Abstract
OBJECTIVES
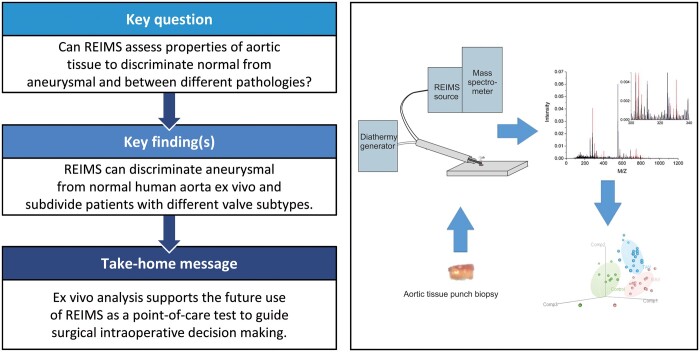
Many intraoperative decisions regarding the extent of thoracic aortic surgery are subjective and are based on the appearance of the aorta, perceived surgical risks and likelihood of early recurrent disease. Our objective in this work was to carry out a cross-sectional study to demonstrate that rapid evaporative ionization mass spectrometry (REIMS) of electrosurgical aerosol is able to empirically discriminate ex vivo aneurysmal human thoracic aorta from normal aorta, thus providing supportive evidence for the development of the technique as a point-of-care test guiding intraoperative surgical decision-making.
METHODS
Human aortic tissue was obtained from patients undergoing surgery for thoracic aortic aneurysms (n = 44). Normal aorta was obtained from a mixture of post-mortem and punch biopsies from patients undergoing coronary surgery (n = 13). Monopolar electrocautery was applied to samples and surgical aerosol aspirated and analysed by REIMS to produce mass spectral data.
RESULTS
Models generated from REIMS data can discriminate aneurysmal from normal aorta with accuracy and precision of 88.7% and 85.1%, respectively. In addition, further analysis investigating aneurysmal tissue from patients with bicuspid and tricuspid aortic valves was discriminated from normal tissue and each other with accuracies and precision of 93.5% and 91.4% for control, 83.8% and 76.7% for bicuspid aortic valve and 89.3% and 86.0% for tricuspid aortic valve, respectively.
CONCLUSIONS
Analysis of electrosurgical aerosol from ex vivo aortic tissue using REIMS allowed us to discriminate aneurysmal from normal aorta, supporting its development as a point-of-care test (Intelligent Knife) for guiding surgical intraoperative decision-making.
Keywords: Thoracic aortic aneurysm, Rapid evaporative ionization mass spectrometry, Bicuspid aortic valve, Tricuspid aortic valve
INTRODUCTION
Indications for surgery on thoracic aortic aneurysms are well described in European and American Society Guidelines [1, 2] and are heavily weighted towards symptomology and cross-sectional dimensions. Many intraoperative decisions regarding the extent of thoracic aneurysm surgery are subjective and are based on the appearance of the vessel (including subjective assessment of the aneurysm size, aortic wall thickness and appearance of the tunica media), perceived surgical risks and likelihood of early recurrent disease. To date, technological innovation has not provided an intraoperative point-of-care test, which helps guide these decisions, which may have significant consequences for patients if incorrect. Recent advances in oncological surgery have shown the utility of analysing electrosurgical aerosol with rapid evaporative ionization mass spectrometry (REIMS) or so-called ‘Intelligent Knife’ (i-knife), in guiding surgeons on tumour resection margins [3]. The technique involves intraoperative mass spectrometric analysis of tissue diathermy fumes which provides an empirical measure of normal versus tumour cells. Our objective is to share our preliminary data from using REIMS to analyse ex vivo normal and aneurysmal human aortic tissue, and comparison to previously reported supportive biomechanical and biochemical variables, as a prelude to intraoperative human studies.
METHODS
Tissue and patient characteristics
Aortic tissue samples were obtained from patients undergoing elective proximal thoracic aortic surgery with aneurysmal pathology at Liverpool Heart and Chest Hospital between 2016 and 2019. Control tissues were obtained from patients undergoing elective coronary artery bypass surgery at Liverpool Heart and Chest Hospital or snap frozen cadaveric samples from National Health Service Blood and Transplant service (NHS-BT). All tissue was stored at −80°C prior to testing. As this was a preliminary investigation study size was determined by tissue availability at time of study. Tissue samples originated from the root and ascending aorta, the nature of the leaflet configuration of the aortic valve was noted. Patients with known syndromic connective tissue disorders, aortic dissection or atherosclerotic aneurysms were excluded from the study. Tissue from surgery was frozen in super-cooled liquid nitrogen immediately after collection. Surgical patient demographic and clinical characteristics were stored in hospital Electronic Patient Records, age and gender information for the cadaveric tissue was obtained from NHS-BT records. The study was ethically approved by Liverpool Bio-Innovation Hub (project approval references 15-06 and 19-09). The study conformed to the principles outlined in the Declaration of Helsinki and followed Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. Informed consent was obtained for all participants. Cadaveric samples were removed from donors for transplant but not subsequently utilized for this purpose for various reasons. Consent was obtained to use this tissue for research with National Research Ethics Service approval (REC Reference 11/EE/0528).
REIMS
Tissue samples were thawed and a 5-mm punch removed and placed onto glass microfiber paper (GFP, GE Healthcare Whatman). Samples were wetted with 100 µl of milli Q water (Merck Millipore). Sampling used a monopolar diathermy electrosurgical pencil (Erby Medical UK LTD, Leeds) (i-knife). A power of 35 W was applied across the tissue punch at room temperature, and the aerosol was aspirated and directed into the REIMS source (Waters, UK). At the same time, a lockmass solution of 5 pmol/µl leu-enkephalin in propan-2-ol at a flow rate of 50 µl/min was delivered to the REIMS source attached to a Synapt G2-Si (Waters, UK) in resolution mode. Spectra were recorded in negative ion mode from m/z 50 to 1200 at a scan rate of 1 Hz. Burn events lasted from 10 to 30 s. To avoid pseudoreplication caused by segmentation of burn events, the raw data were first processed using Progenesis Bridge to convert any uneven burn events into a single pseudo-Gaussian [time, total ion current (TIC)]. TIC is the cumulative effect of ions hitting the detector regardless of m/z throughout a single scan event, i.e. the sum of all m/z intensities of the spectra at the specific scan. The processed TIC trace was always 35 scans across, with the burn event covering 12 scans reaching a maximum at scan 18. This processed TIC trace was then imported into LiveID ensuring that there was only one datum per burn event in all subsequent analysis. Where sufficient tissue was available, samples were subject to 2 REIMS analyses on different days.
Data processing and statistical analyses
Raw data were pre-processed using Progenesis Bridge, a feature within the MassLynx Software (Waters, Manchester), with a threshold of 0, to normalize the TIC trace and combine the burn events enabling the comparison of a single spectrum from each punch. Data were binned with an m/z window of 0.01 and data matrix exported.
All data transformations and modelling were undertaken in the statistical software R [4] with graphical representations using the packages rgl [5], ggplot2 [6] and ggpubr [7]. Zero values were replaced for very small positive numbers to avoid indeterminacy calculations (i.e. 10−8). Data were log transformed and Pareto scaled. Partial least squares-discriminant analysis (PLS-DA) models were used to determine the suitability of REIMS data to predict different aetiologies as well as select relevant features with predictive capabilities. PLS-DA models were created using the package mixOmics [8]. Data were split in 80/20 proportions for training/test. The training data were used to build a PLS-DA model. The number of components chosen for the model was determined using a two-fold cross-validation and choosing the number of components that minimize the misclassification error. The test data were used to assess the model and calculate accuracy, sensitivity, recall and f1 for the model (see Supplementary Material for definitions of these terms). Important features of the models were extracted and reported [variable importance projection (VIP) plots]. This process was repeated 50 times generating 50 models with average accuracy and sensitivity reported. A second model was created in the same manner by selecting a subset of data based on m/z values with a VIP mean and median of >1. Representative score plots and model accuracies are reported in the results section. Receiver operating characteristic (ROC) and area under the curve were used to provide graphical representation of the model diagnostics. They were calculated based on the predicted scores obtained from the models on the test data. For each class the prediction is made of the class itself versus the others, allowing more than binary models to be appraised.
RESULTS
Control and aneurysmal tissues can be discriminated using REIMS
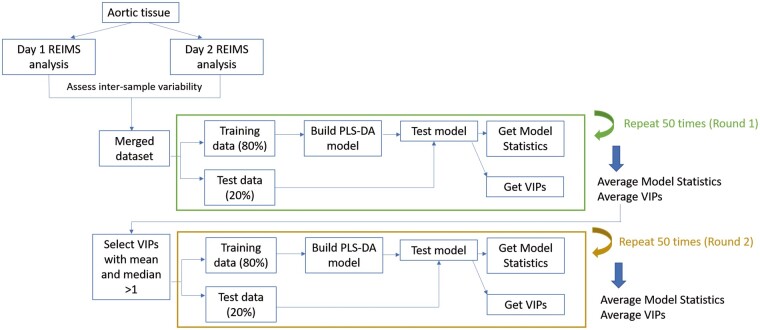
An overview of the experimental data collection, data processing and analyses is shown in Fig. 1. Table 1 shows clinical characteristics for all patients. Cohorts had no significant difference in age (P = 0.67), or indexed size for aneurysmal cohorts (P = 0.15). REIMS data were collected from aortic tissue for control (n = 13) and aneurysm (n = 44) patients over 2 separate days with patients analysed on both days if sufficient tissue was available. Variability between multiple samples from the same patient was minimal compared with variability between patient samples as can be seen in Supplementary Material, Fig. S1. Datasets were combined ensuring that all patients were only represented once.
Figure 1:
Workflow for the study. Aortic tissue was obtained during aortic replacement surgery or from post-mortem tissue for control (n = 13) or aneurysm patients (n = 44). Rapid evaporative ionization mass spectrometry data were acquired over 2 days with samples measured on both days (where sufficient tissue was available) to test intra- and inter-sample reproducibility (see Supplementary Material, Fig. S1). A merged dataset was produced with ∼50% of samples from each rapid evaporative ionization mass spectrometry dataset. Partial least squares-discriminant analysis modelling was performed and tested using randomly generated training (80%) and test (20%) data (round 1). Important features of the models were extracted (variable importance of projections) and variables selected from these for further modelling (round 2). Models from round 2 showed increased accuracy and precision.
Table 1:
Aortic pathology and summary patient clinical characteristics grouped by clinical condition collated from electronic patient records and NHS blood and transplant service records
| Control | BAV | TAV | |
|---|---|---|---|
| Pathology: aetiology | |||
| Normal: coronary artery disease/healthy post-mortem tissue (n) | 4/9 | ||
| Aneurysm: bicuspid aortic valve (n) | 21 | ||
| Aneurysm: tricuspid aortic valve (n) | 23 | ||
|
Age (years), median (IQR) |
67 (19) |
66 (7) |
72 (15) |
| Sex | |||
| Male (n) | 9 | 12 | 12 |
| Female (n) | 4 | 9 | 11 |
|
Indexed aorta size, median (IQR) |
– |
11.5 (3.0) |
13.2 (8.0) |
| Diabetic | 0/4 | 0/21 | 1/23 |
| Hypertensive | 3/4 | 13/21 | 17/23 |
| Hypercholesterolemia | 3/4 | 11/21 | 17/23 |
| Family history of aneurysm | 0/4 | 3/21 | 3/23 |
Indexed aorta size is not available for control patients. Clinical characteristics for control patients reflect CABG patients only as this information is not available for NHS-BT tissue. Quantitative values are expressed as median and interquartile range (IQR).
BAV: bicuspid aortic valve; CABG: coronary artery bypass graft; NHS-BT: NHS blood and transplant service; SD: standard deviation; TAV: tricuspid aortic valve.
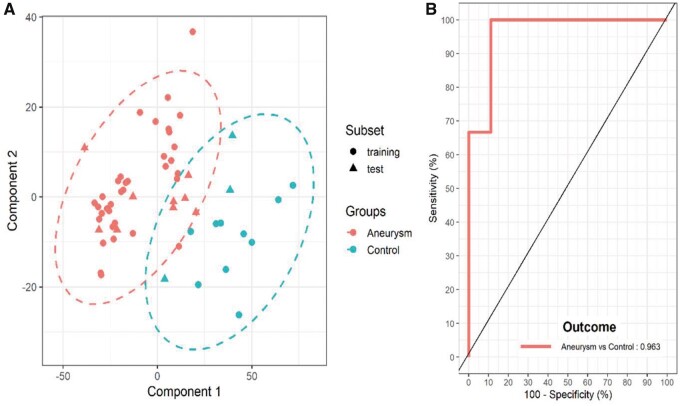
An initial round of modelling was carried out using 11 500 m/z values. This resulted in 2 component models that were able to discriminate between control and aneurysm patient tissues with 85.8% mean accuracy and 78.9% mean precision (Supplementary Material, Table S1). To improve the models further, important features of the models (VIPs) with mean and median score larger than 1 were selected for a second round of modelling (see Fig. 1). This generated a model that included 3236 m/z values and was able to discriminate patients with improved accuracy of 88.7% and precision 85.1% (Supplementary Material, Table S2). A representative score plot is shown in Fig. 2A accompanied by the accompanying ROC curve (Fig. 2B). Full model statistics for both models are reported in Supplementary Material, Tables S1 and S2. Interrogation of the models generated showed that gender (Supplementary Material, Fig. S2A) or source of control tissue (Supplementary Material, Fig. S2B) were not contributing to the observed discrimination.
Figure 2:
Partial least squares-discriminant analysis representative model for rapid evaporative ionization mass spectrometry data between control (n = 13) and aneurysm patients (n = 44). (A) Score plot of the two-component model. Points represented for both training (circles) and test (triangles) for both groups control (cyan) and aneurysm (red) with ellipse representative of the 95% region around the means of each group. It is clear that the groups can be separated with this model. (B) Receiver operating curve of the model representing an area under the curve value of 0.963. Fifty models were fitted with average statistics reported in Supplementary Material, Table S2.
Aneurysmal aortic tissues derived from patients with bicuspid and tricuspid valves can be discriminated using REIMS
Following this initial assessment, aneurysm patients were sub-divided according to their aortic valve morphology into bicuspid aortic valve (BAV) (n = 21) and tricuspid aortic valve (TAV) (n = 23) cohorts and the modelling process repeated. PLS-DA models to discriminate between control, BAV and TAV resulted in 3 component models. The models from round 1, built using 11 500 m/z values resulted in mean accuracies of 81.3%, 57.0% and 70.3% and mean precision values of 68.8%, 40.0% and 67.3% for the discrimination of control, BAV and TAV tissues from the other groups, respectively (Supplementary Material, Table S3). A tota of 3351 m/z variables with a mean and median VIP score of larger than 1 were selected for a second round of modelling. A representative score plot for one of the models is shown in Fig. 3. An additional 2D plot showing 2 of the 3 components indicating training and test data and accompanying ROC curve is shown in Supplementary Material, Fig. S3. The mean accuracies of this model increased to 93.5%, 83.8% and 89.3% and mean precision values increased to 91.4%, 76.7% and 86.0% for control, BAV and TAV, respectively. All values for model assessment are reported in Supplementary Material, Tables S3 and S4, with area under the curve assessment reported in Supplementary Material, Table S5. Hence, the techniques are able to discriminate tissues from patients with various cardiovascular aetiologies.
Figure 3:
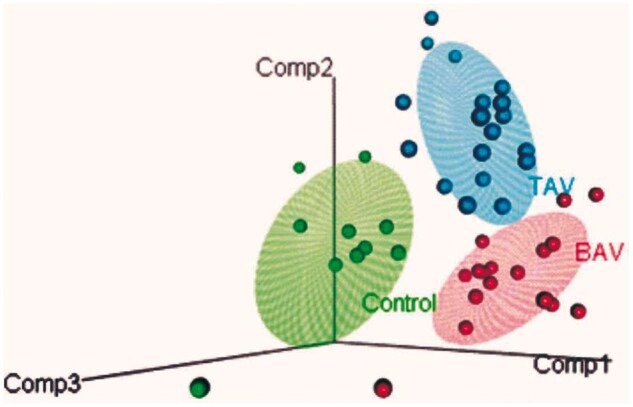
Partial least squares-discriminant analysis representative model 3D plot for rapid evaporative ionization mass spectrometry data between control, bicuspid aortic valve and tricuspid aortic valve patients. Three-component model generated, each patient is represented as a circle for control (green, n = 13), bicuspid aortic valve (red, n = 21) and tricuspid aortic valve (blue, n = 23), with ellipse representative of the 95% region around the means of each group. Model statistics are reported in Supplementary Material, Table S4.
DISCUSSION
REIMS coupled with a diathermy (i-knife) has the potential to aide to surgical decision-making in aortic surgery. The principles of the technique are that surgical aerosol emanating from electrocautery and destruction of tissues is aspirated into a mass spectrometry producing a signal by measuring the mass to charge ratio of molecular ions and their charged fragments [9]. REIMS has been explored in a number of clinical [10, 11] and non-clinical [12–14] areas; however, i-knife is currently being explored, and has achieved some notoriety, as an aide to identifying tumour margins in breast surgery [3]. We have hypothesized that REIMS may be of use in aortic surgery by providing some objectivity to the subjective intraoperative decision-making commonly required in the management of borderline aneurysms. As a preliminary explorative approach, we conducted batch laboratory REIMS analysis of aortic tissue from patients which included normal and aneurysmal proximal aorta (ascending aorta). Our methodology was able to clearly discriminate aneurysmal aorta from normal aorta (Fig. 2) with accuracy of 88.7% and precision of 85.1%. We have previously reported differences in biomechanical and biochemical properties of aortic tissue between normal, BAV and TAV [15–17]. We have also observed distinct patterns of matrix degradation in aortic tissue between the 3 groups [15]. Detailed assessment of histological staining identified a decrease in elastin content across all 3 layers of the aortic wall that followed the trend; control > BAV > TAV. In addition, we found that TAV patient tissue had highly fragmented elastin with thinner elastic lamellae compared to BAV and control. Other studies have also shown altered matrix architecture for aneurysmal tissue [18, 19] with a similar trend of BAV fibre orientation more similar to control tissue than TAV [20]. The current study compares tissue from patients with the same aortic pathologies used in these previous studies and shows that REIMS can discriminate aortic tissue from the 3 patient cohorts (Fig. 3). While the 3D model can clearly discriminate between the 3 aetiologies, we can see that in 2 dimensions TAV and controls have the most differences while BAV tissue properties seem to sit in the interphase of both clusters (Supplementary Material, Fig. S3), consistent with previously reported histological observations [15]. This observation could explain why precision and accuracy values discriminating BAV from other pathologies are lower (83.8% and 76.7%, respectively) than those for control (93.5% and 91.4%, respectively) or TAV (89.3% and 86.0%, respectively).
All of the techniques utilized in previous studies highlighted above require ex vivo tissue and are often time-consuming processes and can detect a very limited number of variables at a time. Here, we show that REIMS can discriminate between control, and BAV and TAV aneurysmal tissue with excellent accuracy and precision. REIMS generates data virtually instantaneously, within seconds making it ideally suited for intraoperative use without causing a delay to the operating procedure. We therefore propose REIMS as a near real-time technique with potential as a future point-of-care technique (Fig. 4). The guidance for surgical intervention on the thoracic aorta is dominated by preoperative imaging measurements with adjustments for syndromic disease, symptomology and concomitant cardiac disease [1, 2]. In reality, final decisions on resection or the longitudinal extent of resection are based on the subjective decisions of the surgeon. In non-syndromic aneurysms the most significant extent of a proximal aneurysm is principally the ascending aneurysm. In borderline proximal and distal disease, decisions are made on the risk of extending the resection to include the aortic root and proximal aortic arch versus the risk of early recurrent disease. This will be based on factors such as patient age, comorbidity and frailty but also on the surgeon’s opinion on the appearances of the aorta, the thinness of the wall and the lamination of the media. Such decisions have significant consequences and to-date are based on a surgeon’s experience, with few, if any, options for objective measures.
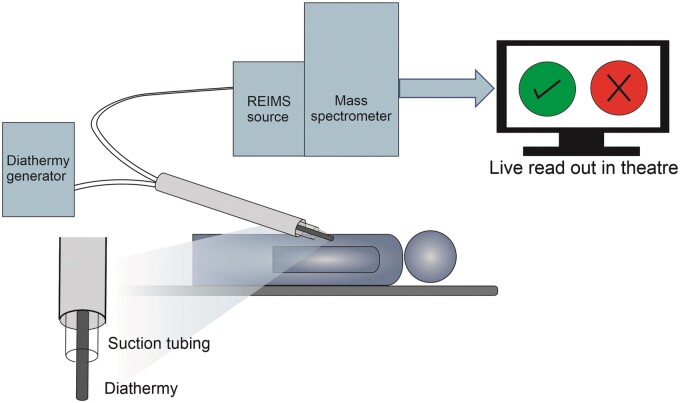
Figure 4:
Schematic displaying potential rapid evaporative ionization mass spectrometry set-up for use in aortic surgery to assist with intraoperative decision-making.
Our intention is to conduct future studies including a systematic prospective study of ex vivo fresh aorta examining the longitudinal REIMS signal from normal, borderline and aneurysmal aorta, indexed to body surface area along with a mechanistic study examining REIMS signal and aetiology of aneurysmal formation. Using this approach, intraoperative data obtained in near real-time (seconds) could be employed to guide surgeons on the extent of resection required at the margins on patients with borderline aneurysmal ascending aorta and in other interventions including valve and coronary surgery.
Currently REIMS technology is not commercially available for clinical application and remains in development. The diathermy is comparable with a typical electrosurgical tool. However, the attached mass spectrometer would require situating in an adjacent room with a computer monitor in theatre to report the results. Therefore, the hardware needs miniaturizing and commercializing. This study is based on laboratory analysis of ex vivo aorta; however, other oncological studies have shown that it may pragmatically be deployed in the operating theatre. Our hope is that future studies will allow us to deploy this REIMS, and other spectroscopic methods such as Raman spectroscopy in the operating room, recording in vivo measurements.
Limitations
There were several limitations to this study. First, analysis was carried out retrospectively following freezing, storage and thawing of tissue. Future studies should aim to conduct in vivo analysis. Second, normal or control aorta was a mixture of punch biopsy from patients undergoing coronary artery by-pass graft and post-mortem tissue from NHS-BT. However, no statistical differences were observed during data analysis between these 2 different sources enhancing our confidence that differences between control and aneurysmal tissue were not due to differences in storage or tissue collection processes. Third, we have not systematically sampled longitudinally from ‘normal’, ‘borderline’ or ‘peri-aneurysmal penumbra’ and aneurysm. This is required as the next stage in technique development. Fourth, patients with connective tissue disorders were excluded from the study. In future, other pathologies (Marfan, Loeys–Dietz syndrome, Ehlers–Danlos syndrome) will be addressed systematically with the aim of producing a library of biosignatures. Fifth, we have not determined the origin/identity of the key spectrometry signals (VIPs) within this study. The latter remains one of the caveats of this technique: there are very limited and poorly annotated database entries or software to undertake signal identification.
CONCLUSION
Analysis of electrosurgical aerosol from ex vivo aortic tissue using REIMS allows us to discriminate aneurysmal from normal aorta and sub-divide patients with different valve sub-types, supporting its development as a point-of-care test (i-knife) for guiding surgical intraoperative decision-making.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
DATA AVAILABILITY
Raw or processed REIMS data are available upon request to the corresponding author.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Deborah Harrington, Manoj Kuduvalli, Ahmed Othman at Liverpool Heart and Chest Hospital and Ya Hua Chim (University of Liverpool) for their assistance in collecting tissue samples. We also acknowledge the Technology Directorate at University of Liverpool for their facilities support and Philip Brownridge for instrument maintenance.
Funding
This work was supported by the British Heart Foundation (FS/12/61/29877 to Jillian Madine PG/16/107/32681 to Riaz Akhtar, Jillian Madine and Mark Field) and Royal Academy of Engineering/Leverhulme Trust (LTSRF1617/13/76 to Riaz Akhtar).
Conflict of interest: None declared.
ABBREVIATIONS
- BAV
Bicuspid aortic valve
- i-knife
Intelligent Knife
- NHS-BT
NHS blood and transplant service
- PLS-DA
Partial least squares-discriminant analysis
- REIMS
Rapid evaporative ionization mass spectrometry
- ROC
Receiver operating curve
- TAV
Tricuspid aortic valve
- TIC
Total ion current
- VIP
Variable importance of projection
Author contributions
Hannah A. Davies: Conceptualization; Data curation; Formal analysis; Writing—original draft; Writing—review & editing. Eva Caamano-Gutierrez: Data curation; Formal analysis; Validation; Writing—original draft. Joscelyn Sarsby: Data curation; Formal analysis; Methodology; Writing—review & editing. Omar Nawaytou: Investigation; Resources; Visualization; Writing—review & editing. Amer Harky: Data curation; Resources; Writing—review & editing. Riaz Akhtar: Funding acquisition; Resources; Visualization; Writing—review & editing. Mark Field: Conceptualization; Investigation; Project administration; Resources; Visualization; Writing—original draft; Writing—review & editing. Jillian Madine: Conceptualization; Data curation; Formal analysis; Funding acquisition; Project administration; Supervision; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Emmanouil Ioannis Kapetanakis, Mario Giovanni Gerardo D'Oria and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD. et al. ; members ATF. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–926. [DOI] [PubMed] [Google Scholar]
- 2.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE. et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 3.St John ER, Balog J, McKenzie JS, Rossi M, Covington A, Muirhead L. et al. Rapid evaporative ionisation mass spectrometry of electrosurgical vapours for the identification of breast pathology: towards an intelligent knife for breast cancer surgery. Breast Cancer Res 2017;19:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. https://www.R-project.org/. [Google Scholar]
- 5.Adler D, Murdoch D. rgl: 3D Visualization Using OpenGL. R package version 010026, 2019. https://CRAN.R-project.org/package=rgl.
- 6.Wickham H.ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag, 2016. [Google Scholar]
- 7.Kassambara A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 025, 2020. https://CRAN.R-project.org/package=ggpubr.
- 8.Rohart F, Gautier B, Singh A, Lê Cao K-A.. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol 2017;13:e1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schäfer K-C, Dénes J, Albrecht K, Szaniszló T, Balog J, Skoumal R. et al. In vivo, in situ tissue analysis using rapid evaporative ionization mass spectrometry. Angew Chem Int Ed Engl 2009;48:8240–2. [DOI] [PubMed] [Google Scholar]
- 10.Tzafetas M, Mitra A, Paraskevaidi M, Bodai Z, Kalliala I, Bowden S. et al. The intelligent knife (iKnife) and its intraoperative diagnostic advantage for the treatment of cervical disease. Proc Natl Acad Sci U S A 2020;117:7338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balog J, Sasi-Szabó L, Kinross J, Lewis MR, Muirhead LJ, Veselkov K. et al. Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci Transl Med 2013;5:194ra93. [DOI] [PubMed] [Google Scholar]
- 12.Balog J, Perenyi D, Guallar-Hoyas C, Egri A, Pringle SD, Stead S. et al. Identification of the species of origin for meat products by rapid evaporative ionization mass spectrometry. J Agric Food Chem 2016;64:4793–800. [DOI] [PubMed] [Google Scholar]
- 13.Black C, Chevallier OP, Haughey SA, Balog J, Stead S, Pringle SD. et al. A real time metabolomic profiling approach to detecting fish fraud using rapid evaporative ionisation mass spectrometry. Metabolomics 2017;13:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strittmatter N, Jones EA, Veselkov KA, Rebec M, Bundy JG, Takats Z.. Analysis of intact bacteria using rapid evaporative ionisation mass spectrometry. Chem Commun (Camb) 2013;49:6188–90. [DOI] [PubMed] [Google Scholar]
- 15.Chim YH, Davies HA, Mason D, Nawaytou O, Field M, Madine J. et al. Bicuspid valve aortopathy is associated with distinct patterns of matrix degradation. J Thorac Cardiovasc Surg 2020;160:e239–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies HA, Caamaño-Gutiérrez E, Chim YH, Field M, Nawaytou O, Ressel L. et al. Idiopathic degenerative thoracic aneurysms are associated with increased aortic medial amyloid. Amyloid 2019;26:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung JCY, Wong E, Tang M, Eliathamby D, Forbes TL, Butany J. et al. Biomechanics of aortic dissection: a comparison of aortas associated with bicuspid and tricuspid aortic valves. J Am Heart Assoc 2020;9:e016715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsamis A, Krawiec JT, Vorp DA.. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface 2013;10:20121004–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisano C, Maresi E, Balistreri CR, Candore G, Merlo D, Fattouch K. et al. Histological and genetic studies in patients with bicuspid aortic valve and ascending aorta complications. Interact CardioVasc Thorac Surg 2012;14:300–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillippi JA, Green BR, Eskay MA, Kotlarczyk MP, Hill MR, Robertson AM. et al. Mechanism of aortic medial matrix remodeling is distinct in patients with bicuspid aortic valve. J Thorac Cardiovasc Surg 2014;147:1056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw or processed REIMS data are available upon request to the corresponding author.