Abstract
A growing body of literature implicates noradrenergic (NE) signaling in the modulation of ethanol consumption. However, relatively few studies have detailed specific brain pathways that mediate NE-associated binge-like ethanol consumption. To begin to fill this gap in the literature, male and female C57BL6/J and TH-ires-cre mice underwent pharmacological and chemogenetic testing, respectively, in combination with “drinking in the dark” procedures to model binge-like consumption of ethanol or sucrose solutions. First, we showed that intraperitoneal administration of the NE reuptake inhibitor, reboxetine, blunted binge-like ethanol intake in C57BL6/J mice. Chemogenetic activation of locus coeruleus (LC) tyrosine hydroxylase (TH)-expressing neurons blunted binge-like ethanol intake regardless of sex. Chemogenetic activation of LC projections to the lateral hypothalamus (LH), a region implicated in ethanol consumption, blunted binge-like ethanol drinking without altering sucrose intake in ethanol-experienced or ethanol-naïve mice. In C57BL/6J mice, LH-targeted microinfusion of an α1- adrenergic receptor (AR) agonist blunted binge-like ethanol intake across both sexes, while LH infusion of a β-AR agonist blunted binge-like ethanol intake in females exclusively. Finally, in mice with high baseline ethanol intake both an α1- AR agonist and an α-2 AR antagonist blunted binge-like ethanol intake. The present results provide novel evidence that increased NE tone in a circuit arising from the LC and projecting to the LH reduces binge-like ethanol drinking in mice, and may represent a novel approach to treating binge or heavy drinking prior to the development of dependence.
Keywords: Norepinephrine, Lateral Hypothalamus, Chemogenetic, Binge-Like Drinking, Drinking in the Dark, Mice
1. Introduction
Alcohol abuse poses significant health risks and economic costs to individuals around the world (Lim et al., 2012; Rehm et al., 2009). Repeated bouts of binge drinking, defined as a pattern of drinking yielding blood ethanol concentrations (BECs) achieving the U.S. legal limit of 80 mg/dl within a short (two-hour) time window (NIAAA, 2004), are thought to contribute to the development of later-life alcohol dependence (Hingson et al., 2006). Recent estimates indicate that nearly 90% of U.S. adults that drink excessively consume alcohol in the form of a binge (Esser et al., 2014). Thus, understanding the neurochemical signaling systems that modulates binge-like ethanol consumption is critical for identification of potential therapies for reducing this dangerous pattern of behavior. To this end, the 4-day “drinking-in-the-dark” (DID) paradigm and its variants are among the more popular paradigms for modeling voluntary binge-like ethanol intake in mice (Rhodes et al., 2005; Thiele and Navarro, 2014) and rats (Bell et al., 2011; Holgate et al., 2017), promoting ingestion of large quantities of ethanol and reliably generating BECs exceeding 80 mg/dl. Using DID procedures, researchers have begun to examine roles for neurochemical signaling systems and circuitry that modulate binge-like ethanol intake (Sprow and Thiele, 2012). Interestingly, there is evidence that overlapping neurochemical pathways in the brain modulate both alcohol use disorders and eating disorders (Thiele et al., 2003), suggesting that at least in some circumstances common therapeutic strategies may be identified.
Norepinephrine (NE) is centrally synthesized in brainstem nuclei, with the majority of its synthesis occurring via the locus coeruleus (LC) (Sawchenko and Swanson, 1982), and has long been implicated in ethanol ingestion (Arango et al., 1994; Gilpin and Koob, 2010; Lu et al., 1997; O’Neil et al., 2013; Rasmussen et al., 2014a; Rasmussen et al., 2009; Rasmussen et al., 2014b; Simpson et al., 2009; Verplaetse et al., 2012) as well as feeding behavior (Bello et al., 2019). In fact, the NE/dopamine (DA) reuptake inhibitor bupropion (BUP), when combined with the non-selective opioid antagonist, naltrexone (NAL), has been used successfully to treat binge eating in human (Halseth et al., 2018), and we have recently showed that BUP alone and in combination with NAL blunts binge-like ethanol intake in mice (Navarro et al., 2019). Further, in a preliminary open-label study we recently reported that BUP + NAL therapy blunts the frequency of binge ethanol drinking in humans (Walter et al., 2020). These observations suggest that blunted NE tone contributes to binge behavior, and increasing NE tone is protective. However, there is a gap in the literature on the specific NE neurocircuitry that modulates binge ethanol drinking.
Insight into the NE mechanisms involved in modulating binge behavior comes from studies that have used c-Fos immunoreactivity (IR) as a marker of neuronal activation. It has been shown that the LC is activated following voluntary binge-like ethanol consumption (Burnham and Thiele, 2017). Interestingly, i.p. injection of ethanol into rats bred for high versus low ethanol consumption revealed reduced ethanol-induced c-Fos IR in the LC among rats bred for high levels of intake (Thiele et al., 1997). More recently, we showed that an inbred line of mice that was selectively bred to achieve binge-like blood ethanol levels (the iHDID-1 line) failed to exhibit ethanol-induced c-Fos expression in the LC at doses that induced c-Fos expression in the control HS/Npt line (Robinson et al., 2020). These observations suggest that LC activity is triggered by ethanol, and the observation that this activity is blunted in high drinking lines of rats and mice supports a role for ethanol-induced LC activity as a protective mechanism to limit ethanol intake.
In rodents, the lateral hypothalamus (LH) has been implicated in modulation of many behaviors including ethanol consumption (Chen et al., 2013; Chen et al., 2014; Navarro et al., 2016; Sprow et al., 2016; Wayner et al., 1971) and seeking (Marchant et al., 2009; Marchant et al., 2014). Though brainstem NE nuclei innervate numerous regions (Robertson et al., 2016), the LH connects reciprocally with the LC (Jones and Moore, 1977; Papp and Palkovits, 2014) and contains rich populations of α-1, α-2, and β-adrenergic receptors (ARs) (Leibowitz et al., 1982). Thus, the LC may signal through the LH to regulate binge-like ethanol consumption. Experiments herein elucidated the role of the LC in binge-like ethanol intake. First, we show that peripheral administration of a NE reuptake inhibitor (NRI) significantly blunted binge-like ethanol consumption without altering overall motor behavior or anxiety-like behavior, consistent with our previous studies using BUP. Using excitatory Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), we demonstrated that general activation the LC blunted binge-like ethanol and sucrose consumption. Specific activation of TH-expressing LC neuron projecting to the LH similarly reduced binge-like ethanol consumption without altering sucrose drinking in ethanol-experienced or ethanol-naïve mice. Finally, we show that LH-directed adrenergic receptor (AR) manipulations reduced ethanol consumption that were compound-specific, and in some cases sex-specific and dependent on baseline levels of ethanol intake. The present results provide novel evidence that increased NE tone in a circuit arising from the LC and projecting to the LH reduces binge-like ethanol drinking, and may represent a novel strategy for treating binge drinking disorders prior to the development of ethanol dependence.
2. Methods and Materials
2.1. Animals
Male and female TH-ires-Cre mice (Savitt et al., 2005), bred in house and backcrossed on a C57BL6/J strain, were utilized for DREADD manipulations and tracing studies. Male and female C57BL6/J mice (stock # 000664, Jackson Laboratory), 6 – 8 weeks old upon arrival were utilized for pharmacological manipulations. All mice were individually housed at least 1 week prior to testing onset with ad libitum access to Prolab® RMH 3000 (Purina labDiet®; St. Louis, MO) and water except where noted. The animal vivarium was maintained at 22 °C on a 12:12 h reverse light/dark cycle, with lights off at 0830 h. All protocols were conducted under National Institute of Health guidelines and were approved by the University of North Carolina Institutional Animal Care and Use Committee.
2.2. Surgery
Mice were anesthetized via an intraperitoneal ketamine (66.7 mg/kg; Henry Schein, Dublin, OH) and xylazine (6.67 mg/kg; Henry Schein) cocktail. 0.1 mL 1% lidocaine HCl (Hospira, Inc., Lake Forest, IL) was subcutaneously applied above the skull. For chemogenetic manipulations, TH-ires-Cre mice received bilateral infusions of the excitatory DREADD, AAV8-hSyn-DIO-hM3d(Gq)-mcherry (6 × 1012 vg/ml), or control vector lacking the DREADD construct, AAV8-hSyn-DIO-mcherry (8 × 1012), targeting the LC (AP: −5.40 mm, ML: ±0.85 mm, DV: −3.85mm). For all DREADD experiments, 0.3 μL virus/hemisphere was infused over five minutes, and infusion cannula were left in place for ten minutes post-infusion to discourage virus transport up the cannula tract. For pharmacological and chemogenetic pathway manipulations, 26-G cannula (Double guide cannula, 2.2mm width, 5mm below pedestal; internal: 0.5mm projection; Plastics One, Roanoke, VA) were permanently affixed to the skull, targeting the LH (AP: −1.10 mm, ML: ±1.10 mm, DV: −5.10 mm).
2.3. Drinking in the dark paradigm (DID)
The DID procedure is commonly used to model voluntary binge-like ethanol consumption in mice (Albrechet-Souza et al., 2015; McCall et al., 2013; Patkar et al., 2016; Rinker et al., 2016). Thorough procedural details have been described elsewhere (Thiele et al., 2014). Briefly, DID is a four-day procedure wherein standard mouse water bottles were removed and replaced with a 10 ml pipette containing 20% (v/v) ethanol beginning 3 hours into the dark cycle, and mice were permitted two-hours of ethanol access. Ethanol bottles were removed, and water was returned after the two-hour test each day. On the fourth day (test day) experimental manipulation occurred approximately 30-min before ethanol access and at the conclusion of testing approximately 10 mL of tail blood were acquired from each mouse and processed as described previously (Burnham and Thiele, 2017). Mice undergoing DID procedures commonly achieve blood ethanol concentrations (BECs) exceeding the NIAAA-defined 80 mg/dl threshold for a binge episode (Thiele et al., 2014). Each four-day testing period constituted a binge episode or cycle.
2.4. Open-Field Testing (OFT)
OFT was used to assess whether changes in consumption were potentially influenced by compound-driven alterations in locomotor and/or anxiety-like behavior. In OFT, mice were pretreated with either drug or vehicle 20 minutes prior to placement in the center of the testing chamber (42cm × 42 cm × 30cm; catalog #71-SFAC; Omnitech Electronics, Inc., Columbus, OH). Doses used for OFT were identical to doses used in DID testing except where otherwise noted in figures. Testing began 3-h into the dark cycle, and for the duration of a 2-h test period, animal movements were tracked using VersaMax (AccuScan Instruments, Inc., Columbus, OH) and quantified using VersaDat Version 4.00 (AccuScan Instruments, Inc.). Differences in total distance traveled and time spent in center of test chamber defined drug-elicited effects in locomotor activity and anxiety-like behavior (Simon et al., 1994), respectively.
2.5. DREADD and TH-ires-Cre mouse verification
Functional validation of DREADDs was performed via examination of c-Fos activity. Specifically, hM3Dq-expressing mice were pretreated with either vehicle or CNO (i.p.) 90 min prior to sacrifice. To validate the Cre line, immunohistochemistry for TH expression (green; see immunohistochemistry below) and viral vector expression (mCherry tag) images were acquired and subsequently overlayed in Adobe Photoshop. Given previous research indicating back-metabolism of CNO to clozapine (Manvich et al., 2018; Raper et al., 2017), utilization of a DREADD-deficient control vector was critical to determining whether effects of CNO were specific to DREADD-expressing constructs. Across our chemogenetic studies, CNO (3.0 mg/kg i.p. or 900 pmol microinfusions) failed to alter behavior relative to vehicle in control-DREADD cohorts, suggesting behavioral alterations occurring post-CNO exposure in hM3Dq-transfected mice were products of anticipated compound and virus interaction. Indeed, it has been shown that 3.0 – 3.5 mg/kg CNO doses fail to alter behavior in the absence of a DREADD construct (Jendryka et al., 2019; Mazzone et al., 2018; Vardy et al., 2015)
2.6. Immunohistochemistry
At the completion of the study, mice were overdosed with 0.1 mL i.p. ketamine/xylazine (6.67 mg/0.1 mL; 0.67 mg/01. mL; in 0.9% saline) and transcardially perfused with 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS (pH = 7.4). Brains were collected and processed according to previously established protocols (Burnham and Thiele, 2017). Rabbit anti-TH (1:20k; catalog #AB1542, EMD Millipore, Billerica, MA) and rabbit anti-c-Fos (1:1k; catalog #226003, Synaptic Systems, Goettingen, Germany) primary antibodies and a 488-conjugated goat anti-Rb (1:500; catalog #111-545-144, Jackson Immunoresearch, West Grove, PA) secondary antibody were utilized where indicated. Virus and cannulae placements were determined via fluorescent and bright field microscopes, respectively.
2.6. Experimental Design
2.6.1. Experiment 1: Effects of Systemic Administration of a Peripherally Bioavailable NRI on Binge-Like Ethanol Intake
All mice were habituated to handling and gentle restraint for several minutes prior to ethanol access on days 1–3 of DID procedures. On day 4, the selective NRI, reboxetine mesylate (Tocris, catalog #1982), was dissolved in 0.9% saline and administered intraperitoneally 30-min prior to testing onset on the fourth day of the DID cycle. Each animal received vehicle (0.9% saline) or 10 mg/kg reboxetine (10 ml/kg body weight) in a Latin square design over 2-DID cycles. Vehicle or drug administration order was determined based on mouse drinking pattern observed on days 1 – 3 of the first DID cycle such that average baseline consumption between vehicle and drug groups were approximately equal (n=21: 11 female, 10 male). After 1-week of recovery from ethanol testing, mice underwent additional DID testing using the same Latin square design described above but with 3% sucrose in place of 20% ethanol to determine if drug effects on behavior were specific to ethanol consumption, followed by OFT as described above. Dosing based upon previous literature in C57BL6/J mice (Roni and Rahman, 2015).
2.6.2. Experiment 2: Effects of Chemogenetic Activation of LC on Binge-Like Ethanol Intake
Following 6-weeks of recovery/virus transduction, mice underwent 2-consecutive DID ethanol cycles wherein vehicle (saline+DMSO) or 3.0 mg/kg clozapine-N-oxide (CNO; in 0.5% DMSO+saline solution, injected intraperitoneally (i.p) at 5.0 ml/kg; National Institute of Health, Lot# 13626-76, SAF# 27709) was administered 30-min prior to ethanol access on test days. On days 1–3 of DID procedures mice were habituated to handling and gentle restraint for several minutes prior to ethanol access. Vehicle or CNO administration order was determined based on mouse drinking pattern observed on days 1 – 3 of the first DID cycle such that average baseline consumption between vehicle and CNO groups were approximately equal, and one group was randomly selected to receive CNO week 1. Over 2-DID cycles each mouse experienced vehicle and CNO in a counterbalanced Latin square design (hM3Dq n=15, control viral vector n=8). CNO dosage was based upon previous literature (Navarro et al., 2016; Vardy et al., 2015). After 1-week of recovery from ethanol testing, mice underwent additional DID testing using the same Latin square design described above but with 3% sucrose in place of 20% ethanol to determine if drug effects on behavior were specific to ethanol consumption.
2.6.3. Experiment 3: Effects of Chemogenetic Activating the LC ➔ LH TH+ Pathway on Binge-Like Ethanol Intake
Following 6-weeks of recovery/virus transduction, mice underwent 2-consecutive DID ethanol cycles wherein vehicle (saline+1% DMSO; 0.3 μl/1min infusion/hemisphere) or CNO (900 pmol/0.3 μl/1 min infusion/hemisphere; dose based on our recent work (Rinker et al., 2016)) was infused bi-laterally into LH-targeted cannulae 30 min prior to ethanol access on test days. On days 1–3 of DID procedures mice were habituated to handling, gentle restraint, and cannulae head-cap manipulations for several minutes prior to ethanol access. As above, vehicle or CNO administration order was determined based on mouse drinking pattern observed on days 1 – 3 of the first DID cycle such that average baseline consumption between vehicle and CNO groups were approximately equal, and one group was randomly selected to receive CNO week 1. Over 2-DID cycles each mouse experienced vehicle and CNO in a counterbalanced Latin square design (hM3Dq n=10, control viral vector n=6). After 1-week of recovery from ethanol testing, mice underwent additional DID testing using the same Latin square design described above but with 3% sucrose in place of 20% ethanol to determine if drug effects on behavior were specific to ethanol consumption, followed by OFT as described above.
2.6.4. Experiment 4: Effects of LH α1 AR Agonism on Binge-Like Ethanol Intake
The selective α1 AR agonist, phenylephrine (PHEN; catalog # 2838, R&D Systems, Minneapolis, MN), was dissolved in 0.9% saline and injected bilaterally into the LH 15 – 25 minutes prior to the start of testing on DID day 4. Each animal received vehicle (0.9% saline/0.3 μl/hemisphere over 1 min) and PHEN (20 nmol/0.3μl/hemisphere over 1 minute) on DID day 4 in a Latin square design over 2-DID cycles. On days 1–3 of DID procedures mice were habituated to handling, gentle restraint, and cannulae head-cap manipulations for several minutes prior to ethanol access. Vehicle or drug administration order was determined based on mouse drinking pattern observed on days 1 – 3 of the first DID cycle such that average baseline consumption between vehicle and drug groups were approximately equal, and one group was randomly selected to receive CNO week 1 (n=17: 9 female, 8 male). After 1-week of recovery from ethanol testing, mice underwent additional DID testing using the same Latin square design described above but with 3% sucrose in place of 20% ethanol to determine if drug effects on behavior were specific to ethanol consumption. Dosing was based upon previous literature (Kochenborger et al., 2012; Mansur et al., 2010, 2011).
2.6.5. Experiment 5: Effects of LH β AR Agonism on Binge-Like Ethanol Intake
The β agonist, xamoterol hemifumarate (XAM; product # ab145957, Abcam, Cambridge, MA), was dissolved in 0.9% saline and injected bilaterally into the LH 15 – 25 minutes prior to testing onset on DID day 4. Each mouse received vehicle (0.9% saline/0.5 μl/hemisphere over 1 min) and xamoterol (0.5 μg/0.5 μl/hemisphere over 1 minute) in a Latin square design over 2-DID cycles. On days 1–3 of DID procedures mice were habituated to handling, gentle restraint, and cannulae head-cap manipulations for several minutes prior to ethanol access. Vehicle or drug administration order was determined based on mouse drinking pattern observed on days 1 – 3 of the first DID cycle such that average baseline consumption between vehicle and drug groups were approximately equal, and one group was randomly selected to receive CNO week 1 (n=13: 6 female, 7 male). After 1-week of recovery from ethanol testing, mice underwent additional DID testing using the same Latin square design described above but with 3% sucrose in place of 20% ethanol to determine if drug effects on behavior were specific to ethanol consumption. Dosing was based upon previous literature (Torkaman-Boutorabi et al., 2014).
2.6.6. Experiment 6: Effects of LH α2 Antagonism on Binge-Like Ethanol Intake
The selective α2 antagonist, atipamezole (catalog # A9611, Sigma-Aldrich), was dissolved in a 25% DMSO, 75% dH20 solution and was injected bilaterally into the LH 15 – 25 minutes prior to the start of testing on DID day 4. On days 1–3 of DID procedures mice were habituated to handling, gentle restraint, and cannulae head-cap manipulations for several minutes prior to ethanol access. Vehicle or drug administration order was determined based on mouse drinking pattern observed on days 1 – 3 of the first DID cycle such that average baseline consumption between vehicle and drug groups were approximately equal. Each animal received vehicle (25% DMSO, 75% dH20 solution/0.3 μl/hemisphere/1 minute), 1.5 μg (in 0.3 μl/hemisphere over 1 minute), and 15 μg (in 0.3 μl/hemisphere over 1 minute) in a Latin square design over 3-DID cycles. Vehicle or drug administration order was determined based on mouse drinking pattern observed on days 1 – 3 of the first DID cycle such that average baseline consumption between vehicle and drug groups were approximately equal, and one group was randomly selected to receive CNO week 1 (n=21: 11 female, 10 male). After 1-week of recovery from ethanol testing, mice underwent additional DID testing using a similar Latin square design described above but with 3% sucrose in place of 20% ethanol to determine if drug effects on behavior were specific to ethanol consumption. In this study, mice were given vehicle or the 15 μg dose of atipamezole. Dosing was based upon previous literature in rats (Ostock et al., 2015).
2.7. Statistical Analyses
For DREADD manipulations, three-way ANOVA analysis failed to detect a significant effect of treatment order, so data were collapsed and analyzed via two-way ANOVA. Two-way ANOVA failed to detect significant effect of sex, so data were collapsed across sexes and analyzed via paired t-tests (treatment; total consumption and BECs). For pharmacological manipulations, repeated-measures ANOVAs (sex × treatment) were used to assess total consumption and BECs, respectively. When significant main effects or interactions were obtained, Bonferroni-corrected t-tests were performed such that statistical significance was accepted based on the formula of α = 0.05/n where n = the number of comparisons conducted. All other manipulations utilized paired t-tests (when comparing pre/post treatment within animals) or independent t-tests (when comparing animals in different conditions), and analyses were performed using GraphPad Prism 7 (La Jolla, CA) or SPSS 25 (IBM Analytics, Armonk, New York). Findings are presented as mean ± standard error of the mean (SEM) and considered significant if p < 0.05 (two-tailed).
3. Results
3.1. Experiment 1: Effects of Systemic Administration of a Peripherally Bioavailable NRI on Binge-Like Ethanol Intake
Data from this experiment are presented in Fig. 1. A repeated-measures ANOVA (sex × treatment) failed to reveal a main effect of sex or sex by treatment interaction, so data from males and females were collapsed. Paired t-test for total ethanol intake revealed a significant reduction in intake following 10 mg/kg reboxetine pretreatment (Fig. 1A) [t test: t(20)=4.199, p<0.001]. 10 mg/kg reboxetine similarly blunted BECs (Fig. 1B) [t test: t(20)=3.473, p=0.002]. Fig. 1C shows a reboxetine-driven reduction in sucrose consumption [t test: t(20)=4.451, p=0.002]. Unpaired t-tests failed to find significant differences in total distance traveled (Fig. 1D) [t(12)=1.116, p=0.29] or time spent in chamber center in the first 5 minutes of testing (Fig. 1E) [t(12)=0.335, p=0.74]. Since we hypothesized that binge-induced activation of NE is protective against ethanol intake, we hypothesized that mice with high ethanol intake would be more sensitive to reductions in ethanol intake following reboxetine administration. To this end, we performed a median-split of mice based on their consumption during vehicle infusion, and re-analyzed consumption data with a two-way, 2 × 2 (treatment: vehicle versus reboxetine × drinker: low drinkers versus high drinking) ANOVA (Fig. 1F). We found a significant main effects of treatment [F(1, 19)=22.31, p<0.001], drinker [F(1, 19)=17.17, p=0.001], and treatment × drinker interaction [F(1, 19)=7.81, p=0.012]. Bonferroni corrected t-tests indicated a significant differences in binge-like ethanol intake between vehicle and reboxetine in high drinkers [t-test t(10)=4.92, p=0.002], but not in levels of binge-like ethanol intake between vehicle and reboxetine in low drinkers [t-test t(9)=1.54, p=0.16].
Figure 1. Systemic administration of reboxetine, a noradrenaline reuptake inhibitor, blunts binge-like ethanol intake in C57BL6/J mice.
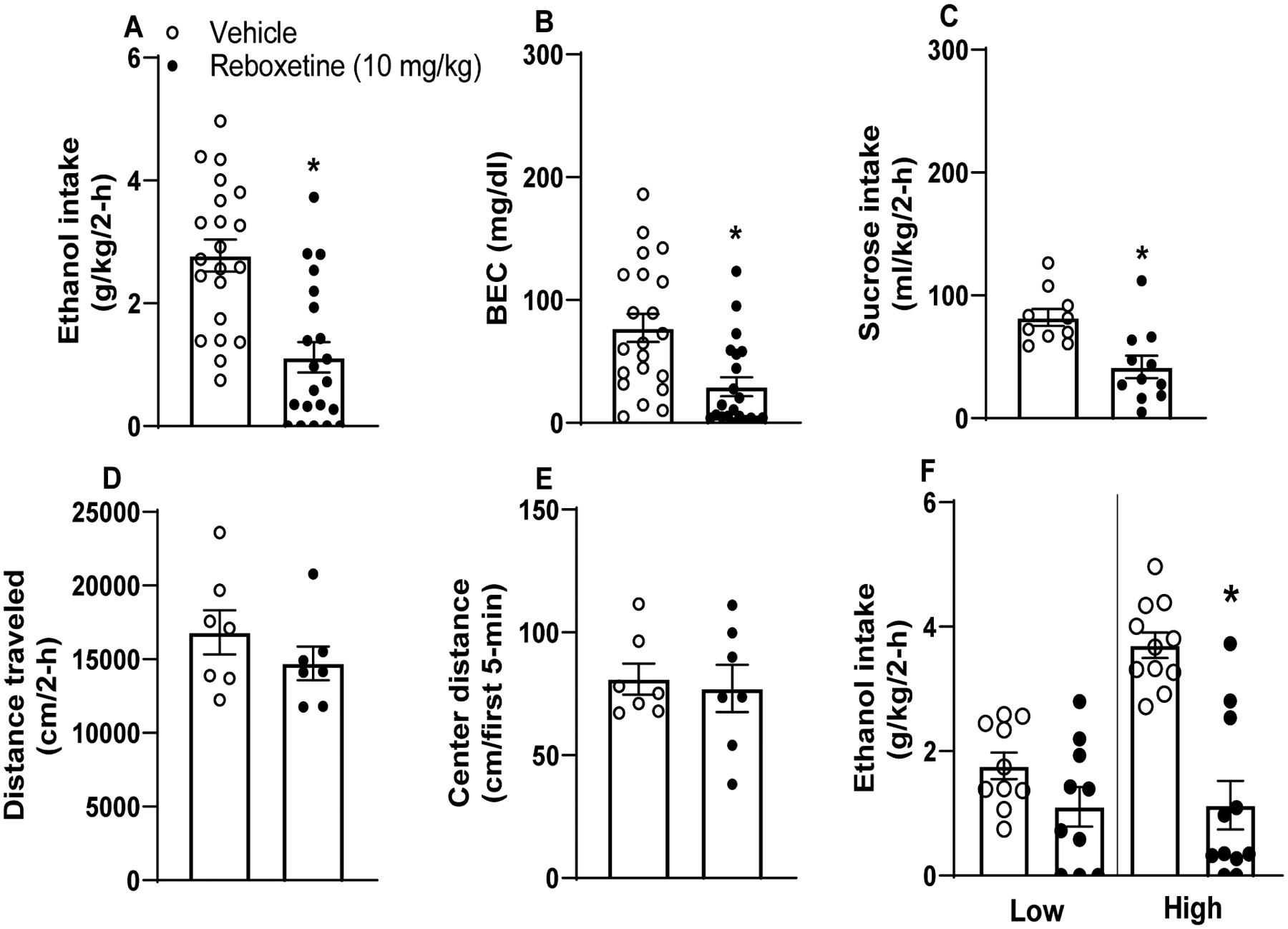
10 mg/kg i.p. reboxetine blunted binge-like ethanol (A; n=21) intake and BECs (B; n=21). Following ethanol testing, these mice underwent sucrose testing wherein 10 mg/kg reboxetine blunted intake of a 3% sucrose solution (C; n=19). Alterations in intake patterns were not secondary to altered locomotor or anxiety-like states, as 10 mg/kg reboxetine pretreatment prior to OFT failed to alter total distance traveled (D; n=7) and center time (E; n=7) relative to vehicle-injected cohorts (n=7). Data collapsed across males and females due to absence of sex effects. (F) Further analyses of ethanol intake data indicated that reboxetine blunted intake in mice with High baseline ethanol intake (upper 50% of a median-split) and not in mice with Low baseline intake (lower 50% of a median-split). values represent mean ± SEM. * indicates p<0.05 in paired t-test.
3.2. Experiment 2: Effects of Chemogenetic Activation of LC on Binge-Like Ethanol Intake
Data from this experiment are presented in Fig. 2. As seen in Fig. 2A, among TH-ires-cre mice expressing AAV8-hSyn-DIO-hM3D(Gq)-mcherry virus in the LC, 3.0 mg/kg i.p. CNO administration blunted binge-like ethanol intake [t-test t(14)=3.34, p=0.005]. As seen in Fig. 2B, BECs (n=10; 5 blood samples were lost due to mechanical error) are blunted following CNO administration relative to vehicle treatment [t test t(9)=2.811, p=0.02]. Conversely, in TH-ires-cre mice expressing AAV8-hSyn-DIO-mcherry in the LC, i.p. CNO failed to alter ethanol consumption as shown in Fig. 2C [t-test t(7)=0.06, p=0.96] and associated BECs shown in Fig. 2D (n=6; 2 blood samples were lost due to mechanical error) [t-test t(5)=0.54, p=0.61]. A previous report has shown that chemogenetic activation of the LC complex induces an anxiogenic-like effect as measured via OFT in a CNO dose-dependent manner (Sciolino et al., 2016). The induction of anxiety-like behavior could be a potential explanation for blunted ethanol consumption stemming from LC activation, as a general reduction of consummatory behavior may have been secondary to increased anxiety. To test this possibility, we assessed sucrose consumption. As seen in Fig. 2E, CNO pretreatment in a randomly selected subset of TH-ires-cre mice expressing AAV8-hSyn-DIO-hM3D(Gq)-mcherry virus in the LC also blunted intake of a 3% sucrose solution [t test t(10)=3.818, p=0.003], suggesting that chemogenetic activation of TH+ LC neurons may nonspecifically blunt consumption, at least in mice with histories of binge-like ethanol intake. As seen in Fig. 2F, this effect is not likely a product of CNO given that CNO pretreatment in TH-ires-cre mice expressing AAV8-hSyn-DIO-mcherry in the LC failed to alter consumption of 3% sucrose [t test t(5)=0.086, p=0.93]. Representative photomicrographs show hM3Dq DREADD expression (red; Fig. 2G), TH IHC (green; Fig. 2H), hM3Dq DREADD/TH IHC overlay (yellow; Fig. 2I). Approximate spread of DREADD expression in the LC is shown in Fig. 2J. To verify hM3Dq DREADD function, a randomly-selected subset of mice was pretreated with 3.0 mg/kg CNO or vehicle prior to sacrifice, and neuronal activation determined via c-Fos immunohistochemistry (Suppl. Fig. 1A–H). 3.0 mg/kg CNO pretreatment significantly increased c-Fos expression in mice expressing hM3Dq in the LC relative to vehicle pretreated controls [t test t(6)=7.174, p<0.001]. CNO pretreatment had no effect in mice expressing the empty vector control [t test t(7)=0.52, p=0.619]. As above, we performed a follow-up assessment of ethanol consumption data by performing a median-split of mice based on their consumption during vehicle infusion, and we then re-analyzed consumption data with a two-way, 2 × 2 (treatment: vehicle versus CNO × drinker: low drinkers versus high drinking) ANOVA (Fig. 2K). We found a significant main effects of treatment [F(1, 13)=13.76, p=0.003], drinker [F(1, 13)=7.97, p<0.014], and treatment × drinker interaction [F(1, 13)=5.25, p=0.039]. Bonferroni corrected t-tests indicated a significant differences in binge-like ethanol intake between vehicle and CNO in high drinkers [t-test t(7)=3.36, p=0.012], but not in levels of binge-like ethanol intake between vehicle and CNO in low drinkers [t-test t(6)=2.03, p=0.088].
Figure 2. Chemogenetic activation of the LC blunts binge-like ethanol intake in TH-ires-cre mice.
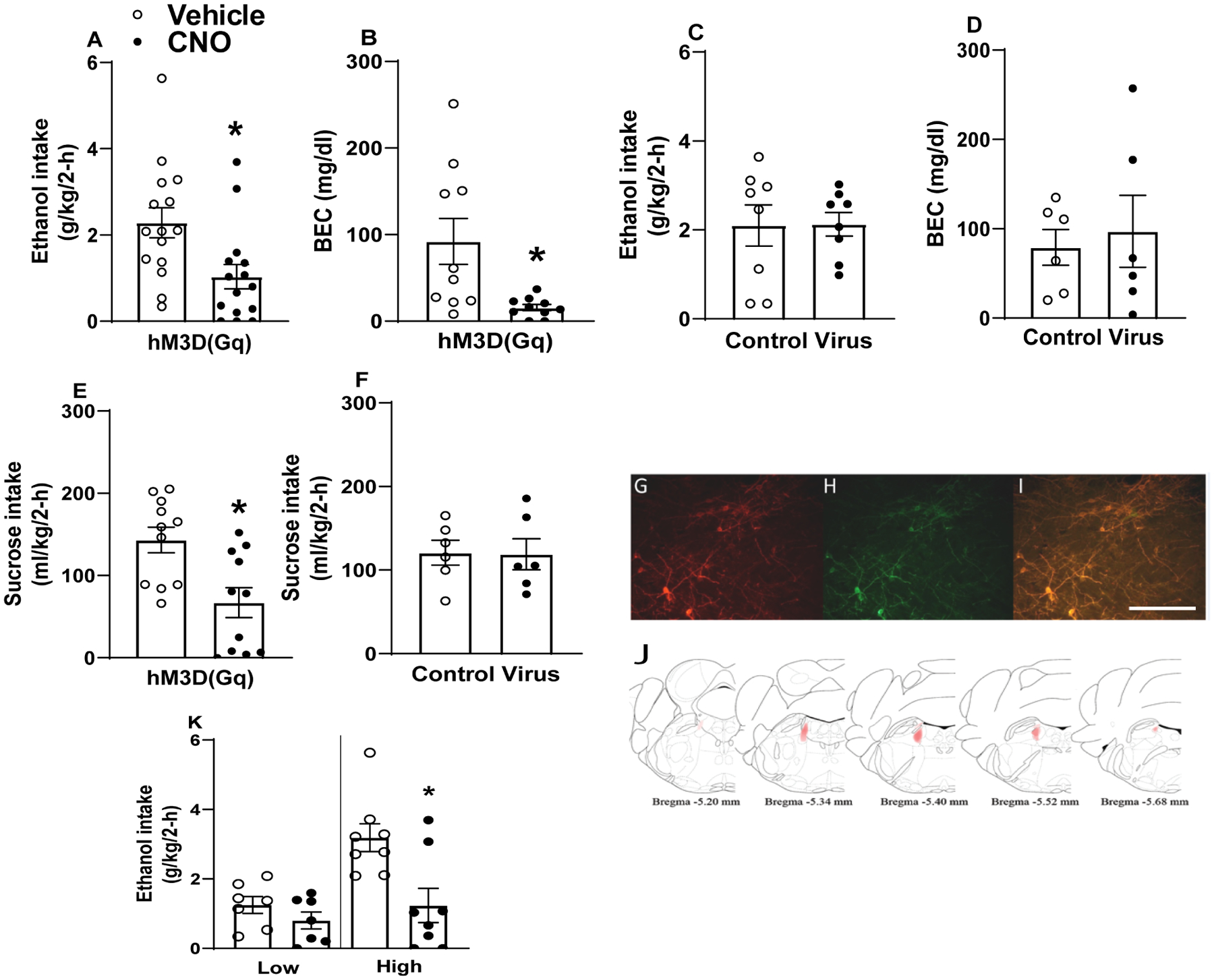
I.p. CNO, but not vehicle, blunts binge-like ethanol intake in TH-ires-cre mice expressing hM3Dq DREADD (A; n=15) but not control AAV (C; n=8). I.p. CNO, but not vehicle, blunts BECs in TH-ires-cre mice expressing hM3Dq DREADD (B; n=10) but not control AAV (D; n=6). I.p. CNO, but not vehicle, blunts intake of a 3% sucrose solution in TH-ires-cre mice expressing hM3Dq DREADD (E; n=11) but not control AAV (F; n=6). Representative photomicrographs of hM3Dq DREADD virus (red, G), tyrosine hydroxylase (TH) immunohistochemistry (green, H), and hM3Dq/TH coexpression (yellow, I). For zoomed out images of DREADD expression in the LC please see Suppl. Fig. 1. Heat map of hM3Dq DREADD virus spread, with darker areas indicating similar virus expression across mice and lighter areas indicating less-common expression patterns (J). (K) Further analyses of ethanol intake data indicated that chemogenetic activation of LC TH+ neurons blunted intake in mice with High baseline ethanol intake (upper 50% of a median-split) and not in mice with Low baseline intake (lower 50% of a median-split). All values indicate mean ± SEM. Data collapsed across sexes due to absence of statistically significant sex differences. * indicates p < 0.05. Scale bar = 100 μm.
3.3. Experiment 3: Effects of Chemogenetic Activating the LC ➔ LH TH+ Pathway on Binge-Like Ethanol Intake
Given the reciprocal signaling between the LC and LH regions detailed in literature as well as LC➔LH tracing in TH-ires-cre mice (Suppl. Fig. 2A–D) and results from NE nuclei chemogenetic activation, we sought to manipulate LC ➔ LH NE neurocircuitry to determine the role of the pathway in modulating binge-like ethanol consumption. Results from this experiment are presented in Fig. 3. As seen in Fig. 3A, relative to vehicle injection days, LH-targeted CNO microinfusion blunted binge-like ethanol intake (n=10) [t test: t(9)=3.61, p=0.006]. There were also blunted BECs (Fig. 3B) [t(9)=2.801, p=0.02] in TH-ires-cre mice expressing hM3Dq DREADD in the LC. Conversely, in TH-ires-cre mice expressing AAV8-hSyn-DIO-mcherry, LH-targeted CNO microinfusions failed to alter ethanol intake (Fig. 3C) [t test: t(5)=0.134, p=0.90] or associated BECs (Fig. 3D; 1 sample was lost due to mechanical error) [t(4)=0.544, p=0.62]. As is evident in Fig. 3E, CNO failed to alter intake of a 3% sucrose solution in mice expressing the hM3Dq DREADD in the LC [t test: t(9)=0.119, p=0.91] or mice expressing the empty vector control in the LC (Fig. 3F) [t test: t(4)=0.75, p=0.50] relative to vehicle. In a separate experiment, chemogenetic activation of the LC ➔ LH TH+ pathway also failed to blunt sucrose consumption in ethanol-naïve mice (Suppl. Fig. 3A–B).
Figure 3. Chemogenetic activation of the LC ➔ LH pathway blunts binge-like ethanol intake in TH-ires-cre mice.
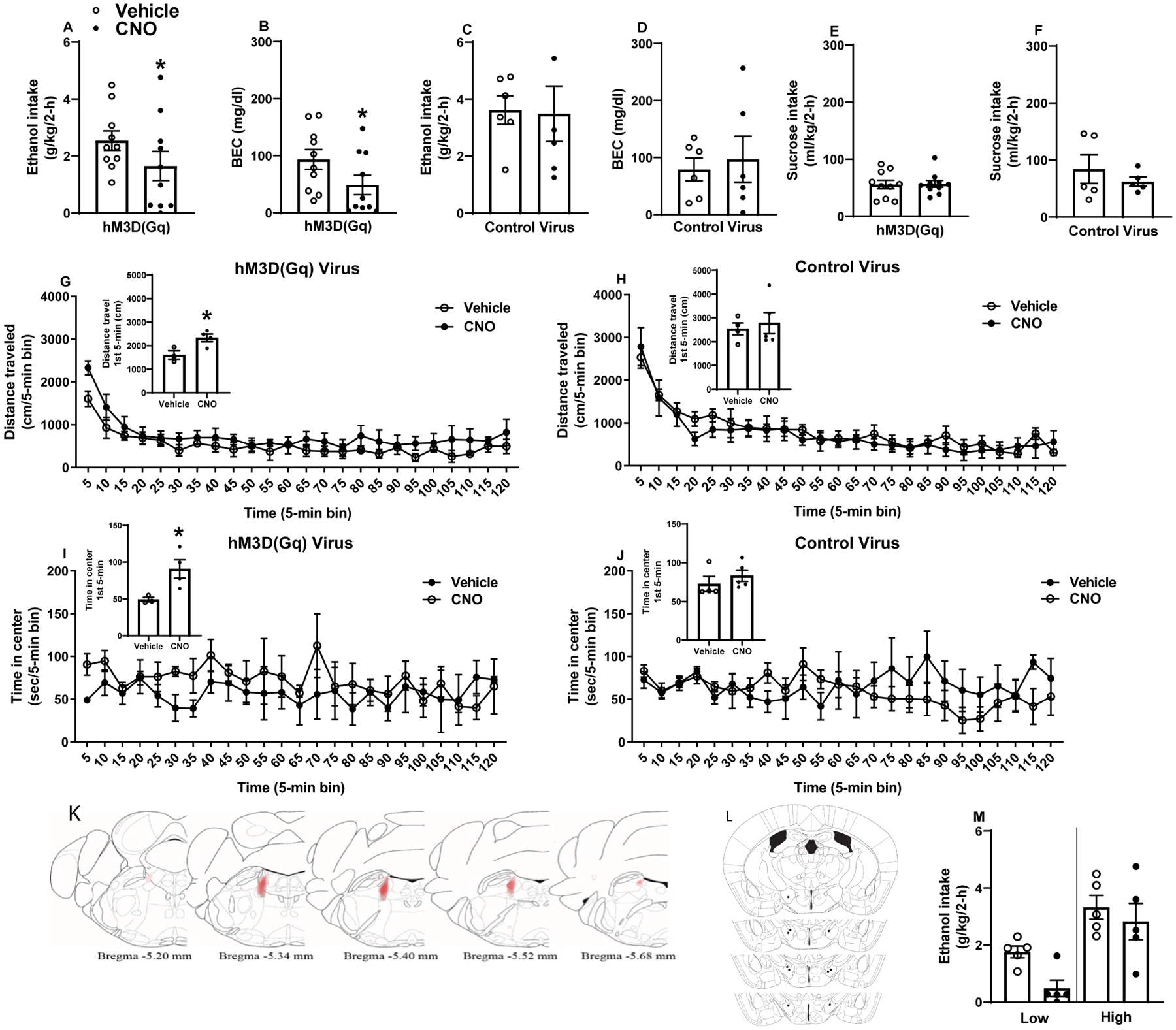
LH-targeted CNO, but not vehicle, blunts binge-like ethanol intake in TH-ires-cre mice expressing hM3Dq DREADD (A; n=10) but not control AAV (C; n=6) in LC. LH-targeted CNO, but not vehicle, blunts BECs in TH-ires-cre mice expressing hM3Dq DREADD (B; n=10) but not control AAV (D; n=5) in LH. In TH-ires-cre mice with a history of ethanol consumption in the DID paradigm, LH-targeted CNO failed to alter intake of a 3% sucrose solution in mice expressing hM3Dq DREADD (E; n=10) or control AAV (F; n=5) in the LC. In the OFT, LH-targeted CNO failed to alter total distance travelled in the 2-h test regardless of virus condition (G & H), though distance travelled in the first 5 minutes of testing as increased among mice expressing hM3Dq (G inset). Pathway activation in hM3D(Gq) treated mice increased time spent in chamber center during the first 5 minutes of testing (I inset), but we failed to observe a significant main effect of pathway activation on center time across the 2-h test (I). CNO alone failed to alter center time in control virus treated mice (J). Heat map of hM3Dq DREADD virus spread, with darker areas indicating similar virus expression across mice and lighter areas indicating less-common expression patterns (K). Approximate cannula placements at various anterior-posterior positions within the LH indicated by black dots (L). (M) Further analyses of ethanol intake data indicated that chemogenetic activation of LC TH+ neurons blunted intake in mice with no significant differences between mice with High baseline ethanol intake (upper 50% of a median-split) versus mice with Low baseline intake (lower 50% of a median-split). Data collapsed across sexes due to absence of statistically significant sex differences across experiments. All values indicate mean ± SEM. * indicates p<0.05 in paired t-test.
Separate, ethanol-naïve TH-ires-cre mice were utilized OFT experiments. Chemogenetic activation of the LC➔LH pathway increased distance travelled in the first 5 minutes of testing [t(5)=2.977, p=0.03] (Fig. 3G, inset) but did not alter distance travelled over the entire two-hour test [treatment: F(1,5)=1.021, p=0.36; time: F(23,115)=18.08, p<0.01; interaction: F(23,115)=1.17, p=0.29] (Fig. 3G). In mice expressing the empty vector control, CNO microinfusion did not alter distance travelled in the first 5 minutes of testing [t(7)=0.386, p=0.71] (Fig. 3H, inset) or distance travelled over the entire two-hour test (treatment: F(1,7)=0.14, p=0.71; time: F(23,161)=21.16, p<0.01; interaction: F(23,161)=0.68, p=0.86] (Fig. 3H). LC➔LH projection activation increased time spent in OFT chamber center in the first 5 minutes of testing [t(12)=3.718, p=0.003] (Fig. 3I, inset) but did not yield a significant effect across the duration of the two-hour test [treatment: F(1,5)=0.75, p=0.42; time: F(23,115)=0.83, p<0.69; interaction: F(23,115)=0.70, p=0.84] (Fig. 3I). CNO did not alter center time in the first 5 minutes of testing in control mice [t(7)=0.888, p=0.40] (Fig. 3J, inset) or over the two-hour test [treatment: F(1,7)=0.20, p=0.66; time: F(23,161)=1.09, p=0.36; interaction: F(23,161)=1.63, p=0.04] (Fig. 3J). Representative DREADD virus expression in the LC, and cannula placements within the LH are presented in Figs. 3K and 3L, respectively. As above, we performed a follow-up assessment of ethanol consumption data by performing a median-split of mice based on their consumption during vehicle infusion, and we then re-analyzed consumption data with a two-way, 2 × 2 (treatment: vehicle versus CNO × drinker: low drinkers versus high drinking) ANOVA (Fig. 3M). We found significant main effects of treatment [F(1, 8)=16.02, p=0.004], drinker [F(1, 8)=12.57, p=0.008], and but the treatment × drinker interaction effect was not significant [F(1, 8)=3.09, p=0.12].
3.4. Experiment 4: Effects of LH α1 AR Agonism on Binge-Like Ethanol Intake
Data from each of the pharmacological experiments are presented in Fig. 4. A two-way ANOVA failed to detect an effect of sex on any data set, so data from males and females were collapsed. Paired t-tests were used to assess total consumption. Relative to vehicle injections, 20 nmol PHEN/hemisphere blunted total consumption (Fig. 4A) [t test: t(16)=2.391, p=0.03] but not associated BECs (Fig. 4B) [t(16)=1.03, p=0.32]. Following 2 weeks of ethanol DID testing, mice experienced 2 consecutive weeks, in a Latin square design, of 3% sucrose testing to determine if 20 nmol PHEN effects were specific to ethanol. Relative to vehicle, 20 nmol PHEN failed to alter total intake of a 3% sucrose solution (Fig. 4C) (n=15; 2 animals had clogged cannulae) [t test: t(14)=0.826, p=0.42]. Approximate cannula placements are denoted in Fig. 4D. As above, we performed a follow-up assessment of ethanol consumption data by performing a median-split of mice based on their consumption during vehicle infusion, and we then re-analyzed consumption data with a two-way, 2 × 2 (treatment: vehicle versus PHEN × drinker: low drinkers versus high drinking) ANOVA (Fig. 4E). We found significant effects of treatment [F(1, 15)=8.38, p=0.011], drinker [F(1, 15)=8.72, p<0.01], and treatment × drinker interaction [F(1, 15)=11.97, p=0.004]. Bonferroni corrected t-tests indicated a significant differences in binge-like ethanol intake between vehicle and PHEN in high drinkers [t-test t(8)=4.90, p=0.001], but not in levels of binge-like ethanol intake between vehicle and PHEN in low drinkers [t-test t(7)=−0.37, p=0.73].
Figure 4. LH α1 adrenergic receptor activation blunts binge-like ethanol intake in C57BL6/J mice.
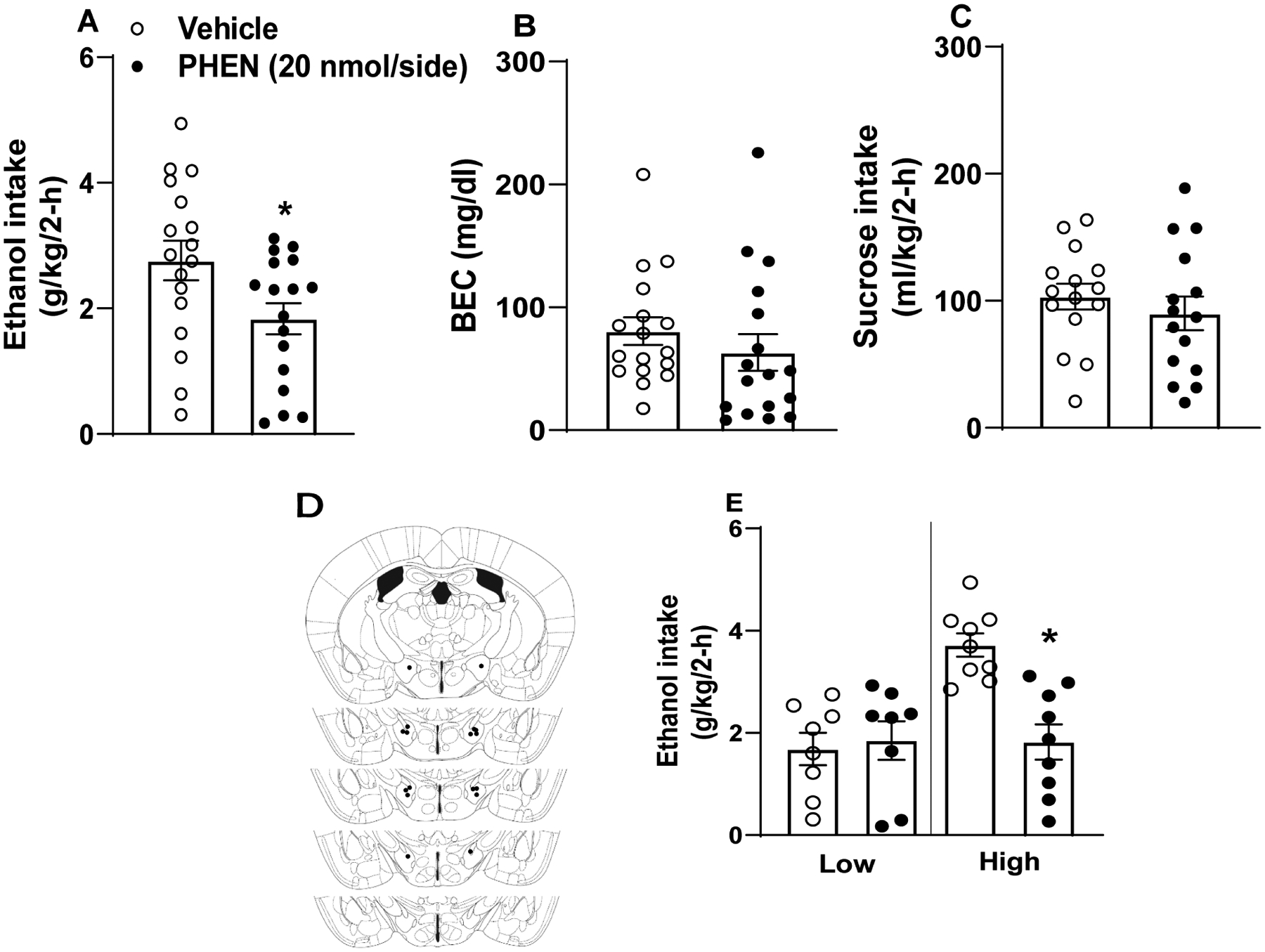
α1 agonism via LH-targeted phenylephrine (PHEN) blunted total ethanol intake (A; n=17) but not overall BECs (B; n=17) in the DID paradigm while failing to alter intake of a 3% sucrose solution (C; n=15). Male and female data are collapsed as we failed to observe an interaction of sex and drug during LH α1 manipulation. Approximate cannula placements (D). (E) Further analyses of ethanol intake data indicated that PHEN blunted intake in mice with High baseline ethanol intake (upper 50% of a median-split) and not in mice with Low baseline intake (lower 50% of a median-split). All values represent mean ± SEM. * represents p<0.05 in paired t-test.
3.5. Experiment 5: Effects of LH β AR Agonism on Binge-Like Ethanol Intake
Repeated-measures two-way ANOVA (sex × treatment) revealed a significant interaction of sex × treatment [F(1,11)=7.796, p=0.018], with 0.5 μg XAM/hemisphere significantly reducing total binge-like ethanol consumption in females (Fig. 5A). Main effects of sex [F(1,11)=2.546, p=0.139] and treatment [F(1,11)=2.309, p=0.239] were not detected. XAM similarly blunted BECs in females but not males (Fig. 5B) [treatment: F(1,11)=0.462, p=0.51; sex: F(1,11)=0.421, p=0.53; interaction: F(1,11)=10.56, p<0.01]. However, 0.5 μg/hemisphere XAM failed to similarly alter sucrose consumption (Fig. 5C), as we failed to detect significant main effects [sex: F(1,11)=1.188, p=0.299; treatment: F(1,11)=1.02, p=0.334] or a sex × treatment interaction [F(1,11)=0.008, p=0.932]. Approximate cannula placements are denoted in Fig. 5D. As above, we performed a follow-up assessment of ethanol consumption data by performing a median-split of mice based on their consumption during vehicle infusion, and we then re-analyzed consumption data with a two-way, 2 × 2 (treatment: vehicle versus XAM × drinker: low drinkers versus high drinking) ANOVA (Fig. 5E). While the treatment effect was not significant [F(1, 11)=1.13, p=0.311], drinker [F(1, 11)=13.04, p=0.004], and the treatment × drinker interaction [F(1, 11)=6.43, p=0.028] were both statistically significant. Bonferroni corrected t-tests failed to reveal significant differences in binge-like ethanol intake between vehicle and XAM in high drinkers [t-test t(6)=2.42, p=0.052], as well as binge-like ethanol intake between vehicle and XAM in low drinkers [t-test t(5)=−1.15, p=0.302].
Figure 5. LH β adrenergic receptor activation blunts binge-like ethanol intake in female C57BL6/J mice.
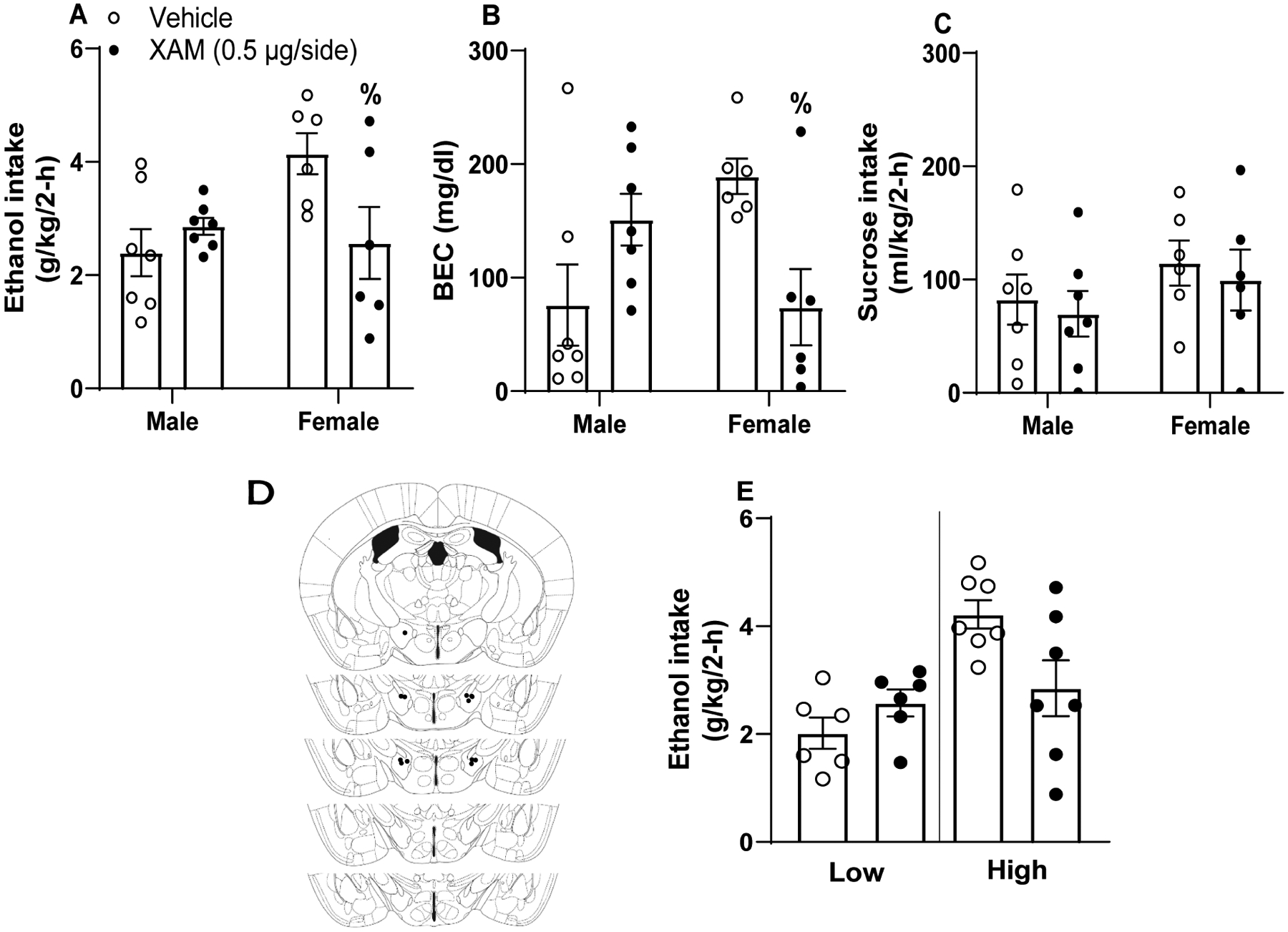
β agonism via xamoterol hemifumerate (XAM) blunted binge-like ethanol intake (A) and BECs (B) in females exclusively without altering sucrose intake (C; n=6 female, 7 male). Approximate cannula placements within the LH (D). (E) Further analyses of ethanol intake data indicated, when collapsed across sexes, no significant differences between vehicle and XAM treatment in mice with High baseline ethanol intake (upper 50% of a median-split) or mice with Low baseline intake (lower 50% of a median-split). All values represent mean ± SEM. % represents significant interaction following two-way ANOVA as determined via Bonferroni post-hoc testing.
3.6. Experiment 6: Effects of LH α2 Antagonism on Binge-Like Ethanol Intake
A repeated-measures two-way ANOVA (treatment × sex) revealed a significant interaction of sex × dose [F(2, 38)=3.245, p=0.05] on total ethanol consumption but failed to reveal significant main effects of sex [F(1,19)=0.985, p=0.33] or dose [F(2,38)=0.16, p=0.85]. However, Bonferroni post-hoc testing failed to reveal group differences, thus the sexes are collapsed in the Fig. 6A. Further, we failed to detect significant differences in BECs (Fig. 6B) [treatment: F(2,34)=0.399, p=0.67; sex: F(1,17)=0.354, p=0.56; interaction: F(2,34)=0.722, p=0.49]. Animals were repurposed for 3% sucrose testing to determine specificity of drug effects, comparing vehicle and 1.50 μg atipamazole/hemisphere treatments. Fig. 6C shows results from a repeated-measures ANOVA (treatment × sex), where 1.50 μg atipamezole(APZ)/hemisphere microinfusion revealed an interaction of drug and sex on intake of a 3% sucrose solution (1 female was lost between studies due to clogged cannula) [treatment: F(1,18)=0.987, p=0.33; sex: F(1,18)=0.007, p=0.93; interaction: F(1,18)=7.451, p=0.014], but Bonferroni post-hoc testing failed to reveal group differences, thus the sexes are collapsed in Fig. 6C. Approximate cannula placements are defined in Fig. 6D. As above, we performed a follow-up assessment of ethanol consumption data by performing a median-split of mice based on their consumption during vehicle infusion, and we then re-analyzed consumption data with a two-way, 3 × 2 (treatment: vehicle versus APZ × drinker: low drinkers versus high drinking) ANOVA (Fig. 5E). While the treatment effect was not significant [F(2, 38)=0.38, p=0.152], drinker [F(1, 19)=8.84, p=0.008], and the treatment × drinker interaction [F(2, 38)=6.33, p=0.004] were both statistically significant. Bonferroni corrected t-tests indicated a non-significant reduction of binge-like ethanol intake following the 1.5 μg dose [t-test t(10)=2.3, p=0.045] but a significant reduction following the 15 μg dose [t-test t(10)=3.24, p=0.009] of APZ relative to vehicle treatment in the high drinkers. Conversely, in the low drinkers, relative to vehicle treatment the 1.5 μg dose of APZ increased ethanol intake [t-test t(9)=−3.13, p=0.012] while the and 15 μg dose of APZ did not significantly alter intake [t-test t(9)=−1.91, p=0.089].
Figure 6. LH α2 adrenergic receptor blockade fails to blunt binge-like ethanol intake in C57BL6/J mice.
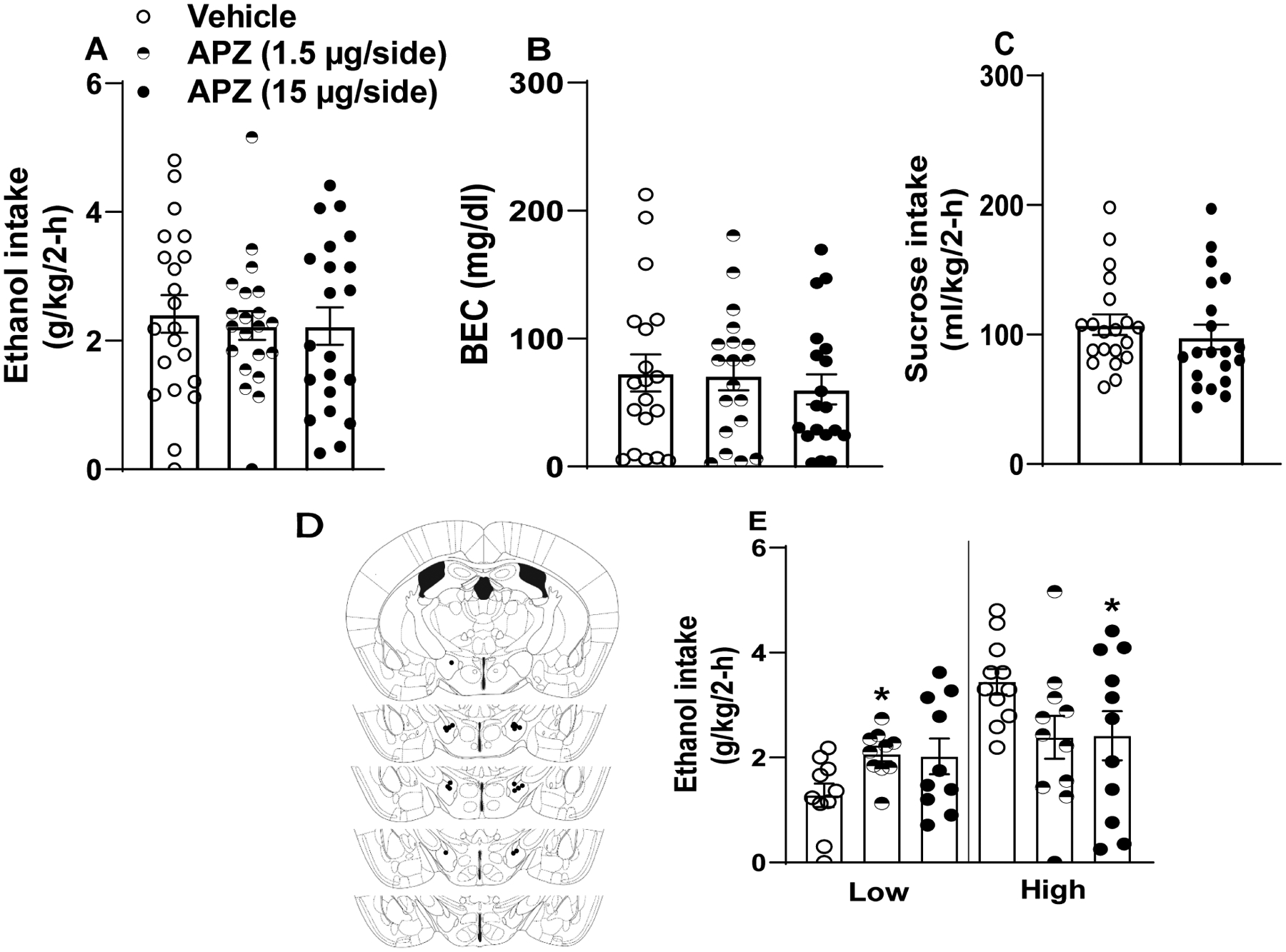
α2 antagonism via APZ failed to significantly alter total ethanol intake (A; n=21), BECs (B; n=21), or intake of a 3% sucrose solution (C; n=20). Approximate cannula placements within the LH (D). (E) Further analyses of ethanol intake data indicated that the high dose of APZ blunted intake in mice with High baseline ethanol intake (upper 50% of a median-split) and that the low dose of APZ increased ethanol intake in mice with Low baseline intake (lower 50% of a median-split). All values represent mean ± SEM.
4. Discussion
This series of studies sought to uncover the roles of the LC and its projection to the LH in modulation of voluntary binge-like ethanol consumption. First, we first showed that systemic administration of the NRI, reboxetine, blunted binge-like ethanol intake in both sexes without altering locomotor or anxiety-like behavior. These findings are consistent with our previous observation that the NE and dopamine re-uptake inhibitor, BUP, significantly blunts binge-like ethanol drinking mice (Navarro et al., 2019). Next, we showed that general chemogenetic activation of NE neurons in the LC blunted binge-like ethanol, but also reduced and sucrose intake suggesting non-specific effects on consummatory behavior. These non-specific effects on behavior may be secondary to the increased anxiety-like behavior, which is also triggered by general chemogenetic activation of these neurons (Sciolino et al., 2016). On the other hand, chemogenetic activation specifically of the NE LC➔LH projection blunted binge-like ethanol consumption without altering sucrose consumption in both ethanol experienced and ethanol naïve mice. Interestingly, while general activation of the NE neurons in the LC is anxiogenic (Sciolino et al., 2016), we found that activation of NE LC ➔ LH was anxiolytic, reflected as increased center time during the first 5-minutes in the OFT. There was also an increase in distance traveled during the first 5-minute of the OFT following activation of the NE LC ➔ LH pathway, though this transient increase in locomotor activity is unlikely to have impacted the 2-hour ethanol intake test, reinforced by the lack of an effect of this manipulation on 2-hour sucrose drinking. In our pharmacological studies with C57BL/6J mice, at the doses tested we found that LH infusion of the α1 AR agonist, PHEN, blunted binge-like ethanol intake independent of sex and without altering sucrose intake, the β1 AR agonist, XAM, blunted ethanol intake without altering sucrose intake in female mice, and the a high dose of the α2 AR antagonist, ATP, blunted binge-like ethanol intake in mice with high baseline ethanol intake but a low dose of ATP increased ethanol intake in mice with low baseline ethanol intake.
NE binds to α2 ARs to decrease NE release from presynaptic terminals (Aghajanian and VanderMaelen, 1982; Starke et al., 1974) and engages post-synaptic Gi-signaling cascades (Wang et al., 2007) while engagement of postsynaptic Gq-coupled α1 ARs (Piascik and Perez, 2001) and Gs-coupled β ARs (Molinoff, 1984) increase neuronal activity. Our findings that elevated NE signaling via systemic NRI, LH-localized α1 AR activation and α2 AR blockade, chemogenetic activation of NE neurons in the LC or LC➔LH projection, blunts binge-like ethanol intake appears incongruent with literature showing reduced ethanol intake following systemic administration of drugs that blunt, rather than increase, NE signaling. For example, the α2 AR agonist, clonidine (Rasmussen et al., 2014a), the α1 AR antagonist, prazosin (Froehlich et al., 2013; Rasmussen et al., 2009; Verplaetse et al., 2012), or the β AR antagonist, propranolol (Verplaetse and Czachowski, 2015) all reduce ethanol intake. There are at least two possible explanations for these differences. First, systemic administration of AR-selective pharmacological compounds detailed in the literature inherently modifies signaling throughout the central and peripheral nervous systems, so altered intake patterns resulting from systemic administration reflect a net effect of system-wide receptor interactions. However, our chemogenetic and pharmacological manipulations were brain region- and circuit-specific and suggest that increasing NE tone in these regions is protective against excessive consumption. One caveat to this reasoning is our finding that systemic treatment with the NRI, reboxetine, blunted binge-like ethanol intake, though the actions of NRIs are mechanistically distinct from AR agonists or antagonists, as they increase global NE availability for all ARs (Dostert et al., 1997). Secondly, previous studies showing that reduced NE tone blunts ethanol intake utilized rodent strains bred for high levels of ethanol consumption (Froehlich et al., 2013; O’Neil et al., 2013; Rasmussen et al., 2014a; Rasmussen et al., 2009; Rasmussen et al., 2014b; Verplaetse and Czachowski, 2015; Verplaetse et al., 2012), ethanol-dependent rats (Gilpin and Koob, 2010), or alcohol-dependent humans (Simpson et al., 2009) while we studied non-dependent C57BL6/J and TH-ires-cre mice. Thus, the contributions of NE signaling may differ between subjects that are ethanol-dependent or selectively bred for high ethanol intake versus non-dependent binge drinking subjects. Overall, our data suggest that treatments that increase NE tone may have therapeutic value for treating binge or heavy alcohol abuse, prior to the development of dependence. Following the development of ethanol dependence, reducing NE tone may be protective, particularly against the adverse symptoms of withdrawal, such as anxiety, which drive high ethanol intake through negative reinforcement (Koob and Le Moal, 2001).
We previously found that ethanol-induced c-Fos expression in the LC was significantly lower among rats selectively bred for high ethanol intake relative to low ethanol drinking lines (Thiele et al., 1997), and more recently we found that an inbred line of mice that was selectively bred to achieve binge-like blood ethanol levels (the iHDID-1 line) failed to exhibit ethanol-induced c-Fos expression in the LC at doses that induced c-Fos expression in the control HS/Npt line (Robinson et al., 2020). Together these results suggest that the LC may be part of a mechanism that protects against ethanol intake and which is compromised in high drinking lines. Additionally, high-alcohol drinking (HAD) and alcohol-preferring (P) rats each demonstrate fewer NE transporter sites within the LC relative to low-alcohol drinking (LAD) and alcohol non-preferring (NP) rats, possibly as a compensatory mechanism for reduced NE release (Hwang et al., 2000). Thus, dysregulated NE activity in the LC may contribute to a pattern of high ethanol consumption observed in ethanol-preferring and high drinking rodent lines. Consistent with our chemogenetic manipulations, increasing or decreasing NE activity via LC-targeted microinfusion of substance P or clonidine, respectively, suppresses or increases voluntary ethanol consumption in rats (West et al., 2015). Collectively, these data suggest that ethanol-induced activation of NE signaling in the LC may represent a protective “braking” mechanism against high ethanol intake. This conclusion is bolstered by our observations with chemogenetic and pharmacological manipulations that increased NE/AR signaling was most effective in blunting binge-like ethanol intake in mice with higher levels of ethanol intake in most cases. We speculate that this signaling becomes blunted over repeated ethanol use, which in turns leaves individuals vulnerable to increased levels of drinking and ultimately dependence. Ongoing research within our lab is exploring this possibility.
Activation of the NE LC➔LH pathway blunted binge-like ethanol intake without altering the consumption of sucrose in ethanol-experience or ethanol naïve-mice. These observations suggest that the effects of activating this pathway are specific to binge-like ethanol intake. Similarly, our pharmacological manipulations blunted binge-like ethanol intake without altering sucrose intake. It is important to keep in mind that the LC and other NE nuclei (Ahlskog, 1974; Redmond et al., 1977; Rinaman, 2003; Rinaman et al., 1998; Roman et al., 2016; Sahakian et al., 1983; Schreihofer et al., 1997; Uemura et al., 1997) and the LH (Jennings et al., 2013; Navarro et al., 2016; Stamatakis et al., 2016) as well as LH ARs (Ferrari et al., 1990; Leibowitz, 1970) have been implicated in consummatory behavior encompassing ethanol as well as biologically significant rewards, such as food. What is more, the LH is critical for reward processing, as rodents will work for stimulation of the LH (Hoebel and Teitelbaum, 1962; Jennings et al., 2015; Margules and Olds, 1962). Interestingly, pharmacotherapies that increase NE tone are used to treat disorder of excessive eating (Carbone et al., 2020; Greenway et al., 2009; Greenway et al., 2010). And as noted below, we are currently investigating similar approaches to treating binge drinking prior to dependence (Navarro et al., 2019; Walter et al., 2020), and we are more thoroughly examining the potential role of the NE+ LC ➔ LH circuit in modulating the consumption of various natural rewards in ethanol-naïve subjects.
The LH is a neurochemically diverse region, producing GABA, glutamate, orexin/hypocretin, dynorphin, neurotensin, galanin, and cocaine and amphetamine-regulated transcript, each of which has been implicated in the consumption of ethanol (Olney et al., 2015; Salinas et al., 2014; Sprow et al., 2016). Future investigations aimed at identifying LH postsynaptic contacts of NE axons stemming from the LC will help unpack the mechanisms by which NE LC➔LH neurons, and AR signaling in the LH, modulate binge-like ethanol intake. Interestingly, we have previously shown that chemogenetic manipulation of GABAergic neurons in the LH modulates binge-like ethanol intake (Navarro et al., 2016), making innervation of LH GABAergic neurons by NE neurons arising from the LC as an important future research direction.
Utilization of both sexes in our studies was critical given literature implicating estrogen in modulation of NE synthesis and LH AR signaling (Alfinito et al., 2009; Etgen et al., 2001; Lubbers et al., 2010; Selmanoff et al., 1976; Vathy and Etgen, 1988), and sex differences in LC structure (Guillamon et al., 1988; Luque et al., 1992; Pinos et al., 2001). Our pharmacological manipulations within the LH revealed sex-specific effects of the β AR agonist, which may stem from estrogen modulation of NE synthesis and LH AR signaling as noted above. However, any sex differences in chemogenetic manipulations of the NE LC➔LH projection were likely masked given that LC NE activation likely influences all ARs. Further, additional sex effects may have surfaced had we examined full dose-response curves in our pharmacology studies. Future research is needed to more clearly establish potential sex differences in the role that AR signaling in the LH plays in modulating binge-like ethanol intake.
Importantly, while we did remove animals with cannulae placements that were determined to have missed the LH (see Suppl. Fig. 4 for photomicrograph of cannulae placement histology), one caveat worth noting is the possibility that during in vivo pharmacological studies compounds may have moved beyond the LH and impacted AR signaling in adjacent brain regions. While we cannot completely rule out this possibility, we did note that TH+ fibers arising from the LC and innervating the hypothalamus appeared to be specific to LH innervation, and were not evident in nearby regions such as the dorsal zona incerta (ZI; see Suppl. Fig. 5). Thus, we are confident in the specificity of the TH+ LC ➔ LH circuit in the modulating of binge-like ethanol intake. A second caveat is that since TH+ neurons of the LC have been shown to co-express glutamate (Fung et al., 1994), we cannot definitively conclude that chemogenetic activation of TH+ neurons in the LC or LC ➔ LH circuit blunted binge-like ethanol intake exclusively by stimulation of NE release, and thus we must temper this conclusion. That being said, we did find that treatment with the NRI reboxetine, and that LH-infusion of the high dose of the α2 AR antagonist APZ, blunted binge-like ethanol intake in mice with high baseline levels of ethanol intake, presumably by increase synaptic levels of NE and thus implicating endogenous NE signaling. The latter observation specifically points to a role for endogenous NE signaling in the LH in the modulation of binge-like ethanol intake.
5. Conclusions
Though well-established as playing a critical role in depression and anxiety disorders (Montoya et al., 2016), a growing body of literature is unpacking a role for central NE signaling in the modulation of drug and alcohol intake. Our results indicate that elevated NE signaling in the NE LC➔LH circuit plays protective roles against binge-like ethanol intake. We recently reported that the dopamine/norepinephrine reuptake inhibitor, bupropion, blunts binge-like ethanol intake in mice (Navarro et al., 2019), and reduced the frequency of binge alcohol drinking in a phase II clinical trial study (Walter et al., 2020). These observations are consistent with our current finding that the NRI, reboxetine, blunted binge-like ethanol drinking in mice. Taken together, these results suggest potential therapeutic value for drugs that increase NE tone, such as reboxetine and bupropion, in minimizing binge-like ethanol drinking in humans prior to the development of ethanol dependence.
Supplementary Material
Highlights.
Peripheral administration of the NE reuptake inhibitor, reboxetine, blunts binge-like ethanol intake, suggesting a potential therapeutic target.
Chemogenetic activation of the locus coeruleus blunts binge-like ethanol intake.
Chemogenetic activation of a norepinephrine circuit from the LC to lateral hypothalamus (LH) selectively blunts binge-like ethanol intake but not sucrose drinking.
Pharmacological manipulation of adrenergic receptor in the LH selectively blunts binge-like ethanol intake.
Acknowledgments:
We thank Rhiannon Thomas, Timothy Gilliam, Sophie Bendrath, and Sonia Sabater for their expert assistance with the present projects. This research was funded by NIH grants AA013573, AA022048, & AA025809.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare no competing financial interests. Dr. Thiele owns shares of Glauser Life Sciences, a copy the aims to develop therapeutics for mental health disorders. The work that is presented in this paper is not directly related to the scientific aims of Glauser Life Sciences.
References
- Aghajanian GK, VanderMaelen CP, 1982. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science 215, 1394–1396. [DOI] [PubMed] [Google Scholar]
- Ahlskog JE, 1974. Food intake and amphetamine anorexia after selective forebrain norepinephrine loss. Brain Res 82, 211–240. [DOI] [PubMed] [Google Scholar]
- Albrechet-Souza L, Hwa LS, Han X, Zhang EY, DeBold JF, Miczek KA, 2015. Corticotropin Releasing Factor Binding Protein and CRF2 Receptors in the Ventral Tegmental Area: Modulation of Ethanol Binge Drinking in C57BL/6J Mice. Alcohol Clin Exp Res 39, 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfinito PD, Chen X, Mastroeni R, Pawlyk AC, Deecher DC, 2009. Estradiol increases catecholamine levels in the hypothalamus of ovariectomized rats during the dark-phase. Eur J Pharmacol 616, 334–339. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ, 1994. Fewer pigmented neurons in the locus coeruleus of uncomplicated alcoholics. Brain Res 650, 1–8. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ, 2011. Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav 100, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Yeh CY, James MH, 2019. Reduced Sensory-Evoked Locus Coeruleus-Norepinephrine Neural Activity in Female Rats With a History of Dietary-Induced Binge Eating. Front Psychol 10, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham NW, Thiele TE, 2017. Voluntary Binge-like Ethanol Consumption Site-specifically Increases c-Fos Immunoexpression in Male C57BL6/J Mice. Neuroscience 367, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone EA, Caroleo M, Rania M, Calabro G, Staltari FA, de Filippis R, Aloi M, Condoleo F, Arturi F, Segura-Garcia C, 2020. An open-label trial on the efficacy and tolerability of naltrexone/bupropion SR for treating altered eating behaviours and weight loss in binge eating disorder. Eat Weight Disord. [DOI] [PubMed] [Google Scholar]
- Chen YW, Barson JR, Chen A, Hoebel BG, Leibowitz SF, 2013. Opioids in the perifornical lateral hypothalamus suppress ethanol drinking. Alcohol 47, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Morganstern I, Barson JR, Hoebel BG, Leibowitz SF, 2014. Differential role of D1 and D2 receptors in the perifornical lateral hypothalamus in controlling ethanol drinking and food intake: possible interaction with local orexin neurons. Alcohol Clin Exp Res 38, 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert P, Benedetti MS, Poggesi I, 1997. Review of the pharmacokinetics and metabolism of reboxetine, a selective noradrenaline reuptake inhibitor. Eur Neuropsychopharmacol 7Suppl 1, S23–35; discussion S71–23. [DOI] [PubMed] [Google Scholar]
- Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS, 2014. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev Chronic Dis 11, E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A, 2001. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav 40, 169–177. [DOI] [PubMed] [Google Scholar]
- Ferrari AC, Camargo LA, Saad WA, Renzi A, De Luca Junior LA, Menani JV, 1990. Clonidine and phenylephrine injected into the lateral hypothalamus inhibits water intake in rats. Brain Res 522, 125–130. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fischer SM, Rasmussen DD, 2013. Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcohol Clin Exp Res 37, 1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Reddy VK, Zhuo H, Liu RH, Wang Z, Barnes CD, 1994. Anatomical evidence for the presence of glutamate or enkephalin in noradrenergic projection neurons of the locus coeruleus. Microsc Res Tech 29, 219–225. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF, 2010. Effects of beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl) 212, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, Cowley MA, Group NBS, 2009. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab 94, 4898–4906. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E, Group C-IS, 2010. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 376, 595–605. [DOI] [PubMed] [Google Scholar]
- Guillamon A, de Blas MR, Segovia S, 1988. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res 468, 306–310. [DOI] [PubMed] [Google Scholar]
- Halseth A, Shan K, Gilder K, Malone M, Acevedo L, Fujioka K, 2018. Quality of life, binge eating and sexual function in participants treated for obesity with sustained release naltrexone/bupropion. Obes Sci Pract 4, 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR, 2006. Age of alcohol-dependence onset: associations with severity of dependence and seeking treatment. Pediatrics 118, e755–763. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Teitelbaum P, 1962. Hypothalamic control of feeding and self-stimulation. Science 135, 375–377. [DOI] [PubMed] [Google Scholar]
- Holgate JY, Shariff M, Mu EW, Bartlett S, 2017. A Rat Drinking in the Dark Model for Studying Ethanol and Sucrose Consumption. Front Behav Neurosci 11, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BH, Wang GM, Wong DT, Lumeng L, Li TK, 2000. Norepinephrine uptake sites in the locus coeruleus of rat lines selectively bred for high and low alcohol preference: a quantitative autoradiographic binding study using [3H]-tomoxetine. Alcohol Clin Exp Res 24, 588–594. [PubMed] [Google Scholar]
- Jendryka M, Palchaudhuri M, Ursu D, van der Veen B, Liss B, Katzel D, Nissen W, Pekcec A, 2019. Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep 9, 4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD, 2013. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA, Aita M, Shilling-Scrivo K, Ramakrishnan C, Deisseroth K, Otte S, Stuber GD, 2015. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Moore RY, 1977. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res 127, 25–53. [PubMed] [Google Scholar]
- Kochenborger L, Zanatta D, Berretta LM, Lopes AP, Wunderlich BL, Januario AC, Neto JM, Terenzi MG, Paschoalini MA, Faria MS, 2012. Modulation of fear/anxiety responses, but not food intake, following alpha-adrenoceptor agonist microinjections in the nucleus accumbens shell of free-feeding rats. Neuropharmacology 62, 427–435. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2001. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24, 97–129. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, 1970. Reciprocal hunger-regulating circuits involving alpha- and beta-adrenergic receptors located, respectively, in the ventromedial and lateral hypothalamus. Proc Natl Acad Sci U S A 67, 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Jhanwar-Uniyal M, Dvorkin B, Makman MH, 1982. Distribution of alpha-adrenergic, beta-adrenergic and dopaminergic receptors in discrete hypothalamic areas of rat. Brain Res 233, 97–114. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, ak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA, 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Jaatinen P, Rintala J, Sarviharju M, Kiianmaa K, Hervonen A, 1997. Effects of lifelong ethanol consumption on rat locus coeruleus. Alcohol Alcohol 32, 463–470. [DOI] [PubMed] [Google Scholar]
- Lubbers LS, Zafian PT, Gautreaux C, Gordon M, Alves SE, Correa L, Lorrain DS, Hickey GJ, Luine V, 2010. Estrogen receptor (ER) subtype agonists alter monoamine levels in the female rat brain. J Steroid Biochem Mol Biol 122, 310–317. [DOI] [PubMed] [Google Scholar]
- Luque JM, de Blas MR, Segovia S, Guillamon A, 1992. Sexual dimorphism of the dopamine-beta-hydroxylase-immunoreactive neurons in the rat locus ceruleus. Brain Res Dev Brain Res 67, 211–215. [DOI] [PubMed] [Google Scholar]
- Mansur SS, Terenzi MG, Neto JM, Faria MS, Paschoalini MA, 2010. Changes in food intake and anxiety-like behaviors after clonidine injected into the median raphe nucleus. Behav Brain Res 212, 71–77. [DOI] [PubMed] [Google Scholar]
- Mansur SS, Terenzi MG, Neto JM, Faria MS, Paschoalini MA, 2011. Phenylephrine into the median raphe nucleus evokes an anxiolytic-like effect in free-feeding rats but does not alter food intake in free feeding rats. Behav Brain Res 217, 209–214. [DOI] [PubMed] [Google Scholar]
- Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH, Weinshenker D, 2018. The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci Rep 8, 3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP, 2009. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci 29, 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Rabei R, Kaganovsky K, Caprioli D, Bossert JM, Bonci A, Shaham Y, 2014. A critical role of lateral hypothalamus in context-induced relapse to alcohol seeking after punishment-imposed abstinence. J Neurosci 34, 7447–7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margules DL, Olds J, 1962. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science 135, 374–375. [DOI] [PubMed] [Google Scholar]
- Mazzone CM, Pati D, Michaelides M, DiBerto J, Fox JH, Tipton G, Anderson C, Duffy K, McKlveen JM, Hardaway JA, Magness ST, Falls WA, Hammack SE, McElligott ZA, Hurd YL, Kash TL, 2018. Acute engagement of Gq-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Mol Psychiatry 23, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall NM, Sprow GM, Delpire E, Thiele TE, Kash TL, Pleil KE, 2013. Effects of sex and deletion of neuropeptide Y2 receptors from GABAergic neurons on affective and alcohol drinking behaviors in mice. Front Integr Neurosci 7, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinoff PB, 1984. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs 28Suppl 2, 1–15. [DOI] [PubMed] [Google Scholar]
- Montoya A, Bruins R, Katzman MA, Blier P, 2016. The noradrenergic paradox: implications in the management of depression and anxiety. Neuropsychiatr Dis Treat 12, 541–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Luhn KL, Kampov-Polevoy AB, Garbutt JC, Thiele TE, 2019. Bupropion, Alone and in Combination with Naltrexone, Blunts Binge-Like Ethanol Drinking and Intake Following Chronic Intermittent Access to Ethanol in Male C57BL/6J Mice. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Olney JJ, Burnham NW, Mazzone CM, Lowery-Gionta EG, Pleil KE, Kash TL, Thiele TE, 2016. Lateral Hypothalamus GABAergic Neurons Modulate Consummatory Behaviors Regardless of the Caloric Content or Biological Relevance of the Consumed Stimuli. Neuropsychopharmacology 41, 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA, 2004. NIAAA Council Approves Definition of Binge Drinking. National Institute of Health Winter edition. [Google Scholar]
- O’Neil ML, Beckwith LE, Kincaid CL, Rasmussen DD, 2013. The alpha1-adrenergic receptor antagonist, doxazosin, reduces alcohol drinking in alcohol-preferring (P) Rats. Alcohol Clin Exp Res 37, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE, 2015. Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity. Alcohol Clin Exp Res 39, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostock CY, Hallmark J, Palumbo N, Bhide N, Conti M, George JA, Bishop C, 2015. Modulation of L-DOPA’s antiparkinsonian and dyskinetic effects by alpha2-noradrenergic receptors within the locus coeruleus. Neuropharmacology 95, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp RS, Palkovits M, 2014. Brainstem projections of neurons located in various subdivisions of the dorsolateral hypothalamic area-an anterograde tract-tracing study. Front Neuroanat 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar OL, Belmer A, Holgate JY, Tarren JR, Shariff MR, Morgan M, Fogarty MJ, Bellingham MC, Bartlett SE, Klenowski PM, 2016. The antihypertensive drug pindolol attenuates long-term but not short-term binge-like ethanol consumption in mice. Addict Biol. [DOI] [PubMed] [Google Scholar]
- Piascik MT, Perez DM, 2001. Alpha1-adrenergic receptors: new insights and directions. J Pharmacol Exp Ther 298, 403–410. [PubMed] [Google Scholar]
- Pinos H, Collado P, Rodriguez-Zafra M, Rodriguez C, Segovia S, Guillamon A, 2001. The development of sex differences in the locus coeruleus of the rat. Brain Res Bull 56, 73–78. [DOI] [PubMed] [Google Scholar]
- Raper J, Morrison RD, Daniels JS, Howell L, Bachevalier J, Wichmann T, Galvan A, 2017. Metabolism and Distribution of Clozapine-N-oxide: Implications for Nonhuman Primate Chemogenetics. ACS Chem Neurosci 8, 1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander L, Malone J, Federoff D, Froehlich JC, 2014a. The alpha2-adrenergic receptor agonist, clonidine, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol 48, 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC, 2009. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res 33, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Beckwith LE, Kincaid CL, Froehlich JC, 2014b. Combining the alpha1 - adrenergic receptor antagonist, prazosin, with the beta-adrenergic receptor antagonist, propranolol, reduces alcohol drinking more effectively than either drug alone. Alcohol Clin Exp Res 38, 1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond DE Jr., Huang YH, Snyder DR, Maas JW, Baulu J, 1977. Hyperphagia and hyperdipsia after locus coeruleus lesions in the stumptailed monkey. Life Sci 20, 1619–1628. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J, 2009. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373, 2223–2233. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC, 2005. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84, 53–63. [DOI] [PubMed] [Google Scholar]
- Rinaman L, 2003. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci 23, 10084–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG, 1998. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am J Physiol 275, R262–268. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, Kash TL, Navarro M, Thiele TE, 2016. Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, Jensen P, 2016. Uncovering diversity in the development of central noradrenergic neurons and their efferents. Brain Res 1641, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SL, Dornellas APS, Burnham NW, Houck CA, Luhn KL, Bendrath SC, Companion MA, Brewton HW, Thomas RD, Navarro M, Thiele TE, 2020. Distinct and overlapping patterns of acute ethanol-induced c-Fos activation in two inbred replicate lines of mice selected for drinking to high blood ethanol concentrations. Brain Sci 10, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman CW, Derkach VA, Palmiter RD, 2016. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun 7, 11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roni MA, Rahman S, 2015. Effects of lobeline and reboxetine, fluoxetine, or bupropion combination on depression-like behaviors in mice. Pharmacol Biochem Behav 139, 1–6. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Winn P, Robbins TW, Deeley RJ, Everitt BJ, Dunn LT, Wallace M, James WP, 1983. Changes in body weight and food-related behaviour induced by destruction of the ventral or dorsal noradrenergic bundle in the rat. Neuroscience 10, 1405–1420. [DOI] [PubMed] [Google Scholar]
- Salinas AG, Nguyen CT, Ahmadi-Tehrani D, Morrisett RA, 2014. Reduced ethanol consumption and preference in cocaine- and amphetamine-regulated transcript (CART) knockout mice. Addict Biol 19, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM, 2005. Bcl-x is required for proper development of the mouse substantia nigra. J Neurosci 25, 6721–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, 1982. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 257, 275–325. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Cameron JL, Verbalis JG, Rinaman L, 1997. Cholecystokinin induces Fos expression in catecholaminergic neurons of the macaque monkey caudal medulla. Brain Res 770, 37–44. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Plummer NW, Chen YW, Alexander GM, Robertson SD, Dudek SM, McElligott ZA, Jensen P, 2016. Recombinase-Dependent Mouse Lines for Chemogenetic Activation of Genetically Defined Cell Types. Cell Rep 15, 2563–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmanoff MK, Pramik-Holdaway MJ, Weiner RI, 1976. Concentrations of dopamine and norepinephrine in discrete hypothalamic nuclei during the rat estrous cycle. Endocrinology 99, 326–329. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J, 1994. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res 61, 59–64. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M, 2009. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res 33, 255–263. [DOI] [PubMed] [Google Scholar]
- Sprow GM, Rinker JA, Lowery-Gointa EG, Sparrow AM, Navarro M, Thiele TE, 2016. Lateral hypothalamic melanocortin receptor signaling modulates binge-like ethanol drinking in C57BL/6J mice. Addict Biol 21, 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprow GM, Thiele TE, 2012. The neurobiology of binge-like ethanol drinking: evidence from rodent models. Physiol Behav 106, 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Van Swieten M, Basiri ML, Blair GA, Kantak P, Stuber GD, 2016. Lateral Hypothalamic Area Glutamatergic Neurons and Their Projections to the Lateral Habenula Regulate Feeding and Reward. J Neurosci 36, 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K, Montel H, Gayk W, Merker R, 1974. Comparison of the effects of clonidine on pre- and postsynaptic adrenoceptors in the rabbit pulmonary artery. Alpha-sympathomimetic inhibition of Neurogenic vasoconstriction. Naunyn Schmiedebergs Arch Pharmacol 285, 133–150. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL 2nd, 2014. “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci 68, 9 49 41–49 49 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, 2014. “Drinking in the dark” (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol 48, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, Sparta DR, Fee JR, Knapp DJ, Cubero I, 2003. Alcoholism and obesity: overlapping neuropeptide pathways? Neuropeptides 37, 321–337. [DOI] [PubMed] [Google Scholar]
- Thiele TE, van Dijk G, Bernstein IL, 1997. Ethanol-induced c-Fos expression in rat lines selected for low and high alcohol consumption. Brain Res 756, 278–282. [DOI] [PubMed] [Google Scholar]
- Torkaman-Boutorabi A, Hashemi-Hezaveh SM, Sheidadoust H, Zarrindast MR, 2014. The possible role of medial prefrontal cortex beta-1-adrenoceptors in morphine-induced amnesia. Pharmacology 93, 272–277. [DOI] [PubMed] [Google Scholar]
- Uemura N, Hisano S, Fukui Y, 1997. Induction of Fos-like immunoreactivity in the lower brainstem and the spinal cord of the rat by intraperitoneal administration of an endogenous satiety substance, 2-buten-4-olide. Neurosci Lett 227, 131–134. [DOI] [PubMed] [Google Scholar]
- Vardy E, Robinson JE, Li C, Olsen RHJ, DiBerto JF, Giguere PM, Sassano FM, Huang XP, Zhu H, Urban DJ, White KL, Rittiner JE, Crowley NA, Pleil KE, Mazzone CM, Mosier PD, Song J, Kash TL, Malanga CJ, Krashes MJ, Roth BL, 2015. A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron 86, 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vathy I, Etgen AM, 1988. Ovarian steroids and hypothalamic norepinephrine release: studies using in vivo brain microdialysis. Life Sci 43, 1493–1499. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Czachowski CL, 2015. Low-dose prazosin alone and in combination with propranolol or naltrexone: effects on ethanol and sucrose seeking and self-administration in the P rat. Psychopharmacology (Berl) 232, 2647–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL, 2012. Effects of prazosin, an alpha1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res 36, 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, Navarro M, Thiele TE, Pedersen C, Kampov-Polevoy A, Garbutt JC, 2020. A Preliminary, Open-Label Study of Naltrexone and Bupropion Combination Therapy for Treating Binge Drinking in Human Subjects. Alcohol Alcohol 55, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF, 2007. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129, 397–410. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I, Carey RJ, Nolley D, 1971. Ethanol drinking elicited during electrical stimulation of the lateral hypothalamus. Physiol Behav 7, 793–795. [DOI] [PubMed] [Google Scholar]
- West CH, Boss-Williams KA, Ritchie JC, Weiss JM, 2015. Locus coeruleus neuronal activity determines proclivity to consume alcohol in a selectively-bred line of rats that readily consumes alcohol. Alcohol 49, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


