Abstract
Background:
Breastfeeding mothers with HIV infection not qualifying for antiretroviral therapy (ART) based on country-specific guidelines at the time of the PROMISE trial and their uninfected neonates were randomized to maternal ART (mART) or infant nevirapine prophylaxis (iNVP) postpartum. HIV transmission proportions were similar (< 1%) in the two arms. We assessed whether maternal viral load (MVL) or CD4 cell counts were associated with breastfeeding HIV transmission.
Methods:
MVL was collected at entry (7-14 days postpartum) and weeks 6, 14, 26, and 50 postpartum. CD4 cell counts were collected at entry and weeks 14, 26, 38, and 50 postpartum. Infant HIV-1 nucleic acid tests (NAT) were obtained at weeks 1, 6, every 4 weeks until week 26, then every 12 weeks. The associations of baseline and time-varying MVL and CD4 with transmission risk were assessed using time-to-event analyses by randomized treatment arm.
Results:
2431 mother-infant pairs enrolled. Baseline MVL (p= 0.11) and CD4 (p=0.51) were not significantly associated with infant HIV-1 infection. Time-varying MVL was significantly associated with infant HIV-1 infection (hazard ratio (95% CI): 13.96 (3.12, 62.45)) in the mART arm but not in the iNVP arm (hazard ratio (95% CI): 1.04 (.20, 5.39)). Time-varying CD4 cell counts were also significantly associated with infant HIV-1 infection in the mART arm but not in the iNVP arm (hazard ratio, 95% CI: 0.18 (0.03, 0.93) in mART arm and 0.38 (0.08, 1.77) in iNVP arm).
Conclusions:
In women receiving mART, increased MVL and decreased CD4 count during breastfeeding were associated with increased risk of infant HIV-1 infection.
INTRODUCTION
Breastfeeding (BF) by infants born to most women living with HIV is critical to infant survival in resource limited countries and protects the infant against common infectious causes of infant mortality such as diarrhea and pneumonia. In a meta-analysis by the WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality, BF was associated with a 6-fold (95% confidence interval [CI] 6-10) decrease in mortality due to infectious diseases for infants less than two months of age. Protection persisted but declined with age during infancy with a 4-fold (95% CI 3-6) decrease for ages 2 to 3 months, a 3-fold (95% CI 2-4) decrease for ages 4 to 5 months, a 2-fold (95% CI 1-3) decrease for ages 6 to 8 months and 1.4-fold (95% CI 1-3) decrease for ages 9 to 11 months.1 However, breastfeeding also carries the risk of HIV transmission to her infant. Prior to antiretroviral (ARV) interventions, the risk of transmission during breastfeeding after 1 month of age is 0.6% to 0.9% per month, with mixed feeding associated with higher and exclusive BF associated with lower rates.2 With prolonged BF into the second year of life, acquisition of infant HIV infection through breast milk contributes an estimated 30- 50% of all mother-to-child HIV transmission (MTCT) among untreated HIV-infected mothers.
Prior to universal recommendations for use of ART (generally includes three ARVs from at least two different HIV drug classes) at time of diagnosis, two general strategies had been proposed to reduce the risk of postpartum breastfeeding HIV transmission: 1) use of infant antiretroviral prophylaxis during BF3–7 and 2) use of maternal triple antiretroviral therapy (ART) during breastfeeding.8–15 The infant antiretroviral prophylaxis strategy aims to protect the infant during the period of HIV exposure during BF while maternal prophylaxis aims to reduce the risk of transmission primarily by lowering viral load in breast milk, although transfer of antiretrovirals to the infant through breast milk ingestion also occurs, which could provide some indirect antiretroviral prophylaxis to the infant.
If the adult treatment tenet U=U (Undetectable is Untransmittable), which applies to sexual transmission, is indeed applicable to breastfeeding HIV-infected women and risk of transmission to their infants, successful maternal ART that results in undetectable maternal HIV-1 plasma viral load (MVL) would prevent infant infection.16 At this time, however, there is insufficient evidence to demonstrate whether this assumption holds true in the breastfeeding population, whether undetectable MVL means undetectable breast milk viral load, and whether detectable versus undetectable viral levels in breastmilk may be an important risk factor for transmission during the period of lactation.
The Postpartum component of the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) network’s Promoting Maternal-Infant Survival Everywhere (PROMISE) trial (NCT01061151) compared these two strategies and demonstrated similar proportions of HIV-transmission during breastfeeding (0.57% vs. 0.58% of analyzed infants in the maternal ART and infant prophylaxis arms, respectively, hazard ratio [HR] 1.0, 96% repeated confidence interval 0.3-3.1); and safety profile.17 In this secondary analysis, we sought to describe the relationship between MVL, CD4 count and HIV transmission during breastfeeding among women and their breastfeeding infants enrolled in the PROMISE study.
METHODS
IMPAACT PROMISE was a multi-component randomized, open-label strategy trial sponsored by the U.S. National Institutes of Health conducted at 14 health-facility-based research sites in seven countries: India (1 site), Malawi (2), South Africa (5), Tanzania (1), Uganda (1), Zambia (1) and Zimbabwe (3). The PROMISE Postpartum Component was designed to compare the relative efficacy and safety of maternal ART (mART) or infant nevirapine (iNVP) prophylaxis for prevention of breastmilk HIV-1 transmission. Eligible women included those continuing from the Antepartum Component of PROMISE and those who did not receive medical care or infant HIV prevention interventions prior to active labor (Late-Presenters). All women were in good health, had CD4 ≥ 350 cells/mm3 or local standard to begin ART (if higher), and had never received ART for treatment of HIV-related conditions. ARV and ART use for the prevention of infant infection in prior pregnancies was allowed. Additional details of the study and efficacy and safety outcomes have been published.17
Enrollment of eligible mothers and their infants into the Postpartum Component occurred 1 week after delivery of the infant (days 7-14 of life) after confirming that the infant had a negative HIV nucleic acid test (NAT). Mother-infant pairs were randomized to one of two prophylaxis regimens, mART or iNVP in a 1:1 ratio. Randomized regimen was continued until 42 days after last breastmilk exposure (two weeks following breastfeeding cessation, defined as no breastmilk exposure for >28 days) or age 18 months, whichever came first. Participants were followed in the Postpartum Component through 104 weeks postpartum or until randomization to stop or continue mART (but not iNVP) in the subsequent PROMISE Maternal Health Component, whichever was earlier. MVL was determined at entry and weeks 6, 14, 26, 50 and 74 postpartum, with quantification performed using polymerase-chain-reaction assay in real-time in the mART arm and by batch testing of stored samples in the iNVP arm. Maternal CD4 was collected at entry and weeks 14, 26, 38, 50, 62, 74, 86 and 98 postpartum. However, MVL and CD4 measurements after week 50 postpartum were not included in the analyses because a substantial subgroup of women in the mART arm, but not the iNVP arm (those in the mART arm who still had CD4 ≥ 350 cells/mm3 or local standard to begin ART, if higher), were randomized to stop mART after week 50, making interpretation of the post-week 50 MVL data difficult. Infant HIV-1 NAT was obtained at weeks 1, 6, every 4 weeks until week 26, then every 12 weeks during breastfeeding. Confirmed infant HIV-1 infection was defined as positive HIV-1 NAT from a specimen drawn at any post-randomization visit, confirmed by a positive HIV-1 NAT on a second specimen drawn at a subsequent time point.
Virology
All laboratories performing maternal and infant virologic testing were certified by the Virology Quality Assurance Program of the Division of AIDS (DAIDS) of the National Institute of Allergy and Infectious Diseases. Among infants with confirmed HIV, the Roche Amplicor HIV-1 DNA test v1.5 was used to detect and confirm infection.
Selected infants with apparent transmission despite MVL reported as “undetected” or “detected but below 40 copies/mL” had additional virologic testing. DNA was extracted from Dried Blood Spots (DBS) specimens collected prior to the first positive Roche 1.5 DNA PCR using the Chelex-100 method. The number of nucleated cells assayed was determined by human βeta-globin quantitative PCR. To test for potential low-level infection, DNA from >300,000 cells from each 50μl DBS was submitted to nested PCR targeting the RT region of HIV pol(primers: first round forward CCTACACCTGTCAACATAATTGG and reverse AAYTTCTGTATATCATTGACAGTCCA; second round forward AATTAAAGCCAGGAATGGATGG and reverse CAYTTGTCAGGATGGAGTTCATA); DNA was divided between multiple ≤1μg reactions to avoid inhibition of PCR.
Statistical analysis:
The associations of receipt of ART during pregnancy, baseline MVL and CD4 cell count, and time-varying MVL and CD4 cell count with transmission risk were assessed using Time to Event analyses using Cox proportional hazards models with time-varying and non-time- varying co-variates (randomized treatment arm, receipt of ART during pregnancy). MVL was categorized into a 2-category variable (<1,000 copies/mL and ≥1,000 copies/mL); a 3-category model with categories of HIV-1 RNA <400 copies/mL, HIV-1 RNA between 400 and 1,000 copies/mL and HIV-1 RNA ≥1,000 copies/mL) was attempted but could not be analyzed due to the very low event rates. CD4 was categorized as a 2-category variable (<500 or ≥ 500 cells/mm3). Missing maternal HIV-1 plasma RNA and CD4 at a visit week was handled using a Last Observation Carried Forward (LOCF) approach. The analyses were censored in the same way as the previously reported time-to-event analyses of the primary efficacy outcome (confirmed infant HIV-1 infection) with follow-up time censored at the earliest of either the recommended minimum duration of BF at the site or the specimen draw date of the last negative infant HIV-1 NAT result drawn during the transmission risk period.17 Separate analyses were done for each arm for analysis because the post-randomization visits show little overlap between the 2 arms with respect to maternal HIV-1 plasma RNA and CD4 and it could not be assumed that the effect of a specific change in maternal RNA (e.g. an increase of 500 copies/ml) was the same in the two arms.
A two-sided p-value <0.05 was considered statistically significant.
RESULTS
2431 mother-infant pairs were enrolled into the PROMISE postpartum component between June 6, 2011 and October 1, 2014, with 1,220 women in the mART arm and 1,211 women in the iNVP arm. As reported previously, there were seven confirmed infant HIV-1 infections in each treatment arm in the primary postpartum efficacy analysis. MVL and CD4 counts are shown in Table 1.
Table 1.
Postpartum Maternal HIV-1 plasma viral load and CD4 count (Note that undetectable maternal viral loads are set to the assay lower limit of detection (LLD) minus 1 copy/mL.
| Randomized Treatment Arm | ||||
|---|---|---|---|---|
| Maternal ART (N=1,220) | Infant Nevirapine (N=1,211) | Total (N=2,431) | ||
| Baseline | ||||
| Maternal Viral Load | N | 1,220 | 1,211 | 2,431 |
| Median (range) | 222(20-2,177,097) | 400 (20-445,765) | 325 (20-2,177,097) | |
| <1,000 copies/mL | 911 (75%) | 814 (67%) | 1,725 (70%) | |
| ≥1,000 copies/mL | 309 (25%) | 397 (33%) | 706 (29%) | |
| CD4 Count | N | 1,220 | 1,211 | 2,431 |
| Median (range) | 706 (296-2,094) | 720 (353-2,353) | 712 (296-2,353) | |
| < 500 cells/mm3 | 162 (13%) | 170 (14%) | 332 (14%) | |
| ≥ 500 cells/mm3 | 1,058 (87%) | 1,041 (86%) | 2,099 (86%) | |
| Week 6 | ||||
| Maternal Viral Load | N | 1,189 | 1,150 | 2,339 |
| Median (range) | 39 (19-1,126,783) | 7,528 (19-1,747,043) | 399 (19-1,747,043 | |
| <1,000 copies/mL | 1,089 (91%) | 252 (22%) | 1,341 (58%) | |
| ≥1,000 copies/mL | 100 (8%) | 898 (78%) | 998 (43%) | |
| Week 14 | ||||
| Maternal Viral Load | N | 1,156 | 1,152 | 2,308 |
| Median (range) | 39 (19-1,177,352) | 5,288 (39-1,472,415) | 399 (19-1,472,415) | |
| <1,000 copies/mL | 1,028 (89%) | 269 (23%) | 1,297 (56%) | |
| ≥1,000 copies/mL | 128 (11%) | 883 (77%) | 1,011 (44%) | |
| CD4 Count | N | 1,139 | 1,153 | 2,292 |
| Median (range) | 801 (303-1,990) | 657 (210-2,068) | 733 (580-925) | |
| <500 cells/mm3 | 49 (4%) | 244 (21%) | 293 (13%) | |
| ≥ 500 cells/mm3 | 1,090 (96%) | 909 (79%) | 1,999 (87%) | |
| Week 26 | ||||
| Maternal Viral Load | N | 1,122 | 1,164 | 2,286 |
| Median (range) | 39 (19-857,075) | 5,893 (19-1,290,490) | 399 (19-1,290,490) | |
| <1,000 copies/mL | 982 (88%) | 282 (25%) | 1,264 (55%) | |
| ≥1,000 copies/mL | 140 (12%) | 882 (76%) | 1,022 (45%) | |
| CD4 Count | N | 1,113 | 1,169 | 2,282 |
| Median (range) | 824 (662-1,014) | 620 (503-816) | 719 (562-928) | |
| <500 cells/mm3 | 63 (6%) | 288 (25%) | 351 (15%) | |
| ≥ 500 cells/mm3 | 1,050 (94%) | 881 (75%) | 1,931 (85%) | |
| Week 38 | ||||
| CD4 Count | N | 1,051 | 1,138 | 2,189 |
| Median (range) | 844 (309-7,071) | 622 (495-797) | 729 (559-930) | |
| <500 cells/mm3 | 56 (5%) | 296 (26%) | 352 (16%) | |
| ≥ 500 cells/mm3 | 995 (95%) | 842 (74%) | 1,837 (84%) | |
| Week 50 | ||||
| Maternal Viral Load | N | 962 | 1,077 | 2,039 |
| Median (range) | 39 (19-1,240,535) | 5,319 (19-1,832,639) | 447 (19-1,832,639) | |
| <1,000 copies/mL | 814 (85%) | 313 (29%) | 1,127 (55%) | |
| ≥1,000 copies/mL | 148 (15%) | 764 (71%) | 912 (45%) | |
| CD4 Count | N | 957 | 1,077 | 2,034 |
| Median (range) | 848(282-1,941) | 597 (191-2,814) | 703 (191-2,814) | |
| <500 cells/mm3 | 65 (7%) | 305 (28%) | 370 (18%) | |
| ≥ 500 cells/mm3 | 892 (93%) | 772 (72%) | 1,664 (82%) | |
Effect of baseline maternal plasma HIV-1 viral load and CD4 cell count
In the primary analysis, neither baseline (obtained at the time of randomization into the Postpartum Component) MVL (p= 0.10) nor CD4 cell count (p=0.41) were significantly associated with the incidence of infant HIV-1 infection. The incidence rates (per 100 person-years) of infant infection was greater among mothers with baseline MVL ≥ 1,000 copies/mL [1.2 (0.9,1.7) among women in the mART group and 0.7 (0.5, 1.0) among women in the iNVP group] when compared to the women with baseline MVL < 1,000 copies/mL [0.3 (0.3,0.4) among women in the mART group and 0.5 (0.4,0.6) among women in the iNVP group]. Incidence rates were similar regardless of baseline maternal CD4 cell count (data not shown).
Effect of time-varying maternal plasma HIV-1 viral load and CD4 cell count
Time to Event analyses were performed separately for each treatment arm and included time-varying MVL and CD4 cell counts, and ART use during pregnancy as co-variates. Models fitted included individual and combined co-variates. Parameter estimates for the models are shown in Table 2. Time-varying MVL was significantly associated with infant HIV-1 infection (hazard ratio (95% CI): 13.96 (3.12, 62.45)) in the mART arm but not in the iNVP arm (hazard ratio (95% CI): 1.04 (0.20 – 5.39)) (Model 1). Similar results were obtained when time-varying MVL and ART use during pregnancy were included in the model (Model 2), suggesting that the use of ART during pregnancy does not confound the relationship between infant infection and time-varying MVL.
Table 2.
Summary of time-to-event analyses for infant HIV-1 transmission during breastfeeding with time-varying maternal plasma HIV-1 viral load and CD4 count and receipt of ART during pregnancy as co-variates
| Treatment Arm | |||||
|---|---|---|---|---|---|
| Maternal ART | Infant Nevirapine | ||||
| Model Co-Variate(s) | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| Model 1 | Maternal Viral Loada | 13.96 (3.12, 62.45) | 0.0006 | 1.04 (.20, 5.39) | 0.9673 |
| Model 2 | Maternal Viral Load | 14.66 (3.27, 65.76) | 0.0005 | 1.14 (0.22, 5.97) | 0.8777 |
| No ART during Pregnancyb | 0.17 (0.02, 1.44) | 0.1041 | 0.18 (0.02, 1.53) | 0.1172 | |
| Model 3 | Maternal CD4c | 0.18 (0.03, 0.93) | 0.0413 | 0.38 (0.08, 1.77) | 0.2206 |
| Model 4 | Maternal CD4 | 0.15 (0.03, 0.78) | 0.0241 | 0.33 (0.07, 1.55) | 0.1609 |
| No ART during Pregnancy | 0.16 (0.02, 1.36) | 0.0943 | 0.17 (0.02, 1.41) | 0.1003 | |
| Model 5 | Maternal Viral Load | 11.57 (2.45, 54.68) | 0.0020 | 0.95 (0.18, 5.01) | 0.9541 |
| Maternal CD4 | 0.34 (0.06, 1.88) | 0.2168 | 0.38 (0.08, 1.78) | 0.2205 | |
| Model 6 | Maternal Viral Load | 11.91 (2.52, 56.39) | 0.0018 | 1.04 (0.20, 5.51) | 0.9648 |
| Maternal CD4 | 0.29 (0.05, 1.57) | 0.1502 | 0.33 (0.07, 1.57) | 0.1639 | |
| No ART during Pregnancy | 0.16 (0.02, 1.32) | 0.0881 | 0.17 (0.02, 1.41) | 0.1002 | |
Maternal plasma HIV-1 viral load ≥ 1,000 relative to < 1,000;
Did not receive ART during pregnancy relative to receiving ART during pregnancy;
Maternal CD4 ≥ 500 relative to CD4 <500 cells/mm3
Time-varying CD4 cell count was also significantly associated with infant HIV-1 infection in the mART arm but not the iNVP arm (Model 3, hazard ratio, 95% CI: 0.18, 0.03-0.93 in mART arm and 0.38, 0.08-1.77 in iNVP arm). This association persisted when time-varying CD4 cell count and use of ART during pregnancy were included in the model (Model 4) but was not observed when time-varying MVL and CD4 cell count and ART during pregnancy were included in the models (Models 5 and 6); only MVL remained significantly associated with infant HIV-1 infection in these models. Maternal time-varying CD4 cell count was not associated with infant infection in the iNVP arm in any of the models tested.
Timing of infant infection
Among the seven HIV-1-infected infants in the mART arm, the first positive HIV-1 NAT tests were at weeks 13, 14, 26, 38, 38, 38, and 50, respectively (Table 3). In the iNVP arm, infant infections were first detected at week 6, 6, 14, 26, 50, 51, and 74. MVL measurements closest (prior to or on the same day) to the first infant positive NAT ranged from not detected to 52,002 copies/mL with a median of 6,627 in the mART arm. In the iNVP arm, the median MVL was 59,816 copies/mL with a range of 815 to 153,963 copies/mL.
Table 3.
Timing of Infant Infection and Maternal Viral Load
| Postpartum Randomization Arm | Infant Age at First Positive HIV-1 NAT (weeks) | Maternal Viral Load Closest to and Prior to Infant Positive HIV-1 NAT (copies/mL) | Days between Infant Positive HIV-1 NAT and Reported Maternal Viral Loada |
|---|---|---|---|
| mART | 13b | < 40 | 0 |
| 14 | 20,331 | 0 | |
| 26 | 6,627 | 81 | |
| 38 | 39,228 | 0 | |
| 38 | 6.107 | 84 | |
| 38c | Not detected | 0 | |
| 50 | 52,002 | 0 | |
| iNVP | 6 | 59,278 | 26 |
| 6 | 153,963 | 0 | |
| 14 | 59,816 | 0 | |
| 26 | 815 | 0 | |
| 50 | 124,886 | 0 | |
| 51 | 64,132 | 7 | |
| 74 | 4,369 | 158 |
Two cases were notable as the mothers had MVL measured as “non-detected” or “detected but below 40 copies/mL” on the date that their infant’s first sample tested positive for HIV RNA. The pattern of MVL and infant NAT testing are shown in Figure 1. In the first case (Figure 1A), the mother had been randomized to receive maternal ART during the antepartum component of PROMISE and then to continue in the mART arm of the postpartum component. This mother’s MVL at 13 weeks postpartum, when the first infant NAT was positive, was < 40 copies/mL. PCR testing of 390,000 cells from her infant’s DBS specimen collected when 6 weeks of age was negative for HIV DNA. In the second case (Figure 1B), the mother entered the postpartum randomization as a late-presenter, meaning that she had received no antenatal antiretroviral agents. She was randomized to the mART arm in the postpartum component and quickly achieved and maintained undetectable MVL. Her infant’s first test positive for HIV RNA was at 38 weeks of age. HIV DNA was undetectable in tests of a total of 440,000 cells from a DBS specimen collected when this infant was 26 weeks of age.
Figure 1.
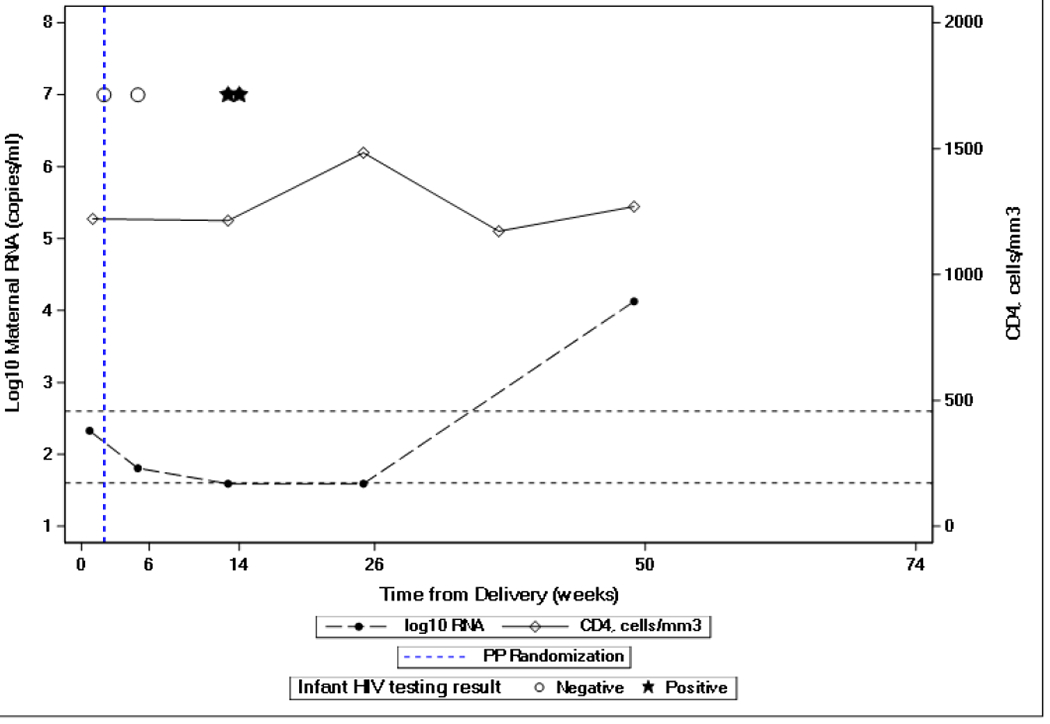
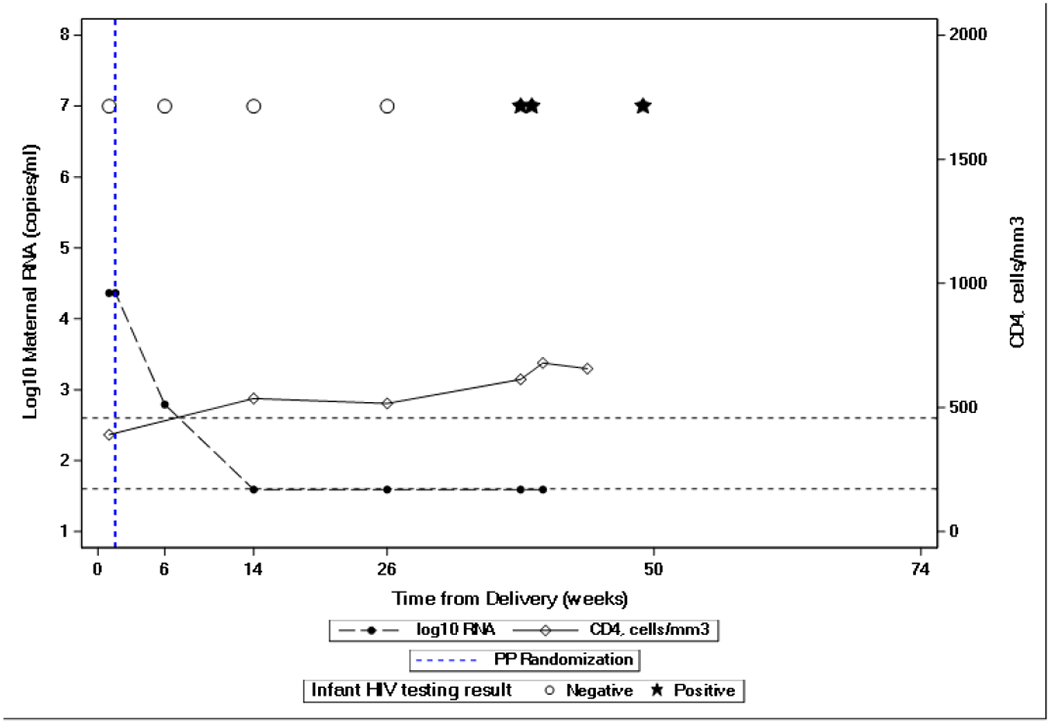
Maternal plasma HIV-1 viral load and infant NAT testing of two infants with maternal HIV-1 viral load “non-detected” or “detected but below 40 copies/mL” prior to positive infant HIV-1 NAT testing. Dashed lines show maternal HIV-1 viral load results. Solid lines show maternal CD4 cell count. Open circles represent negative infant HIV-1 NATs and black stars represent positive infant HIV-1 NAT.
DISCUSSION
In this analysis of factors influencing HIV-1 transmission during breastfeeding in a large multi-site perinatal trial, we demonstrated a role for MVL and CD4 count among women randomized to the mART arm but not among women and infants who were randomized to the iNVP arm. When MVL and CD4 count were both included in models, CD4 count did not remain a significant finding suggesting that MVL is a more sensitive indicator of HIV-1 transmission risk. MVL is a well-known risk factor for HIV-1 transmission in multiple settings. A study of heterosexual partners discordant for HIV infection (HPTN 052) randomized the HIV-1-infected partner to receive either delayed or immediate ART. No HIV transmissions within these couples occurred when the HIV-positive partner had a suppressed viral load (less than 400 copies/mL).18 Among linked infections in the immediate treatment group, both baseline CD4 cell count and viral load were associated with partner infection. Similarly among discordant couples of men who have sex with men, no genetically linked HIV-1 transmission occurred when the HIV-1-infected partner had virologic suppression.19–21 Multiple studies of pregnant women with HIV-1 infection have also demonstrated that the level of MVL at or near the time of delivery is associated with risk of HIV-1 transmission to the infant. In a study of 12,486 infants delivered in the United Kingdom and Ireland, the transmission risk was significantly lower in women with MVL <50 copies/mL (0.09%) than in women with higher MVLs.22 Similar findings were reported from the multicenter French Perinatal Cohort where the perinatal transmission rate was higher for women with MVL of 50 to 400 copies/mL near delivery than for those with <50 copies/mL, and higher still for women with MVL >400 copies/mL at delivery (4.4% for women initiating ART in the third trimester and with MVL >400 copies/mL at delivery).23
MVL in plasma and breastmilk was reported from the Breastfeeding, Antiretroviral and Nutrition (BAN) study conducted in Malawi. In this study, breastfeeding women with HIV infection and their infants were randomized to one of three arms postpartum: maternal NVP-based ART, infant NVP or no intervention. Similar to findings in our study, among mothers randomized to the ART arm, the overall median log10 maternal plasma VL was two logs less among non-transmitting mothers compared to transmitting mothers.24 Based on the collective data supporting a relationship between higher MVL and increased risk of transmission, the finding in the mART arm of our study are not surprising.
The lack of association between MVL and infant HIV-1 transmission in the setting of infant prophylaxis and no maternal ART has also been reported in the BAN study. In this Malawi randomized trial among late presenting women who received no antepartum intervention, and were then randomized to receive either maternal ART or infant NVP prophylaxis during up to 28 weeks of breastfeeding, the investigators reported that among those mother-infant pairs randomized to the infant NVP prophylaxis arm, there were no differences in the MVL for those who did or didn’t transmit. The mechanism of this NVP protection is not well understood; it is not clear if NVP is providing protection as pre-exposure or post-exposure prophylaxis or both.
The two infants in our cohort who had acquisition of HIV-1 despite apparent undetectable or detectable but <40 copies/mL MVL may represent true episodes of transmission in the presence of undetectable viral replication. Previous studies have observed similar events and hypothesized that HIV-specific or nonspecific factors led to the undetectable results in already infected infants until breastfeeding ended.25 However, these findings may alternatively be explained by peripartum or breastfeeding transmission and transient suppression of infant viral replication via maternal ART that passes in breastmilk to the infant26–27 or by 6 weeks of NVP prophylaxis given to all infants in the PROMISE study, regardless of randomized arm, as recommended by the WHO. No infant infection was detected at the 6 weeks visit with the earliest confirmed infection occurring at 13 weeks. It is also possible that, even in the presence of undetectable MVL, there was detectable HIV-1 virus in breastmilk. In the BAN study, there were two instances when MVL was undetectable yet cell-free RNA was detected in breastmilk.24 Alternatively, it is possible that infection could occur due to cell-associated HIV-1 DNA in breastmilk, levels of which may not be affected by maternal ART.28 Additional studies that may address these questions are planned.
In conclusion, in the iNVP arm of the PROMISE study, MVL was not significantly associated with HIV-1 transmission during breastfeeding. However, in women receiving mART, increased MVL during breastfeeding was associated with increased risk of infant HIV-1 infection. These data emphasize the important role of viral suppression in breastfeeding women, especially if the infant is not receiving prophylaxis. Infant NVP should be considered in situations with breastfeeding mothers who do not achieve viral suppression. Additionally, even in light of the two infants who apparently had HIV transmission despite undetectable or <40 copies/mL MVL, these data add to the growing body of information that support low risk of transmission with undetectable MVL throughout breastfeeding. Overall these findings from PROMISE underscore the importance of counseling about the risk of HIV transmission and importance of maternal ART adherence to attain and/or sustain undetectable MVL in preventing HIV transmission during breastfeeding.
Funding:
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Study drugs were provided by AbbVie, Boehringer-Ingelheim, Gilead Sciences, and ViiV/GlaxoSmithKline.
Footnotes
Conflict of Interest:
Dr. Flynn is a consultant for Merck. No other authors declare conflict of interest.
Prior presentation:
Data was presented in part at the 22nd International AIDS Conference, July 23-27, 2018, Amsterdam, the Netherlands and the 10th International Workshop of HIV Pediatrics, July 20-21, Amsterdam, the Netherlands
Registration:
ClinicalTrials.gov: NCT01061151; Completed
References
- 1.Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet. 2000. Feb 5;355(9202):451–5. Erratum in: Lancet 2000 Mar 25;355(9209):1104. [PubMed] [Google Scholar]
- 2.The Breastfeeding and HIV International Transmission Study Group. Late postnatal transmission of HIV in breast-fed children: an independent patient data meta-analysis. J Infect Dis 2004; 189:2154–66. [DOI] [PubMed] [Google Scholar]
- 3.Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372 (9635):300–12. [DOI] [PubMed] [Google Scholar]
- 4.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV transmission. N Engl J Med. 2008;359(2):119–29. [DOI] [PubMed] [Google Scholar]
- 5.Nagot N, Kankasa C, Meda N, et al. Lopinavir/Ritonavir versus Lamivudine peri-exposure prophylaxis to prevent HIV-1 transmission by breastfeeding: the PROMISE-PEP trial Protocol ANRS 12174. BMC Infect Dis. 2012;12:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilewo C, Karlsson K, Massawe A, et al. Prevention of mother-to-child transmission of HIV-1 through breast-feeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania: the Mitra Study. JAIDS. 2008;48 (3):237–40. [DOI] [PubMed] [Google Scholar]
- 7.Coovadia HM, Brown ER, Fowler MG, et al. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomized, double-blind, placebo-controlled trial. Lancet. 012;379:221- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas T, Masaba R, Borkowf CB, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding--the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLosMed. 2011;8 (3):e1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilewo C, Karlsson K, Ngarina M, et al. Prevention of mother to child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania – the MITRA-PLUS study. JAIDS. 2009;52:406–16. [DOI] [PubMed] [Google Scholar]
- 10.Peltier CA, Ndayisaba GF, Lepage P, et al. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother to child transmission in Rwanda. AIDS 2009;23:2415–23.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palombi L, Marazzi MC, Voetberg A, et al. Treatment acceleration and the experience of the DREAM program in PMTCT of HIV. AIDS. 2007;21 Suppl 4:S65–71. [DOI] [PubMed] [Google Scholar]
- 12.Zeh C, Weidle P, Nafisa L, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple antiretroviral prophylaxis for prevention of mother to child transmission: a secondary analysis. PLosMed. 2011;8 (3):e1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chasela CS, Hudgens MG, Jamieson DJ et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breastfeeding in Botswana. N Engl J Med. 2010;362:2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis 2011;11:171–80. 10. [DOI] [PubMed] [Google Scholar]
- 16.Waitt C, Low N, Van de Perre P, Lyons F, Loutfy M, Aebi-Popp K. Does U=U for breastfeeding mothers and infants? Breastfeeding by mothers on effective treatment for HIV infection in high-income settings. Lancet HIV. 2018. Sep;5(9):e531–e536. [DOI] [PubMed] [Google Scholar]
- 17.Flynn PM, Taha TE, Cababasay M, et al. Prevention of HIV-1 Transmission Through Breastfeeding: Efficacy and Safety of Maternal Antiretroviral Therapy Versus Infant Nevirapine Prophylaxis for Duration of Breastfeeding in HIV-1-Infected Women With High CD4 Cell Count (IMPAACT PROMISE): A Randomized, Open-Label, Clinical Trial. J Acquir Immune Defic Syndr. 2018;77(4):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016;375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–81. [DOI] [PubMed] [Google Scholar]
- 20.Bavinton BR, Pinto AN, Phanuphak N, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet. 2018. Jul 16. [DOI] [PubMed] [Google Scholar]
- 21.Rodger AJ. Risk of HIV transmission through condomless sex in MSM couples with suppressive ART: The PARTNER2 Study extended results in gay men. Presented at the 22nd International AIDS Conference; July 23-27, 2018; Amsterdam, the Netherlands. [Google Scholar]
- 22.Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 20002011. AIDS. 2014;28(7):1049–1057. [DOI] [PubMed] [Google Scholar]
- 23.Mandelbrot L, Tubiana R, Le Chenadec J, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis. 2015. [DOI] [PubMed] [Google Scholar]
- 24.Davis NL, Miller WC, Hudgens MG, et al. Maternal and breastmilk viral load: Impacts of adherence on peripartum HIV infections averted-The Breastfeeding, Antiretrovirals, and Nutrition Study. J Acquir Immune Defic Syndr. 2016. Dec 15;73(5):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strehlau R, Paximadis M, Patel F, et al. HIV Diagnostic Challenges in Breast-Fed Infants of Mothers on Antiretroviral Therapy. AIDS. 2019. Sep 1:33(11):1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King CC, Kourtis AP, Persaud D, et al. Delayed HIV Detection Among Infants Exposed to Postnatal Antiretroviral Prophylaxis During Breastfeeding. AIDS. 2015. Sep 24:29(15):1953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgard M, Blanche S, Jasseron C, et al. Performance of HIV-1 DNA or HIV-1 RNA tests for early diagnosis of perinatal HIV-1 infection during anti-retroviral prophylaxis. J Pediatr. 2012. Jan;160(1):60–6.e1. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro RL, Ndung’u T, Lockman S et al. Highly active antiretroviral therapy started during pregnancy or postpartum suppresses HIV-1 RNA but not DNA in breast milk. J Infect Dis. 2005. Sep 1;192 (50):713–9. [DOI] [PubMed] [Google Scholar]


