Abstract
Spinal cord stimulation has seen unprecedented growth in new technology in the 50 years since the first subdural implant. As we continue to grow our understanding of spinal cord stimulation and relevant mechanisms of action, novel questions arise as to electrical dosing optimization. Programming adjustment — dose titration — is often a process of trial and error that can be time-consuming and frustrating for both patient and clinician. In this report, we review the current preclinical and clinical knowledge base in order to provide insights that may be helpful in developing more rational approaches to spinal cord stimulation dosing. We also provide key conclusions that may help in directing future research into electrical dosing, given the advent of newer waveforms outside traditional programming parameters.
Keywords: neural dosing, pharmacology, neuromodulation, spinal cord stimulation, electrical dosing
Introduction
For pain medicine, optimizing the dosage of pharmaceuticals means that the medication choice and amount, route of administration, and frequency are carefully titrated to maximize efficacy while minimizing side effects.1–3 Generally, this means using the lowest effective dose. In intrathecal drug delivery, this concept has gained traction in that an equivalent or perhaps superior clinical outcome can be achieved with a substantially lower daily dose of drug.4
Spinal cord stimulation (SCS) is a medical option within interventional pain medicine. Although used clinically for more than 50 years, SCS has gained a great deal of interest in recent years, as it represents a drug-free option for ongoing management of chronic pain, such as back pain, radicular pain, complex regional pain syndrome (which includes causalgia), peripheral neuropathies, and other neuropathic pain conditions.5–7 Traditionally, for SCS therapy, electrodes are implanted in the epidural space overlying the portions of the dorsal column of the spinal cord that somatotopically correspond to the painful dermatomes of the body. An electrical current is then delivered to the electrodes by means of an implanted pulse generator that often includes an implanted battery. The electricity induces changes in the electrical potential of the cell membranes of axons in the spinal cord, in many cases generating action potentials that propagate anti- and orthodromically to modulate neurons in the gray matter of the spinal cord, as well as brain sites. On a basic level, this is thought to modulate neural function within pain pathways in various ways depending on the pattern of stimulation to help mask or relieve pain. SCS is often considered successful when pain is reduced by 50% or more. Importantly, SCS can reduce pain severity sufficiently to allow some pain patients to reduce their use of medications and break free of opioid dependence.8,9 A landmark randomized controlled trial comparing SCS with conventional medical management showed that the former provided better leg pain relief, quality of life, and functional capacity and could be sustained through 24 months.10
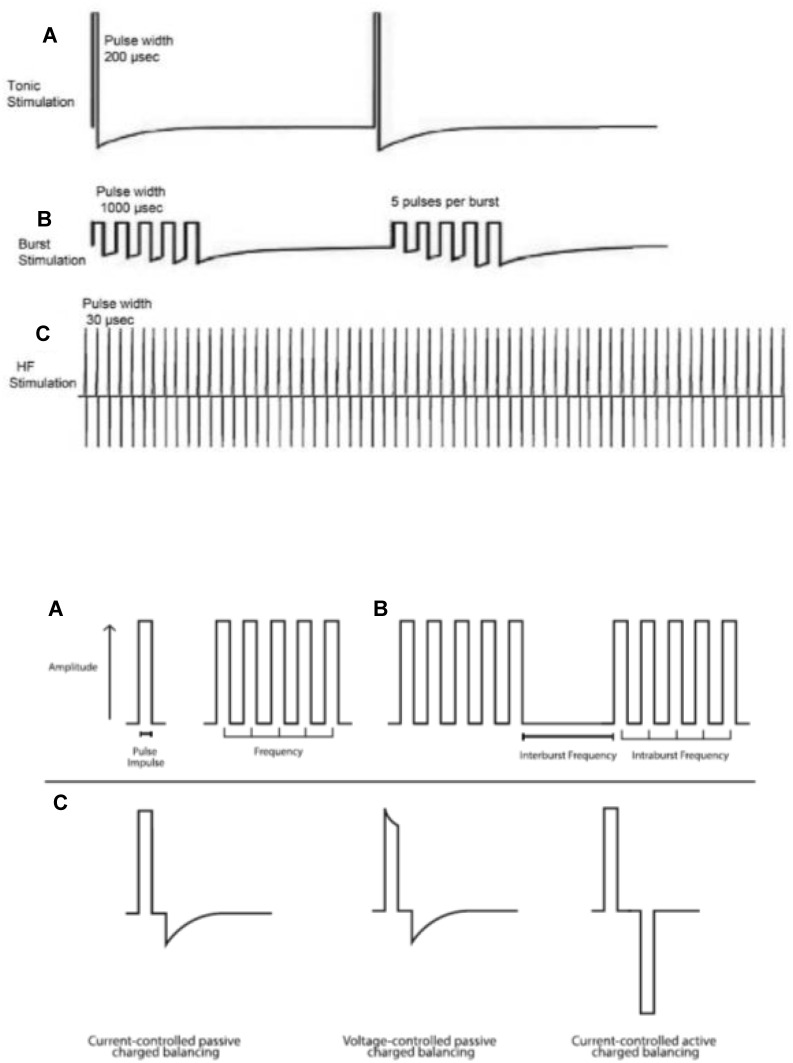
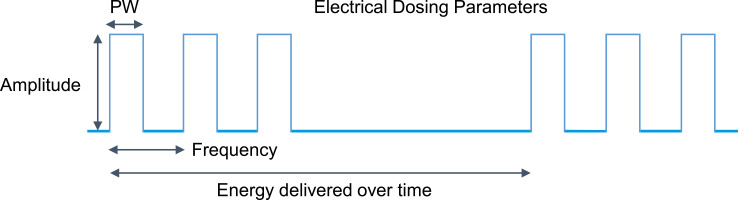
In the last decade, innovations in SCS have led to the development of new electrical waveforms. Where SCS was once limited to square pulses delivered in a consistent stream at a set frequency (2–1,200 Hz), pulse width (1–1,000 microseconds), and amplitude (0–20 mA), these programming parameters are still commonly used today, but are now often referred to as “traditional tonic stimulation.” New stimulation patterns and frequencies now include burst SCS (pulsatile packets of stimulation with different charge recovery strategies) and high-frequency SCS, the definition of which is contentious: some claim it to have a low range of 1kHz, while others claim it needs to be >1.2 kHz, the traditional upper limit of most commercially available implantable pulse generators, yet others claim it refers only to stimulation of 5–10kHz. Examples are shown in Figure 1. High-density or high-dose SCS, in which longer pulse widths and/or higher frequencies are used, has also shown clinical utility.11–14 Beyond just programming parameter changes, devices that target novel anatomical structures, such as the dorsal root ganglion, have been approved by regulatory bodies all over the world.15,16 Multiple detailed reviews have been published on putative mechanisms of action specific to these various approaches.17–20 With this range of interventions and programming strategies available, clinicians must stay abreast of the comprehensive body of literature supporting each therapeutic approach to guide selection of the most appropriate therapy for a given patient. Once a therapy is chosen, similar to pharmacological treatment, the appropriate electrical dosage must be prescribed/titrated by programming the device with the correct number and polarity of active electrodes along with the appropriate settings for pulse width, frequency, amplitude, and duty cycling (the percentage of “stimulation-on” time).21 This concept is summarized in Figure 2.
Figure 1.
Top: Waveforms for tonic (A), burst (B), and high-frequency (C) SCS, allowing comparison of relative pulse widths, frequencies, and amplitudes. Reprinted with permission from Reprinted with permission from Taylor and Francis. Ahmed S, Yearwood T, De Ridder D, Vanneste S. Burst and high frequency stimulation: underlying mechanism of action. Expert Rev Med Devices. 2018;15(1):61–70.67 Bottom: Schematic illustrating pulse width, frequency, and burst variables (A and, B), as well as factors that create the electrical properties of a single pulse within a waveform (C). Reproduced from Reproduced from Caylor J, Reddy R, Yin S, et al. Spinal cord stimulation in chronic pain: evidence and theory for mechanisms of action. Bioelectron Med. 2019;5(1):12. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.38
Figure 2.
Key parameters in dose programming for dorsal column stimulation.
Clinical observations and studies have emerged from the SCS industry and the implanter community that clinically meaningful pain relief and avoidance of side effects may be achieved by minimizing the electrical charge that is delivered to the neural interface. Clearly, this mirrors the approach for pharmaceuticals. Unfortunately, the field of SCS has yet to adequately describe an electrical equivalent of pharmacokinetics/pharmacodynamics that can be used as a guide for dosing. Instead, dosing decisions are usually empirical, based on observations in the programming clinic and feedback from the patient. Programming adjustments — dose titration — are often a process of trial and error that can be time-consuming and frustrating for both patient and clinician. In this report, we review the current preclinical and clinical knowledge base to provide insights that may be helpful in developing more rational approaches to SCS dosing. We also provide key conclusions that may help in directing future research into electrical dosing.
Insights Gained into Dosing from Preclinical Research
Tonic Paresthesia-Based SCS
While initially discussed within the context of the gate-control theory of pain, there have been significant advances in understanding the mechanisms of action of paresthesia-based SCS. Among the best-studied mechanisms is attenuation of the activity of wide dynamic range neurons (pathologically activated in chronic pain states) via increased activity of inhibitory interneurons through both spinal and supraspinal mediators.22 These mediators include GABA, glycine, serotonin, and norepinephrine.22–24 Yakhnitsa et al demonstrated that following paresthesia-based SCS in an animal model of painful mononeuropathy, there was a reduction in dorsal horn neuronal hyperexcitability that corresponded with improvement in tactile allodynia.25 An additional proposed mechanism is the reduction of glial cell activation at the level of the spinal cord. An animal-model study completed by Sato et al examined the use of paresthesia-based SCS utilizing sham, 4 Hz, or 60 Hz treatment of varying durations and amplitudes in rats with peripheral nerve injury.26 They found that greater analgesia was seen with 6 hours of 60 Hz than 30 minutes, and that not at 90% amplitude of motor threshold (energy level at which motor activation occurs) was significantly more efficacious than 75%. Of note, 50% of motor activation saw no changes in paw-withdrawal threshold, essentially equivalent to sham treatment. The authors also found that there were robust reductions in microglial and astrocyte activation markers following both 4 Hz and 60 Hz paresthesia-based stimulation. These results have recently been replicated in a spared nerve injury model in rodents using 60 Hz stimulation, again showing reductions in microglial activation and pain responses with application of either minocycline, a glial inhibitor, or SCS.27
Burst SCS
Burst SCS utilizes a paradigm of pulse trains of high frequency (typically 500 Hz) stimulation in five 1-millisecond pulses at an overall interburst frequency of 40 Hz.28 This therapy was born of observations that many neurons in the nervous system fire in phasic or burst patterns.29 Intracellular recordings of these burst/phasic firing neurons have shown that the burst firing typically rides on a plateau of slow calcium-mediated depolarization. Snider et al examined burst signaling within the visual cortex of a cat model and found that spatially optimal longer bursts were more effective in eliciting neuronal responses than shorter spatially nonoptimal bursts.30 Crosby et al investigated the parameters involved in burst SCS in a rat model with cervical nerve root injury and neuropathic pain, and found that pulse number, pulse width, and amplitude were significantly correlated with suppression of neuronal firing.31 A comparison to paresthesia-based SCS in an animal model was done by Gong et al.32 Rats with spared nerve injury were implanted and treated with sham, 16 Hz, 60 Hz, and 140 Hz paresthesia-based frequencies and four burst therapies with varying burst frequency, pulse frequency, and pulse width. They found that all but sham and 16 Hz SCS had statistically significant improvement in paw withdrawal threshold, and that burst parameters of 2, 3, and 4 were statistically superior to 60 Hz. Within the burst paradigms, there was no difference between stimulation frequency, pulse width, or pulse frequency.
Unique to the targeting of burst SCS to the mediothalamic and limbic components of pain processing is improvement in the affective and emotional components of pain. Although difficult to study, this has been partially demonstrated by Meuwissen et al using a rat model comparing paresthesia-based and burst SCS.33 This study suggested that more than paresthesia-based SCS, burst did in fact modulate the affective–emotional components of pain. At this time, the effect of burst SCS on the biochemical mediators of the pain pathway, such as serotonin, norepinephrine, or opioids is unknown.
High-Frequency SCS
As opposed to traditional paresthesia-based SCS, paresthesia-free SCS uses significantly higher-frequency electrical impulses (10 kHz) and lower amplitudes, with ultimately no sensations felt by the patient.34 This combination of high pulse width and low amplitude ultimately increases the duty cycle. While overall understanding of the mechanism of action of paresthesia-free SCS is lacking, there have been several proposed mechanisms. Linderoth et al proposed a neuronal blockade, desynchronization of neural signals, and membrane integration leading to the equivalent of temporal summation.35 Neuronal blockade has been somewhat supported by Lee et al, in which an ex vivo patch clamp of spinal cord neurons demonstrated suppressed firing with high-frequency stimulation.35 It has also been theorized that the temperature increases related to the higher power of high-frequency SCS delivered into the tissue may also be a mechanism of action, or at the least a factor in lead positioning and programming. Energy delivery that is sufficient to induce heating (ie, as may be observed in kilohertz-frequency waveforms) may modulate spinal activity to harness the gate-control theory of pain control via a novel heat mechanism.36,37 Although theoretical, heating-based SCS therapy would be influenced by the percentage of stimulation-on time and tissue impedance. Changes in complex cerebrospinal fluid (CSF)–temperature homeostasis can alter cellular function and metabolism, in addition to vascular function. Furthermore, it has been hypothesized that temperature changes affecting heat shock protein that then modulate neuroinflammation may be an additional mechanism of action.36,38 The supraspinal effect of paresthesia-free SCS is unknown at this time, but has been hypothesized to be negligible, due to the primarily superficial neuronal tracts affected.34
Insights Gained into Dosing from Clinical Studies
Subperception SCS
Thompson et al demonstrated significant clinically equivalent pain reduction compared to baseline at sequentially decreasing frequencies from 1,000 Hz to 10 Hz.39 At the same time, the pulse width of the stimulating waveform was increased, maintaining subparesthesia stimulation therapy. This study also suggested that high frequencies are not needed to produce noteworthy subparesthesia analgesia in SCS. The clinical results are compelling, but they are due not so much to frequency per se, but profoundly more to the technique of field application, incorporating both area distribution enhancement and field vector orientation. Pain relief was 48%–52% with all frequencies.39
Burst SCS
In nonlinear burst, charge accumulates over exposed tissue, increasing incrementally with each successive stimulatory phase, and is maintained at that level by the prolonged interphase delay. Some of this charge tends to drift, especially within the CSF, significantly increasing the effective pulse width of the compound stimulatory waveform. As the charge accumulates, it spreads preferentially along the surface of the spinal cord much more than it penetrates the surface to deeper levels, as it follows the path of least resistance. This effectively enlarges the surface area of the applied field and affects a much larger volume of neural elements in the process, thus having a larger field of tissue engagement. These multiple depolarizations, sequentially increasing in number and intensity throughout the burst train, have powerful global influence in both orthodromic action potentials to cortical areas and antidromic action potentials to the dorsal horn and beyond.40 An early report in patients who had previously received tonic SCS showed incremental improvements when using burst stimulation.41 Later, a randomized controlled trial showed that 60% of participants had at least 30% pain relief in response to burst SCS vs 51% with tonic SCS.42
Work by Vesper et al employed the use of duty cycling in what came to be termed microdosing.43 In these 5 seconds on–5 seconds off and 5 seconds on–10 seconds off doses, it was concluded that therapy was not compromised, and less total energy was required. This work was expanded by Deer et al, albeit with more nuanced duty cycle interval programming that involved alternating 30 seconds on with up to 360 seconds off.44 After 6 months, nearly half the patients selected the 30:360 ratio of stimulation and had good therapeutic outcomes.
In these studies, the concept of dosing using the least possible exposure to SCS therapy built on previous concepts described by Miller et al in their attempts to define neural dosing in terms of total charge delivery per second.21 The authors for both the aforementioned microburst studies chose their settings somewhat arbitrarily, and considerations of optimal number of pulses per burst, optimal amplitudes, and intraburst and interburst frequencies were lacking; however, the underlying effects on human neurophysiology are being explored and have been described in recent studies utilizing neuromonitoring as an objective proxy for postsynaptic neural effects.45,46 These variables affect both the device’s battery life and the biological impact of the applied electrical field, and while some of these neural effects are being uncovered using human neurophysiological measures, future studies will continue to build on this knowledge.
High-Frequency SCS
The literature supports the notion there are minimal supraspinal effects of high-frequency SCS.47 Specifically, there is no evidence that evoked compound action potentials (ECAPs) are produced by this stimulatory waveform, and to date there have been no reported PET studies that would indicate direct supraspinal mechanisms playing a measurable role.
Recently, al-Kaisy et al described the use of cascade programming for high-frequency SCS at their clinic in the UK.48,49 Specifically, cascade programming is based on duty cycling sequentially through four bipole contact configurations using all eight contacts of the lead (1–2, 3–4, 5–6, and 7–8). Each bipole of one anode and one cathode is activated for 5 seconds and then deactivated as the next bipole in the sequence is activated for 5 seconds. As such, the entire lead is covered in 20 seconds, during which time each bipole is active for 5 seconds and inactive for 15 seconds. This design mitigates against small lead migration, long reprogramming sessions to search for optimal targeting, and potential overstimulation. Every 20 seconds, 32 mm is treated in this manner. The choice of a 5-second activation time was based on in vitro studies of astrocyte-depolarization times during external electrical stimulation,50 and this fits within the framework of focusing on glial cell dynamics as part of the overall stimulation paradigm. Built within this programming paradigm is the duty cycling concept such that no region of the cord is exposed to >5 seconds of stimulation every 20 seconds. Using cascading in the study, >50% pain reduction was achieved in back pain for 57% of patients at 6 months and 56% at last follow-up, and 60% or patients with leg pain at 6 months and 59% at last follow-up.48,49
Provenzano et al reported a retrospective review over the first 12 months after implantation of 42 patients with a high-frequency SCS device for treatment of neuropathic pain from a large variety of pain conditions.51 Interestingly, despite impressive trial successes with a trial:implant ratio of 89%, only 39% of patients continued to have their best pain relief at the same contact configuration as used in the trial. Additionally, 73% of patients who used duty cycling (switching between on and off for set durations) were considered treatment responders. One patient was noted to have responded equally well at both the T9/10 and T10/11 disk spaces, while others required stimulation of multiple areas within the programming paradigm to achieve suitable relief. Of note, the recharge burdens reported in this study were substantial, with an average of 2.1 hours daily. This level of energy use is a hallmark of high-frequency stimulation programming strategies, with reported recharge times from 45 minutes to >2.5 hours per day.13,52
ECAP-Controlled Closed-Loop SCS
The ability to measure ECAPs is a more recent advancement within the field of neuromodulation that allows for both the study and modification of the delivery of SCS; however their use in neuromodulation devices goes back to the late 1990s in the cochlear implant field. ECAPs are the population response of nerve fibers.53 This is achieved through the measurement of the electrical field created by the movement of ions across the membrane of the nerve axon during an action potential from the initial stimulus.54 The neurophysiological recordings can then be used to determine the recruitment of a subset of fibers in response to dorsal column stimulation by utilizing nonstimulation recording electrodes to measure ECAPs. Animal studies initially demonstrated this in 2012, confirming the theory that SCS primarily recruits large-diameter Aβ fibers and that the ECAP amplitude increases with increasing current level.55 This has also been shown in a human model.56 The prospective open-label AVALON study using a closed-loop SCS device for patients with chronic back and/or leg pain was published in 2018 and with follow-up in 2020, showing excellent pain relief that was durable over time.53,57 Importantly, this demonstrated that the device was able both to deliver treatment and measure ECAP to adjust therapy by adjusting dorsal column–fiber recruitment levels. A randomized controlled study comparing ECAP-controlled closed-loop SCS with open-loop SCS by Mekhail et al showed that pain outcomes were better in the closed-loop group after 3 and 12 months of treatment (72% overall VAS reduction at 12 months in the closed-loop group and 56% reduction in the open-loop group). Furthermore, patients in the closed-loop group had ECAP amplitudes that were within the therapeutic range a much greater proportion of the time than patients in the open-loop group; however, the proportion of increased time spent in this paresthesia-inducing window (12 months closed loop 95%, open loop 48%) did not equate to an equivalent ratio of pain relief.58 This discrepancy will need to be explored in future studies.
Dosing in SCS: The Mechanisms
The dose of an administered electrical field to a neural target is a very complex combination of variables: polarity of the electrical field; frequency; amplitude; shape of the stimulatory waveform; effective impedance of spinal tissue and impedance of internal elements of the device, both implantable pulse generator and leads; effective pulse width; distribution of charge over the targeted area; instantaneous rate of change of this charge distribution; three-dimensional vector properties of the field; and mass and complexity of neuronal tissue exposed to applied electrical fields. In addition, the stimulation-on vs -off ratio, CSF thickness and other complex spinal anatomy, circulation of CSF, lead encapsulation, lead positioning, and electrode configurations affect how much stimulus is delivered to the spinal target.21,59–64 Changes in any one variable may affect overall dosing. An anatomically realistic computational model of SCS has been developed and can help researchers understand the interactions of complex tissue compartments in terms of impedance.62 It has also been noted that actual stimulation output may be lower than the as-programmed stimulation:37 a “governor” function established by regulatory requirements to maintain safe levels of electrical stimulation. If unaware of this, clinicians could dramatically misinterpret the implications of their programming choices.
The field polarity is either negative (cathode) or positive (anode), and the applied field voltage repels the negative charge from the anode toward the cathode where it accumulates simultaneously with the positive charge accumulating under the anode. This interphase delay is the period when no electrical charge is moving relative to the lead contacts of the device. This certainly does not mean the charge is not undergoing tissue penetration or slight dispersion in the CSF at the surfaces of exposed tissue. We have yet to fully understand exactly how the charge accommodates itself along the surface(s) of the biological tissue to which it has been applied.59
The shape of the stimulatory waveform represents this instantaneous variation in field intensity. The recruitment of neurons under the influence of a sinusoidal waveform will be quite different from the recruitment expected under a square waveform. Both these waveforms will recruit different-size axons or glial elements in a manner different from an exponential stimulatory phase or a randomly applied series of square waves of randomly generated frequencies and amplitudes. It is reasonable to think that various waveforms could achieve differential tissue responses in neurons and glial cells. To prove the point, a very simplistic set of waveforms was applied by Vallejo et al in an animal model of chronic neuropathic pain.65 They then examined the genomic consequences of these various waveforms in different types of glial and neuronal cells to determine differences in transcription and production of proteins caused by the waveforms that showed clear differences. While this work helped expand knowledge in this field, the glial and neuronal structures examined resided directly below the applied electric fields, and typical SCS-electrode placement in the spinal canal (T7–T10) is not located above the corresponding neuronal and glial cells’ receptive fields for low-back and leg pain (lumbar enlargement, T12–L2). This anatomical distance between electrical field and target tissue must be explored and accounted for before these preclinical results can be generalized to the clinical population. The prevalent theme in work across multiple SCS waveforms is that higher doses of electricity significantly increase the surface area of the spinal cord covered by the stimulatory waveform. Together with the biological and clinical status of the targeted field, this can greatly influence the efficacy of the therapy.
PET CT or MRI have been used to image activated microglia with radioligands targeting the transporter protein TSPO in the spinal cord, and may provide a guide to determining optimal field targeting for paresthesia-free stimulatory waveforms.66 The maximal area of neuroinflammation in the spinal cord may not be located at the anatomical site determined by paresthesia-guided targeting. The area of neuroinflammation in the cord of greatest concern for neuropathic pain is within the gray matter of the dorsal horn. This is where the multiple areas of neural circuitries involved in central sensitization and wind-up phenomena are most likely to occur. Because all SCS is a surface-related phenomenon, sensory fibers must gain dorsal exposure to be recruited by the applied electrical field, and this may require as much as the width of a whole vertebral body in the lower area of the thoracic spinal cord. The sensory perception of paresthesia-guided targeting over the dorsal columns is often removed at a distance from the region of the dorsal horn where neuroinflammatory processes associated with neuropathic pain are concentrated, as noted previously.
Briefer exposure has a lower risk of overstimulation. This may explain why the outcomes of the Sunburst study were not as robust as had been expected and did improve with decreasing target amplitudes.42 Further studies are under way to more fully evaluate the use of this technique and other techniques to reduce stimulation dose. The use of on–off stimulation patterns has now become prevalent in all the paresthesia-free waveforms as a means to avoid potential overstimulation.
Another theme has been to incorporate a global therapy by combining both orthodromic and antidromic SCS therapy. The bursting paradigm creates orthodromic modulation at the cortical level, capable of affecting both the discriminative and affective components of chronic neuropathic pain. On the other hand, 10 kHz SCS has not specifically demonstrated any direct neurophysiological supraspinal activity,47 its effects appear to be much more local, and there is a trend within the industry to promote the blending of paresthesia-based sensory stimulation with paresthesia-free stimulation. Because stimulation can be incremental, with periods of stimulator activation alternating with periods of inactivity (“off” periods), there is ample time to add a bit of paresthesia-based sensory stimulation to distract pain awareness in the hope of bringing on more rapid pain relief. Paresthesia-free stimulation tends to be notoriously slow in onset, especially if its primary modus operandi is local, as it should be for purposes of safety and durability of therapy. However, in an industry that developed during the age of paresthesia-based sensory stimulation, it can be culturally burdensome to wait for the onset of efficacy. There is no rigorous medical evidence to pursue this approach. Instead, there is evidence to the contrary. Subjects exposed to conventional paresthesia-based therapy fared less well with subsequent paresthesia-free therapy during a crossover clinical trial.42 It should also be noted that blending these stimulation modalities is at odds with the concept of lower neural doses, as they often use much higher levels of energy to interleave or simultaneously stimulate at multiple frequencies, pulse widths, and amplitudes. These strategies should not be confused with strategies that attempt to lower the electrical energy applied to the target tissue. Another significant difficulty with the concept of blending paresthesia-free therapy with paresthesia-based therapy is the inability to separate the true responders from the placebo responders using this programming paradigm. Also, the effects of antidromic stimulation from paresthesia-based therapy could drastically counteract the effects of paresthesia-free therapies, as these interactions have not been adequately studied. Additionally, the combination of therapies could result in overstimulation, due to neuroplasticity and higher energy use. As such, until the nature of cognitive distraction, potential for placebo response, and counterproductive potential of paresthesia-based treatments if blended with paresthesia-free therapy are understood, this integrated trend requires more research.
Each of these trends impact considerably on our notions of “neural dosing” and must be considered as we further develop a neural dosing approach to therapy.
Acknowledgments
Third-party editing assistance was provided by Allison Foster, PhD of Foster Medical Communications.
Funding Statement
Support for development of this manuscript was provided by Abbott.
Disclosure
Dr Krishnan Chakravarthy is the founder of NXTStim and a consultant for Medtronic, Bioness, Nalu Medical, and Mainstay Medical outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Daughton CG, Ruhoy IS. Lower-dose prescribing: minimizing “side effects” of pharmaceuticals on society and the environment. Sci Total Environ. 2013;443:324–337. doi: 10.1016/j.scitotenv.2012.10.092 [DOI] [PubMed] [Google Scholar]
- 2.Donagher J, Martin JH, Barras MA. Individualised medicine: why we need Bayesian dosing. Intern Med J. 2017;47(5):593–600. doi: 10.1111/imj.13412 [DOI] [PubMed] [Google Scholar]
- 3.Bielinski SJ, St Sauver JL, Olson JE, et al. Cohort profile: the right drug, right dose, right time: using genomic data to individualize treatment protocol (RIGHT Protocol). Int J Epidemiol. 2020;49(1):23k–24k. doi: 10.1093/ije/dyz123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grider JS, Etscheidt MA, Harned ME, et al. Trialing and maintenance dosing using a low-dose intrathecal opioid method for chronic nonmalignant pain: a prospective 36-month study. Neuromodulation. 2016;19(2):206–219. doi: 10.1111/ner.12352 [DOI] [PubMed] [Google Scholar]
- 5.Lee AW, Pilitsis JG. Spinal cord stimulation: indications and outcomes. Neurosurg Focus. 2006;21(6):1–6. doi: 10.3171/foc.2006.21.6.6 [DOI] [PubMed] [Google Scholar]
- 6.Dones I, Levi V. Spinal cord stimulation for neuropathic pain: current trends and future applications. Brain Sci. 2018;8(8):138. doi: 10.3390/brainsci8080138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mekhail N, Visnjevac O, Azer G, Mehanny DS, Agrawal P, Foorsov V. Spinal cord stimulation 50 years later: clinical outcomes of spinal cord stimulation based on randomized clinical trials—a systematic review. Reg Anesth Pain Med. 2018;43(4):391–406. doi: 10.1097/AAP.0000000000000744 [DOI] [PubMed] [Google Scholar]
- 8.Adil S, Charalambous L, Spears C, et al. Impact of spinal cord stimulation on opioid dose reduction: a nationwide analysis. Neurosurgery. 2020;88(1):193–201. doi: 10.1093/neuros/nyaa353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labaran L, Aryee JNA, Bell J, et al. Opioids and spinal cord stimulators: pre- and postoperative opioid use patterns and predictors of prolonged postoperative opioid use. Neurospine. 2020;17(1):246–253. doi: 10.14245/ns.1938308.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762–770. doi: 10.1227/01.NEU.0000325731.46702.D9 [DOI] [PubMed] [Google Scholar]
- 11.Sweet J, Badjatiya A, Tan D, Miller J. Paresthesia-free high-density spinal cord stimulation for postlaminectomy syndrome in a prescreened population: a prospective case series. Neuromodulation. 2015;19(3):260–267. doi: 10.1111/ner.12357 [DOI] [PubMed] [Google Scholar]
- 12.Provenzano DA, Rebman J, Kuhel C, Trenz H, Kilgore J. The efficacy of high-density spinal cord stimulation among trial, implant, and conversion patients: a retrospective case series. Neuromodulation. 2017;20(7):654–660. doi: 10.1111/ner.12612 [DOI] [PubMed] [Google Scholar]
- 13.Benyamin R, Galan V, Hatheway J, et al. Options: a prospective, open-label study of high-dose spinal cord stimulation in patients with chronic back and leg pain. Pain Physician. 2020;23(1):87–98. doi: 10.36076/ppj.2020/23/87 [DOI] [PubMed] [Google Scholar]
- 14.Goudman L, De Smedt A, Eldabe S, et al. High-dose spinal cord stimulation for patients with failed back surgery syndrome: a multicenter effectiveness and prediction study. PAIN. 2021;162(2):582–590. doi: 10.1097/j.pain.0000000000002035 [DOI] [PubMed] [Google Scholar]
- 15.Lempka SF, Patil PG. Innovations in spinal cord stimulation for pain. Curr Opin Biomed Eng. 2018;8:51–60. doi: 10.1016/j.cobme.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maheshwari A, Pope JE, Deer TR, Falowski S. Advanced methods of spinal stimulation in the treatment of chronic pain: pulse trains, waveforms, frequencies, targets, and feedback loops. Expert Rev Med Devices. 2019;16(2):95–106. doi: 10.1080/17434440.2019.1567325 [DOI] [PubMed] [Google Scholar]
- 17.Heijmans L, Joosten EA. Mechanisms and mode of action of spinal cord stimulation in chronic neuropathic pain. Postgrad Med. 2020;132(sup3):17–21. doi: 10.1080/00325481.2020.1769393 [DOI] [PubMed] [Google Scholar]
- 18.Joosten EA, Franken G. Spinal cord stimulation in chronic neuropathic pain: mechanisms of action, new locations, new paradigms. Pain. 2020;161(1):S104–S113. doi: 10.1097/j.pain.0000000000001854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goudman L, De Groote S, Linderoth B, et al. Exploration of the supraspinal hypotheses about spinal cord stimulation and dorsal root ganglion stimulation: a systematic review. J Clin Med. 2021;10(13):2766. doi: 10.3390/jcm10132766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia K, Wray JK, Kumar S. Spinal Cord Stimulation. In: StatPearls. Treasure Island (FL):StatPearls Publishing; 2021. Available from:. http://www.ncbi.nlm.nih.gov/books/NBK553154/. Accessed July26, 2021. [PubMed] [Google Scholar]
- 21.Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: a review. Neuromodulation. 2016;19(4):373–384. doi: 10.1111/ner.12438 [DOI] [PubMed] [Google Scholar]
- 22.Zhang TC, Janik JJ, Grill WM. Mechanisms and models of spinal cord stimulation for the treatment of neuropathic pain. Brain Res. 2014;1569:19–31. doi: 10.1016/j.brainres.2014.04.039 [DOI] [PubMed] [Google Scholar]
- 23.Cui M, Feng Y, McAdoo DJ, Willis WD. Periaqueductal gray stimulation-induced inhibition of nociceptive dorsal horn neurons in rats is associated with the release of norepinephrine, serotonin, and amino acids. J Pharmacol Exp Ther. 1999;289(2):868–876. [PubMed] [Google Scholar]
- 24.Cui JG, O’Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73(1):87–95. doi: 10.1016/S0304-3959(97)00077-8 [DOI] [PubMed] [Google Scholar]
- 25.Yakhnitsa V, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates dorsal horn neuronal hyperexcitability in a rat model of mononeuropathy. Pain. 1999;79(2–3):223–233. doi: 10.1016/S0304-3959(98)00169-9 [DOI] [PubMed] [Google Scholar]
- 26.Sato KL, Johanek LM, Sanada LS, Sluka KA. Spinal cord stimulation reduces mechanical hyperalgesia and glial cell activation in animals with neuropathic pain. Anesth Analg. 2014;118(2):464–472. doi: 10.1213/ANE.0000000000000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinoda M, Fujita S, Sugawara S, et al. Suppression of superficial microglial activation by spinal cord stimulation attenuates neuropathic pain following sciatic nerve injury in Rats. Int J Mol Sci. 2020;21(7):2390. doi: 10.3390/ijms21072390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirketeig T, Schultheis C, Zuidema X, Hunter CW, Deer T. Burst spinal cord stimulation: a clinical review. Pain Med. 2019;20(Suppl 1):S31–S40. doi: 10.1093/pm/pnz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo RE, Hounsgaard J. Burst-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. J Physiol. 1996;493(Pt 1):55–66. doi: 10.1113/jphysiol.1996.sp021364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snider RK, Kabara JF, Roig BR, Bonds AB. Burst firing and modulation of functional connectivity in cat striate cortex. J Neurophysiol. 1998;80(2):730–744. doi: 10.1152/jn.1998.80.2.730 [DOI] [PubMed] [Google Scholar]
- 31.Crosby ND, Goodman Keiser MD, Smith JR, Zeeman ME, Winkelstein BA. Stimulation parameters define the effectiveness of burst spinal cord stimulation in a rat model of neuropathic pain. Neuromodulation. 2015;18(1):1–8. doi: 10.1111/ner.12221 [DOI] [PubMed] [Google Scholar]
- 32.Gong W-Y, Johanek LM, Sluka KA. A comparison of the effects of burst and tonic spinal cord stimulation on hyperalgesia and physical activity in an animal model of neuropathic pain. Anesth Analg. 2016;122(4):1178–1185. doi: 10.1213/ANE.0000000000001161 [DOI] [PubMed] [Google Scholar]
- 33.Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Burst spinal cord stimulation in peripherally injured chronic neuropathic rats: a delayed effect. Pain Pract. 2018;18(8):988–996. doi: 10.1111/papr.12701 [DOI] [PubMed] [Google Scholar]
- 34.Chakravarthy K, Richter H, Christo P, Williams K, Guan Y. Spinal cord stimulation for treating chronic pain: reviewing preclinical and clinical data on paresthesia-free high-frequency therapy. Neuromodulation. 2018;21(1):10–18. doi: 10.1111/ner.12721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linderoth B, Foreman RD. Conventional and novel spinal stimulation algorithms: hypothetical mechanisms of action and comments on outcomes. Neuromodulation. 2017;20(6):525–533. doi: 10.1111/ner.12624 [DOI] [PubMed] [Google Scholar]
- 36.Zannou AL, Khadka N, Truong DQ, et al. Temperature increases by kilohertz frequency spinal cord stimulation. Brain Stimul. 2019;12(1):62–72. doi: 10.1016/j.brs.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zannou AL, Khadka N, FallahRad M, Truong DQ, Kopell BH, Bikson M. Tissue temperature increases by a 10 kHz spinal cord stimulation system: phantom and bioheat model. Neuromodulation. 2019. doi: 10.1111/ner.12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caylor J, Reddy R, Yin S, et al. Spinal cord stimulation in chronic pain: evidence and theory for mechanisms of action. Bioelectron Med. 2019;5(1):12. doi: 10.1186/s42234-019-0023-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson SJ, Tavakkolizadeh M, Love‐Jones S, et al. Effects of rate on analgesia in kilohertz frequency spinal cord stimulation: results of the PROCO randomized controlled trial. Neuromodulation. 2018;21(1):67–76. doi: 10.1111/ner.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Ridder D, Vanneste S, Plazier M, van der Loo E, Menovsky T. Burst spinal cord stimulation. Neurosurgery. 2010;66(5):986–990. doi: 10.1227/01.NEU.0000368153.44883.B3 [DOI] [PubMed] [Google Scholar]
- 41.de Vos CC, Bom MJ, Vanneste S, Lenders MWPM, de Ridder D. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation. 2014;17(2):152–159. doi: 10.1111/ner.12116 [DOI] [PubMed] [Google Scholar]
- 42.Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56–66. doi: 10.1111/ner.12698 [DOI] [PubMed] [Google Scholar]
- 43.Vesper J, Slotty P, Schu S, et al. Burst SCS microdosing is as efficacious as standard burst scs in treating chronic back and leg pain: results from a randomized controlled trial. Neuromodulation. 2019;22(2):190–193. doi: 10.1111/ner.12883 [DOI] [PubMed] [Google Scholar]
- 44.Deer TR, Patterson DG, Baksh J, et al. Novel intermittent dosing burst paradigm in spinal cord stimulation. Neuromodulation. 2020;24(3):566–573. doi: 10.1111/ner.13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falowski SM, Benison A. Prospective analysis utilizing intraoperative neuromonitoring for the evaluation of inter-burst frequencies. J Pain Res. 2021;14:703–710. doi: 10.2147/JPR.S298797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falowski SM. An observational case series of spinal cord stimulation waveforms visualized on intraoperative neuromonitoring. Neuromodulation. 2019;22(2):219–228. doi: 10.1111/ner.12781 [DOI] [PubMed] [Google Scholar]
- 47.Bocci T, De Carolis G, Paroli M, et al. Neurophysiological comparison among tonic, high frequency, and burst spinal cord stimulation: novel insights into spinal and brain mechanisms of action. Neuromodulation. 2018;21(5):480–488. doi: 10.1111/ner.12747 [DOI] [PubMed] [Google Scholar]
- 48.Al-Kaisy A, Markham K, Harris S, Costanzi M, Pang D, Palmisani S. Multilevel sequential Cascade 10kHz spinal cord stimulation for chronic neuropathic pain. In: International Neuromodulation Society Conference; 2017. [Google Scholar]
- 49.Al-Kaisy A, Royds J, Al‐Kaisy O, et al. Cascade programming for 10 kHz spinal cord stimulation: a single center case series of 114 patients with neuropathic back and leg pain. Neuromodulation. 2020;24(3):488–498. doi: 10.1111/ner.13219 [DOI] [PubMed] [Google Scholar]
- 50.Rouach N, Duc KD, Sibille J, Holcman D. Dynamics of ion fluxes between neurons, astrocytes and the extracellular space during neurotransmission. bioRxiv. 2018;4(1):305706. doi: 10.1101/305706 [DOI] [Google Scholar]
- 51.Provenzano DA, Lauer Z, Kilgore J. Understanding programming parameters for 10-kHz SCS trial and implant patients. In: North American Neuromodulation Society Conference; 2019. [Google Scholar]
- 52.Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851–860. doi: 10.1097/ALN.0000000000000774 [DOI] [PubMed] [Google Scholar]
- 53.Russo M, Cousins MJ, Brooker C, et al. Effective relief of pain and associated symptoms with closed-loop spinal cord stimulation system: preliminary results of the Avalon study. Neuromodulation. 2018;21(1):38–47. doi: 10.1111/ner.12684 [DOI] [PubMed] [Google Scholar]
- 54.Laird JH, Parker JL. A model of evoked potentials in spinal cord stimulation. In: Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS; July2013:6555–6558. doi: 10.1109/EMBC.2013.6611057. [DOI] [PubMed] [Google Scholar]
- 55.Parker JL, Karantonis DM, Single PS, Obradovic M, Cousins MJ. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain. 2012;153(3):593–601. doi: 10.1016/j.pain.2011.11.023 [DOI] [PubMed] [Google Scholar]
- 56.Parker JL, Karantonis DM, Single PS, et al. Electrically evoked compound action potentials recorded from the sheep spinal cord. Neuromodulation. 2013;16(4):295–303. doi: 10.1111/ner.12053 [DOI] [PubMed] [Google Scholar]
- 57.Russo M, Brooker C, Cousins MJ, et al. Sustained long-term outcomes with closed-loop spinal cord stimulation: 12-month results of the prospective, multicenter, open-label Avalon study. Neurosurgery. 2020;87(4):E485–E495. doi: 10.1093/neuros/nyaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123–134. doi: 10.1016/S1474-4422(19)30414-4 [DOI] [PubMed] [Google Scholar]
- 59.Benyamin R, Grider JS, Motejunas MW, et al. Spinal cord stimulation: principles and applications. In: Davis SF, Kaye AD, editors. Principles of Neurophysiological Assessment, Mapping, and Monitoring. Springer International Publishing; 2020:285–300. doi: 10.1007/978-3-030-22400-4_22 [DOI] [Google Scholar]
- 60.Paz-Solís J, Thomson S, Jain R, Chen L, Huertas I, Doan Q. Exploration of high and low frequency options for subperception spinal cord stimulation using neural dosing parameter relationships: the HALO study. Neuromodulation;2021. doi: 10.1111/ner.13390 [DOI] [PubMed] [Google Scholar]
- 61.Alo K, Varga C, Krames E, et al. Factors affecting impedance of percutaneous leads in spinal cord stimulation. Neuromodulation. 2006;9(2):128–135. doi: 10.1111/j.1525-1403.2006.00050.x [DOI] [PubMed] [Google Scholar]
- 62.Khadka N, Liu X, Zander H, et al. Realistic anatomically detailed open-source spinal cord stimulation (RADO-SCS) model. J Neural Eng. 2020;17(2):026033. doi: 10.1088/1741-2552/ab8344 [DOI] [PubMed] [Google Scholar]
- 63.Šarolić A, Škalic I, Deftu A, Sapunar D. Impedance measurement of bipolar stimulation electrodes immersed in medium. In: 2018 EMF-Med 1st World Conference on Biomedical Applications of Electromagnetic Fields (EMF-Med); 2018: 1–2. doi: 10.23919/EMF-MED.2018.8526008. [DOI] [Google Scholar]
- 64.Zander HJ, Graham RD, Anaya CJ, Lempka SF. Anatomical and technical factors affecting the neural response to epidural spinal cord stimulation. J Neural Eng. 2020;17(3):036019. doi: 10.1088/1741-2552/ab8fc4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vallejo R, Gupta A, Kelley Ca, et al. Effects of phase polarity and charge balance spinal cord stimulation on behavior and gene expression in a rat model of neuropathic pain. Neuromodulation. 2019;23(1):26–35. doi: 10.1111/ner.12964 [DOI] [PubMed] [Google Scholar]
- 66.Tronel C, Largeau B, Santiago Ribeiro MJ, Guilloteau D, Dupont A-C, Arlicot N. Molecular targets for PET imaging of activated microglia: the current situation and future expectations. Int J Mol Sci. 2017;18(4):802. doi: 10.3390/ijms18040802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed S, Yearwood T, De Ridder D, Vanneste S. Burst and high frequency stimulation: underlying mechanism of action. Expert Rev Med Devices. 2018;15(1):61–70. doi: 10.1080/17434440.2018.1418662 [DOI] [PubMed] [Google Scholar]