Abstract
Blood-brain barrier (BBB) disruption is one of the most important pathological changes following cerebral ischemia-reperfusion. We tested whether inhibition of the serum and glucocorticoid regulated kinase 1 (SGK1) would decrease BBB disruption and contribute to decreasing infarct size in the first few hours of cerebral ischemia-reperfusion within the thrombolysis therapy time window. After transient middle cerebral artery occlusion (MCAO), an SGK1 inhibitor GSK650394, or vehicle was administered into the lateral ventricle of rats. After one hour of MCAO and two hours of reperfusion, we determined BBB disruption using the transfer coefficient (Ki) of 14C-α-aminoisobutyric acid, and also determined infarct size, phosphorylation of NDRG1, and MMP2 protein level. Ischemia-reperfusion increased (+34%, p < 0.05) and GSK650394 decreased (−25%, p < 0.05) the Ki in the ischemic-reperfused cortex. GSK650394 decreased the percentage of cortical infarct (−31%, p < 0.001). At the same time GSK650394 reduced NDRG1 phosphorylation and MMP2 protein level in the ischemic-reperfused cortex suggesting that SGK1 was inhibited by GSK650394 and that lower MMP2 could be one of the mechanisms of decreased BBB disruption. Collectively our data suggest that GSK650394 could be neuroprotective and one of the mechanisms of the neuroprotection could be decreased BBB disruption. SGK1 inhibition within the thrombolysis therapy time window might reduce cerebral ischemia-reperfusion injury.
Keywords: Blood-brain barrier, Brain protection, Cerebral ischemia-reperfusion, SGK1, GSK650394
1. Introduction
Even though numerous regimens have been reported to be neuroprotective in stroke in the experimental animals, the only clinical treatment for stroke victims is to restore perfusion as soon as possible by tissue plasminogen activator (tPA) or endovascular thrombectomy. However, they have a very narrow therapeutic time window: 3 - 4.5 or 6 hours respectively [1]. During the first few hours of cerebral ischemia and reperfusion, cells may undergo necrosis and/or apoptosis due to various factors such as excitatory neurotransmitters, Ca++influx, platelet activation, blood-brain barrier (BBB) disruption, nitric oxide, oxidative stress, and inflammation [2,3].
BBB disruption is one of the most important pathological changes in ischemia-reperfusion injury [2,4] and following thrombolysis therapy [5]. BBB disruption can aggravate the untoward effects of reperfusion therapy [4,5]. New agents that could ameliorate BBB disruption during this early stage of cerebral ischemia-reperfusion could be beneficial for the neuronal survival as well as reducing complications of the thrombolytic therapy.
Serum and glucocorticoid regulated kinase 1 (SGK1) is a serine-threonine kinase that is induced by serum and glucocorticoid, and activated by insulin and growth factors via phosphoinositide 3 kinase (PI3K), phosphoinositide-dependent kinase-1 (PDK1), and mechanistic target of rapamycin complex 2 (mTORC2) [6]. During stress conditions such as starvation, the increased mTORC2 activation promotes flux through critical metabolic pathways to maintain cell survival [7,8]. However, sustained PI3K/mTORC2 signaling is also undesirable since it may lead to diseases such as metabolic disorders, cancer, and neurodegenerative diseases [9]. Previous studies on the PI3K-Akt-mTOR pathway suggested involvement of mTORC1 and mTORC2 in altering BBB permeability and infarct size in early cerebral ischemia-reperfusion [10,11]. SGK1 has two distinctive functions in neuronal survival [12]. Activation of SGK1 could be neuroprotective since SGK1 is one of the main substrates of mTORC2 and a homologue of Akt, and share downstream targets with anti-apoptotic PI3K-Akt signaling, supporting cell survival [12,13]. However, SGK1 activation could also jeopardize neuronal survival since SGK1 upregulates various ion channels including ENaC, TRPV4-6 and GluR6, and Na+/K+-ATPase which consumes high energy, disrupting the energy balance in cerebral ischemia [12,14,15]. The energy imbalance may cause depolarization of various cells, opening of calcium channels, cell swelling, excitotoxicity, and BBB disruption which are the main reasons of neuronal death in early cerebral ischemia [2,3,12,14].
The action of SGK1 on ion channels suggests that SGK1 activation may increase BBB disruption since excitatory neurotransmitters and Ca++ flux are associated with increased BBB disruption in cerebral ischemia [16,17]. Cytoplasmic Ca++ concentration is an important factor in determining the BBB permeability and elevation of cytosolic calcium level (Ca++) can compromise BBB integrity as previously reported [18,19]. However, no studies are available about the effects of SGK1 on BBB disruption in cerebral ischemia-reperfusion. Furthermore, the data of SGK1 on neuronal survival in cerebral ischemia is contradictory to each other. Induction of SGK1 in cerebral ischemia is reported to be neuroprotective in some studies [20,21]. In contrast, other researchers reported that inhibition of SGK1 is neuroprotective [15]. In this current study, we hypothesized that inhibition of SGK1 would decrease BBB disruption and decrease infarct in early cerebral ischemia-reperfusion especially within thrombolysis therapy time window
To assess the degree of BBB disruption we used a small (14C-α-aminoisobutyric acid (14C-AIB)) and a large molecular weight (3H-dextran) tracer to measure BBB transfer quantitatively. Infarct size was obtained with tetrazolium staining. We checked if GSK650394 inhibited SGK1 by determining its effect on N-Myc downstream regulated gene 1 (NDRG1), since NDRG1 is specifically targeted by SGK1 [22,23]. We determined matrix metalloproteinase 2 (MMP2) protein level as a molecular marker of BBB disruption. In cerebral ischemia, MMPs break down extracellular matrix such as tight junctions and basal lamina which are part of the BBB [24]. All the parameters were evaluated at two hours of reperfusion after one hour of middle cerebral artery occlusion (MCAO), which is within the thrombolysis therapy time window. We found that SGK1 inhibition decreased BBB disruption and reduced infarct in cerebral ischemia-reperfusion suggesting that SGK1 inhibition may reduce cerebral ischemia-reperfusion injury.
2. Materials and methods
2.1. Animals
The US Public Health Service Guidelines and the Guide for the Care of Laboratory Animals (DHHS Publication No. 85-23, revised 1996) were followed. Our Institutional Animal Care and Use Committee approved our research.
Male Fischer 344 rats weighing 220 - 250 g were randomly divided into the following groups: (1) MCAO/reperfusion (Control Group, n = 17), (2) MCAO/reperfusion + GSK650394 (GSK650394 Group, n = 17), and (3) sham (Sham Group, n = 4). All rats were ventilated through tracheal tubes with 2% isoflurane in an air-oxygen mixture. A femoral arterial catheter was inserted to connect to Statham P23Db pressure transducer and an Iworx data acquisition system to monitor heart rate and blood pressure. Blood samples were obtained for analysis of hemoglobin, blood gases and pH using a Radiometer blood gas analyzer (ABL80). A femoral venous catheter was used to administer radioactive tracer and normal saline. Body temperature was monitored with a servo-controlled rectal thermistor probe and was maintained at 37°C ± 0.5 with a heating lamp. As a representative pericranial temperature, temporalis muscle temperature was monitored using a thermocouple probe (Omega Engineering, Inc., Stamford, CT), which was 36.8°C ± 0.4. After MCAO and a burr hole placement, the isoflurane concentration was reduced to 1.4%. For the GSK650394 Group, 5 μL of 2mM GSK650394, an SGK1 inhibitor, was administered in the left lateral ventricle 10 min after transient left MCAO. For the Control Group, after left MCAO, the same volume of vehicle (DMSO + saline) was administered in the left lateral ventricle at the same time point. In the Sham Group the internal and external carotid artery dissection and burr hole were performed in the same way but neither MCAO nor intraventricular injection was performed. After one hour of MCAO and two hours of reperfusion, parameters of BBB permeability, size of infarct and Western blot were determined.
2.2. Transient middle cerebral artery occlusion (MCAO)
Transient occlusion of the MCA was performed as previously reported but modified for this experiment [10,25]. Through a midline ventral cervical incision, the left common carotid artery was exposed. A 4.0 monofilament thread with silicone covered tip was inserted into the stump of the left external carotid artery and advanced approximately 1.7 cm into the left internal carotid artery until resistance was met. The filament was kept in place for 60 min to block the MCA. Then it was removed to allow reperfusion and the external carotid artery was closed. Measurements of BBB permeability, size of infarct and Western blot were performed after 120 min of reperfusion. BBB permeability parameters were determined in the ischemic-reperfused cortex (IR-C), contralateral cortex (CC), ipsilateral hippocampus (IH), contralateral hippocampus (CH), cerebellum (CBLL), and pons.
2.3. Cerebral intraventricular injection
Rats were placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA). A cranial burr hole (1 mm) was drilled 0.8 mm posterior and 1.5 mm lateral to the bregma and 3.6 mm ventral to the surface of the skull. A 26-gauge needle was inserted perpendicularly through the burr hole into the left lateral ventricle [26], and 5 μL of 2 mM GSK650394 or vehicle was injected into the left lateral ventricle.
2.4. Blood-brain barrier permeability
After one hour of MCA occlusion and two hours of reperfusion, 20 μCi of 14C-AIB (molecular weight 104 Da, Amersham, Arlington Heights, Illinois) was rapidly injected intravenously and flushed with 0.5 mL of normal saline as published in our previous studies [10]. Blood samples were collected from the femoral arterial catheter at 20-second intervals for the first 2 min and then, every min for the next 8 min. Five min after injecting 14C-AIB, 20 μCi of 3H-dextran (molecular weight 70,000 Da, Amersham, Arlington Heights, IL) was injected intravenously and flushed with 0.5 mL of normal saline. After collecting the ten-min arterial blood sample, the rats were decapitated. The following brain regions were dissected: IR-C, CC, IH, CH, CBLL, and pons. Brain samples were solubilized in Soluene™ (Packard, Downers Grove, IL). Arterial blood samples were centrifuged, and the plasma was separated. Plasma and brain samples were counted on a liquid scintillation counter that was equipped for dual label counting. Quench curves were prepared using carbon tetrachloride. All samples were automatically corrected for quenching. The blood-brain transfer coefficient for 14C-AIB was determined assuming a unidirectional transfer of 14C-AIB over a ten-min period of the experiment using the equation described previously [10,11,27]:
2.5. Size of infarction
For tetrazolium staining, 2,3,5-triphenyltetrazolium chloride (Sigma) 0.05% solution in PBS was prepared and warmed to 37°C [28]. As described previously, each brain was sliced in coronal sections using a straight edge razor blade resulting 3-4 slices of approximately 2-3 mm thick [10]. After incubating the slices in the tetrazolium for 30-min, they were washed three times in PBS, one min per wash. Each slice was then placed in a small weighing dish pre-filled with PBS. The dish was placed on a dissection microscope and a clean slide was placed over it. The cortical region of each slice was traced onto the slide using a 0.3 mm marker. Any infarcted areas were marked by cross-hatching over any areas not well marked with tetrazolium stain. The slides were scanned and the images were measured for total and infarcted areas using ImageJ to determine the percentage of infarcted areas.
2.6. Western blot
In three rats from the Control and GSK Group, brain tissue was lysed in a radioimmunoprecipitation assay buffer (RIPA buffer) which is made of 150 mM NaCl, 25 mM Tris pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 1 mM EDTA, 1 mM Na3PO4, 1 mM NaF, and protease inhibitor mixture. The tissue lysate was centrifuged at 15000g for 30 min at 4°C. Bradford assay was used to quantitate protein levels. Equal amounts of total extracts were loaded in each lane and proteins were resolved by SDS-PAGE, then subjected to immunoblotting using antibodies to NDRG1, pNDRG1 (Thr346) (Cell Signaling, Danvers, MA), and MMP2 (Abcam, Cambridge, MA) followed by Western blot analysis.
2.7. Statistical analysis
A two-way analysis of variance was performed using the general linear model (PROG GLM) from the SAS Institute (Cary, NC) to assess the differences in Ki, volume of dextran distribution and vital signs between the experimental groups and among the various examined regions. The statistical significance of differences was determined using the Tukey test. The differences in the size of cortical infarct and protein levels were analyzed with an unpaired Student t-test. All data were expressed as mean ± standard error, and the significance was defined as p < 0.05.
3. Results
3.1. Hemodynamic parameters and blood gases
Hemodynamic variables and blood gases of the experimental groups prior to measuring BBB permeability are presented in Table 1. All the parameters were within physiological ranges for rats. There were no statistically significant differences in any of the parameters between the GSK650394 treated and untreated rats. The vital signs and blood gas values of the rats that were used to determine the size of cortical infarct and Western blot were similar to the corresponding group of the rats used to determine BBB permeability.
Table 1.
Hemodynamic and blood gas parameters for the control and the GSK650394 treated group just before determination of blood-brain barrier permeability.
| Group | MCAO/reperfusion (n = 8) |
MCAO/reperfusion + GSK650394 (n = 8) |
|---|---|---|
| Mean blood pressure (mm Hg) | 87 ± 6 | 90 ± 5 |
| Heart rate (beats/min) | 344 ± 15 | 322 ± 17 |
| Arterial PO2 (mmHg) | 105 ± 6 | 112 ± 6 |
| Arterial PCO2 (mmHg) | 32 ± 3 | 34 ± 3 |
| pH | 7.36 ± 0.02 | 7.32 ± 0.03 |
| Hemoglobin (g/100 mL) | 10.4 ± 0.7 | 10.3 ± 0.6 |
Values are mean ± SEM. MCAO/reperfusion: Control Group, MCAO/reperfusion + GSK650394: GSK650394 Group.
3.2. Transfer coefficient (Ki)
In the MCAO/reperfusion group, the Ki of the ischemic-reperfused cortex was significantly higher (+34%, p < 0.05) than the contralateral cortex. The Ki of ipsilateral and contralateral hippocampus was lower than the ischemic-reperfused cortex (p < 0.05). The Ki of the contralateral hippocampus was lower than pons (p < 0.01). Administration of GSK650394 decreased the Ki of 14C-AIB in the ischemic-reperfused cortex (IR-C, −25%, p < 0.05) compared with the Control Group. In the GSK650394 treated rats there was no significant difference in Ki between the IR-C and CC, and the Ki of all other non-ischemic brain regions was similar to the contralateral cortex (Fig. 1 (A)). In the Sham Group, there were no statistical differences in the Ki as well as dextran distribution in any brain regions when compared with other groups except that the Ki of the IR-C of the Sham Group was lower than that of the Control Group (p < 0.05). The Ki of each brain region of the Sham Group was as following: IR-C, 4.23 ± 0.82; CC, 3.50 ± 0.38; IH, 2.43 ± 0.51; CH, 3.20 ± 0.71; CBLL, 4.00 ± 0.75; Pons, 4.50 ± 0.88 μL/g/min respectively. The volume of dextran distribution of each brain region of the Sham Group was as following: IR-C, 2.30 ± 0.09; CC, 1.83 ± 0.23; IH, 2.05 ± 0.27; CH, 1.88 ± 0.23; CBLL, 1.98 ± 0.06; Pons, 2.35 ± 0.25 mL/100g respectively.
Fig. 1.
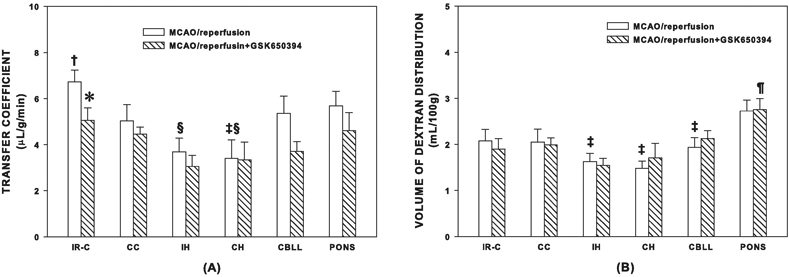
Transfer coefficient (Ki) of 14C-AIB (A) and volume of dextran distribution (B) in various brain regions of the MCAO/reperfusion (Control Group) and MCAO/reperfusion+ GSK650394 (GSK650394 Group) after one hour of middle cerebral artery occlusion and two hours of reperfusion. n=8 in each group. A significant decrease (−25%) in Ki was noted with GSK650394 treatment in the IR-C. No significant difference was noted in the volume of dextran distribution with GSK650394 treatment in the IR-C. IR-C: Ischemic-reperfused cortex. CC: Contralateral cortex. IH: Ipsilateral hippocampus. CH: Contralateral hippocampus. CBLL: Cerebellum. *: P < 0.05 vs the MCAO/reperfusion group (Control Group), †: P < 0.05 vs CC. ‡: P < 0.05 vs pons. §: P < 0.05 vs IR-C. ¶: P < 0.05 vs all other brain regions. Values are means ± SEM.
3.3. Volume of dextran distribution
There were no statistical differences in the volume of dextran distribution in any brain regions that were examined between these two experimental groups with or without GSK650394 treatment. In the MCAO/reperfusion group, the volume of dextran distribution of the IH, CH and CBLL were smaller than the pons. The volume of dextran distribution of the pons was greater than any other brain regions that were studied in the GSK650394 treated group (Fig. 1 (B)).
3.4. Size of infarction
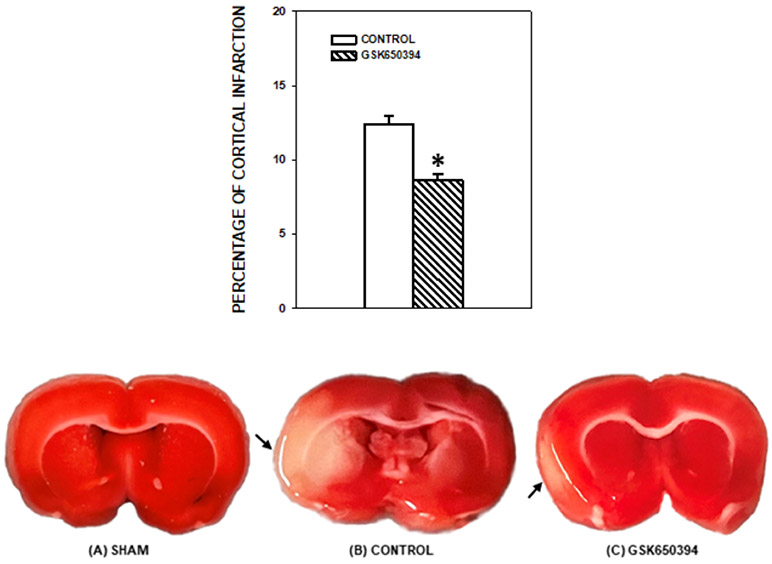
An infarcted area was observed in the affected cortex of each rat in both groups. The percentage of cortical infarct out of total cortical area was significantly smaller in the rats with GSK650394 treatment than without GSK650394 (−31%, p < 0.001, Fig. 2).
Fig. 2.
Top: Percentage of cortical infarcted area compared to total cortical area in the experimental groups after one hour of middle cerebral artery occlusion and two hours of reperfusion. A significant decrease in cortical infarct (−31%) was noted with GSK650394 treatment. n = 6 in each group. * P < 0.001 vs the MCAO/reperfusion (Control Group). Values are means ± SEM. Bottom: The representative photographs of the coronal section of the brain of a rat from the Sham (A), the Control (B), and the GSK650394 (C) Groups. The tissue samples were photographed right after PBS washing without any fixatives. The infarcted area is indicated by an arrow. A reduced cortical infarcted area with GSK650394 treatment is clearly noted at this early stage of cerebral ischemia-reperfusion. Sham: No MCAO/reperfusion group, Control: MCAO/reperfusion group, GSK650394: MCAO/reperfusion + GSK650394 group.
3.5. Western blot
Phosphorylation of NDRG1 (pNDRG1) and MMP2 protein levels were diminished by treatment with GSK650394 in the ischemic-reperfused cortex indicating that the drug inhibited SGK1 and that the lower MMP2 could be one of the mechanisms of the attenuated BBB disruption. After normalizing protein loading (actin), NDRG1 and pNDRG1 protein levels were obtained. The ratio of pNDRG1/NDRG1 in each brain region was quantitated and plotted relative to the value of the contralateral cortex of the non-treated control rats as relative fold phosphorylation in Fig. 3.
Fig. 3.
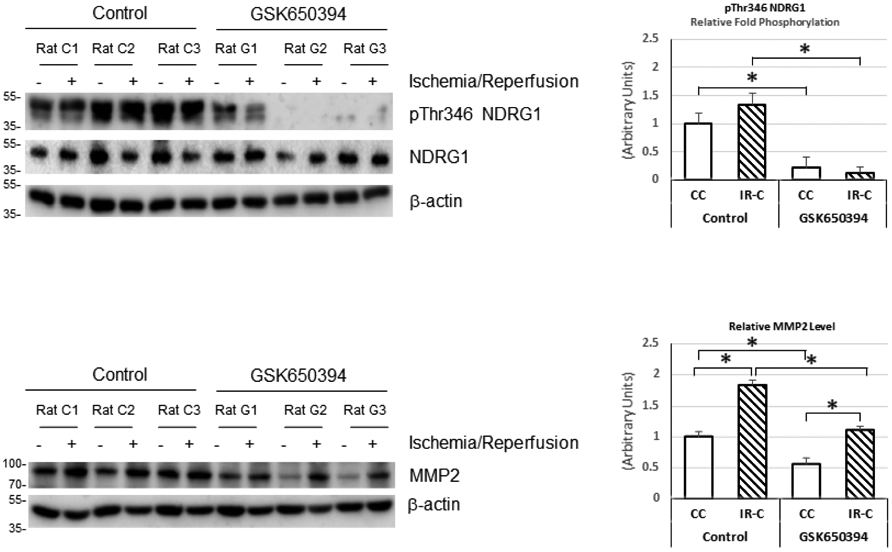
The representative Western blots of pNDRG1, NDRG1 and MMP2 and their quantitation after one hour of middle cerebral artery occlusion and two hours of reperfusion. pNDRG1 and MMP2 were diminished by treatment with GSK650394 in the ischemic-reperfused cortex, indicating that the drug inhibited SGK1 and the lower MMP2 could be one of the mechanisms of the attenuated BBB disruption. After normalizing protein loading (actin), NDRG1 and pNDRG1 protein levels were obtained. The ratio of pNDRG1/NDRG1 in each brain region was quantitated and plotted relative to the value of the contralateral cortex of the non-treated control rats as Relative Fold Phosphorylation. CC: Contralateral cortex, IR-C: Ischemic-reperfused cortex, Control: MCAO/reperfusion group, GSK650394: MCAO/reperfusion+GSK650394 group. *: P < 0.05. Values are means ± SEM.
4. Discussion
Our study is the first to demonstrate a decrease in BBB disruption with a smaller infarct and lower MMP2 level following inhibition of SGK1 with GSK650394 during the first few hours of cerebral ischemia-reperfusion and within the thrombolysis therapy time window. Our data indicate that the reduced BBB disruption could be one of the contributing factors for a smaller infarct with inhibition of SGK1.
There was a marked reduction in phosphorylation of NDRG1 with GSK650394 in the ischemic-reperfused cortex as well as in the contralateral cortex indicating a significant inhibition of SGK1 activity. We measured phosphorylation of NDRG1 since SGK1 is rapidly degraded and NDRG1 serves as a more stable marker of SGK1 activation [22,23]. Since active SGK1 is involved in anti-apoptotic pathways as well as in activation of various ion channels which may cause BBB disruption and excitotoxicity, our data may reflect the net results of inhibition of those two contradictory functions for neuronal survival in cerebral ischemia. Our data indicated that GSK650394 might have played a bigger role in inhibiting ion channels than inhibition of SGK1 induced anti-apoptotic pathway resulting in smaller infarct size at two hours of reperfusion after one hour of MCAO.
Our study suggests that SGK1 activation might be harmful for neuronal survival during the first few hours of reperfusion after focal cerebral ischemia. Inoue et al. demonstrated that SGK1 inhibitors reduced neurotoxicity induced by NMDA and decreased Ca++ increase in cultured neurons, and suggested these as related mechanisms for neuroprotection with SGK1 inhibitors [15]. The decrease in Ca++ flux and NMDA induced neurotoxicity with inhibition of SGK1 could have also contributed to increased neuronal survival with decreased BBB disruption in our current study.
In contrast to the benefits of inhibiting SGK1, there are reports that overexpression or induction of SGK1 is neuroprotective in cerebral ischemia-reperfusion [20,21]. Serum and glucocorticoid regulated kinase has three isoforms: SGK1, SGK2, and SGK3, all of which are present in the brain but activation, induction, and distribution are different. SGK1 mRNA is strongly and rapidly induced by serum or dexamethasone but SGK3 mRNA is not affected significantly. The expression of SGK2 mRNA is much lower than SGK1 in the brain [29]. Sherk et al. reported that GSK650394 inhibited the enzymatic activity of not only SGK1 but also SGK2. Therefore, the involvement of other SGK isoforms beside SGK1 cannot be ruled out in the neuroprotective effects of GSK650394 in our current study [30].
At two hours of reperfusion after one hour of MCAO, there was a trend of increasing pNDRG1 in the ischemic-reperfused cortex of the control rats, which indicates increased activity of SGK1 by the ischemia-reperfusion. Our data are similar to the reports that SGK1 gene expression is upregulated in the brain global ischemia [31]. Since one of the main substrates of mTORC2 is SGK1, our current study suggests that mTORC2 could have been activated in the stress condition such as ischemia-reperfusion where there is a shortage of energy and imbalance of energy supply and demand [32]. Using Akt HM phosphorylation (pS473Akt) as a readout of mTORC2 activity, our previous studies have already shown that mTORC2 activation is enhanced during cerebral ischemia-reperfusion [33,34].
As we predicted, our data demonstrated a decrease in BBB disruption with SGK1 inhibition when the Ki of 14C-AIB was determined to reflect BBB permeability in the ischemic-reperfused cortex. 14C-AIB is an inert, hydrophilic, small molecule (molecular weight 104 Da). The volume of dextran distribution reflects dextran leaked into the brain tissue as well as dextran in the plasma. Since the volume of distribution of dextran in the ischemic-reperfused cortex was similar to that in the contralateral cortex in both groups of rats, we concluded that the leakage of dextran into the brain tissue was minimal. At the same degree of BBB disruption, permeability of dextran (70,000 Da), which is a larger molecular tracer, was not significantly altered whereas the transfer of a smaller molecule (14C-AIB, 104 Da) was decreased with GSK650394. Even at the degree of BBB disruption that could be detected with only 14C-AIB, circulating neurotransmitters, toxins, ions, and chemicals could get into the brain tissue and could alter O2 consumption [35]. There are some regional differences in the Ki in the same group of rats. In normal conditions there is little leakage through BBB except circumventricular areas [36]. Systemic studies are needed to understand the mechanisms of the regional differences in the BBB permeability. Since the volume of dextran distribution represents dextran in the plasma as well as dextran that has leaked into the brain tissue, our data suggest that the plasma volume of hippocampus was lower than pons in the control rats and the plasma volume of pons was highest among the brain regions in the GSK650394 treated rats. This plasma volume could be changed due to multiple factors like anesthetic agents, CO2, and pathological factors such as ischemia, hypoxia and trauma.
Taken together it is important to minimize BBB disruption to reduce cerebral ischemia-reperfusion injury. Our data which showed an increase in MMP2 protein level in the ischemic-reperfused cortex are similar to other previous studies [24,37]. Among MMPs, MMP2 was a major enzyme that mediated occludin degradation and caveolin-1 mediated claudin-5 redistribution, which can cause BBB disruption in the early stage of cerebral ischemia [24]. Our data for pNDRG1 or MMP2 expressions are from the whole brain tissue that includes neurons, glia, and endothelial cells. Zhang et al. reported that vascular endothelial SGK1 was associated with BBB dysfunction in permanent brain ischemic model. It is conceivable that the increase of MMP2 in our current study could be due to endothelial SGK1 [38]. In our current study, inhibition of SGK1 could have reduced MMP2 as well as Ca++ induced aggravation of BBB disruption [16,18,19]. This decrease in BBB disruption by GSK650394 could also have contributed to the decrease in infarct size by SGK1 inhibition. Our data suggest that SGK1 inhibition would decrease the complications of tPA such as hemorrhage and cerebral edema in stroke.
5. Conclusions
In conclusion, our data demonstrated a reduced cortical infarct with decrease in BBB disruption with inhibition of SGK1 by GSK650394 when the parameters were determined in the early stage of cerebral ischemia-reperfusion. Our data suggest that inhibition of SGK1 is beneficial for neuronal survival in the first few hours of cerebral ischemia-reperfusion especially within the thrombolysis therapy time window and that a decrease of BBB disruption could be one of the contributory factors to reduced infarct by GSK650394. Inhibition of SGK1 might reduce ischemia-reperfusion injury in early cerebral ischemia-reperfusion.
Highlights.
Inhibition of SGK1 decreased infarct in early cerebral ischemia-reperfusion.
Inhibition of SGK1 decreased BBB disruption in early cerebral reperfusion.
Decreased MMP2 by GSK650394 could be one of the mechanisms of the BBB protection.
Inhibition of SGK1 may be neuroprotective within the first few hours of stroke.
Acknowledgements
This research was supported by intramural departmental sources and partially supported by the National Institutes of Health [E. Jacinto #GM079176].
Footnotes
Declaration of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Boulanger JM, Lindsay MP, Gubitz G, et al. , Canadian Stroke Best Practice Recommendations for Acute Stroke Management: Prehospital, Emergency Department, and Acute Inpatient Stroke Care, 6th Edition, Update 2018, Int. J. Stroke Off. J. Int. Stroke Soc 13 (2018) 949–984. 10.1177/1747493018786616. [DOI] [PubMed] [Google Scholar]
- [2].Lin L, Wang X, Yu Z, Ischemia-reperfusion Injury in the Brain: Mechanisms and Potential Therapeutic Strategies, Biochem. Pharmacol. Open Access 5 (2016) 1–16. 10.4172/2167-0501.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mayor D, Tymianski M, Neurotransmitters in the mediation of cerebral ischemic injury, Neuropharmacology. 134 (2018) 178–188. 10.1016/j.neuropharm.2017.11.050. [DOI] [PubMed] [Google Scholar]
- [4].Andjelkovic AV, Xiang J, Stamatovic SM, Hua Y, Xi G, Wang MM, Keep RF, Endothelial Targets in Stroke: Translating Animal Models to Human, Arterioscler. Thromb. Vasc. Biol 39 (2019) 2240–2247. 10.1161/ATVBAHA.119.312816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jin X, Liu J, Liu W, Early ischemic blood brain barrier damage: a potential indicator for hemorrhagic transformation following tissue plasminogen activator (tPA) thrombolysis?, Curr. Neurovasc. Res 11 (2014) 254–262. 10.2174/1567202611666140530145643. [DOI] [PubMed] [Google Scholar]
- [6].García-Martínez JM, Alessi DR, mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1), Biochem. J 416 (2008) 375–385. 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- [7].Kazyken D, Magnuson B, Bodur C, Acosta-Jaquez HA, Zhang D, Tong X, Barnes TM, Steinl GK, Patterson NE, Altheim CH, Sharma N, Inoki K, Cartee GD, Bridges D, Yin L, Riddle SM, Fingar DC, AMPK directly activates mTORC2 to promote cell survival during acute energetic stress, Sci. Signal 12 (2019). 10.1126/scisignal.aav3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moloughney JG, Kim PK, Vega-Cotto NM, Wu C-C, Zhang S, Adlam M, Lynch T, Chou P-C, Rabinowitz JD, Werlen G, Jacinto E, mTORC2 Responds to Glutamine Catabolite Levels to Modulate the Hexosamine Biosynthesis Enzyme GFAT1, Mol. Cell 63 (2016) 811–826. 10.1016/j.molcel.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Szwed A, Kim E, Jacinto E, Regulation and metabolic functions of mTORC1 and mTORC2, Physiol. Rev (2021). 10.1152/physrev.00026.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chi OZ, Kiss GK, Mellender SJ, Liu X, Liu S, Jacinto E, Weiss HR, Inhibition of p70 ribosomal S6 kinase 1 (S6K1) by PF-4708671 decreased infarct size in early cerebral ischemia-reperfusion with decreased BBB permeability, Eur. J. Pharmacol 855 (2019) 202–207. 10.1016/j.ejphar.2019.05.010. [DOI] [PubMed] [Google Scholar]
- [11].Liu X, Kiss GK, Mellender SJ, Weiss HR, Chi OZ, Activation of Akt by SC79 decreased cerebral infarct in early cerebral ischemia-reperfusion despite increased BBB disruption, Neurosci. Lett 681 (2018) 78–82. 10.1016/j.neulet.2018.05.046. [DOI] [PubMed] [Google Scholar]
- [12].Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V, (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms, Physiol. Rev 86 (2006) 1151–1178. 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- [13].Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME, Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a), Mol. Cell. Biol 21 (2001) 952–965. 10.1128/MCB.2L3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lang F, Shumilina E, Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1, FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 27 (2013) 3–12. 10.1096/fj.12-218230. [DOI] [PubMed] [Google Scholar]
- [15].Inoue K, Leng T, Yang T, Zeng Z, Ueki T, Xiong Z-G, Role of serum- and glucocorticoid-inducible kinases in stroke, J. Neurochem 138 (2016) 354–361. 10.1111/jnc.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brown RC, Davis TP, Calcium modulation of adherens and tight junction function: a potential mechanism for blood-brain barrier disruption after stroke, Stroke. 33 (2002) 1706–1711. 10.1161/01.str.0000016405.06729.83. [DOI] [PubMed] [Google Scholar]
- [17].Chi OZ, Hunter C, Liu X, Weiss HR, Effects of exogenous excitatory amino acid neurotransmitters on blood-brain barrier disruption in focal cerebral ischemia, Neurochem. Res 34 (2009) 1249–1254. 10.1007/s11064-008-9902-7. [DOI] [PubMed] [Google Scholar]
- [18].Berrout J, Jin M, O’Neil RG, Critical role of TRPP2 and TRPC1 channels in stretch-induced injury of blood-brain barrier endothelial cells, Brain Res. 1436 (2012) 1–12. 10.1016/j.brainres.2011.11.044. [DOI] [PubMed] [Google Scholar]
- [19].De Bock M, Culot M, Wang N, Bol M, Decrock E, De Vuyst E, da Costa A, Dauwe I, Vinken M, Simon AM, Rogiers V, De Ley G, Evans WH, Bultynck G, Dupont G, Cecchelli R, Leybaert L, Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability, J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 31 (2011) 1942–1957. 10.1038/jcbfm.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McCaig C, Ataliotis P, Shtaya A, Omar AS, Green AR, Kind CN, Pereira AC, Naray-Fejes-Toth A, Fejes-Toth G, Yáñez-Muñoz RJ, Murray JT, Hainsworth AH, Induction of the cell survival kinase Sgk1: A possible novel mechanism for α-phenyl-N-tert-butyl nitrone in experimental stroke, J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 39 (2019) 1111–1121. 10.1177/0271678X17746980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang W, yun Qian C, qing Li S, Protective Effect of SGK1 in Rat Hippocampal Neurons Subjected to Ischemia Reperfusion, Cell. Physiol. Biochem 34 (2014) 299–312. 10.1159/000363000. [DOI] [PubMed] [Google Scholar]
- [22].Inglis SK, Gallacher M, Brown SG, McTavish N, Getty J, Husband EM, Murray JT, Wilson SM, SGK1 activity in Na+ absorbing airway epithelial cells monitored by assaying NDRG1-Thr346/356/366 phosphorylation, Pflugers Arch. 457 (2009) 1287–1301. 10.1007/s00424-008-0587-1. [DOI] [PubMed] [Google Scholar]
- [23].McCaig C, Potter L, Abramczyk O, Murray JT, Phosphorylation of NDRG1 is temporally and spatially controlled during the cell cycle, Biochem. Biophys. Res. Commun 411 (2011) 227–234. 10.1016/j.bbrc.2011.06.092. [DOI] [PubMed] [Google Scholar]
- [24].Liu J, Jin X, Liu KJ, Liu W, Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage, J. Neurosci. Off. J. Soc. Neurosci 32 (2012) 3044–3057. 10.4172/2167-0501.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Longa EZ, Weinstein PR, Carlson S, Cummins R, Reversible middle cerebral artery occlusion without craniectomy in rats, Stroke. 20 (1989) 84–91. 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [26].Seyer B, Pham V, Albiston AL, Chai SY, Cannula implantation into the lateral ventricle does not adversely affect recognition or spatial working memory, Neurosci. Lett 628 (2016) 171–178. 10.1016/j.neulet.2016.06.034. [DOI] [PubMed] [Google Scholar]
- [27].Gross PM, Blasberg RG, Fenstermacher JD, Patlak CS, The microcirculation of rat circumventricular organs and pituitary gland, Brain Res. Bull 18 (1987) 73–85. 10.1016/0361-9230(87)90035-9. [DOI] [PubMed] [Google Scholar]
- [28].Joshi CN, Jain SK, Murthy PSR, An optimized triphenyltetrazolium chloride method for identification of cerebral infarcts, Brain Res. Protoc 13 (2004) 11–17. 10.1016/j.brainresprot.2003.12.001. [DOI] [PubMed] [Google Scholar]
- [29].Kobayashi T, Deak M, Morrice N, Cohen P, Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase, Biochem. J 344Pt 1 (1999) 189–197. [PMC free article] [PubMed] [Google Scholar]
- [30].Sherk AB, Frigo DE, Schnackenberg CG, Bray JD, Laping NJ, Trizna W, Hammond M, Patterson JR, Thompson SK, Kazmin D, Norris JD, McDonnell DP, Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic, Cancer Res. 68 (2008) 7475–7483. 10.1158/0008-5472.CAN-08-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nishida Y, Nagata T, Takahashi Y, Sugahara-Kobayashi M, Murata A, Asai S, Alteration of serum/glucocorticoid regulated kinase-1 (sgk-1) gene expression in rat hippocampus after transient global ischemia, Brain Res. Mol. Brain Res 123 (2004) 121–125. 10.1016/j.molbrainres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- [32].Weiss HR, Grayson J, Liu X, Barsoum S, Shah H, Chi OZ, Cerebral ischemia and reperfusion increases the heterogeneity of local oxygen supply/consumption balance, Stroke. 44 (2013) 2553–2558. 10.1161/STROKEAHA.113.001172. [DOI] [PubMed] [Google Scholar]
- [33].Chi OZ, Barsoum S, Vega-Cotto NM, Jacinto E, Liu X, Mellender SJ, Weiss HR, Effects of rapamycin on cerebral oxygen supply and consumption during reperfusion after cerebral ischemia, Neuroscience. 316 (2016) 321–327. 10.1016/j.neuroscience.2015.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Weiss HR, Chi OZ, Kiss GK, Liu X, Damito S, Jacinto E, Akt activation improves microregional oxygen supply/consumption balance after cerebral ischemia-reperfusion, Brain Res. 1683 (2018) 48–54. 10.1016/j.brainres.2018.01.019. [DOI] [PubMed] [Google Scholar]
- [35].Chi OZ, Wei HM, Lu X, Weiss HR, Increased blood-brain permeability with hyperosmolar mannitol increases cerebral O2 consumption and O2 supply/consumption heterogeneity, J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 16 (1996) 327–333. 10.1097/00004647-199603000-00019. [DOI] [PubMed] [Google Scholar]
- [36].Wilhelm I, Nyúl-Tóth Á, Suciu M, Hermenean A, Krizbai IA, Heterogeneity of the blood-brain barrier, Tissue Barriers. 4 (2016) e1143544. 10.1080/21688370.2016.1143544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shen Y, Gu J, Liu Z, Xu C, Qian S, Zhang X, Zhou B, Guan Q, Sun Y, Wang Y, Jin X, Inhibition of HIF-1α Reduced Blood Brain Barrier Damage by Regulating MMP-2 and VEGF During Acute Cerebral Ischemia, Front. Cell. Neurosci 12 (2018) 288. 10.3389/fncel.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang T, Fang S, Wan C, Kong Q, Wang G, Wang S, Zhang H, Zou H, Sun B, Sun W, Zhang Y, Mu L, Wang J, Wang J, Zhang H, Wang D, Li H, Excess salt exacerbates blood-brain barrier disruption via a p38/MAPK/SGK1-dependent pathway in permanent cerebral ischemia, Sci. Rep 5 (2015) 16548. 10.1038/srep16548. [DOI] [PMC free article] [PubMed] [Google Scholar]