Abstract
During myogenesis, proliferating myoblasts withdraw from the cell cycle, acquire an apoptosis-resistant phenotype, and differentiate into myotubes. Previous studies indicate that myogenic induction of the cyclin-dependent kinase inhibitor p21 results in an inhibition of apoptotic cell death in addition to its role as a negative cell cycle regulator. Here we demonstrate that the protein encoded by the Akt proto-oncogene is induced in C2C12 cells during myogenic differentiation with a corresponding increase in kinase activity. In differentiating cultures, expression of dominant-negative forms of Akt increase the frequency of cell death whereas expression of wild-type Akt protects against death, indicating that Akt is a positive modulator of myocyte survival. Antisense oligonucleotides against p21 block cell cycle withdrawal, inhibit Akt induction, and enhance cell death in differentiating myocyte cultures. Adenovirus-mediated transfer of wild-type or constitutively active Akt constructs confer partial resistance to cell death under conditions where cell cycle exit is blocked by the antisense oligonucleotides. Collectively, these data indicate that cell cycle withdrawal facilitates the induction of Akt during myogenesis, promoting myocyte survival.
During myogenesis, proliferating myoblasts irreversibly withdraw from the cell cycle and differentiate into myotubes. The cyclin-dependent kinase (CDK) inhibitor p21 and the retinoblastoma protein (pRb) appear to be critical in establishing the postmitotic state during myogenesis (55). p21 is markedly induced in differentiating C2C12 cells and in 10T1/2 fibroblasts that are induced to differentiate following transformation with MyoD (23, 24, 40, 42). Bromodeoxyuridine-labeling experiments have shown that upregulation of p21 correlates with the initiation of cell cycle exit, an early event in the myogenic differentiation pathway (4). Myocytes lacking pRb, a downstream target of CDK inhibitors, are incapable of irreversible cell cycle exit upon differentiation (41, 46). The transcription of muscle-specific genes can be inhibited by the forced expression of cyclins and CDKs, or E2F1, and this inhibition is largely reversed by the expression of constitutively active mutants of pRb (22). It is reported that the myocyte differentiation and cell cycle-regulatory functions of pRb and E2F1 require different domains within these proteins (22, 48).
A number of early studies described embryonic muscle precursor cells that undergo temporally regulated disintegration (reviewed in reference 21), a process that has more recently been referred to as programmed cell death or apoptosis. In previous studies, we found that a significant fraction of myoblasts undergo apoptosis during the differentiation of the C2C12 myogenic cell line, while differentiated C2C12 myotubes are relatively resistant to apoptosis (56, 57). Coimmunolocalization experiments with temporal markers of myogenesis revealed that acquisition of the apoptosis-resistant phenotype coincided with induction of the p21 CDK inhibitor but not with the appearance of myogenin, an earlier marker of myogenic differentiation (4). In addition, forced expression of the CDK inhibitors p21 or p16 blocked apoptosis during C2C12 differentiation (56, 57). The effects of CDK inhibitors on myocyte proliferation and survival are likely determined by their ability to modulate the state of pRb phosphorylation and cell growth. Consistent with this hypothesis, the CC42 pRb-deficient myogenic cell line undergoes a relatively high frequency of apoptosis during differentiation (56). These pRb−/− myocytes display a normal time course of p21 induction during differentiation, and forced expression of the p21 or p16 CDK inhibitors has no effect on the frequency of apoptosis. However, forced expression of pRb suppresses apoptosis in both pRb−/− and wild-type cell lines during differentiation. Consistent with these observations, transgenic mice expressing low levels of pRb display substantial cell death in muscle masses occurring prior to the onset of terminal differentiation (59). In these mice, surviving myocytes accumulate large polyploid nuclei, indicating a defect in the permanent withdrawal from the cell cycle. Collectively, these studies suggest that cell cycle activity markedly influences the susceptibility of differentiating myoblasts to apoptosis. However, the mechanisms by which perturbations in cell cycle activity induce apoptosis are essentially unknown for any cell type.
Akt is a proto-oncogene encoding a serine-threonine kinase whose amino terminus contains a pleckstrin homology (PH) domain (53). Various extracellular stimuli activate Akt through the phosphoinositide 3-kinase (PI 3-kinase) pathway (12, 20, 30). The lipid products of the PI 3-kinase reaction may activate Akt either by binding to the Akt PH domain (19, 33) or by activating a protein kinase that phosphorylates Akt (34, 52). Activation of Akt inhibits apoptosis induced by growth factor withdrawal or irradiation in neural cells, fibroblasts, and lymphocytes (11, 25). Recently, it has been shown that Akt phosphorylates the proapoptotic proteins Bad and caspase 9 leading to their inactivation and cell survival (8, 13, 43).
In the present study, we demonstrate that Akt protein and kinase activities are markedly upregulated during myogenic differentiation. We also show that myocytes die at a high frequency when cell cycle exit is blocked by treatment with antisense oligonucleotides to p21 mRNA. Under these conditions, endogenous Akt induction is suppressed, but forced Akt expression protects mitotic cells from death under conditions that promote myogenic differentiation. Thus, we propose that myocyte survival can be controlled through the ability of cell cycle activity to modulate Akt induction during myogenesis.
MATERIALS AND METHODS
Cell culture.
C2C12 myoblasts (American Type Culture Collection) were cultured as described elsewhere (4). Cells were maintained in growth medium (Dulbecco’s modified Eagle medium [DMEM] supplemented with 20% fetal bovine serum). To induce differentiation, cells at 40 to 50% confluence were shifted to differentiation medium (DMEM supplemented with 2% heat-inactivated horse serum). In some experiments, cells were switched to DMEM containing 0.5% heat-inactivated horse serum or no serum. Higher frequencies of cell death were achieved with the lower serum levels in the media.
Western immunoblot analysis.
Cells were washed with phosphate-buffered saline (PBS) twice on ice and harvested by scraping. Cell lysates were prepared in cell lysis buffer (1% Nonidet P-40, 10% glycerol, 137 mM NaCl, 20 mM Tris-HCl [pH 7.4], 20 mM NaF, 2 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride) by rotating for 15 min at 4°C, followed by centrifugation at 14,000 rpm for 10 min. Protein concentration was determined with a bicinchoninic acid kit (Pierce). Twenty micrograms of protein was separated on a sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Millipore). The membrane was blocked with T-PBS (1× PBS, 0.2% Tween) containing 5% dry milk and incubated with primary antibody. After three washes with T-PBS, the blots were incubated with secondary antibody (donkey anti-rabbit [Amersham] or donkey anti-goat [Santa Cruz Biotechnology] immunoglobulin G conjugated with horseradish peroxidase) and developed with the Amersham enhanced chemiluminescence detection system. Anti-Akt, anti-p21, anti-Cdc2, anti-Cdk4, and antimyogenin antibodies were purchased from Santa Cruz. Phospho-Akt-specific antibody was purchased from New England Biolabs, anti-β-galactosidase (β-Gal) antibody was from Sigma, and rhodamine-labeled antibody was from Pierce.
Akt half-life determinations.
C2C12 cultures were incubated in growth medium or shifted to differentiation medium for 2 days. At this time, cultures were exposed to 35.5 μM cycloheximide, and cells were harvested at 2, 4, or 6 h for Western immunoblot analysis as described above. Levels of Akt were analyzed relative to those of Cdc2, which is not degraded under these conditions (50). Akt and Cdc2 expression levels were determined by using Scanalytics software. N-acetyl-leucyl-leucyl-norleucinal (ALLN) was from Sigma.
In vitro kinase assay.
Akt kinase assays were performed as described previously (20), with minor modifications. Briefly, 100 μg of protein in 500 μl of the cell lysis buffer described above was preincubated with protein G-agarose for 30 min at 4°C. After centrifugation, anti-Akt or anti-hemagglutinin epitope (HA) antibody and protein G-agarose (Boehringer) were added to the supernatant in the presence of bovine serum albumin (2 mg/ml). Immunoprecipitation was performed at 4°C for 2 h. In control experiments, 8 μg of immunogenic Akt peptide was included in the immunoprecipitation reaction to determine the specificity of the H2B kinase reaction. After being washed twice with cell lysis buffer, once with H2O, and twice with kinase buffer (20 mM HEPES [pH 7.2], 10 mM MgCl2, 10 mM MnCl2), immunoprecipitates were incubated in 50 μl of kinase buffer containing 2 μg of histone H2B (Boehringer) and [γ-32P]ATP (5 μM, 10 μCi; Dupont) at room temperature for 30 min. Kinase reactions were terminated by adding SDS-sample solution. After electrophoresis on 15% polyacrylamide gels, gels were dried and exposed to X-ray film.
Northern blot analysis.
Northern blotting was performed as described elsewhere (2). In brief, total RNA was prepared with acid guanidium thiocyanate-phenol-chloroform method (10). Total RNA (25 μg) was separated in formaldehyde gel and transferred to Hybond N membrane (Amersham). After cross-linking by irradiation with UV light, the membrane was prehybridized in Rapid-hyb buffer (Amersham) for 2 h at 65°C, followed by hybridization overnight. The probe was a cDNA fragment corresponding to the PH domain of mouse Akt, which was radiolabeled with a random primer labeling kit (Multiprime; Amersham) and purified with a Nick column (Pharmacia Biotech). The membrane was washed twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) solution containing 0.1% SDS at room temperature for 10 min, twice 2× SSC solution containing 0.1% SDS at 50°C for 10 min, twice in 0.5× SSC solution containing 0.1% SDS at 65°C for 10 min, and twice in 0.2× SSC solution containing 0.1% SDS at 65°C for 10 min. The membrane was exposed to X-ray film at −70°C.
Antisense oligonucleotide experiments.
Antisense phosphorothioate oligonucleotides against p21 (5′-TGT CAG GCT GGT CTG CCT CC-3′) and the control (5′-TGG ATC CGA CAT GTC AGA-3′) were designed and were transfected into the cells with Lipofectin (Gibco) as described elsewhere (44). Cells were cultured overnight in growth media at a density of 3 × 105 cells in 100-mm-diameter dishes. Cells were washed in differentiation medium after the growth medium was removed and exposed to oligonucleotide solution. The oligonucleotide solution was prepared by incubating oligonucleotides with 75 μg of Lipofectin in 1.5 ml of OptiMEM for 15 min at room temperature. Then the oligonucleotide-Lipofectin mixture was diluted with differentiation medium and added to the cells. For cell survival analysis, cells were plated at a density of 104 cells per well in a 24-well plate. Treatment with oligonucleotides was performed as described above. Flow cytometry analyses were performed as described previously (56). In brief, C2C12 cells were cultured in differentiation medium with the antisense or control oligonucleotides mixed with Lipofectin, as described previously, for 24 h. Cells were washed with PBS and fixed with 70% ethanol. Samples were stained with propidium iodide. DNA content was measured using a FACScan flow cytometer (Becton Dickson) as described elsewhere (56).
Adenovirus vector construction and infection.
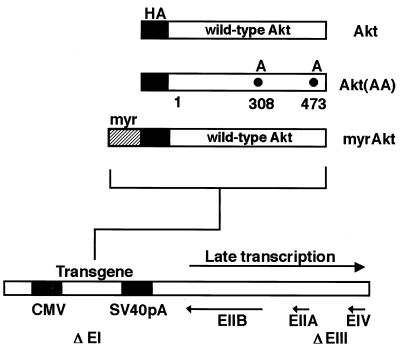
Replication-defective adenovirus vectors expressing mouse Akt proteins under the control of the cytomegalovirus promoter were constructed (Fig. 1). An HA tag was fused in frame to the N termini of the Akt coding sequences. The dominant-negative mutant Adeno-Akt(AA) expressing the mutant Akt [T308A, S473A] construct cannot be activated by phosphorylation (1). The constitutively active Akt construct (Adeno-myrAkt) has the c-Src myristoylation sequence fused in frame to the N terminus of the HA-Akt (wild-type) coding sequence. DNA fragments of each Akt fusion gene were inserted into the EcoRI/XbaI site of pACCMVpLpA, and the resulting plasmid was cotransfected with pJM17 into 293 cells to allow for homologous recombination (7). Recombinant adenovirus constructs expressing β-Gal (Adeno-βgal) and p27 (Adeno-p27) were described previously (9, 49). All constructs were amplified in 293 cells and purified by ultracentrifugation in the presence of CsCl. For transfection, C2C12 cells were incubated with the adenovirus at a multiplicity of infection (MOI) of 250 in growth medium for 15 h. Virus was removed when the medium was replaced. Under these conditions, transfection efficiency was greater than 90%.
FIG. 1.
Structures of replication-defective adenoviral vectors that express wild-type and mutated Akt. CMV, cytomegalovirus promoter/enhancer; SV40 pA, simian virus 40 polyadenylation site.
Plasmid transfection and cell viability analysis.
The empty expression vector (pcDNA3) or an expression vector encoding wild-type Akt tagged with HA or a dominant-negative form of Akt (Akt [K179M] or Akt [T308A, S473A]) was cotransfected with an expression vector encoding enhanced green fluorescent protein (GFP) (Clontech) at a ratio of 9:1 by the LipofectAmine method (Gibco) according to the manufacturer’s protocol. In brief, C2C12 cells were plated in 24-well plates overnight and incubated in a mixture of each individual plasmid (2 μg per well) and LipofectAmine (1:5 [wt/wt]) in growth medium for 6 h. After an 18-h incubation in growth medium, cells were changed to low-mitogen medium. After incubating for 24 h, cells were fixed with 3.7% formaldehyde and stained with Hoechst 33342. Floating and adherent GFP-positive cells with pyknotic or normal nuclei were determined by visual examination, and the fraction of the cells with pyknotic nuclei was calculated.
Alternatively, immunofluorescent microscopic analysis was performed as described by Andrés and Walsh (4). Briefly, cells were fixed with 3.7% formaldehyde in PBS for 20 min. Cells were washed with PBS twice and permeabilized with 0.2% Triton X-100 in PBS for 3 min. After three washes with PBS, cells were incubated with the first antibody in PBS containing bovine serum albumin (10 mg/ml) at 30°C for 1 h. After three more washes with PBS, cells were incubated with secondary antibody labeled with rhodamine at 37°C for 1 h. TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) staining was performed as specified by the manufacturer (Boehringer Mannheim). Briefly, cells were fixed with 3.7% formaldehyde in PBS for 20 min. Cells were washed with PBS twice and permeabilized with 0.2% Triton X-100 in PBS for 3 min. After three washes with PBS, cells were incubated in a solution of terminal deoxynucleotidyltransferase with nucleotide mixture at 37°C for 1 h.
Statistical analyses.
Data are presented as means ± standard errors of the means (SEM). Differences between data groups were assessed for statistical significance by using an unpaired t test.
RESULTS
Akt upregulation during muscle differentiation.
Changes in Akt protein expression during murine C2C12 myogenesis were examined by Western immunoblot analysis. C2C12 myoblasts cultured in high-mitogen growth medium expressed low levels of Akt protein, but Akt was induced following exposure of cultures to low-mitogen differentiation medium (Fig. 2A). Consistent with previous reports (23, 24, 40), the CDK inhibitor p21 was induced by differentiation, while the levels of Cdk4 did not change.
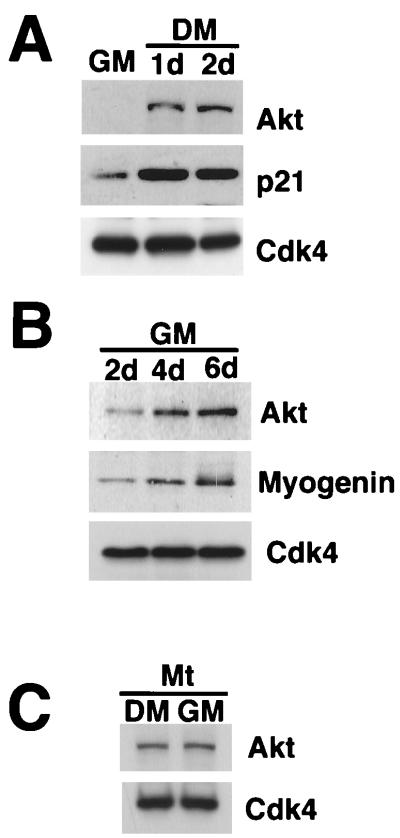
FIG. 2.
Akt is induced upon myocyte differentiation. (A) Akt protein upregulation during myogenesis induced by mitogen deprivation. C2C12 cells were cultured in growth medium (GM) or in differentiation medium (DM) for 24 h (1d) or for 48 h (2d). Cell lysates containing 20 μg protein were subjected to Western immunoblot analysis using anti-Akt, p21, and Cdk4 antibodies. (B) Akt protein upregulation induced by prolonged cultivation of confluent cultures in high-mitogen medium. C2C12 cells were cultured in growth medium (GM) for 2, 4, and 6 days. Cell lysates were prepared, and 20 μg of extract protein was immunoblotted with anti-Akt, myogenin, and Cdk4. (C) Akt protein expression is sustained in terminally differentiated cells following serum restimulation. C2C12 cells were induced to differentiate into myotubes by incubating cultures for 4 days in differentiation medium. These cultures were then cultured in fresh differentiation medium (DM) or growth medium (GM) for 24 h. Cell lysates (20 μg) were immunoblotted with anti-Akt and Cdk4 antibodies.
Experiments were performed to examine whether Akt induction was a consequence of myogenic induction or due solely to changes in the mitogen content of the media. Though high-mitogen growth medium inhibits myogenesis, a low rate of differentiation can be detected in highly confluent cultures of C2C12 cells (6, 58). Induction of Akt was also detected under these conditions, but it occurred more slowly than in differentiation medium (compare Fig. 2A and 2B). Furthermore, once Akt was expressed by C2C12 myotubes (derived from incubation in differentiation medium), restimulation with growth medium did not suppress Akt levels (Fig. 2C). Similar regulatory behavior has also been described for p21 and myogenin induction during C2C12 differentiation (4), and these data indicate that Akt induction is not a simple consequence of differences in the mitogen content of the media but a feature of irreversible myogenic differentiation.
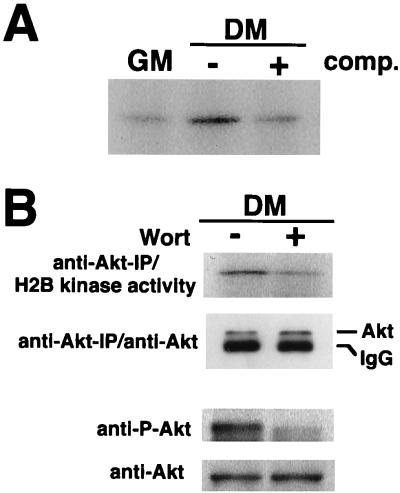
Akt activity was assessed by measuring H2B kinase activity in anti-Akt immunoprecipitates of extracts prepared from cultures exposed to growth and differentiation media (Fig. 3A). Akt activity was low in proliferating myoblasts but was induced in cultures exposed to low-mitogen differentiation media. Inclusion of immunogenic peptide during the Akt immunoprecipitation step reduced histone H2B kinase activity to background levels in extracts from differentiated cells, indicating the specificity of the assay. To elucidate the role of PI 3-kinase in Akt activation during myogenesis, differentiating cultures of C2C12 cells were incubated with wortmannin (Fig. 3B). Wortmannin suppressed Akt-associated histone H2B kinase activity. Wortmannin treatment also reduced the level of Akt phosphorylation, assessed with an antibody that is specific for Akt phosphorylation at residue 473 and indicative of the status of Akt activation (1).
FIG. 3.
Akt kinase activity in differentiating myocyte cultures is sensitive to inhibition by wortmannin. (A) Cell lysates were prepared from C2C12 cells cultured in growth medium (GM) or differentiation medium (DM) for 48 h. Kinase activity was determined in anti-Akt immunoprecipitates, using histone H2B as a substrate. Specificity of the kinase reaction was analyzed by inclusion of 8 μg of Akt peptide (comp.) in the immunoprecipitation reaction with the extract prepared from differentiating cells. (B) Akt activity in differentiating myocytes is inhibited by wortmannin. C2C12 cells were cultured in differentiation medium (DM) for 48 h. Wortmannin (Wort) was added to the medium at final concentration of 200 nM, and cells were harvested after 1 h of incubation. Dimethyl sulfoxide was used as a vehicle at final concentration 0.1%. In the upper panel, Akt kinase activity immunoprecipitated (IP) with anti-Akt antibody was analyzed with histone H2B as a substrate. Immunoblot analysis with anti-Akt was also performed on anti-Akt-immunoprecipitated materials. In the lower panel, cell lysates were immunoblotted with anti-Akt and anti-phospho-Akt (anti-P-Akt) antibodies. IgG, immunoglobulin G.
Northern blot analyses were performed to elucidate the mechanism of Akt upregulation. These assays revealed no significant changes in Akt mRNA levels between cultures of proliferative myoblast and differentiating cells (Fig. 4A). Akt mRNA was also not upregulated in MyoD-transformed 10T1/2 cells exposed to differentiation medium (data not shown). In contrast, the Akt-related gene Akt2 is upregulated by myogenic differentiation at the level of mRNA (2, 3). Upregulation of Akt2 protein was also detected in the differentiating C2C12 cultures, but in contrast to Akt, Akt2 upregulation appeared less pronounced and was not sustained at later time points (not shown). Thus, we focused on understanding the functional significance of Akt induction during myogenic differentiation.
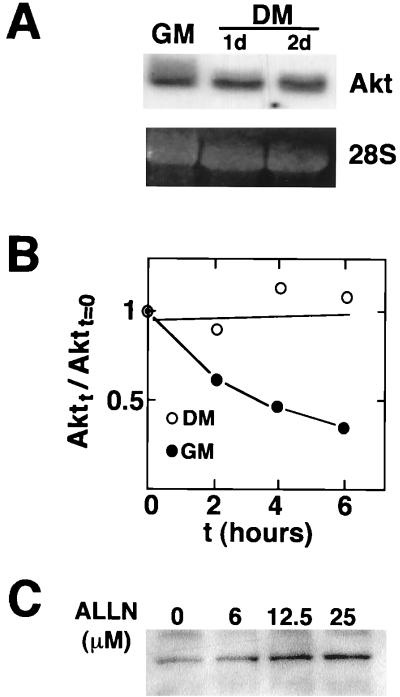
FIG. 4.
Myogenic induction of Akt occurs through protein stabilization. (A) Akt mRNA is not upregulated during myogenesis. Total RNAs were prepared from the C2C12 cells in growth medium (GM) or in differentiation medium (DM) for 24 h (1d) or for 48 h (2d). Northern blot analysis was performed with a cDNA probe to the PH domain of Akt. The 28S RNA band is shown to indicate equal loading of the gel. (B) Akt protein stability increases upon myogenic differentiation. Cycloheximide was added to cultures exposed to growth medium (GM) or differentiation medium (DM) (2 days), and Western immunoblot analysis was performed on Akt and Cdc2 at the indicated time points (t). Cdc2 levels do not change significantly under the conditions of this assay (50), and it is used to control for differences in sample loading. The ratio of Akt to Cdc2 signal in the absence of cycloheximide is assigned a value of 1.0. (C) Proteasome inhibitor ALLN promotes Akt protein expression. C2C12 cells were cultured with the indicated concentrations of ALLN in growth medium for 12 h. Dimethyl sulfoxide was used as a vehicle at a final concentration of 0.1%. Cell lysates were immunoblotted with anti-Akt antibody.
To further delineate the mechanism of Akt upregulation, Akt protein half-life was examined in cultures exposed to cycloheximide in growth medium or differentiation medium (Fig. 4B). Akt protein levels remained stable in cultures exposed to differentiation medium over the 6-h time course of the assay, while Akt half-life was approximately 3 h in the cultures exposed to high-mitogen growth medium. Thus, an increase in the stability of the Akt peptide appears to contribute to the induction of Akt during myogenic differentiation. To determine whether the short half-life of Akt in myoblast is dependent on proteasome activity, cultures were incubated with the proteasome inhibitor ALLN (47, 50). Incubation with ALLN led to a dose-dependent accumulation of Akt (Fig. 4C), suggesting that Akt turnover is dependent on the action of the 26S proteasome.
Akt promotes myocyte viability.
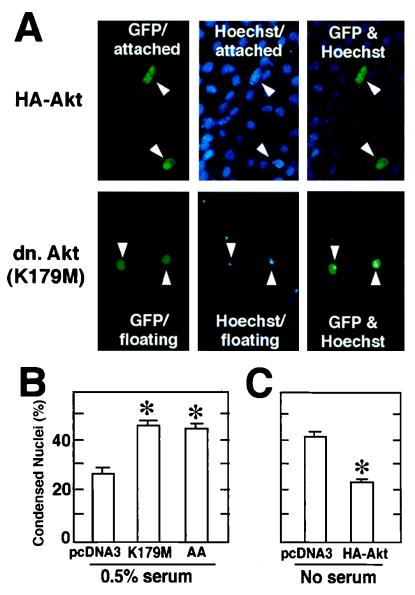
Plasmid vectors expressing wild-type and dominant-negative forms of Akt were used to assess the role of Akt in myocyte survival during differentiation. Akt expression plasmids were initially cotransfected with a plasmid expressing GFP into C2C12 cells in growth medium, and cultures were shifted to medium containing 0.5% horse serum or no serum to induce differentiation. In these assays, serum levels were lowered to enhance the rate of differentiation and frequency of apoptosis. Apoptosis was assessed in the differentiating cultures by analyzing the fraction of floating and adherent GFP-positive cells with condensed chromatin following staining with Hoechst 33342 (15) (Fig. 5A). Most cells with condensed chromatin were floating, but adherent cells with condensed chromatin were also noted. For quantitative analyses, all GFP-positive cells, both floating and attached, were analyzed for each experimental condition.
FIG. 5.
Effects of expression of wild-type or mutant Akt on the frequency of myocytes displaying nuclear condensation during differentiation. Differentiating C2C12 cells were cotransfected with plasmids encoding dominant-negative (dn.) or wild-type Akt along with a plasmid encoding GFP as a transfection marker at a ratio of 9:1. (A) Representative photomicrographs showing adherent (attached) and floating cells in myocyte cultures transfected with the GFP expression plasmid and plasmids encoding wild-type Akt or the K179M Akt mutant. Note the condensed Hoechst 33342-stained nuclei that correspond to the floating GFP-positive cells in cultures transfected with K179M Akt. In contrast, cultures cotransfected with an expression plasmid encoding wild-type Akt revealed a high frequency of adherent GFP-positive cells with normal-appearing nuclei. (B) Dominant-negative Akt expression plasmids promote myocyte apoptosis during differentiation. Cells were cotransfected with empty vector (pcDNA3) or plasmids expressing the dominant-negative forms of Akt, using the LipofectAmine method. After transfection, cells were incubated in growth medium for 18 h and then in DMEM containing 0.5% horse serum. Low concentrations of serum were used for these assays to increase the frequency of apoptosis. After 24 h of incubation, cells were fixed and stained with Hoechst 33342 as described in Materials and Methods. The adherent and floating transfected GFP-positive cells were scored for normal or pyknotic nuclei. Data are shown as mean ± SEM (∗, P < 0.01). (C) Wild-type Akt promotes myocyte survival. Cells were cotransfected with the indicated expression plasmids and the GFP expression plasmid. After transfection and incubation in growth medium for 18 h, the medium was changed to DMEM without serum. Serum-free medium was used to maximize the number of cells undergoing apoptosis, such that the protective effects of wild-type Akt could be determined more accurately. After 24 h of incubation, the transfectants were scored as described for panel B. Data are shown as mean ± SEM (∗, P < 0.01).
The mutant Akt [K179M] has a mutation in the ATP-binding site and is catalytically inactive (5), while the mutant Akt [T308A, S473A] has alanine substituted for serine/threonine at the indicated residues and cannot be activated by phosphorylation (1). Cotransfection with either dominant-negative Akt construct markedly increased the fraction of GFP-positive cells that were floating and displayed chromatin condensation when differentiating cultures were incubated in 0.5% horse serum (Fig. 5B). In contrast, forced expression of wild-type Akt in the cotransfection assay reduced the fraction of floating GFP-positive cells with condensed chromatin when differentiating cultures were incubated in serum-free medium (Fig. 5C). Collectively, these data suggest that Akt upregulation is essential for full myocyte viability during serum deprivation-induced myocyte differentiation and that elevated Akt levels are sufficient to confer partial resistance to apoptosis under these conditions.
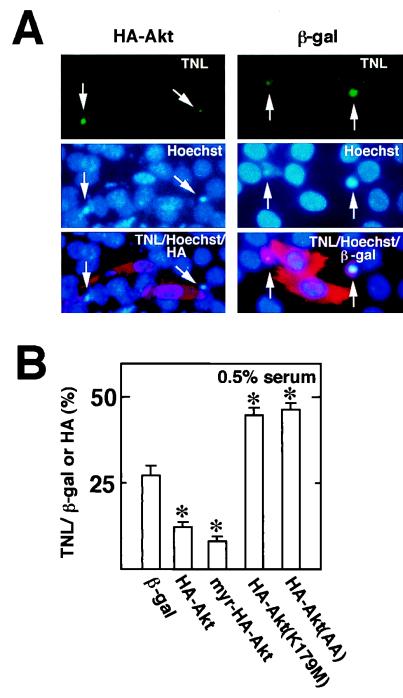
To further document the effect of Akt on cell survival, cultures were transfected with plasmids expressing HA-tagged, wild-type, constitutively active, or dominant-negative Akt constructs, and frequencies of apoptosis were assessed by comparing the percentages of HA-positive cells that were positive for TUNEL staining. TUNEL-positive cells typically displayed chromatin condensation in the Hoechst stain (Fig. 6A). Cells positive for plasmid-encoded wild-type or constitutively active Akt displayed a reduced frequency of TUNEL-positive nuclei than the control plasmid expressing lacZ (Fig. 6B). In contrast, cells positive for expression of either dominant-negative Akt construct displayed higher frequencies of TUNEL-positive nuclei. In these assays, the effects of active and inactive Akt constructs were compared in parallel and under identical medium conditions, demonstrating that Akt can be sufficient and essential for myocyte survival independent of culture conditions.
FIG. 6.
Effects of expression of wild-type or mutant Akt on the frequency of TUNEL-positive cells during myogenic differentiation. Differentiating myocytes were transfected with plasmids encoding wild-type, constitutively active, or dominant-negative Akt tagged with HA. The plasmid encoding β-Gal (β-gal) was used as a control. After transfection, cells were incubated in growth medium for 18 h and then in DMEM containing 0.5% horse serum for 24 h. Transfected cells were identified by immunostaining with anti-HA or β-Gal antibodies. Apoptotic cells were detected by TUNEL (TNL). (A) Representative photomicrographs showing TUNEL-positive cells (arrows). Cells were treated as described above and stained by TUNEL (TNL; green), Hoechst 33342 (Hoechst; blue), and anti-HA or β-Gal antibody (HA or β-gal; red). (B) Percentage of cells that stained positive for plasmid-encoded transgene expression. For each condition, four separate cultures were transfected with the indicated plasmid, and at least 50 HA- or β-Gal-positive cells were counted on each plate. Data are shown as mean ± SEM.
p21-mediated cell cycle withdrawal is coordinated with Akt induction and cell survival.
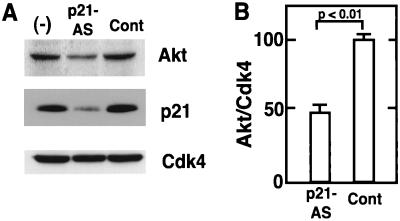
To investigate the relationship between p21 and Akt in myocytes, we investigated the effects of p21-antisense oligonucleotides on Akt induction, cell cycle, and apoptosis. C2C12 cultures were treated with p21-antisense or control oligonucleotides and induced to differentiate. Western immunoblot analysis of cultures harvested after 24 h of treatment revealed that the p21-antisense oligonucleotides markedly inhibited the induction of p21 (Fig. 7A). The p21-antisense oligonucleotides had no effect on Cdk4 levels, but there was a reproducible reduction in Akt expression. A statistically significant reduction in Akt expression, normalized to Cdk4 expression, was noted in multiple experiments (Fig. 7B).
FIG. 7.
Antisense oligonucleotides against p21 inhibit the induction of p21 and Akt during myogenic differentiation. (A) C2C12 cells were cultured in standard differentiation medium (−) or in differentiation medium containing Lipofectin with 0.5 μM p21-antisense (p21-AS) or control (Cont) oligonucleotides for 24 h. After 24 h of incubation, cells were harvested and cell lysates (20 μg protein) were immunoblotted with anti-Akt, anti-p21, and anti-Cdk4 antibodies. Representative data from one of five assays are shown. (B) Quantitative analysis of reduced Akt expression. The intensities of the Akt and Cdk4 bands in immunoblots from five independent experiments were quantified by gel densitometry. The ratios of Akt band intensity relative to Cdk4 band intensity were calculated and normalized to the ratios of band intensities in parallel cultures that were incubated in differentiation medium with no oligonucleotide or Lipofectin. Data are shown as mean ± SEM.
Flow cytometric analyses were performed to determine the effect of the p21-antisense oligonucleotides on cell cycle progression during differentiation. As shown in Table 1, cultures treated with p21-antisense oligonucleotides for 24 h had a greater percentage of cells in the S and G2/M phases of the cell cycle than cultures treated with control oligonucleotides or with differentiation medium alone. These data provide causal evidence that p21 induction mediates cell cycle exit during myogenic differentiation.
TABLE 1.
Antisense oligonucleotides against p21 mRNA block cell cycle exit upon myogenic differentiation
C2C12 cells were cultured in differentiation medium in the presence or absence (none) of 0.5 μM phosphorothioate antisense oligonucleotides to p21 (p21-AS) or control oligonucleotides for 24 h.
Determined by fluorescence-activated cell sorting analysis.
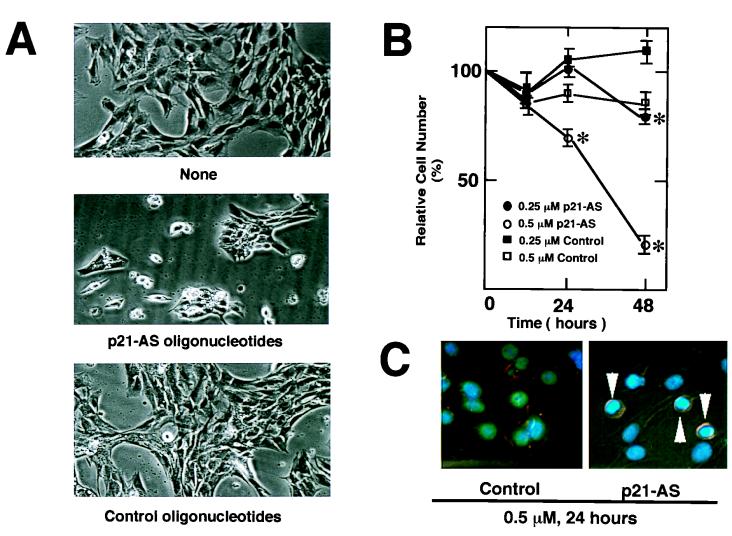
Previously, it has been shown that induction of p21 coincides with the acquisition of an apoptosis-resistant phenotype and that forced expression of p21 can block apoptosis in differentiating cultures of C2C12 myocytes (56, 57). Thus, we analyzed the effects of the p21-antisense oligonucleotides on myocyte survival. As shown in Fig. 8A, markedly enhanced cell death was observed in differentiating cultures treated with p21-antisense oligonucleotides than in control cultures. The frequency of cell death was dependent on the dose of antisense oligonucleotides, and cell death was most notable between 24 and 48 h in differentiation medium (Fig. 8B). Cultures treated with p21-antisense oligonucleotide contained high frequencies of mononuclear cells displaying cytoplasmic shrinkage and chromatin condensation (Fig. 8C), indicators of apoptotic death. These data suggest that cell cycle withdrawal is essential for the suppression of apoptotic cell death during myogenesis.
FIG. 8.
Antisense oligonucleotides against p21 promote cell death during differentiation. The cells were plated and transfected with the oligonucleotides. C2C12 cells were cultured in standard differentiation medium or in differentiation medium containing Lipofectin with the indicated oligonucleotides for 24 or 48 h. (A) Representative light photomicrographs of cultures exposed to differentiation medium in the absence (none) or presence of 0.5 μM p21-antisense (p21-AS) or control oligonucleotides for 48 h were obtained by a Nikon Diaphot light microscope at a magnification of ×100. (B) Cultures were washed with PBS and harvested by trypsinization. Cell number was determined with a hemacytometer. Data are shown as mean ± SEM of four parallel cultures that were treated according to the indicated conditions (∗, P < 0.01). (C) Representative photomicrographs showing Hoechst 33342-staining patterns of differentiating myocyte cultures exposed to 0.5 μM control or p21-antisense oligonucleotides for 24 h in medium containing 2% horse serum. Note the condensation of chromatin in cells exposed to p21-antisense oligonucleotides (arrowheads).
Adenovirus-mediated Akt gene transfer can protect mitotic cells from death during myogenic differentiation.
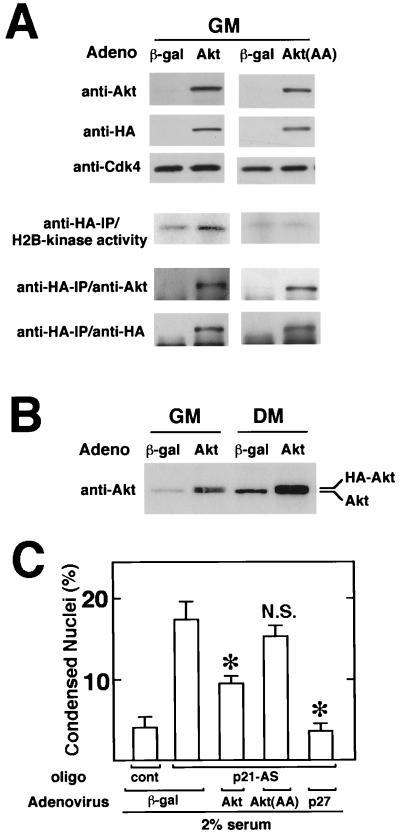
To examine the functional significance of suppressed Akt expression in p21-deficient myocytes, p21-antisense oligonucleotide-treated cultures were infected with replication-defective adenoviral vectors expressing wild-type Akt (Adeno-Akt) or dominant-negative Akt(AA) [Adeno-Akt(AA)], and the frequency of cell death during differentiation was assessed. These vectors express the Akt transgene fused to HA to distinguish virus-encoded and endogenous proteins (Fig. 1). Adenovirus transduction efficiencies in C2C12 cell culture exceeds 90% under the conditions of these assays. Myoblast cultures infected with Adeno-Akt or Adeno-Akt(AA) express transgene proteins, as detected by Western immunoblot analysis using either anti-Akt or anti-HA antibodies (Fig. 9A). H2B kinase activity was detected in anti-HA immunoprecipitates of myoblasts infected with Adeno-Akt but not Adeno-Akt(AA). Immunoprecipitation-coupled immunoblot analyses with anti-HA or anti-Akt antibodies confirmed the presence of transgene-encoded Akt proteins in the anti-HA immunoprecipitates. Collectively, these assays show that Adeno-Akt encodes a functional protein kinase, while Adeno-Akt(AA) encodes a kinase-deficient protein.
FIG. 9.
Adenovirus-mediated Akt gene transfer partially inhibits apoptosis in differentiating myocytes following p21 ablation. (A) Adeno-Akt expresses functional Akt, but the Adeno-Akt(AA)-encoded protein is kinase deficient. C2C12 cells were infected in growth medium (GM) with the adenoviral vectors expressing β-Gal or HA-tagged Akt or Akt(AA) at an MOI of 250 for 24 h prior to harvest. Cell lysates (10 μg of protein) were subjected to Western immunoblot analysis using anti-Akt, anti-HA, or anti-Cdk4 antibody. Histone H2B kinase activity in anti-HA immunoprecipitates (IP) was determined as described in Materials and Methods. Immunoblot analysis, with anti-Akt or anti-HA antibody was performed on the anti-HA-immunoprecipitated material to confirm the presence of transgene-encoded protein. (B) Akt overexpression following infection with Adeno-Akt. C2C12 cultures in growth medium were infected in parallel with Adeno-Akt or Adeno-βgal at an MOI of 250. After 15 h, the media was replaced with fresh growth medium (GM) or differentiation medium (DM) containing 2% horse serum for 24 h. Cell lysates (20 μg) were then analyzed by Western immunoblotting using anti-Akt antibody. (C) Akt expression partially inhibits p21-antisense-induced cell death. C2C12 cells were cultured in growth medium and infected with Adeno-Akt, Adeno-Akt(AA), Adeno-p27, or Adeno-βgal at an MOI of 250 for 15 h. Cultures were then transferred to 2% horse serum differentiation medium in the presence of 0.5 μM p21-antisense (p21-AS) or control (cont) oligonucleotide for 24 h. Frequency of apoptosis was determined by staining with Hoechst 33342 and scoring for nuclei with normal or condensed chromatin. Data are shown as mean ± SEM (n = 4). (∗, P < 0.01; NS, nonsignificant compared to Adeno-βgal-treated cultures exposed to p21-antisense oligonucleotides).
Western immunoblot analysis with anti-Akt antibodies revealed that the level of Akt overexpression resulting from Adeno-Akt infection was fivefold in myoblast cultures and threefold in cultures exposed to differentiation media (Fig. 9B). In these experiments, we noted that virus-encoded Akt protein, expressed from a cassette containing heterologous 5′ and 3′ noncoding sequences, was also induced upon exposure to differentiation medium. These data further suggest that myogenic differentiation increases the stability of the Akt peptide, and they are consistent with the results of protein half-life and proteasome inhibition experiments reported above (Fig. 4B and C).
To test whether Adeno-Akt can protect against p21-antisense-induced death, myocyte cultures were infected with adenoviral constructs and exposed to differentiation medium containing 2% horse serum and 0.5 μM p21-antisense oligonucleotides for 24 h (Fig. 9C). Control cultures were infected with an adenoviral vector expressing β-galactosidase (Adeno-βgal), which does not affect C2C12 cell viability (56). Treatment of control cultures with the p21-antisense oligonucleotide increased the fraction of cells undergoing apoptosis by a factor of 4, as assessed by analyses of chromatin condensation. Infection with Adeno-Akt significantly reduced the frequency of apoptosis in differentiating cultures exposed to p21-antisense oligonucleotides, suggesting that Akt can protect mitotic cells against death under conditions that promote differentiation. Of note, the adenoviral construct expressing the Akt(AA) mutant, which enhances death in the absence of p21-antisense oligonucleotides (Fig. 5B and 6B), did not cause further death in the p21-deficient cells (Fig. 9C). Since p21 ablation and Akt(AA) do not have an additive effect on cell death, these results suggest that Akt participates in the antiapoptotic signaling pathway that is activated upon cell cycle withdrawal.
Adenovirus-mediated overexpression of the CDK inhibitor p27 (Adeno-p27) almost completely reversed the apoptosis induced by a p21-antisense oligonucleotide (Fig. 9C). These data further suggest that the cell death induced by the p21-antisense oligonucleotides is a consequence of its effect on cell cycle activity and not due to nonspecific cytotoxicity by this agent. Infection with Adeno-p27 also abrogated Akt downregulation by p21-antisense oligonucleotides (data not shown), consistent with the hypothesis that Akt expression is negatively regulated by cell cycle activity.
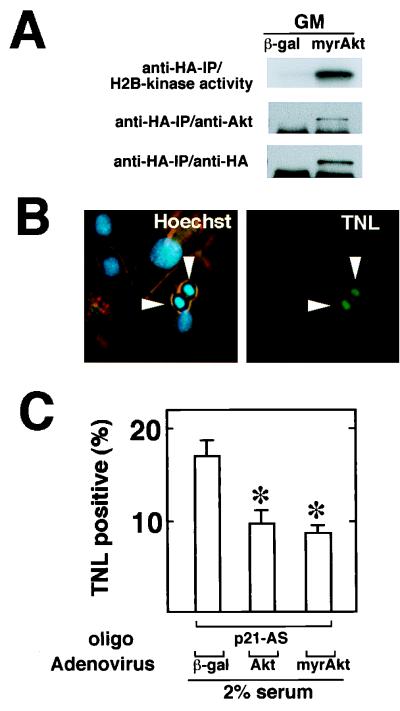
The experiments described above demonstrate that adenovirus-encoded p27 more effectively suppressed cell death induced by the p21-antisense oligonucleotides than adenovirus-encoded wild-type Akt (Fig. 9C) despite its ability to overexpress Akt between three- and fivefold (Fig. 9B). These data suggested that either upstream signaling pathways may become limiting with respect to Akt activation (particularly in the low-mitogen medium) or other cell cycle-dependent survival pathways independent of Akt may be required for full myocyte viability. To address this issue further, an adenoviral vector expressing constitutively active Akt (Adeno-myrAkt) was constructed (Fig. 1). Adeno-myrAkt expressed similar levels of HA-tagged recombinant protein as the other adenoviral Akt vectors (Fig. 10A). However, in marked contrast to wild-type Akt, the protein encoded by Adeno-myrAkt displayed high levels of activity in the absence of mitogen stimulation (not shown). We calculated 3- and 15-fold higher levels of Akt-associated H2B kinase activity in Adeno-myrAkt-infected cultures of differentiating C2C12 cells relative to the activities seen following infection with Adeno-Akt or the endogenous activity, respectively. Differentiating cultures of C2C12 cells infected with Adeno-myrAkt also displayed lower frequencies of apoptosis, as assessed by TUNEL staining for DNA fragmentation or Hoechst staining for chromatin condensation (Fig. 10B and C). However, infection with Adeno-myrAkt did not provide significantly more protection from cell death than infection with Adeno-Akt.
FIG. 10.
Constitutively active Akt partially inhibits apoptosis in differentiating myocytes following p21 ablation. (A) Adeno-myrAkt expresses functional Akt. C2C12 cells were infected in growth medium (GM) with the adenoviral vectors expression β-Gal or HA-tagged myrAkt at an MOI of 250 for 24 h prior to harvest. Histone H2B kinase activity in anti-HA immunoprecipitates (IP) was determined as described in Materials and Methods, and immunoblot analysis with anti-Akt and anti-HA antibodies was performed on the anti-HA-immunoprecipitated material to confirm the presence of transgene-encoded protein. (B) Analysis of apoptosis by TUNEL staining (TNL) in differentiating cultures of C2C12 cells following infection with adenoviral vectors. Note that many TUNEL-positive cells also display chromatin condensation when stained with Hoechst 33342. (C) Akt expression partially inhibits p21-antisense-induced cell death. C2C12 cells were cultured in growth media and infected with Adeno-myrAkt, Adeno-Akt, or Adeno-βgal at an MOI of 250 for 15 h. Cultures were then transferred to 2% horse serum differentiation medium in the presence of 0.5 μM p21-antisense (p21-AS) or control oligonucleotide for 24 h. Frequency of apoptosis was determined by scoring for nuclei that were TUNEL positive. Data are shown as mean ± SEM (n = 4). (∗, P < 0.01 compared to Adeno-βgal-infected cultures exposed to p21-antisense oligonucleotides.)
DISCUSSION
In many systems, aberrant cell cycle activity can induce apoptosis, and cell cycle activity can markedly influence the susceptibility of cells to apoptosis as induced by a variety of stimuli (29). However, the mechanisms by which cells sense perturbations in cell cycle activity and induce apoptosis have not been defined. Myogenic precursor cells irreversibly withdraw from the cell cycle as they differentiate into multinucleated myotubes. As differentiation progresses, myogenic cells display a reduced propensity to undergo apoptotic cell death in low-mitogen differentiation media (56, 57), possibly through induction of a survival factor. In this study, we have defined one component of the mechanism by which proliferation and apoptosis are coordinated during myogenic differentiation in vitro. We show that kinase Akt is induced during myogenic differentiation, with consequences on myocyte survival. The functional significance of Akt induction in differentiating cultures is indicated by the observations that dominant-negative Akt mutants promote apoptotic cell death while apoptosis is suppressed by the forced expression of wild-type or constitutively active Akt. Constitutively active Akt suppressed apoptosis by 83% relative to cells transfected with dominant-negative Akt (Fig. 6), indicating the importance of Akt as a regulator of cellular survival during myogenic differentiation. In addition to its protective effects, myogenic activation of Akt may also facilitate the differentiation process (28).
Previously, phosphorylation of Akt has been shown to mediate the antiapoptotic activity of mitogen survival factors in neuronal cells, lymphocytes, fibroblasts, and other cell types (11). Here, it is shown that developmental regulatory signals can also control Akt expression through stabilization of the Akt peptide in postmitotic myocytes. Protein stabilization may represent a common mechanism through which cell cycle activity regulates the expression of key myogenic factors. Similarly to Akt, MyoD has recently been shown to have a short half-life in proliferating myoblasts, but it is stabilized by cell cycle exit during differentiation (32, 50). Stability of the Myf-5 protein, another myogenic factor, is also governed by cell cycle activity in myocytes (38).
In many cell types it is established that Akt activity is regulated by mitogen-dependent signals via the PI 3-kinase pathway (18, 34, 52). As shown here, the PI 3-kinase inhibitor wortmannin also inhibits Akt activity in differentiating myocyte cultures. Though differentiation is typically induced by incubating proliferative myoblast cultures with low-mitogen medium, differentiating myocytes produce insulin-like growth factor I (IGF-I) and IGF-II, activators of the PI 3-kinase pathway (16, 17, 35, 45, 54). Thus, in addition to Akt protein induction, autocrine stimulation by factors that activate PI 3-kinase signaling may be required to complete a regulatory circuit that favors myocyte survival. Consistent with this hypothesis, IGF-II has been reported to function as an autocrine survival factor during myogenic differentiation (51).
Multiple lines of correlative evidence suggest that CDK inhibitor induction initiates cell cycle withdrawal during myogenic differentiation and that cell cycle exit promotes myocyte survival (reviewed in reference 55). Here it is shown that ablation of p21 induction with antisense oligonucleotides blocks the cell cycle exit that usually occurs early in myogenic differentiation, providing causal evidence that this CDK inhibitor is critical for the establishment of the postmitotic state in vitro. Cultures treated with p21-antisense oligonucleotides also exhibited high rates of apoptotic cell death that was particularly notable at later time points during the differentiation time course. Conversely, cell cycle inhibition by the forced expression of pRB, p16, or p21 suppresses apoptosis during myogenesis (56, 57). These data provide strong evidence that apoptosis and mitotic activity are coupled during myogenic differentiation in vitro. Similar regulatory pathways may also be operational during myogenesis in vivo, as mice expressing reduced levels of pRb contain muscle groups that undergo a high frequency of apoptosis at an early stage in the differentiation process and display cell cycle defects (59). On the other hand, mice lacking individual CDK inhibitors develop normally, with no obvious muscle defects. Presumably, CDK inhibitors are functionally redundant during embryonic development (60). Consistent with this hypothesis, we show here that forced expression of p27 can compensate for p21 in suppressing apoptosis when p21 is ablated by the antisense oligonucleotide.
To elucidate the relationship between cell cycle withdrawal and Akt-mediated cell survival, Akt levels were assessed in differentiating myocytes blocked from cell cycle withdrawal by treatment with p21-antisense oligonucleotides. Akt levels are suppressed in the p21-deficient cells, indicating a functional interaction between cell cycle progression and Akt expression during myocyte differentiation. Akt gene transfer experiments were performed on these p21-deficient cells to determine the functional significance of Akt suppression by mitotic activity. Adenovirus-mediated Akt gene transfer reduced the high frequency of apoptosis induced by p21-antisense oligonucleotides, suggesting that elevated Akt expression can partially compensate for the deficiency arising from the absence of efficient cell cycle exit. In contrast, adenovirus-mediated expression of a dominant-negative Akt did not increase the frequency of cell death above what occurs when differentiating cells are exposed to p21-antisense oligonucleotides alone. Since these agents are not additive, these data suggest that Akt functions in the antiapoptotic pathway induced by cell cycle exit.
Although Akt appears to function downstream from cell cycle exit to protect against death during myogenesis, forced expression of Akt cannot fully suppress apoptosis in mitotic myocytes. In contrast, adenovirus-mediated overexpression of p27 was much more effective at inhibiting apoptosis caused by the p21 deficiency. The lack of complete protection by Akt overexpression could result from other components of the Akt pathway that become limiting under these conditions. To test whether activation of Akt is limiting in mitotic cells during myogenic differentiation, cultures were infected with an adenoviral construct expressing constitutively active Akt. No enhancement of cell survival was observed despite a 15-fold increase in overall Akt activity relative to endogenous levels. These data suggest that other cell cycle-regulated pathways may work in conjunction with Akt to ensure myocyte survival during differentiation. Consistent with this hypothesis, it has recently been shown that IGF-I promotes survival of Rat-1 cells through Akt-dependent and -independent pathways (37). The identity of this alternative survival pathway is unknown, but it could involve Akt2, a homologue of Akt that displays a highly tissue-specific pattern of expression and is expressed in muscle (2). The apoptosis-regulatory properties of Akt2 are unknown at this time. Alternatively, mechanisms that involve the direct association of cell cycle proteins with apoptosis-regulatory factors may exist, as has recently been described for pRb, MDM2, and p53 (27). Of note, it has been shown that Bcl-2 can impart a selective advantage to proliferative myoblasts, leading to their clonal expansion (14), although levels of Bcl-2 are downregulated at middle or late stages of myogenesis when cell cycle exit and Akt protein stabilization occur.
Here we document an upregulation of Akt in a developmental system with consequences on apoptosis. While it is well established that cell cycle exit is required for the activation of contractile protein genes during myogenesis, we propose that cell cycle exit is required for efficient induction of Akt and cell survival. The experimental observations described here provide a foundation for understanding the mechanisms that coordinate apoptosis and cell cycle activity during myogenesis and may broadly relate to other systems where proliferating cells undergo apoptosis. Links between cell cycle and apoptosis have also been observed in differentiating neuronal cells, which undergo apoptosis at a high frequency when exposed to antisense oligonucleotides to p21 (44) and are protected from death by the overexpression of the CDK inhibitor p16 (36). Mesangial cells deficient in p27 also undergo elevated rates of apoptosis and viability can be restored by cell cycle exit (26). Finally, it has been shown that terminally differentiated cardiomyocytes express lower levels of tissue-specific proteins and undergo apoptosis in response to cell cycle progression induced by forced expression of E2F1 or E1a (31, 39). Thus, in several differentiation systems, cell cycle activity may suppress antiapoptotic genes, such as Akt, required for survival as cellular differentiation progresses.
ACKNOWLEDGMENTS
This work was supported by NIH grants HD23681, HL50692, and AG15052 to K.W.
We thank Roy C. Smith for carefully reading the manuscript. We thank Linda Whittaker for preparing the manuscript.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Altomare D A, Guo K, Cheng J Q, Sonoda G, Walsh K, Testa J R. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene. 1995;11:1055–1060. [PubMed] [Google Scholar]
- 3.Altomare D A, Lyons G E, Mitsuuchi Y, Cheng J Q, Testa J R. Akt2 mRNA is highly expressed in embryonic brown fat and AKT2 kinase is activated by insulin. Oncogene. 1998;16:2407–2411. doi: 10.1038/sj.onc.1201750. [DOI] [PubMed] [Google Scholar]
- 4.Andrés V, Walsh K. Myogenin expression, cell cycle withdrawal and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellacosa A, Franke T F, Gonzalez-Portal M E, Datta K, Taguchi T, Gardner J, Cheng J Q, Testa J R, Tsichlis P N. Structure, expression and chromosomal mapping of c-akt: relationship to v-akt and its implications. Oncogene. 1993;8:745–754. [PubMed] [Google Scholar]
- 6.Bennett A M, Tonks N K. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- 7.Bett A J, Haddara W, Prevec L, Graham F L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 9.Chen D, Krasinski K, Chen D, Sylvester A, Chen J, Nisen P D, Andrés V. Down-regulation of cyclin-dependent kinase activity and cyclin A promoter activity in vascular smooth muscle cells by p27 (KIP-1), an inhibitor of neointima formation in the rat carotid artery. J Clin Investig. 1997;99:2334–2341. doi: 10.1172/JCI119414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Coffer P J, Jin J, Woodgett J R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta K, Bellacosa A, Chan T O, Tsichlis P N. Akt is a direct target of the phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:30835–30839. doi: 10.1074/jbc.271.48.30835. [DOI] [PubMed] [Google Scholar]
- 13.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 14.Dominov J A, Dunn J J, Miller J B. Bcl-2 expression identifies an early stage of myogenesis and promotes clonal expansion of muscle cells. J Cell Biol. 1998;142:537–544. doi: 10.1083/jcb.142.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 16.Florini J R, Ewton E Z, Magri K A. Hormones, growth factors and myogenic differentiation. Annu Rev Physiol. 1991;53:201–216. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- 17.Florini J R, Magri K A, Ewton D Z, James P L, Grindstaff K, Rotwein P S. “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J Biol Chem. 1991;266:15917–15923. [PubMed] [Google Scholar]
- 18.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 19.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-biphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 20.Franke T F, Yang S-I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phophatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 21.Glücksmann A. Cell deaths in normal vertebrate ontogeny. Biol Rev Cambridge Philos Soc. 1951;26:59–86. doi: 10.1111/j.1469-185x.1951.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 22.Guo K, Walsh K. Inhibition of myogenesis by multiple cyclin/cdk complexes: coordinate regulation of myogenesis and cell cycle activity at the level of E2F. J Biol Chem. 1997;272:791–797. doi: 10.1074/jbc.272.2.791. [DOI] [PubMed] [Google Scholar]
- 23.Guo K, Wang J, Andrés V, Smith R C, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol. 1995;15:3823–3829. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 25.Hemmings B A. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 26.Hiromura K, Pippin J W, Fero M L, Roberts J M, Shankland S J. Modulation of apoptosis by the cyclin-dependent kinase inhibitor p27Kip1. J Clin Investig. 1999;103:597–604. doi: 10.1172/JCI5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh J-K, Chan F S G, O’Connor D J, Mittnacht S, Zhong S, Lu X. RB regulates the stability and the apoptotic function of p53 via MDM2. Mol Cell. 1999;3:181–193. doi: 10.1016/s1097-2765(00)80309-3. [DOI] [PubMed] [Google Scholar]
- 28.Jiang B-H, Aoki M, Zheng J Z, Li J, Vogt P K. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc Natl Acad Sci USA. 1999;96:2077–2081. doi: 10.1073/pnas.96.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King K L, Cidlowski J A. Cell cycle regulation and apoptosis. Annu Rev Physiol. 1998;60:601–617. doi: 10.1146/annurev.physiol.60.1.601. [DOI] [PubMed] [Google Scholar]
- 30.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirshenbaum L A, Abdellatif M, Chakraborty S, Schneider M D. Human E2F-1 reactivates cell cycle progression in ventricular myocytes and represses cardiac gene transcription. Dev Biol. 1996;179:402–411. doi: 10.1006/dbio.1996.0270. [DOI] [PubMed] [Google Scholar]
- 32.Kitzmann M, Vandromme M, Schaeffer V, Carnac G, Labbé J-C, Lamb N, Fernandez A. cdk1- and cdk2-mediated phosphorylation of MyoD Ser200 in growing C2 myoblasts: role in modulating MyoD half-life and myogenic activity. Mol Cell Biol. 1999;19:3167–3176. doi: 10.1128/mcb.19.4.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohn A D, Takeuchi F, Roth R A. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 35.Kou K, Rotwein P S. Transcriptional activation of the insulin-like growth factor-II gene during myoblast differentiation. Mol Endocrinol. 1993;7:291–302. doi: 10.1210/mend.7.2.8469241. [DOI] [PubMed] [Google Scholar]
- 36.Kranenburg O, van der Eb A J, Zantema A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J. 1996;15:46–54. [PMC free article] [PubMed] [Google Scholar]
- 37.Kulik G, Weber M J. Akt-dependent and -independent survival signaling pathways utilized by insulin-like growth factor I. Mol Cell Biol. 1998;18:6711–6718. doi: 10.1128/mcb.18.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindon C, Montarras D, Pinset C. Cell cycle-regulated expression of the muscle determination factor Myf5 in proliferating myoblasts. J Cell Biol. 1998;140:111–118. doi: 10.1083/jcb.140.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Kitsis R N. Induction of DNA synthesis and apoptosis in cardiac myocytes by E1A oncoprotein. J Cell Biol. 1996;133:325–334. doi: 10.1083/jcb.133.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Missero C, Calautti E, Eckner R, Chin J, Tsai L H, Livingston D M, Dotto G P. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 chain protein in terminal differentiation. Proc Natl Acad Sci USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novitch B G, Mulligan G J, Jacks T, Lassar A B. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J Cell Biol. 1996;135:441–456. doi: 10.1083/jcb.135.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker S B, Eichele G, Zhang P, Rawls A, Sands A T, Bradley A, Olson E N, Harper J W, Elledge S J. p53-independent expression of p21cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 43.Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 44.Poluha W, Poluha D K, Chang B, Crosbie N E, Schonhoff C M, Kilpatrick D L, Ross A H. The cyclin-dependent kinase inhibitor p21WAF1 is required for survival of differentiating neuroblastoma cells. Mol Cell Biol. 1996;16:1335–1341. doi: 10.1128/mcb.16.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen K M, Wentworth B M, Rosenthal N, Villa-Komaroff L. Specific, temporally regulated expression of the insulin-like growth factor II gene during muscle cell differentiation. Endocrinology. 1993;133:474–481. doi: 10.1210/endo.133.2.8393762. [DOI] [PubMed] [Google Scholar]
- 46.Schneider J W, Gu W, Zhu L, Mahdavi V, Nadal-Ginard B. Reversal of terminal differentiation mediated by p107 in Rb−/− muscle cells. Science. 1994;264:1467–1471. doi: 10.1126/science.8197461. [DOI] [PubMed] [Google Scholar]
- 47.Seemüller E, Lupas A, Stock D, Löwe J, Huber R, Baumeister W. Proteasome from Thermoplasma acidophilum: a threonine protease. Science. 1995;268:579–582. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- 48.Sellers W R, Novitch B G, Miyake S, Heith A, Otterson G A, Kaye F J, Lassar A B, Kaelin W G., Jr Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev. 1998;12:95–106. doi: 10.1101/gad.12.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith R C, Branellec D, Gorski D H, Guo K, Perlman H, Dedieu J-F, Pastore C, Mahfoudi A, Denèfle P, Isner J M, Walsh K. p21CIP1-mediated inhibition of cell proliferation by overexpression of the gax homeodomain gene. Genes Dev. 1997;11:1674–1689. doi: 10.1101/gad.11.13.1674. [DOI] [PubMed] [Google Scholar]
- 50.Song A, Wang Q, Goeble M G, Harrington M A. Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol Cell Biol. 1998;18:4994–4999. doi: 10.1128/mcb.18.9.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart C E H, Rotwein P. Insulin-like growth factor-II is an autocrine survival factor for differentiating myoblasts. J Biol Chem. 1996;271:11330–11338. doi: 10.1074/jbc.271.19.11330. [DOI] [PubMed] [Google Scholar]
- 52.Stokoe D, Stephens L R, Copeland T, Gaffney P R J, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 53.Testa J R, Bellacosa A. Membrane translocation and activation of the Akt kinase in growth factor-stimulated hematopoietic cells. Leuk Res. 1997;21:1027–1031. doi: 10.1016/s0145-2126(97)00093-3. [DOI] [PubMed] [Google Scholar]
- 54.Tollefsen S E, Sadow J L, Rotwein P. Coordinate expression of insulin-like growth factor II and its receptor during muscle differentiation. Proc Natl Acad Sci USA. 1989;86:1543–1547. doi: 10.1073/pnas.86.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh K, Perlman H. Cell cycle exit upon myogenic differentiation. Curr Opin Genet Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Guo K, Wills K N, Walsh K. Rb functions to inhibit apoptosis during myocyte differentiation. Cancer Res. 1997;57:351–354. [PubMed] [Google Scholar]
- 57.Wang J, Walsh K. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science. 1996;273:359–361. doi: 10.1126/science.273.5273.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshida S, Fujisawa-Sehara A, Taki T, Arai K, Nabeshima Y. Lysophosphatidic acid and bFGF control different modes in proliferating myoblasts. J Cell Biol. 1996;132:181–193. doi: 10.1083/jcb.132.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zacksenhaus E, Jiang Z, Chung D, Marth J D, Phillips R A, Gallie B L. pRb controls proliferation, differentiation, and death of skeletal muscle cells and other lineages during embryogenesis. Genes Dev. 1996;10:3051–3064. doi: 10.1101/gad.10.23.3051. [DOI] [PubMed] [Google Scholar]
- 60.Zhang P, Wong C, Liu D, Finegold M, Harper J W, Elledge S J. p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]