Abstract
Background.
Persistent inflammation in HIV infection is associated with elevated cardiovascular disease risk, even with viral suppression. Identification of novel surrogate biomarkers can enhance cardiovascular disease risk stratification and suggest novel therapies. We investigated the potential of IL-32, a proinflammatory multi-isoform cytokine, as a biomarker for subclinical carotid artery atherosclerosis in virologically-suppressed women living with HIV (WLWH).
Methods and Results.
Nested within the Women’s Interagency HIV Study (WIHS), we conducted a cross-sectional comparison of IL-32 between 399 WLWH and 100 women without HIV, followed by a case-control study of 72 WLWH (36 carotid artery plaque cases vs. 36 age-matched controls without plaque). Plasma IL-32 protein was measured by ELISA, and mRNA of IL-32 isoforms (IL-32α, β, γ, D, ε, θ) was quantified by RT-PCR from peripheral blood mononuclear cells (PBMCs). Plasma IL-32 protein levels were higher in WLWH compared to women without HIV (p=0.02). Among WLWH, while plasma IL-32 levels did not differ significantly between plaque cases and controls, expression of IL-32 isoforms α, β and ε mRNA was significantly higher in PBMCs from cases (p=0.01, p=0.005, and p=0.018, respectively). Upregulation of IL-32β and IL-32ε among WLWH with carotid artery plaque persisted after adjustment for age, race/ethnicity, smoking, systolic blood pressure, body mass index, and history of hepatitis C virus (p=0.04 and p=0.045); the adjusted association for IL-32α was marginally significant (p=0.07).
Conclusion.
IL-32 isoforms should be studied further as potential cardiovascular disease biomarkers. This is of particular interest in WLWH by virtue of altered IL-32 levels in this population.
Keywords: HIV, cardiovascular disease, atherosclerosis, carotid artery, IL-32
Introduction
Unresolved low-grade inflammation in HIV infection, even with antiretroviral therapy (ART), contributes to cardiovascular disease (CVD) 1. Multiple factors are believed to contribute to this chronic inflammation, including persistent immune stimulation fuelled by residual HIV viremia and possibly antigens from other co-infections such as cytomegalovirus (CMV) or hepatitis C virus (HCV), imbalance of intestinal microbiota composition towards pathogenic bacteria (i.e., gut dysbiosis), and bacterial and fungal translocation as a result of compromised gut mucosal barrier integrity 2–10. These mediators may sustain overt inflammation by upregulating a large number of inflammatory factors, including TNF-α, IL-1β, VCAM-1, hsCRP, D-dimer, sCD14, sCD163, and IL-6 11–15. Some of these factors, such as hsCRP, are used as biomarkers to enhance risk stratification of CVD and associated mortality 16. However, the role of hsCRP as an inflammatory marker is limited to prognosis and risk prediction since it does not seem to represent a causal factor for CVD 17, and moreover the association of hsCRP with atherosclerosis is blunted in the setting of HIV infection 18. Other factors, including IL-6, have the potential to be used as both biomarkers and therapeutic targets 19. However, clinical trials with tocilizumab, a monoclonal antibody targeting the IL-6 receptor, have reported considerable side effects such as increased total and low-density cholesterol levels 20. Thus, the identification of novel inflammatory factors, especially candidates upstream of IL-6 signaling, may provide better alternatives for CVD risk stratification as well as lead to potential treatment targets. In this regard, we have recently reported that IL-32, a proinflammatory cytokine that is expressed in multiple isoforms (α, β, γ, D, ε, θ, ζ, η, and small/sm) 21–23, is upregulated in HIV infection and its expression is not normalized with ART 24. We have also demonstrated that IL-32 induces a strong inflammatory response in T-cells by enhancing the production of IL-6, TNF-α and IFNγ, and further induces HIV transcription from latently infected cells 24,25. These observations suggest that IL-32 may contribute to the persistent immune activation and inflammation that are the major etiologic mediators of atherosclerosis 26 and may represent a biomarker of future CVD. Here, we investigated this hypothesis by studying expression of IL-32 and its isoforms in a case-control study of ART-treated, virally suppressed women living with HIV (WLWH), comparing those with and without subclinical carotid artery atherosclerosis.
Methods
Study design and population
We conducted a study nested within the Women’s Interagency HIV Study (WIHS), a long-standing prospective multi-center cohort of WLWH and women at risk for HIV infection from the same communities 27,28. The WIHS is now part of the MACS/WIHS Combined Cohort Study29. To study expression of total IL-32 protein by HIV serostatus, we randomly selected 499 participants (399 WLWH and 100 without HIV) with stored plasma and peripheral blood mononuclear cells (PBMCs) collected during WIHS Visit 34 (April to September 2011). WLWH selected for this study were required to be taking ART and virally suppressed with <100 HIV RNA copies/mL at the time of the visit (COBAS TaqMan v2.0 HIV-1, Roche). Despite good adherence to ART, viral blips <100 copies/mL are relatively common among people with HIV without being associated with clinical variables 30.
Next, we conducted a case-control study among WLWH examining expression of IL-32 isoforms based on presence or absence of subclinical carotid artery atherosclerosis. This study was nested within a vascular substudy of the WIHS. Briefly, starting in 2004, WIHS participants were invited to undergo high-resolution B-mode ultrasound every two to three years to image the carotid artery 31,32. Among participants in the fourth wave (2010–2012) of the vascular substudy, we selected 36 WLWH with subclinical atherosclerosis (cases), and matched them by age with 36 WLWH without subclinical atherosclerosis (controls). WLWH were also required to be taking ART and virologically suppressed (<100 copies/mL) at the time of the most recent scan, as well as free of coronary heart disease (self-report of angina, myocardial infarction, or coronary revascularization) at the baseline vascular substudy visit.
Case definition
As part of the vascular substudy, six locations in the right carotid artery were imaged: the near and far walls of the common carotid artery, carotid bifurcation, and internal carotid artery 31,32. A standardized protocol was used at all centers, 33 and measurements were obtained at a centralized reading center (University of Southern California). Cases of subclinical atherosclerosis had plaque, defined as a focal wall protuberance into the lumen of the artery with a minimal diameter of 1.5 mm at its maximum point, measured in at least one of the six aforementioned artery locations. Controls were found to not have plaque at any of the imaged locations. While mean intima-media thickness (IMT) was also assessed from standardized ultrasound images by automated computerized edge detection at the far walls of the common carotid artery and the carotid bifurcation, our previous studies have not found these measurements to be positively associated with HIV serostatus 31, and therefore we did not design our study to examine IL-32 isoforms in relation to mean IMT.
IL-32 measures
Total IL-32 protein was quantified from stored plasma using the human IL-32 ELISA kit (R&D System, Cat #DY3040–05). Total RNA was isolated from cryopreserved human PBMCs using the RNeasy plus mini kit from Qiagen as per the manufacturer’s protocol (Catalog #74134). Quantification of IL-32 isoforms (α, β, γ, D, ε and θ) was performed using One-step SYBR Green reverse transcription quantitative PCR (RT-qPCR) performed on LightCycler 480II machine (Roche) with QIAGEN QuantiTect (Catalog #204243). Relative expression of IL-32 RNA was normalized to the housekeeping gene β-glucuronidase. Primer sets for the different IL-32 isoforms and β-glucuronidase, conditions for the quantitative PCR and analysis were done as recently reported24.
Potential confounders
All comparisons in the case-control study accounted for age as part of the matched design. We considered the following variables as additional potential confounders: race/ethnicity; education; current crack/cocaine or alcohol use; history of injection drug use; smoking history; body mass index (BMI); systolic blood pressure; total, LDL, and HDL-cholesterol levels (Quest standard lipid panel); history of diabetes mellitus; menopause status; and current use of medications for hypertension, hyperlipidemia, or diabetes. History of hepatitis C virus (HCV) infection was defined by presence of antibody to HCV by second-generation or third-generation ELISA (Ortho-Diagnostic Systems) or presence of HCV-RNA by HCV-branched DNA (Quantiplex 2.0, Bayer-Versant Diagnostics) and RT-PCR (COBAS Amplicor HCV detection kit, Roche). We also considered current and nadir CD4+ T-cell count, history of clinical AIDS, CD4:CD8 ratio, and hsCRP levels.
Statistical analysis
Data were analyzed using GraphPad Prism 8 (GraphPad Software, San Diego, CA) and SAS 9.4 (SAS Institute, Cary, NC). Differences by HIV serostatus or case status were assessed by the Mann-Whitney U test or chi-square test, as appropriate. Correlations were assessed with the non-parametric Spearman test. Multivariable logistic regression analyses controlled for potential confounders, including those significantly associated with carotid artery plaque in bivariate analyses (P<0.10), as well as age and smoking status based on a priori knowledge 34. Levels of IL-32 isoforms were log-transformed when not normally distributed, and scaled to Z-score prior to model fitting. We used alpha<0.05 to determine statistical significance.
Ethical considerations
Participants provided written informed consent, and all analyses were performed in accordance with the guidelines and regulations approved by the Institutional Review Boards (IRBs) of the “Centre Hospitalier de l’Université de Montréal” Research Center (approval #CE.11.063) and each participating WIHS center.
Results
Total IL-32 protein was quantified in plasma from n=399 ART-treated, virologically suppressed WLWH and n=100 women without HIV. While characteristics between WLWH and women without HIV were generally similar, WLWH were slightly older (median age 50 vs. 47.5), had higher total cholesterol levels (median 185.5 vs. 177.5 mg/dL), lower systolic blood pressure (median 119 vs. 124 mm Hg), lower CD4 counts (median 598 vs. 989 cells/mm3), and had fewer behavioral risk factors like smoking history (66% vs. 80%), current crack/cocaine use (4% vs. 10%), and current alcohol use (33% vs. 59%). Plasma samples were blinded to presence of subclinical atherosclerosis to confirm the upregulation of IL-32 in HIV infection as we previously reported 24, replicated here in an independent sample.
As shown in Figure 1A, plasma IL-32 protein levels were significantly higher in WLWH compared to women without HIV (p=0.02). Among WLWH, IL-32 showed a positive correlation with participant age (r=0.13, p=0.0084, Figure 1B) and, similar to our previous findings 25, were negatively correlated with CD4 count (r=−0.1, p=0.04, Figure 1C), albeit weakly. After restricting the analysis to the 36 atherosclerosis cases and 36 age-matched controls (Table 1), total IL-32 plasma protein did not differ significantly between the two groups (p=0.35) (Figure 1D).
Figure 1: Total IL-32 protein levels and isoforms mRNA expression in PBMCs of WLWH with or without subclinical atherosclerosis.
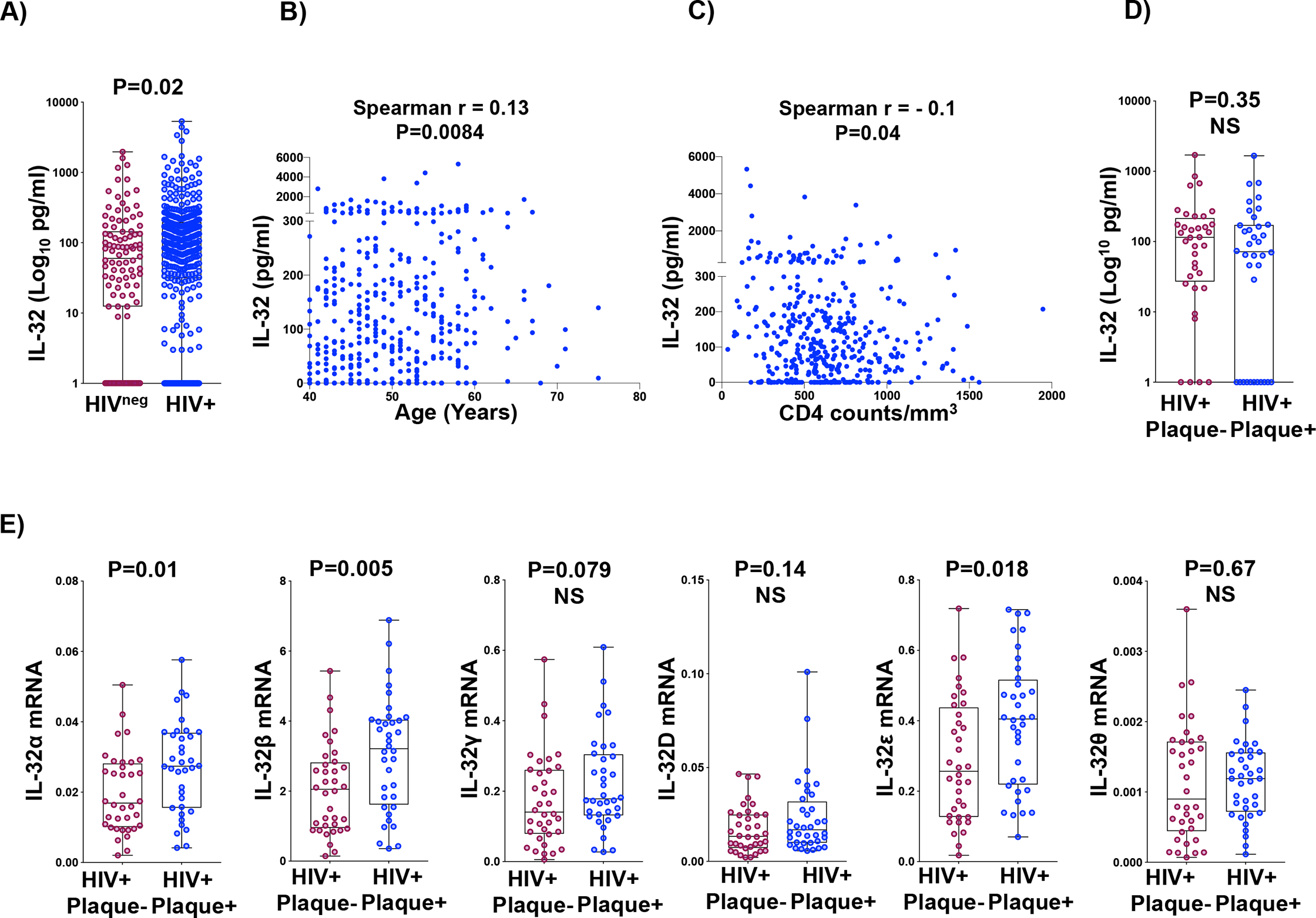
A) Total IL-32 protein measured by ELISA in plasma from n=100 women without HIV and n=399 WLWH. B) Correlations of IL-32 protein in WLWH (n=399) with participant age. C) Correlations of IL-32 protein in WLWH (n=399) with participant CD4 T-cell count. D) IL-32 protein measured by ELISA in plasma from WLWH with (n=36) or without (n=36) subclinical atherosclerosis (HIV+plaqueneg and HIV+plaque+, respectively). E) RT-qPCR data for IL-32 isoforms (α, β, γ, D, ε and θ) amplified from total PBMCs of HIV+plaqueneg (n=36) compared to HIV+plaque+ (n=36). IL-32 mRNA levels were normalized to the housekeeping gene β-glucuronidase. P values are calculated with the two-tailed non-parametric Mann-Whitney test in A, D and E and Spearman correlations in B&C. NS: Non-Significant.
Table 1.
Demographic and clinical parameters of ART-treated virally suppressed case-control study participants.
| Controls (no plaque, N=36) N (%) or median (IQR) | Cases (plaque, N=36) N (%) or median (IQR) | P-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | 55 (51–58.5) | 55 (51–58.5) | 0.92 |
| Race/ethnicity | |||
| Black, non Hispanic | 15 (42) | 21 (58) | 0.08 |
| Hispanic | 19 (53) | 10 (28) | |
| White, non Hispanic or other | 2 (6) | 5 (14) | |
| Education | |||
| Did not complete high school | 17 (47) | 18 (50) | 0.99 |
| Completed high school | 11 (31) | 8 (22) | |
| At least some college | 8 (22) | 10 (28) | |
| Behaviour-related characteristics | |||
| Current crack/cocaine use | 0 (0) | 3 (8) | 0.24 |
| Current alcohol use | 8 (22) | 8 (22) | 0.99 |
| History of injection drug use | 12 (33) | 18 (50) | 0.23 |
| History of HCV infection | 12 (33) | 20 (56) | 0.10 |
| History of smoking Cardiometabolic risk factors |
21 (58) | 27 (75) | 0.21 |
| Body mass index, kg/m2 | 30.1 (26.6–38.9) | 26.9 (24.3–31.0) | 0.01 |
| Systolic blood pressure, mm Hg | 116 (108–125.5) | 123.5 (113.5–137) | 0.049 |
| Current use of anti-hypertensive medications | 16 (44) | 22 (61) | 0.24 |
| Total cholesterol, mg/dL | 184.5 (164.5–224) | 185 (150–221) | 0.59 |
| LDL cholesterol, mg/dL | 103 (77–125) | 96.5 (78–124) | 0.79 |
| HDL cholesterol, mg/dL | 59 (47–70.5) | 53 (42–68) | 0.30 |
| Current use of lipid-lowering medications | 12 (33) | 13 (36) | 0.99 |
| History of diabetes mellitus | 9 (25) | 12 (33) | 0.60 |
| Current use of diabetes medications | 7 (19) | 5 (14) | 0.75 |
| Menopausal (includes surgical) | 25 (69) | 27 (75) | 0.79 |
| Current use of anti-inflammatory medications | 7 (19) | 7 (19) | 0.99 |
| IMT*, common carotid artery (mm) | 0.733 (0.662–0.803) | 0.830 (0.738–0.954) | 0.0003 |
| IMT, bifurcation (mm) | 0.816 (0.739–0.888) | 0.884 (0.788–1.003) | 0.02 |
| HIV-specific characteristics and biomarkers | |||
| CD4+ count, cells/uL | 627 (520.5–779) | 600.5 (452.5–796) | 0.74 |
| Current ART regimen | 0.67 | ||
| Integrase inhibitor-based NNRTI-based** PI-based*** Other |
6 (17) 15 (42) 13 (36) 2 (6) |
7 (19) 11 (31) 17 (47) 1 (3) |
|
| History of clinical AIDS | 20 (56) | 17 (47) | 0.64 |
| Nadir CD4+ count, cells/uL | 194.5 (131.5–353) | 241.5 (105–348.5) | 0.82 |
| CD4:CD8 ratio | 0.82 (0.49–1.03) | 0.67 (0.49–0.91) | 0.32 |
| hsCRP, ug/mL | 1.9 (1.0–4.2) | 1.9 (1.0–6.8) | 0.82 |
IMT: Intima-media thickness
NNRTI: Non-nucleoside reverse transcriptase inhibitor
PI: Protease inhibitor.
Given the multitude of IL-32 isoforms and their differential functions as we and others have previously shown 22,24, we aimed to investigate whether these isoforms are differentially expressed based on atherosclerosis status. As shown in Figure 1E, levels of the IL-32α, β and ε isoforms were significantly higher in PBMCs isolated from WLWH with subclinical atherosclerosis (p=0.01, p=0.005, and p=0.018, respectively) compared to age-matched controls without atherosclerosis. Upregulation of the IL-32β and ε isoforms persisted after additional adjustment for age, race/ethnicity, smoking status, systolic blood pressure, BMI, and history of hepatitis C virus (p=0.04 and p=0.045, respectively). After adjustment, every Z-score increase in IL-32β was associated with a 96% higher odds of plaque (adjusted odds ratio [aOR] 1.96, 95% confidence interval [CI] 1.05–3.68) Similarly, every Z-score increase in IL-32ε was associated with a 92% higher odds of plaque (aOR 1.92, 95% CI 1.01–3.63). The adjusted association for IL-32α was marginally significant (aOR 1.80, 95% CI 0.96–3.37, p=0.07). Levels of IL-32γ and IL-32D were higher in plaque cases compared with controls, but these differences were not statistically significant.
Discussion
Our study provides evidence for a potential role of the proinflammatory cytokine IL-32 as a biomarker for subclinical atherosclerosis in well-controlled WLWH. This role was restricted to cell-associated IL-32 mRNA that distinguished differentially expressed isoforms, rather than circulating total IL-32 proteins detected by a common set of antibodies. Of note, we previously showed in a separate sample that mRNA levels of IL-32 isoforms positively correlate with cell-associated IL-32 proteins but not with plasma IL-32 24. These data suggest that differences in mRNA expression observed in the current study between WLWH with or without subclinical atherosclerosis are likely to be translated into functional proteins at the cellular level.
We showed here that the IL-32α, β and ε isoforms were highly expressed (and IL-32β and ε significantly so) in WLWH with subclinical atherosclerosis, independent of age, smoking status, and other CVD risk factors including BMI. Of note, higher BMI is known to be associated with low-grade inflammation, due in part to the production and secretion of proinflammatory cytokines in adipose tissue 35. However, in the current study, average BMI was lower among cases with subclinical atherosclerosis compared with controls (Table 1). Despite this, expression of IL-32 isoforms was significantly higher among cases, bolstering the link between IL-32 and subclinical atherosclerosis and suggesting the potential for use of these isoforms as biomarkers of CVD. However, we acknowledge the limitation of the relatively small sample size used in the current study. Thus, replication of these observations in larger cohorts of both men and women living with HIV is warranted.
Moreover, the functional consequence for the simultaneous co-expression of the three IL-32 isoforms (IL-32α, β and ε) remains to be determined. While IL-32β plays a proinflammatory role by inducing IL-6 and IFNγ in activated T cells 24 (likely inducing a Th1 phenotype) and similarly IL-32ε induces a distinct form of caspase-independent apoptosis 36, IL-32α shows anti-inflammatory potential since it induces IL-10 expression but not IL-6, as we have previously shown 24. However, IL-32β and IL-32ε are expressed at a relatively higher ratio compared to IL-32α (100 and 10 fold more, respectively24) and therefore the overall dominant function of IL-32 expression is likely to be inflammatory, which favors atherogenesis with plaque development and growth.
In conclusion, our observations align with mounting evidence for a potential role of IL-32 as a key player in vascular inflammation and CVD 37–39 and warrant further investigations to build the case for this novel proinflammatory cytokine as a CVD biomarker and therapeutic target.
Acknowledgements
We would like also to thank Stéphanie Matte and Daniel Tremblay-Sher for their help with administrative duties and cohort database. This work was supported by funds through the Canadian Institutes of Health Research, CIHR [grant number PJT 148482], National Institutes of Health, NIH [grant number R01AG054324], Fonds de Recherche Santé du Québec, FRQS [grant number 35381] and FRQS SIDA-MI network. DBH is supported by NIH K award (K01-HL-137557). NC is supported by a Research Scholar Career Award of the Quebec Health Research Fund (FRQ-S, #253292). RCK was supported by 5R01MD011389, 5R01HL140976 and 1R01HL148094 from the National Institutes of Health. Data in this manuscript were collected by the Women’s Interagency HIV Study, now the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). Contributing MWCCS sites (Principal Investigators): Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01- HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205.
Footnotes
Competing financial interests
Dr. Jorge R. Kizer has stock ownership in Bristol-Myers Squibb, Johnson & Johnson, Medtronic, Merck and Pfizer. All other co-authors have no conflict of interest to declare.
References
- 1.Triant VA. Cardiovascular disease and HIV infection. Curr HIV/AIDS Rep. 2013;10(3):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramendra R, Isnard S, Mehraj V, et al. Circulating LPS and (1-->3)-beta-D-Glucan: A Folie a Deux Contributing to HIV-Associated Immune Activation. Front Immunol. 2019;10:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehraj V, Ramendra R, Isnard S, et al. Circulating (1-->3)-beta-D-glucan Is Associated With Immune Activation During Human Immunodeficiency Virus Infection. Clin Infect Dis. 2020;70(2):232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massanella M, Fromentin R, Chomont N. Residual inflammation and viral reservoirs: alliance against an HIV cure. Curr Opin HIV AIDS. 2016;11(2):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan TP, Woods JO, Sedaghat AR, Siliciano JD, Siliciano RF, Wilke CO. Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J Virol. 2009;83(17):8470–8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naeger DM, Martin JN, Sinclair E, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5(1):e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negredo E, Massanella M, Puig J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clin Infect Dis. 2010;50(9):1300–1308. [DOI] [PubMed] [Google Scholar]
- 9.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10(2):e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna DB, Lin J, Post WS, et al. Association of Macrophage Inflammation Biomarkers With Progression of Subclinical Carotid Artery Atherosclerosis in HIV-Infected Women and Men. J Infect Dis. 2017;215(9):1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shan Z, Clish CB, Hua S, et al. Gut Microbial-Related Choline Metabolite Trimethylamine-N-Oxide Is Associated With Progression of Carotid Artery Atherosclerosis in HIV Infection. J Infect Dis. 2018;218(9):1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libby P, Ridker PM. Novel inflammatory markers of coronary risk: theory versus practice. Circulation. 1999;100(11):1148–1150. [DOI] [PubMed] [Google Scholar]
- 15.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott P, Chambers JC, Zhang W, et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moran CA, Sheth AN, Mehta CC, et al. The association of C-reactive protein with subclinical cardiovascular disease in HIV-infected and HIV-uninfected women. AIDS. 2018;32(8):999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grund B, Baker JV, Deeks SG, et al. Relevance of Interleukin-6 and D-Dimer for Serious Non-AIDS Morbidity and Death among HIV-Positive Adults on Suppressive Antiretroviral Therapy. PLoS One. 2016;11(5):e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacchiega BC, Bacchiega AB, Usnayo MJ, Bedirian R, Singh G, Pinheiro GD. Interleukin 6 Inhibition and Coronary Artery Disease in a High-Risk Population: A Prospective Community-Based Clinical Study. J Am Heart Assoc. 2017;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JD, Bae SY, Hong JW, et al. Identification of the most active interleukin-32 isoform. Immunology. 2009;126(4):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro-Dias F, Saar Gomes R, de Lima Silva LL, Dos Santos JC, Joosten LA. Interleukin 32: a novel player in the control of infectious diseases. J Leukoc Biol. 2017;101(1):39–52. [DOI] [PubMed] [Google Scholar]
- 23.Xin T, Chen M, Duan L, Xu Y, Gao P. Interleukin-32: its role in asthma and potential as a therapeutic agent. Respir Res. 2018;19(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaidan SM, Leyre L, Bunet R, et al. Upregulation of IL-32 Isoforms in Virologically Suppressed HIV-Infected Individuals: Potential Role in Persistent Inflammation and Transcription From Stable HIV-1 Reservoirs. J Acquir Immune Defic Syndr. 2019;82(5):503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Far M, Kouassi P, Sylla M, et al. Proinflammatory isoforms of IL-32 as novel and robust biomarkers for control failure in HIV-infected slow progressors. Sci Rep. 2016;6:22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaccarezza M, Balla C, Rizzo P. Atherosclerosis as an inflammatory disease: Doubts? No more. Int J Cardiol Heart Vasc. 2018;19:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adimora AA, Ramirez C, Benning L, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol. 2018;47(2):393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Souza G, Bhondoekhan F, Benning L, et al. Characteristics Of The Macs-Wihs Combined Cohort Study: Opportunities For Research On Aging With Hiv In The Longest Us Observational Study Of HIV. Am J Epidemiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conway JM, Coombs D. A stochastic model of latently infected cell reactivation and viral blip generation in treated HIV patients. PLoS Comput Biol. 2011;7(4):e1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22(13):1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanna DB, Post WS, Deal JA, et al. HIV Infection Is Associated With Progression of Subclinical Carotid Atherosclerosis. Clin Infect Dis. 2015;61(4):640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135(11):939–953. [DOI] [PubMed] [Google Scholar]
- 34.Calabrese F, Baraldo S, Bazzan E, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(9):894–901. [DOI] [PubMed] [Google Scholar]
- 35.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–2135. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Wang Y, Liu X, Xing X, Zhang Y. Interleukin-32epsilon induces caspase-independent apoptosis mediated by N-Myc interactor in macrophages infected with Mycobacterium tuberculosis. FEBS J. 2019;286(3):572–583. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi H, Huang J, Ye F, Shyr Y, Blackwell TS, Lin PC. Interleukin-32beta propagates vascular inflammation and exacerbates sepsis in a mouse model. PLoS One. 2010;5(3):e9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Shi L, Xue Y, et al. Interleukin-32 increases in coronary arteries and plasma from patients with coronary artery disease. Clin Chim Acta. 2019;497:104–109. [DOI] [PubMed] [Google Scholar]
- 39.Vallejo J, Saigusa R, Gulati R, et al. Combined protein and transcript single cell RNA sequencing reveals cardiovascular disease and HIV signatures. bioRxiv. 2020:2020.2009.2010.292086. [Google Scholar]


