Abstract
Objective:
Among older adults, the ability to stand or walk while performing cognitive tasks (i.e., dual-tasking) requires coordinated activation of several brain networks. In this multi-center, double-blinded, randomized, and sham-controlled study, we examined the effects of modulating the excitability of the left dorsolateral prefrontal cortex (L-DLPFC) and the primary sensorimotor cortex (SM1) on dual-task performance ‘costs’ to standing and walking.
Methods:
Fifty-seven older adults without overt illness or disease completed four separate study visits during which they received 20-minutes of transcranial direct current stimulation (tDCS) optimized to facilitate the excitability of the L-DLPFC and SM1 simultaneously, each region separately, or neither region (sham). Before and immediately after stimulation, participants completed a dual-task paradigm in which they were asked to stand and walk with and without concurrent performance of a serial-subtraction task.
Results:
tDCS simultaneously targeting the L-DLPFC and SM1, as well as tDCS targeting the L-DLPFC alone, mitigated dual-task costs to standing and walking to a greater extent than tDCS targeting SM1 alone or sham (p<0.02). Blinding efficacy was excellent and participant subjective belief in the type of stimulation received (real or sham) did not contribute to the observed functional benefits of tDCS.
Interpretation:
These results demonstrate that in older adults, dual-task decrements may be amenable to change and implicate L-DPFC excitability as a modifiable component of the control system that enables dual-task standing and walking. tDCS may be used to improve resilience and the ability of older results to walk and stand under challenging conditions, potentially enhancing everyday functioning.
Introduction
Standing and walking are two complex tasks that are essential to many activities of daily living. While each task is executed under different biomechanical constraints, both tasks depend upon control systems requiring activation of distributed cognitive and sensorimotor cortical networks1,2. Moreover, both tasks are often performed concurrently with cognitive tasks such as talking, reading, or problem-solving3. This ‘dual-tasking’ thus competes for shared neural resources4 and may impair performance in one or both tasks5. Older adults who exhibit greater dual-task decrements (i.e., costs) to standing or walking performance exhibit worse ‘executive’ function and are more likely to fall6 and suffer cognitive decline7. We thus need to better understand the neural underpinnings of dual-task standing and walking, and subsequently, identify strategies to enhance this important capacity.
Transcranial direct current stimulation (tDCS) selectively modulates cortical excitability by sending low-level currents between electrodes placed on the scalp. The generated cortical electric field polarizes neuronal populations and modulates resting membrane potentials8. Pilot work suggests that a single exposure to traditional tDCS that putatively targets the left dorsolateral prefrontal cortex (L-DLPFC)—a brain region subserving executive function—may mitigate the dual-task costs to standing and walking in older adults9–13. tDCS targeting the primary sensorimotor cortex (SM1) has also been reported to improve walking speed and/or dynamic balance in healthy younger adults14 and in those suffering from multiple sclerosis15. In these earlier investigations, however, the current was transferred between one large (e.g., 35 cm2) positive electrode placed over the target region and one negative electrode placed elsewhere on the scalp. While this approach modulates cortical excitability, the generated electric field is diffuse and influences numerous brain regions well beyond the target16. It thus remains unclear whether modulation of the excitability of the L-DLPFC or SM1, in particular, was the driving force behind observed functional benefits.
Advances in tDCS technology and electrical field modeling now afford administration of current through an array of smaller gel-based electrodes, via montages designed to optimize specific aspects of the induced electric field over multiple cortical regions within one or more functional brain networks16,17. This approach may be particularly advantageous for augmenting dual-task standing and walking since both the L-DLPFC and SM1 likely contribute18,19. We thus aimed to examine the impact of tDCS optimized to facilitate the excitability of the L-DLPFC alone, the bilateral leg region of SM1 alone, and both of these regions simultaneously, on dual-task standing and walking in older adults. We hypothesized that tDCS simultaneously targeting both regions (i.e., L-DLPFC+SM1) would reduce dual-task costs and that this reduction would be larger than tDCS designed to target either of these regions separately, and larger than that after an “active-sham” stimulation protocol20,21. Secondarily, we hypothesized that the tDCS-induced reduction in dual-task cost would be correlated with the enhancement of executive function as measured by two common neuropsychological tests.
Methods
We completed a multi-site, double-blinded, randomized, sham-controlled ‘within-subject’ cross-over study (NCT03191812). Participants completed a screening and baseline visit and were then exposed to four different stimulation conditions (i.e., L-DLPFC+SM1, L-DLPFC only, SM1 only, sham), in random order, for four subsequent study visits separated by at least three days between consecutive visits22. Each visit was completed at approximately the same time of day. Participants completed a 30-minute functional assessment before and immediately after exposure to each stimulation condition. Functional assessments included dual-task gait and balance, and several aspects of executive function measured by the Stroop Color and Word Test and the Digit Symbol Substitution Test.
Participants
Sample size was determined based upon the results of our pilot study12 and the conservative assumption that the standard deviation of within-subject change in dual-task costs to standing and walking performance would be as high as 5%. We estimated that complete datasets on 61 participants, accounting for 20% attrition, would provide 90% power to detect a mean difference of 5% between any two intervention states with 0.05 type I error rate.
Sixty-one participants were recruited across two sites: 33 at Hebrew SeniorLife (Boston, MA, U.S) and 28 at Tel Aviv Sourasky Medical Center (Tel Aviv, Israel) (Figure 1). Inclusion criteria were age ≥65 years and the ability to stand or ambulate unassisted for at least 25 feet. Exclusion criteria were a Montreal Cognitive Assessment (MoCA) score <24, self-reported diagnosis of Parkinson’s disease or other neurodegenerative disorder; self-report of acute illness, injury or unstable medical condition or hospitalization within the past three months; self-reported active cancer or terminal diseases; any report of severe lower-extremity arthritis, pain, or orthopedic problems likely to affect gait and balance; self-report of peripheral neuropathy, or other peripheral neuromuscular disease; use of antipsychotics, anti-seizure, or other neuroactive medications; report or physician-diagnosis of schizophrenia or other psychiatric illness, or contraindications to tDCS (e.g., eczema, reported seizure within the past two years, use of neuroactive drugs, risk of metal objects or implanted devices in the brain, skull, or head).
Figure 1.
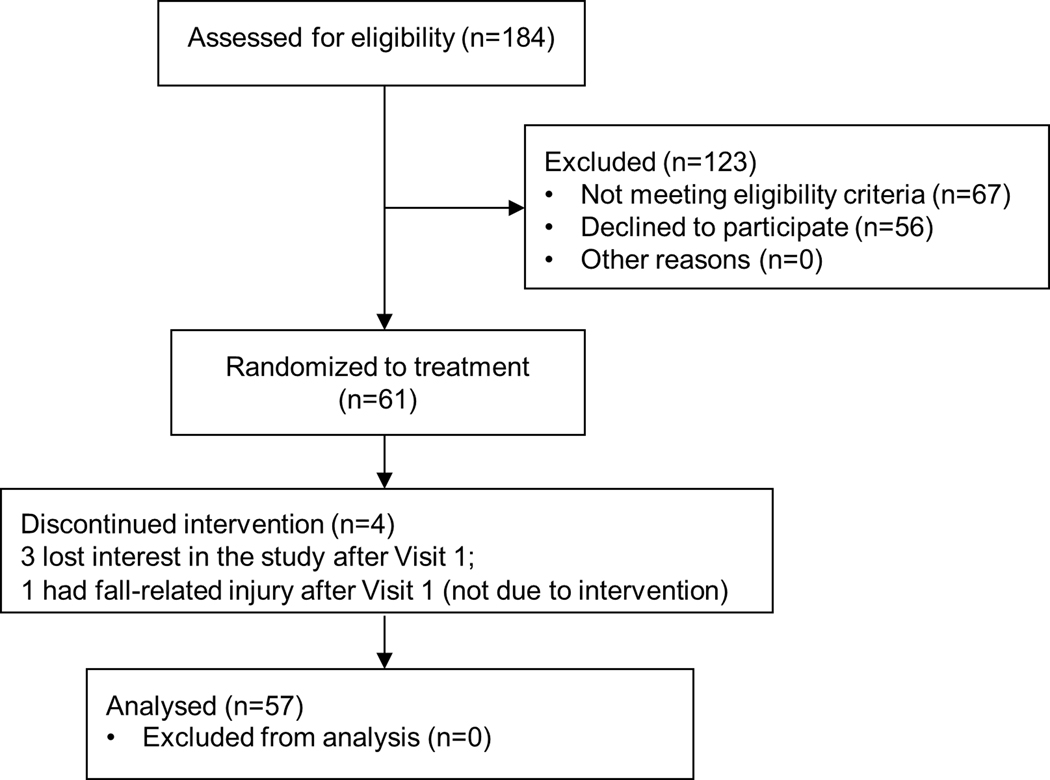
The CONSORT diagram.
Protocol
During the first visit (i.e., screening), individuals provided written informed consent of participation approved by the Institutional Review Boards of both sites. Demographics, height, weight, medical history, and medications were recorded and the MoCA was completed. Eligible participants completed the timed-up-and-go test of mobility23. A familiarization protocol was completed during which participants tried on the Neoprene cap used for tDCS. The dual-task paradigm was also described to participants before they practiced one, 60-second trial of serial subtractions while seated, standing, and walking. On each subsequent visit, brain stimulation and functional assessments at each site were administered by personnel formally trained by the study’s Project Director and blinded to stimulation condition.
Pre/Post stimulation functional assessments
Dual-task standing and walking
Standing was assessed by having participants complete two, 30-second trials in each of two conditions: eyes open (i.e., single task) and eyes-open while performing a serial subtraction task (i.e., dual-task)24–26; specifically, audibly counting backward by 3’s from a random 3-digit number (200~999) provided immediately before each trial. During each trial, participants were instructed to keep arms at side and feet shoulder-width apart. Foot placement was traced on the first trial and this tracing was used in all subsequent trials. Before each trial, participants were reminded to avoid extraneous movements and focus their vision on a small “X” drawn on a wall at eye-level approximately three meters away.
After finishing standing trials, participants completed two walking trials in each of two conditions: usual walking (i.e., single-task) and walking while performing the same serial-subtraction task described above (i.e., dual-task). Each trial consisted of walking down and back one time along a 20-meter straight walkway with a 180 degree turn at the end.
Within the standing and walking tests, trial condition order was randomized both before and after stimulation and across study visits. Standing postural sway speed and area, and gait speed and stride time variability when walking, which are linked to mobility, cognitive status, and falls risk6,7,12,13, were derived from kinematics acquired with a wearable system (APDM, Portland, OR)27. The sensors of this system were secured with Velcro straps over the left and right insteps (i.e., the dorsal surface of the foot in front of the ankle and above the arch), and the lumber spine. Stride time variability (STV) was defined as the coefficient of variation about the mean stride time of each trial. Stride times were defined by the elapsed time between consecutive heel strikes of each leg. Outcomes derived from the two trials of each condition were averaged. The dual-task cost (DTC) to each outcome was computed by calculating the percent change from single to dual-task conditions.
Within dual-task trials, the total number of verbalized serial subtraction responses and the number of errors were recorded. The error rate (%) was calculated by dividing the number of errors by the total responses and multiplying by 100.
Neuropsychological tests
The performance of dual-task standing and walking is linked to several aspects of executive function, including processing speed, attention, and inhibitory control28–30. We, therefore, used two neuropsychological tests to measure these aspects. The Stroop Color and Word Test (SCWT) is a common test of executive function that assesses the effect of processing a specific stimulus feature that interferes with the simultaneous processing of a second stimulus feature and demands inhibitory control31,32. The interference score of SCWT was calculated as described in previous studies31,32, with greater scores reflecting better ability to appropriately inhibit cognitive interference (i.e., cognitive inhibitory control). Four different validated versions of SCWT were used across stimulation visits and the cognitive inhibitory function was assessed pre- and post-tDCS testing within each stimulation visit. The Digit Symbol Substitution Test (DSST) assesses motor speed, attention, and visuo-perceptual function33. Participants were first given a key grid of numbers and matching symbols. During the 90-second test, participants filled in as many empty boxes with the numbers corresponding to each symbol from the key grid. The number of total responses and the incorrect responses was recorded. The error rate (i.e., ratio of incorrect to total responses) was used in analyses. Different test versions of DSST were used, in random order, to eliminate potential learning effects.
tDCS and sham stimulation
tDCS and sham stimulation were delivered with the participant sitting and resting. The Starstim® system (Neuroelectrics Inc., Barcelona, Spain) was connected to six round 3.14cm2 Ag/AgCl electrodes (NGPiStim electrodes, Neuroelectrics Inc.) positioned on the scalp and secured in place with a Neoprene cap. Conductive gel was placed below each electrode. Stimulation sessions were 20 minutes in duration, plus a 59-second ramp-up and ramp-down to maximize participant comfort.
The montage (electrode placement and current parameters) for each stimulation condition was developed using the Stimweaver® optimization technique17 on a realistic template head model34. The stimulation regions-of-interest (ROIs) (i.e., L-DLPFC and SM1) were determined via parcellation of Brodmann areas (i.e., L-DLPFC: BA 46; SM1: BA 1–4, within the leg area) (Figure 2). Each montage was designed to facilitate the excitability of the designated ROI, while optimally distributing the injected currents to minimize potential effects elsewhere in the brain. To do so, the Stimweaver algorithm was used to optimize the component of the electrical field normal to the cortical surface (i.e., En) over each ROI, as this component of the electric field polarizes cortical pyramidal cells17 and is linked to the effects of stimulation on cortical excitability. The target En-field was set to +0.25 V/m over each designated ROI, and 0 V/m over the remaining regions. To ensure safety, the current delivered by any one electrode did not exceed 1.5 mA and the total amount of current injected by all electrodes (i.e., anodes) was below 4 mA. Optimizations were further constrained such that the same placement of the six electrodes was used for all conditions to facilitate blinding of study personnel and participants. The above optimization procedure resulted in electrodes placed over AF4, CP1, FC1, Cz, F3, and FC5 positions of 10/20 EEG electrode system.
Figure 2. The targets of tDCS (A) and modeling of the normal component of the electric field (En) over the cortex as induced by montages targeting the left dorsolateral prefrontal cortex (L-DLPFC) and the primary sensorimotor cortex (SM1) (B), SM1 only (C), L-DLPFC only (D), and neither of these regions (i.e., active sham control) (E).
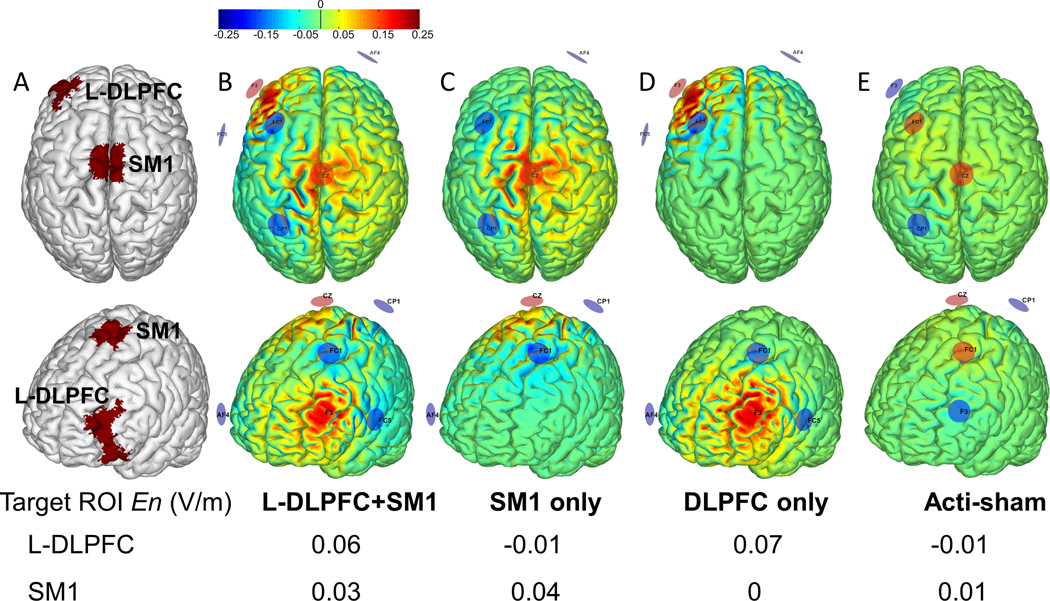
The SM1 and L-DPFC, as shown in red in A, were the target ROIs of tDCS. Modeling was carried out with the ‘Stimweaver’ algorithm on a standard brain to visualize the distribution of the component of the tDCS-induced electric field normal to the cortical surface. Warmer and cooler colors in B-E represent greater positive or negative normal component (En) of electrical field, respectively. Positive (current injecting, anodes) electrodes are in red; negative electrodes (cathodes) are in blue. The average En over the cortical surface of each ROI is presented in the table. Due to the constraints of location, size of the ROI, as well as the safety issue, the average En of SM1 leg region, which is located much deeper into the inter-hemispheric fissure as compared to L-DLPFC, was lower (See Discussion).
Sham stimulation was developed using the “acti-sham” approach19. Briefly, we used the same optimization procedure used to develop the active tDCS montages described above, but used a much smaller value for the target normal component of the electrical field, that is, 10−4 V/m. To avoid the trivial solution (all currents set to zero), the current in the electrodes at FC1 and Cz was set to a minimal intensity of 0.25 mA. The electrode pool involved in the optimization was restricted to the six electrode positions used in the active montages. The optimized montage thus employed current shunting to maximize current flow through cutaneous tissue (thereby inducing sensation) and achieve a relatively small electrical field at the level of the cortex throughout the 20-minute session.
Personnel uninvolved in tDCS administration preconfigured tDCS and sham stimulation parameters for each of the four montages. Administrators were blinded to stimulation type by using a password-protected “blinded” mode. Immediately after each stimulation session, participants completed a blinding efficacy questionnaire. They were asked to state whether they received tDCS, sham intervention, or were unsure of the type of stimulation they received on that day. If the participant believed that they received tDCS or sham, they were asked to state their confidence in this belief on a scale of 1 (not confident) to 3 (very confident). At the beginning and end of each visit, participants completed a side effects questionnaire35 (Table 2) to assess if they experienced any pre-to-post abnormal sensations or changes (e.g., sleepiness) potentially attributed to stimulation.
Table 2.
Number of self-reported side-effect events of tDCS and sham stimulation.
| tDCS condition | ||||
|---|---|---|---|---|
| Side effects† | Sham | SM1 only | L-DLPFC only | L-DLPFC+SM1 |
| Sensations under electrode | 5 (8.5%) | 2 (3.4%) | 5 (8.5%) | 1 (1.7%) |
| Skin redness | 0 | 0 | 0 | 0 |
| Headache | 0 | 0 | 0 | 0 |
| Neck pain | 1 (1.7%) | 0 | 0 | 0 |
| Sleepiness | 2 (3.4%) | 1 (1.7%) | 1 (1.7%) | 1 (1.7%) |
| Trouble concentrating | 0 | 0 | 0 | 1 (1.7%) |
| Acute mood change | 0 | 1 (1.7%) | 0 | 0 |
Side effects were recorded at the beginning and the end of each tDCS visits using a questionnaire adapted from Brunoni et al (2011). A side-effect event related to tDCS/sham was counted when the event was reported only at the end, but not at the beginning of each visit, or when the severity of the event was increased after stimulation as compared to before stimulation.
Statistical analysis
Statistical analyses were performed using JMP Pro 14 software (SAS Institute, Cary NC). Significance level was set to 0.05 for all analyses. Descriptive statistics (i.e., mean, standard deviation (SD)) were used to summarize demographic characteristics of participants and study outcomes. Shapiro-Wilk tests were used to examine if the data was normally distributed.
Primary analyses utilized two-way repeated-measures ANOVAs to examine the effects of tDCS on functional outcomes. The dependent variable of each model was the outcome of standing (i.e., sway speed and area) or walking (i.e., walking speed and stride time variability). Similar analyses were applied to the secondary outcomes including the error rate of the counting task during dual-task standing and walking, SCTW interference score, and the DSST error rate. Model effects included age, sex, site, stimulation condition (i.e., SM1 only, L-DLPFC only, L-DLPFC+SM1, sham), time (pre-, post-stimulation), and their interaction. Age and sex were included because each was associated with one or more metrics of standing or walking during pre-tDCS assessments; for example, women walked faster than men (F>4.8, p<0.02) and participants with older age had greater dual-task cost to walking speed (r>0.38, p<0.0001). Though we assumed no carryover effect of tDCS on functional performance owing to the 3-day washout periods, we examined potential period effects by also including visit order (i.e., Visit 1, 2, 3 and 4) and the interaction between visit order and time as model effects. Motivated by the results of the primary analyses, we conducted a secondary exploratory analysis using two-way ANOVAs to compare the effects of the stimulation conditions that included the left DLPFC (L-DLPFC only, L-DLPFC+SM1) to those that did not (SM1 only, sham). Tukey’s post-hoc testing was used to compare factor means of significant models. The effect size of tDCS was expressed as Cohen’s d.
Blinding efficacy and its effect on functional outcomes were determined in multiple steps. First, we utilized Kruskal-Wallis tests to determine whether the distribution of participants’ guesses of tDCS condition (i.e., real, sham, or unsure) differed between tDCS conditions. For this analysis, we only included guesses from the first stimulation visit, as participant guesses in subsequent visits may have been influenced by subjective experiences of the preceding visits. Second, we examined the effects of stimulation condition on the confidence of subjective guesses of real or sham stimulation using ANOVA models. Separate models were used to examine the effect of condition on the confidence of guessing real and sham within the first visit only. The dependent variable was the confidence scale respectively related to the subjective guess of real and sham. The model factor was stimulation condition. Third, we examined if the order in which type of stimulation was administered (i.e., visit order) influenced the proportion of participants who guessed ‘real’ using Kruskal-Wallis test. Fourth, for those stimulation conditions that led to improved functional performance, we examined the effects of subjective guess on the tDCS-induced change in functional outcome using repeated-measures ANOVA models. The model factor was the guess of stimulation type (i.e., real, sham, or unsure), and the dependent variables were the percent changes in functional outcomes from pre- to post-stimulation.
Results
Fifty-seven of 61 participants completed all study visits and were included in the analysis (Figure 1). The other four participants withdrew before completing any of the stimulation visits (three lost interest; one suffered a fall-related injury) and we thus excluded them from the analyses. Participants from the two study sites were similar in age, sex, body weight, height, MoCA score, and timed-up-and-go performance (p>0.71, Table 1). The percentages of reported tDCS side effects were small (Table 2), transient, and mild. All outcomes were normally distributed (p>0.11). Cohort means and standard deviations of functional outcomes, separated by both stimulation condition and time (pre/post stimulation), are presented in Table 3.
Table 1:
Participant characteristics
| Site | ||
|---|---|---|
| Tel Aviv | Boston | |
| Sample (n) | 26 | 31 |
| Sex (n, female) | 22 | 21 |
| Age range (years) | 65–86 | 66–91 |
| Age means (SD) (years) | 75±5 | 75±5 |
| Height (m) | 1.6 ± 0.1 | 1.6 ± 0.1 |
| Body mass (kg) | 71 ± 15 | 77 ± 18 |
| MoCA | 26 ± 3 | 27 ± 3 |
| TUG (sec) | 12 ± 3 | 12 ± 3 |
Table 3.
The effects of tDCS on cognitive, standing and walking performance (mean (SD)).
| tDCS condition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sham | SM1 only | L-DLPFC only | L-DLPFC+SM1 | p | ||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||
| Standing | ||||||||||
| Dual-task cost | ||||||||||
| -Sway speed (%) | 29±67B | 35±68B | 34±62B | 41±54B | 36±69B | 11±43A | 29±63B | 2±39A | 0.0001 | |
| -Sway area (%) | 72±81B | 75±69B | 64±79B | 76±91B | 63±68B | 27±49A | 67±71B | 21±43A | 0.006 | |
| Single task | ||||||||||
| -Sway speed (m/s) | 0.25±0.13 | 0.33±0.21 | 0.30±0.18 | 0.31±0.18 | 0.33±0.36 | 0.30±0.18 | 0.32±0.25 | 0.32±0.19 | 0.46 | |
| -Sway area (mm2/s4) | 35.8±42.1 | 34.7±23.7 | 39.2±29.9 | 44.5±43.7 | 34.6±19.3 | 40.1±25.7 | 44.6±36.8 | 37.2±18.4 | 0.51 | |
| Dual-task | ||||||||||
| -Sway speed (m/s) | 0.31±0.23B | 0.40±0.29B | 0.40±0.26B | 0.45±0.35B | 0.32±0.22B | 0.28±0.21A | 0.31±0.16B | 0.29±0.19A | 0.01 | |
| -Sway area (mm2/s4) | 57.5±5.5B | 58.7±48.1B | 56.8±43.7B | 62.7±45.7B | 59.8±46.9B | 45.0±27.3A | 70.2±53.2B | 46.2±24.4A | 0.02 | |
| Walking | ||||||||||
| Dual-task cost | ||||||||||
| -Speed (%) | −10±10B | −9±8B | −10±9B | −8±11B | −11±9B | −5±7A | −11±7B | −5±6A | 0.01 | |
| -ST variability (%) | 20±40 | 24±63 | 26±52 | 24±54 | 28±45 | 12±37 | 21±52 | 12±36 | 0.51 | |
| Single task | ||||||||||
| -Speed (m/s) | 1.08±0.18 | 1.09±0.19 | 1.08±0.19 | 1.09±0.2 | 1.10±0.2 | 1.10±0.2 | 1.11±0.2 | 1.09±0.2 | 0.96 | |
| ST variability | 3.2±1.2 | 3.2±1.2 | 2.9±1 | 2.9±0.8 | 2.8±1 | 2.8±0.7 | 3.2±1.6 | 2.7±0.8 | 0.59 | |
| Dual-task | ||||||||||
| -Speed (m/s) | 1.0±0.21 | 1.01±0.2 | 1.01±0.2 | 1.01±0.2 | 1.0±0.22 | 1.05±0.2 | 0.97±0.2 | 1.05±0.2 | 0.51 | |
| -ST variability | 3.8±1.8 | 3.6±1.6 | 3.7±1.7 | 3.5±1.9 | 3.5±1.7 | 3.4±1.4 | 3.8±2.0 | 3.3±1.5 | 0.87 | |
| Cognitive performance | ||||||||||
| DSST (%) | 1.6±2.7 | 2.3±3.5 | 1.6±2.4 | 1.8±3.3 | 2±4 | 1.4±2 | 2.1±3.5 | 1.3±1.6 | 0.22 | |
| SCWT | 0.1±10.5 | −1.2±10.5 | −0.2±8.9 | −1.1±10.1 | −2.9±10.2 | 1.4±8.6 | −1.1±10.4 | 0.2±9.7 | 0.18 | |
| Counting during standing | Total # | 22.8±12.9 | 22.5±11 | 22.7±11.1 | 22.5±11 | 21.8±11 | 21.9±12 | 21.1±11 | 22.3±10 | 0.83 |
| Error # | 0.9±1 | 0.9±1 | 1±1.2 | 0.8±1.1 | 0.9±0.9 | 0.6±0.7 | 0.8±1 | 0.8±1 | 0.81 | |
| Error rate (%) | 5.2±6.1 | 6.4±8.3 | 4.7±6.4 | 3.6±5.6 | 5.2±6.4 | 3.5±5.6 | 5±6.3 | 3.5±4.5 | 0.43 | |
| Counting during walking | Total # | 33.3±10.5 | 33.5±10.5 | 32.2±11 | 32.4±10.6 | 32.4±10.5 | 32.1±10.8 | 32.8±10.5 | 32.5±11 | 0.99 |
| Error # | 1.3±1.5 | 1.5±1.7 | 1.5±1.8 | 1.7±2.2 | 1.7±2 | 1.7±2.1 | 1.6±2.1 | 1.5±2.1 | 0.85 | |
| Error rate (%) | 4.4±5.7 | 4.6±5.7 | 5.8±6.9 | 5.5±7.2 | 6.5±8.3 | 4.4±5.4 | 4.7±5.3 | 4.8±6.4 | 0.63 | |
SM1 = targeting only sensorimotor cortex; DLPFC = targeting dorsolateral prefrontal cortex only; DLPFC+SM1: targeting both DLPFC and SM1 simultaneously; ST variability = coefficient of variation of stride time variability.
p value: tDCS condition and time interaction of 2 X 4 ANOVA adjusted for site.
Total #: total number of digits counted in dual-task condition;
Error #: total number of counting errors in dual-task condition;
Different superscript letters (A and B) within each row indicate mean that were significantly different from one another as determined by Tukey’s post-hoc testing of ANOVA models with a significant tDCS condition by time (pre-post tDCS) interaction term.
The effects of tDCS on standing performance
Two-way repeated-measures ANOVA models adjusted for age, sex, and site indicated significant interactions between stimulation condition and time (Table 3) for dual-task postural sway speed (F=3.4, p=0.01, Cohen’s d=0.68), dual-task postural sway area (F=3.5, p=0.02, Cohen’s d=0.8), and the dual-task costs to both sway speed (F=6.1, p=0.0001, Cohen’s d=0.84 Figure 3A) and area (F=4.6, p=0.006, Cohen’s d=0.7, Figure 3B). No effect of visit order or interaction between visit order and time were observed (F<0.64, p>0.4), indicating that the order in which stimulation conditions were administered did not influence the effects of tDCS on sway outcomes. Tukey’s post-hoc analyses revealed that dual-task postural sway speed (p<0.02) and area (p<0.01), and the dual-task costs to these two outcomes (p<0.003), were lower following both L-DLPFC only and L-DLPFC+SM1 tDCS, as compared to all other pre- and post-stimulation means (which were not significantly different from one another). On the other hand, tDCS did not influence postural sway speed or area in the single task standing condition (F<0.9, p>0.46). Within dual-task standing trials, serial subtraction performance was excellent (overall error rate: 5±6% with 95% confidence interval: 2–9%) and was unaffected by tDCS (F=0.9, p=0.43).
Figure 3. The effect of tDCS on the dual-task costs to metrics of standing (A and B) and walking (C and D) performance.
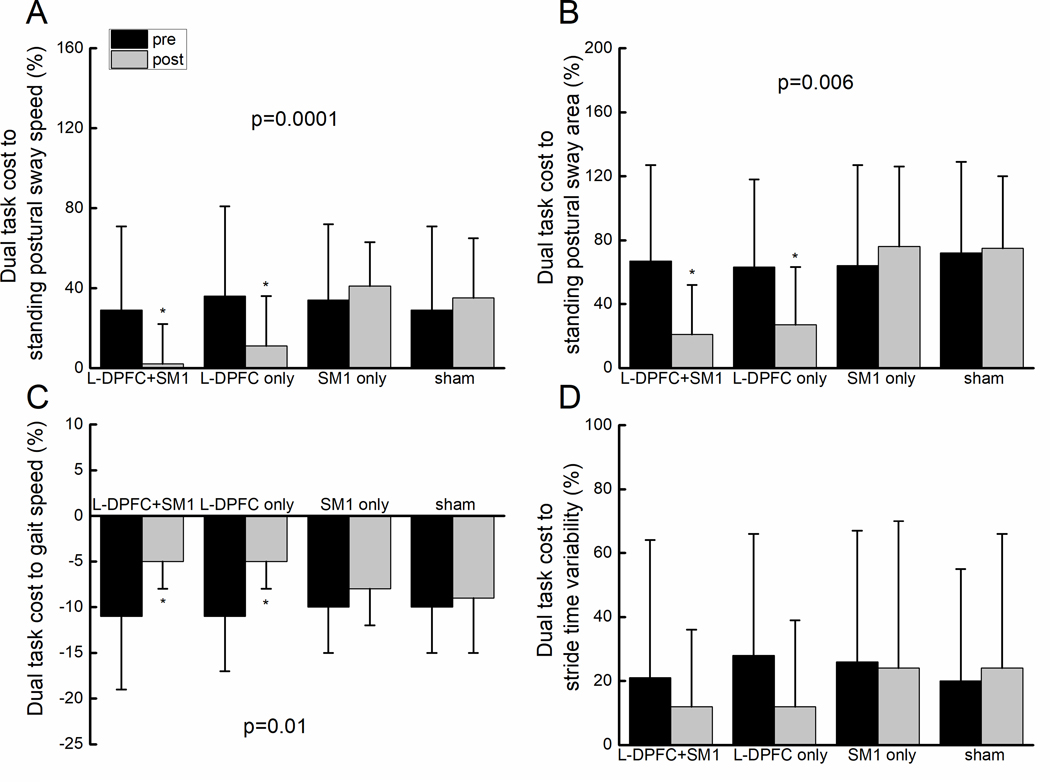
On Figures A, B and D, larger positive values on the Y-axis reflect greater dual task costs. On Figure C, larger negative values on the Y-axis reflect greater dual task costs; i.e., greater reduction in gait speed induced by dual tasking. Error bars reflect the 95% confidence interval. For each outcome, bars marked with an asterisk represent means that were different from all other means (yet not different from one another), as determined by Tukey’s post-hoc testing of ANOVA models with a significant interaction between tDCS condition and time (pre-post stimulation).
The effects of tDCS on walking performance
Two-way repeated-measures ANOVA models adjusted for age, sex and site reveal a significant interaction between stimulation condition and time (Table 3) on the dual-task cost to gait speed (F=3.8, p=0.01, Cohen’s d=0.81, Figure 3C). No effects of visit order or its interaction with time were observed (F<0.74, p>0.53), again suggesting that the order in which the stimulation conditions were administered did not influence the effects of tDCS on this outcome (i.e., no period effects). Tukey’s post-hoc analysis revealed that the dual-task cost to gait speed (p<0.03) was lower following DLPFC-only and L-DLPFC+SM1 tDCS, as compared to all other pre- and post-stimulation means. tDCS did not significantly influence gait speed or stride time variability in either single and dual-task conditions, or the dual-task cost to stride time variability (F<0.76, p>0.51, Figure 3D). Within dual-task walking trials, serial subtraction performance was excellent (overall error rate: 5±6%; range 0–30%) and was unaffected by tDCS (F=0.58, p=0.63).
The effects of tDCS on cognitive performance
Two-way repeated-measures ANOVA models revealed no significant interaction of stimulation condition and time (Table 3) on the SCWT interference score (F=1.6, p=0.18), or the DSST error rate (F=1.5, p=0.22). A significant effect of visit order on SCTW interference score (but not on DSST or counting task error rate) was observed (F=3.1, p=0.03), such that performance during Visit 2, 3 and 4 was significantly better than performance during Visit 1.
Comparison of stimulation that did and did not include the left DLPFC
Similar to the results of the primary analysis, two-way repeated-measures ANOVA models comparing L-DLPFC-targeted to L-DLPFC-not-targeted stimulation indicated that L-DLPFC-targeted stimulation reduced dual-task postural sway speed and area, dual-task costs to these two outcomes, and the dual-task cost to gait speed (F>6.8, p<0.009, Cohen’s d>0.6; Tukey’s post-hoc test: p<0.01). This model also revealed a significant interaction between stimulation category and time for the dual-task cost to stride time variability (F=4.2, p=0.04, Cohen’s d=0.32). Tukey’s post-hoc analyses indicated that the dual-task costs to stride time variability were lower following L-DLPFC-targeted stimulation (p<0.01), as compared to all other pre- and post-stimulation means (which did not differ from one another). No effects of visit order were observed (F<0.76, p>0.52). A trend towards significant interaction between stimulation category and time was observed for SCWT interference score (F=2.7, p=0.09), such that L-DLPFC-targeted stimulation appeared to induce greater improvement in SCWT performance. The stimulation category did not influence DSST or serial subtraction performance (F=0.94, p=0.33).
Blinding efficacy
The number and percentages of participant guesses of stimulation condition, by stimulation condition, are presented in Table 4. Kruskal-Wallis exact test based upon participant guesses following stimulation on the first visit indicated that the proportion of guesses for each intervention type were not significantly different from that expected by chance (p=0.29). Moreover, on the first visit, there was no difference in the confidence of guesses (real tDCS: p=0.14; sham: p=0.97) between stimulation conditions. Within each condition of stimulation, the order in which it was administered did not influence the number of participants who guessed ‘real’ (p>0.31).
Table 4.
Blinding efficacy of tDCS and sham stimulation.
| tDCS condition | |||||
|---|---|---|---|---|---|
| Within first visit | Sham (n=15) | SM1 only (n=16) | DLPFC only (n=14) | L-DLPFC+SM1 (n=12) | |
| Participant Guess | |||||
| - tDCS | Number of guesses | 7 (46.7%) | 7 (43.8%) | 11 (78.6%) | 8 (66.6%) |
| Confidence in guess† | 2.1±0.9 | 2.4±0.8 | 1.7±0.9 | 2.6±0.5 | |
| - Sham | Number of guesses | 2 (13.3%) | 1 (6.2%) | 1 (7.1%) | 3 (25%) |
| Confidence in guess† | 2±1.4 | 2 | 2 | 1.6±0.6 | |
| - Unsure | Number of guesses | 6 (40%) | 8 (50%) | 2 (14.3%) | 1 (8.4%) |
| Across four visits | |||||
| Participant Guess | Sham | SM1 only | DLPFC only | DLPFC+SM1 | |
| - tDCS | Number of guesses | 30 (52.6%) | 34 (59.6%) | 39 (68.4%) | 38 (66.6%) |
| Confidence in guess† | 2.3±0.7 | 2.3±0.8 | 2.2±0.8 | 2.4±0.7 | |
| - Sham | Number of guesses | 12 (21.1%) | 7 (12.2%) | 5 (8.8%) | 8 (14%) |
| Confidence in guess† | 2±0.9 | 1.8±1 | 2.4±0.5 | 1.8±0.7 | |
| - Unsure | Number of guesses | 15 (26.3%) | 16 (28.1%) | 13 (22.8%) | 11 (19.3%) |
The confidence scale ranged from 1 (not very confident) to 3 (highly confident)
Motivated by the observation that both L-DLPFC-only and L-DLPFC+SM1 tDCS improved dual-task performance, we examined the effects of subjective guess (i.e., real, sham, unsure) on the improvement of functional performance. Each stimulation condition was analyzed separately. ANOVA models revealed no effect of subjective guess on the improvement in standing dual-task postural sway speed or area, the dual-task cost to either of these outcomes or the dual-task cost to gait speed (L-DLPFC-only: F<1.1, p>0.35, L-DLPFC+SM1: F<0.94, p>0.39).
Discussion
This multi-center, randomized, double-blind study of multi-channel tDCS demonstrated that tDCS simultaneously targeting the L-DLPFC and SM1, and targeting only the L-DLPFC, both mitigated the dual-task costs to standing and walking in a relatively large sample of older adults without overt disease, compared to stimulation of SM1 or sham. In contrast to our expectations, the results did not support the hypothesis that the multi-target tDCS montage would induce greater benefit than the single-target montages. Instead, as tDCS targeting SM1 only did not induce benefits, our results indicate that facilitation of L-DLPFC excitability is critical to improving dual-task performance in this population. This conclusion was strengthened by results indicating that acti-sham stimulation effectively blinded participants to condition, and, that belief in the type of stimulation received did not contribute to observed improvements.
Dual-task costs to performance arise from insufficient and/or inappropriate allocation of neural resources shared by the two tasks36. Neuroimaging evidence indicates that activation of L-DLPFC increases during the performance of two concurrent cognitive tasks, especially when one or both tasks require verbalization37–40. Here, we employed neuro-modeling techniques and delivered tDCS via multiple gel-electrodes to not only control the current flow to target regions but also to dilute the electric field over non-target regions. Our results thus suggest that observed benefits to dual-tasking stemmed from modulating the neurons within the L-DLPFC region, as opposed to changing the brain state in general, or modulating of excitability within off-target regions. Future neuroimaging investigations into the functional changes within the L-DLPFC and its connected neural networks that resulted in improved dual-task performance are thus warranted.
“Resilience” is used in the physical sciences to describe the capacity of a strained body to recover its size and shape after stress-caused deformation41. Within the field of aging research, resilience has been used to describe the ability of older adults to cope and maintain performance when the bio-physiological system is stressed or demands on the system are increased41. A high level of resilience has been linked to successful aging42. As compared to standing or walking in single-task conditions, dual-tasking adds cognitive stress to the motor control system. The dual-task cost, which captures the impact of that stress on task performance, may thus be used to assess one’s resilience of cognitive-motor function43. The current results suggest that this resilience is modifiable via tDCS-induced modulation of L-DLPFC excitability in older adults, and warrant investigation into the underlying mechanisms through which tDCS induces such benefit.
tDCS targeting the SM1 leg area did not affect single- or dual-task standing and walking performance. Previous studies reported on the benefits of traditional tDCS over the motor cortex on motor learning performance of hand or lower extremities and mobility in older adults18,44. Conversely, a recent study reported that a 3-week tDCS intervention targeting M1 in combination with gait training did not improve walking performance in patients with Parkinson’s disease45. Such inconsistencies, along with the current results, suggest that dual-task standing and walking performance may not be substantially influenced by the excitability of the SM1 or its modulation via tDCS, at least in some settings.
The tDCS montages designed to target the L-DLPFC alone and the SM1 alone likely induced different electric field properties over each target. This is because the montages were optimized by placing similar weights (i.e., 0.25 V/m) to each anatomically-defined target region within the Stimweaver algorithm while the two regions differ in size, structure, and location within the brain. Electric field modeling of the optimized montages indicated that these differences, together with the other constraints placed on the optimization algorithm, resulted in montages that induced electric fields that were weaker over SM1 than L-DLPFC. In other words, the tDCS “dose” delivered to the SM1 was likely less than that of the L-DLPFC. Future research is thus needed to compare the effects of tDCS montages that induce similar doses over these two target regions. It should be considered, however, that adding this constraint to the optimization would presumably increase off-target electric field differences and/or require different electrode placements across montages that might hinder the ability to effectively double-blind to stimulation condition.
Primary and secondary analyses indicated that tDCS did not influence performance on the SDMT, SCWT, or the dual-task counting task. Lack of an effect on these tasks may have been due to ceiling effects, as baseline error rates were relatively low. Secondary analyses indicated a non-significant trend (p=0.09) towards improved performance in the SCWT following the two montages that targeted the L-DLPFC, as compared to the two montages that did not target this region. This result was likely confounded by an observed practice effect on this particular outcome, despite employing multiple versions of the test and administering them in random order across visits.
The regulation of standing and walking in relatively healthy older adults is dependent upon distributed sensorimotor networks that likely include regions not targeted in the current study46,47. tDCS targeting the premotor or supplementary motor cortices, for example, have also been reported to improve gait or balance in people with stroke48 or Parkinson’s disease49. It may, therefore, be worthwhile to test the effects of tDCS targeting these other regions on gait and balance, either alone or in combination with the L-DLPFC, in older adults without overt disease. At the same time, studies are needed to directly compare the effects of the multi-channel, model-driven approach to targeting the L-DLPFC, as used in the current study, with the traditional ‘low-resolution’ sponge-based approach to targeting this region.
tDCS targeting the L-DLPFC appeared to have a greater positive impact on standing as compared to walking. It is unclear if this result suggests that standing, relative to walking, is more dependent upon L-DLPFC function in the studied population, or if the metrics chosen to assess performance during standing were more sensitive to tDCS-induced changes in brain function. The current study focused on the immediate after-effects of tDCS. Studies are thus needed to examine the duration of benefit from one exposure, as well as the potential longer-term benefits of repeated exposures. As only one type of lab-based dual-tasking was used and a relatively healthy older adult cohort was included in this study, future work is also needed to examine the effects of tDCS targeting L-DLPFC on different types of dual-tasking, as well as other metrics of executive function included resource allocation when dual-tasking50, in both healthier older adults and those with more severe cognitive-motor limitations.
Acknowledgements
This work was supported by grants from the U.S.-Israel Binational Science Foundation (2015271) and the Boston Claude D. Pepper Older Americans Independence Center (P30-AG013679). Dr. Junhong Zhou was supported by a Hebrew SeniorLife Marcus-Applebaum pilot grant; Drs. Brad Manor and Junhong Zhou were supported by grants from the National Institutes of Health (R21 AG064575; R01 AG059089–01). Dr. Lipsitz holds the Irving and Edyth S. Usen Chair in Geriatric Medicine at Hebrew SeniorLife. Dr. Hausdorff was supported in part by Mobilise-D, which has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 820820. Dr. Hausdorff receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Footnotes
Potential Conflict of Interest
The noninvasive brain stimulation device used in this study was produced by Neuroelectrics Corp. and Starlab Neuroscience. Dr. Giulio Ruffini is a co-founder of these companies, Dr. Ricardo Salvador works for this company, and Dr. Pascual-Leone serves on their scientific advisory board. All the other authors state there is no conflict of interest.
References
- 1.Yogev‐Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Movement Disord 2008;23:329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou J, Poole V, Wooten T, Lo OY, et al. Multiscale dynamics of spontaneous brain activity is associated with walking speed in older adults. J Gerontol A-Biol 2020;75:1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillel I, Gazit E, Nieuwboer A, et al. Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur Rev Aging Phys Act 2019;16:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li KZ, Bherer L, Mirelman A, et al. Cognitive involvement in balance, gait and dual-tasking in aging: a focused review from a neuroscience of aging perspective. Front Neurol 2018;9:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srygley JM, Mirelman A, Herman T, et al. When does walking alter thinking? Age and task associated findings. Brain Res 2009;1253:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muir-Hunter SW, Wittwer JE . Dual-task testing to predict falls in community-dwelling older adults: a systematic review. Physiotherapy 2016;102:29–40. [DOI] [PubMed] [Google Scholar]
- 7.Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, et al. Association of dual-task gait with incident dementia in mild cognitive impairment: results from the gait and brain study. JAMA Neurol 2017;74:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swank C, Mehta J, Criminger C. Transcranial direct current stimulation lessens dual task cost in people with Parkinson’s disease. Neurosci Lett 2016;626:1–5. [DOI] [PubMed] [Google Scholar]
- 10.Wrightson JG, Twomey R, Ross EZ, et al. The effect of transcranial direct current stimulation on task processing and prioritisation during dual-task gait. Exp Brain Res 2015;233:1575–1583. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Hao Y, Wang Y, et al. Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. Eur J Neurosci. 2014;39:1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manor B, Zhou J, Jor’dan A, et al. Reduction of dual-task costs by noninvasive modulation of prefrontal activity in healthy elders. J Cog Neurosci 2016;28:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manor B, Zhou J, Harrison R, et al. Transcranial direct current stimulation may improve cognitive-motor function in functionally limited older adults. Neurorehab Neural Re 2018;32:788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaminski E, Steele CJ, Hoff M, et al. Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clin Neurophysiol 2016;127:2455–2462. [DOI] [PubMed] [Google Scholar]
- 15.Pilloni G, Choi C, Shaw MT, et al. Walking in multiple sclerosis improves with tDCS: a randomized, double-blind, sham-controlled study. Ann Clin Trans Neurol 2020;7:2310–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer DB, Fried PJ, Ruffini G, et al. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage 2017;157:34–44. [DOI] [PubMed] [Google Scholar]
- 17.Ruffini G, Fox MD, Ripolles O, et al. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage. 2014;89:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rostami M, Mosallanezhad Z, Ansari S, et al. Multi-session anodal transcranial direct current stimulation enhances lower extremity functional performance in healthy older adults. Exp Brain Res 2020;238:1–12. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Zhang M, Bu L, et al. Posture-related changes in brain functional connectivity as assessed by wavelet phase coherence of NIRS signals in elderly subjects. Behav Brain Res 2016;312:238–245. [DOI] [PubMed] [Google Scholar]
- 20.Fonteneau C, Mondino M, Arns M, et al. Sham tDCS: A hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul 2019;12:668–673. [DOI] [PubMed] [Google Scholar]
- 21.Neri F, Mencarelli L, Menardi A, et al. A novel tDCS sham approach based on model-driven controlled shunting. Brain Stimul 2020;13:507–516. [DOI] [PubMed] [Google Scholar]
- 22.Sohn MK, Jee SJ, Kim YW. Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Ann Rehab Med 2013;37:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 24.Hausdorff JM, Schweiger A, Herman T, et al. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A-Biol 2008;63:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirelman A, Maidan I, Bernad-Elazari H, et al. Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J NeuroEng Rehabil 2014;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beauchet O, Freiberger E, Annweiler C, et al. Test-retest reliability of stride time variability while dual tasking in healthy and demented adults with frontotemporal degeneration. J NeuroEng Rehabil 2011;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollman JH, Watkins MK, Imhoff AC, et al. A comparison of variability in spatiotemporal gait parameters between treadmill and overground walking conditions. Gait Posture 2016;43:204–209. [DOI] [PubMed] [Google Scholar]
- 28.Doi T, Shimada H, Makizako H, et al. Cognitive function and gait speed under normal and dual-task walking among older adults with mild cognitive impairment. BMC Neurol 2014;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wild LB, de Lima DB, Balardin JB, et al. Characterization of cognitive and motor performance during dual-tasking in healthy older adults and patients with Parkinson’s disease. J Neurol 2013;260:580–589. [DOI] [PubMed] [Google Scholar]
- 30.Doi T, Makizako H, Shimada H, et al. Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: a fNIRS study. Aging Clin Exp Res 2013;25:539–544. [DOI] [PubMed] [Google Scholar]
- 31.Scarpina F, Tagini S. The Stroop Color and Word Test. Front Psychol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verhaeghen P, De Meersman L. Aging and the Stroop effect: A meta-analysis. Psychol Aging 1998;13:120. [DOI] [PubMed] [Google Scholar]
- 33.Smith A. Symbol Digit Modalities Test. Los Angeles, LA: Western Psychological Services; 1973. [Google Scholar]
- 34.Miranda PC, Mekonnen A, Salvador R, Ruffini G. The electric field in the cortex during transcranial current stimulation. Neuroimage. 2013;70:48–58. [DOI] [PubMed] [Google Scholar]
- 35.Brunoni AR, Amadera J, Berbel B, et al. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 2011;14:1133–1145. [DOI] [PubMed] [Google Scholar]
- 36.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc 2012;60:2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirelman A, Maidan I, Bernad-Elazari H, et al. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cog 2017;115:41–46. [DOI] [PubMed] [Google Scholar]
- 38.Holtzer R, Mahoney JR, Izzetoglu M, et al. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A-Bio 2011;66:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maidan I, Nieuwhof F, Bernad-Elazari H, et al. The role of the frontal lobe in complex walking among patients with Parkinson’s disease and healthy older adults: an fNIRS study. Neurorehab Neural Re 2016;30:963–971. [DOI] [PubMed] [Google Scholar]
- 40.Nóbrega-Sousa P, Gobbi B, Orcioli-Silva D, et al. Prefrontal cortex activity during walking: Effects of aging and associations with gait and executive function. Neurorehab Neural Re 2020; 10.1177/1545968320953824 [DOI] [PubMed] [Google Scholar]
- 41.Angevaare MJ, Roberts J, van Hout HP, et al. Resilience in older persons: A systematic review of the conceptual literature. Ageing Res Rev 2020;63:101144. [DOI] [PubMed] [Google Scholar]
- 42.Moore RC, Eyler LT, Mausbach BT, et al. Complex interplay between health and successful aging: role of perceived stress, resilience, and social support. Am J Geriat Psychiatr 2015;23:622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitson HE, Duan-Porter W, Schmader KE, et al. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A-Bio 2016;71:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hummel FC, Heise K, Celnik P, et al. Facilitating skilled right hand motor function in older subjects by anodal polarization over the left primary motor cortex. Neurobiol Aging 2010;31:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schabrun SM, Lamont RM, Brauer SG. Transcranial direct current stimulation to enhance dual-task gait training in Parkinson’s disease: a pilot RCT. PloS One 2016;11:e0158497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurz MJ, Wilson TW, Arpin DJ. Stride-time variability and sensorimotor cortical activation during walking. Neuroimage 2012;59:1602–1607. [DOI] [PubMed] [Google Scholar]
- 47.Harada T, Miyai I, Suzuki M, et al. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res 2009;193:445–454. [DOI] [PubMed] [Google Scholar]
- 48.Kaski D, Dominguez RO, Allum JH, et al. Improving gait and balance in patients with leukoaraiosis using transcranial direct current stimulation and physical training: an exploratory study. Neurorehabil Neural Re 2013;27:864–871. [DOI] [PubMed] [Google Scholar]
- 49.Lu C, Huffmaster SLA, Tuite PJ, et al. The effects of anodal tDCS over the supplementary motor area on gait initiation in Parkinson’s disease with freezing of gait: a pilot study. J Neurol 2018;265:2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gage WH, Sleik RJ, Polych MA, et al. The allocation of attention during locomotion is altered by anxiety. Exp Brain Res 2003;150:385–394. [DOI] [PubMed] [Google Scholar]


