Abstract
Tumor immunotherapy is rapidly evolving as one of the major pillars of cancer treatment. Cell-based immunotherapies, which utilize patient’s own immune cells to eliminate cancer cells, have shown great promise in treating a range of malignancies, especially those of hematopoietic origins. However, their performance on a broader spectrum of solid tumor types still fall short of expectations in the clinical stage despite of the promising preclinical assessments. In this review, we briefly introduce cell-based immunotherapies and the inhibitory mechanisms in tumor microenvironments that may have contributed to this discrepancy. Specifically, a major obstacle to the clinical translation of cell-based immunotherapies is in the lack of preclinical models that can accurately assess the efficacies and mechanisms of these therapies in a (patho-)physiologically relevant manner. Lately, tissue engineering and organ-on-a-chip tools and microphysiological models have allowed for more faithful recapitulation of the tumor microenvironments, by incorporating crucial tumor tissue features such as cellular phenotypes, tissue architecture, extracellular matrix, physical parameters, and their dynamic interactions. This review summarizes the existing engineered tumor models with a focus on tumor immunology and cell-based immunotherapy. We also discuss some key considerations for the future development of engineered tumor models for immunotherapeutics.
Graphical abstract
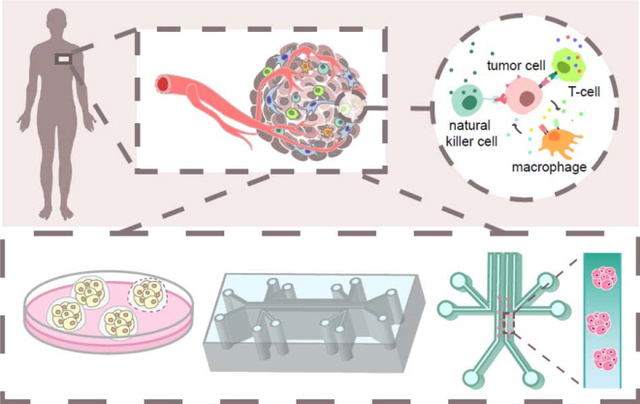
Introduction
Immunotherapy is arguably the most rapidly evolving and promising cancer therapeutics in the past decade [1] and regarded as the fourth pillar of cancer treatment [2]. Unlike conventional therapeutic strategies, immunotherapy aims to boost the functions of a patient’s own immune system to recognize and attack the cancer cells, which can generate durable remission even in advanced tumors with relatively few side effects [3]. Immune checkpoint inhibitors, which target the negative regulators of the immune system, have been integrated in the standard anti-cancer therapies as single agents or in combination with chemotherapies [4] The emerging cancer viruses and personalized cancer vaccines are also showing promising results in clinical trials [5, 6]. Most notably, cell-based immunotherapies are taking the center stage of immunotherapy in recent years. For example, Sipuleucel-T (Provenge) and three chimeric antigen receptor (CAR) T cell therapies, Tisagenlecleucel (Kymriah), Axicabtagene ciloleucel (Yescarta), and Brexucabtagene autoleucel (Tecartus), were approved by the US Food and Drug Administration (FDA). Many others including CAR and T cell receptor (TCR)-engineered T cells are in the preclinical and clinical pipelines [7, 8]. However, the success of these therapies has largely been limited to a few types of malignancies, particularly those of hematopoietic origin, and their efficacies still fall short of expectations in most solid tumors [9], which account for over 90% of all cancer types [10]. There is also tremendous variability in the efficacy of immunotherapy across patients, therapies, and cancer types [11].
Part of this disappointment is attributed to the staggering complexity and heterogeneity of the tissue microenvironment in solid tumors, or the tumor microenvironment (TME). The TME impose physical, chemical, molecular, and cellular barriers to infiltrating therapeutic immune cells [12, 13]. Preclinical in vitro and in vivo models are crucial for understanding the working mechanisms and evaluating the efficacy of cell-based immunotherapies in the context of the TME. However, it remains challenging to recapitulate the immune TME in most existing in vivo models, which typically involve immunodeficient mice and/or TME components with little/limited resemblance to human pathophysiology [14]. Thus, the mechanistic insights derived from these models often fail to predict the outcomes in clinical trials. Engineered in vitro models have emerged to mimic the essential elements of the TME using human cell lines or patient-derived cells. With the assistance of modern techniques such as micro-fabrication, organoid culture, and bioprinting, a new generation of in vitro tumor models are emerging that recapitulate the human TME with unprecedented control. They allow for precise mechanistic evaluation of individual TME components in immunotherapy, while also enabling assessment of therapeutic efficacy in a more (patho)physiologically relevant manner, and potentially on an individual patient basis for personalized medicine. In this review, we discuss the unique aspects of cell-based immunotherapy, the major challenges for their efficacy, and recent advances in engineered tumor models in the context of cellular immunotherapies. We also examine the additional crucial aspects of TME that need to be incorporated in future models to further accelerate immunotherapeutic discoveries at the molecular scale and the systems level and to enable faithful clinical translation.
Cell-Based Cancer Immunotherapy
Cell-based cancer immunotherapy focuses on using living immune cells as a therapeutic agent to kill or aid the killing of tumor cells. In most cases, immune cells are extracted and isolated from patient’s body, expanded ex vivo with or without modifications, and reinfused back into the patient in a process known as adoptive cell transfer (ACT) [15] (Figure 1). In addition to increasing their number and immune potency, adoptively transferred immune cells can be modified to selectively target tumor-specific or tumor-associated antigens.
Figure 1.
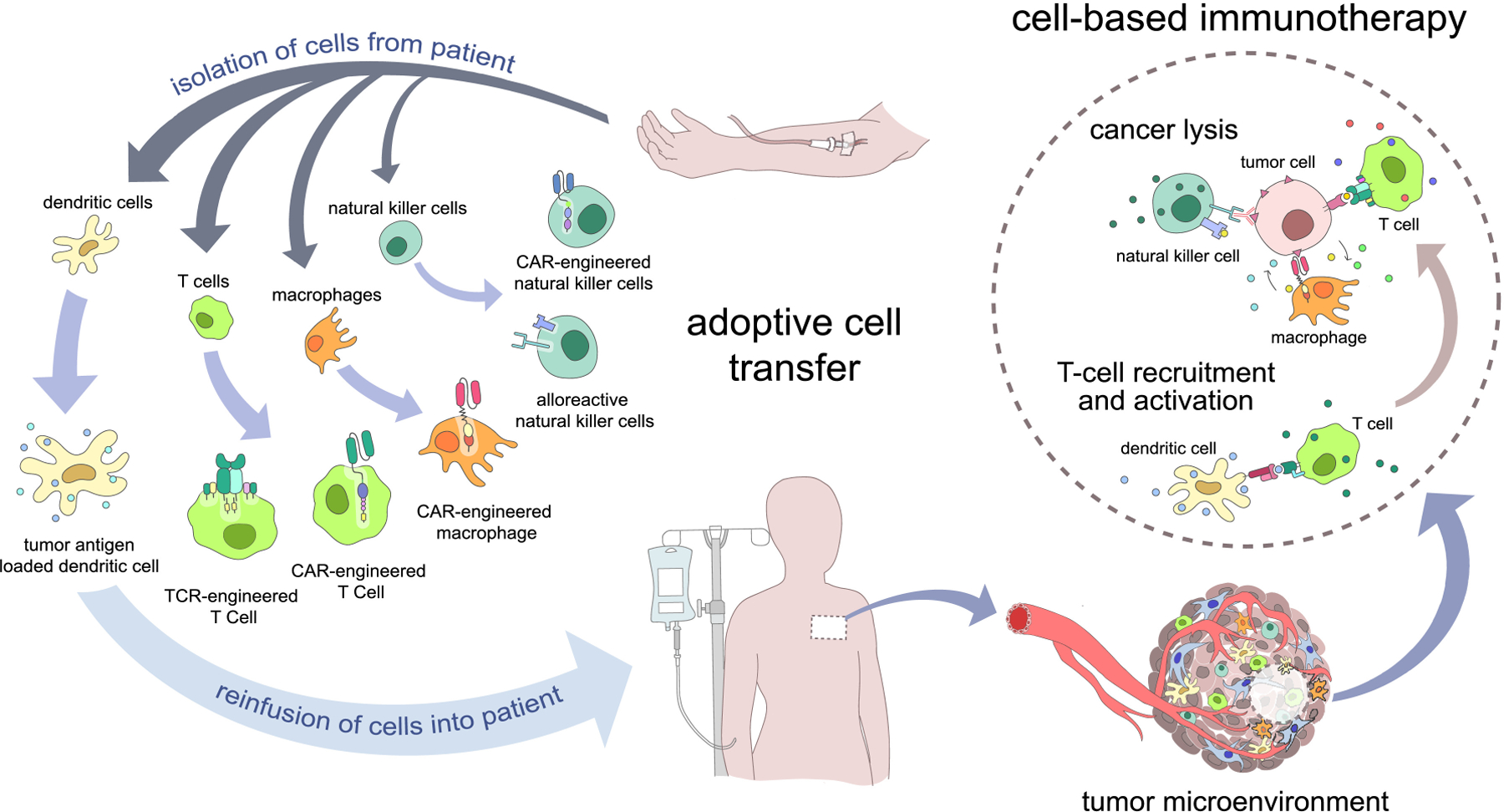
A brief overview of cell-based immunotherapy. In a process known as adoptive cell transfer, cells are extracted from patient blood, engineered, and reinfused into the patient. Engineered immune cells circulate in the bloodstream until they eventually arrive in the tumor site, resulting in cancer killing or T cell recruitment and activation.
Among the many cell types involved in tumor immunity (Figure 1), dendritic cells and T cells have been the most successful and extensively used for cell-based immunotherapies. Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that mediate the innate immune response and induction of the adaptive immune responses. Adoptively transferred DCs are used for cancer vaccination: DCs loaded with cancer-specific antigens recruit and activate endogenous antigen-specific cytotoxic T cells to induce anti-cancer immunity [16, 17]. Clinical trials against cervical, ovarian, and colorectal cancer have shown DC vaccine-induced recruitment of CD4+ and CD8+ T cells in tumors [18–20]. Provenge (Sipuleucel-T) was the first FDA-approved cell-based immunotherapeutic vaccine against advanced prostate cancer [21, 22]. It utilizes APCs isolated from patient’s white blood cells (consisting of mainly DCs) that are cultured ex vivo with a recombinant fusion protein containing a prostate tumor antigen and granulocyte-macrophage colony stimulating factor (GM-CSF) [17]. T cells play a central role in anti-tumor immunity. Tumor infiltrating lymphocytes (TILs), which contain T cell clones that recognize tumor antigens, have been expanded ex vivo and reinfused into patients with metastatic melanoma [23–25]. T cells without tumor-specific TCRs can also be endowed with anti-tumor immunity by cloning the TCR gene from TILs (also known as the TCR-engineered T cells), which overcomes the dependence of TIL therapy on the preexistence or identification of tumor-reactive lymphocytes [26].
Another form of genetically engineered T cell is CAR engineered T cells. CARs are synthetic receptors that recognize tumor-associated antigens (TAAs) expressed on target cell surfaces, most often composed of an extracellular single chain variable fragment (scFv) from a clinical antibody and intracellular signaling domains that activate T cells [27, 28]. Three CAR T cell therapies have been approved by the FDA. Novatis’ Tisagenlecleucel (Kymriah) was the first approved CAR T therapy against B-cell precursor acute lymphoblastic leukemia (B-ALL) in 2017. Strikingly, up to 90% complete remission was observed in B-ALL patients in earlier clinical trials [29]. In the same year, Kite’s Axicabtagene ciloleucel (Yescarta) was approved for treating adult patients with relapsed or refractory large B-cell lymphoma. Most recently, in July 2020, the FDA approved Kite’s Brexucabtagene autoleucel (Tecartus) for treating adults with relapsed or refractory mantle cell lymphoma. All three CAR T cell therapies are based on CD19, which is a target for B cell malignancies [30], despite reports of on-target, off-tumor effects and neurotoxicity [31]. In August 2019, the FDA granted breakthrough therapy designation to an experimental CAR T cell therapy targeting surface molecule CD22 in children and young adults with relapsed of CD19-resistant B-ALL [32]. Following these incredible clinical developments, CAR T cells targeting multiple tumor antigens have been actively investigated for treatment of several solid cancer types. Some identified targets include EGFRvIII for glioblastoma, HER2 for sarcomas, MET for melanoma and breast cancer, PSMA for prostate cancer, and mesothelin for metastatic breast cancer. Nevertheless, the preclinical and clinical results have been disappointing so far [33–36].
Several other immune cell types are also gaining attention in cell-based immunotherapies. Macrophages are highly plastic immune cells that regulate tissue homeostasis, wound healing, and innate immune responses [37]. Tumor-associated macrophages (TAMs) are often correlated with poor patient prognosis, and exhibit bipolar effects promoting and inhibiting tumor immunosuppression [38]. Modifying macrophages to express CARs that specifically target tumor antigens resulted in phagocytosis of antigen-expressing cancer cells [39]. On the other hand, TAM depletion is a common approach to lower the immunosuppressive TME and synergistically enhance immune checkpoint blockade, DC vaccines, and ACT of cytotoxic T cells [40, 41]. Natural killer (NK) cells are another innate immune cell capable of eradicating tumor cells. Adoptive transfer of alloreactive NK cells has shown promising results in treating a range of malignancies including acute myeloid leukemia and non-small cell lung cancer [42–45]. Engineering NK cells with CAR further enables their tumor targeting through both the native receptors and the CAR, thus reducing the chance of immune escape by cancer cells [46].
TME as a Barrier to Cell-based Immunotherapy
Cell-based cancer immunotherapy shows tremendous promise for targeted and durable remission of cancer. However, much of the exciting success in preclinical studies and clinical applications with certain cancers have not translated well to a broader range of cancer types, especially in solid tumors [47, 48]. While the reason is most likely multifactorial, the immunosuppressive TME has been recognized as a major player [49]. The TME consists of intricate interactions between its cellular and acellular components. The cellular players include myofibroblasts, immune cells, endothelial cells, adipocytes, etc.; the acellular players include the extracellular matrix (ECM), cell-secreted soluble factors, metabolites, nutrients, oxygen, as well as physicochemical parameters such as pH, ECM stiffness, and mechanical forces [50].
The architecture of solid cancer consists of the parenchyma (neoplastic cells) and the stroma (all other cellular and extracellular components) [51]. Nests of parenchyma are in complex interplay with the stroma, molding phenotypic heterogeneity and immunosuppressive factors [52, 53]. Elevated ECM production and stiffness/density impose physical barrier to therapeutic immune cell infiltration [54]. Recruitment or differentiation of regulatory T cells (Tregs), TAMs, myeloid-derived suppressor cells (MDSCs) and cancer-associated fibroblasts (CAFs) can establish an immunosuppressive TME that compromise cytotoxic T cell cytokine secretion, growth, and cytotoxicity against tumor cells [55]. Increased expression of suppressive ligands and soluble factors/cytokines by cancer and stromal cells in TME, such as PD-L1/L2 [56, 57], prostaglandin E2 (PGE2), adenosine, and TGF-β function against anti-tumor immune cells [58–60].
The metabolic landscape in TME has lately emerged as one of the crucial regulators of immunotherapeutic resistance. Nearly all cancers exhibit shifted energy metabolism from oxidative phosphorylation (OXPHOS) to aerobic glycolysis (also known as Warburg effect) [61]. Meanwhile, hypoxia is a common feature of most solid tumors, especially in those with high malignancy [62]. Hypoxia signaling pathways, particularly that of the hypoxia inducible factor (HIF), overlap strongly with those implicated in the altered cancer and immune cell metabolism, where the action of HIF is involved in almost every step of glycolysis [63]. In cell-based immunotherapies, the competition for glucose between cancer cells and the glycolytic anti-tumor leukocytes has been proposed to hinder the penetration and functionality of these therapeutic cells, and to activate the stress responses that lead to their exhaustion or death [64]. On the other hand, hypoxia has been shown to impair CAR T cell expansion in vitro and their differentiation into effector memory cells [65]. Hypoxia can also upregulate the expression of some antigens in tumors such as carbonic anhydrase IX [66]. These evidences suggest opposing roles of hypoxia in cell-based immunotherapy which require further investigation.
Major Limitations with Conventional Tumor Models
The integrated, heterogeneous facets of the TME leads to immune evasion and immunotherapeutic failures [67]. Currently, no tumor model has fully addressed the complexity of the TME. In this section, we will briefly review the challenges of conventional approaches to model the immune microenvironment in the TME.
Traditional in vitro cell culture still serve as mainstream screening platforms for drug discovery and therapy evaluation [68]. In cell-based immunotherapies, random mixture of therapeutic immune cells with target cancer cells is often used to evaluate the efficacy of targeted cell killing. For epithelial cancers, this usually involves culturing immune cells on a two-dimensional (2-D) monolayer of cancer cells in a dish. The advantages of the random mixture or 2-D studies include high turnover rate, relatively cheap costs, and facile maintenance. However, clinical translation remains a challenge, which is largely due to the lack of tissue-level characteristics like tissue morphology, vasculature, cell-cell/cell-matrix interactions, and diffusion-limited processes in the TME [69, 70].
In vivo models allow investigation of whole-body systemic response to treatment [14]. Mouse syngeneic tumor models involve implantation of MHC-matched cancer cell lines into subcutaneous or orthotopic sites of recipient mice. Since the implanted cancer cells are compatible with the host immune system, these models have been widely used to assess anti-tumor immunotherapies [71]. Yet, such tumors usually lack genetic heterogeneity and do not truly capture the morphology and microenvironment of the tumors they represent from the original organs. Genetically engineered mouse models (GEMMs) carry oncogenes that are induced/activated in specific organs, which then develop de novo tumors with a natural immune microenvironment [72]. Some advanced GEMMs display genetic heterogeneity, mimic the histopathological and molecular features of the corresponding human tumors, and can spontaneously progress into metastatic disease [73]. GEMMs are thus of great importance in understanding the biological mechanisms underlying tumor immunology. However, both mouse syngeneic models and GEMMs come with high costs, slow turnover rate, inability to control selected processes of the TME [74], and most importantly, lack of consistency with human immune system [75]. Thus, these models have limited utility in predicting the immunotherapeutic outcomes in human tumors.
Xenograft models have been developed to improve human relevance of mouse tumor models, by implanting human cell lines or patient-derived tissues in immunodeficient mice [76]. Notably, cell-line and patient-derived xenograft (PDX) models have been utilized for preclinical evaluation of CAR T cell therapies [77, 78]. Nonetheless, cell-line xenografts often do not accurately model the true behaviors of the human tumors due to the adaptation of cell lines to in vitro growth conditions and a lack of human stroma. Similarly, the preservation of the TME in PDX models is also incomplete and temporary, and tend to deviate from the patient’s original tumor [79], such as with murine stroma replacing human stroma over time [80, 81]. Furthermore, the hosts of cell-line xenografts and PDX models are immunocompromised to prevent rejection of human tumor tissue. The lack immune infiltrate renders the platform inadequate to assess immunotherapy in the context of human immune system, particularly those associated with the adaptive immune functions. Even in humanized mouse models that allow investigation of xenografts with an intact human immune system (i.e. through transplantation of human hematopoietic stem cells, HSCs [82]), there are significant hurdles such as obtaining HSCs from the same patient to avoid immune rejection [83]. Existing models, therefore, are insufficient for efficient clinical translation. The advantages and disadvantages of these models are outlined in Table 1.
Table 1.
A brief summary of the advantages and disadvantages of existing tumor models.
| Model Type | Advantages | Disadvantages | |
|---|---|---|---|
| Cell monolayer | Uses human source of cancer cells; relatively cheap costs; high throughput | Ignores the TME and its effects; cancer cell lines fail to capture phenotypic and genotypic heterogeneity | |
| Mouse models (syngeneic models and genetically engineered mouse models (GEMM)) | Recapitulates the TME; allows systemic evaluation of therapies | Very limited human relevance; difficult to control selected aspects of the TME; high costs; low throughput | |
| Cell-line xenografts and patient-derived xenografts (PDX) | Uses human source of cancer cells; recapitulates the TME (i.e. cancer-stromal interaction) | Limited human relevance when studying cancer-immune interactions; high costs; low throughput | |
| Engineered | Spheroid | Uses human cancer cells; recapitulates selected aspects of the TME; easily controllable; relatively cheap costs; medium throughput | Lacks the extracellular architecture; usually lacks parenchyma-stroma organization; lack clonal and genetic heterogeneity |
| Organoid | Uses human cell sources; recapitulates selected aspects of the TME | Difficulty with sourcing cells | |
| Organotypic models | Uses human source of cells; includes parenchyma and stroma cells | Difficulty with sourcing cells and long-term maintenance | |
| Microfluidics | Uses human source of cells; able to control local concentrations of soluble factors; includes connectivity | Requires expert handling | |
Engineered Models for Tumor Immunology
Engineered in vitro tumor models can recapitulate specific features of the TME while maintaining relatively low costs and medium to high throughput for rapid investigation. These models enable unprecedented spatiotemporal control of microenvironment factors that play major roles in tumor biology [84, 85]. A major advancement in the in vitro tumor modeling has come from replicating the 3-D tumor architecture in vivo. Compared to traditional 2-D assays, cancer cells in the 3-D spatial architecture exhibit distinct behaviors [86–88] that mimic signaling pathways in primary tumor samples [89] or those from in vivo models [90]. These include decreased tumor proliferation [91, 92], loss of cell polarity [93], up-regulation of angiogenic factors such as hypoxia-inducible protein 2 and VEGF 1/2 [86, 94], and increased reliance on glycolysis [95–97]. Such features directly influence the assessment of various therapies at the preclinical stage [98]. For example, cells in 3-D exhibit decreased sensitivity to radiation therapy, death receptor ligation, interferons, or chemotherapeutic reagents [91, 94, 99]. For cell-based immunotherapy, 3-D microenvironment also poses physical, chemical, and cellular barriers to the infiltration of therapeutic immune cells that 2-D cultures cannot recapitulate.
Another major feature of the TME is the heterogeneity of cellular types that interact with each other. Co-culture models that interrogate the interactions of different cell types are crucial to provide a systemic study of the complex interchange of cellular communication during immunotherapeutic regimens. Therapeutic outcome has been associated with the existence of certain immune cell types such as macrophages inhibiting cytotoxic T cell activity through engagement of PD-L1/PD-L2 [100] or through immunosuppressive molecules such as IL-10, TGF-β, and prostaglandins [101]. Therefore, the inclusion of innate and adaptive immune cells in preclinical tumor models can provide a more wholesome outlook on the TME and its potential impact on cell-based immunotherapy.
In addition, like the other organs-on-a-chip models [85], engineered tumor models have exploited self-assembly and/or microfabrication to facilitate mass production of highly customizable and tunable platforms. Miniaturization further improves the high-through capacity to screen various therapies and developing novel pharmaceuticals. Here we divide the section into reviews of tumor spheroids, organoids, and microfluidic models, with a focus on their adaptation in evaluating immunotherapies.
Tumor spheroids
The first generation of bio-inspired cell culture involved cancer cells that spontaneously formed cell-cell interactions under non-adherent conditions [102]. These structures, or tumor spheroids, depict dense cancer cells in 3-D (Figure 2a) [102]. The spatial configuration limits passive diffusion of oxygen, nutrients, metabolites, waste products, and other signaling molecules, resulting in a heterogeneous landscape of soluble factors much like the TME (Figure 2b) [103, 104]. Tumor heterogeneity in the 3-D space have provided important insight into mechanisms of immunotherapy. Tumor spheroids down-regulated human leukocyte antigen (HLA) expression and antigen presentation, effectively evading T cell treatment as compared to monolayer studies [105, 106]. Glioblastoma spheroids, in drastic contrast to cell monolayers, resembled patient phenotypes (Figure 2c) and significantly impaired anti-cancer IFN-γ production from DCs and T cells [107]. Spheroids produced similar results as in a mice xenograft model when assessing combinatorial synergy of bispecific antibodies and PD-1 inhibition in activating T cells [108]. Examples like these suggest that spheroids are representative of how in vivo tumors evade current immunotherapies.
Figure 2.
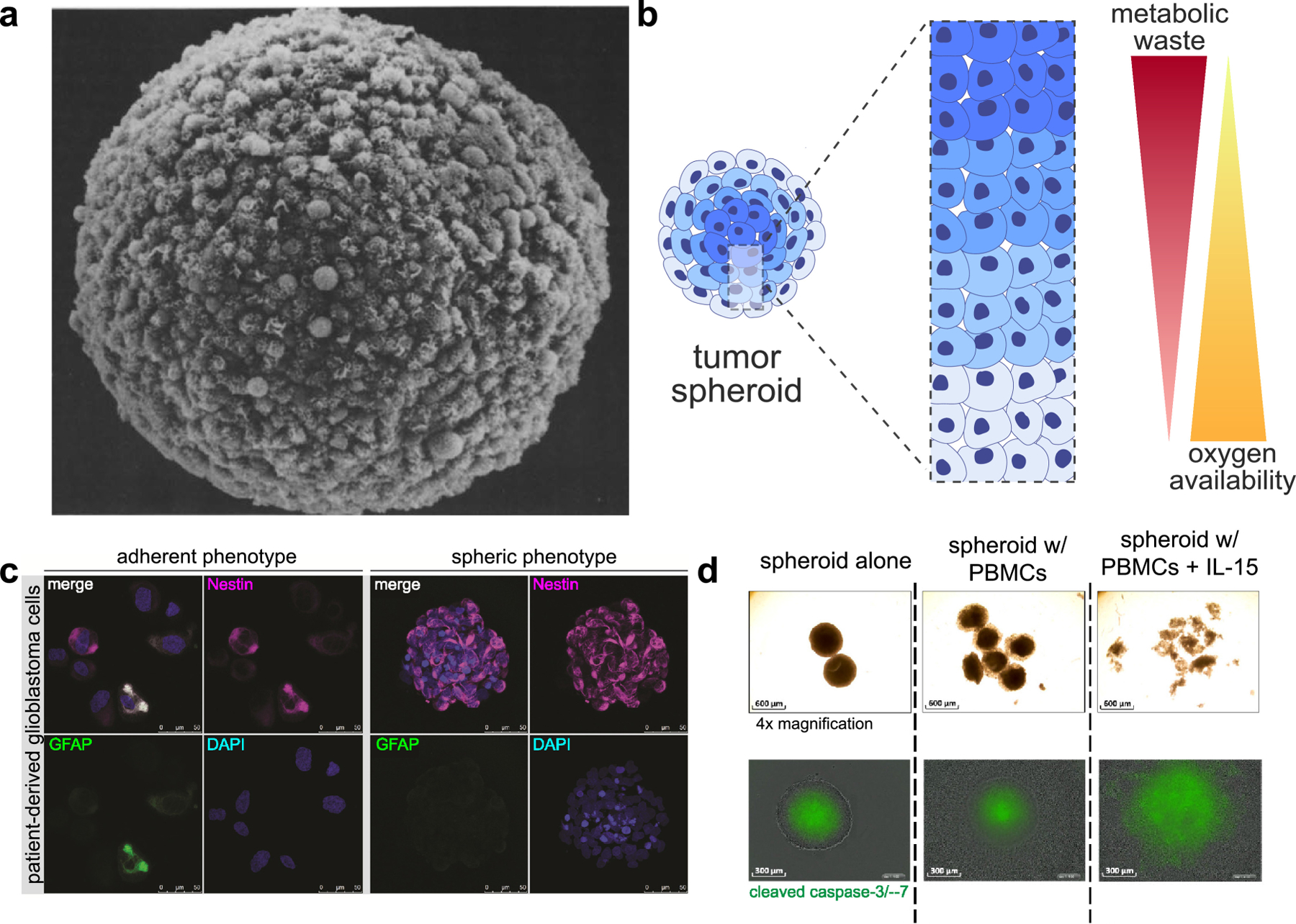
(a) A representation of a multicellular tumor spheroid (ref [102]). (b) Tumor spheroids naturally form a gradient of soluble factors, with a core that is oxygen-deficient and rich in metabolic waste products. (c) Culture conditions (2-D versus spheroid culture) change phenotypic expression of patient-derived glioblastoma cells (ref [107]). (d) PBMC-induced tumor apoptosis was monitored by evaluating spheroid volume (top) and expression of cleaved caspase-3/−7 (bottom) (ref [110]).
In addition, spheroids provide visual confirmation of immune cell infiltration and the resulting cytotoxicity; for example, CAR T cell infiltration and cytotoxicity were quantified in spheroids of HCT 116 colorectal cancer cells [109]. Courau et al. recently reported an interesting comparison of the infiltration efficiency of activated T cells and NK cells in HT29 colon cancer spheroids. NK cells infiltrated better than T cells, while CD8+ CD45RO+ memory T cells were up-regulated within the infiltrated T cell population (Figure 2d) [110], which have been implied to correlate with better patient prognosis [111, 112]. On the other hand, NK cells consistently show efficient infiltration despite the dense cell-cell barriers of tumor spheroids [113, 114], while antibodies failed to penetrate spheroids in 72 hours [114]. Further, immune stimulation with IL-15 or anti-MHC class I chain-related protein A and B (anti-MICA/B) antibodies induced an almost 3-fold increase in infiltration by both T and NK cells, and the effect was reversed upon the addition of IFN-γ or anti-natural killer group 2D (anti-NKG2D) antibodies [110]. SDF-1α has been confirmed as NK cell attractants in a lung carcinoma spheroid model [115]. Interestingly, inhibition of CXCL12 with NOX-A12 (Olaptesed pegol) was found to enhance infiltration of T and NK cells (B cells to a lesser extent, and no enhancement of monocyte infiltration) into spheroids of colorectal carcinoma, pancreatic adenocarcinoma, glioblastoma, and non-small cell lung cancers (mixed with murine stromal MS-5 cells). Activation of T cells was enhanced through PD-1 inhibition as well, exemplifying the ability for spheroids to assess combinatorial effects from multiple immunotherapeutic agents [116].
The relevance of immunotherapy infiltration studies further depends on the depiction of a dense stroma and ECM network in patient tumors. Spheroids, however, often lack scaffolds that resemble in vivo ECM architecture. To remedy this, 3-D culture of spheroids embedded in ECM materials like Matrigel have been explored [117]. In spheroids cocultured with stromal cells, bispecific antibodies targeting the tumor (carcinoembryonic antigen, or CEA) or fibroblasts (fibroblast activation protein, or FAP) increased T cell infiltration and tumor cell lysis, especially when coupled with T cell activation the interleukin-2 variant (IgG-IL2v) which selectively activates CD8+ cytotoxic, CD 4+ helper T cells, and NK cells [118]. The addition of human SV80 stromal cell line into human A549 and Calu-6 tumor spheroids confined lymphocyte infiltration to the boundaries as compared to spheroids consisting of only cancer cells, but influenced chemokine secretion and increased the portion of activated T cells into the tumor bulk [119]. Nevertheless, spheroid models rarely depict clonal and genetic heterogeneity, as they are often derived from immortalized cancer cell lines. It is thus difficult to evaluate immunotherapeutic efficacies associated with intratumor heterogeneity and tumor mutational burden [120].
Tumor organoids
Organoids are 3-D in vitro tissue cultures embedded in an ECM hydrogel with organ-specific cell types derived from patient tissues, embryonic stem cells, or induced pluripotent stem cells [121, 122]. In their seminal work in 2009, Sato et al. first demonstrated a crypt-villus organoid formed through differentiation and self-organization of intestinal stem cells [121], which has since transformed the in vitro studies of human tissues and organs. Organoids offer unique advantages over spheroids due to the more complex, heterogeneous, and 3-D architectural and physiological functions [123]. Tumor organoid cultures have been established from cancer biopsy samples from various cancer types, which resemble the tumor epithelium they were derived from both phenotypically and genetically [124]. Compared to PDX models, organoids can be more efficiently cultured and expanded [83]. A major advantage of tumor organoids is its ability to capture self-organization of cells and cell-ECM interactions in the 3-D space (Figure 3a,b). Further, tissue-derived organoids have recently been shown to preserve the complex immune profile of the TME, allowing ex vivo evaluation of a variety of anticancer agents [125–129]. Ex vivo analysis has been carried out in multiple functional readouts, including histology staining, high-content imaging after immunostaining, gene expression and sequencing, immune profiling with flow cytometry, and cytokine profiling with immunoassays. Interestingly, patient-derived tumor organoids were used as a platform to obtain tumor-reactive T cells from the same patient’s peripheral blood lymphocyte population (Figure 3c) [130, 131]. Tumor organoids have also been propagated using an air-liquid interface (ALI) method, which successfully modeled infiltrated immune cell species as well as response to immune checkpoint blockade with anti-PD-1 and/or anti-PD-L1 [127]. The Hedgehog pathway inducing PD-L1 expression in organoids of gastric cancer also led to assessment of anti-PD-L1 therapy in combination with CD8+ CTLs [132]. In a similar manner, effector immune cells and CAR-targeting capabilities were assessed against colorectal cancer organoids as a proof-of-concept preclinical model that simultaneously allows studies of CAR-targeting and safety assessment [133]. In a more recent case study, organoids of patient-derived non-small cell lung cancer (NSCLC) were studied ex vivo in parallel to clinical evaluation of chemotherapy (cisplatin and vinorelbine) and immunotherapy (pembrolizumab) in the patient [134]. In addition to immunofluorescence of immunosuppression markers, the authors quantified mutational burden with next-generation sequencing and tumor proliferation rate with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. While the organoids were cultured in Matrigel (derived from a non-human source), the study demonstrated the potential for personalized clinical translation of immunotherapy using organoids [134]. Synthetic designer matrices have also been explored as an alternative to animal-derived matrices, potentially increasing human-relevance in organoid cultures [135]. However, organoids are less common as a tool to evaluate immunotherapy due to its difficulty in logistics (establishing a clinical collaboration and a need for consent to access patient tumor and blood) and costs [134]. Although there is an improvement of architectural complexity from spheroids, organoids still incompletely capture genotypic and phenotypic heterogeneity [136].
Figure 3.
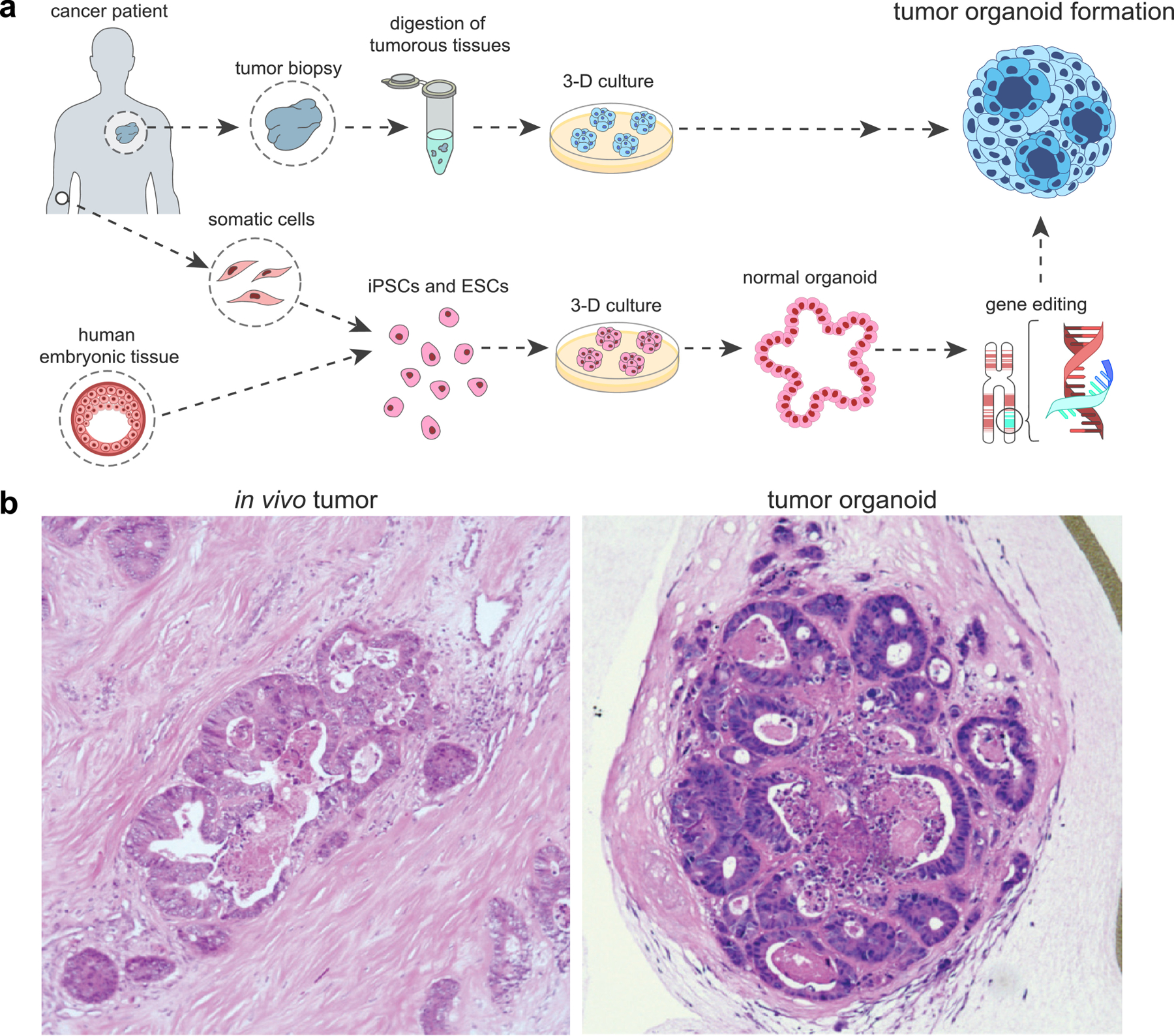
(a) A schematic of tumor organoid formation. Organoids can be formed from digested tumor tissue or through self-assembly of differentiated stem cells. (b) Tumor organoids recapitulate in vivo histology (ref [195]).
It is worth noting that another intermediate culture type, namely the organotypic tumor slice culture, has been developed to examine tumor response to therapeutics. In contrast to the self-assembled tumor organoids, these cultures are derived from sliced primary tumor tissue in varying thicknesses, which can capture the phenotypic and genetic heterogeneity as well as maintain TME conditions in vitro for a short period of time. For instance, organotypic tumor slices closely maintain architecture and cell composition of a primary tumor [137, 138]. Immunophenotyping has been conducted in tissue slices using microscopy techniques based on immunofluorescent staining or immunohistochemistry [139–141], or with multicolor flow cytometry after tissue dissociation [138]. Tracking tumor slices over time has revealed the importance of ECM components in T cell migration [142] and macrophage in the efficacy of anti-PD-1 therapy [41]. In a separate study, organotypic tumor slices also reliably predicted tumor response to immunotherapeutic agents like IFN-γ, an anti-PD-L1 antibody, and an anti-CTLA4 antibody, although cytotoxic effects from pharmacological agents exhibited some variability compared to those observed in organoids [138]. While still unknown, such organotypic cultures offer exciting potential to the assessment of cell-based immunotherapies in a patient-specific manner.
Microphysiological models
Microfabrication and microfluidics are a popular approach to study the interaction of multiple cell types from tissues and organs in the same system. This is often achieved by connecting different cell compartments with microfluidics with physiological flow, or interfacing them through medium/ECM-filled conduits, mechanical structures, or direct physical contact. Such models are also known/referred to as the organ-on-a-chip or microphysiological models [143, 144]. In most microfluidic designs, cancer cells are confined to a compartment connected to a channel that serves to create flow, establish gradients of soluble factors, and/or deliver immunotherapeutic agents (Figure 4a). These models are often fabricated in polydimethylsiloxane (PDMS), a transparent polymer material; the transparency allows real time imaging to study cell migration and interactions [145]. Immune cell response and motility can be studied in microfluidics because of its ability to establish gradients of soluble factors and chemokines, allowing for in vitro observations of cytokine-dependent immune cell recruitment [146]. The ability to optically track immune cells in microfabricated devices is an important advantage in resolving temporal and spatial dynamics. For example, live-cell microscopy based cell tracking revealed a heavy dependence of dendritic cell migration on CXCR4 [147]. Chemotactic gradients were also shown to guide directional NK cell recruitment and penetration into breast cancer through the CXCL12-CXCR4, CCL28-CCR3, and CXCL8-CXCR1/2 axes [114]. Conversely, the effects of immune cells on tumor behavior can also be visualized; a three-compartment microfluidics with pneumatic control over compartment connectivity revealed that myofibroblasts enhanced breast cancer cell migration, while the effect was reversed through TNF-α production from macrophages [148]. Bai et al. showed a contact-dependent mechanism (through ICAM-1 and β2 integrins) of M2a macrophage stimulating lung adenocarcinoma dispersion [149]. Yet another microfluidics device incorporated cancer, PBMCs, endothelial cells, and fibroblasts, to reveal the systemic effects, including enhanced cancer-immune interactions, when treated with an anti-HER2 receptor antibody, trastuzumab [150]. Microfluidic models have also reported on the differential cytotoxic effects of TCR-engineered T cells that have been prepared by viral transduction or mRNA electroporation against tumor compartments (Figure 4b) [151, 152], as well as the effect of anti-PD-1 therapy on TILs infiltration into murine and human tumor fragments cultured ex vivo [153]. Moreover, microfluidics have the added advantage of investigating delivery of immunotherapeutics through channels mimicking the vasculature [143, 154–156]. For instance, macrophages increased the permeability of endothelial-lined fluidics channels, resulting in enhanced tumor intravasation (Figure 4c) [143], which corroborates with in vivo data on M2 macrophages stimulating tumor relapse resulting from revascularization and increased likelihood of metastasis [157]. Vascularized microfluidics have the potential to provide insight into the delivery mechanisms of a variety of immunotherapeutic agents. As such, tumor modeling in microfluidics platforms offer approaches to study underlying mechanisms of tumor immunotherapy that are difficult to examine in animal models [158].
Figure 4.
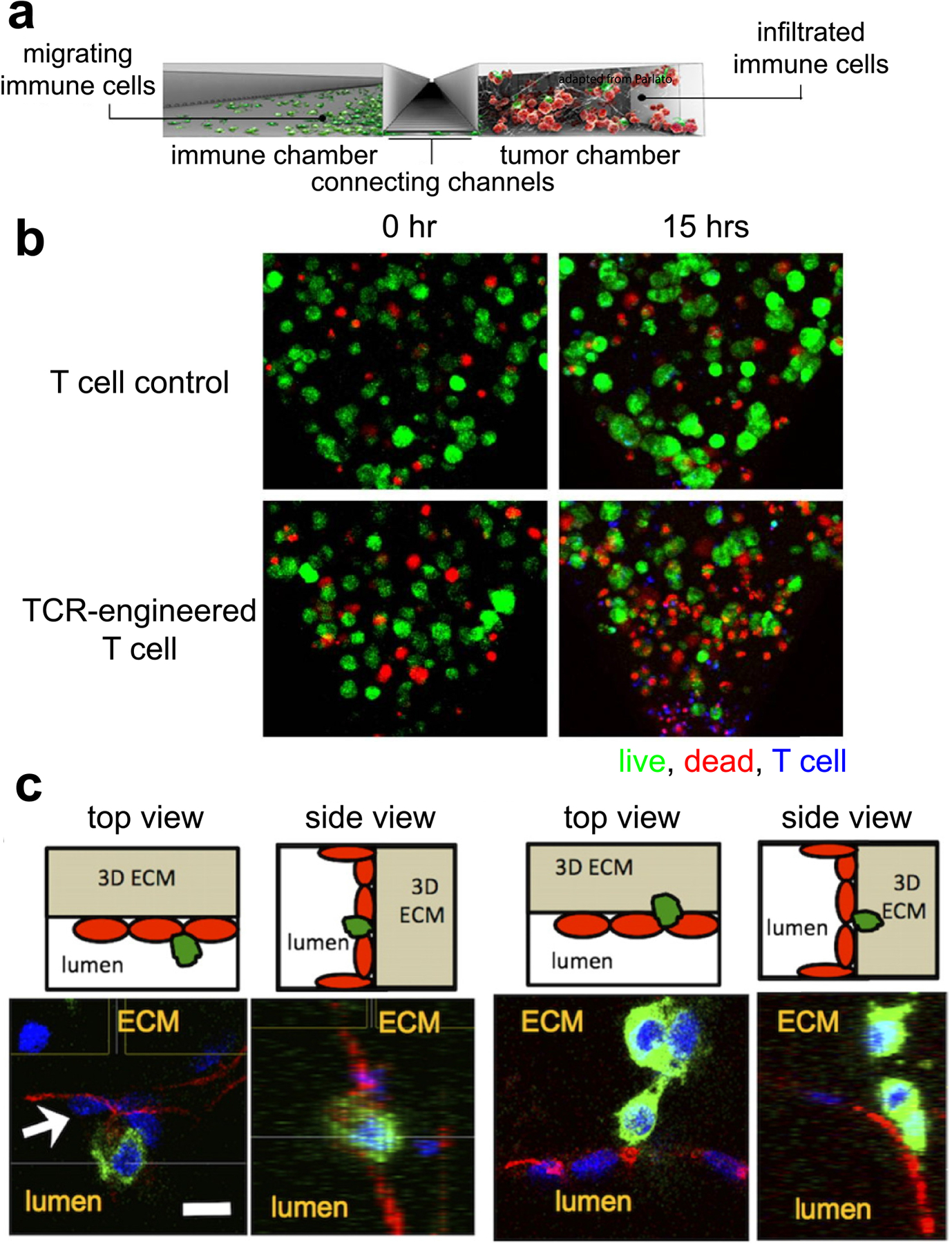
(a) A side section of a typical microfluidics device. A channel connects the immune and tumor chamber, allowing for crosstalk between the two cell types. (b) TCR-engineered T cells (blue) showed tumor (green)-specific cytotoxicity (red) over time, compared to control T cells (ref [151]). (c) Endothelial cells (red) lining the surfaces of a microfluidics channel interacted with tumor cells (green) extravasating (ref [143]).
Towards capturing the increasingly complex TME landscape
Novel platforms that combine components from different engineering strategies provide a more complex microenvironmental landscape. Scaffolding approaches, where cancer cells are embedded in a matrix of biological and/or synthetic materials, is one approach to controlling 3-D cell-ECM architecture. For example, CAR T cell infiltration and cytotoxicity was assessed in lung and breast cancer cells cultured in a 3-D matrix of the basement membrane from decellularized jejunum [159]. Another popular approach is hybrid modeling of spheroids and microfluidics consist of a fluidic chamber that embeds spheroids in a hydrogel material, reminiscent of parenchyma nests in a dense matrix of ECM (Figure 5a) [160, 161]. Organoids derived from mouse and patient tumors were embedded in a microfluidics compartment and successfully demonstrated recapitulation and ability to predict outcome to PD-1 blockade therapy [125, 162]. The same group identified CDK4/6 inhibition and PD-1 blockade as a potentially promising combinatorial approach to augment anti-cancer T cell activation [163]. In a similar manner, the antitumor activity of TCR-engineered T cells were assessed in a microfluidics device that compartmentalized spheroids of liver carcinoma cells embedded in a collagen gel (Figure 5b) [151]. A higher throughput approach of cytotoxicity assay using an injection molded plastic array culture (CACI-IMPACT) device has been explored to assess NK cell-induced cytotoxicity in cancer cells embedded in a 3-D ECM matrix [164]. We have previously engineered a unique hypoxic tumor model that integrated micromilling, micropatterning, and fluidics technology. Using this model, we assessed the infiltration of Her2-targeting CAR T cells, and their induced cytotoxicity against cancer cells in a solid tumor model with a gradient of oxygen and metabolism (Figure 5c) [165]. Bioprinting, or extrusion of cells embedded in bioinks such as hydrogels, ECM proteins, and various polymers, offer precision over the spatial architecture of multiple cell types [166]. Co-extrusion of cancer cell-embedded alginate and CaCl2 solution created a hollow lumen, with geometrical control of the lumen. Macrophages were then introduced into the lumen, where it was observed that cancer-macrophage interactions increased with serpentine lumens compared to straight lumens, implying the impact of geometry-mediated gradients of biochemical factors [167]. These tools evaluate mechanisms of immunotherapy efficacy while considering multiple influential TME factors, potentially improving clinical translatability.
Figure 5.
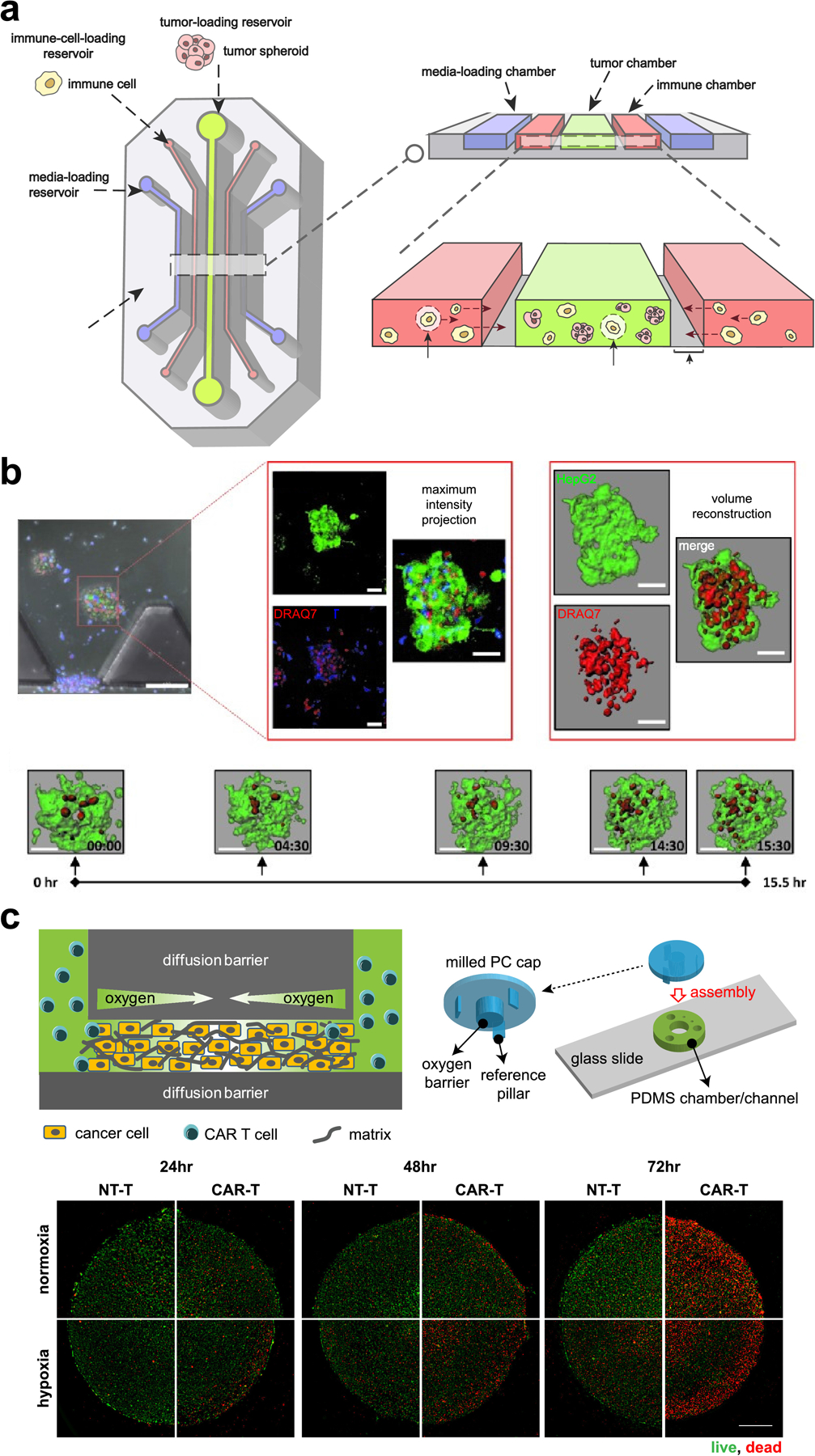
(a) Multicellular structures like spheroids and organoids can be embedded in a hydrogel-filled chamber of a microfluidics device. (b) A microfluidics device with TCR-engineered T cells (blue) and tumor spheroids (green) was imaged over time to assess the degree of T cell-induced cytotoxicity (red) (ref [151]). (c) 3-D tumor micropatterns cultured under a gradient of hypoxia were treated with CAR T cells to resolve spatial and temporal cytotoxicity patterns (ref [165]).
While this review does not focus on computational and mathematical studies of tumor immunology, it is worth mentioning that system biology approaches provide powerful methodologies that elucidate the complex interactions of TME players and predict emergent behaviors that arise from the complex, interconnected network. Mathematical models of tumor-immune microenvironments have contributed meaningful advanced in understanding cancer immunotherapy, especially by enabling predictions based on situations that are difficult to test experimentally or by integrating different time and spatial complexities [168, 169].
Important Considerations
Engineered models, or microphysiological systems, are rapidly emerging as novel tools for biology and medicine [170]. These models allow for controlled recreation of key aspects of the TME for better predictability and translatability of therapeutic regimens. As the field of tissue engineering advances, microphysiological systems have shown great promise in addressing the complexity and multi-dimensionality of biology. For further progress, multiple considerations must be addressed in the design of engineered models for cell-based immunotherapies (Figure 6).
Figure 6.
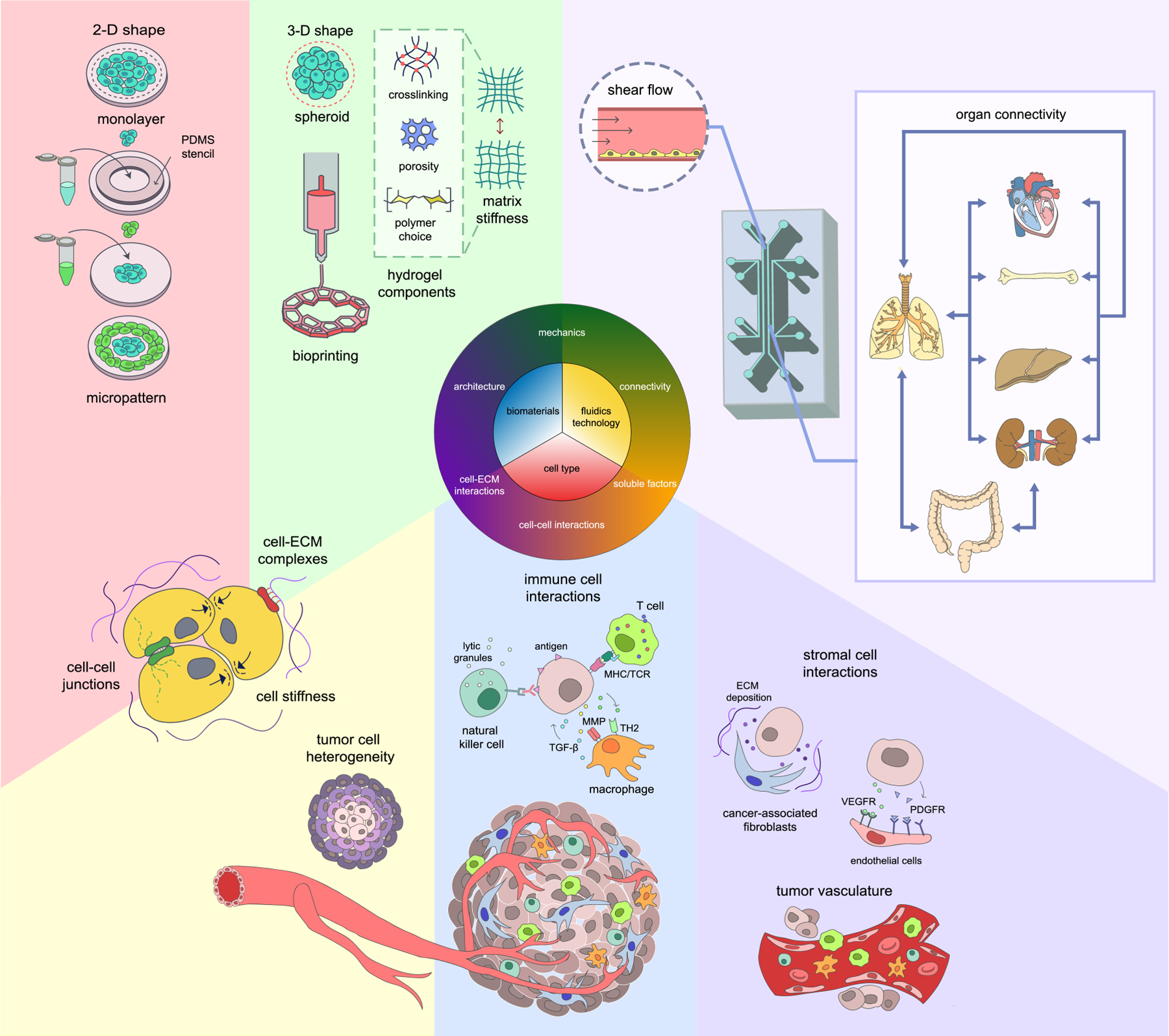
Engineered tumor models assessing tumor immunology and immunotherapy require smart designs. Special considerations should be made in cell-cell interactions of different cell types, connectivity of cell-secreted factors, biomechanics such as fluid pressure and ECM stiffness, and accurate depiction of a 3-D architecture.
ECM in a 3-D arrangement is an important factor that regulates accurate tumor biology [171, 172]. Specifically, different biomaterials have been exploited to modulate the 3-D architecture [173]. Biomaterials range from natural, synthetic, or a hybrid of the two, with various methods of modulating cell-ECM interactions as well as mechanical tunability [174]. Scaffolds and matrices derived from biomaterials provide complex biochemical and mechanical cues, further increasing the relevance and translatability of preclinical tumor models.
Another crucial parameter is the spatial organization of various cell types. Solid tumors comprise of two interdependent compartments: the parenchyma and the stroma. The parenchyma, or neoplastic cells organized into nests, while stroma surround these nests. The spatial architecture of different cell players give rise to complex cell-cell interplay and environmental cues (e.g. mechanical and chemical). Endothelial cells and fibroblastic cells (such as CAFs) can form conduits or physical barriers to immune cell infiltration. Additionally, the incorporation of CAFs and bone marrow stromal cells (BMSCs) in tumor models show spatially and temporally altered cancer phenotype and response to therapy [175–177]. Moreover, systemic integration of engineered tumor models with microphysiological models of other healthy tissues/organs can potentially reveal unexpected life-threatening side effects before therapies are introduced in clinical studies.
Recently, the effects of soluble factors in giving rise to differential therapeutic outcomes is increasingly appreciated. In addition to cytokines and chemokines, soluble factors from external sources include oxygen and nutrients can also play significant roles in immunotherapy [178]. As a hallmark of solid tumors, tumor hypoxia enhance chemoresistance [179] and immunosuppression [57, 180], and correlate with poor patient outcomes [181]. Most hypoxia models induce a uniform degree of hypoxia with enclosed chambers or through chemical induction [182–184]. For a true bioinspired approach, soluble factors must be controlled spatially and temporally, with similar methods of induction (most often driven by a combination of cellular production/consumption and limitation to diffusion through solid tumor bulks). Our group has engineered platforms with a diffusion-limited gradient of oxygen in a 2-D [185] and 3-D tumor models with cell-cell and cell-ECM communications in order to elucidate CAR T cell function against solid tumors [165]. Cellular response against hypoxia is reflected in cell proliferation, metabolic state, and survival [165, 185]. As a result, the metabolic demands of tumor cells exist in a spectrum, creating a complex landscape of nutrients such as glucose, lactate, and amino acids [178]. The pH of the TME also share similarities with lactate production, correlating with the metabolic status of cancer. Fluidics technology enables controlled gradients of soluble factors. It also adds further dimensions of complexity such as shear stress from fluid flow, and allows for connectivity between different tissue compartments in the engineered models [186].
While integrating multiple TME parameters, engineered tumor models must retain a smart design that allows for isolation of individual components for effective readout and accurate assessment. A common approach is to compartmentalize each cell type, with the flexibility to add or remove each compartment. Stackable tissue blocks of tumors, monocytes, ECM, and vasculature, for instance, allowed spatiotemporal investigation of monocyte activation and macrophage-endothelial interactions that resulted in distinct morphological characteristics [187]. Engineered models that allow heterotypic cell co-cultures may predict unwanted side effects in immunotherapies. Future models should also integrate sensors/biosensors to monitor soluble factors such as glucose [188, 189], lactate [190, 191], and pH levels [192], e.g. like microparticle-based oxygen sensors [165, 185, 193]. On the other hand, the entire complexity of the TME cannot, and probably should not, be incorporated in a single model [84, 85]. Just as organ-on-chips aim to recapitulate the basic functional units of functional tissues/organs, engineered tumor models should focus on evaluating the crucial interactions in TME with well-designed controls. Moreover, the design of engineered models should facilitate the observation and quantification of the interested cells, factors, or interactions [194].
Today, various groups are developing state-of-the-art engineered models through rapid iterations and validations for their functional and biological relevance. For translational purposes, however, additional consideration should be taken to standardize critical engineering techniques and analytical tools across these platforms. They should also be simplified to ensure adoption across fields and disciplines, to supplement existing in vitro and in vivo studies and integrate in the pipeline of immunotherapeutic discoveries.
Conclusion and Future Outlook
Despite its promising outlook, cell-based immunotherapy still faces multiple difficulties. Like the organ-on-a-chip systems, engineered in vitro tumor models allow rapid assessment of novel cell-based immunotherapeutic strategies for solid tumors. They are powerful tools capable of delineating therapy efficacy through multidimensional incorporation of TME components. Strategically combining insight from multiple engineered tumor models may also provide more accurate mechanistic insights and therapeutic predictions. At present, it is important to note that most existing engineered models incorporate only a few key TME parameters at best, while tumor progression and immunotherapeutic outcomes depend on a milieu of local and systemic factors that interconnect in a complex manner. By integrating key cellular and molecular components, physical and chemical factors, spatiotemporal biosensing and analysis, as well as next-generation single-cell and omics technologies, future engineered tumor models will have the potential to provide more powerful insights and accurate therapeutic predictions for cell-based immunotherapies and personalized medicine.
statement of significance.
Cell-based immunotherapies have shown great promise in treating hematological malignancies and some epithelial tumors. However, their performance on a broader spectrum of solid tumor types still fall short of expectations. Major obstacles include the inhibitory mechanisms in tumor microenvironments (TME) and the lack of preclinical models that can accurately assess the efficacies and mechanisms of cellular therapies in a (patho-)physiologically relevant manner. In this review, we introduce recent progress in tissue engineering and microphysiological models for more faithful recapitulation of TME for cell-based immunotherapies, and some key considerations for the future development of engineered tumor models. This overview will provide a better understanding on the role of engineered models in accelerating immunotherapeutic discoveries and clinical translations.
Acknowledgements
This work was supported in part by the NIH NCI grant R01CA220012, NIH NIBIB Trailblazer Award R21EB024748, USC Viterbi School of Engineering, and the STOP CANCER Marni Levine Memorial Research Career Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Kruger S, Ilmer M, Kobold S, Cadilha BL, Endres S, Ormanns S, Schuebbe G, Renz BW, D’Haese JG, Schloesser H, Heinemann V, Subklewe M, Boeck S, Werner J, Bergwelt-Baildon M, Advances in cancer immunotherapy 2019 – latest trends, Journal of Experimental & Clinical Cancer Research 38(1) (2019) 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Scheetz L, Park KS, Li Q, Lowenstein PR, Castro MG, Schwendeman A, Moon JJ, Engineering patient-specific cancer immunotherapies, Nature Biomedical Engineering 3(10) (2019) 768–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lesterhuis WJ, Haanen JBAG, Punt CJA, Cancer immunotherapy--revisited, Nature Reviews. Drug Discovery 10(8) (2011) 591–600. [DOI] [PubMed] [Google Scholar]
- [4].Robert C, A decade of immune-checkpoint inhibitors in cancer therapy, Nature Communications 11(1) (2020) 3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD, Recurrent Glioblastoma Treated with Recombinant Poliovirus, New England Journal of Medicine 379(2) (2018) 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shemesh CS, Hsu JC, Hosseini I, Shen B-Q, Rotte A, Twomey P, Girish S, Wu B, Personalized Cancer Vaccines: Clinical Landscape, Challenges, and Opportunities, Molecular Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, C.R.d. Vries, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA, Cancer Regression in Patients After Transfer of Genetically Engineered Lymphocytes, Science 314(5796) (2006) 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mo Z, Du P, Wang G, Wang Y, The Multi-Purpose Tool of Tumor Immunotherapy: Gene-Engineered T Cells, Journal of Cancer 8(9) (2017) 1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF, Understanding the tumor immune microenvironment (TIME) for effective therapy, Nature Medicine 24(5) (2018) 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA: A Cancer Journal for Clinicians 70(1) (2020) 7–30. [DOI] [PubMed] [Google Scholar]
- [11].Hegde PS, Chen DS, Top 10 Challenges in Cancer Immunotherapy, Immunity 52(1) (2020) 17–35. [DOI] [PubMed] [Google Scholar]
- [12].Giraldo NA, Sanchez-Salas R, Peske JD, Vano Y, Becht E, Petitprez F, Validire P, Ingels A, Cathelineau X, Fridman WH, Sautès-Fridman C, The clinical role of the TME in solid cancer, British Journal of Cancer 120(1) (2019) 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rafei H, Mehta RS, Rezvani K, Editorial: Cellular Therapies in Cancer, Front Immunol 10 (2019) 2788–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Decker WK, Silva RF, Sanabria MH, Angelo LS, Guimarães F, Burt BM, Kheradmand F, Paust S, Cancer Immunotherapy: Historical Perspective of a Clinical Revolution and Emerging Preclinical Animal Models, Front Immunol 8 (2017) 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Farkona S, Diamandis EP, Blasutig IM, Cancer immunotherapy: the beginning of the end of cancer?, BMC Medicine 14(1) (2016) 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huber A, Dammeijer F, Aerts JGJV, Vroman H, Current State of Dendritic Cell-Based Immunotherapy: Opportunities for in vitro Antigen Loading of Different DC Subsets?, Front Immunol 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sabado RL, Balan S, Bhardwaj N, Dendritic cell-based immunotherapy, Cell Research 27(1) (2017) 74–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ramanathan P, Ganeshrajah S, Raghanvan RK, Singh SS, Thangarajan R, Development and clinical evaluation of dendritic cell vaccines for HPV related cervical cancer--a feasibility study, Asian Pac J Cancer Prev 15(14) (2014) 5909–16. [DOI] [PubMed] [Google Scholar]
- [19].Sarivalasis A, Boudousquié C, Balint K, Stevenson BJ, Gannon PO, Iancu EM, Rossier L, Martin Lluesma S, Mathevet P, Sempoux C, Coukos G, Dafni U, Harari A, Bassani-Sternberg M, Kandalaft LE, A Phase I/II trial comparing autologous dendritic cell vaccine pulsed either with personalized peptides (PEP-DC) or with tumor lysate (OC-DC) in patients with advanced high-grade ovarian serous carcinoma, Journal of Translational Medicine 17(1) (2019) 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kajihara M, Takakura K, Kanai T, Ito Z, Saito K, Takami S, Shimodaira S, Okamoto M, Ohkusa T, Koido S, Dendritic cell-based cancer immunotherapy for colorectal cancer, World journal of gastroenterology 22(17) (2016) 4275–4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer, New England Journal of Medicine 363(5) (2010) 411–422. [DOI] [PubMed] [Google Scholar]
- [22].Cheever MA, Higano CS, PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine, Clin Cancer Res 17(11) (2011) 3520–6. [DOI] [PubMed] [Google Scholar]
- [23].Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA, Cancer Regression and Autoimmunity in Patients After Clonal Repopulation with Antitumor Lymphocytes, Science 298(5594) (2002) 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE, Use of Tumor-Infiltrating Lymphocytes and Interleukin-2 in the Immunotherapy of Patients with Metastatic Melanoma, New England Journal of Medicine 319(25) (1988) 1676–1680. [DOI] [PubMed] [Google Scholar]
- [25].Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP, Haworth LR, Levy C, Mavroukakis SA, Nichol G, Yellin MJ, Rosenberg SA, Autoimmunity Correlates With Tumor Regression in Patients With Metastatic Melanoma Treated With Anti–Cytotoxic T-Lymphocyte Antigen-4, Journal of Clinical Oncology 23(25) (2005) 6043–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sadelain M, Rivière I, Riddell S, Therapeutic T cell engineering, Nature 545(7655) (2017) 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gross G, Waks T, Eshhar Z, Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity, Proc Natl Acad Sci U S A 86(24) (1989) 10024–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M, Chimeric antigen receptors combining 4–1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication, Mol Ther 18(2) (2010) 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA, Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia, New England Journal of Medicine 371(16) (2014) 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC, CAR T cell immunotherapy for human cancer, Science 359(6382) (2018) 1361–1365. [DOI] [PubMed] [Google Scholar]
- [31].Parker KR, Migliorini D, Perkey E, Yost KE, Bhaduri A, Bagga P, Haris M, Wilson NE, Liu F, Gabunia K, Scholler J, Montine TJ, Bhoj VG, Reddy R, Mohan S, Maillard I, Kriegstein AR, June CH, Chang HY, Posey AD Jr., Satpathy AT, Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies, Cell 183(1) (2020) 126–142e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, Mackall CL, CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy, Nat Med 24(1) (2018) 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Beatty GL, O’Hara MH, Nelson AM, McGarvey M, Torigian DA, Lacey SF, Melenhorst JJ, Levine B, Plesa G, June CH, Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemotherapy refractory metastatic pancreatic cancer, Journal of Clinical Oncology 33(15_suppl) (2015) 3007–3007. [Google Scholar]
- [34].Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P, A Phase I Study on Adoptive Immunotherapy Using Gene-Modified T Cells for Ovarian Cancer, Clinical Cancer Research 12(20) (2006) 6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Newick K, O’Brien S, Moon E, Albelda SM, CAR T Cell Therapy for Solid Tumors, Annual Review of Medicine 68(1) (2017) 139–152. [DOI] [PubMed] [Google Scholar]
- [36].Zhang C, Wang Z, Yang Z, Wang M, Li S, Li Y, Zhang R, Xiong Z, Wei Z, Shen J, Luo Y, Zhang Q, Liu L, Qin H, Liu W, Wu F, Chen W, Pan F, Zhang X, Bie P, Liang H, Pecher G, Qian C, Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA(+) Metastatic Colorectal Cancers, Mol Ther 25(5) (2017) 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gordon S, Plüddemann A, Tissue macrophages: heterogeneity and functions, BMC Biology 15(1) (2017) 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Henze A-T, Mazzone M, The impact of hypoxia on tumor-associated macrophages, The Journal of Clinical Investigation 126(10) (2016) 3672–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Morrissey MA, Williamson AP, Steinbach AM, Roberts EW, Kern N, Headley MB, Vale RD, Chimeric antigen receptors that trigger phagocytosis, eLife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG, CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models, Cancer Research 74(18) (2014) 5057–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J, Ouakrim H, Régnier F, Lupo A, Alifano M, Damotte D, Donnadieu E, Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment, Proceedings of the National Academy of Sciences of the United States of America 115(17) (2018) E4041–E4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB, Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer, Blood 105(8) (2005) 3051–3057. [DOI] [PubMed] [Google Scholar]
- [43].Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, Rigatos G, Papamichail M, Perez SA, A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer, Cancer Immunology, Immunotherapy 59(12) (2010) 1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui C-H, Leung W, NKAML: A Pilot Study to Determine the Safety and Feasibility of Haploidentical Natural Killer Cell Transplantation in Childhood Acute Myeloid Leukemia, Journal of Clinical Oncology 28(6) (2010) 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Curti A, Ruggeri L, D’Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G, Paolini S, Fruet F, Isidori A, Parisi S, Bandini G, Baccarani M, Velardi A, Lemoli RM, Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients, Blood 118(12) (2011) 3273–3279. [DOI] [PubMed] [Google Scholar]
- [46].Mehta RS, Rezvani K, Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer, Front Immunol 9(283) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M, Safety, activity, and immune correlates of anti-PD-1 antibody in cancer, The New England Journal of Medicine 366(26) (2012) 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tucker MD, Zhu J, Marin D, Gupta RT, Gupta S, Berry WR, Ramalingam S, Zhang T, Harrison M, Wu Y, Healy P, Lisi S, George DJ, Armstrong AJ, Pembrolizumab in men with heavily treated metastatic castrate-resistant prostate cancer, Cancer Medicine 8(10) (2019) 4644–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hegde S, Pahne J, Smola-Hess S, Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression, FASEB J 18(12) (2004) 1439–41. [DOI] [PubMed] [Google Scholar]
- [50].Balkwill FR, Capasso M, Hagemann T, The tumor microenvironment at a glance, Journal of Cell Science 125(23) (2012) 5591–5596. [DOI] [PubMed] [Google Scholar]
- [51].Clark WH, Tumour progression and the nature of cancer, Br J Cancer 64(4) (1991) 631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Seip K, Jorgensen K, Haselager MV, Albrecht M, Haugen MH, Egeland EV, Lucarelli P, Engebraaten O, Sauter T, Maelandsmo GM, Prasmickaite L, Stroma-induced phenotypic plasticity offers phenotype-specific targeting to improve melanoma treatment, Cancer Lett 439 (2018) 1–13. [DOI] [PubMed] [Google Scholar]
- [53].Behren A, Thompson EW, Anderson RL, Ferrao PT, Editorial: Cancer Plasticity and the Microenvironment: Implications for Immunity and Therapy Response, Frontiers in Oncology 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E, Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors, J Clin Invest 122(3) (2012) 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ahirwar DK, Nasser MW, Ouseph MM, Elbaz M, Cuitiño MC, Kladney RD, Varikuti S, Kaul K, Satoskar AR, Ramaswamy B, Zhang X, Ostrowski MC, Leone G, Ganju RK, Fibroblast-derived CXCL12 promotes breast cancer metastasis by facilitating tumor cell intravasation, Oncogene 37(32) (2018) 4428–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N, Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade, Proceedings of the National Academy of Sciences 99(19) (2002) 12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S, PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation, Journal of Experimental Medicine 211(5) (2014) 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Inada M, Takita M, Yokoyama S, Watanabe K, Tominari T, Matsumoto C, Hirata M, Maru Y, Maruyama T, Sugimoto Y, Narumiya S, Uematsu S, Akira S, Murphy G, Nagase H, Miyaura C, Direct Melanoma Cell Contact Induces Stromal Cell Autocrine Prostaglandin E2-EP4 Receptor Signaling That Drives Tumor Growth, Angiogenesis, and Metastasis, J Biol Chem 290(50) (2015) 29781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Blay J, White TD, Hoskin DW, The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine, Cancer Res 57(13) (1997) 2602–5. [PubMed] [Google Scholar]
- [60].Hannon GJ, Beach D, p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest, Nature 371(6494) (1994) 257–61. [DOI] [PubMed] [Google Scholar]
- [61].Vander Heiden MG, Cantley LC, Thompson CB, Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation, Science 324(5930) (2009) 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wilson WR, Hay MP, Targeting hypoxia in cancer therapy, Nat Rev Cancer 11(6) (2011) 393–410. [DOI] [PubMed] [Google Scholar]
- [63].Ai M, Budhani P, Sheng J, Balasubramanyam S, Bartkowiak T, Jaiswal AR, Ager CR, Haria DD, Curran MA, Tumor hypoxia drives immune suppression and immunotherapy resistance, Journal for ImmunoTherapy of Cancer 3(2) (2015) 1–1.25648675 [Google Scholar]
- [64].Newick K, Moon E, Albelda SM, Chimeric antigen receptor T-cell therapy for solid tumors, Molecular Therapy - Oncolytics 3 (2016) 16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Berahovich R, Liu X, Zhou H, Tsadik E, Xu S, Golubovskaya V, Wu L, Hypoxia Selectively Impairs CAR-T Cells In Vitro, Cancers 11(5) (2019) 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cui J, Zhang Q, Song Q, Wang H, Dmitriev P, Sun MY, Cao X, Wang Y, Guo L, Indig IH, Rosenblum JS, Ji C, Cao D, Yang K, Gilbert MR, Yao Y, Zhuang Z, Targeting hypoxia downstream signaling protein, CAIX, for CAR T-cell therapy against glioblastoma, Neuro-Oncology 21(11) (2019) 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dagogo-Jack I, Shaw AT, Tumour heterogeneity and resistance to cancer therapies, Nature Reviews. Clinical Oncology 15(2) (2018) 81–94. [DOI] [PubMed] [Google Scholar]
- [68].Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC, In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform, Frontiers in Bioengineering and Biotechnology 4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ, Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology, Proc Natl Acad Sci U S A 95(25) (1998) 14821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Amatangelo MD, Bassi DE, Klein-Szanto AJ, Cukierman E, Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts, Am J Pathol 167(2) (2005) 475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ, Therapeutic cell engineering with surface-conjugated synthetic nanoparticles, Nat Med 16(9) (2010) 1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P, Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo, Cell 49(4) (1987) 465–75. [DOI] [PubMed] [Google Scholar]
- [73].Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G, Klein CA, Systemic spread is an early step in breast cancer, Cancer Cell 13(1) (2008) 58–68. [DOI] [PubMed] [Google Scholar]
- [74].Cheon D-J, Orsulic S, Mouse Models of Cancer, Annual Review of Pathology: Mechanisms of Disease 6(1) (2011) 95–119. [DOI] [PubMed] [Google Scholar]
- [75].Mestas J, Hughes CCW, Of mice and not men: differences between mouse and human immunology, Journal of Immunology (Baltimore, Md.: 1950) 172(5) (2004) 2731–2738. [DOI] [PubMed] [Google Scholar]
- [76].Cassidy JW, Caldas C, Bruna A, Maintaining Tumor Heterogeneity in Patient-Derived Tumor Xenografts, Cancer Research 75(15) (2015) 2963–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jiang Z, Jiang X, Chen S, Lai Y, Wei X, Li B, Lin S, Wang S, Wu Q, Liang Q, Liu Q, Peng M, Yu F, Weng J, Du X, Pei D, Liu P, Yao Y, Xue P, Li P, Anti-GPC3-CAR T Cells Suppress the Growth of Tumor Cells in Patient-Derived Xenografts of Hepatocellular Carcinoma, Front Immunol 7 (2017) 690–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li S, Siriwon N, Zhang X, Yang S, Jin T, He F, Kim YJ, Mac J, Lu Z, Wang S, Han X, Wang P, Enhanced Cancer Immunotherapy by Chimeric Antigen Receptor–Modified T Cells Engineered to Secrete Checkpoint Inhibitors, Clinical Cancer Research 23(22) (2017) 6982–6992. [DOI] [PubMed] [Google Scholar]
- [79].Morgan KM, Riedlinger GM, Rosenfeld J, Ganesan S, Pine SR, Patient-Derived Xenograft Models of Non-Small Cell Lung Cancer and Their Potential Utility in Personalized Medicine, Frontiers in Oncology 7 (2017) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Julien S, Merino-Trigo A, Lacroix L, Pocard M, Goéré D, Mariani P, Landron S, Bigot L, Nemati F, Dartigues P, Weiswald L-B, Lantuas D, Morgand L, Pham E, Gonin P, Dangles-Marie V, Job B, Dessen P, Bruno A, Pierré A, De Thé H, Soliman H, Nunes M, Lardier G, Calvet L, Demers B, Prévost G, Vrignaud P, Roman-Roman S, Duchamp O, Berthet C, Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 18(19) (2012) 5314–5328. [DOI] [PubMed] [Google Scholar]
- [81].Peng S, Creighton CJ, Zhang Y, Sen B, Mazumdar T, Myers JN, Lai SY, Woolfson A, Lorenzi MV, Bell D, Williams MD, Johnson FM, Tumor grafts derived from patients with head and neck squamous carcinoma authentically maintain the molecular and histologic characteristics of human cancers, Journal of Translational Medicine 11 (2013) 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M, Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice, Blood 106(5) (2005) 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bleijs M, Wetering M, Clevers H, Drost J, Xenograft and organoid model systems in cancer research, The EMBO Journal 38(15) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Esch EW, Bahinski A, Huh D, Organs-on-chips at the frontiers of drug discovery, Nature Reviews Drug Discovery 14(4) (2015) 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhang B, Korolj A, Lai BFL, Radisic M, Advances in organ-on-a-chip engineering, Nature Reviews Materials 3(8) (2018) 257. [Google Scholar]
- [86].Ghosh S, Spagnoli GC, Martin I, Ploegert S, Demougin P, Heberer M, Reschner A, Three-dimensional culture of melanoma cells profoundly affects gene expression profile: A high density oligonucleotide array study, Journal of Cellular Physiology 204(2) (2005) 522–531. [DOI] [PubMed] [Google Scholar]
- [87].Liu H, Lin J, Roy K, Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells, Biomaterials 27(36) (2006) 5978–5989. [DOI] [PubMed] [Google Scholar]
- [88].Kim H, Phung Y, Ho M, Changes in Global Gene Expression Associated with 3D Structure of Tumors: An Ex Vivo Matrix-Free Mesothelioma Spheroid Model, PLOS ONE 7(6) (2012) e39556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, Lorenz K, Lee EH, Barcellos-Hoff MH, Petersen OW, Gray JW, Bissell MJ, The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression, Molecular Oncology 1(1) (2007) 84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Pickl M, Ries CH, Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab, Oncogene 28(3) (2009) 461–468. [DOI] [PubMed] [Google Scholar]
- [91].Görlach A, Herter P, Hentschel H, Frosch PJ, Acker H, Effects of nIFN β and rifn γ on growth and morphology of two human melanoma cell lines: Comparison between two-and three-dimensional culture, International Journal of Cancer 56(2) (1994) 249–254. [DOI] [PubMed] [Google Scholar]
- [92].Chignola R, Schenetti A, Andrighetto G, Chiesa E, Foroni R, Sartoris S, Tridente G, Liberati D, Forecasting the growth of multicell tumour spheroids: implications for the dynamic growth of solid tumours, Cell Proliferation 33(4) (2000) 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Yamada KM, Cukierman E, Modeling Tissue Morphogenesis and Cancer in 3D, Cell 130(4) (2007) 601–610. [DOI] [PubMed] [Google Scholar]
- [94].Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ, Engineering tumors with 3D scaffolds, Nature Methods 4(10) (2007) 855–860. [DOI] [PubMed] [Google Scholar]
- [95].Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M, Tumor-derived lactic acid modulates dendritic cell activation and antigen expression, Blood 107(5) (2006) 2013–2021. [DOI] [PubMed] [Google Scholar]
- [96].Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M, Inhibitory effect of tumor cell–derived lactic acid on human T cells, Blood 109(9) (2007) 3812–3819. [DOI] [PubMed] [Google Scholar]
- [97].Feder-Mengus C, Ghosh S, Weber WP, Wyler S, Zajac P, Terracciano L, Oertli D, Heberer M, Martin I, Spagnoli GC, Reschner A, Multiple mechanisms underlie defective recognition of melanoma cells cultured in three-dimensional architectures by antigen-specific cytotoxic T lymphocytes, British Journal of Cancer 96(7) (2007) 1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jang M, Kim SS, Lee J, Cancer cell metabolism: implications for therapeutic targets, Experimental & Molecular Medicine 45(10) (2013) e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Faute MA, Laurent L, Ploton D, Poupon M-F, Jardillier J-C, Bobichon H, Distinctive alterations of invasiveness, drug resistance and cell-cell organization in 3D-cultures of MCF-7, a human breast cancer cell line, and its multidrug resistant variant, Clinical & Experimental Metastasis 19(2) (2002) 161–168. [DOI] [PubMed] [Google Scholar]
- [100].Kuang D-M, Zhao Q, Peng C, Xu J, Zhang J-P, Wu C, Zheng L, Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1, The Journal of Experimental Medicine 206(6) (2009) 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Aras S, Zaidi MR, TAMeless traitors: macrophages in cancer progression and metastasis, British Journal of Cancer 117(11) (2017) 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Sutherland RM, MacDonald HR, Howell RL, Multicellular Spheroids: A New Model Target for In Vitro Studies of Immunity to Solid Tumor Allografts: Brief Communication, JNCI: Journal of the National Cancer Institute 58(6) (1977) 1849–1853. [DOI] [PubMed] [Google Scholar]
- [103].Groebe K, Vaupel P, Evaluation of oxygen diffusion distances in human breast cancer xenografts using tumor-specific in vivo data: Role of various mechanisms in the development of tumor hypoxia, International Journal of Radiation Oncology • Biology • Physics 15(3) (1988) 691–697. [DOI] [PubMed] [Google Scholar]
- [104].Nunes AS, Barros AS, Costa EC, Moreira AF, Correia IJ, 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs, Biotechnology and Bioengineering 116(1) (2019) 206–226. [DOI] [PubMed] [Google Scholar]
- [105].Dangles V, Validire P, Wertheimer M, Richon S, Bovin C, Zeliszewski D, Vallancien G, Bellet D, Impact of human bladder cancer cell architecture on autologous T-lymphocyte activation, International Journal of Cancer 98(1) (2002) 51–56. [DOI] [PubMed] [Google Scholar]
- [106].Busse A, Letsch A, Fusi A, Nonnenmacher A, Stather D, Ochsenreither S, Regenbrecht CRA, Keilholz U, Characterization of small spheres derived from various solid tumor cell lines: are they suitable targets for T cells?, Clinical & Experimental Metastasis 30(6) (2013) 781–791. [DOI] [PubMed] [Google Scholar]
- [107].Erhart F, Weiss T, Klingenbrunner S, Fischhuber K, Reitermaier R, Halfmann A, Blauensteiner B, Lötsch D, Spiegl-kreinecker S, Berger W, Sialana FJ, Lubec G, Felzmann T, Dohnal A, Visus C, Spheroid glioblastoma culture conditions as antigen source for dendritic cell-based immunotherapy: spheroid proteins are survival-relevant targets but can impair immunogenic interferon γ production, Cytotherapy 21(6) (2019) 643–658. [DOI] [PubMed] [Google Scholar]
- [108].Chang C-H, Wang Y, Li R, Rossi DL, Liu D, Rossi EA, Cardillo TM, Goldenberg DM, Combination Therapy with Bispecific Antibodies and PD-1 Blockade Enhances the Antitumor Potency of T Cells, Cancer Research 77(19) (2017) 5384–5394. [DOI] [PubMed] [Google Scholar]
- [109].Dillard P, Köksal H, Inderberg E-M, Wälchli S, A Spheroid Killing Assay by CAR T Cells, JoVE (Journal of Visualized Experiments) (142) (2018) e58785. [DOI] [PubMed] [Google Scholar]
- [110].Courau T, Bonnereau J, Chicoteau J, Bottois H, Remark R, Assante Miranda L, Toubert A, Blery M, Aparicio T, Allez M, Le Bourhis L, Cocultures of human colorectal tumor spheroids with immune cells reveal the therapeutic potential of MICA/B and NKG2A targeting for cancer treatment, Journal for Immunotherapy of Cancer 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].de Jong R, Brouwer M, Miedema F, van Lier RA, Human CD8+ T lymphocytes can be divided into CD45RA+ and CD45RO+ cells with different requirements for activation and differentiation, J Immunol 146(7) (1991) 2088–94. [PubMed] [Google Scholar]
- [112].Hu G, Wang S, Tumor-infiltrating CD45RO + Memory T Lymphocytes Predict Favorable Clinical Outcome in Solid Tumors, Scientific Reports 7(1) (2017) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lanuza PM, Vigueras A, Olivan S, Prats AC, Costas S, Llamazares G, Sanchez-Martinez D, Ayuso JM, Fernandez L, Ochoa I, Pardo J, Activated human primary NK cells efficiently kill colorectal cancer cells in 3D spheroid cultures irrespectively of the level of PD-L1 expression, Oncoimmunology 7(4) (2018) e1395123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ayuso JM, Truttschel R, Gong MM, Humayun M, Virumbrales-Munoz M, Vitek R, Felder M, Gillies SD, Sondel P, Wisinski KB, Patankar M, Beebe DJ, Skala MC, Evaluating natural killer cell cytotoxicity against solid tumors using a microfluidic model, OncoImmunology 8(3) (2019) 1553477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sherman H, Gitschier HJ, Rossi AE, A Novel Three-Dimensional Immune Oncology Model for High-Throughput Testing of Tumoricidal Activity, Front Immunol 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zboralski D, Hoehlig K, Eulberg D, Frömming A, Vater A, Increasing Tumor-Infiltrating T Cells through Inhibition of CXCL12 with NOX-A12 Synergizes with PD-1 Blockade, Cancer Immunology Research 5(11) (2017) 950–956. [DOI] [PubMed] [Google Scholar]
- [117].Lee GY, Kenny PA, Lee EH, Bissell MJ, Three-dimensional culture models of normal and malignant breast epithelial cells, Nat Methods 4(4) (2007) 359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Herter S, Morra L, Schlenker R, Sulcova J, Fahrni L, Waldhauer I, Lehmann S, Reisländer T, Agarkova I, Kelm JM, Klein C, Umana P, Bacac M, A novel three-dimensional heterotypic spheroid model for the assessment of the activity of cancer immunotherapy agents, Cancer Immunology, Immunotherapy 66(1) (2017) 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Koeck S, Kern J, Zwierzina M, Gamerith G, Lorenz E, Sopper S, Zwierzina H, Amann A, The influence of stromal cells and tumor-microenvironment-derived cytokines and chemokines on CD3+CD8+ tumor infiltrating lymphocyte subpopulations, OncoImmunology 6(6) (2017) e1323617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr., Italiano A, Kao S, Piha-Paul SA, Delord J-P, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jin F, Norwood K, Bang Y-J, Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study, The Lancet Oncology 21(10) (2020) 1353–1365. [DOI] [PubMed] [Google Scholar]
- [121].Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H, Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche, Nature 459(7244) (2009) 262–5. [DOI] [PubMed] [Google Scholar]
- [122].Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM, Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro, Nature 470(7332) (2011) 105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Porter RJ, Murray GI, McLean MH, Current concepts in tumour-derived organoids, British Journal of Cancer 123(8) (2020) 1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Drost J, Clevers H, Organoids in cancer research, Nature Reviews Cancer 18(7) (2018) 407–418. [DOI] [PubMed] [Google Scholar]
- [125].Jenkins RW, Aref AR, Lizotte PH, Ivanova E, Stinson S, Zhou CW, Bowden M, Deng J, Liu H, Miao D, He MX, Walker W, Zhang G, Tian T, Cheng C, Wei Z, Palakurthi S, Bittinger M, Vitzthum H, Kim JW, Merlino A, Quinn M, Venkataramani C, Kaplan JA, Portell A, Gokhale PC, Phillips B, Smart A, Rotem A, Jones RE, Keogh L, Anguiano M, Stapleton L, Jia Z, Barzily-Rokni M, Cañadas I, Thai TC, Hammond MR, Vlahos R, Wang ES, Zhang H, Li S, Hanna GJ, Huang W, Hoang MP, Piris A, Eliane J-P, Stemmer-Rachamimov AO, Cameron L, Su M-J, Shah P, Izar B, Thakuria M, LeBoeuf NR, Rabinowits G, Gunda V, Parangi S, Cleary JM, Miller BC, Kitajima S, Thummalapalli R, Miao B, Barbie TU, Sivathanu V, Wong J, Richards WG, Bueno R, Yoon CH, Miret J, Herlyn M, Garraway LA, Allen EMV, Freeman GJ, Kirschmeier PT, Lorch JH, Ott PA, Hodi FS, Flaherty KT, Kamm RD, Boland GM, Wong K-K, Dornan D, Paweletz CP, Barbie DA, Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids, Cancer Discovery 8(2) (2018) 196–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, Rata M, Koh D-M, Tunariu N, Collins D, Hulkki-Wilson S, Ragulan C, Spiteri I, Moorcraft SY, Chau I, Rao S, Watkins D, Fotiadis N, Bali M, Darvish-Damavandi M, Lote H, Eltahir Z, Smyth EC, Begum R, Clarke PA, Hahne JC, Dowsett M, Bono J, Workman P, Sadanandam A, Fassan M, Sansom OJ, Eccles S, Starling N, Braconi C, Sottoriva A, Robinson SP, Cunningham D, Valeri N, Patient-derived organoids model treatment response of metastatic gastrointestinal cancers, Science 359(6378) (2018) 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, Liu IH, Chiou S-H, Salahudeen AA, Smith AR, Deutsch BC, Liao L, Zemek AJ, Zhao F, Karlsson K, Schultz LM, Metzner TJ, Nadauld LD, Tseng Y-Y, Alkhairy S, Oh C, Keskula P, Mendoza-Villanueva D, De La Vega FM, Kunz PL, Liao JC, Leppert JT, Sunwoo JB, Sabatti C, Boehm JS, Hahn WC, Zheng GXY, Davis MM, Kuo CJ, Organoid modeling of the tumor immune microenvironment, Cell 175(7) (2018) 1972–1988.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Tsai S, McOlash L, Palen K, Johnson B, Duris C, Yang Q, Dwinell MB, Hunt B, Evans DB, Gershan J, James MA, Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models, BMC cancer 18(1) (2018) 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kong JCH, Guerra GR, Millen RM, Roth S, Xu H, Neeson PJ, Darcy PK, Kershaw MH, Sampurno S, Malaterre J, Liu DSH, Pham TD, Narasimhan V, Wang M, Huang Y-K, Visvanathan K, McCormick J, Lynch AC, Warrier S, Michael M, Desai J, Murray W, Mitchell C, Ngan S, Phillips WA, Heriot AG, Ramsay RG, Tumor-Infiltrating Lymphocyte Function Predicts Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer, JCO Precision Oncology (2) (2018) 1–15. [DOI] [PubMed] [Google Scholar]
- [130].Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, Haar J, Fanchi LF, Slagter M, Velden DL, Kaing S, Kelderman S, Rooij N, Leerdam ME, Depla A, Smit EF, Hartemink KJ, Groot R, Wolkers MC, Sachs N, Snaebjornsson P, Monkhorst K, Haanen J, Clevers H, Schumacher TN, Voest EE, Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids, Cell 174(6) (2018) 1586–1598.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cattaneo CM, Dijkstra KK, Fanchi LF, Kelderman S, Kaing S, Rooij N, Brink S, Schumacher TN, Voest EE, Tumor organoid–T-cell coculture systems, Nature Protocols 15(1) (2020) 15–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Chakrabarti J, Holokai L, Syu L, Steele NG, Chang J, Wang J, Ahmed S, Dlugosz A, Zavros Y, Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer, Oncotarget 9(100) (2018) 37439–37457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Schnalzger TE, de Groot MHP, Zhang C, Mosa MH, Michels BE, Röder J, Darvishi T, Wels WS, Farin HF, 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids, The EMBO Journal 38(12) (2019) e100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Liello RD, Ciaramella V, Barra G, Venditti M, Corte CMD, Papaccio F, Sparano F, Viscardi G, Iacovino ML, Minucci S, Fasano M, Ciardiello F, Morgillo F, Ex vivo lung cancer spheroids resemble treatment response of a patient with NSCLC to chemotherapy and immunotherapy: case report and translational study, ESMO Open 4(4) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordonez-Moran P, Clevers H, Lutolf MP, Designer matrices for intestinal stem cell and organoid culture, Nature 539(7630) (2016) 560–564. [DOI] [PubMed] [Google Scholar]
- [136].Arnadottir SS, Jeppesen M, Lamy P, Bramsen JB, Nordentoft I, Knudsen M, Vang S, Madsen MR, Thastrup O, Thastrup J, L.A. C, Characterization of genetic intratumor heterogeneity in colorectal cancer and matching patient-derived spheroid cultures, Mol Oncol 12(1) (2018) 132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Vaira V, Fedele G, Pyne S, Fasoli E, Zadra G, Bailey D, Snyder E, Faversani A, Coggi G, Flavin R, Bosari S, Loda M, Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors, Proceedings of the National Academy of Sciences 107(18) (2010) 8352–8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sivakumar R, Chan M, Shin JS, Nishida-Aoki N, Kenerson HL, Elemento O, Beltran H, Yeung R, Gujral TS, Organotypic tumor slice cultures provide a versatile platform for immuno-oncology and drug discovery, Oncoimmunology 8(12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Jiang X, Seo YD, Chang JH, Coveler A, Nigjeh EN, Pan S, Jalikis F, Yeung RS, Crispe IN, Pillarisetty VG, Long-lived pancreatic ductal adenocarcinoma slice cultures enable precise study of the immune microenvironment, Oncoimmunology 6(7) (2017) e1333210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Peranzoni E, Bougherara H, Barrin S, Mansuet-Lupo A, Alifano M, Damotte D, Donnadieu E, Ex Vivo Imaging of Resident CD8 T Lymphocytes in Human Lung Tumor Slices Using Confocal Microscopy, Journal of Visualized Experiments : JoVE (130) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Finnberg NK, Gokare P, Lev A, Grivennikov SI, MacFarlane AW, Campbell KS, Winters RM, Kaputa K, Farma JM, Abbas AE-S, Grasso L, Nicolaides NC, El-Deiry WS, Application of 3D tumoroid systems to define immune and cytotoxic therapeutic responses based on tumoroid and tissue slice culture molecular signatures, Oncotarget 8(40) (2017) 66747–66757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Bougherara H, Mansuet-Lupo A, Alifano M, Ngô C, Damotte D, Le Frère-Belda M-A, Donnadieu E, Peranzoni E, Real-Time Imaging of Resident T Cells in Human Lung and Ovarian Carcinomas Reveals How Different Tumor Microenvironments Control T Lymphocyte Migration, Front Immunol 6 (2015) 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD, Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function, Proceedings of the National Academy of Sciences of the United States of America 109(34) (2012) 13515–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Agliari E, Biselli E, Ninno AD, Schiavoni G, Gabriele L, Gerardino A, Mattei F, Barra A, Businaro L, Cancer-driven dynamics of immune cells in a microfluidic environment, Scientific Reports 4 (2014) 6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Kim C, Kasuya J, Jeon J, Chung S, Kamm RD, A Quantitative Microfluidic Angiogenesis Screen for Studying Anti-Angiogenic Therapeutic Assay, Lab on a chip 15(1) (2015) 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Nandagopal S, Wu D, Lin F, Combinatorial Guidance by CCR7 Ligands for T Lymphocytes Migration in Co-Existing Chemokine Fields, PLOS ONE 6(3) (2011) e18183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Parlato S, Ninno AD, Molfetta R, Toschi E, Salerno D, Mencattini A, Romagnoli G, Fragale A, Roccazzello L, Buoncervello M, Canini I, Bentivegna E, Falchi M, Bertani FR, Gerardino A, Martinelli E, Natale C, Paolini R, Businaro L, Gabriele L, 3D Microfluidic model for evaluating immunotherapy efficacy by tracking dendritic cell behaviour toward tumor cells, Scientific Reports 7(1) (2017) 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Hsu T-H, Kao Y-L, Lin W-L, Xiao J-L, Kuo P-L, Wu C-W, Liao W-Y, Lee C-H, The migration speed of cancer cells influenced by macrophages and myofibroblasts co-cultured in a microfluidic chip, Integrative Biology: Quantitative Biosciences from Nano to Macro 4(2) (2012) 177–182. [DOI] [PubMed] [Google Scholar]
- [149].Bai J, Adriani G, Dang T-M, Tu T-Y, Leong Penny H-X, Wong S-C, Kamm RD, Thiery J-P, Contact-dependent carcinoma aggregate dispersion by M2a macrophages via ICAM-1 and β2 integrin interactions, Oncotarget 6(28) (2015) 25295–25307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Nguyen M, De Ninno A, Mencattini A, Mermet-Meillon F, Fornabaio G, Evans SS, Cossutta M, Khira Y, Han W, Sirven P, Pelon F, Di Giuseppe D, Bertani FR, Gerardino A, Yamada A, Descroix S, Soumelis V, Mechta-Grigoriou F, Zalcman G, Camonis J, Martinelli E, Businaro L, Parrini MC, Dissecting Effects of Anti-cancer Drugs and Cancer-Associated Fibroblasts by On-Chip Reconstitution of Immunocompetent Tumor Microenvironments, Cell Reports 25(13) (2018) 3884–3893.e3. [DOI] [PubMed] [Google Scholar]
- [151].Pavesi A, Tan AT, Koh S, Chia A, Colombo M, Antonecchia E, Miccolis C, Ceccarello E, Adriani G, Raimondi MT, Kamm RD, Bertoletti A, A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors, JCI Insight 2(12) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lee SWL, Adriani G, Ceccarello E, Pavesi A, Tan AT, Bertoletti A, Kamm RD, Wong SC, Characterizing the Role of Monocytes in T Cell Cancer Immunotherapy Using a 3D Microfluidic Model, Front Immunol 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Moore N, Doty D, Zielstorff M, Kariv I, Moy LY, Gimbel A, Chevillet JR, Lowry N, Santos J, Mott V, Kratchman L, Lau T, Addona G, Chen H, Borenstein JT, A multiplexed microfluidic system for evaluation of dynamics of immune-tumor interactions, Lab on a Chip 18(13) (2018) 1844–1858. [DOI] [PubMed] [Google Scholar]
- [154].Myers DR, Sakurai Y, Tran R, Ahn B, Hardy ET, Mannino R, Kita A, Tsai M, Lam WA, Endothelialized Microfluidics for Studying Microvascular Interactions in Hematologic Diseases, JoVE (Journal of Visualized Experiments) (64) (2012) e3958–e3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bischel LL, Young EWK, Mader BR, Beebe DJ, Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels, Biomaterials 34(5) (2013) 1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Nagaraju S, Truong D, Mouneimne G, Nikkhah M, Microfluidic Tumor–Vascular Model to Study Breast Cancer Cell Invasion and Intravasation, Advanced Healthcare Materials 0(0) (2018) 1701257. [DOI] [PubMed] [Google Scholar]
- [157].Hughes R, Qian B-Z, Rowan C, Muthana M, Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons M, Gonzalez-Angulo AM, Joyce JA, De Palma M, Pollard JW, Lewis CE, Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy, Cancer research 75(17) (2015) 3479–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Adriani G, Pavesi A, Tan AT, Bertoletti A, Thiery JP, Kamm RD, Microfluidic models for adoptive cell-mediated cancer immunotherapies, Drug Discovery Today 21(9) (2016) 1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wallstabe L, Göttlich C, Nelke LC, Kühnemundt J, Schwarz T, Nerreter T, Einsele H, Walles H, Dandekar G, Nietzer SL, Hudecek M, ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models, JCI Insight 4(18) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sun D, Lu J, Chen Z, Yu Y, Li Y, A novel three-dimensional microfluidic platform for on chip multicellular tumor spheroid formation and culture, Microfluidics and Nanofluidics 17(5) (2014) 831–842. [Google Scholar]
- [161].Patra B, Peng C-C, Liao W-H, Lee C-H, Tung Y-C, Drug testing and flow cytometry analysis on a large number of uniform sized tumor spheroids using a microfluidic device, Scientific Reports 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Aref AR, Campisi M, Ivanova E, Portell A, Larios D, Piel BP, Mathur N, Zhou C, Coakley RV, Bartels A, Bowden M, Herbert Z, Hill S, Gilhooley S, Carter J, Cañadas I, Thai TC, Kitajima S, Chiono V, Paweletz CP, Barbie DA, Kamm RD, Jenkins RW, 3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade, Lab on a Chip 18(20) (2018) 3129–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, Ivanova E, Paweletz CP, Bowden M, Zhou CW, Herter-Sprie GS, Sorrentino JA, Bisi JE, Lizotte PH, Merlino AA, Quinn MM, Bufe LE, Yang A, Zhang Y, Zhang H, Gao P, Chen T, Cavanaugh ME, Rode AJ, Haines E, Roberts PJ, Strum JC, Richards WG, Lorch JH, Parangi S, Gunda V, Boland GM, Bueno R, Palakurthi S, Freeman GJ, Ritz J, Haining WN, Sharpless NE, Arthanari H, Shapiro GI, Barbie DA, Gray NS, Wong K-K, CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation, Cancer Discovery 8(2) (2018) 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Park D, Son K, Hwang Y, Ko J, Lee Y, Doh J, Jeon NL, High-Throughput Microfluidic 3D Cytotoxicity Assay for Cancer Immunotherapy (CACI-IMPACT Platform), Front Immunol 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Ando Y, Siegler EL, Ta HP, Cinay GE, Zhou H, Gorrell KA, Au H, Jarvis BM, Wang P, Shen K, Evaluating CAR-T Cell Therapy in a Hypoxic 3D Tumor Model, Advanced Healthcare Materials 0(0) (2019) 1900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Kim HN, Habbit NL, Su C-Y, Choi N, Ahn EH, Lipke EA, Kim D-H, Microphysiological Systems as Enabling Tools for Modeling Complexity in the Tumor Microenvironment and Accelerating Cancer Drug Development, Advanced Functional Materials 29(22) (2019) 1807553. [Google Scholar]
- [167].Grolman JM, Zhang D, Smith AM, Moore JS, Kilian KA, Rapid 3D Extrusion of Synthetic Tumor Microenvironments, Advanced Materials (Deerfield Beach, Fla.) 27(37) (2015) 5512–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Konstorum A, Vella AT, Adler AJ, Laubenbacher RC, Addressing current challenges in cancer immunotherapy with mathematical and computational modelling, Journal of the Royal Society, Interface 14(131) (2017) 20170150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Mahlbacher GE, Reihmer KC, Frieboes HB, Mathematical modeling of tumor-immune cell interactions, J. Theor. Biol 469 (2019) 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Wikswo JP, The relevance and potential roles of microphysiological systems in biology and medicine, Experimental biology and medicine (Maywood, N.J.) 239(9) (2014) 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Riedl A, Schlederer M, Pudelko K, Stadler M, Walter S, Unterleuthner D, Unger C, Kramer N, Hengstschläger M, Kenner L, Pfeiffer D, Krupitza G, Dolznig H, Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT–mTOR–S6K signaling and drug responses, Journal of Cell Science 130(1) (2017) 203–218. [DOI] [PubMed] [Google Scholar]
- [172].Han K, Pierce SE, Li A, Spees K, Anderson GR, Seoane JA, Lo Y-H, Dubreuil M, Olivas M, Kamber RA, Wainberg M, Kostyrko K, Kelly MR, Yousefi M, Simpkins SW, Yao D, Lee K, Kuo CJ, Jackson PK, Sweet-Cordero A, Kundaje A, Gentles AJ, Curtis C, Winslow MM, Bassik MC, CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities, Nature 580(7801) (2020) 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Gu L, Mooney DJ, Biomaterials and Emerging Anticancer Therapeutics: Engineering the Microenvironment, Nature reviews. Cancer 16(1) (2016) 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].El-Sherbiny IM, Yacoub MH, Hydrogel scaffolds for tissue engineering: Progress and challenges, Global Cardiology Science & Practice 2013(3) (2013) 316–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Shen K, Luk S, Hicks DF, Elman JS, Bohr S, Iwamoto Y, Murray R, Pena K, Wang F, Seker E, Weissleder R, Yarmush ML, Toner M, Sgroi D, Parekkadan B, Resolving cancer–stroma interfacial signalling and interventions with micropatterned tumour–stromal assays, Nature Communications 5 (2014) 5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Shen K, Luk S, Elman J, Murray R, Mukundan S, Parekkadan B, Suicide Gene-Engineered Stromal Cells Reveal a Dynamic Regulation of Cancer Metastasis, Scientific Reports 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Begum HM, Ta HP, Zhou H, Ando Y, Kang D, Nemes K, Mariano CF, Hao J, Yu M, Shen K, Spatial Regulation of Mitochondrial Heterogeneity by Stromal Confinement in Micropatterned Tumor Models, Scientific Reports 9(1) (2019) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Anastasiou D, Tumour microenvironment factors shaping the cancer metabolism landscape, British Journal of Cancer 116(3) (2017) 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Eales KL, Hollinshead KER, Tennant DA, Hypoxia and metabolic adaptation of cancer cells, Oncogenesis 5(1) (2016) e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Barsoum IB, Smallwood CA, Siemens DR, Graham CH, A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells, Cancer Research 74(3) (2014) 665–674. [DOI] [PubMed] [Google Scholar]
- [181].Harris AL, Hypoxia — a key regulatory factor in tumour growth, Nature Reviews Cancer 2(1) (2002) 38–47. [DOI] [PubMed] [Google Scholar]
- [182].Wu D, Yotnda P, Induction and Testing of Hypoxia in Cell Culture, JoVE (Journal of Visualized Experiments) (54) (2011) e2899–e2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Wang R, Jin F, Zhong H, A novel experimental hypoxia chamber for cell culture, American Journal of Cancer Research 4(1) (2014) 53–60. [PMC free article] [PubMed] [Google Scholar]
- [184].Bakmiwewa SM, Heng B, Guillemin GJ, Ball HJ, Hunt NH, An effective, low-cost method for achieving and maintaining hypoxia during cell culture studies, BioTechniques 59(4) (2015) 223–224, 226, 228–229. [DOI] [PubMed] [Google Scholar]
- [185].Ando Y, Ta HP, Yen DP, Lee S-S, Raola S, Shen K, A Microdevice Platform Recapitulating Hypoxic Tumor Microenvironments, Scientific Reports 7(1) (2017) 15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Abaci HE, Shuler ML, Human-on-a-chip design strategies and principles for physiologically based pharmocokinetics/pharmacodynamics modeling, Integrative biology : quantitative biosciences from nano to macro 7(4) (2015) 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Yu J, Berthier E, Craig A, Groot TE, Sparks S, Ingram PN, Jarrard DF, Huang W, Beebe DJ, Theberge AB, Reconfigurable open microfluidics for studying the spatiotemporal dynamics of paracrine signalling, Nature Biomedical Engineering 3(10) (2019) 830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Lin Y, Lu F, Tu Y, Ren Z, Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles, Nano Letters 4(2) (2004) 191–195. [Google Scholar]
- [189].Lin Z, Cherng-Wen T, Roy P, Trau D, In-situ measurement of cellular microenvironments in a microfluidic device, Lab on a Chip 9(2) (2009) 257–262. [DOI] [PubMed] [Google Scholar]
- [190].Ges IA, Baudenbacher F, Enzyme-coated microelectrodes to monitor lactate production in a nanoliter microfluidic cell culture device, Biosensors & bioelectronics 26(2) (2010) 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Biswas A, Bornhoeft LR, Banerjee S, You Y-H, McShane MJ, Composite Hydrogels Containing Bioactive Microreactors for Optical Enzymatic Lactate Sensing, ACS Sensors 2(11) (2017) 1584–1588. [DOI] [PubMed] [Google Scholar]
- [192].Wu M-H, Lin J-L, Wang J, Cui Z, Cui Z, Development of high throughput optical sensor array for on-line pH monitoring in micro-scale cell culture environment, Biomedical Microdevices 11(1) (2009) 265–273. [DOI] [PubMed] [Google Scholar]
- [193].Acosta MA, Ymele-Leki P, Kostov YV, Leach JB, Fluorescent microparticles for sensing cell microenvironment oxygen levels within 3D scaffolds, Biomaterials 30(17) (2009) 3068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Villasante A, Vunjak-Novakovic G, Tissue-engineered models of human tumors for cancer research, Expert opinion on drug discovery 10(3) (2015) 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Neal JT, Kuo CJ, Organoids as Models for Neoplastic Transformation, Annu Rev Pathol 11 (2016) 199–220. [DOI] [PubMed] [Google Scholar]


