of TACE | 3.9±1.6 | 3.8±2.0 | 0.833 | |
| No. of cTACE | 1 (0,5) | 2 (0,10) | 0.493 | |
| No. of DEB-TACE | 2 (0,4) | 1 (0,5) | 0.131 | |
| TBIL (µmol/L) | 16.4 (9.2,37.5) | 19.5 (7.0,45.2) | 0.336 | |
| ALT (U/L) | 30.0 (3.0,100.0) | 64.0 (10.0,127.0) | 0.007 | |
| AST (U/L) | 57.0 (20.0,98.0) | 54.0 (18.0,134.0) | 0.594 |
Notes: The categorical variables were reported as numbers (percentages) and all the continuous variables as means ± standard error or median (minimum, maximum), depending on variable distribution.
Abbreviations: MTGs, molecular targeted agents; ICIs, immune checkpoint inhibitors; LN, liquefactive necrosis; TACE, transarterial chemoembolization; DEB-TACE, drug-eluting bead (DEB)-TACE; cTACE, conventional TACE; TBIL, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha-fetoprotein.
Discussion
Most recently, more and more studies were conducted to evaluate the efficacy and safety of combination therapeutic modalities for treatment of HCC, and several studies, including TACE plus MTGs, ICIs plus ICIs, TACE combined with ICIs plus MTGs, had shown the synergistic effect of the combination therapy.12,17 However, immunotherapy is a newly developed therapeutic modality, and studies about combination ICIs with local treatment or MTGs are still under investigation; thus, the efficacy and safety of these combination strategies need to be evaluated in the future. In the present study, we reported that obvious liquefactive necrosis formation with or without bacterial infection was more frequent occurrence when treated HCC by TACE combined with MTGs plus ICIs, which could be considered as a side effect of this combination strategy, and we also identified that the larger tumor size and higher AFP level were associated with more liquefactive necrosis formation within the tumor. To the best of my knowledge, this is the first related report in the literature.
The results of this study demonstrated that HCC patients who received TACE combined with MTGs plus ICIs had increased liquefactive necrosis formation within the tumor than patients who received TACE combined with MTGs. It should be the results of tumor tissue liquefaction necrosis formation were not coordinated with its absorption. Generally, the tumor necrotic tissue was dissolved and liquefaction by the action hydrolase released by neutrophil or engulfed by the macrophages, and then both of them were removed by the peripheral veins and lymphatics.20 The TACE induces hypoxic response and may embolize small veins8 and the MTGs inhibit angiogenesis,14 both of which result in the decrease of veins. The ICIs enhanced the anti-cancer effect, and the combination of it with TACE and MTGs results in liquefactive necrosis formation in a short time, which exceeds the velocity of removal by the decreased vein and lymphatics. Wu et al have reported that HCC patients who underwent single TACE treatment can also develop liquefactive necrosis.19 Therefore, to identify the effect of MTGs and ICIs on the development of liquefactive necrosis, we excluded the patients who developed liquefactive necrosis before the administration of MTGs and ICIs in the present study. Also, the number of TACE in each group has been compared and the result was not statistically significant. Based on the above analysis, maybe a low dose of ICIs could decrease the liquefactive necrosis formation; thus, more studies should be conducted to investigate the optimal dose of ICIs in this combination therapeutic modality.
The present study results revealed that the tumor size and AFP level were associated with the liquefactive necrosis formation within the tumor. The possible reason may be that the bigger tumor has a more hypoxic tumor microenvironment and often lead to more necrosis within the tumor tissue, which is often seen in clinical practice. Based on our study results, it should be paid more attention when treating HCC with large size and high AFP level by this combination therapeutic modality.
All the patients with liquefactive necrosis presented with clinical symptoms including abdominal pain and fever. The former is resulted from the increasing tension by the liquefactive necrotic tumor and the latter results from bacterial infection. In the present study, most of the cases (81.8%) were aseptic liquefaction, but it still had the possibility of bacterial infection. Thus, clinicians should consider this condition when treating HCC with this combination therapeutic modality. Catheter drainage with or without anhydrous alcohol ablation seemed effective in our cases, but the exact effect needs more studies to confirm.
Limitation
Firstly, the results of this study should be viewed with an inherent bias due to the respective nature, and prospective studies or randomized controlled trials are needed to identify the results. Secondly, the study was conducted with a small sample size and a relatively short follow-up, so it warrants further large-sample studies to study. Finally, the MTGs used in this present study were different, which may interfere with the results. We still cannot make relevant statistical analyses because of the small sample size, and thus further studies are needed.
Conclusion
Patients with HCC who received TACE combined with MTGs plus ICIs have increased liquefactive necrosis formation, and the larger tumor size and higher AFP level were associated with more liquefactive necrosis formation within the tumor. Therefore, it is important to investigate the optimal using method of this combination therapeutic modality and perform intensive follow-up for patients with high risk factors.
Acknowledgments
This work was funded by grants from the National Natural Science Foundation of China [81873917] and China Health Promotion Foundation [XM_2018_011_0006_01].
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin Mol Hepatol. 2016;22(1):7–17. doi: 10.3350/cmh.2016.22.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HY, Park JW. Clinical trials of combined molecular targeted therapy and locoregional therapy in hepatocellular carcinoma: past, present, and future. Liver Cancer. 2014;3(1):9–17. doi: 10.1159/000343854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:v238–v255. doi: 10.1093/annonc/mdy308 [DOI] [PubMed] [Google Scholar]
- 6.Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona clinic liver cancer staging system. J Hepatol. 2006;44(4):723–731. doi: 10.1016/j.jhep.2005.12.015 [DOI] [PubMed] [Google Scholar]
- 7.Prince D, Liu K, Xu W, et al. Management of patients with intermediate stage hepatocellular carcinoma. Ther Adv Med Oncol. 2020;12:1758835920970840. doi: 10.1177/1758835920970840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakurai M, Okamura J, Kuroda C. Transcatheter chemo-embolization effective for treating hepatocellular carcinoma. A histopathologic study. Cancer. 1984;54(3):387–392. doi: [DOI] [PubMed] [Google Scholar]
- 9.Xie DY, Ren ZG, Zhou J, et al. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacco R, Bargellini I, Bertini M, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22(11):1545–1552. doi: 10.1016/j.jvir.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 11.Shimose S, Iwamoto H, Tanaka M, et al. Increased arterio-portal shunt formation after drug-eluting beads TACE for hepatocellular carcinoma. Oncology. 2020;98(8):558–565. doi: 10.1159/000507262 [DOI] [PubMed] [Google Scholar]
- 12.Girardi DM, Pacífico J, Guedes de Amorim F, Dos Santos Fernandes G, Teixeira MC, Pereira A. Immunotherapy and targeted therapy for hepatocellular carcinoma: a literature review and treatment perspectives. Pharmaceuticals. 2021;14(1):28. doi: 10.3390/ph14010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Doorn DJ, Takkenberg RB, Klümpen H. Immune checkpoint inhibitors in hepatocellular carcinoma: an overview. Pharmaceuticals. 2021;14(1):3. doi: 10.3390/ph14010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu X, Tang Z, Sun H. Targeting angiogenesis for liver cancer: past, present, and future. Genes Dis. 2020;7(3):328–335. doi: 10.1016/j.gendis.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindson J. Combined TACE and sorafenib for HCC treatment. Nat Rev Gastroenterol Hepatol. 2020;17(2):66. doi: 10.1038/s41575-020-0264-1 [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Jeong SW, Young Jang J, Kim YJ. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. 2020;21(21):8165. doi: 10.3390/ijms21218165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viveiros P, Riaz A, Lewandowski RJ, Mahalingam D. Current state of liver-directed therapies and combinatory approaches with systemic therapy in hepatocellular carcinoma (HCC). Cancers. 2019;11(8):1085. doi: 10.3390/cancers11081085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng L, Fang S, Wu F, et al. Efficacy and safety of TACE combined with sorafenib plus immune checkpoint inhibitors for the treatment of intermediate and advanced TACE-refractory hepatocellular carcinoma: a Retrospective Study. Front Mol Biosci. 2021;7:609322. doi: 10.3389/fmolb.2020.609322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Xie Y, Chang X, et al. Type of necrosis influences prognosis in hepatocellular carcinoma after the first transarterial chemoembolization. Med Sci Monit. 2021;27:e929884. doi: 10.12659/MSM.929884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei Y, Jianquan Z, Hongqiong C, et al. Cause analysis and management of liquefactive necrosis of thyroid nodules after microwave ablation. Acad J Second Mil Med Univ. 2018;39(12):1343–1347. [Google Scholar]