Abstract
Objective
To evaluate the long-term consequences of COVID-19 survivors one year after recovery, and to identify the risk factors associated with abnormal patterns in chest imaging manifestations or impaired lung function.
Methods
COVID-19 patients were recruited and prospectively followed up with symptoms, health-related quality of life, psychological questionnaires, 6-minute walking test, chest computed tomography (CT), pulmonary function tests, and blood tests. Multivariable logistic regression models were used to evaluate the association between the clinical characteristics and chest CT abnormalities or pulmonary function.
Results
Ninety-four patients with COVID-19 were recruited between January 16 and February 6, 2021. Muscle fatigue and insomnia were the most common symptoms. Chest CT scans were abnormal in 71.28% of participants. The results of multivariable regression showed an increased odds in age. Ten patients had diffusing capacity of the lung for carbon monoxide (DLCO) impairment. Urea nitrogen concentration on admission was significantly associated with impaired DLCO. IgG levels and neutralizing activity were significantly lower compared with those in the early phase.
Conclusions
One year after hospitalization for COVID-19, a cohort of survivors were mainly troubled with muscle fatigue and insomnia. Pulmonary structural abnormalities and pulmonary diffusion capacities were highly prevalent in surviving COVID-19 patients. It is necessary to intervene in the main target population for long-term recovery.
Keywords: COVID-19, CT abnormalities, Lung function, Neutralizing antibodies, IgG antibodies
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, arising from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in more than 190 million confirmed cases and more than 4.0 million deaths (WHO, 2021). Survivors with COVID-19 are frequently reported to have persistent symptoms, and pulmonary function and psychological problems. It is challenging and necessary to evaluate the long-term sequelae of COVID-19.
Persistent pulmonary function impairment and health status were demonstrated in SARS survivors up to 1 year following hospital discharge (Hui et al., 2005; Ong et al., 2005; Ruhl et al., 2017). Higher titers of antibodies against SARS, MERS, and H7N9 continued to persist for 1 year (Choe et al., 2017; Ma et al., 2018; Xie et al., 2005). There are several reports of long-term consequences of COVID-19 at 3 months and 6 months after discharge (Gonzalez et al., 2021; Huang et al., 2021; Qin et al., 2021; Tarsitani et al., 2021; Zhao et al., 2020), but the prevalence and severity of the long-term sequelae of COVID-19 have remained largely unknown.
This study systematically assessed the long-term health consequences of COVID-19 survivors 1 year after discharge. Participants in this study underwent an evaluation of health status, involving the 36-Item Short-Form Health Survey (SF-36), the 14-item Hamilton Anxiety Rating Scale (HAMA-14), the 24-item Hamilton Depression Rating Scale (HAMD-24), the modified British Medical Research Council (mMRC), and 6-minute walking test (6MWT). The characterization of chest computed tomography (CT), lung function, and titers of antibodies were also examined.
Materials and Methods
Study design and participants
This prospective observational study included six cohorts of adult inpatients (aged ≥ 18 years). All adult patients with laboratory-confirmed SARS-CoV-2 infection, and subsequently admitted to the designated local hospitals in Henan Province, were enrolled. This study was approved by the Institutional Review Board of the relevant centers. All participants remained anonymous, and written informed consent was obtained. This study was registered with the Chinese Clinical Trial Registry, ChiCTR2000033186. The World Health Organization's (WHO) interim guidance diagnosis for adults with COVID-19 was used (WHO, 2020).
Data collection
Baseline and hospital stay: the clinical data of all participants were extracted from electronic medical records, including sociodemographic information, time of admission, length of hospital stay, and comorbidities. Clinical classification of COVID-19, blood routine outcomes, and therapeutics were also recorded. All data were checked by three physicians.
12-month follow-up: follow-up consultations were conducted in the outpatient clinic of the relevant centers. Face-to-face interviews were performed by trained physicians and all participants were asked to complete a series of questionnaires. For the symptom questionnaire, participants were asked to report new symptom onset after COVID-19. All participants received 6MWT, pulmonary function tests (PFTs), and high-resolution CT of the chest.
For general and respiratory symptoms, participants were asked to report persistent symptoms after COVID-19. Items such as fatigue, muscle weakness, joint paint, sleeping difficulties, headache, hair loss, chest pain, smell or taste disorder, myalgia, palpitations, dizziness, sore throat or difficulty swallowing, diarrhea or nausea, and skin rash were assessed. Furthermore, the Chinese versions of HAMA-14 and HAMD-24 were used to evaluate signs and symptoms of anxiety and depression (Lu et al., 2020). Overall, participants with HAMA scores of 0-6, 7-13 and ≥ 14 points were categorized as having no anxiety, mild/moderate anxiety, and severe anxiety, respectively (Qin et al., 2020). The total score of HAMD was operationally categorized as follows: normal (score 0-6), mild or probable depression (score 7-17), moderate or definite depression (score 18-24), and severe depression (score ≥ 25) (Zhuang et al., 2018). The SF-36 is a well-known health-related quality of life questionnaire that comprehensively measures eight aspects to access physical and mental health: physical function (PF), role physical (RP), body pain (BP), general health perceptions (GH), vitality (VT), social function (SF), role emotional (RE), and mental health (MH) (Apolone and Mosconi, 1998) it presents a score of 0-100, with a higher score indicating better health status.
Chest CT acquisition and image analysis
Each subject underwent an initial chest CT examination and follow-up examinations during a single-breath at full inspiration. All CT scans were acquired with the patients in the supine position with both limbs raised above the head. The whole-lung spiral CT scan was performed from the apex to the base of the lungs. The CT scanner models from the hospitals involved in this multicenter study were listed as following: Somatom Definition AS 128, Philips Brilliance 16, Philips Brilliance 64, and Philips Incisive 64. All images were then reconstructed with a 1.0-5.0 mm slice with the same increment.
Two radiologists, who were blinded to the clinical information, independently reviewed and scored the CT images. When there was a divergent opinion, they made the finial decision via a view console. The radiologists assessed the following eight characteristics (Guler et al., 2021): ground glass opacities (GGO), consolidation, nodule, reticulation, interlobular septal thickening, crazy-paving pattern, subpleural curvilinear line, and pulmonary fibrosis. The CT score was derived from abnormal pulmonary involvement based on a 5-point scale: 0, normal; 1, < 5%; 2, 5-25%; 3, 26-50%; 4, 51-75%; 5, > 75%). A total score was eventually recorded via the addition of the score of an individual segment.
Pulmonary function tests
Outpatient PFTs were conducted in the Lung Function Laboratory of the Guangshan People's Hospital and Xixian People's Hospital, using MasterScreen PFT (Jaeger, Germany) or MasterScreen (Jaeger, Germany) according to ATS-ERS guidelines (Graham et al., 2019). The PFTs yielded the following parameters: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC, FVC% pred, and diffusing capacity of the lung for carbon monoxide (DLCO).
Dynamic changes of SARS-CoV-2 IgG, IgM, and neutralizing antibodies
Serum IgM and IgG antibodies against the SARS-CoV-2 spike protein (S) and the nucleocapsid protein (N) were measured by the commercial kit provided by YHLO biotechnology (Catalog number, G86095M/G86095G), which has previously been described (Zhao et al., 2020). The cut-off for positivity was equal to 10.0 AU/mL for both IgM and IgG, according to the manufacturer. The SARS-CoV-2 neutralizing antibodies (NAb) were measured by the SARS-CoV-2 sVNT kit (Catalog number, L00847, GenScript), according to the manufacturer instructions (Tan et al., 2020). The inhibition of the sample was proportional to the titer of the anti-SARS-CoV-2 neutralizing antibodies. There were 55 survivors (including four mild, 47 moderate, and four severe disease) (Zhao et al., 2020) at 3 months after discharge, and 67 survivors (including two mild, 30 moderate, 33 severe, and two critical cases) at 1 year after discharge who were tested for IgM, IgG, and NAb against SARS-CoV-2.
Statistical analysis
Categorical variables were expressed as number (percentage) and compared using the Chi-square test or Fisher exact test. Continuous data were described as mean ± SD (standard deviation), followed by paired or unpaired t-test, or Mann-Whitney test, or Wilcoxon test. Multivariable logistic regression modes were used to explore the risk factors associated with chest CT abnormalities or impaired DLCO. The correlation of different variables was analyzed using Spearman's correlation. All analyses were performed using SPSS 21.0 and GraphPad Prism 8.0. Two-sided P < 0.05 was considered as statistically significant.
Results
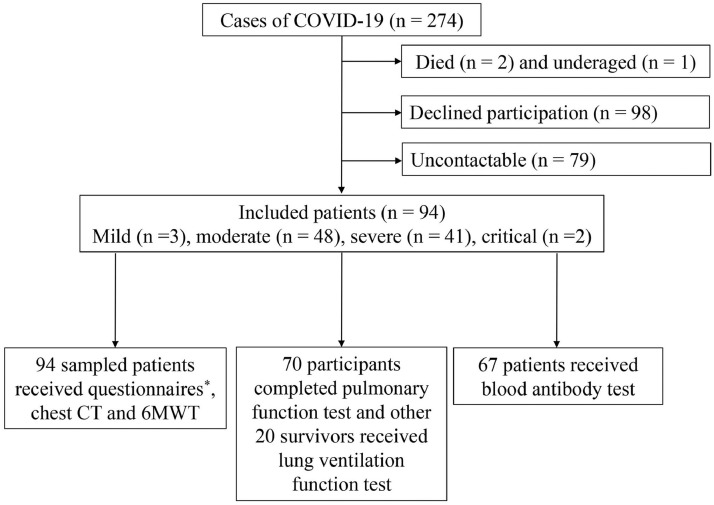
A total of 272 patients with COVID-19 were discharged from the relevant hospitals and the follow-up study was conducted from January 16 to February 6, 2021. Of these, 180 survivors did not attend follow-up study for several reasons, which are outlined in Figure 1 . Finally, 94 adult participants, who included 3 cases of mild pneumonia, 48 cases of pneumonia, 41 cases of severe pneumonia, and 2 critical cases, were enrolled for questionnaire interview, chest CT, and 6MWT. For lung function test, 70 sampled patients ascertained as eligible received complete PFTs. Twenty survivors refused to complete the lung diffusion function test. Moreover, sixty-seven survivors received a blood antibody test.
Figure 1.
Flow chart of patients with COVID-19 at 1 year after hospital discharge between January 23 and February 27, 2020.
*Questionnaires included general and respiratory symptoms, 36-Item Short-Form Health Survey (SF-36), 14-item Hamilton Anxiety Rating Scale (HAMA-14), 24-item Hamilton Depression Rating Scale-24 (HAMD-24), and the modified British Medical Research Council (mMRC).
6MWT = 6-minute walking test; CT = computed tomography
The demographics and characteristics of the study population are shown in Table S1. The mean age of these cases was 48.11 years, and 40 (42.55%) of them were females. Seven of them were former smokers or current smokers. The most common comorbidity was hypertension (16 cases, 17.02%), followed by diabetes mellitus (9 cases, 9.57%), chronic heart disease (4 cases, 4.26%), and asthma (2 cases, 2.13%). Although 11 (11.70%) survivors were transferred to ICU, none of them required invasive mechanical ventilation. The overall duration of hospital stay was (15.08 ± 5.71) days. With regard to treatment, patients were mostly treated with antibacterial agents (82.98%), interferon (81.91%), corticosteroids (30.85%), and immunoglobulins (10.64%). All patients received antiviral treatment. The median duration from symptom onset to follow-up visit was 366.0 (355.0, 376.0) days, and the median time from hospital discharge to follow-up visit was 345.0 (333.0, 349.0) days.
Symptoms, HAMA, HAMD, mMRC, and SF-36 questionnaires at 1-year follow-up
At 1-year follow-up, 61.70% of patients (58 of 94) reported at least one symptom that did not exist before COVID-19 infection, including muscle fatigue (39.36%), insomnia (22.34%), joint paint (20.21%), headache (14.89%), hair loss (13.83%), and chest pain (13.83%) (Table 1 ). Eleven patients (11.70%) still experienced a smell or taste disorder. The frequency of muscle fatigue in severe/critical COVID-19 was higher than that of mild/moderate COVID-19 (P < 0.05, Table 1). According to the results (Table S2), persistent symptoms, anxiety or depression, and the mMRC dyspnea scale of COVID-19 patients had no relation to age, which was consistent with previous reports (Hui et al., 2005; Qin et al., 2021).
Table 1.
Symptoms, quality of life, and anxiety/depression questionnaires results at 1-year follow-up.
| Symptoms | ||||
| Total | Mild/moderateN = 51 | Severe/criticalN = 43 | P | |
| Any one of the following symptoms, N (%) | ||||
| Muscle fatigue | 37 (39.36) | 15 (29.41) | 22 (51.16) | 0.032 |
| Insomnia | 21 (22.34) | 10 (19.61) | 11 (25.58) | 0.488 |
| Joint paint | 19 (20.21) | 7 (13.73) | 12 (27.91) | 0.088 |
| Headache | 14 (14.89) | 9 (17.65) | 5 (11.63) | 0.414 |
| Hair loss | 13 (13.83) | 5 (9.80) | 8 (18.60) | 0.218 |
| Chest pain | 13 (13.83) | 5 (9.80) | 8 (18.60) | 0.218 |
| Palpitations | 11 (11.70) | 6 (11.76) | 5 (11.63) | 0.984 |
| Smell or taste disorder | 11 (11.70) | 6 (11.76) | 5 (11.63) | 0.984 |
| Myalgia | 11 (11.70) | 7 (13.73) | 4 (9.30) | 0.506 |
| Dizziness | 10 (10.64) | 4 (7.84) | 6 (13.95) | 0.534 |
| Sore throat or difficulty swallowing | 9 (9.57) | 5 (9.80) | 4 (9.30) | 1.000 |
| Diarrhea or nausea | 9 (9.57) | 6 (11.76) | 3 (6.98) | 0.664 |
| Skin rash | 2 (2.13) | 2 (3.92) | 0 | 0.498 |
| Questionnaires | Mild/moderateN = 51 | Severe/criticalN = 43 | P | |
| HAMA | 0.370 | |||
| No anxiety (≤ 6), N (%) | 55 (58.51) | 30 (58.82) | 25 (58.14) | |
| Mild/moderate anxiety (7-13), N (%) | 30 (31.91) | 18 (35.29) | 12 (27.91) | |
| Severe anxiety (≥ 14) | 9 (9.57) | 3 (5.88) | 6 (13.95) | |
| HAMD | 0.646 | |||
| Normal (≤ 6), N (%) | 54 (57.45) | 32 (62.75) | 22 (51.16) | |
| Mild/probable depression (7-17), N (%) | 30 (31.91) | 15 (29.41) | 15 (34.88) | |
| Moderate/definite depression (18-24), N (%) | 7 (7.45) | 3 (5.88) | 4 (9.30) | |
| Severe depression (≥ 25), N (%) | 3 (3.19) | 1 (1.96) | 2 (4.65) | |
| mMRC score | 0.344 | |||
| 0, N (%) | 72 (76.60) | 41 (80.39) | 31 (72.09) | |
| ≥ 1, N (%) | 22 (23.40) | 10 (19.61) | 12 (27.91) | |
| SF-36 | ||||
| Physical function (PF) | 95 (90, 100) | 95 (90, 100) | 95 (85, 100) | 0.150 |
| Role-physical (RP) | 100 (75, 100) | 100 (75, 100) | 100 (25, 100) | 0.037 |
| Body pain (BP) | 74 (61.75, 100) | 74 (52, 100) | 74 (64, 100) | 0.418 |
| General health perceptions (GH) | 66 (47, 80) | 65.49 ± 20.55 | 58.88 ± 25.81 | 0.179 |
| Vitality (VT) | 75 (63.75, 90) | 80 (65, 90) | 70 (60, 85) | 0.108 |
| Social function (SF) | 70 (40, 80) | 70 (50, 80) | 60 (40, 80) | 0.740 |
| Role-emotional (RE) | 100 (66.67, 100) | 100 (66.67, 100) | 100 (66.67, 100) | 0.502 |
| Mental health (MH) | 76 (60, 92) | 76 (60, 92) | 76 (64, 92) | 0.846 |
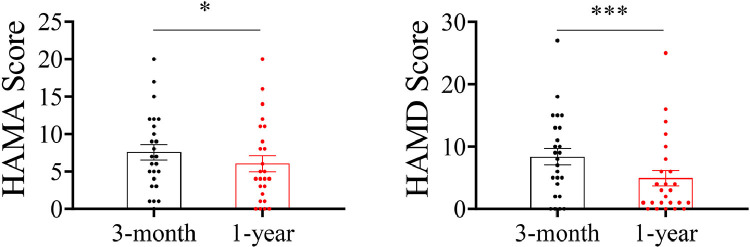
For anxiety symptoms, 30 (31.91%) patients were evaluated as mild/moderate anxiety and 9 (9.57%) patients were severe. For depression symptoms, 42.55% of patients presented altered depression scores, including mild/probable depression (30 cases, 31.91%), moderate/definite depression (7 cases, 7.45%), and severe depression (3 cases, 3.19%). Although there were 25 patients who participated in both the 3-month and 1-year follow-ups, the HAMA and HAMD scores of 25 enrolled survivors at 1-year follow-up were significantly lower than those of patients at the 3-month follow-up (Figure 2 ). In addition, the prevalence of mMRC score ≥ 1 was 22 (23.40%). The SF-36 revealed that PF, RP, BP, VT, RE, and MH reached the highest scores (95, 100, 74, 75, 100, and 76, respectively), while GH and SF reached the lowest scores (66 and 70, respectively).
Figure 2.
Comparison of the results of HAMA and HAMD scores between the 25 COVID-19 survivors at the 3-months and 1-year follow-ups.
* P < 0.05; *** P < 0.001
Lung function, 6MWT, and chest CT at 1-year follow-up
The pulmonary function, 6MWT, and chest CT results are shown in Table 2 . Anomalies were noted in FEV1% predicted in 16 of 90 cases (17.78%), FEV1/FVC in 9 (10%), total lung capacity (TLC%) predicted in 4 cases (5.71%), and DLCO% predicted in 10 cases (14.29%). One year after discharge, 20% and 35.29% of mild/moderate COVID-19 patients developed impaired pulmonary diffusion capacities and abnormal chest imaging manifestations (Table 2). Lung function tests of 25 patients who participated in both the 3-month (Zhao et al., 2020) and 1-year follow-ups were collected. There was no significant difference in FVC%, FEV1% pred, FEV1/FVC, and TLC% between patients at the 3-month and 1-year follow-ups. The diffusing capacity in COVID-19 patients 1 year after discharge was higher than that at the 3-month follow-up, even though there was no significant between-group difference (Table S3). All of these results indicate that CT patterns of abnormalities may contribute to pulmonary interstitial damage. The median (IQR) distance in the exercise test was 504.00 (486.36, 540.00) meters, with a median oxygen saturation of 97%; there was no oxygen saturation < 90% (data not shown). The difference in the distance of the 6MWT between the current cohort and healthy population was calculated adjusted by sex, age, weight, and height (Enright and Sherrill, 1998). The distance of the sampled participants showed a significant decrease compared with the healthy population (median: 596.45, IQR: 514.50-635.19; P < 0.0001, Table S4).
Table 2.
Pulmonary function, 6MWT, and chest CT scan findings in all patients at 1-year follow-up.
| Pulmonary function | ||||
| Mild/moderate(n = 50) | Severe/critical(n = 40) | P | ||
| FVC%, (n = 90)Normal range ≥ 80% | 101.17 ± 16.60 | 102.59 ± 14.71 | 99.38 ± 18.73 | 0.364 |
| FEV1% pred, (n = 90)Normal range ≥ 80% | 100.85 (87.88, 108.68) | 101 (88.55, 107.92) | 99.7 (84.88, 110.18) | 0.881 |
| ≥ 80%, N (%) | 74 (82.22) | 42 (84) | 32 (80) | 0.622 |
| < 80%, N (%) | 16 (17.78) | 8 (16) | 8 (20) | |
| FEV1/FVC, (n = 90)Normal range ≥ 70% | 79.74 (75.86, 84.23) | 79.37 (75.75, 85.19) | 79.94 (76.47, 83.22) | 0.951 |
| ≥ 70%, N (%) | 81 (90) | 46 (92) | 35 (87.5) | 0.724 |
| < 70%, N (%) | 9 (10) | 4 (8) | 5 (12.5) | |
| Mild/moderate(n = 35) | Severe/critical(n = 35) | P | ||
| TLC%, (n = 70)Normal range ≥ 80% | 98.86 ± 12.24 | 100.34 (94.9, 108) | 94.98 (87.1, 106.5) | 0.079 |
| ≥ 80%, N (%) | 66 (94.29) | 33 (94.29) | 33 (94.29) | 1.000 |
| 50-80%, N (%) | 4 (5.71) | 2 (5.71) | 2 (5.71) | |
| RV%, (n = 70)Normal range ≥ 65% | 105.96 (93.78, 117.96) | 114.2 (95.3, 124.26) | 102.1 (89.6, 114.49) | 0.113 |
| DLCO%, (n = 70)Normal range ≥ 80% | 99.50 ± 18.82 | 99.54 ± 21.62 | 99.46 ± 15.84 | 0.856 |
| ≥ 80%, N (%) | 60 (85.71) | 28 (80) | 32 (91.43) | 0.172 |
| 60-80%, N (%) | 10 (14.29) | 7 (20) | 3 (8.57) | |
| 6MWT (n = 94) | Mild/moderate(n = 51) | Severe/critical(n = 43) | P | |
| Distance (m) | 504 (486.36, 540) | 504 (498, 546) | 500 (468, 528) | 0.248 |
| Minimal oxygen saturation (%) | 97 (95, 98) | 98 (96, 99) | 96 (94, 98) | 0.001 |
| Chest CT (n = 94) | Mild/moderate(n = 51) | Severe/critical(n = 43) | P | |
| Density | ||||
| Ground-glass, N (%) | 38 (40.43) | 18 (35.29) | 20 (46.51) | 0.270 |
| Volume of GGO, cm3 | 0.00 (0.00, 0.32) | 0.00 (0.00, 0.12) | 0.00 (0.00, 0.88) | 0.033 |
| Consolidation, N (%) | 2 (2.13) | 0 | 2 (4.65) | 0.207 |
| Internal structures | ||||
| Interlobular septal thickening, N (%) | 10 (10.64) | 3 (5.88) | 7 (16.28) | 0.196 |
| Subpleural lines, N (%) | 14 (14.89) | 6 (11.76) | 8 (18.60) | 0.353 |
| Nodule, N (%) | 28 (29.79) | 14 (27.45) | 14 (32.56) | 0.590 |
| Linear opacities, N (%) | 13 (13.83) | 9 (17.65) | 4 (9.30) | 0.243 |
| Lesions | ||||
| Reticulation, N (%) | 4 (4.26) | 1 (1.96) | 3 (6.98) | 0.492 |
| Fibrotic, N (%) | 8 (8.51) | 2 (3.92) | 6 (13.95) | 0.172 |
| CT score | ||||
| Score, mean (SD) | 1.50 (0.00, 3.25) | 1 (0, 2) | 2 (1, 6) | 0.002 |
| Number of lobes involved, median (IQR) | 1.50 (0.00, 3.00) | 1 (0, 2) | 2 (1, 3) | 0.005 |
Overall, many abnormalities in chest CT were detected in 67 survivors at 1-year follow-up, including 38 with local GGO (40.43%) and two with consolidation (2.13%). GGO, nodule, and subpleural lines were the most frequent abnormalities in chest CT (40.43%, 29.79%, and 14.89%, respectively). Fibrotic lesions were observed in 13.83% of these 94 patients. The median total CT score was 1.50 (IQR 0.00-3.25) and the median number of segments involved was 1.50 (IQR 0.00-3.00). According to Table 2, survivors with severe/critical cases showed a lower level of minimal oxygen saturation in 6MWT and a significantly higher CT score (P < 0.05). Furthermore, the follow-up CT in severe/critical patients showed a greater number of involved lobes (mild/moderate patients 1 [0-2] vs. severe/critical patients 2 [1-3]; P < 0.05). Chest imaging manifestations of 25 survivors who participated in both the 3-month (Zhao et al., 2020) and 1-year follow-ups were collected. Additionally, the patients at the 3-month follow-up had higher total scores of chest CT compared with those in the late convalescence phase (Figure S1).
Comparison of clinical characteristics between normal and abnormal chest CT
As shown in Table 3 , the clinical characteristics of patients between the normal and abnormal chest CT groups were compared. Chest CT scan was performed for 94 patients and showed abnormalities in 67 survivors at 1-year follow-up. The median age for participants with abnormal CT was 52 (IQR 46-58), much older than that of the normal CT group (median: 40; IQR: 28-50). Furthermore, 79.10% of patients in the abnormal CT group had symptoms of cough, and this rate was remarkably higher than that of the normal CT group (55.56%). The median CXR peak score evaluated during hospital stay was 6.00 (IQR, 3.00-12.00) for the abnormal CT group and 2.00 (IQR, 1.00-4.00) for the normal CT group.
Table 3.
Univariate analysis of predictors of abnormal CT score.
| Parameters | Normal range | Normal CT group(N = 27) | Abnormal CT group(N = 67) | P value |
|---|---|---|---|---|
| Age | ≥ 18 | 40 (28, 50) | 52 (46, 58) | 0.000 |
| Sex, female (%) | 11 (40.74) | 29 (43.28) | 0.504 | |
| Incubation period, d | 5 (2, 8) | 5 (3, 8) | 0.241 | |
| Hospital period, d | 12 (10, 17) | 15 (12, 18) | 0.079 | |
| Temperature, ℃ | 38.12 ± 0.81 | 38.14 ± 0.68 | 0.891 | |
| History of smoking | 1 (3.7) | 6 (8.96) | 0.657 | |
| Comorbidities | ||||
| Hypertension | 3 (11.11) | 13 (19.40) | 0.509 | |
| Diabetes mellitus | 3 (11.11) | 6 (8.96) | 1.000 | |
| Chronic heart disease | 1 (3.70) | 3 (4.48) | 1.000 | |
| Severe/critical | 1 (3.70) | 10 (14.93) | 0.239 | |
| Signs and symptoms at admission | ||||
| Fever, No. (%) | 22 (81.48) | 61 (91.04) | 0.342 | |
| Cough, No. (%) | 15 (55.56) | 53 (79.10) | 0.021 | |
| Weakness, No. (%) | 9 (33.33) | 20 (29.85) | 0.741 | |
| Chest tightness, No. (%) | 4 (14.81) | 18 (26.87) | 0.212 | |
| CXR peak score | 2.00 (1.00, 4.00) | 6.00 (3.00, 12.00) | 0.007 | |
| Laboratory data | ||||
| Blood routine | ||||
| Leucocyte count (× 109/L) | 4-10 | 5.48 (4.75, 6.36) | 4.97 (3.92, 6.04) | 0.180 |
| Neutrophil count (× 109/L) | 2-7 | 3.74 (2.29, 4.78) | 3.28 (2.36, 4.69) | 0.649 |
| Lymphocyte count (× 109/L) | 0.8-4.0 | 1.69 (1.09, 1.97) | 1.18 (0.91, 1.56) | 0.014 |
| NLR | 2.08 (1.31, 4.16) | 2.73 (1.73, 3.92) | 0.169 | |
| Monocyte count (× 109/L) | 0.12-0.80 | 0.37 (0.31, 0.45) | 0.30 (0.19, 0.41) | 0.036 |
| Eosinophil count (× 109/L) | 0.02-0.50 | 0.03 (0.01, 0.10) | 0.01 (0.00, 0.04) | 0.003 |
| Red blood cell count (× 109/L) | 3.50-5.50 | 4.70 ± 0.45 | 4.56 ± 0.57 | 0.286 |
| Hemoglobin concentration (g/L) | 110-160 | 138.78 ± 16.94 | 135.02 ± 19.33 | 0.380 |
| Platelet count (× 1012/L) | 100-300 | 171.22 ± 59.28 | 169.87 ± 63.05 | 0.924 |
| Blood biochemistry | ||||
| AST, U/L | 0-40 | 24.00 (17.00, 29.00) | 25.00 (19.60, 37.00) | 0.224 |
| ALT, U/L | 0-40 | 18.00 (16.00, 37.10) | 21.60 (13.10, 40.10) | 0.454 |
| Albumin, g/L | 35-55 | 42.94 ± 5.07 | 39.25 ± 3.87 | 0.000 |
| TP, g/L | 60-85 | 67.42 ± 4.45 | 65.23 ± 5.83 | 0.082 |
| GGT, U/L | 0-47 | 21.00 (15.50, 35.60) | 26.00 (16.00, 52.40) | 0.547 |
| ALP, U/L | 20-150 | 60.00 (55.00, 76.20) | 61.00 (47.90, 74.50) | 0.655 |
| TBA, μmol/L | 0-15 | 3.50 (2.30, 5.00) | 2.80 (2.00, 4.40) | 0.652 |
| Total bilirubin, μmol/L | 0-24 | 10.20 (7.70, 15.60) | 9.70 (7.40, 13.44) | 0.590 |
| Direct bilirubin, μmol/L | 0.00-9.50 | 2.50 (1.52, 4.90) | 2.92 (2.02, 4.50) | 0.264 |
| Indirect bilirubin, μmol/L | 0-17.1 | 7.80 (5.70, 11.20) | 6.70 (5.10, 9.90) | 0.185 |
| Urea nitrogen, μmol/L | 1700-8300 | 3.86 (2.80, 4.92) | 4.03 (3.33, 5.17) | 0.188 |
| Creatinine, μmol/L | 20.00-106.00 | 63.00 (50.00, 75.80) | 69.00 (56.30, 76.00) | 0.245 |
| UA, μmol/L | 200-428 | 272.10 ± 69.78 | 239.62 ± 73.35 | 0.052 |
| Glucose, mmol/L | 3.89-6.11 | 5.45 (4.84, 6.27) | 5.81 (5.16, 7.23) | 0.131 |
| TG, mmol/L | 0.00-1.70 | 1.59 (1.00, 2.00) | 1.14 (0.85, 1.58) | 0.059 |
| LDH, U/L | 100-240 | 171.7 (141.2, 227.0) | 217.2 (170.0, 268.4) | 0.012 |
| Infection-associated | ||||
| CRP, mg/L | 5-10 | 5.00 (2.00, 21.62) | 15.00 (8.00, 30.27) | 0.003 |
| Blood coagulation | ||||
| Prothrombin time, s | 11-15 | 13.20 (11.70, 14.40) | 13.10 (11.70, 14.70) | 0.584 |
| INR | 0.8-1.5 | 1.06 ± 0.19 | 1.10 ± 0.18 | 0.367 |
| APTT, s | 14-21 | 27.80 (22.00, 35.20) | 28.10 (22.10, 35.70) | 0.848 |
| Thrombin time, s | 22-38 | 14.80 (12.40, 18.20) | 16.50 (12.60, 18.20) | 0.598 |
| Fibrinogen, g/L | 2-4 | 3.68 ± 1.18 | 3.91 ± 1.07 | 0.352 |
| D-dimer, μg/L | 0-500 | 290.00 (130, 390) | 290.00 (120, 410) | 0.987 |
| Treatment | ||||
| Corticosteroids, No. (%) | 4 (14.81) | 25 (37.31) | 0.009 | |
| Interferon beta, No. (%) | 22 (81.48) | 55 (82.09) | 1.000 | |
| Immunoglobulins, No. (%) | 1 (3.7) | 9 (13.43) | 0.166 |
Data are expressed as mean ± SD, median (IQR), and No. (%). Comparisons were determined by Student's t-test, Mann-Whitney U test, or χ2 test, as appropriate.
Abbreviations: NLR, neutrophil-lymphocyte ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; TBA, total bile acids; GLO, globulin; UA, uric acid; TG, triglyceride; CRP, C-reactive protein; LDH, lactate dehydrogenase; INR, international normalized ratio; APTT, active partial thrombin time
There were plenty of differences in laboratory findings between the normal and abnormal chest CT groups. At hospital admission, patients had decreased lymphocyte count (P = 0.014). For blood biochemistry, a lower level of albumin (P = 0.000) and higher level of LDH (P = 0.012) in patients with abnormal chest CT were evidenced compared with those in the normal CT group. The level of CRP was much higher in the abnormal CT group (P = 0.003), indicating a more serious infection. With regard to treatment, participants in the abnormal CT group were more likely to receive corticosteroids (37.31% vs 14.81%, P = 0.009) than those in the normal CT group. After multivariable adjustment, older participants showed an OR 1.080 (95% CI: 1.013, 1.153) for abnormal CT at 1-year follow-up (Table 4 ).
Table 4.
Multivariate analysis of predictors of abnormal CT score.
| β | P value | OR (95% CI) | βa | P valuea | OR (95% CI)a | |
|---|---|---|---|---|---|---|
| Cough | -1.150 | 0.069 | 0.317 (0.092, 1.095) | -1.070 | 0.098 | 0.343 (0.097, 1.218) |
| Age | 0.072 | 0.024 | 1.075 (1.009, 1.144) | 0.077 | 0.019 | 1.080 (1.013, 1.153) |
| CXR peak score | 0.085 | 0.221 | 1.089 (0.950, 1.248) | 0.093 | 0.194 | 1.097 (0.954, 1.218) |
| Lymphocyte count | -0.060 | 0.604 | 0.942 (0.756, 1.173) | -0.061 | 0.606 | 0.941 (0.747, 1.185) |
| CRP | -0.009 | 0.557 | 0.991 (0.963, 1.021) | -0.008 | 0.588 | 0.992 (0.963, 1.022) |
| LDH | -0.001 | 0.840 | 0.999 (0.990, 1.008) | -0.002 | 0.741 | 0.998 (0.989, 1.008) |
| Albumin | -0.094 | 0.244 | 0.910 (0.776, 1.066) | -0.088 | 0.290 | 0.916 (0.779, 1.078) |
| Corticosteroids | -1.093 | 0.134 | 0.335 (0.080, 1.398) | -0.969 | 0.206 | 0.380 (0.085, 1.701) |
Abbreviations: CI, confidence interval; CXR, chest x-ray; CRP, C-reactive protein; LDH, lactate dehydrogenase
Logistic regression analysis for sex, history of smoking, and symptoms of chest tightness at admission
Lung function sequelae in COVID-19 patients 1 year after hospital discharge
Ten of 70 survivors with COVID-19 had impaired DLCO% predicted at 1-year follow-up. To figure out the differences between normal and impaired DLCO survivors, this study compared demographics, clinical characteristics, and laboratory parameters between the two groups in Table 5 . It found that laboratory parameters including red blood cell count, hemoglobin concentration, ALT and TP on admission were lower in the impaired DLCO group, and the difference between the two cohorts was statistically significant. The level of urea nitrogen in the DLCO-impaired group was higher than in the DLCO-normal group. Other variables between the impaired DLCO group and normal DLCO group showed no significant difference. Finally, age, sex, and a history of smoking were imputed into the multivariable logistic regression model. It was found that the higher level of urea nitrogen at admission was associated with DLCO% predicted < 80% (OR 1.004, 95% CI 1.001-1.006, P = 0.021, Table 6 ).
Table 5.
Univariate analysis of predictors of abnormal DLCO% predicted.
| Parameters | Normal range | DLCO normal group (N = 60) | DLCO impaired group (N = 10) | P value |
|---|---|---|---|---|
| Age | ≥ 18 | 47.33 ± 12.29 | 48.90 ± 15.90 | 0.722 |
| Sex, female (%) | 25 (41.67) | 6 (60) | 0.461 | |
| Incubation period, d | 5.00 (3.00, 7.00) | 4.00 (3.00, 6.00) | 0.494 | |
| Hospital period, d | 13.00 (10.00, 18.00) | 16.00 (11.75, 18.00) | 0.337 | |
| Temperature, ℃ | 38.20 (37.50, 38.68) | 38.00 (38.00, 38.35) | 0.880 | |
| History of smoking | 3 | 1 | 1.000 | |
| Comorbidities | ||||
| Hypertension | 9 (15) | 3 (30) | 0.476 | |
| Diabetes mellitus | 3 (5) | 1 (10) | 1.000 | |
| Chronic heart disease | 3 (5) | 1 (10) | 1.000 | |
| Signs and symptoms at admission | ||||
| Fever, N (%) | 53 (83.33) | 9 (90) | 1.000 | |
| Cough, N (%) | 45 (75) | 8 (80) | 1.000 | |
| Weakness, N (%) | 19 (31.67) | 0 | 0.089 | |
| Chest tightness, N (%) | 15 (25) | 0 | 0.171 | |
| CXR peak score | 4.00 (1.00, 12.00) | 6.00 (3.00, 9.00) | 0.602 | |
| CXR score | 1.00 (0.00, 3.00) | 2.00 (0.00, 4.00) | 0.774 | |
| Laboratory data | ||||
| Blood routine | ||||
| Leucocyte count (× 109/L) | 4-10 | 5.45 ± 1.85 | 4.74 ± 1.00 | 0.242 |
| Neutrophil count (× 109/L) | 2-7 | 3.73 ± 1.64 | 3.06 ± 1.13 | 0.215 |
| Lymphocyte count (× 109/L) | 0.8-4.0 | 1.23 (0.96, 1.71) | 1.13 (0.95, 1.64) | 0.724 |
| NLR | 2.72 (1.99, 4.10) | 2.13 (1.61, 4.08) | 0.470 | |
| Monocyte count (× 109/L) | 0.12-0.80 | 0.33 (0.26, 0.44) | 0.34 (0.23, 0.46) | 0.968 |
| Eosinophil count (× 109/L) | 0.02-0.50 | 0.01 (0.00, 0.04) | 0.02 (0.01, 0.05) | 0.378 |
| Red blood cell count (× 109/L) | 3.50-5.50 | 4.72 ± 0.51 | 4.12 ± 0.46 | 0.001 |
| Hemoglobin concentration (g/L) | 110-160 | 139.16 ± 18.47 | 122.70 ± 14.58 | 0.009 |
| Platelet count (× 1012/L) | 100-300 | 160.00 (124.00, 195.50) | 155.00 (124.50, 199.25) | 0.987 |
| Blood biochemistry | ||||
| AST, U/L | 0-40 | 26.00 (17.08, 36.10) | 22.80 (19.10, 30.78) | 0.737 |
| ALT, U/L | 0-40 | 20.75 (15.13, 41.38) | 16.50 (7.75, 22.40) | 0.041 |
| Albumin, g/L | 35-55 | 41.06 ± 4.75 | 41.64 ± 2.87 | 0.711 |
| TP, g/L | 60-85 | 66.86 ± 5.19 | 63.25 ± 5.34 | 0.046 |
| GGT, U/L | 0-47 | 30.00 (16.55, 43.15) | 17.60 (13.00, 28.20) | 0.113 |
| TBA, μmol/L | 0-15 | 3.50 (2.30, 5.08) | 2.40 (1.98, 2.85) | 0.102 |
| Total bilirubin, μmol/L | 0-24 | 9.15 (7.11, 12.95) | 8.33 (6.15, 13.25) | 0.795 |
| Direct bilirubin, μmol/L | 0.00-9.50 | 2.85 (1.90, 4.58) | 2.30 (1.35, 2.99) | 0.129 |
| Indirect bilirubin, μmol/L | 0-17.1 | 6.26 (4.68, 8.38) | 5.57 (5.03, 10.03) | 0.699 |
| Urea nitrogen, μmol/L | 1700-8300 | 4024.33 ± 1183.33 | 5837.00 ± 1549.66 | 0.000 |
| Creatinine, μmol/L | 20.00-106.00 | 65.80 ± 11.60 | 68.73 ± 16.16 | 0.489 |
| UA, μmol/L | 200-428 | 252.82 ± 71.61 | 256.34 ± 75.65 | 0.887 |
| Glucose, mmol/L | 3.89-6.11 | 5.77 (5.07, 6.49) | 5.79 (5.33, 6.62) | 0.873 |
| TG, mmol/L | 0.00-1.70 | 1.12 (0.82, 1.61) | 1.36 (1.09, 1.71) | 0.343 |
| LDH, U/L | 100-240 | 195.80(149.60-261.53) | 217.30(141.00-260.75) | 0.887 |
| Infection associated | ||||
| CRP, mg/L | 5-10 | 11.15 (5.10, 28.53) | 9.45 (2.88, 23.06) | 0.615 |
| Blood coagulation | ||||
| Prothrombin time, s | 11-15 | 13.20 (11.18, 14.78) | 14.45 (11.48, 15.55) | 0.373 |
| INR | 0.8-1.5 | 1.09 ± 0.21 | 1.09 ± 0.20 | 0.991 |
| APTT, s | 14-21 | 25.10 (20.13, 28.88) | 28.55 (25.40, 35.63) | 0.055 |
| Thrombin time, s | 22-38 | 17.65 (15.35, 24.73) | 14.80 (14.18, 19.13) | 0.113 |
| Fibrinogen, g/L | 2-4 | 3.92 ± 1.15 | 3.78 ± 1.41 | 0.730 |
| D-dimer, μg/L | 0-500 | 225.00 (82.50, 400.00) | 280.00 (255.00, 360.00) | 0.306 |
| Treatment | ||||
| Corticosteroids, No. (%) | 20 (33.33) | 0 (0) | 0.075 | |
| Interferon beta, No. (%) | 57 (95) | 8 (80) | 0.297 | |
| Immunoglobulins, No. (%) | 3 (5) | 1 (10) | 1.000 |
Data are expressed as mean ± SD, median (IQR), and No. (%). Comparisons were determined by Student's t-test, Mann-Whitney U test, or χ2 test, as appropriate.
Abbreviations: NLR, neutrophil-lymphocyte ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; TBA, total bile acids; GLO, globulin; UA, uric acid; TG, triglyceride; CRP, C-reactive protein; LDH, lactate dehydrogenase; INR, international normalized ratio; APTT, active partial thrombin time
Table 6.
Multivariate analysis of predictors of abnormal DLCO.
| β | P value | OR (95% CI) | βa | P valuea | OR (95% CI)a | |
|---|---|---|---|---|---|---|
| Red blood cell count | -4.253 | 0.169 | 0.014 (0.000, 6.063) | -4.884 | 0.127 | 0.008 (0.000, 4.037) |
| Hemoglobin concentration | -0.095 | 0.318 | 0.909 (0.754, 1.096) | 0.002 | 0.987 | 1.002 (0.784, 1.281) |
| ALT | -0.249 | 0.096 | 0.780 (0.582, 1.045) | -0.227 | 0.139 | 0.797 (0.590, 1.076) |
| TP | -0.121 | 0.523 | 0.886 (0.611, 1.285) | -0.304 | 0.189 | 0.738 (0.468, 1.162) |
| Urea nitrogen | 0.004 | 0.032 | 1.004 (1.000, 1.007) | 0.003 | 0.021 | 1.004 (1.001, 1.006) |
Abbreviations: CI, confidence interval; TP, total protein; ALT, alanine aminotransferase
Logistic regression analysis adjusted for age, sex, and history of smoking
Dynamic changes of antibodies
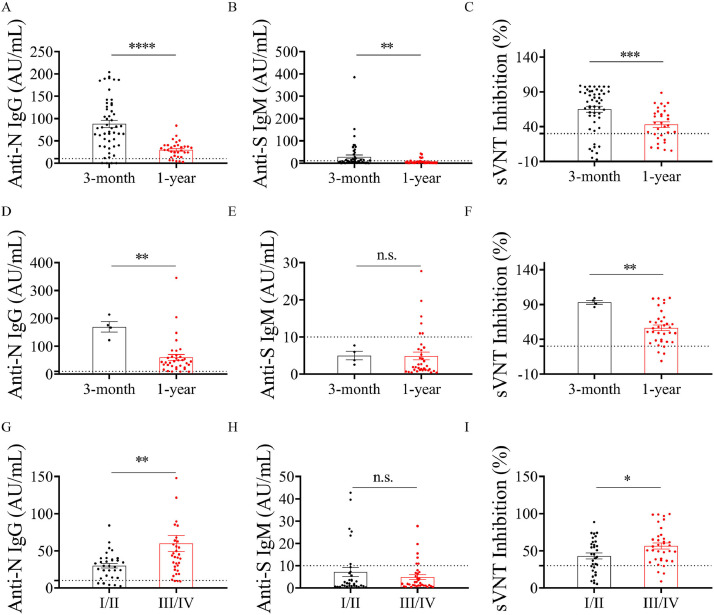
There were 55 and 67 survivors tested for SARS-CoV-2 IgG, IgM antibodies, and NAbs against SARS-CoV-2 at 3 months and 1 year after discharge, respectively. The negative rates of SARS-CoV-2 IgG were 7.27% and 11.94% at the 3-month and 1-year follow-ups, respectively. SARS-CoV-2 IgM became negative in 60.00% (33 of 55 patients) 3 months after discharge. At the 1-year follow-up after hospital discharge, the negative rate of IgM was 82.09% (55 of 67 patients). It was observed that the concentrations of SARS-CoV-2 IgG and IgM antibodies in the early convalescence phase were higher than those of survivors in the late convalescence phase. Application of the manufacturer's advised cut-off of 30% resulted in 47 samples (85.45%) reporting as unambiguously positive for ‘neutralization’ at the 3-month follow-up, whilst 55 of 67 participants (82.09%) who presented for follow-up displayed an efficient neutralization 1 year after hospital discharge. As shown in Figure 3 , there was no difference in serum anti-S IgM level between the mild/moderate and severe/critical groups (P > 0.05) 1 year after discovery. The anti-N IgG level of participants was 29.73 (IQR: 14.92-39.56) for the mild/moderate group and 46.76 (IQR: 24.59-63.78) for the severe/critical group. A significant difference was observed between the mild/moderate and severe/critical groups (P < 0.05). The neutralizing activity in sVNT was higher than that in the severe/critical group (P < 0.05). The same phenomenon was noticed in the anti-N IgG and SARS-CoV-2 sVNT level during follow-ups of survivor patients, but not in anti-S IgM (Figure 3).
Figure 3.
Anti-SARS-CoV-2 IgG and IgM antibodies, and neutralizing activity kinetics in the serum of patients with SARS-CoV-2 infection. The serum of 55 participants who participated in the 3-months follow-up were collected, including 4 mild cases, 47 moderate cases, and 4 severe cases. Of 67 survivors 1 year after discovery, 2.99% (2 cases) were classified as mild, 44.78% (30 cases) moderate, 49.25% (33 cases) severe, and 2.99% (2 cases) critical. Comparison of anti-N IgG, anti-S IgM antibody concentration or neutralizing activity of patient serum in different COVID-19 groups in recovering status (mild/moderate: A, B, and C; severe/critical: D, E, and F). Distribution of anti-N IgG (D), anti-S IgM (E) antibodies, and sVNT inhibition (F) in different COVID-19 groups 1 year post discharge (I/II = mild/moderate group; III/IV = severe/critical group).
n.s. = not significant
* P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001
A significant correlation between the potent neutralizing activity in the SARS-CoV-2 sVNT and anti-N IgG antibodies was observed. The neutralizing activity in sVNT was not significantly correlated with the level of anti-S IgM antibodies at the 1-year follow-up (Figure 4 ). It is worth noting that patients produced robust NAb responses after SARS-CoV-2 infection, and the majority of antibody neutralizing activity persisted for more than 1 year after infection.
Figure 4.
Neutralizing activity in sVNT correlates with anti-N IgG antibodies. (A and B) Serum from 55 individuals was tested for antibodies against SARS-CoV-2 N IgG and neutralizing activity response at 3 months (A) and 1 year (B) after hospital discharge. (C and D) Serum from 55 or 67 individuals was tested for antibodies against SARS-CoV-2 S protein (IgM) and sVNT against neutralizing antibodies against SARS-CoV-2 that block the interaction with ACE2 cell surface receptor at 3 months (C) and 1 year (D) after discharge.
Discussion
Plenty of studies have been performed to describe the sequalae of COVID-19 survivors after hospital discharge. This study performed the first study with a duration of 1-year follow-up exploring the clinical consequences of adult patients recovering from SARS-CoV-2. It found that at 1 year after hospital discharge, a high proportion of survivors endorsed at least one symptom, particularly muscle fatigue, insomnia, and joint paint. The most striking finding was the high proportion of patients with lung injury (71.28%) and DLCO impairment (14.29%) 1 year after discharge, although the severity of COVID-19 had no relation to abnormality of CT and DLCO. The levels of SARS-CoV-2 IgG, IgM, and neutralizing activity were significantly lower than those in the early convalescence phase.
It was found that muscle fatigue and sleep difficulties were most common even at 1 year after hospital discharge. The rates were lower than those reported in the 1-year follow-up study of SARS survivors (Tansey et al., 2007). A follow-up study of COVID-19 survivors showed that 29.5% of patients still had muscular fatigue at the 3-month follow-up (Gonzalez et al., 2021). A previous study reported that the most common 6-month consequences of COVID-19 in patients discharged from hospital were muscle fatigue (63%) and sleep difficulties (26%), whilst age was the risk factor for fatigue (Huang et al., 2021). However, age had no relationship with the symptoms in COVID-19 survivors in the current study. Additionally, the results of questionnaires in this study showed that a considerable proportion of participants had persistent psychological symptoms. This is consistent with data from previous COVID-19 survivors at 1-month follow-up after hospital treatment (Mazza et al., 2020). The 6MWT distances were shorter than the reference values. The muscle fatigue and psychiatric consequences were likely caused by the immune response, virus infection, social isolation, a potentially fatal illness, and stigma.
A recent meta-analysis of CT imaging of COVID-19 patients showed that 91.6% of patients showed an abnormal pattern in chest imaging manifestations, and patchy or GGO were the most common findings in the acute phase (Zhu et al., 2020). Two studies including some critical COVID-19 patients showed that the prevalence of chest CT abnormalities ranged from 80.7%-53.91% at the 3-month and 6-month follow-ups (Gonzalez et al., 2021; Huang et al., 2021). A recent study found that the rate of radiological anomalies was 39% and the median of CT score was 0.0 (IQR: 0.0-1.0) 7 months after recovery (Liu et al., 2021). The rate of radiographic anomalies and fibrosis was 71.28% and 8.51% in the current cohort. Even with the high rate of lung injury on chest imaging, the median CT score was 1.5 (IQR: 0.00, 3.25) at the 1-year follow-up. The severe/critical COVID-19 patients showed significant increases in CT abnormalities compared with the mild/moderate patients at the 1-year follow-up. Therefore, it can be inferred that chest CT imaging abnormalities caused by SARS-CoV-2 could gradually be resolved over time. Furthermore, the factor associated with lung damage on chest CT was age, which was consistent with previous studies on SARS and MERS (Antonio et al., 2003; Chan et al., 2003; Chang et al., 2005; Feikin et al., 2015). It could be speculated that age might be a predictor of radiological damage in patients who have recovered from COVID-19. Whether the remaining radiological anomalies completely resolve needs to be investigated in longer-term and further large-scale studies.
A similar phenomenon could be noticed with pulmonary function during follow-ups of survivor patients with COVID-19. At the time of hospital discharge, the findings from 110 patients with mild (n = 24), moderate (n = 67), and severe (n = 19) disease showed that DLCO anomalies were noted in 47.2% of patients (Mo et al., 2020). The rate of impaired DLCO remained high 6 months after discharge (34.13%), although it was lower than that at 3 months (54%) (Huang et al., 2021; Qin et al., 2021). A recent study showed that 82% of ICU patients with ARDS secondary to COVID-19 had impaired DLCO at the 3-months follow-up (Gonzalez et al., 2021). The result of lung function assessment in this study showed that 14.29% of participants had a lung carbon monoxide diffusion dysfunction 1 year after hospital discharge; this is consistent with data from previous SARS 1-year follow-up studies (Hui et al., 2005; Ong et al., 2005). The severity of pulmonary inflammation in the acute phase might be the reason for fibroblast activation and impaired DLCO in the convalescence phase (Qin et al., 2021). The current study also found that the level of urea nitrogen was an independent factor of abnormal DLCO, which is in agreement with previous studies (Izcovich et al., 2020; Mudatsir et al., 2020). Thus, this study will help clinicians and policymakers in tailoring management strategies for COVID-19 survivors to identify impaired DLCO as early as possible, and to develop better centralized management and pulmonary rehabilitation.
Previous studies have shown that serum IgG and neutralizing antibodies against SARS-CoV and MERS-CoV can persist for an average of 2 years (Cao et al., 2007; Choe et al., 2017; Payne et al., 2016; Wu et al., 2007). Recent studies have shown that approximately 90% of the patient cohort remained SARS-CoV-2 IgG-positive 3-6 months following symptom onset (Maine et al., 2020; Rodda et al., 2021; Zhao et al., 2020). Regarding NAbs, 85% of patients had a high NAbs titer 3-4 months post-symptom onset (Jiang et al., 2021). SARS-CoV-2 IgG titers and NAbs neutralizing activity at 1-year follow-up in recovered individuals in the current cohort exhibited a significant decrease compared with those at 3 months after hospital discharge. The current cohort found no difference in the seropositivity of the antibodies among survivors with COVID-19 between 3 months and 1 year after discharge. The decline of serum IgG and neutralizing antibodies observed in the present study indicates re-infection among recovered COVID-19 patients. Taken together, the findings from this study suggest rising antibody levels 1 year after hospital discharge in patients with COVID-19 and this will have important implications regarding monitoring the immune response against SARS-CoV-2 and establishing vaccination strategies.
There were several limitations to this study. First, the cohort with confirmed SARS-CoV-2 infection was small, whilst a larger sample size would be more ideal for this type of study. Second, the baseline data of PFTs and 6MWT were unavailable, so it was unknown whether the observed abnormalities were already present prior to diagnosis with COVID-19. Third, since only two patients with critical COVID-19 symptoms were enrolled, further efforts are needed to assess the long-term outcomes of critical COVID-19 survivors.
Conclusions
In conclusion, a cohort of patients were mainly troubled with muscle fatigue, insomnia, anxiety or depression 1 year after being in hospital for COVID-19. Pulmonary structural abnormalities and functional impairment were common among those who were tested. The high level of urea nitrogen on hospital admission due to COVID-19 could effectively predict impaired DLCO after 1 year of discharge. COVID-19 elicits immune responses that persist and display functional hallmarks of antiviral immunity.
Ethical approval and participation consent
The study was approved by the First Affiliated Hospital of Zhengzhou University (2020-KY-61) and was registered with the Chinese Clinical Trial Registry, ChiCTR2000033186. Written informed consent was obtained from all the patients.
Conflict of interest
The authors declare no competing interests.
Funding source
The Science & Technological Project of Henan Province (No. 212102310208), The Major Project of Medical Science and Technology of Henan Province (No. SBGJ202001006) and The Youth Project of Medical Science and Technology of Henan Province (No. SBGJ202003022), Youth innovation fund of the First Affiliated Hospital of Zhengzhou University (No. YNQN2017169 and No. YNQN2017171), and Shenzhen Science and Technology Program (No. JSGG20200225153121723).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.09.017.
Appendix. Supplementary materials
References
- Antonio GE, Wong KT, Hui DS, Wu A, Lee N, Yuen EH, et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003;228(3):810–815. doi: 10.1148/radiol.2283030726. [DOI] [PubMed] [Google Scholar]
- Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51(11):1025–1036. doi: 10.1016/s0895-4356(98)00094-8. [DOI] [PubMed] [Google Scholar]
- Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357(11):1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- Chan KS, Zheng JP, Mok YW, Li YM, Liu YN, Chu CM, et al. SARS: prognosis, outcome and sequelae. Respirology. 2003;8(l):S36–S40. doi: 10.1046/j.1440-1843.2003.00522.x. Supp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Yu CJ, Chang SC, Galvin JR, Liu HM, Hsiao CH, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236(3):1067–1075. doi: 10.1148/radiol.2363040958. [DOI] [PubMed] [Google Scholar]
- Choe PG, Perera R, Park WB, Song KH, Bang JH, Kim ES, et al. MERS-CoV Antibody Responses 1 Year after Symptom Onset, South Korea, 2015. Emerg Infect Dis. 2017;23(7):1079–1084. doi: 10.3201/eid2307.170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. Pt 1. [DOI] [PubMed] [Google Scholar]
- Feikin DR, Alraddadi B, Qutub M, Shabouni O, Curns A, Oboho IK, et al. Association of Higher MERS-CoV Virus Load with Severe Disease and Death, Saudi Arabia, 2014. Emerg Infect Dis. 2015;21(11):2029–2035. doi: 10.3201/eid2111.150764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J, Benitez ID, Carmona P, Santisteve S, Monge A, Moncusi-Moix A, et al. Pulmonary Function and Radiological Features in Survivors of Critical Covid-19: A 3-Month Prospective Cohort. Chest 2021. [DOI] [PMC free article] [PubMed]
- Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler SA, Ebner L, Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C, et al. Pulmonary function and radiological features four months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021 doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DS, Wong KT, Ko FW, Tam LS, Chan DP, Woo J, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XL, Wang GL, Zhao XN, Yan FH, Yao L, Kou ZQ, et al. Lasting antibody and T cell responses to SARS-CoV-2 in COVID-19 patients three months after infection. Nat Commun. 2021;12(1):897. doi: 10.1038/s41467-021-21155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Lv F, Huang Y, Xiao K. Follow-Up Study of the Chest CT Characteristics of COVID-19 Survivors Seven Months After Recovery. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.636298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Wang H, Lin Y, Li L. Psychological status of medical workforce during the COVID-19 pandemic: A cross-sectional study. Psychiatry Res. 2020;288 doi: 10.1016/j.psychres.2020.112936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma MJ, Liu C, Wu MN, Zhao T, Wang GL, Yang Y, et al. Influenza A(H7N9) Virus Antibody Responses in Survivors 1 Year after Infection, China, 2017. Emerg Infect Dis. 2018;24(4):663–672. doi: 10.3201/eid2404.171995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine GN, Lao KM, Krishnan SM, Afolayan-Oloye O, Fatemi S, Kumar S, et al. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott Architect. J Clin Virol. 2020;133 doi: 10.1016/j.jcv.2020.104663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudatsir M, Fajar JK, Wulandari L, Soegiarto G, Ilmawan M, Purnamasari Y, et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Res. 2020;9:1107. doi: 10.12688/f1000research.26186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KC, Ng AW, Lee LS, Kaw G, Kwek SK, Leow MK, et al. 1-year pulmonary function and health status in survivors of severe acute respiratory syndrome. Chest. 2005;128(3):1393–1400. doi: 10.1378/chest.128.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DC, Iblan I, Rha B, Alqasrawi S, Haddadin A, Al Nsour M, et al. Persistence of Antibodies against Middle East Respiratory Syndrome Coronavirus. Emerg Infect Dis. 2016;22(10):1824–1826. doi: 10.3201/eid2210.160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Chen S, Zhang Y, Dong F, Zhang Z, Hu B, et al. Diffusion capacity abnormalities for Carbon Monoxide in patients with COVID-19 at three-month follow-up. Eur Respir J. 2021 doi: 10.1183/13993003.03677-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Sun J, Wang M, Lu X, Dong Q, Zhang L, et al. Gender differences in dysfunctional attitudes in major Depressive Disorder. Front Psychiatry. 2020;11:86. doi: 10.3389/fpsyt.2020.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184(1):169–183. doi: 10.1016/j.cell.2020.11.029. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl AP, Huang M, Colantuoni E, Karmarkar T, Dinglas VD, Hopkins RO, et al. Healthcare utilization and costs in ARDS survivors: a 1-year longitudinal national US multicenter study. Intensive Care Med. 2017;43(7):980–991. doi: 10.1007/s00134-017-4827-8. [DOI] [PubMed] [Google Scholar]
- Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- Tansey CM, Louie M, Loeb M, Gold WL, Muller MP, de Jager J, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167(12):1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- Tarsitani L, Vassalini P, Koukopoulos A, Borrazzo C, Alessi F, Di Nicolantonio C, et al. Post-traumatic Stress Disorder among COVID-19 survivors at 3-month follow-up after hospital discharge. J Gen Intern Med. 2021 doi: 10.1007/s11606-021-06731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Clinical management of COVID-19: interim guidance, 27 May 2020. https://apps.who.int/iris/handle/10665/332196.
- WHO. WHO Coronavirus (COVID-19) Dashboard. Accessed July 20, 2021. https://covid19.who.int/.
- Wu LP, Wang NC, Chang YH, Tian XY, Na DY, Zhang LY, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Liu Y, Fan B, Xiao Y, Tian Q, Chen L, et al. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir Res. 2005;6:5. doi: 10.1186/1465-9921-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. E Clin Med. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhong Z, Li H, Ji P, Pang J, Li B, et al. CT imaging features of 4121 patients with COVID-19: A meta-analysis. J Med Virol. 2020;92(7):891–902. doi: 10.1002/jmv.25910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xu H, Fang Z, Xu C, Xue C, Hong X. Platelet serotonin and serotonin transporter as peripheral surrogates in depression and anxiety patients. Eur J Pharmacol. 2018;834:213–220. doi: 10.1016/j.ejphar.2018.07.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.