The TTR V142I variant (previously known as V122I), associated with hereditary transthyretin amyloidosis (hATTR), is the most common TTR variant worldwide, with recent prevalence estimates of 1 in 330 individuals (1). Up to 4% of African American (AAs) in the U.S. are heterozygous for V142I (i.e., V142I+) (2), which, in part, is thought to explain the observation that cardiac amyloidosis (CA) is four times more common among AA than European descent patients over 60 years of age (3). .Non-cardiac clinical features of hATTR such as carpal tunnel syndrome, spinal stenosis and polyneuropathy (“red flags”) can precede CA by several years, and have been observed in undiagnosed V142I+ individuals absent heart failure (HF) symptoms (4). Nevertheless, the clinical diagnosis of hATTR in V142I+ individuals is typically only made after they develop overt CA and HF.
Retinol binding protein 4 (RBP4) is a lipocalin retinol carrier that binds 1:1 with transthyretin in serum, functioning as an endogenous transthyretin stabilizer (5). Circulating RBP4 levels depend on the interaction of RBP4 and transthyretin, and are reduced in hATTR. RBP4 levels have been shown to discriminate between V142I CA and non-CA HF in AA patients 60 years or older when integrated with other clinical parameters (6). However, RBP4 has not been studied in undiagnosed or younger V142I+ individuals without CA to inform preclinical hATTR. Here, we hypothesized that RBP4 levels would differentiate younger undiagnosed V142I+ individuals without overt CA from V142I- individuals, indicating circulating destabilized transthyretin and potentially enabling earlier risk prediction for hATTR.
V142I+ and V142I- individuals were identified from the BioMe Biobank in NYC, which links exome sequencing to longitudinal electonic health record (EHR) data (2, 4), and includes banked plasma samples from participants at enrollment. We obtained plasma from 40 self-reported AA V142I+ individuals without any diagnosis codes related to cardiomyopathy, HF, or amyloidosis (heart healthy). V142I+ individuals were aged 40–60 years at enrollment, were 60% female, and 50% had at least one red flag (based on diagnosis codes related to carpal tunnel syndrome, spinal stenosis, and polyneuropathy). V142I+ individuals were age-, sex-, and race-matched with 80 heart-healthy V142I- individuals from BioMe. We conducted manual chart reviews blinded to V142I status, and excluded 9 individuals (2 V142I+ and 7 V142I-) who had evidence of HF, significant valvular disease, or reduced (≤ 50%) ejection fraction. Plasma samples were randomized and analyzed in duplicate for target protein concentrations using a solid phase sandwich enzyme linked immunosorbent assay for human RBP4 (Quantikine ELISA kit, R&D Systems, Minneapolis, MN, USA). All samples were assayed in technical duplicate and standards were included for calibration of readouts against both positive and negative controls. Optical density measurements from ELISA readouts were converted to concentrations (ng/mL) using the standard curve generated during the assay run. Values more than three standard deviations from the mean were excluded from analyses. A two-tailed Student’s t-test (heteroscedastic) was used to compare distributions of protein expression.
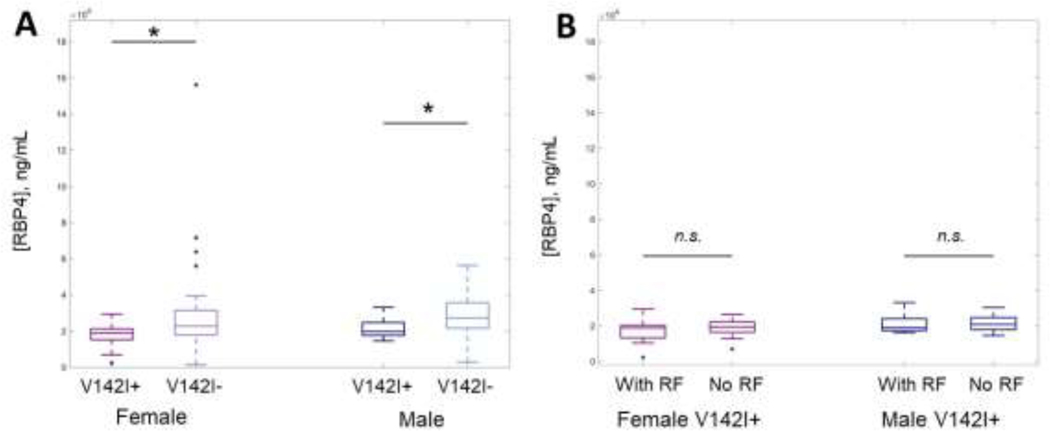
We analyzed RBP4 levels from 111 (38 V142I+ and 73 V142I-) heart-healthy AA individuals aged 40–60 years. Mean RBP4 levels were significantly lower in V142I+ vs. V142I- individuals (N=106; 18.89 μg/mL vs. 24.69 μg/mL, P=1.1×10−4), and this difference was observed in both females (N=61; 17.05 μg/mL vs. 23.54 μg/mL, P=6.8×10−4) and males (N=45; 21.41 μg/mL vs. 26.25 μg/mL, P=3.4×10−2; Figure 1A). Mean RBP4 levels were similarly low among V142I+ individuals with and without red flags (18.12 μg/mL vs. 19.65 μg/mL, P=0.43, Figure 1B). Although the exact relationship between RBP4 and destabilized transthyretin in hATTR is not known, pathogenic variants in TTR such as V142I, which destabilize transthyretin, may increase RBP4 clearance (6). Our findings suggest that circulating destabilized transthyretin may be elevated in young V142I+ individuals preceding CA and even preceding non-cardiac red flags related to hATTR. Given this, RBP4 could be more useful to identify presymptomatic V142I+ individuals at risk of hATTR than clinical screening for red flags alone. This study extends upon previous findings that RBP4 levels can discriminate between hATTR and non-amyloidogenic HF in older AA individuals, and identifies RBP4 as a potential biomarker for hATTR in younger individuals and those without CA.
Figure 1.
A) Retinol binding protein 4 (RBP4) concentrations in African American individuals harboring TTR V142I (V142I+) are significantly lower than in age-, sex- and race-matched V142I-individuals; B) RBP4 levels are similar in V142I+ individuals regardless of presence/absence of red flag (RF) diagnoses. † P < 0.001; * P < 0.05; n.s. = not significant.
hATTR diagnosis is frequently missed or delayed in AA individuals harboring V142I, which potentially contributes to worse HF outcomes. With the emergence of new hATTR therapies, strategies for screening and early detection of disease are paramount. In the ATTR-ACT trial comparing efficacy of the transthyretin stabilizer tafamidis against placebo in CA, the overall positive outcome of reduction in all-cause mortality and cardiovascular-related hospitalizations was driven by the response in the least symptomatic group (those with NYHA Class 1 or 2 HF) (7). These observations have led experts to propose use of tafamidis in V142I+ individuals as early as possible. Noninvasive tools are needed to enable early identification of preclinical hATTR when treatment is likely to be most efficacious. RBP4 levels were previously found to be lower in individuals with hATTR-CA compared to those with non-CA HF; here, we show that RBP4 levels are lower in undiagnosed V142I+ individuals without CA compared to matched V142I- individuals. RBP4 may serve as a useful biomarker to signal preclinical hATTR, permitting early diagnoses and expedited care delivery in patients at high genetic risk of HF, especially in AA populations where there is an urgent need to address existing health disparities. Longitudinal studies are needed to define the discriminative capacity of RBP4 for predicting red flag and CA manifestations of hATTR.
Acknowledgments
Funding: A.R.K received funding from NIH NHLBI (K23 HL140083).
Financial Disclosures: A.R.K has served on the Advisory Board for Alynlam Pharmaceuticals. N.S.A-H was previously employed by Regeneron Pharmaceuticals and has received a speaker honorarium from Genentech.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lahuerta Pueyo C, Aibar Arregui MA, Gracia Gutierrez A, Bueno Juana E, Menao Guillen S. Estimating the prevalence of allelic variants in the transthyretin gene by analysing large-scale sequencing data. Eur J Hum Genet. 2019;27(5):783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damrauer SM, Chaudhary K, Cho JH, Liang LW, Argulian E, Chan L, et al. Association of the V122I Hereditary Transthyretin Amyloidosis Genetic Variant With Heart Failure Among Individuals of African or Hispanic/Latino Ancestry. JAMA. 2019;322(22):2191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson DR, Pastore RD, Yaghoubian R, Kane I, Gallo G, Buck FS, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336(7):466–73. [DOI] [PubMed] [Google Scholar]
- 4.Soper ER, Suckiel SA, Braganza GT, Kontorovich AR, Kenny EE, Abul-Husn NS. Genomic Screening Identifies Individuals at High Risk for Hereditary Transthyretin Amyloidosis. J Pers Med. 2021;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monaco HL, Rizzi M, Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. 1995;268(5213):1039–41. [DOI] [PubMed] [Google Scholar]
- 6.Arvanitis M, Koch CM, Chan GG, Torres-Arancivia C, LaValley MP, Jacobson DR, et al. Identification of Transthyretin Cardiac Amyloidosis Using Serum Retinol-Binding Protein 4 and a Clinical Prediction Model. JAMA Cardiol. 2017;2(3):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018;379(11):1007–16. [DOI] [PubMed] [Google Scholar]