Abstract
Meaningful social interactions are a fundamental human need, the lack of which can pose serious risks to an individual’s physical and mental health. Across species, peer-oriented social behaviors are dramatically reshaped during adolescence, a developmental period characterized by dynamic changes in brain structure and function as individuals transition into adulthood. Thus, the experience of social isolation during this critical developmental stage may be especially pernicious, as it could permanently derail typical neurobiological processes that are necessary for establishing adaptive adult behaviors. The purpose of this review is to summarize investigations in which rodents were isolated during adolescence, then re-housed in typical social groups prior to testing, thus allowing the investigators to resolve the long-term consequences of social adversity experienced during adolescent sensitive periods, despite subsequent normalization of the social environment. Here, we discuss alterations in social, anxiety-like, cognitive, and decision-making behaviors in previously isolated adult rodents. We then explore corresponding neurobiological findings, focusing on the prefrontal cortex, including changes in synaptic densities and protein levels, white matter and oligodendrocyte function, and neuronal physiology. Made more urgent by the recent wave of social deprivation resulting from the COVID-19 pandemic, especially amongst school-aged adolescents, understanding the mechanisms by which even transient social adversity can negatively impact brain function across the lifespan is of paramount importance.
Keywords: adolescent, stress, prefrontal, orbitofrontal, adversity
1. Introduction
The need for meaningful social interaction is fundamental to the human experience [1]. Not only can interaction with peers be intrinsically rewarding and facilitate adaptive cooperation, the lack of social contacts may also negatively impact health and cognitive functioning [2]. Social isolation and subjective loneliness are considered independent risk factors for poorer mental health outcomes and for increased all-cause mortality, comparable in effect size to those of insufficient healthcare access or obesity [3, 4]. Indeed, sociability appears to be tightly regulated by physiologic control mechanisms similar to those that govern other basic biologic drives such as sleep or consummatory behaviors [1, 5]. However, while the neurophysiologic response to, and consequences of, acute social isolation have garnered much attention (reviewed here [5]), the long-term sequelae of social isolation remain relatively under-explored. In particular, periods of social deprivation that coincide with critical developmental stages may be especially pernicious, as they can permanently derail typical maturation processes in the brain.
In this review, we focus on the impact of social isolation during adolescence, a period of dynamic neurobiological and behavioral changes across species [6–8]. Adolescence is generally defined in humans as the period between 10 and 24 years of age, although individual genetic and environmental factors can lead to variation within this range [9]. The onset of adolescence is typically marked by pubertal initiation; however, these processes are distinct. While puberty strictly relates to the development of adult reproductive capacity, adolescence can be understood more broadly to include the longer window in which an organism establishes the skills and behavioral strategies required for survival as an adult, independent of their early-life caregivers [10, 11]. Across species, social interactions during adolescence are fundamentally reshaped to facilitate this transition into adulthood including increased peer-oriented social contacts, play behavior, exploration, and risk-taking [7, 12–16].
While the adolescence-to-adulthood transition represents a critically important and adaptive life epoch, the dynamism of the adolescent period may also open a window of vulnerability to the development of mental health disorders. A marked increase in the incidence of neuropsychiatric diseases, including depression, anxiety, eating disorders, and schizophrenia, occur during adolescence [17, 18]. And although human studies remain largely correlational, negative social experiences have been shown to be a major risk factor for depression during adolescence, and depression that emerges during adolescence is associated with an increased lifetime risk for major depressive disorder (MDD) [19]. This phenomenon is especially salient given the physical distancing requirements enacted in response to the COVID-19 pandemic, which have substantially limited the opportunity for social interaction across all age groups, but which may have long-lasting behavioral effects among adolescents in particular, even after eventual normalization of the social environment [20].
In this review, we will focus on findings related to the effects of social isolation in adolescence on the prefrontal cortex (PFC). The PFC is a critical mediator of social experience and behavior, and it also serves as a “top-down” regulator of various cognitive and emotional processes including attention, decision-making, learning and memory, among others [21, 22], dysregulation of which are key feature of numerous psychiatric disorders [23]. Additionally, unlike functions primarily subserved by other cortical regions such as sensory or motor skills, most PFC-dependent cognitive functions optimize late during mammalian development, thus displaying a relatively protracted maturation extending into adolescence [24].
2. Adolescent Social Isolation and Re-Socialization Procedures in Rodents
In this review, we will be examining findings from experiments using rodents in which subjects are singly housed without access to conspecific counterparts during the adolescent period, then re-housed in typical social groups prior to testing. For the purposes of this review, the period from postnatal day (P)28 to P56 (the beginning of adulthood) will be considered the rodent adolescent period, while the period from P21 (weaning age) to P28 will be considered the pre-adolescent or juvenile period [7, 25–28]. In humans, adolescence is marked by an increased amount of time spent socializing with peers rather than family [12]. Meanwhile in rodents, the period spanning roughly P30 to P40 marks the peak for observed conspecific social play behaviors [29], which then decline as rodents enter adulthood [30]. Thus, while it is often difficult to draw cross-species comparisons for the onset and termination of particular developmental periods, the emergence of more peer-centric social behaviors as individuals transition to independence appears to be a shared developmental milestone characteristic of adolescence across rodents and humans alike. However, it is important to note that the timing of isolation procedures varies widely between studies, so caution is warranted when making direct comparisons between findings. In particular, many studies singly house animals immediately following weaning (P21), and those animals remain isolated for long periods extending into adolescence. As discussed by Lukkes et al. [31], this approach makes it impossible to discern to what extent the long-term effects of isolation are attributable to disruptions of processes during the early adolescent versus pre-adolescent periods. Nevertheless, we will discuss studies in which the isolation period falls entirely within the P28 to P56 adolescence window, as well as those in which the isolation period spans a substantial portion of that range, even if the isolation begins in the juvenile period or extends into the early adult period. Our rationale for this approach is that very few investigations involve isolation specifically during the adolescent and social reintegration.
Finally, in the vast majority of the studies examining the effects of post-weaning social isolation, animals undergo testing while still in isolation conditions [13, 31]. While these studies provide valuable insights, they are not able to disambiguate those effects which are homeostatic (i.e. behavioral and/or neurobiological) responses to the current experience of isolation itself, versus those which are long-term consequences of prior isolation [32] – in particular, those that may have permanently disrupted an important developmental process. In this review, we limit our discussion to studies that test animals following a re-socialization period. The purpose of this review is to summarize investigations in which rodents were isolated during adolescence, then re-housed in typical social groups prior to testing, allowing the investigators to resolve the long-term consequences of social adversity experienced during adolescent sensitive periods.
3. Effect of Adolescent Social Isolation on Adult Behaviors
Long-term behavioral consequences of social isolation during adolescence have been identified in several PFC-dependent domains, including social, anxiety-like, cognitive, and decision-making behaviors (Tables 1–3).
Table 1.
Effect of social isolation during adolescence on social behaviors following resocialization.
| Behavioral test | Species (strain) | Sex | Isolation period | Age of testing | Result (isolated vs. control) | Interpretation (effect of isolation) | Reference |
|---|---|---|---|---|---|---|---|
| Social interaction (free roaming) | Rat (W) | M | P22–35 | P84 | ↓ social approach | ↓ sociability | Hol et al. (1999) [38] |
| P22–28 | ↓ social approach | ||||||
| P29–35 | No effect | No effect | |||||
| Rat (W) | M | P22–35 | P42 | ↓ social approach ↓ anogenital sniffing |
↓ sociability | van den Berg et al. (1999) [33] | |
| Rat (SD) | M | P21–42 | P56 | ↑ approach latency ↓ contact duration |
↓ sociability | Lukkes et al. (2009) [34] | |
| ↑ freezing | ↑ social fear | ||||||
| Rat (SD) | M | P21–42 | P56 | ↑ approach latency ↓ social contacts ↓ contact duration |
↓ sociability | Lukkes et al. (2009) [39] | |
| ↑ freezing | ↑ social fear | ||||||
| Social interaction (fixed) | Mouse (C57BL/6) | M | P21–35 | P50 | ↓ social approach | ↓ sociability | Makinodan et al. (2012) [35] |
| Mouse (C57BL/6) | M | P21–35 | P65 | ↓ social approach | ↓ sociability | Makinodan et al. (2017) [109] | |
| Home cage interaction | Rat (W) | M | P30–40 | P42–64 | ↓ social investigation ↓ social contacts |
↓ sociability | Bator et al. (2018) [37] |
| No effect on play behaviors | Intact social play | ||||||
| Resident-intruder | Rat (W) | M | P22–35 | P42 | ↑ freezing ↓ exploration |
↑ social fear | van den Berg et al. (1999) [33] |
| Sexual behavior* | Rat (W) | M | P22–35 | P42 | No change in sexual behaviors | Intact sexual behaviors | van den Berg et al. (1999) [33] |
Free-roaming behavior following introduction of a sexually receptive female. Measured outcomes include mounting bouts, intromissions, and ejaculations. M: male; P: Postnatal day; SD: Sprague Dawley; W: Wistar.
Table 3.
Effect of social isolation during adolescence on cognitive function and decision-making behaviors following re-socialization.
| Behavioral test | Species (strain) | Sex | Isolation period | Age of testing | Result (isolated vs. control) | Interpretation (effect of isolation) | Reference |
|---|---|---|---|---|---|---|---|
| Rat gambling task | Rat (LH) | M | P21–43 | P84 | ↓ choice accuracy | ↓ instrumental learning | Baarendse et al. (2013) [54] |
| 5-CSRTT | ↑ premature responses (with ↑ task demands) | ↑ impulsive action | |||||
| Delayed reward task | No effect | No effect on impulsive choice | |||||
| Instrumental contingency degradation | Mouse (C57BL/6) | F | P31–60 | P82 | Insensitivity to changes in action-outcome contingency | Deferral to habitual behavior | Hinton et al. (2019) [56] |
| Outcome devaluation |
Insensitivity to changes in outcome value | ||||||
| Morris water maze | Rat (SD) | M | P21–35 | P56 | No effect | Intact spatial memory | Han et al. (2011) [53] |
| Morris water maze (reversal) | ↑ escape time ↑ distance travelled ↓ escape latency |
↓ flexible spatial memory | |||||
| Novel object recognition | Rat (W) | M | P30–40 | P42–64 | No effect | Intact working memory | Bator et al. (2018) [37] |
| Water T-maze | Mouse (FVB/N x C57BL/6) | M | P21–35 | P50 | Impaired acquisition (non-match to place) | Working memory deficit | Makinodan et al. (2012) [35] |
5-CSRTT: 5-choice serial reaction time task; F: female; LH: Lister Hooded; M: male; P: Postnatal day; SD: Sprague Dawley; W: Wistar.
3.1. Social Behaviors
Unsurprisingly, social deprivation during adolescence modifies social behavior in adulthood (Table 1). Socially isolated mice reliably display decreased sociability, as measured by approach toward a conspecific animal in both free-roaming (where both the test animal and conspecific are allowed to freely move within a space) and fixed interaction tests (where the conspecific is constrained to a stationary area, such as under a cup) [33–35]. Interestingly, this reduction in social approach is also seen in animals that have been isolated as adults [36]. Moreover, at least some sociability deficits resulting from isolation during adolescence appear to be reversed by prolonged re-socialization in group housing in adulthood [37]. Additionally, the effect of post-weaning isolation appears to be additive across developmental epochs, as isolation that spans both the pre-adolescent and adolescent periods (P22–35) exerts a more robust impact on adult social interaction than isolation during either the pre-adolescent (P22–28) or early adolescent periods (P29–35) alone [38]. In addition to affiliative social approach, rodents with a history of prior adolescent social isolation also display increased social fear, as measured by freezing behavior when presented with an aggressive resident intruder [33, 34, 39].
3.2. Anxiety-Like Behaviors
Another commonly studied behavioral consequence of post-weaning social isolation is anxiety-like behavior (Table 2). Anxiety states are typically inferred by examining how much time animals spend in the open, uncovered areas of novel environments. In these tests, decreased time spent in the center of an open space (open field test, OFT) versus the sides or corners, or time spent in the two open arms of a plus-shaped apparatus (elevated plus maze, EPM) versus the two covered arms, are considered anxiety-like behaviors. Previously isolated animals generally spend less time in the center or open arms during an OFT [39, 40] or EPM [41–43] test, respectively, suggesting a heightened anxiety-like state.
Table 2.
Effect of social isolation during adolescence on anxiety-like behaviors following re-socialization.
| Behavioral test | Species (strain) | Sex | Isolation period | Age of testing | Result (isolated vs. control) | Interpretation (effect of isolation) | Reference |
|---|---|---|---|---|---|---|---|
| Open field | Rat (W) | M | P22–35 | P42 | No effect | No effect | van den Berg et al. (1999) [33] |
| Rat (SD) | M | P18–32 | P62 | ↑ locomotion | ↑ anxiety | Pascual el al. (2006) [40] | |
| Rat (SD) | M | P21–42 | P56 | ↓ center entries | ↑ anxiety | Lukkes et al. (2009) [39] | |
| Elevated plus maze | Rat (LH) | M | P21–51 | P81 | ↓ open arm time ↓ open arm entries ↓ time spent toward end of open arms |
↑ anxiety | Wright et al. (1991) [42] |
| Rat (W) | M | P22–35 | P42 | No effect | No effect | van den Berg et al. (1999) [33] | |
| Rat (SD) | M | P30–35 | P36–38 | No effect | No effect | Leussis & Andersen (2008) [43] | |
| F | P30–35 | P36–38 | ↓ open arm time | ↑ anxiety | |||
| Rat (LE) | M | P28–74 | P80 | ↓ open arm time ↓ open arm entries |
↑ anxiety | Karkhanis et al. (2014) [41] | |
| Elevated plus maze* | Rat (SD) | M | P30–50 | P80 | ↑ open arm entries ↑ open arm time |
↓ anxiety | Weintraub et al. (2010) [47] |
| F | No effect | No effect |
Following repeated restraint stress. F: female; LE: Long-Evans; LH: Lister Hooded; M: male; P: Postnatal day; SD: Sprague Dawley; W: Wistar
Interestingly, Wright et al. [42] employed a two-phase experimental design in which rats were either isolated or group-housed from P21–51, and then half of isolated mice were re-housed in social groups, while half of group-housed mice were then isolated from P51–81. Rats were tested with the EPM at both P51 and P81, which revealed that adolescent social isolation produces anxiety-like behavior that persists even despite resocialization. In contrast, rats that were initially group-housed, but then isolated for an equivalent duration (P51–81), did not show anxiety-like behaviors, suggesting that the anxiogenic effect of social isolation may be somewhat more pronounced during the adolescent period. However, several other studies have reported an anxiogenic effect of isolation during adulthood, but these studies employed a substantially longer isolation period (10–14 weeks) [44–46] compared to the one used by Wright et al., which matched the adult isolation period to that of the adolescent isolation group (30 days).
Tests of anxiety-like behavior in rodents are quite sensitive to the stressors, which can include variations in husbandry and handling. Thus, behaviors in the OFT or EPM may also reflect differences in stress reactivity among rats. Accordingly, Weintraub et al. [47] found that when previously isolated rats were first subjected to restraint stress as adults and then tested for anxiety-like behaviors, prior isolation was associated with decreased anxiety-like behaviors, and this effect was only observed in males not females, who displayed no difference in EPM behavior. Altogether, it appears that generally, previously isolated rodents display a heightened anxiety-like state, but potentially, resilience to acute stress-induced anxiety.
3.3. Cognitive Function and Decision-Making Behaviors
Cognitive processing and decision making are hallmark PFC-dependent functions [22], which characteristically mature during adolescence [48–51]. The maintenance of social play behaviors during adolescence is thought to be critical for the recruitment and maturation of top-down cortical control of behavior, thus facilitating the development of so-called executive functions and emotional regulation [52].
It appears that adolescent social isolation most often disrupts cognitive functions that require flexible task updating (Table 3), that is, when the set of rules used to determine an optimal behavioral strategy to perform a task are unexpectedly changed. For example, Han et al. [53] report that previously isolated rats display intact spatial memory as adults, as assessed in a Morris water maze task in which animals must swim to find a hidden platform. However, when the location of the platform is changed, previously isolated rats are slower to learn the new location of the platform and adjust their navigation strategy accordingly.
With respect to instrumental behavior (referring to performing a behavior to obtain a desirable outcome), rats with a history of adolescent social isolation are impaired in learning the parameters of a gambling task in which rats must choose between various responses that predict differing rewards or punishments. Specifically, Baarendse et al. [54] report that previously isolated rats fail to discriminate between advantageous and disadvantageous choices. Notably, this deficit was not reversible by pharmacologic manipulation of dopamine, serotonin, or noradrenergic systems, thus emphasizing the durable nature of adolescent isolation-induced deficits in instrumental learning.
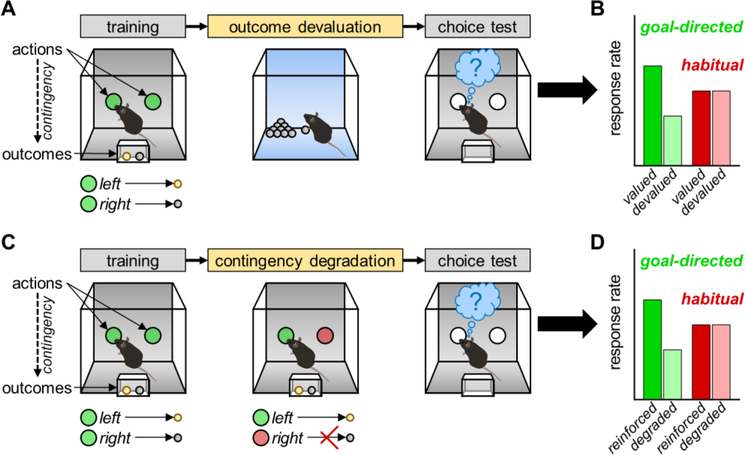
Cognitive flexibility in instrumental conditioning tasks also relies on the ability of animals to learn and update causal associations between actions and their likely outcomes. This capacity can be tested in several ways, each typically requiring animals to first undergo instrumental training to obtain distinct food reinforcers. Then, the experimenter manipulates either the value of one of the reinforcers (commonly via outcome devaluation; see Figure 1A–B) or the predictive relationship between one action and its associated outcome (commonly via contingency degradation; see Figure 1C–D). Here, animals that can flexibly update their behavioral response strategies will preferentially perform actions for higher-value outcomes, or for outcomes that are more likely to be reinforced [55]. Mice that were isolated throughout the adolescent period but subsequently re-socialized as adults display deficits in multiple outcome devaluation and contingency degradation tasks [56]. Importantly, young adolescents are relatively insensitive to changes in action-outcome variables, even though they are sensitive to other outcome predictors such as Pavlovian cues [49]. The ability to flexibly modify and update reward-seeking strategies improves throughout adolescence, and the trajectory of this development (i.e. the rate of improvement) during adolescence predicts flexible task performance in adulthood [51]. Thus, examining behaviors that require processing of action-outcome associations are of particular interest, as this capacity appears to strengthen during the adolescent period and may be particularly vulnerable to social isolation.
Figure 1. Illustration of instrumental tasks used to assess goal-directed decision making.
In both tasks, animals are first trained to associate two actions (left vs. right nose-pokes) with delivery of two distinct reinforcers (yellow vs. grey pellets). (A) Illustration of outcome devaluation. Immediately prior to a choice test, animals are allowed to freely consume one of two the pellets (e.g. grey pellet) thus decreasing the value of that outcome. (B) Choice test responding whereby goal-directed action is defined by a preference for the nose-poke aperture associated with the valued vs. devalued pellet. (C) Illustration of contingency degradation. Animals undergo a session in which one action (e.g. nose-poking on the left) is reinforced as during training, while the other action (e.g. nose-poking on the right) is no longer linked to reinforcer deliver and instead the pellet associated with that action (e.g. grey pellet) is non-contingently (or “for free”). (D) Choice test responding whereby goal-directed action is defined by a preference for the nose-poke aperture associated with the previously reinforced vs. degraded contingency.
Previously isolated rats display impulsivity, a trait which itself is involved in numerous neuropsychiatric conditions [57]. Impulsivity can be experimentally dissociated into two distinct components: 1) impulsive action which refers to the inability to inhibit behaviors and 2) impulsive choice which refers to a preference for smaller, immediate rewards versus larger, delayed ones. Impulsive action can be experimentally assessed using a five-choice serial reaction time task (5-CSRTT) in which a brief light cue is presented at random above one of five ports and responding at the illuminated port produces a food reward. Importantly, the visual cue only appears after a set inter-trial interval (ITI), during which time responding does not produce any rewards and thus animals typically inhibit their responding during this period. Previously isolated rats displayed increased responding during the ITI, thus demonstrating increased impulsive action, but these deficits only emerge when task demands increase (e.g. as the ITI or cue duration shorten) [54]. On the other hand, impulsive choice can be studied using a delayed reward task (DRT) in which rats are allowed to choose between immediate responding for delivery of a small reward or delayed responding for delivery of a large reward (e.g. one vs. four food pellets). Here, previously isolated rats show no differences compared to group-housed controls, even after task demands are increased by increasing the large-reward delay, thus suggesting that isolation results in a selective deficit in impulsive action, but not choice [54].
3.4. Depression-Related Behaviors
MDD is one of the most common and costly disorders worldwide among all health conditions [58]. A history of social adversity during adolescence remains one of the strongest predictors for developing MDD across the lifespan [18]. Investigations examining the impact of social isolation during adolescence on depression-related behavioral phenotypes in adulthood are nevertheless limited. (Of course, it should be noted that social deficits [59], anxiety [18], and instrumental learning deficits [60] are each highly coincident with depressive disorders.) A few studies have specifically examined the effect of adolescent social isolation on putatively depressive-like behaviors. Here, previously isolated animals exhibit decreased active escape behaviors in a forced swim test and learned helplessness assay [43], interpreted as indicators of depressive-like states. Of note, males seem to be more affected [43]. Another strategy to test for depressive-like behaviors is a sucrose consumption task, in which animals are allowed access to a highly palatable sucrose solution. In this task, previously isolated mice consume significantly less of a sucrose solution [61], considered a corollary to anhedonia in human patients.
4. Neurobiological Effects of Social Isolation During Adolescence
4.1. Synaptic and Neuronal Structural Effects of Social Isolation During Adolescence
Adolescence constitutes a period of significant neurodevelopmental changes, with the PFC maturing throughout this period and well into early adulthood [62]. Structural imaging studies in humans have shown that frontal cortical gray matter undergoes significant thinning during adolescence. Studies across species provide evidence that this reduction in gray matter volume, particularly in the PFC, may be attributable to synapse elimination [62–67]. This pattern is also evident in rodents, as dendritic spines, the primary sites of excitatory neurotransmission in the brain [68], are significantly pruned in the frontal cortex of both rats and mice during adolescence [69–72]. Of note, there may exist meaningful sex differences with regard to the mechanisms underlying these maturation processes, as the concurrent decrement in synaptic marker density appears to be more marked in females than males [73], while gonadal hormones are required for this pruning in males but not females [74, 75]. Overall, these processes are thought to refine synaptic connections to facilitate more efficient neurotransmission and performance of adult behaviors critical for survival [76, 77]. However, this structural instability during adolescence may also constitute a vulnerability to the development of neuropsychiatric disease [67, 78, 79]. Here, we review studies examining the neurobiological consequences of isolation in adolescence, which may provide insights into the mechanisms driving the emergence and persistence of isolation-induced behavioral deficits (Table 4).
Table 4.
Long-term neurobiological effects of social isolation during adolescence.
| Reference | Species, Strain | Sex | Isolation period | Age at testing | Analysis method (PFC region) | Result |
|---|---|---|---|---|---|---|
| Bator et al. (2018) [37] | Rat (W) | M | P30–40 | P45–48 | WB (mPFC) | ↑ GAD65 ↑ cell surface GAD65 ↓ GAD67 |
| P70–75 | No effect on GAD65 or GAD67 | |||||
| Leussis & Andersen (2008) [43] | Rat (SD) | M+F | P30–35 | P36–38 | WB (IL) | ↓ spinophilin ↓ synaptophysin No effect on TH |
| Leussis et al. (2008) [80] | Rat (SD) | M | P21–35 | P60 | WB (IL, Cg1, Cg3) | ↓ synaptophysin |
| Hinton et al. (2019) [56] | Mouse (C57BL/6) | F | P31–60 | P82 | IHC (OFC, Cg1) | ↓ CNPase levels |
| IHC (OFC) | ↑ PSD-95 puncta | |||||
| WB (OFC) | ↑ PSD-95 levels | |||||
| Mackowiak et al. (2019) [100] | Rat (W) | M | P30–40 | P46–47 | WB (mPFC) | ↑ PV levels |
| P70–71 | ↓ PV levels | |||||
| Pascual et al. (2006) [40] | Rat (SD) | M | P18–32 | P62 | IHC (mPFC) | ↑ VIPergic neurons |
| Han et al. (2011) [53] | Rat (SD) | M | P21–35 | P56 | IHC (mPFC) | ↑ BDNF+ neurons |
| Makinodan et al. (2012) [35] | Mouse (FVB/N x C57BL/6) | M | P21–35 | P65 | Stereology (mPFC L5–6) | ↓ OL process length ↓ OL arborization No effect on OL number |
| qRT-PCR (mPFC L5–6) | ↓ Mbp mRNA ↓ Mag mRNA |
|||||
| Makinodan et al. (2017) [109] | Mouse (C57BL/6) | M | P21–35 | P65 | TEM (mPFC L5) | ↓ myelin thickness |
| Leussis & Andersen (2008) [43] | Rat (SD) | M | P30–35 | P36–38 | WB (Cg3) | ↓ MBP |
| Pascual et al. (2006) [40] | Rat (SD) | M | P18–32 | P62 | Golgi staining (mPFC) | ↓ dendritic processes ↓ dendritic arborization |
| Hinton et al. (2019) [56] | Mouse (C57BL/6) | F | P31–60 | P82 | Stereology (mPFC L5) | ↑ dendritic spine density ↑ “spine rich” dendrites |
| Makinodan et al. (2017) [109] | Mouse (C57BL/6) | M | P21–35 | P65 | qRT-PCR (mPFC) | ↓ c-Fos mRNA ↓ Arc mRNA ↓ Npas4 mRNA |
| Makinodan et al. (2017) [109] | Mouse (C57BL/6) | M | P21–35 | P65 | EEG (mPFC L5–6) | ↓ γ-oscillation power ↑ β-oscillation power |
| Baarendse et al. (2013) [54] | Rat (LE) | M | P21–43 | P84 | Ex vivo patch clamp (mPFC L5) | No effect on membrane properties (RMP, rheobase, input resistance) |
| Absent dopamine receptor modulation of EPSCs |
CNPase: 2’,3’-Cyclic-nucleotide 3’-phosphodiesterase; EPSC: excitatory post-synaptic current; EEG: electroencephalogram; F: female; IHC: immunohistochemistry; L5: layer 5; L5–6 layer 5–6; LE: Long-Evans; LH: Lister Hooded; M: male; MAG: myelin associated glycoprotein; MBP: myelin basic protein; OL: oligodendrocyte; P: Postnatal day; PV: parvalbumin; RMP: resting membrane potential; qRT-PCR: quantitative reverse transcription PCR; SD: Sprague Dawley; TEM: transmission electron microscopy; VIP: vasopressin intestinal peptide; W: Wistar; WB: western blot.
Hinton et al. [56] utilized transgenic mice that express yellow fluorescent protein (YFP) on layer V pyramidal neurons to examine neuronal morphologies in mice that had been socially isolated throughout the adolescent period (P31–60) and were then re-socialized for several weeks (until P82). Here, mice with a history of isolation displayed elevated dendritic spine densities in the PFC both during the isolation period and following resocialization in adulthood, and in particular, these neurons had a higher proportion of dendrites that were considered “spine rich.” These findings suggest that social deprivation during adolescence results in a failure in the dendritic spine pruning process that typically occurs during that period, resulting in a lasting spine hyper-density into adulthood.
Studies by Leussis and colleagues [43, 80] used immunohistochemistry (IHC) and western blotting (WB) from whole tissue punches and demonstrated a loss of the pre-synaptic marker synaptophysin in the medial prefrontal cortex (mPFC), specifically the infralimbic (IL) and cingulate (Cg) regions, in rodents isolated for a brief period during early adolescence (P30–35). Thus, these findings by Leussis et al. describing an isolation-induced loss of synaptic markers are apparently discordant with the dendritic spine abundance described by Hinton et al. [56]. However, there are numerous parsimonious explanations for these findings, including that the isolation-induced failure of dendritic spine pruning observed by Hinton et al. were observed in a specific subset of deep layer glutamatergic neurons, while synaptophysin loss as evidenced by IHC or WB by Leussis et al. could reflect mixed structural patterns across many cell types.
Interestingly, these findings may also suggest that the consequences of social isolation during adolescence are highly dependent on the isolation period. Specifically, it is possible that early adolescent isolation results in synaptic loss in the short term (e.g. P30–35 as per Leussis and colleagues [43, 80]), but if isolation then extends into later adolescent periods (e.g. P31–60 as per Hinton et al. [56]), typical pruning is halted, thus producing a spine overabundance in adulthood. Notably, Hinton et al. also reported that spine hyper-density was accompanied by increased levels of PSD-95, an excitatory post-synaptic marker. PSD-95 can reflect synaptic number or strength [81], raising the possibility that the spines that remain following the early isolation may undergo precocious strengthening, which could render them resistant to later elimination [82].
In addition to modifying dendritic spine densities, social isolation during adolescence appears to diminish dendritic arborization and reduce dendritic branches, resulting in simplified dendritic morphologies among neurons in the PFC [40, 83]. Interestingly, these dendritic morphologic deficits were reversed in one report by a monoamine oxidase inhibitor administered during the subsequent resocialization period in early adulthood [83]. Monoaminergic tone, especially in the PFC, is also highly dynamic during adolescence and plays an important role in PFC development [13]. These findings suggest that the critical window for monoamine-triggered structural maturation in the PFC, typically characteristic of adolescence, may not be closed by adulthood, and thus offers a potential therapeutic mechanism for correcting deficits in neuronal structure and morphology in adults with a history of social adversity during adolescence.
4.2. Cellular and Molecular Effects of Adolescent Social Isolation
Given the dramatic structural maturation of PFC neurons during adolescence, it is reasonable to hypothesize that structural and synaptic changes due to social isolation are driven by likewise dynamic changes in the cellular and molecular processes within these brain regions. A recent study utilizing single-cell RNA-sequencing to examine the transcriptional and epigenetic profile of neurons from the mPFC revealed that excitatory neurons across adolescence and into early adulthood undergo large-magnitude changes in pathways related to Rho signaling, actin cytoskeleton, membrane signaling, and cell adhesion [84], processes necessary for dendritic spine turnover, which peaks during adolescence [85].
While the levels of single gene transcripts or products have seldom been tracked through adolescence, a few proteins were recently measured: Levels of the cytoskeletal regulatory proteins p190RhoGAP and Rho-kinase 2 (ROCK2) increase between P35 and P42 before decreasing again by P56 across PFC regions in mice [71]. The effect of social isolation on these signaling factors has not yet been established, but correcting dendritic architecture via ROCK2 inhibition remedied reward-related decision-making deficits following adolescent isolation [56]. Pharmacologic inhibition of ROCK2 also has anti-depressant-like efficacy, potentially via dendritic and dendritic spine remodeling in multiple brain regions [56, 86, 87]. Additionally, Leussis et al [43] demonstrated decreased levels of spinophillin, another actin-interacting regulator of dendritic spines [88], specifically in the IL of previously isolated mice. Notably, both ROCK2 and spinophillin are enriched in the dendritic spines of neurons, where they are most closely associated with dynamic cytoskeleton-mediated changes in spine morphology [88, 89]. It seems likely that they are directly involved in dendritic spine maturation during specific developmental epochs; their disruption (in levels or function) by social isolation could thereby redirect maturational trajectories.
Han et al [53] reported elevations in brain-derived neurotrophic factor (BDNF), a potent and ubiquitous modulator of neuronal plasticity [90], in the mPFC following adolescent social isolation. Notably, this effect appears to be brain region specific, as BDNF declined in the nucleus accumbens and the CA1 and dentate gyrus regions of the hippocampus. Notably, levels of the high-affinity BDNF receptor TrkB increase dramatically across adolescence [71]. Given that BDNF and TrkB-mediated signaling robustly stabilize spine structure and promote spine formation [91], this regionally-specific elevation in BDNF levels may provide another explanation for the aberrant synaptic and structural effects observed in the PFC following adolescent social isolation.
4.3. Effects of Adolescent Social Isolation on Myelination
Another mechanism by which isolation could trigger lasting changes in the PFC is through its effects on myelin. A recent study examined a longitudinal dataset collected from human patients across adolescence and into early adulthood using a myelin-sensitive MRI modality and demonstrated that although there is evidence of brain-wide elevation in myelin during adolescence, these changes predominate within Cg, PFC, and temporoparietal areas [92]. Large-magnitude white matter changes in the PFC occur in the adolescent period, and they appear to be key for maturation of adult cognitive functions during development [8, 24, 92–94]. Evidence for this notion has been most extensively characterized in contexts of motor skill learning [95], but is also seen for social behaviors [96]. Also, disruptions of myelination, in particular within fronto-striatal tracts, predict the emergence of compulsivity and impulsivity traits during adolescence [92], which have been linked to the development of psychiatric disorders [57, 97]. Additionally, PFC white matter and myelin gene expression is dysregulated in depressed patients [98]. Thus, the period of accelerated myelination during adolescence appears to be critical for the maturation of adult cognitive and behavioral functions, the perturbation of which may prime the emergence of psychiatric diseases.
Extended social isolation simplifies oligodendrocyte morphology, reduces myelin thickness and myelin gene expression in the mPFC [35, 56]. Notably however, extended social isolation in adulthood, also followed by social re-integration, produces similar results [36]. Thus, social isolation results in robust changes in oligodendrocyte function not specific to the adolescent period. Intriguingly, myelin thickness in the mPFC is reduced in previously-isolated adult mice that were re-housed with previously-isolated cage mates, but not previously-isolated adult animals that were then re-housed with previously-socialized conspecifics [35]. In other words, hypomyelination in the mPFC of adults with a history of isolation could be recovered depending on the cage composition following isolation, suggesting that specific social contexts later in life could buffer against long-term alterations in myelination and myelin-related factors due to early-life social isolation.
4.4. Neurophysiologic Effects of Adolescent Social Isolation
The balance between excitatory and inhibitory signals in the PFC is well-established as a major determinant of social function and processing [99]. Thus, it should not be surprising that a social perturbation like adolescent isolation may durably disrupt this balance.
In addition to factors involved in excitatory neurotransmission (discussed above), isolation may also influence inhibitory synaptic markers in the mPFC. Bator et al. [37] found increased levels of glutamic acid decarboxylase-65 (GAD-65), a facilitator of activity-dependent GABA release from vesicles, in mPFC tissue from rodents at the end of an early isolation period (P31–40). This effect appears to be transient, as GAD-65 levels normalized in adulthood following re-socialization. Adolescent isolation also increases the density of both vasopressin intestinal peptide-expressing (VIP) and parvalbumin-expressing (PV) interneurons in the PFC [40, 100].
Electrophysiologic recordings have also hinted at impaired excitation/inhibition balance in the mPFC following social isolation. Baarendse et al. [54] examined ex vivo whole-cell patch clamp recordings from layer V pyramidal neurons in the mPFC of previously isolated animals. Here, there were no differences in resting membrane properties or intrinsic excitability of these neurons. However, following dopamine receptor stimulation (using bath application of either D1 and D2 receptor agonists), there was a dramatic lack of typical modulation of EPSC amplitude in these neurons, indicating aberrant dopaminergic signaling mechanisms following adolescent isolation and re-socialization. Dopaminergic innervation of the mPFC drastically increases during adolescence [101, 102], and modulation of PFC circuits by D1 and D2 receptors alters excitation/inhibition balance throughout the post-weaning period [103, 104]. Given that the isolation timeline of Baarendse et al. [54] encompassed much of this developmental period, their results suggest that normative dopaminergic system function in the adult mPFC is influenced by the social environment during adolescence via impact on dopamine fiber innervation of, and receptor expression in, the mPFC. Interestingly, these neurophysiologic processes may also play a role in adolescent myelination processes. While adolescent isolation decreased the expression of oligodendrocyte markers in the mPFC [43, 80], those effects were reversible by administration of either MK-801 (a NMDA receptor antagonist) or adinazolam (a benzodiazepine-class GABA receptor agonist) [80].
It should be noted that many of the electrophysiologic studies examining early-life social isolation have mainly focused on the pre-adolescent period. Yamamuro and colleagues [105, 106] performed ex vivo recordings from adult mice that were isolated from P21 to P35. These studies revealed that pyramidal neurons in the mPFC of previously isolated mice display lower intrinsic excitability (lower spike frequency and increment rate, and higher spike threshold) and synaptic excitability (lower sEPSC and mEPSC frequency, and reduced AMPA/NMDA charge ratio) among a set of subcortical-projecting pyramidal neurons [105]. However, these effects were not evident in animals isolated in later adolescence (P35–49). Thus, while the early isolation period in this study encompasses some of early adolescence, caution is warranted in inferring whether this altered neuronal excitability following post-weaning isolation was primarily due to perturbations during the early adolescent or pre-adolescent periods. Notably, these findings were replicated in a specific population of mPFC-to-posterior PVT projecting neurons [106]. Throughout adolescence, projection neurons in the PFC become more strongly linked to subcortical regions, in particular the striatum [107], and social play, which is characteristic of adolescence, appears important for this age-related strengthening of cortico-striatal connectivity [108].
A final piece of evidence that effects of prior social isolation could linger, in terms of mPFC function in adulthood, comes from electrophysiological recordings and analysis of immediate-early gene expression levels in the mPFC evoked by social interaction. Here, Makinodan et al. [109] utilized electroencephalogram recordings to investigate oscillatory activity within layer V of the prelimbic and infralimbic cortices while mice encountered novel conspecifics. Here, the authors observed a decrease in γ-oscillation and c-Fos mRNA in mice with a history of isolation. These losses were partially recovered if isolated mice were subsequently re-housed with group-housed cage mates following the adolescent isolation period.
5. Additional Considerations
Although social isolation is often referred to as “isolation stress,” the extent to which isolated housing constitutes a true stressor remains ambiguous (reviewed here [32, 110]). The impact of post-weaning isolation on the hypothalamic-pituitary-adrenal (HPA) axis is unclear, as most studies report little or no effect on markers of HPA axis function in isolated animals [47, 111]. In adult rodents with a history of social isolation, no abnormalities in blood corticosterone (CORT) or adrenocorticotropic hormone (ACTH) concentration were detected, regardless of whether rodents had been re-socialized or were still experiencing isolation housing [39, 47, 111, 112]. When CORT concentrations were measured longitudinally, beginning from the onset of an adolescent isolation period, isolated mice displayed glucocorticoid insufficiency rather than the heightened CORT tone one would expect for a true stressor, which normalized by adulthood [56]. This pattern may point to mechanisms by which certain isolation-induced behavioral deficits arise. For instance, pharmacologic antagonism of glucocorticoid receptors during adolescence recapitulated deficits in flexible decision making in adulthood that were seen in previously isolated animals [56]. Additionally, when stress reactivity was assessed following an acute stressor, the effects on blood CORT concentrations appeared to diverge based on sex, with previously isolated males exhibiting a diminished CORT response compared to controls, while previously isolated females mounted a more robust response [47].
A final consideration regards the degree to which studies are able to resolve whether the “social” aspect of isolation is a predominant driver of observed effects, given that the absence of conspecifics produces a stimulus-poor environment writ large. To this point, some studies have been conducted to assess to what extent the effects of isolation may be preventable by providing animals with additional environmental enrichment such as frequent introduction of novel toys and platforms. For instance, Hellemans et al. [113] demonstrated that non-social enrichment could partially attenuate the effects of juvenile isolation including anxiety, spatial learning, and cortical thickness. Notably, while many studies have examined the effects of environmental enrichment during adolescence, these studies are typically not conducted in the context of social isolation and use a combination of large social group housing and additional non-social stimuli as “enrichment” (e.g. [35, 114]), thus making these studies difficult to interpret in the context of isolation.
6. Conclusions
Here we have highlighted evidence that even transient social isolation can disrupt the typical development of brain structure and function, and concurrently alter typical behavior and cognition in adulthood. Not only is it imperative to continue to expand our understanding of mechanistic factors, but also consider how new knowledge may reveal therapeutic avenues by which to improve human outcomes.
Acknowledgements
This work was supported by NIH R01MH117103, P50MH100023, and F30MH117873. The Yerkes National Primate Research Center is supported by NIH P51OD011132.
Footnotes
Conflicts of interest: None
Conflict of interest statement for Li et al.
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baumeister RF and Leary MR, The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol Bull, 1995. 117(3): p. 497–529. [PubMed] [Google Scholar]
- 2.Cacioppo JT and Hawkley LC, Perceived social isolation and cognition. Trends Cogn Sci, 2009. 13(10): p. 447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt-Lunstad J, et al. , Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci, 2015. 10(2): p. 227–37. [DOI] [PubMed] [Google Scholar]
- 4.Leigh-Hunt N, et al. , An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health, 2017. 152: p. 157–171. [DOI] [PubMed] [Google Scholar]
- 5.Matthews GA and Tye KM, Neural mechanisms of social homeostasis. Ann N Y Acad Sci, 2019. 1457(1): p. 5–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills KL, et al. , The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci, 2014. 36(3–4): p. 147–60. [DOI] [PubMed] [Google Scholar]
- 7.Spear LP, The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev, 2000. 24(4): p. 417–63. [DOI] [PubMed] [Google Scholar]
- 8.Paus T, Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci, 2005. 9(2): p. 60–8. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer SM, et al. , The age of adolescence. Lancet Child Adolesc Health, 2018. 2(3): p. 223–228. [DOI] [PubMed] [Google Scholar]
- 10.Gopnik A, et al. , Changes in cognitive flexibility and hypothesis search across human life history from childhood to adolescence to adulthood. Proc Natl Acad Sci U S A, 2017. 114(30): p. 7892–7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews JL, Ahmed SP, and Blakemore SJ, Navigating the Social Environment in Adolescence: The Role of Social Brain Development. Biol Psychiatry, 2021. 89(2): p. 109–118. [DOI] [PubMed] [Google Scholar]
- 12.Blakemore SJ and Mills KL, Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol, 2014. 65: p. 187–207. [DOI] [PubMed] [Google Scholar]
- 13.Walker DM, et al. , Long-Term Behavioral Effects of Post-weaning Social Isolation in Males and Females. Front Behav Neurosci, 2019. 13: p. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke AR, et al. , Impact of adolescent social experiences on behavior and neural circuits implicated in mental illnesses. Neurosci Biobehav Rev, 2017. 76(Pt B): p. 280–300. [DOI] [PubMed] [Google Scholar]
- 15.Vanderschuren LJ, Niesink RJ, and Van Ree JM, The neurobiology of social play behavior in rats. Neurosci Biobehav Rev, 1997. 21(3): p. 309–26. [DOI] [PubMed] [Google Scholar]
- 16.Varlinskaya EI and Spear LP, Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res, 2008. 188(2): p. 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalsgaard S, et al. , Incidence Rates and Cumulative Incidences of the Full Spectrum of Diagnosed Mental Disorders in Childhood and Adolescence. JAMA Psychiatry, 2020. 77(2): p. 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler RC, et al. , Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 2005. 62(6): p. 593–602. [DOI] [PubMed] [Google Scholar]
- 19.Thapar A, et al. , Depression in adolescence. Lancet, 2012. 379(9820): p. 1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orben A, Tomova L, and Blakemore SJ, The effects of social deprivation on adolescent development and mental health. Lancet Child Adolesc Health, 2020. 4(8): p. 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlen M, What constitutes the prefrontal cortex? Science, 2017. 358(6362): p. 478–482. [DOI] [PubMed] [Google Scholar]
- 22.Miller EK and Cohen JD, An integrative theory of prefrontal cortex function. Annu Rev Neurosci, 2001. 24: p. 167–202. [DOI] [PubMed] [Google Scholar]
- 23.Chini M and Hanganu-Opatz IL, Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends Neurosci, 2020. [DOI] [PubMed] [Google Scholar]
- 24.Gogtay N, et al. , Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A, 2004. 101(21): p. 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen SL, Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev, 2003. 27(1–2): p. 3–18. [DOI] [PubMed] [Google Scholar]
- 26.Laviola G, et al. , Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev, 2003. 27(1–2): p. 19–31. [DOI] [PubMed] [Google Scholar]
- 27.McCutcheon JE and Marinelli M, Age matters. Eur J Neurosci, 2009. 29(5): p. 997–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick CM and Mathews IZ, HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav, 2007. 86(2): p. 220–33. [DOI] [PubMed] [Google Scholar]
- 29.Thor DH and Holloway WR Jr., Social play in juvenile rats: a decade of methodological and experimental research. Neurosci Biobehav Rev, 1984. 8(4): p. 455–64. [DOI] [PubMed] [Google Scholar]
- 30.Panksepp J, The ontogeny of play in rats. Dev Psychobiol, 1981. 14(4): p. 327–32. [DOI] [PubMed] [Google Scholar]
- 31.Lukkes JL, et al. , Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci, 2009. 3: p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall FS, Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol, 1998. 12(1–2): p. 129–62. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg CL, et al. , Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol, 1999. 34(2): p. 129–38. [PubMed] [Google Scholar]
- 34.Lukkes J, et al. , Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J Neurosci, 2009. 29(32): p. 9955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makinodan M, et al. , A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science, 2012. 337(6100): p. 1357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, et al. , Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci, 2012. 15(12): p. 1621–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bator E, et al. , Adolescent Social Isolation Affects Schizophrenia-Like Behavior in the MAM-E17 Model of Schizophrenia. Neurotox Res, 2018. 34(2): p. 305–323. [DOI] [PubMed] [Google Scholar]
- 38.Hol T, et al. , Isolation during the play period in infancy decreases adult social interactions in rats. Behav Brain Res, 1999. 100(1–2): p. 91–7. [DOI] [PubMed] [Google Scholar]
- 39.Lukkes JL, et al. , Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav, 2009. 55(1): p. 248–56. [DOI] [PubMed] [Google Scholar]
- 40.Pascual R, Zamora-Leon SP, and Valero-Cabre A, Effects of postweaning social isolation and re-socialization on the expression of vasoactive intestinal peptide (VIP) and dendritic development in the medial prefrontal cortex of the rat. Acta Neurobiol Exp (Wars), 2006. 66(1): p. 7–14. [DOI] [PubMed] [Google Scholar]
- 41.Karkhanis AN, et al. , Social isolation rearing increases nucleus accumbens dopamine and norepinephrine responses to acute ethanol in adulthood. Alcohol Clin Exp Res, 2014. 38(11): p. 2770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright IK, Upton N, and Marsden CA, Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav, 1991. 50(6): p. 1129–32. [DOI] [PubMed] [Google Scholar]
- 43.Leussis MP and Andersen SL, Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse, 2008. 62(1): p. 22–30. [DOI] [PubMed] [Google Scholar]
- 44.Barrot M, et al. , Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc Natl Acad Sci U S A, 2005. 102(23): p. 8357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cacioppo S, Capitanio JP, and Cacioppo JT, Toward a neurology of loneliness. Psychol Bull, 2014. 140(6): p. 1464–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace DL, et al. , CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci, 2009. 12(2): p. 200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weintraub A, Singaravelu J, and Bhatnagar S, Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res, 2010. 1343: p. 83–92. [DOI] [PubMed] [Google Scholar]
- 48.Huizinga M, Dolan CV, and van der Molen MW, Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia, 2006. 44(11): p. 2017–36. [DOI] [PubMed] [Google Scholar]
- 49.Naneix F, et al. , Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J Neurosci, 2012. 32(46): p. 16223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blakemore SJ and Robbins TW, Decision-making in the adolescent brain. Nat Neurosci, 2012. 15(9): p. 1184–91. [DOI] [PubMed] [Google Scholar]
- 51.Moin Afshar N, et al. , Reinforcement Learning during Adolescence in Rats. J Neurosci, 2020. 40(30): p. 5857–5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellis SM and Pellis VC, What is play fighting and what is it good for? Learn Behav, 2017. 45(4): p. 355–366. [DOI] [PubMed] [Google Scholar]
- 53.Han X, et al. , Brief social isolation in early adolescence affects reversal learning and forebrain BDNF expression in adult rats. Brain Res Bull, 2011. 86(3–4): p. 173–8. [DOI] [PubMed] [Google Scholar]
- 54.Baarendse PJ, et al. , Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology, 2013. 38(8): p. 1485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balleine BW and O’Doherty JP, Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology, 2010. 35(1): p. 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hinton EA, et al. , Social Isolation in Adolescence Disrupts Cortical Development and Goal-Dependent Decision-Making in Adulthood, Despite Social Reintegration. eNeuro, 2019. 6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moeller FG, et al. , Psychiatric aspects of impulsivity. Am J Psychiatry, 2001. 158(11): p. 1783–93. [DOI] [PubMed] [Google Scholar]
- 58.Whiteford HA, et al. , Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet, 2013. 382(9904): p. 1575–86. [DOI] [PubMed] [Google Scholar]
- 59.Kupferberg A, Bicks L, and Hasler G, Social functioning in major depressive disorder. Neurosci Biobehav Rev, 2016. 69: p. 313–32. [DOI] [PubMed] [Google Scholar]
- 60.Lawlor VM, et al. , Dissecting the impact of depression on decision-making. Psychol Med, 2020. 50(10): p. 1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hinton EA and Gourley SL, Social Adversity During Adolescence Weakens Goal-Directed Decision Making in Adulthood. NEUROPSYCHOPHARMACOLOGY, 2018. 43: p. S115–S115. [Google Scholar]
- 62.Petanjek Z, et al. , Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A, 2011. 108(32): p. 13281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huttenlocher PR, Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res, 1979. 163(2): p. 195–205. [DOI] [PubMed] [Google Scholar]
- 64.Rakic P, et al. , Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science, 1986. 232(4747): p. 232–5. [DOI] [PubMed] [Google Scholar]
- 65.Bourgeois JP, Goldman-Rakic PS, and Rakic P, Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex, 1994. 4(1): p. 78–96. [DOI] [PubMed] [Google Scholar]
- 66.Huttenlocher PR and Dabholkar AS, Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol, 1997. 387(2): p. 167–78. [DOI] [PubMed] [Google Scholar]
- 67.Rakic P, Bourgeois JP, and Goldman-Rakic PS, Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res, 1994. 102: p. 227–43. [DOI] [PubMed] [Google Scholar]
- 68.Berry KP and Nedivi E, Spine Dynamics: Are They All the Same? Neuron, 2017. 96(1): p. 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holtmaat AJ, et al. , Transient and persistent dendritic spines in the neocortex in vivo. Neuron, 2005. 45(2): p. 279–91. [DOI] [PubMed] [Google Scholar]
- 70.Gourley SL, et al. , Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci, 2012. 32(7): p. 2314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shapiro LP, et al. , Differential expression of cytoskeletal regulatory factors in the adolescent prefrontal cortex: Implications for cortical development. J Neurosci Res, 2017. 95(5): p. 1123–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koss WA, et al. , Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse, 2014. 68(2): p. 61–72. [DOI] [PubMed] [Google Scholar]
- 73.Drzewiecki CM, Willing J, and Juraska JM, Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset. Synapse, 2016. 70(9): p. 361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delevich K, et al. , Sex and Pubertal Status Influence Dendritic Spine Density on Frontal Corticostriatal Projection Neurons in Mice. Cereb Cortex, 2020. 30(6): p. 3543–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boivin JR, et al. , Adolescent pruning and stabilization of dendritic spines on cortical layer 5 pyramidal neurons do not depend on gonadal hormones. Dev Cogn Neurosci, 2018. 30: p. 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitaker KJ, et al. , Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc Natl Acad Sci U S A, 2016. 113(32): p. 9105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Selemon LD, A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry, 2013. 3: p. e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keshavan MS, et al. , Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry, 2014. 1(7): p. 549–58. [DOI] [PubMed] [Google Scholar]
- 79.Paus T, Keshavan M, and Giedd JN, Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci, 2008. 9(12): p. 947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leussis MP, et al. , The enduring effects of an adolescent social stressor on synaptic density, part II: Poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse, 2008. 62(3): p. 185–92. [DOI] [PubMed] [Google Scholar]
- 81.Kim E and Sheng M, PDZ domain proteins of synapses. Nat Rev Neurosci, 2004. 5(10): p. 771–81. [DOI] [PubMed] [Google Scholar]
- 82.Whyte AJ, et al. , Reward-Related Expectations Trigger Dendritic Spine Plasticity in the Mouse Ventrolateral Orbitofrontal Cortex. J Neurosci, 2019. 39(23): p. 4595–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pascual R and Zamora-Leon SP, Chronic (−)-deprenyl administration attenuates dendritic developmental impairment induced by early social isolation in the rat. Dev Neurosci, 2007. 29(3): p. 261–7. [DOI] [PubMed] [Google Scholar]
- 84.Bhattacherjee A, et al. , Cell type-specific transcriptional programs in mouse prefrontal cortex during adolescence and addiction. Nat Commun, 2019. 10(1): p. 4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zuo Y, et al. , Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron, 2005. 46(2): p. 181–9. [DOI] [PubMed] [Google Scholar]
- 86.Shapiro LP, et al. , Rho-kinase inhibition has antidepressant-like efficacy and expedites dendritic spine pruning in adolescent mice. Neurobiol Dis, 2019. 124: p. 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fox ME, et al. , Dendritic remodeling of D1 neurons by RhoA/Rho-kinase mediates depression-like behavior. Mol Psychiatry, 2020. 25(5): p. 1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feng J, et al. , Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A, 2000. 97(16): p. 9287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Z, et al. , A critical role of Rho-kinase ROCK2 in the regulation of spine and synaptic function. Neuropharmacology, 2009. 56(1): p. 81–9. [DOI] [PubMed] [Google Scholar]
- 90.Chao MV, Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci, 2003. 4(4): p. 299–309. [DOI] [PubMed] [Google Scholar]
- 91.Koleske AJ, Molecular mechanisms of dendrite stability. Nat Rev Neurosci, 2013. 14(8): p. 536–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ziegler G, et al. , Compulsivity and impulsivity traits linked to attenuated developmental frontostriatal myelination trajectories. Nat Neurosci, 2019. 22(6): p. 992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sowell ER, et al. , In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci, 1999. 2(10): p. 859–61. [DOI] [PubMed] [Google Scholar]
- 94.Miller DJ, et al. , Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A, 2012. 109(41): p. 16480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McKenzie IA, et al. , Motor skill learning requires active central myelination. Science, 2014. 346(6207): p. 318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu J, et al. , Clemastine Enhances Myelination in the Prefrontal Cortex and Rescues Behavioral Changes in Socially Isolated Mice. J Neurosci, 2016. 36(3): p. 957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robbins TW, et al. , Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci, 2012. 16(1): p. 81–91. [DOI] [PubMed] [Google Scholar]
- 98.Zhou B, et al. , Oligodendrocyte lineage cells and depression. Mol Psychiatry, 2021. 26(1): p. 103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yizhar O, et al. , Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature, 2011. 477(7363): p. 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mackowiak M, et al. , Adolescent social isolation affects parvalbumin expression in the medial prefrontal cortex in the MAM-E17 model of schizophrenia. Metab Brain Dis, 2019. 34(1): p. 341–352. [DOI] [PubMed] [Google Scholar]
- 101.Kalsbeek A, et al. , Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol, 1988. 269(1): p. 58–72. [DOI] [PubMed] [Google Scholar]
- 102.Willing J, et al. , Innervation of the medial prefrontal cortex by tyrosine hydroxylase immunoreactive fibers during adolescence in male and female rats. Dev Psychobiol, 2017. 59(5): p. 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brenhouse HC, Sonntag KC, and Andersen SL, Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci, 2008. 28(10): p. 2375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tseng KY and O’Donnell P, Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex, 2007. 17(5): p. 1235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamuro K, et al. , Social Isolation During the Critical Period Reduces Synaptic and Intrinsic Excitability of a Subtype of Pyramidal Cell in Mouse Prefrontal Cortex. Cereb Cortex, 2018. 28(3): p. 998–1010. [DOI] [PubMed] [Google Scholar]
- 106.Yamamuro K, et al. , A prefrontal-paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice. Nat Neurosci, 2020. 23(10): p. 1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liston C, et al. , Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex, 2006. 16(4): p. 553–60. [DOI] [PubMed] [Google Scholar]
- 108.van Kerkhof LW, et al. , Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology, 2013. 38(10): p. 1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Makinodan M, et al. , Effects of the mode of re-socialization after juvenile social isolation on medial prefrontal cortex myelination and function. Sci Rep, 2017. 7(1): p. 5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Green MR and McCormick CM, Effects of stressors in adolescence on learning and memory in rodent models. Horm Behav, 2013. 64(2): p. 364–79. [DOI] [PubMed] [Google Scholar]
- 111.Fone KC and Porkess MV, Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev, 2008. 32(6): p. 1087–102. [DOI] [PubMed] [Google Scholar]
- 112.Weiss IC, et al. , Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res, 2004. 152(2): p. 279–95. [DOI] [PubMed] [Google Scholar]
- 113.Hellemans KG, Benge LC, and Olmstead MC, Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res, 2004. 150(2): p. 103–15. [DOI] [PubMed] [Google Scholar]
- 114.Hoffmann LC, et al. , Effect of “enriched environment” during development on adult rat behavior and response to the dopamine receptor agonist apomorphine. Neuroscience, 2009. 158(4): p. 1589–98. [DOI] [PubMed] [Google Scholar]