Abstract
Study Question:
What is the lowest safe and effective ulipristal acetate (UPA) dose to be released from a copper (Cu) intrauterine system (IUS) that may prevent the copper-induced increase in bleeding and avoid progesterone receptor modulator associated-endometrial changes (PAEC) occurrences in healthy women?
Summary answer:
The 20 μg/d dose meets the criteria of lowest safe and effective UPA dose that can promote a favorable bleeding profile and avoid PAEC occurrences.
What is known already:
UPA is a selective progesterone receptor modulator used to reduce the heavy menstrual bleeding associated with uterine fibroids. However, it induces specific benign and reversible histological endometrial changes (PAEC). Very low levels of UPA may decrease the amount of bleeding associated with copper intrauterine device (Cu-IUD) use, without interfering with ovulation and preventing the occurrence of PAEC.
Study design, size, and duration:
In this single-blinded, randomized, 3-group parallel proof-of-concept study, healthy women were randomized to receive a Cu-IUS releasing a low-dose of UPA (5, 20 and 40 μg/d) for a 12-week treatment period. The study included three periods: baseline, treatment and post-treatment follow-up. Women received a Cu-UPA-IUS between days 2 and 5 of menses and we removed it at 12 weeks of use. The post-treatment follow-up period was equivalent to two cycles starting after IUS removal. The sample size calculation was based on the average bleeding/spotting (B/S) days per cycle expected from ovulatory cycles among women not using any contraceptive and the changes promoted by a Cu-IUD. Considering a relative difference of 40% in the number of B/S days between the baseline period and the end of the third 4-week reference period of Cu-UPA-IUS use, and assuming 80% power, a two-sided 5% level of significance, 8 participants per group were required.
Participants/materials, setting, methods:
We included healthy women, 21–38 years old, with ovulatory cycles, not at risk for pregnancy at the time of inclusion. The primary outcome was the pharmacodynamic effects of a Cu-IUS releasing 5, 20 or 40 μg/d of UPA on bleeding patterns, ovarian function, and the occurrence of PAEC. The secondary outcomes were the pharmacokinetic characteristics and safety profile of the device. Participants filled a daily paper diary about any B/S they experienced. We collected blood and urine samples and performed transvaginal ultrasound. Endometrial biopsies were obtained during the baseline period, at the end of the treatment period and at post-treatment follow-up. For analysis purpose, we divided the 12-week treatment period into three 4-week treatment sequences and called each sequence as “treatment cycle”.
We used generalized linear mixed models with orthogonal contrasts to analyze quantitative variables and Chi-Square/Fisher test for categorial variables. The level of significance was set at 5%.
Results:
We randomized 29 women who had a successful IUS insertion; 27 completed the 12-week treatment period. Compared to baseline, the mean number of B/S days at treatment cycle 3 reduced by 2.6% in the 5 μg/d group (p=0.89), 17.1% in the 20 μg/d group (p=0.24), and 37.8% in the 40 μg/d group (p=0.03). Compared to baseline, the mean number of bleeding-only days at treatment cycle 3 reduced by 16.7% in the 5 μg/d group (p=0.66), 40.5% in the 20 μg/d group (p=0.14), and 77% in the 40 μg/d group (p=0.002). During the 12-week treatment period, ovulation occurred in most of the cycles (5 μg/d: 95.8%, 20 μg/d: 86.7%, 40 μg/d: 81.5%). The frequency of PAECs on the day of IUS removal was 10% (1/10), 10% (1/10) and 44.4% (4/9) in the 5 μg/d, 20 μg/d, and 40 μg/d groups, respectively. Most adverse events (AEs) were mild (87%, 90/104). There were no serious AEs. No AE led to treatment discontinuation.
Limitations, reasons for caution:
The results of this small proof-of-concept study will need to be confirmed in larger and long-term trials with a comparator such as a Cu-IUD.
Wider implications of the findings:
The novel Cu-UPA-IUS appears safe and well-tolerated and highly acceptable across all three UPA doses tested in this short-term proof-of-concept study. By preventing copper-induced increase in bleeding, this new IUS could provide a non-contraceptive benefit, especially for women with a low hemoglobin due to poor nutrition.
Study funding/competing interest(s):
RSW, MP, RM, HS, NK, and DL are employees of the Population Council, a not-for-profit organization that developed UPA for contraception. RSW received a research grant from the National Institute of Child Health and Human Development (NICHD) from the National institute of Health (NIH) as Project Director of a Contraception research center U54 HD 29990. CSV serves on Medical Advisory Boards and gives lectures for Bayer and Merck.
Keywords: ulipristal, copper intrauterine device, bleeding profile, progesterone receptor modulator associated endometrial changes, pharmacokinetic
1. Introduction
Intrauterine contraceptives (IUCs), including copper intrauterine devices (Cu-IUDs) and the levonorgestrel-releasing intrauterine system (LNG-IUS), are highly effective and safe long-acting reversible contraceptive methods (LARCs) [1,2]. Known side-effects represent a major barrier to both uptake of IUCs and method continuation. Uterine bleeding changes are the side-effects most frequently reported by IUC users and the leading cause of premature discontinuation [3–5]. Cu-IUD users often report an increase in the amount and number of days of bleeding [3,4]. In contrast, women using the 52 mg LNG-IUS report a reduction in the amount and number of days of bleeding [3,6]. However, prolonged and frequent bleeding/spotting are common during the first three months following the 52 mg LNG-IUS placement [7].
In order to reduce unfavorable bleeding changes associated with IUC use, we developed a novel IUS that delivers copper to provide its well-described contraceptive effect, in combination with a very low dose of ulipristal acetate (UPA), a selective progesterone receptor modulator (SPRM). We anticipated that very low levels of UPA could act on the endometrium [8] and decrease the amount of bleeding associated with Cu-IUD use, without interfering with ovulation. By maintaining ovulation, the presence of monthly progesterone secretion could prevent progesterone receptor modulator associated endometrial changes (PAEC). Such a novel system preventing copper-induced increase in bleeding could provide a non-contraceptive benefit, especially for women with a low hemoglobin due to poor nutrition.
Therefore, this 12-week proof-of-concept (POC) study was designed to assess the pharmacodynamic and pharmacokinetic outcomes of a novel Cu-IUS releasing 5, 20 and 40 μg/d of UPA in healthy women. We sought to determine the lowest safe and effective UPA dose released by the Cu-IUS to promote a favorable bleeding profile and avoid PAEC occurrences.
2. Material and Methods
2.1. Study design and settings
This was a single-blinded, randomized (ratio 1:1:1), 3-group parallel POC study of a new Cu-IUS releasing UPA.
Participants were consecutively screened and enrolled from April 2017 to January 2018 at one center, PROFAMILIA, in Santo Domingo, Dominican Republic. This study was conducted in accordance with Good Clinical Practice regulations, International Council for Harmonization guidelines, the Declaration of Helsinki, and was registered with clinicaltrials.gov (NCT03230539).
The protocol was approved by the Institutional Review Boards of the Population Council and of PROFAMILIA, as well as by the National Council of Bioethics in Health (CONABIOS) of the Dominican Republic. Each woman provided a written informed consent prior to screening and any study procedures.
2.2. Study population
Participants were healthy women of reproductive age, 21–38 years old, with a prior history of normal ovulatory cycles, i.e, 21–35 days duration when not using hormonal contraception. Eligible participants were not at risk for pregnancy at the time of inclusion (had undergone sterilization or were monogamous and their male partners had undergone sterilization). We excluded women with a known hypersensitivity to any component of the IUS or any contraindication to IUD, UPA and hormonal use according to the World Health Organization (WHO) eligibility criteria for contraceptive use [9]. Women who did not have at least two progesterone measurements ≥10 nmol/l (≥ 3.14 ng/ml) during the baseline cycle demonstrating normal ovulation were excluded from further participation in the study. All exclusion criteria are described in supplementary file 1.
2.3. Intervention
The IUS used in this POC study was developed in collaboration with HRA Pharma France that provided the UPA and a Good Manufacturing Practice manufacturer based in India [Hindustan LifeCare Limited (HLL)] with expertise in development and manufacturing of Cu-IUDs and the LNG-IUS. The novel IUS is a T-shaped polyethylene device, based on the design of an approved Cu-IUD and using the same inserter. The horizontal arms and the upper section of the vertical arm are wrapped in 200 square mm of copper wires and the lower part of the vertical arm includes a small reservoir that can be loaded with up to 36mg of UPA (Supplementary Figure 1).
We targeted UPA delivery doses of 5, 20 and 40 μg/d for the study. While the target duration of use for this Cu-IUS releasing UPA is 3 years, the duration of use for this POC study was 12 weeks.
2.4. Randomization
The sponsor of the study generated a randomization scheme using SAS version 9.4 (SAS Institute, Inc., Cary NC, USA), using a simple randomization procedure (ratio 1:1:1). The 3 types of IUS were consecutively numbered according to the randomization schedule.
2.5. Protocol assessments
The study included three periods: baseline, treatment and post-treatment follow-up (Supplementary file 2). The baseline period began on the first day of participant’s menses during the first cycle following screening. At their next menstrual cycle, women with confirmed ovulation were randomly assigned to have a Cu-IUS releasing 5, 20 or 40 μg of UPA daily. A physician at the study site inserted the IUS between days 2 and 5 of menses and removed it at 12 weeks of use. The post-treatment follow-up period (recovery cycle) was equivalent to 2 cycles starting after IUS removal. Follow-up visits occurred twice a week during the study. For analysis purpose, we divided the 12-week treatment period into three 4-week treatment sequences and called each sequence as “treatment cycle”.
At the screening visit, we assessed hemoglobin, hematocrit, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, cholesterol (total), glucose, blood urea nitrogen, creatinine, and urinalysis. We amended the original protocol to include HIV and hepatitis B testing at screening, following a confirmed case of HIV in one participant who had been enrolled and was then discontinued on day 23 after IUS insertion. At the end of treatment, we measured hemoglobin, hematocrit, AST, and ALT levels. At each visit, we performed a transvaginal ultrasound (TVU) and collected blood samples for estradiol and progesterone measurements. We sampled endometrial biopsies three times: at baseline (follicular phase); on the day of IUS removal (planned at the end of the 12-week treatment period); and at the second recovery cycle (follicular phase) (Supplementary file 2). Each participant had a pregnancy test prior to IUS insertion, before each biopsy and at the end of study visit.
2.6. Outcomes
The primary outcome was the pharmacodynamic effect of a Cu-IUS releasing 5, 20 or 40 μg/d of UPA on bleeding profiles, ovarian function and PAEC occurrences. The secondary outcomes were the pharmacokinetic characteristics and safety profile of the device. As an exploratory objective we investigated UPA concentration in the endometrial tissue.
2.6.1. Bleeding profile
The investigator instructed participants to record any bleeding and/or spotting they experienced daily in a paper diary as one of the following categories: no bleeding; spotting (any bloody vaginal discharge not requiring sanitary protection; use of panty liner permitted); normal (bleeding that requires protection similar to a typical day of the subject’s usual menses); heavy (bleeding that participant considers heavier than her usual menses, requires greater protection, may result in accidents or require use of extra protection to avoid accidents). For analysis purpose, bleeding-only days included normal and heavy bleeding. The diaries were reviewed at each visit.
At the end of the study visit, each participant completed a self-administered questionnaire to describe perception of bleeding patterns during IUS use (Supplementary file 3).
We used the number of bleeding/spotting (B/S) days women recorded during the cycle prior to IUS insertion (baseline period) and during each of the three treatment cycles as the primary bleeding profile outcome. Other analyses included: the number of bleeding-only and spotting-only days at the baseline period and during the treatment cycles; the change in the number B/S days at end of treatment cycle 3 for each participant compared to her baseline cycle; classification of the bleeding patterns using the WHO terminology [10,11] during the 12-week treatment period; and participants’ perceptions of their bleeding patterns during the use of the Cu-UPA-IUS.
2.6.2. Ovarian Function
We measured estradiol (E2) and progesterone (P) levels twice a week during the study. Clinic personnel trained in TVU conducted serial exams to measure the dominant follicle (mean of two largest perpendicular diameters), endometrium thickness, and evidence of follicle rupture (Supplementary file 2). Based on TVU and progesterone levels, we classified each cycle as ovulatory or not. Ovulation was defined as evidence of follicular rupture observed by TVU and two consecutive serum P values ≥ 10 nmol/L (3.14 ng/mL). Non-ovulatory cycles included ovulatory dysfunction, luteinized unruptured follicle (LUF), no follicular development, and follicular development without resolution as previously described [12].
2.6.3. Endometrial assessments
We assessed endometrium thickness by TVU twice a week. We described the percentage of endometrial biopsies with PAEC. Histopathological examination was performed in Edinburgh, Scotland by Dr A Williams. Endometrial aspects at the end of treatment were compared to biopsies from the baseline and the second recovery cycle. The pathologist classified the endometrium in one of the categories: 1. benign; 2. “hyperplasia without atypia” or “atypical hyperplasia/endometrioid intraepithelial hyperplasia”; 3. malignant neoplasm according to WHO 2014 classification [13]. The pathologist also observed whether polyps were absent or present (if present, further classified as benign, hyperplastic or carcinomatous). In addition, he recorded any non-physiological changes which included PAEC features such as: epithelial changes (further classified as secretory, mitotic or apoptotic changes); presence of extensive cysts; unusual vascular changes (further classified as chicken-wire capillaries, thick-walled vessels or ectatic vessels); or any other observation.
We used the immunohistochemical method to assess the Ki67 proliferation marker in glands and stroma. A semi-quantitative histoscore was provided for each endometrial compartment — glands and stroma — according to the percentage of nuclei showing positive staining. Ki67 gland histoscore was the percentage of glands stained x nuclear score (NS) (NS: 0=no staining; 1= < 10% of nuclei show staining in positively stained glands; 2= 10% – 50%; 3 = >50%). Ki67 stroma histoscore was the percentage stromal cells showing staining.
We also determined the expression of a tumor suppressor protein, p53, from a subsample of endometrial specimens taken at baseline, on the day of the Cu-UPA-IUS removal and at the recovery cycle, at Dr I Bagchi’s labs. We measured the quantitation of immunofluorescence intensity in endometrial glands, stroma, and epithelium using ImageJ software v1.52v. To accurately quantify the immunohistochemical findings, histoscores were performed.
2.6.4. Plasma and endometrial UPA concentration
We assessed UPA plasma levels 30 minutes, 2, 4, 8 and 24 hours after IUS insertion. Additional measures occurred on day 5 (±1 day), then weekly during weeks 2 to 12 after IUS insertion. We also assessed UPA levels in the endometrial tissue obtained on the day of IUS removal and during follicular phase of the second recovery cycle.
We measured UPA plasma and endometrial levels by liquid chromatography with tandem mass spectrometry (LC-MS/MS) method at SGS Cephac Europe (France).
2.6.5. Overall safety
Overall safety included hemoglobin and hematocrit levels, endometrial thickness, AST and ALT levels, incidence of PAEC, percentage of participants with adverse events (AEs), serious adverse events (SAEs) and AEs leading to premature discontinuation. We recorded AEs using the Medical Dictionary for Regulatory Activities and reported with the use of the Common Terminology Criteria for Adverse Events, version 4.0. When reports of liver toxicity were disclosed, occurring with the 5 mg tablets of UPA approved in the European Union for uterine fibroid treatment, we modified the protocol to include liver tests and re-consented the participants for this additional measure using saved serum samples at baseline and at end of treatment.
2.7. Sample Size
The primary outcome of this study was the average number of B/S days per cycle associated with the three doses of UPA released by a Cu-IUS. The sample size calculation was based on the average bleeding days per cycle expected from ovulatory cycles among women not using any contraceptive, i.e. 6 days [14]. Previous study results showed that the number of B/S days had increased by 41.7% at 3 months of Cu-IUD use [3]. Hence, a change in 40% in the number of B/S days at the end of treatment cycle 3 of Cu-UPA-IUS use would be relevant [3]. Therefore, considering a relative difference of 40% in the number of B/S days between the baseline cycle and the end of treatment cycle 3 of Cu-UPA-IUS use, and assuming 80% power, a two-sided 5% level of significance and a standard deviation of 1.48 [14], 8 participants per group were required. Considering an anticipated drop-out rate of approximately 20% [15], 10 women per group were enrolled. We calculated the sample size using SAS version 9.4 (SAS Institute, Inc., Cary NC, USA).
2.8. Statistical analysis
All participants who completed the 12-week treatment period were included in the per protocol analysis and all participants who had the IUS inserted at least one day were included in the intention-to-treat analysis (ITT). We used the ITT analysis for describing AEs and the percentage of biopsies with PAEC. In remaining analyses, we used the per protocol population.
For baseline characteristics, we used the Kruskal-Wallis test for quantitative variables and Chi-Square/Fisher tests for categorical variables.
We used generalized linear mixed models with orthogonal contrasts to assess the effect of UPA dose and time on the number of B/S days (Poisson regression), spotting-only days (Poisson regression), and bleeding-only days (exponential regression); on the endometrial thickness (restricted maximum likelihood -REML); endometrial glands and stroma Ki67 histoscore (REML); and levels of hemoglobin (REML), hematocrit (REML), AST (REML), ALT (REML), estrogen (REML), and progesterone (exponential regression).
We used descriptive statistics to summarize the change per subject in B/S days from baseline to the end of treatment cycle 3 (waterfall plot) [16]; the classification of the bleeding patterns using the WHO terminology during the 12-week treatment period; participants’ perception on bleeding patterns associated with the use of the Cu-UPA-IUS; UPA levels; endometrial glands, stroma and epithelial p53 histoscore; frequency of PAEC, AEs, and of ovulatory cycles.
We calculated the following pharmacokinetic parameters: area under the curve (AUC), maximum concentration (Cmax), and the time to reach the maximum concentration (Tmax). We used a noncompartmental approach to assess AUC and assigned a value of zero to levels below the limit of quantification. Statistical analyses were carried out with the use of SAS version 9.4. The level of significance was set at 5% for all comparisons.
3. Results
We screened 49 women and excluded 15 for not meeting eligibility criteria. Of the 34 women eligible to be randomized, 5 withdrew consent before the randomization and we randomized 29 women. All randomized women had a successful IUS insertion and 27 women completed the study (Figure 1). In the 5 μg/d group, 2 participants discontinued early. One participant disclosed she had HIV and was discontinued on day 23 after IUS insertion for a protocol deviation; the other discontinued on day 21 after IUS insertion due to partial expulsion of the device. The ITT included the 29 randomized participants, whereas the per protocol analysis included the 27 participants who completed the 12-week treatment period (Figure 1).
Figure 1 -.

Study flowchart
Table I shows the baseline characteristics of participants included in the ITT analysis. The baseline characteristics did not differ among the groups.
Table I –
Baseline characteristics of the study groups according to the ulipristal acetate dose released by a copper intrauterine system
| Variables | 5 μg UPA/d N = 10 | 20 μg UPA/d N = 10 | 40 μg UPA/d N = 9 | P value |
|---|---|---|---|---|
|
| ||||
| Median (IQR) * | ||||
|
| ||||
| Age (years) | 34.0 (29.0–35.0) | 33.0 (27.0–35.0) | 31.0 (30.0–35.0) | 0.15 |
| BMI (Kg/m2) | 26.3 (21.3–28.1) | 24.8 (21.9–27.8) | 25.3 (23.7–26.5) | 0.10 |
| Schooling (years) | 9.0 (8.0–12.0) | 11.0 (7.0–14.0) | 11.0 (8.0–13.0) | 0.34 |
|
| ||||
| N (%) ** | ||||
|
| ||||
| Parity | 0.75 | |||
| 1–2 | 1 (10) | 2 (20) | 2 (22.2) | |
| ≥ 3 | 9 (90) | 8 (80) | 7 (77.8) | |
| Race | 0.39 | |||
| Black | 1 (10) | 0 | 0 | |
| White | 1 (10) | 0 | 0 | |
| Mixed | 8 (80) | 10 (100) | 9 (100) | |
| Ethnicity | 0.99 | |||
| Latina/Hispanic | 10 (100) | 10 (100) | 9 (100) | |
| Marital Status | 0.29 | |||
| Married/Cohabiting | 10 (100) | 8 (80) | 7 (77.8) | |
| Other1 | 0 | 2 (20) | 2 (22.2) | |
| Non-smokers | 10 (100) | 10 (100) | 9 (100) | 0.99 |
| Previous IUD use | 0 | 1 (10) | 0 | 0.37 |
| Usual volume of menstrual flow | 0.52 | |||
| Scanty (1–2 pads/day) | 2 (20) | 1 (10) | 2 (22.2) | |
| Moderate (3–4 pads/day) | 8 (80) | 8 (80) | 5 (55.6) | |
| Heavy (> 4 pads/day) | 0 | 1 (10) | 2 (22.2) | |
| Usual duration of menses | 0.43 | |||
| < 3 days | 3 (30) | 1 (10) | 3 (33.3) | |
| 4–7 days | 7 (70) | 9 (90) | 6 (66.7) | |
Kruskal-Wallis test
Chi-Square or Fisher’s exact test
IQR: interquartile range; UPA: ulipristal acetate; IUD: intrauterine device; BMI: body mass index
: Includes single, divorced and other.
Intention-to-treat population included the 29 randomized participants
3.1. Bleeding profile
There were no missing data in the bleeding diaries among the 27 women who completed the baseline and 12-week treatment period.
At baseline, the groups had a similar mean number of B/S, bleeding-only and spotting-only days (Table II; Supplementary Figure 2 panels A–C). Compared to baseline, the mean number of B/S days at treatment cycle 3 declined by 2.6% in the 5 μg/d group (p=0.89), 17.1% in the 20 μg/d group (p=0.24), and 37.8% in the 40 μg/d group (p=0.03) (Table II; Supplementary Figure 2 panel A). Compared to baseline, the mean number of bleeding-only days at treatment cycle 3 declined by 16.7% in the 5 μg/d group (p=0.66), 40.5% in the 20 μg/d group (p=0.14), and 77% in the 40 μg/d group (p=0.002). Women in the 40 μg/d group also had a 64% reduction in the mean number of bleeding-only days at treatment cycle 2 compared to baseline (p=0.01) (Table II; Supplementary Figure 2 panel B). Compared to baseline, the mean number of spotting-only days at treatment cycle 3 did not change in any group (Table II; Supplementary Figure 2 panel C).
Table II –
Number of bleeding and/or spotting days according to the ulipristal acetate dose released by a copper intrauterine system during a 12-week treatment period
| UPA dose | Baseline | Treatment cycle 1 | Treatment cycle 2 | Treatment cycle 3 |
|---|---|---|---|---|
|
| ||||
| Bleeding and Spotting days - mean (SD) | ||||
|
| ||||
| 5 μg/d (n= 8) | 6.1 (2.3) | 8.0 (3.9) | 6.4 (3.9) | 6.0 (2.3) |
| 20 μg/d (n=10) | 7.0 (2.4) | 8.3 (4.6) | 6.7 (2.9) | 5.8 (2.7) |
| 40 μg/d (n=9) | 5.9 (1.8)a | 7.0 (2.4) | 4.6 (2.9) | 3.7 (1.7)a |
|
| ||||
| Bleeding-only days - mean (SD) | ||||
|
| ||||
| 5 μg/d (n= 8) | 3.6 (2.7) | 5.1 (5.1) | 3.2 (3.2) | 3.0 (2.7) |
| 20 μg/d (n=10) | 4.2 (1.0) | 4.4 (2.5) | 3.1 (3.5) | 2.5 (3.1) |
| 40 μg/d (n=9) | 3.9 (2.3)b,c | 3.7 (1.4) | 1.4 (1.5)b | 0.9 (1.4)c |
|
| ||||
| Spotting-only days - mean (SD) | ||||
|
| ||||
| 5 μg/d (n= 8) | 3.2 (0.7) | 3.7 (2.1) | 3.6 (2.8) | 3.4 (1.1) |
| 20 μg/d (n=10) | 3.4 (2.2) | 4.9 (3.7) | 4.0 (1.1) | 3.8 (2.1) |
| 40 μg/d (n=9) | 2.6 (2.0) | 3.8 (2.3) | 3.3 (2.4) | 2.8 (1.9) |
UPA: Ulipristal acetate; SD: Standard deviation
Same letters (a, b, c) indicate a comparison with p < 0.05.
Bleeding and spotting, bleeding-only, and spotting-only days analyzed by a generalized linear mixed model with orthogonal contrasts
Per protocol population includes all participants who completed 12 weeks of intrauterine system use (n=27)
We also did a descriptive analysis of each participant’s percentage of change in the number of B/S days from baseline to treatment cycle 3 per study group using the waterfall chart. Most of the participants had either no change or a reduction in the number of B/S days at cycle 3 compared to baseline. Compared to baseline, we observed at least a 20% decrease in the number B/S days at treatment cycle 3 in 37.8% (3/8), 50% (5/10), and 77.8% (7/9) of the participants from the 5 μg/d, 20 μg/d, and 40 μg/d groups, respectively (Figure 2).
Figure 2 –

Percentage of change in the number of bleeding and spotting days from baseline to treatment cycle 3 for each participant according to ulipristal dose released by a copper intrauterine system
The most common bleeding pattern was a normal frequency of bleeding episodes. Participants had 3 or 4 bleeding and/or spotting episodes over a 90-day period. None of the participants reported amenorrhea or frequent or prolonged bleeding episodes (Table III).
Table III -.
Bleeding patterns in a 90-day reference period according to the ulipristal acetate dose released by a copper intrauterine system
| Bleeding episodes in 90-day reference period | 5 μg UPA/d N = 8 | 20 μg UPA/d N=10 | 40 μg UPA/d N=9 |
|---|---|---|---|
|
| |||
| Frequency – n (%) | |||
| Amenorrhea | - | - | - |
| Infrequent | - | 1 (10) | 1 (11) |
| Normal | 8 (100) | 9 (90) | 8 (89) |
| Frequent | - | - | - |
| Duration – n (%) | |||
| Prolonged | - | - | - |
| Not prolonged | 8 (100) | 10 (100) | 9 (100) |
UPA: ulipristal acetate
Per protocol population includes all participants who completed 12 weeks of intrauterine system use (n=27)
Amenorrhea: no bleeding or spotting (B/S) episodes in a 90-day reference period (RP); Infrequent: <3 B/S episodes in a 90-day RP; Normal frequency: 3–5 B/S episodes in a 90-day RP; Frequent: >5 B/S episodes in a 90-day RP.
Based on bleeding questionnaire responses, nearly all participants were satisfied/extremely satisfied with the bleeding pattern they experienced across Cu-UPA-IUS doses (5 μg/d: 75% vs 20 μg/d: 100% vs 40 μg/d: 100%). No participant expressed dissatisfaction with their bleeding pattern. Many participants reported no change in the number of bleeding episodes and of bleeding days with the Cu-UPA-IUS, but the majority reported a reduction in the amount of bleeding they experienced with all UPA doses, i.e., 50% (4/8), 80% (8/10), and 77.8% (7/9) for the 5 μg/d, 20 μg/d, and 40 μg/d groups, respectively (Table IV).
Table IV –
Participants’ perception of the bleeding pattern experienced within 12 weeks of using a copper intrauterine system releasing 5, 20 or 40 μg of ulipristal acetate per day
| 5 μg UPA/d (n= 8) | 20 μg UPA/d (n= 10) | 40 μg UPA/d (n= 9) | |
|---|---|---|---|
|
| |||
| Number of periods experienced compared to usual – n (%) | |||
| Fewer | 1 (12.5) | - | 3 (33.3) |
| No change | 7 (87.5) | 9 (90) | 6 (66.7) |
| More | - | 1 (10) | - |
| Number of bleeding days experienced compared to usual – n (%) | |||
| Fewer | 1 (12.5) | 2 (20) | 4 (44.4) |
| No change | 6 (75) | 5 (50) | 5 (55.6) |
| More | 1 (12.5) | 3 (30) | - |
| Amount of bleeding experienced compared to usual – n (%) | |||
| Decreased a lot | 1 (12.5) | 3 (30) | 3 (33.3) |
| Decreased some | 3 (37.5) | 5 (50) | 4 (44.4) |
| No change | 2 (25) | 1 (10) | 2 (22.2) |
| Increased some | 2 (25) | 1 (10) | - |
| Increased a lot | - | - | - |
| Satisfaction with overall bleeding pattern with the Cu-UPA-IUS use – n (%) | |||
| Extremely satisfied | 1 (12.5) | 3 (30) | 3 (33.3) |
| Satisfied | 5 (62.5) | 7 (70) | 6 (66.7) |
| Neutral | 2 (25) | - | - |
| Dissatisfied /Extremely dissatisfied | - | - | - |
UPA: Ulipristal acetate; Cu-UPA-IUS: copper intrauterine system releasing ulipristal
Per protocol population includes all participants who completed 12 weeks of intrauterine system use (n=27)
3.2. Endometrial assessments
Mean endometrial thickness did not differ across the study periods within each group (Supplementary Table I).
We assessed the frequency of PAEC at baseline (n=29), on the day of the Cu-UPA-IUS removal (n=29) and at the recovery cycle (n=28). There were no PAECs in baseline or recovery cycle samples. On the day of the IUS removal, 6 samples (20.7%) exhibited PAEC. The 40 μg/d group had the highest frequency of PAEC [5 μg/d: 10% (1/10), 20 μg/d: 10% (1/10), 40 μg/d: 44.4% (4/9)]. All PAECs were benign (Table V).
Table V –
Ovulation and histopathological assessment of the endometrium according to the ulipristal dose released by a copper intrauterine system
| Total | 5 μg UPA/d | 20 μg UPA/d | 40 μg UPA/d | |
|---|---|---|---|---|
|
| ||||
| Histopathological assessment of the endometrium (samples) – ITT population | ||||
|
| ||||
| Baseline | N= 29 | N=10 | N= 10 | N=9 |
| Proliferative | 25 (86.2) | 8 (80) | 8 (80) | 9 (100) |
| PAEC | 0 | 0 | 0 | 0 |
| Non-physiological non-PAEC changes 1 | 4 (13.8) | 2 (20) | 2 (20) | 0 |
| At Cu-UPA-IUS removal | N= 29 | N=10 | N= 10 | N=9 |
| Proliferative | 12 (41.4) | 4 (40) | 6 (60) | 2 (22.2) |
| Secretory | 6 (20.7) | 4 (40) | 1 (10) | 1 (11.1) |
| PAEC | 6 (20.7) | 1 (10) | 1 (10) | 4 (44.4) |
| Non-physiological non-PAEC changes 2 | 5 (17.2) | 1 (10) | 2 (20) | 2 (22.2) |
| Recovery cycle * | N=28 * | N=9 * | N=10 | N=9 |
| Proliferative | 27 (96.4) | 9 (100) | 9 (90) | 9 (100) |
| PAEC | 0 | 0 | 0 | 0 |
| Non-physiological non-PAEC changes 3 | 1 (3.6) | 0 | 1 (10) | 0 |
|
| ||||
| Ovarian Function (cycles) – PP population | ||||
|
| ||||
| Baseline | N=27 | N=8 | N= 10 | N=9 |
| Ovulation – n (%) | 27 (100) | 10 (100) | 10 (100) | 9 (100) |
| LUF – n (%) | 0 | 0 | 0 | 0 |
| Follicular development without resolution – n (%) | 0 | 0 | 0 | 0) |
| 12-week treatment period | N=81 | N=24 | N=30 | N=27 |
| Ovulation – n (%) | 71 (87.6) | 23 (95.8) | 26 (86.7) | 22 (81.5) |
| LUF – n (%) | 6 (7.4) | 0 | 1 (3.3) | 5 (18.5) |
| Follicular development without resolution – n (%) | 4 (4.9) | 1 (4.2) | 3 (10) | 0 |
1 participant from the 5 μg/d did not have an endometrial biopsy from the recovery cycle due to partial expulsion of the IUS.
UPA: Ulipristal acetate; PAEC: progesterone receptor modulator associated endometrial changes; LUF: luteinized unruptured follicle.
ITT: Intention-to-treat population included the 29 randomized participants.
PP: Per protocol population includes all participants who completed 12 weeks of intrauterine system use (n=27)
: Asynchronous development with necrotic decidualized stroma (5 μg/d), active chronic endometritis (5 μg/d), 2 samples with mildly disordered proliferation pattern (20 μg/d)
: Active chronic endometritis with a small benign endometrial polyp (5 μg/d), mildly disordered proliferation pattern (20 μg/d), distorted gland architecture with mucinous metaplasia (20 μg/d), asynchronous glandular development (40 μg/d), small benign endometrial polyp seen in background of otherwise normal proliferative endometrium (40 μg/d)
: Disordered proliferation pattern consistent with anovulatory state (20 μg/d)
There were no signs of endometrial hyperplasia in any of the biopsies studied (Table V).
The mean Ki67 gland histoscore was significantly lower at the end of the treatment cycle 3 and at the recovery cycle compared to baseline in all study groups. The mean Ki67 stroma histoscore was significantly lower at the end of the treatment cycle 3 and at the recovery cycle compared to baseline only in the 40 μg/d group. Histoscores from the baseline period and recovery cycle were similar for all groups (Supplementary figure 2).
Histoscores of endometrial p53 immunostaining in stroma, epithelium, and glands were not suppressed at the day of IUS removal and at the recovery cycle compared to baseline in all study groups (Supplementary figure 3).
3.3. Adverse events, liver enzymes, and hemoglobin and hematocrit levels
There were no SAE reports. All participants, except one, experienced at least one AE, with 104 AEs reported overall. The majority were mild (87%, 90/104). The most frequently reported AE was lower abdominal pain (72.4%, 21/29) that was mostly reported on the initial days following IUS insertion and accounted for all AEs related to study product. Eight women (27.6%, 8/29) experienced bacterial vaginosis. No AEs led to treatment discontinuation (Supplementary table II).
Hemoglobin and hematocrit levels did not change during the course of the study except at treatment cycle 3 for women in the 5 μg/d group. Compared to baseline, hemoglobin levels reduced by 6.7% (p<0.001) and hematocrit levels reduced by 6.1% (p=0.001) at treatment cycle 3 in the 5 μg/d group (Supplementary table III).
Compared to baseline, AST levels of the 5 μg/d group reduced by 18% (p=0.04) at treatment cycle 3. In the other groups, AST levels did not present significant changes. Compared to baseline, ALT levels reduced by 36.8% (p=0.02) in the 5 μg/d group and by 41% (p=0.001) in the 40 μg/d group at treatment cycle 3. The other comparisons were not statistically significant (Supplementary table III).
AST and ALT levels remained within the normal range (≤ 33 IU/L) for all participants, except for one participant of the 20 μg/d group who experienced a minor increase in AST/ALT levels at the end of treatment cycle 3 visit. The increase was transient, below 2 times the upper limit of the normal (ULN) range for AST/ALT and resolved without any medical intervention 25 days after IUS removal. Since this participant was an outlier in the 20 μg/d group, we did a sensitivity analysis of AST and ALT levels after removing this participant. Compared to baseline, AST levels did not present significant changes at treatment cycle 3 in the 20 μg/d group. However, as observed for the other groups, ALT levels reduced by 36.2% (p=0.001) in the 20 μg/d group at the treatment cycle 3 compared to baseline period (Supplementary table III).
3.4. Pharmacokinetic assessments
We observed a dose-proportional increase in the mean UPA plasma levels in the first week of IUS placement in all treatment groups, followed by a stabilization of the UPA levels. All plasma UPA levels were below 0.30 ng/mL (Figure 3). The time to reach the maximum UPA concentrations (tmax) was 0.13 days (3 hours) for the 5 and 20 μg/d groups, and 8.5 days for the 40 μg/d group (Supplementary Table IV). Endometrial levels of UPA on the day of IUS removal were high (Figure 3); and endometrium/plasma ratios were 3309.4, 3080.0, and 1529.2 for the 5 μg/d, 20 μg/d, and 40 μg/d groups, respectively.
Figure 3.
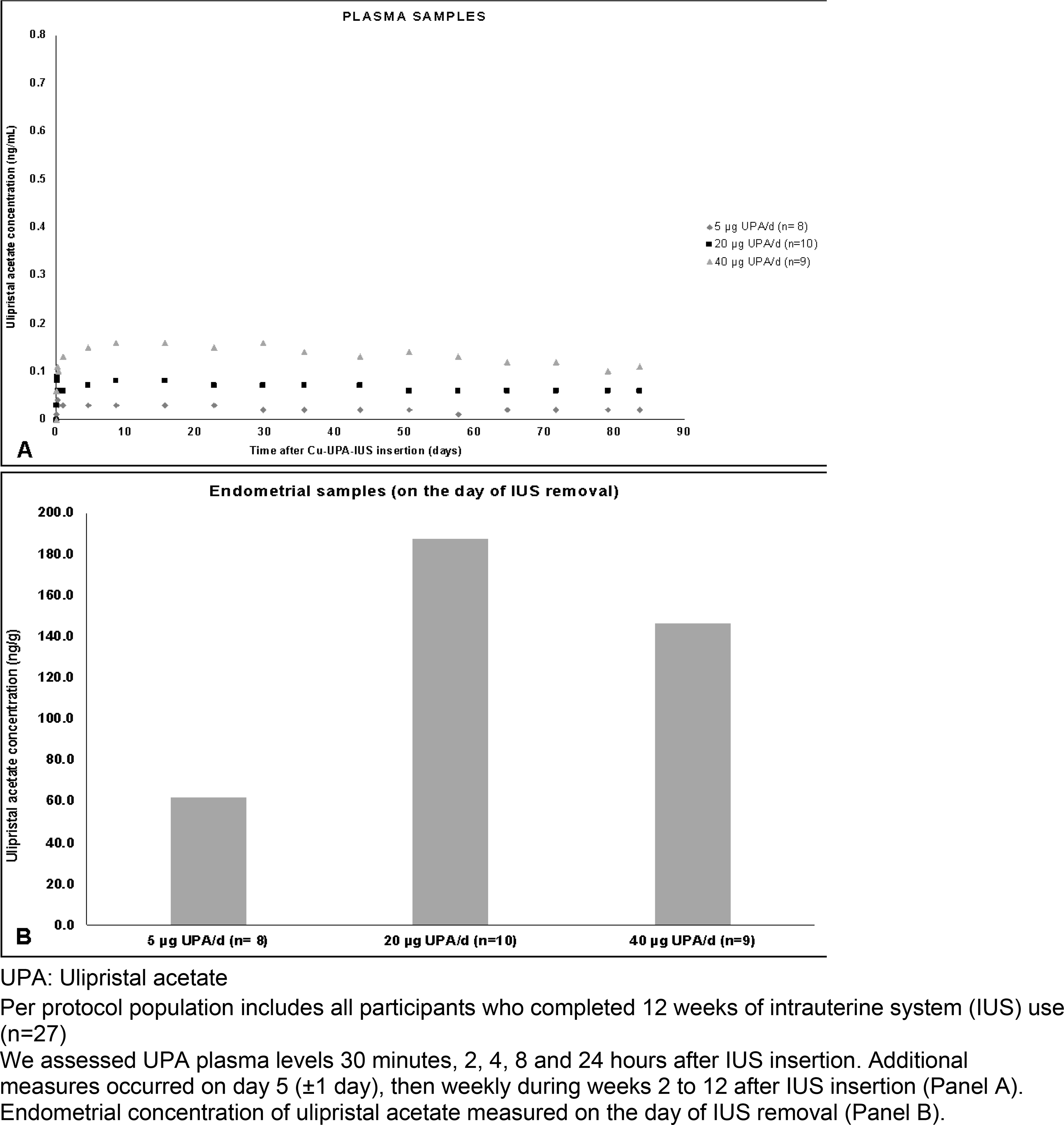
Mean ulipristal acetate concentration according to the ulipristal acetate dose released by a copper intrauterine system (Panel A: Plasma samples; Panel B: Endometrial samples)
3.5. Ovulation and hormone levels
All participants ovulated in the baseline cycle. Ovulation occurred in most of the treatment cycles during the 12-week treatment period (5 μg/d: 95.8%, 20 μg/d: 86.7%, 40 μg/d: 81.5%) (Table V).
Mean estradiol and progesterone levels were similar across the study periods within each group (Supplementary Table I).
4. Discussion
4.1. Pharmacodynamics and effects on bleeding profile
Ovulation occurred in 87.6% of the cycles, confirming our hypothesis that low doses of UPA have little effect on the ovulation. In contrast to the expected copper-induced increases in bleeding, most Cu-UPA-IUS users experienced either no change or a reduction in the number of B/S days over a 12-week treatment period. Women in the 20 μg/d and 40 μg/d groups reported a reduction in the mean number of bleeding-only days of 40.5% and 77%, respectively. A reduction in the mean number of B/S days was most pronounced in the 40 μg/d group.
In response to a bleeding questionnaire, nearly all participants (92.6%) were satisfied or extremely satisfied with the bleeding pattern they experienced; the remaining 7.4% were neutral. No participant expressed dissatisfaction with their bleeding pattern. Additional questionnaire responses indicated another positive change in the bleeding profile, namely, a reduction in the amount of bleeding as reported by 50%, 80%, and 77.8% of participants using the Cu-UPA-IUS containing 5 μg/d, 20 μg/d, and 40 μg/d, respectively. While this reduced bleeding finding will require further study to confirm these results, it differs markedly from the experience of Cu-IUD users who usually report a significant increase in the number of B/S days and in amount of the menstrual blood loss, especially in the first 6 months of IUD use [3,16]. Increase in bleeding is the most common reason cited for early discontinuation of the Cu-IUD [3–5].
Hemoglobin levels did not decline in the 20 μg/d and 40 μg/d groups. Although the 5 μg/d group was not associated with an increase in bleeding, this UPA dose may not be sufficient to avert the reduction in hemoglobin levels caused by the Cu-IUD [17].
The main effect on bleeding profiles observed in most studies with SPRM and oral UPA is the induction of amenorrhea [7,18,19]. In those studies, the doses of SPRM were much higher than those we used in the IUS and ovulation was impaired. In our Cu-UPA-IUS study, amenorrhea did not occur at any dose tested. Nearly all participants experienced normal frequency of bleeding episodes without prolonged bleeding. The absence of irregular bleeding within 3 months following device placement is likely related to the maintenance of monthly ovulation; this may represent an advantage of the Cu-UPA-IUS over the LNG-IUS [3,7].
The mechanisms by which low UPA doses promote bleeding control are unclear. It was previously speculated that changes in vessels, effects on endometrial stroma, as well as the antiproliferative effect of UPA on endometrial glands could explain the decrease in bleeding [8,20]. The antiproliferative effect described with SPRMs has been related to an overexpression of the androgen receptor in endometrial tissue [8,21]. Other mechanisms have also been proposed such as changes in angiogenic factors [22], metalloproteinases [23], and endometrial Natural Killer cells [24]. These findings suggest that UPA may maintain matrix and vessel stability, hence playing a key role in the homeostasis of the endometrium and, in consequence, preventing a copper-induced increase in uterine bleeding.
4.2. Safety profile
The Cu-UPA-IUS was safe and well-tolerated during this 12-week short-term use. No serious AEs occurred during the study period. The 40 μg/d group was not associated with a higher frequency or severity of AEs. Lower abdominal pain accounted for all AEs related to the study product and was mostly mild. Lower abdominal pain is a commonly anticipated product-related AE for intrauterine contraceptives [4,25].
Liver enzymes decreased or did not change over 12 weeks of Cu-UPA-IUS use. One participant in the 20 μg/d dose group experienced a minor and transient increase in liver enzymes (AST and ALT) that remained lower than 2xULN and did not meet the definition of drug-induced liver injury [26].
The occurrence of PAECs is another finding of special concern for SPRM users. UPA and other SPRM studies induce specific histological endometrial changes (PAECs) that are benign and reversible within 2 months after the end of treatment [27]. The incidence of PAECs seems to be lower in women exposed to low UPA doses who still ovulate, as compared with women with ovulatory suppression [12]. The Cu-UPA-IUS, at all doses tested, produced very low plasma concentrations of UPA in our study population (< 0.30 ng/mL vs 15 ng/mL in oral UPA 5 mg) [28]. In our study, the overall incidence of PAECs was 20.7%, lower than the incidence reported for oral UPA (70–75%) [29–31] and for the UPA contraceptive vaginal ring 800 μg/d (40%) [12]. On the day of the IUS removal, the 40 μg/d dose presented the highest frequency of PAECs among the three groups (44.4%). In contrast, 10% of the samples from the other groups exhibited PAEC on day of the IUS removal.
The Ki67 antigen is a well-established cell proliferation marker in clinical assessment of the endometrium [32]. In our study, there was no evidence that rates of cell proliferation increased during Cu-UPA-IUS use, in keeping with findings reported among users of oral UPA for uterine fibroids [33]. Mutation and suppression of the p53 gene has been shown to be one of the most frequently observed features in human endometrial carcinogenesis [34]. As a preliminary analysis, Cu-UPA-IUS use does not seem to suppress or overexpress p53 immunohistochemical staining.
Ovulation occurred in 87.6% of the treatment cycles, confirming our hypothesis that low doses of UPA have little effect on the ovulation.
4.3. Strengths and limitations of the study
Our study has strengths. There were no missing data in the bleeding diaries and no participants were lost to follow-up. In addition, we had no discontinuations related to AEs. Limitations included its single-blinded design which may have caused a bias in reported outcomes. However, since bleeding patterns were self-reported by participants who were blinded to the UPA dose released by the Cu-IUS, the effect of self-reporting on the primary outcome is limited. The short-term treatment period reduced the probability of capturing the maximum effect of the Cu-UPA-IUS on bleeding profile, as well as any possible long-term effect on the endometrium. The reduction in the amount of bleeding was a patient-reported outcome captured by the bleeding questionnaire completed at the end of IUS use and should be measured by objective tools in future studies [35]. Finally, our study lacked a comparator such as a Cu-IUD.
4.4. Clinical and research implications
Our study is the first report of the use of a Cu-UPA-IUS. The 40 μg/d group had the best performance on bleeding reduction; however, this group had the highest frequency of PAECs. The 20 μg/d also promoted a clinically relevant effect in the bleeding profile outcomes, but with a lower incidence of PAECs. Hence, the 20 μg/d dose meets the criteria of lowest safe and effective UPA dose able to promote a favorable bleeding profile and avoid PAEC occurrence. In addition, testing an intermediate dose of 10 μg/d of UPA may be pertinent.
In conclusion, the novel Cu-UPA-IUS seems safe and well-tolerated and highly acceptable across all three UPA doses tested in this short-term POC study. Future studies addressing questions of efficacy and safety following long-term exposure are required. In September 2020, the safety committee of the European Medicines Agency recommended revoking marketing authorization of oral UPA (5 mg/day) for the treatment of uterine fibroids due to post-marketing rare occurrences of serious liver injury, including the need for liver transplantation [36]. A causal link to UPA is inconclusive and no serious liver injury has been observed in any clinical study or toxicology study of UPA. Uncertainty regarding a potential causal link of UPA to liver damage has resulted in regulatory clinical holds for studies that could reveal additional therapeutic benefits of the drug. With respect to potential development of the novel Cu-UPA-IUS, the system delivers very low levels of UPA through an intrauterine route of administration, thus, minimizing any effect on hepatic tissue. Further discussion is warranted to consider potential risk/benefit for therapeutic indications of UPA in order to allow future development of this novel IUS that could benefit many women worldwide.
Supplementary Material
Acknowledgments
The study was funded by NICHD grant U54 HD 29990 and HRA Pharma, France.
Footnotes
Trial registration number:Clinicaltrials.gov, NCT03230539
References
- [1].Committee on Gynecologic Practice Long-Acting Reversible Contraception Working Group. Committee Opinion No. 642: Increasing Access to Contraceptive Implants and Intrauterine Devices to Reduce Unintended Pregnancy. Obstet Gynecol 2015;126:e44–48. 10.1097/AOG.0000000000001106. [DOI] [PubMed] [Google Scholar]
- [2].Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012;366:1998–2007. 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- [3].Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994;49:56–72. [DOI] [PubMed] [Google Scholar]
- [4].Rowe P, Farley T, Peregoudov A, Piaggio G, Boccard S, Landoulsi S, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception 2016;93:498–506. 10.1016/j.contraception.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sivin I, el Mahgoub S, McCarthy T, Mishell DR, Shoupe D, Alvarez F, et al. Long-term contraception with the levonorgestrel 20 mcg/day (LNg 20) and the copper T 380Ag intrauterine devices: a five-year randomized study. Contraception 1990;42:361–78. 10.1016/0010-7824(90)90046-x. [DOI] [PubMed] [Google Scholar]
- [6].Lethaby A, Hussain M, Rishworth JR, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev 2015:CD002126. 10.1002/14651858.CD002126.pub3. [DOI] [PubMed] [Google Scholar]
- [7].Goldthwaite LM, Creinin MD. Comparing bleeding patterns for the levonorgestrel 52 mg, 19.5 mg, and 13.5 mg intrauterine systems. Contraception 2019;100:128–31. 10.1016/j.contraception.2019.03.044. [DOI] [PubMed] [Google Scholar]
- [8].Brenner RM, Slayden OD, Nath A, Tsong YY, Sitruk-Ware R. Intrauterine administration of CDB-2914 (Ulipristal) suppresses the endometrium of rhesus macaques. Contraception 2010;81:336–42. 10.1016/j.contraception.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].World Health Organization. WHO | Medical eligibility criteria for contraceptive use. 5th ed.Geneva: WHO; 2015. WHO n.d. http://www.who.int/reproductivehealth/publications/family_planning/MEC-5/en/ (accessed January 5, 2018). [PubMed] [Google Scholar]
- [10].Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception 1986;34:253–60. [DOI] [PubMed] [Google Scholar]
- [11].Mansour D, Korver T, Marintcheva-Petrova M, Fraser IS. The effects of Implanon on menstrual bleeding patterns. Eur J Contracept Reprod Health Care 2008;13Suppl 1:13–28. 10.1080/13625180801959931. [DOI] [PubMed] [Google Scholar]
- [12].Brache V, Sitruk-Ware R, Williams A, Blithe D, Croxatto H, Kumar N, et al. Effects of a novel estrogen-free, progesterone receptor modulator contraceptive vaginal ring on inhibition of ovulation, bleeding patterns and endometrium in normal women. Contraception 2012;85:480–8. 10.1016/j.contraception.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kurman R, Carcangiu M, Herrington C, Young R. WHO Classification of Tumours of Female Reproductive Organs. vol. 6. 4th ed.2014. [Google Scholar]
- [14].Belsey EM, Pinol AP. Menstrual bleeding patterns in untreated women. Task Force on Long-Acting Systemic Agents for Fertility Regulation. Contraception 1997;55:57–65. 10.1016/s0010-7824(96)00273-9. [DOI] [PubMed] [Google Scholar]
- [15].Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359:781–5. 10.1016/S0140-6736(02)07882-0. [DOI] [PubMed] [Google Scholar]
- [16].Larsson G, Milsom I, Jonasson K, Lindstedt G, Rybo G. The long-term effects of copper surface area on menstrual blood loss and iron status in women fitted with an IUD. Contraception 1993;48:471–80. 10.1016/0010-7824(93)90136-u. [DOI] [PubMed] [Google Scholar]
- [17].Lowe RF, Prata N. Hemoglobin and serum ferritin levels in women using copper-releasing or levonorgestrel-releasing intrauterine devices: a systematic review. Contraception 2013;87:486–96. 10.1016/j.contraception.2012.09.025. [DOI] [PubMed] [Google Scholar]
- [18].Donnez J, Courtoy GE, Donnez O, Dolmans M-M. Ulipristal acetate for the management of large uterine fibroids associated with heavy bleeding: a review. Reprod Biomed Online 2018;37:216–23. 10.1016/j.rbmo.2018.04.040. [DOI] [PubMed] [Google Scholar]
- [19].Murji A, Whitaker L, Chow TL, Sobel ML. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst Rev 2017;4:CD010770. 10.1002/14651858.CD010770.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kannan A, Bhurke A, Sitruk-Ware R, Lalitkumar PG, Gemzell-Danielsson K, Williams ARW, et al. Characterization of Molecular Changes in Endometrium Associated With Chronic Use of Progesterone Receptor Modulators: Ulipristal Acetate Versus Mifepristone. Reprod Sci 2018;25:320–8. 10.1177/1933719117746764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nayak NR, Slayden OD, Mah K, Chwalisz K, Brenner RM. Antiprogestin-releasing intrauterine devices: a novel approach to endometrial contraception. Contraception 2007;75:S104–111. 10.1016/j.contraception.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ravet S, Munaut C, Blacher S, Brichant G, Labied S, Beliard A, et al. Persistence of an intact endometrial matrix and vessels structure in women exposed to VA-2914, a selective progesterone receptor modulator. J Clin Endocrinol Metab 2008;93:4525–31. 10.1210/jc.2008-0731. [DOI] [PubMed] [Google Scholar]
- [23].Li A, Felix JC, Yang W, Xiong DW, Minoo P, Jain JK. Effect of mifepristone on endometrial matrix metalloproteinase expression and leukocyte abundance in new medroxyprogesterone acetate users. Contraception 2007;76:57–65. 10.1016/j.contraception.2007.03.005. [DOI] [PubMed] [Google Scholar]
- [24].Wilkens J, Male V, Ghazal P, Forster T, Gibson DA, Williams ARW, et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J Immunol 2013;191:2226–35. 10.4049/jimmunol.1300958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Diedrich JT, Madden T, Zhao Q, Peipert JF. Long-term utilization and continuation of intrauterine devices. Am J Obstet Gynecol 2015;213:822.e1–6. 10.1016/j.ajog.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, Clinical Practice Guideline Panel: Chair:, Panel members, EASL Governing Board representative: EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol 2019;70:1222–61. 10.1016/j.jhep.2019.02.014. [DOI] [PubMed] [Google Scholar]
- [27].Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol 2008;21:591–8. 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- [28].Pohl O, Zobrist RH, Gotteland J-P. The clinical pharmacology and pharmacokinetics of ulipristal acetate for the treatment of uterine fibroids. Reprod Sci 2015;22:476–83. 10.1177/1933719114549850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012;366:409–20. 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- [30].Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012;366:421–32. 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- [31].Williams ARW, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol 2012;31:556–69. 10.1097/PGP.0b013e318251035b. [DOI] [PubMed] [Google Scholar]
- [32].Sivalingam VN, Kitson S, McVey R, Roberts C, Pemberton P, Gilmour K, et al. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br J Cancer 2016;114:281–9. 10.1038/bjc.2015.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Whitaker LHR, Murray AA, Matthews R, Shaw G, Williams ARW, Saunders PTK, et al. Selective progesterone receptor modulator (SPRM) ulipristal acetate (UPA) and its effects on the human endometrium. Hum Reprod 2017;32:531–43. 10.1093/humrep/dew359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Köbel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of P53 Immunohistochemistry in Endometrial Carcinomas: Toward Increased Reproducibility. Int J Gynecol Pathol 2019;38Suppl 1:S123–31. 10.1097/PGP.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Magnay JL, O’Brien S, Gerlinger C, Seitz C. A systematic review of methods to measure menstrual blood loss. BMC Womens Health 2018;18:142. 10.1186/s12905-018-0627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].EMA. Ulipristal acetate 5mg medicinal products. European Medicines Agency; 2020. https://www.ema.europa.eu/en/medicines/human/referrals/ulipristal-acetate-5mg-medicinal-products (accessed September 14, 2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


