Abstract
There is a need to develop a novel analgesic for pain associated with interstitial cystitis/painful bladder syndrome (IC/PBS). The use of the conventional μ-opioid receptor agonists to manage IC/PBS pain is controversial due to adverse CNS effects. These effects are attenuated in benzylideneoxymorphone (BOM), a low-efficacy μ-opioid receptor agonist/δ-opioid receptor antagonist that attenuates thermal pain and is devoid of reinforcing effects. We hypothesize that BOM will inhibit bladder pain by attenuating responses of urinary bladder distension (UBD)-sensitive afferent fibers. Therefore, the effect of BOM was tested on responses of UBD-sensitive afferent fibers in L6 dorsal root from inflamed and non-inflamed bladder of rats. Immunohistochemical (IHC) examination reveals that following the induction of inflammation there were significant high expressions of μ, δ, and μ-δ heteromer receptors in DRG. BOM dose-dependently (1–10mg/Kg, i.v) attenuated mechanotransduction properties of these afferent fibers from inflamed but not from non-inflamed rats. In behavioral model of bladder pain, BOM significantly attenuated visceromotor responses (VMRs) to UBD only in inflamed group of rats when injected either systemically (10mg/Kg, i.v.) or locally into the bladder (0.1ml of 10mg/ml). Furthermore, oxymorphone (OXM), a high-efficacy μ-opioid receptor agonist, attenuated responses of mechanosensitive bladder afferent fibers and VMRs to UBD. Naloxone (10mg/Kg, i.v.) significantly reversed the inhibitory effects of BOM and OXM on responses of bladder afferent fibers and VMRs suggesting μ-opioid receptor-related analgesic effects of these compounds. The results reveal that a low-efficacy, bifunctional opioid-based compound can produce analgesia by attenuating mechanotransduction functions of afferent fibers innervating the urinary bladder.
Keywords: Cystitis, Opioids, Analgesic, Bladder pain, Bladder Afferents
1. Introduction
Interstitial cystitis/painful bladder syndrome (IC/PBS), a common urologic disorder, is poorly managed by multiple drugs including pentosan polysulfate (Elmiron®), amitriptyline, hydroxyzine cimetidine, and cyclosporine A (Giusto et al., 2018). Of these options, only the coating agent Elmiron® is FDA approved for the treatment of IC/PBS. However, adequate analgesic effects are reached up to about 6 months after initiation and is effective in only approximately 50% of patients (Anderson and Perry, 2006; Hanno, 1997; Mulholland et al., 1990; Parsons et al., 1993). Amitriptyline has also been used long-term for the treatment of IC/PBS (Foster et al., 2010; van Ophoven and Hertle, 2005); however, sedation, drowsiness, dizziness, and nausea are adverse central nervous system (CNS) effects that compound its use as a general therapy (Generali and Cada, 2014).
Although a significant number of IC patients are treated with opioid analgesics as a “gold standard”, the opioid treatment for IC/PBS pain remains controversial (Lusty et al., 2018; Pape et al., 2019). Despite current survey revealing a decline of conventional opioid use, the prescription rate per IC diagnosis has not decreased (Zillioux et al., 2020). Therefore, a safer treatment strategy for the use of opioids needs to be developed for bladder pain management. A bifunctional class of agents bears a pharmacodynamic profile of high-efficacy μ opioid receptor agonism, with lower-potency agonist effects at the related κ and δ opioid receptors. Morphine, fentanyl and oxymorphone (OXM) are clinically used examples of high efficacy μ opioid receptor agonists that cause life-threatening respiratory depression, constipation, tolerance, and dependence (Burns et al., 2018; Devereaux et al., 2018; Foley, 1993; Way, 1993). Analgesic tolerance, physical dependence, and constipation are significant adverse effects that plague the chronic use of high efficacy μ opioid receptor agonists as analgesics for chronic pain conditions. The ongoing opioid crisis in the United States necessitates the need to develop analgesics that can be used to manage chronic pain without manifesting adverse CNS effects (Madras, 2018). An ideal therapeutic agent to manage visceral pain should have (1) low abuse liability, (2) no tolerance development, and (3) no respiratory depression.
Bifunctional opioid receptor ligands offer potential advantages over high-efficacy μ opioid receptor-preferring agonists as analgesics (Ananthan, 2006; Cunningham et al., 2019). For example, buprenorphine, a partial μ opioid receptor agonist/κ opioid receptor antagonist, helps in recovery from opioid abuse by attenuating μ opioid receptor-induced withdrawal symptoms and blocking the effect of endogenous κ opioid receptor agonist dynorphins that causes dysphoria and hallucinations (Lutfy and Cowan, 2004). Eluxadoline (Viberzi®) is a peripherally restricted μ opioid receptor agonist/δ opioid receptor antagonist that manages the symptoms of diarrhea-predominant irritable bowel syndrome (IBS-D) without producing constipation. Here, δ opioid receptor antagonism slows gastrointestinal motility without causing constipation (Breslin et al., 2012; Wade et al., 2012). Combining the pharmacodynamic profiles of buprenorphine and eluxadoline, a centrally-bioavailable μ opioid receptor agonist/δ opioid receptor antagonist can produce analgesia without manifesting tolerance and dependence (Cunningham et al., 2019). Further, low-efficacy MOR partial agonists generate significantly less respiratory depression (Dahan, 2006). Thus, these types of compounds can be considered potential therapeutics for chronic visceral pain management. It is also favorable if such compound can produce analgesia by acting peripherally by modulating the functions of sensory afferent fiber innervating the visceral organs.
We previously reported the development of benzylideneoxymorphone (BOM), which is a low-efficacy μ opioid receptor partial agonist/δ opioid receptor antagonist that exhibits partial efficacy in hot plate and tail-flick latency antinociception tests in mice (Healy et al., 2017). More recently, we reported that the discriminative stimulus effects of BOM are similar to morphine and mediated by μ opioid receptors (Mada et al., 2020).
Although the “ceiling effect” of BOM in severe thermal pain tests makes this a suboptimal medication for severe pain, these behavioral effects suggest that BOM would have limited abuse liability and is therefore a promising lead for medications development. We hypothesize that this pharmacodynamic profile might be beneficial in treating inflammatory bladder pain conditions that cause upregulation of opioid receptor expression in bladder tissues and sensory neurons allowing the effective target for peripherally-restricted opioid agonists (Stein, 2013).
The main objective of this study is to examine the peripheral analgesic effect of BOM in bladder pain following the induction of cystitis in rats. Experiments include (1) validation of bladder inflammation (cystitis) in rats following injection of protamine sulfate and zymosan into the bladder and examination of histopathology of bladder tissue, (2) immunohistochemical (IHC) staining for μ opioid receptors, δ opioid receptors, and μ-δ opioid receptor heteromer expression in lumbar 6 (L6) dorsal root ganglion (DRG) cells, (3) electrophysiological recordings of nerve action potentials from UBD-sensitive L6 spinal dorsal root afferent fibers and (4) objective measurement of bladder pain in awake rats by recording visceromotor responses (VMRs) to UBD.
2. Methods
2.1. Animals
The study was performed in adult female Sprague-Dawley rats (Harlan Laboratories, Indianapolis, USA). A total number of 125 rats were used to undertake all experimental procedures. Rats weighing 250–300g were housed in pairs. For behavioral studies after the surgery rats were housed in separate cages. Rats were kept under controlled conditions with a 12 hours light/dark schedule and had free access to both food and water. All procedures were performed in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of Medical College of Wisconsin’s (AUA 000355). In addition, we followed the humane pain management recommendation of Office of Laboratory Animal Welfare (OLAW).
2.2. Chemicals and solutions
The synthesis and pharmacological efficacy of BOM has been described previously (Healy et al., 2017). The compound was synthesized in the laboratory of Dr. Cunningham (fig. 1). Structurally, the compound is μ opioid receptor agonist, that has been modified to enhance the δ opioid receptor affinity and to lower the efficacy at μ and δ opioid receptors. The drug was first dissolved in 100μl of 100% of ethanol and 100μl of Tween-80 and then diluted in sterile water to make the final concentration of 1% alcohol and Tween-80. Oxymorphone hydrochloride (OXM, Mallinckrodt, Inc.) and naloxone hydrochloride (NLX), protamine sulfate, and zymosan (Sigma Aldrich Chem Co.) OXM, and NLX were dissolved in a sterile saline solution.
Figure 1:
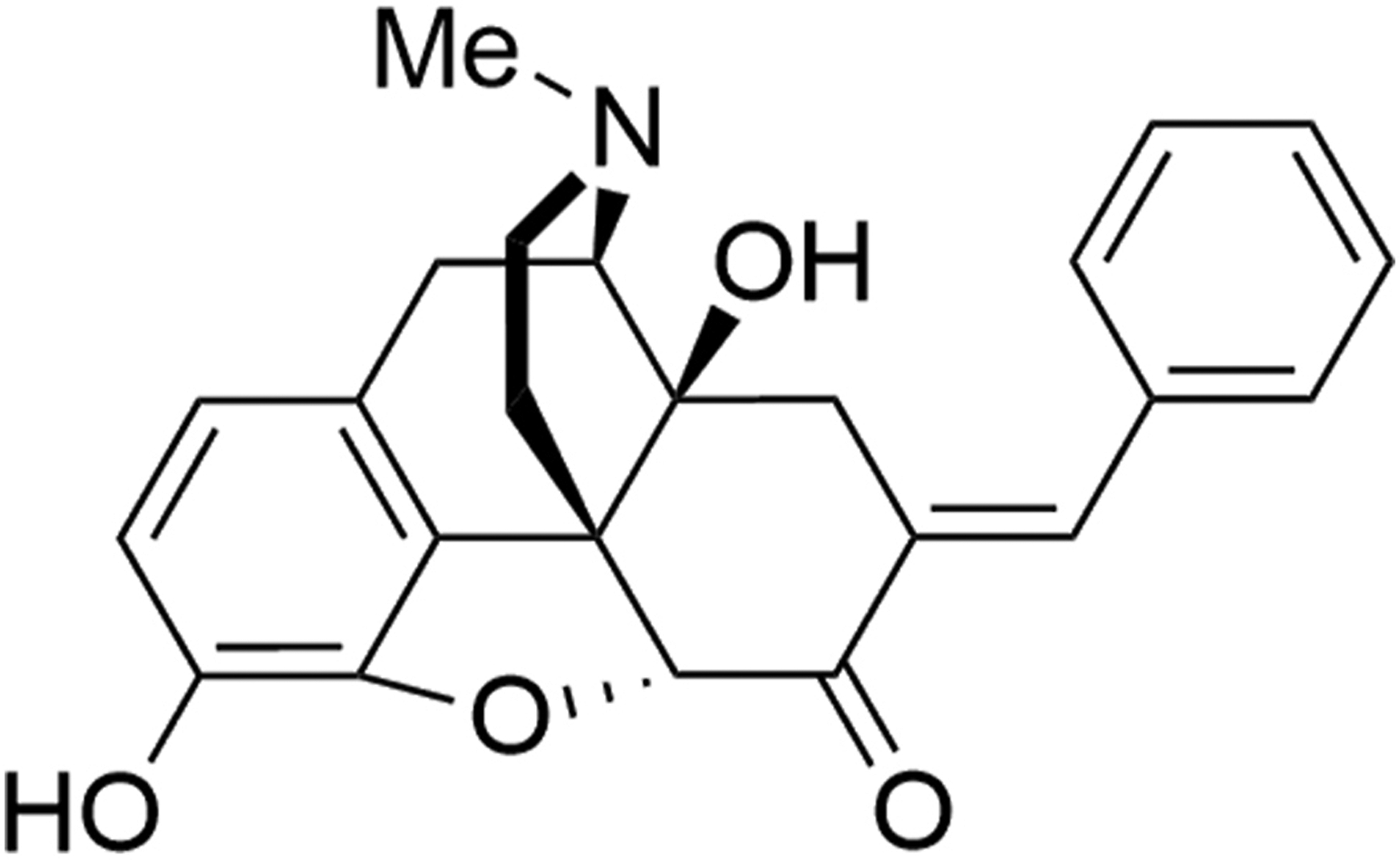
Illustrates the chemical structure of benzylideneoxymorphone (BOM).
2.3. Induction of cystitis
Bladder inflammation was produced by intravesical injection of protamine sulfate (PS, 10% in sterile saline) followed by zymosan (5% in sterile saline) into the bladder. The rationale for intravesical instillation of protamine sulfate, an arginine-rich protein, was to break the urothelial barrier to increase the permeability so that zymosan can act effectively to produce inflammation (Chuang et al., 2003; Niku et al., 1994; Tzan et al., 1993). Zymosan, a yeast wall derived glucan, produces bladder tissue inflammation (Kannampalli et al., 2017; Miranda et al., 2011; Ness and Randich, 2010; Sengupta et al., 2013). Rats were anesthetized with isoflurane (2% induction and 1.5% maintenance with flow rate 1 ml/min), the vaginal area was swabbed clean with betadine and protamine sulfate and zymosan were transurethrally injected into the bladder by gently inserting a shielded i.v. catheter (BD Insyte-N Autoguard, 24GA). Protamine sulfate solution (0.3ml) was first injected into the bladder and left inside the bladder for 20 minutes followed by slow aspiration. Zymosan solution (0.3ml) was then instilled into the bladder and left inside for 20 minutes. The control group rats went through similar instillation procedure but received only two doses of sterile saline (0.3ml per dose) into the bladder. This procedure was repeated for three days. The behavioral and electrophysiology studies were performed 24 hours (Kannampalli et al., 2017) following the induction of cystitis.
2.4. Bladder tissue histology
Bladder tissues from protamine sulfate+zymosan- and saline-treated rats were removed after euthanizing and fixed in 4% paraformaldehyde. For quantification of mast cells, the bladder tissue was stored overnight in 4% paraformaldehyde, the tissues were dehydrated in graded alcohol and paraffin embedded. Serial sections of 4μm thickness were cut, and the slides were oven dried at 60°C. Sections were de-paraffinized by two times wash (5 min each) with xylene followed by two times wash with 100% ethanol and a 5 min wash in 95% ethanol and hydrated with distilled water. Slides were then placed in 10% modified Toludine Blue, a polychromatic dye, solution in 1% NaCl for 1–2 min followed by 3 min rinses in distilled water (x3) (Miranda et al., 2011; Ribatti, 2018). Slides were quickly dehydrated through 95% and 100% alcohols and cleared in xylene and cover slipped. Mast cell numbers were quantified blinded manner under microscope with X40 magnification (Olympus Corporation, Tokyo, Japan).
2.5. Immunohistochemical (IHC) evaluation of L6 DRG
Non-inflamed (n=4) and inflamed (n=5) rats were deeply anesthetized with sodium pentobarbital (50mg/Kg, i.p.). The rat was placed in supine position and the chest was opened to perfuse trans-cardiac with 300ml of ice-cold 4% paraformaldehyde. Following laminectomy L6 DRG was identified and removed bilaterally and stored overnight in cold (4°C) PFA-lysine-periodate fixative. The following day tissues were incubated in 10% sucrose overnight at 4°C and transferred to 20% sucrose for 8 hours, and then to 30% sucrose overnight on a shaker at 4°C. The DRG was sectioned at a thickness of 25μm using a cryostat (Microm cryostat, Thermo Fisher Scientific, Waltham, MA) and mounted on SuperFrost Plus slides (Fisher Scientific, Hampton, NH) and store at −20°C. For immunostaining, DRG tissue sections mounted on glass slides were washed three times with PBS wash buffer (1X phosphate-buffered saline), 0.1% Triton X-100, 0.01% Sodium azide, 0.01% NGS) and then incubated with target retrieval solution (Dako, Santa Clara, CA) for 30 mins at 70°C in a water bath. This was followed by incubation in blocking buffer containing 10% NGS (Normal Goat Serum, Jackson Immuno Research, West Grove, PA) in wash buffer for 2 hours at room temperature. Subsequently, sections were incubated separately in rabbit anti-μ opioid receptor antibody, (1:500 dilution; Alomone labs, Jerusalem, Israel) and rabbit anti-δ opioid receptor antibody, (1:500 dilution; Alomone labs) along with mouse anti-Neurofilament 200 (1:1000 dilution; Millipore Sigma, Billerica, MA). In another set of experiments, sections were incubated with mouse anti-μ-δ heteromer antibody (1:250 dilution; Kerafast, Inc. Boston, MA) along with either rabbit anti-Neurofilament 200 (1:200 dilution; Millipore Sigma, Billerica, MA) or rabbit anti-TRPV1 antibody (1:250 dilution; Alomone labs) diluted in 5% NGS at 4°C for 48 hours. Tissue sections were then washed 4 times for 15 min each and then incubated with secondary antibodies for 2 hours at room temperature. The following secondary antibodies were used: Alexa Fluor 568 goat anti-rabbit (1:750 dilution) along with Alexa Fluor 488 goat anti-mouse (1:750 dilution) or Alexa Fluor 488 goat anti-rabbit (1:1000 dilution) with Alexa Fluor 568 goat anti-mouse (1:1500 dilution, Invitrogen Inc., Carlsbad, CA). The dilution was made in 5% NGS at room temperature. Finally, the tissues were washed 3 times and cover slipped with mounting medium (Vectasheild, Burlingame, CA).
2.6. Electrophysiological recording from UBD-sensitive afferent fibers in L6 dorsal root.
Electrophysiology recordings were undertaken for non-inflamed and inflamed rats. The surgical procedure and recordings from the L6 dorsal-root was performed as described previously (Sengupta and Gebhart, 1994). Briefly, rats were anesthetized with urethane (1.5 mg/kg, i.p.). The trachea was intubated to mechanically ventilate the rat with room air (55–60 strokes/min and 3–4 ml stroke volume) after paralyzing the rat with Gallamine triethiodide (5mg/Kg, i.v.). A polyethylene catheter (PE-50) was inserted into the femoral vein for injecting test drugs and supplemental dose of anesthesia and muscle relaxant if required. Following a midline laparotomy, a polyethylene catheter (PE-100) and 5F Millar pressure transducer (MPR-500, 5F, AD Instruments, TX, USA) were inserted into the bladder through the dome for UBD and recording of intravesical pressure. The catheter and transducer were tied to secured it in place. The abdominal incision was closed by suturing in layers with 3–0 silk sutures. The external urethral orifice was sealed with a tissue adhesive to stop voiding through urethra. A laminectomy was performed to expose the spinal cord from T13 to S4 and the dorsal aspect of the body was stabilized by clamping the thoracic vertebra and hip bone with spinal clamps. The dorsal incised skin was reflected laterally and tied to the spinal clamps to make a pool for mineral oil. The dura membrane was carefully removed, and the spinal cord was covered with warm (37°C) paraffin oil. The L6 dorsal root was identified and cut at its point of entry to the spinal cord. The nerve action potentials were recorded from the distal cut end of the L6 dorsal rootlet as described previously (Sengupta et al., 2002). The mechanosensitive afferent fibers innervating the bladder was identified by distending the bladder (0.4ml, 10s) with saline (search stimulus). Once a fiber exhibited increase in firing, the action potentials were filtered through a window discriminator (BAK Instrument, MD, USA) preset at specific width of the window for counting and the frequency of discriminated waveform was displayed as histogram of 1s binwidth using Spike 5/CED data acquisition software.
2.7. Experimental protocol for electrophysiology recordings and drug testing
Initially, a baseline nerve action potential was recorded to check the firing pattern of the nerve during spontaneous bladder contractions. The bladder catheter was connected to infusion pump to fill the bladder slowly (flow rate: 0.1ml/min). The Millar pressure transducer was connected to Millar bioamplifier (Mikro-Tip Catheter Transducers; Millar Inc., Texas, USA) to record the intravesical pressure. The bladder was filled with warm (37°C) saline slowly from 0 to 0.8ml and the response patterns of the afferent fiber were recorded to increasing bladder volume (Analytical data not shown).
Following recording of fiber’s responses to slow infusion the bladder catheter was connected to Mariotte’s bottle connected to barostatic distension device to deliver regulated isobaric pressure. The bladder was distended with graded incrementing (10, 20, 30, 40 and 60mmHg) pressure. The duration of each distension was 30 seconds and inter-stimulus time interval was 3 minutes. Prior to testing the effect of BOM or OXM on mechanosensitivity of bladder afferent fibers, a stimulus-response function (SRF) to graded bladder distension was constructed. The test drug was injected either intravenously (i.v.) or locally into the bladder (intravesical). A second SRF was constructed to graded UBD 10 minutes after the injection of test drug. In first set of experiments, dose-dependent effect of BOM was tested by injecting three different doses (1, 5 and 10mg/Kg, i.v.). Each dose was tested on one afferent fiber. All three doses were not tested on single fiber. The most effective dose of BOM (10mg/Kg, i.v. or 0.1ml of 10mg/ml intravesical) was used in subsequent experiments to characterize the mechanism of action of the drug. In another set of experiments, third SRF was constructed following the injection of NLX (10mg/Kg, i.v.) to reverse the effects of test drugs. OXM was used in our experiment as a representative high efficacy μ opioid receptor agonist and as a positive control of BOM, since BOM is synthetically modified derivative of OXM and OXM is available as a therapeutic option to manage chronic pain. We used a single dose (10mg/Kg, i.v.) of OXM to test its effect on the mechanosensitivity of bladder afferent fibers.
2.8. Surgical procedure for recording electromyograph (EMG) to urinary bladder distension (UBD).
After testing the effect of BOM on responses of distension-sensitive bladder afferent fibers we tested if this effect of the drug can attenuate bladder pain in awake rats. The pain evoked by UBD was measured from inflamed (cystitis) and non-inflamed rats. EMG recording electrodes were surgically implanted into the external oblique muscles of the abdomen having the dermatomal motor nerve distribution from the lumbo-sacral spinal cord. Rats were anesthetized with isoflurane (2% induction and 1.5% maintenance with flow rate 1 ml/min). All surgical procedures were undertaken under aseptic condition. The lower abdomen was opened by giving a midline incision (1–1.5cm) to expose the bladder. A small hole was made to the dome of the bladder and a polyethylene catheter (PE-50) was inserted into the bladder. The tip of the catheter was positioned in the middle part of the bladder to make sure that the catheter was not obstructing the neck of the bladder. The hole was closed by tying around the catheter with a silk (3–0) suture and the bladder was slowly distended to check that there was no leak around the ligature and fluid is dripping out freely through the urethra. The abdomen was closed in layers with silk (3–0) suture. A pair of teflon-coated electrodes (Cooner Wire, Part No. A5631, Chatsworth, CA) stripped and flared at the tip were implanted into the external oblique muscle of the abdomen 1–1.5 cm apart to record the EMG of the muscles. The EMG recording electrodes and the bladder catheter were tunneled through the dorsal aspect of the skin and externalized dorsally near the neck. Electrodes and catheter were secured in place by suturing it to the neck muscle. Rats received analgesic (Carprofen, 5mg/kg/day i.m.) for 3 days, and antibiotic (Enrofloxacin, 2.5mg/kg/day) for 2 days and housed separately.
2.9. Measurement of bladder pain to UBD
Following 72 hours’ post-op recovery, rats were placed inside a restraining tube for 2 hours/day for 3 days to acclimatize to experimental conditions. Following restraining training, the visceromotor responses (VMRs) were measured by recording electrical activities (EMG) generating during the contraction of abdominal muscle to UBD, a reflexive muscular response to visceral stimulus. Before recording, the external urethral orifice was sealed with the tissue glue (3M Vetbond, MN, USA) to prevent spontaneous voiding, which allowed us to generate painful intravesical pressure. To distend the bladder, the bladder catheter was connected to Marriotte bottle reservoir containing warm saline via a pressure transducer (Maxxim Medical Disposable Transducer; Texas, USA). The slow filling of the bladder was achieved as described in Section 2.7. For graded isobaric distension, the inlet tube of the Marriotte’s bottle was connected to a barostatic distension device to deliver constant distending pressure (isobaric) to the bladder as described in the previous section (Section 2.7). The EMG signal was amplified using the amplifier (A-M System, model 1700, Sequim, WA) and displayed real-time on a computer screen during the protocol. The data were recorded in real-time using the Spike 5/CED 1401 data acquisition software (CED 1401; Cambridge Electronic Design, Cambridge, UK). A quantitative measurement of increasing VMRs was recorded by distending the bladder to graded intensities (10, 20, 30, 40, and 60 mmHg). The duration of each distension was 30s and the inter-stimulus time interval was three minutes.
2.10. Experimental protocol of VMR recordings and drug testing
Initially, the bladder was filled slowly as described in Section 2.7 to record the progressive increase in VMR during to increasing bladder volume. Subsequently, a SRF to graded distension pressures (10, 20, 30, 40 and 60mmHg) was constructed to record the increasing VMR to increasing bladder pressure. The effects of BOM and OXM were tested in separate experiments. In the first set of experiments, BOM or OXM was injected systemically (i.v) to test for its overall effects on VMRs. To test the peripheral effects of BOM and OXM, in next set of experiments drugs were instilled (0.1ml of 10mg/ml) into the bladder (i.e., intravesical) 10 minutes before repeating the SRF. To reverse the effect of BOM or OXM, NLX (10mg/Kg, i.v.) was injected 2–3 minutes after recording the effects and the SRF was repeated immediately after NLX injection.
2.11. Data analysis
(a). Histology and immunohistology analyses:
The microscopic and histological scoring of Toluidine blue stained bladder tissue was undertaken based on the three basic criteria: (i) presence of no mast cells and no erosion of urothelium was scored as (+), (ii) presence of very few mast cells and low level of edema and vascular congestion was scored as (++); (iii) a high presence of clusters of toluidine blue-stained mast cells and erosion was scored as (+++). The scoring was based on the total number of toluidine blue stained mast cells in each viewing area (x40).
The immunostaining of the sections was examined using a Nikon eclipse 50i microscope (Nikon, Tokyo, Japan) under the red fluorescence filter. Images were captured using similar settings of exposure and gain for non-inflame and inflame samples. The immune-positive cell somas were counted using Image J program software (NIH, Bethesda, MD, USA). For total number of cells in a single tissue section, the background was adjusted to identify cell soma with clear nuclei and cells with positive staining were identified by normalizing the background staining (3 tissue sections/rat). The percentage of positive stained cells was then calculated for all groups. The results are represented as mean ± SEM and p value < 0.05 was considered statistically significant (unpaired student t-test). For illustration, images were background adjusted using Adobe Photoshop CS6 and formatted using CorelDraw X8 software. All statistics were performed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA).
(b). Electrophysiology analysis:
The signal waveform analysis and the measurement of firing frequency of nerve action potentials were performed as described previously (Kannampalli et al., 2017). Briefly, in single fiber recording the nerve action potentials were counted as impulses/second and represented as frequency histogram of 1s binwidth. For multi-fiber recordings, the nerve action potentials were post-processed using Spike 5/CED signal waveform analysis and each UBD-sensitive fiber was separated for subsequent analysis. The total number of action potentials over a 30s resting period prior to bladder distension and during the distension period (30s) were counted and represented as mean impulses/s. To measure the actual changes in response of the neurons to UBD, the mean firing frequency during the resting period (30s pre-distension) was subtracted from the mean firing frequency during the bladder distension (30s distension). The difference was calculated for each distending pressure and the response was divided by the response of the neuron to the maximum distension pressure (60mmHg) before the drug injection to obtain normalized percentile values. For bladder slow infusion (SI) experiments, the action potentials of the fibers were calculated pre-, during and post-SI for a period of 60s and compared with the response after BOM or OXM administration. Statistical analysis was determined using a 2-way Analysis of variance (ANOVA) with Bonferroni’s multiple comparison test. Values are expressed as the mean ± S.E.M. and the p<0.05 was considered as significant.
3.0. Results
3.1. Cellular changes of bladder tissues following instillation of protamine sulfate + zymosan into the bladder.
Following intravesical instillation of protamine sulfate and zymosan into the bladder for three days, the presence of mast cells in the tissue layers was identified and counted. This data was compared to saline-treated bladder tissues. Figure 2A shows examples of microscopical structures of bladder tissues from saline- and protamine sulfate + zymosan-treated rats. In protamine sulfate + zymosan-treated rats, a focal cluster of mast cells was noted in the lamina propria of the bladder (black arrows), whereas in saline-treated rats the cells were sparingly present in the tissue. The analytical data indicate that the total number of mast cells was significantly higher in protamine sulfate + zymosan-treated group (n=3/group) compared to saline-treated group (n=3, t=15.81, df=4; *p < 0.0001 saline- vs PS+Z-treated rats; fig. 2B).
Figure 2:
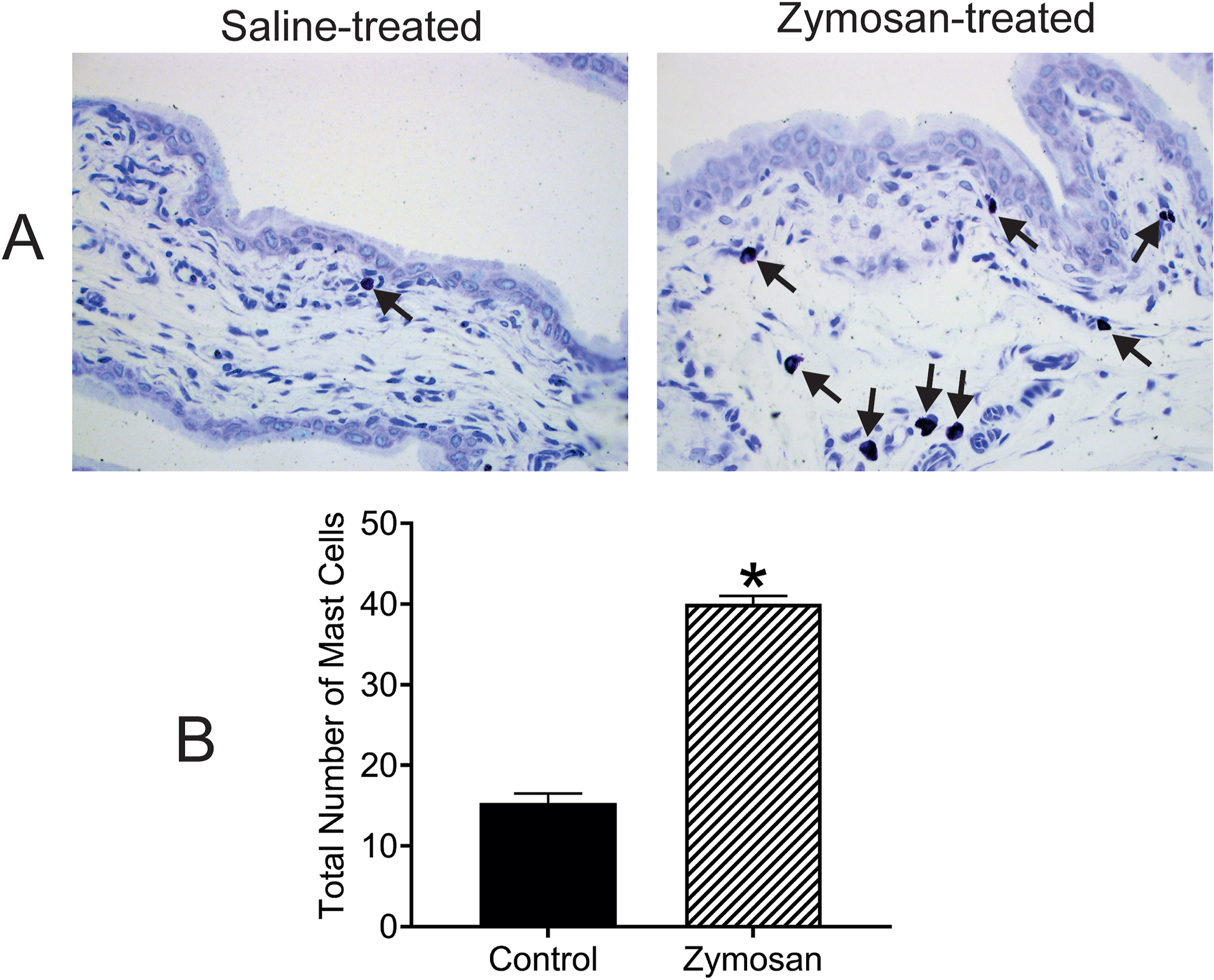
Illustrates cellular changes of bladder tissues intra-vesicle instillation of saline and protamine sulfate (PS)+zymosan (Z) exposure for three days. The bladder tissues were stained with Toluidine blue to assess the effect of treatment on mast cell expression (2A; 40x objective). Focal clusters of mast cells are noted in the lamina propria of the bladder which are significantly higher in PS+Z-treated groups compared to saline-treated control (black arrows). The mast cells were significantly high in PS+Z-treated bladder tissues compared to saline-treated rats (*P < 0.01 saline- vs PS+Z-treated rats, 2B).
3.2. Expression of μ and δ opioid receptors in L6 DRG
The influence of bladder inflammation on expressions of two subtypes of opioid receptors was examined in L6 DRG, which contains the cell bodies of primary sensory neurons innervating the urinary bladder of rats. Using IHC processing the expression of μ and δ opioid receptors were characterized in L6 DRGs from non-inflamed (control) and inflamed bladder. Figure 3 A–D illustrates examples of expressions of μ and δ opioid receptors in the cell somas along with NF200 (neurofilament-200, marker for medium/large diameter neurons). Qualitatively, these figures show that following inflammation of the bladder there were marked increase in number of μ- and δ-opioid receptor (figs 3 A1 and C1) compared to non-inflamed group (figs 3 B1 and D1). Double-labeling study shows that approximately 30±3.3% of μ opioid receptor labeled neurons are positive for NF200 in the inflamed group compared to 19±1.6% in the non-inflamed group (p < 0.05 vs non-inflamed rats). Additionally, about 34±2% of δ opioid receptor labelled neurons are positive for NF200 in the inflamed group compared to 21±1.1% in the non-inflamed group (p < 0.05 vs non-inflamed rats). We further quantified the number μ and δ opioid receptors of positive cells in both inflamed and non-inflamed groups. The statistical analysis reveals that μ and δ opioid receptor expressions are significantly higher in the inflamed rats (μ-opioid receptor expression: 55.3 ± 2.8 vs 42.3 ± 1.3, δ-opioid receptor expression: 48.7 ± 1 vs 39.7 ± 1.5, *p < 0.05 vs non-inflamed rats, fig. 3 E).
Figure 3:
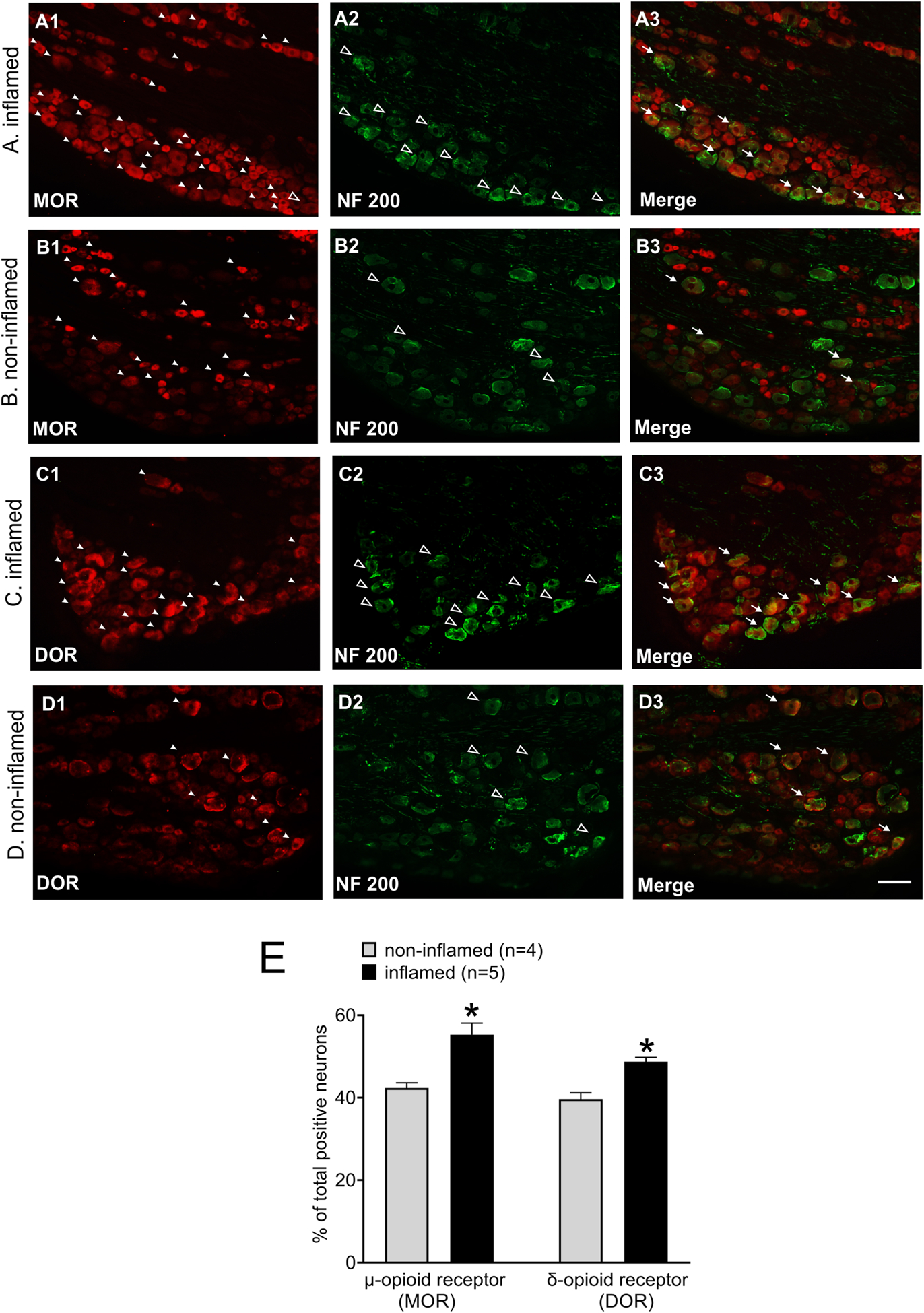
Illustrates immunostaining for μ- and δ-opioid receptor positive cell bodies located in L6 DRGs from inflamed and non-inflamed rats. All tissues were counterstained with NF200 marker for large diameter cell bodies. Panels A and B illustrate immunostaining for μ-opioid receptor and NF200 in DRG cell bodies from inflamed and non-inflamed rats, respectively. In L6 DRG from inflamed rat, A1 shows immunostaining for μ-opioid receptor, A2 shows immunostaining for NF200 and A3 illustrates merged image showing colocalization of μ-opioid receptor and NF200 expression. In L6 DRG of non-inflamed rat, B1 shows μ-opioid receptor staining, B2 is NF200 staining and B3 is merged image showing colocalization of MOR ad NF200 expression. Panels C and D illustrate immunostaining for δ-opioid receptor and NF200 in L6 DRGs from inflamed and non-inflamed rats, respectively. In DRG of inflamed rat, C1 shows immunoreaction for δ-opioid receptor, C2 is for NF200 positive cells and C3 is merged image showing colocalization of δ-opioid receptor and NF200 expression. D1 shows δ-opioid receptor staining, D2 is for NF200 staining and D3 is merged image showing co-expression of δ-opioid receptor and NF200. The white arrow heads indicate μ- and δ-opioid receptor positive cells, the open arrow heads represent NF200 positive cells and the white arrows represent neurons expressing NF200 and μ/δ-opioid receptor. The scale bar is 100μm. E: Shows the quantitative analysis of μ- and δ-opioid receptor expression in non-inflamed and inflamed groups, *p < 0.05.
3.3. Expression of μ-δ opioid receptor heteromers in L6 DRG
For further evaluation of the influence of bladder inflammation on opioid receptor expression, the expression of μ-δ opioid receptor heteromer was examined in L6 DRG. Using IHC the expression of μ-δ opioid receptor heteromer was characterized in L6 DRGs from non-inflamed and inflamed rats. The μ-δ opioid receptor heteromer expressing cells were further characterized using two different neuronal markers, NF200 for large diameter neurons and TRPV1 (transient receptor potential vanilloid receptor 1) small diameter neurons. Figure 4 A–B illustrates examples of double-labeling for μ-δ opioid heteromer and NF200. Figure 4 C–D illustrates examples of double-labeling for μ-δ opioid heteromer and TRPV1 in L6 DRGs. Double-labeling study shows that approximately 44±2.6% of μ-δ opioid receptor heteromer labeled neurons are positive for NF200 in the inflamed group compared to 32±1.5% in the non-inflamed group (p < 0.05 vs non-inflamed rats). Additionally, 43±2.3% of μ-δ opioid receptor heteromer labeled neurons are positive for TRPV1 in the inflamed group compared to 32±1.7% in the non-inflamed group (p < 0.05 vs non-inflamed rats). The statistical analysis reveals that μ-δ opioid receptor heteromer expression is significantly higher in the inflamed rats (inflamed vs non-inflamed: 46 ± 0.8 and 38 ± 1.2, respectively, *p < 0.05, fig. 4 E). We have also noticed an overall increase in TRPV1 positive neurons in inflamed rats (inflamed vs non-inflamed: 200.8 ± 15.3 and 143 ± 6.7, respectively, p < 0.05).
Figure 4:
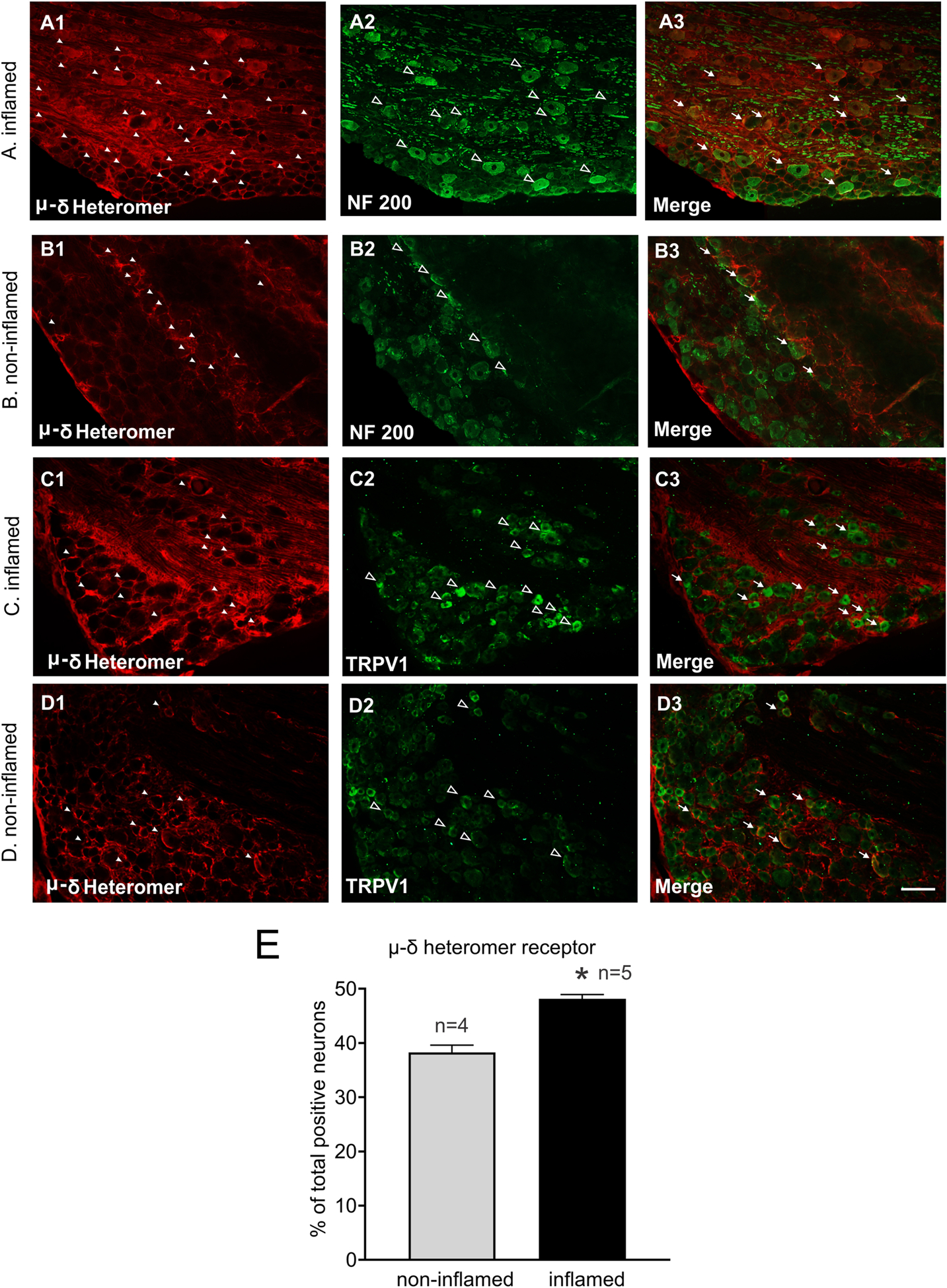
Illustrates immunostaining for μ-δ heteromer-positive cell bodies located in L6 DRGs from inflamed and non-inflamed rats. Panels A and B illustrate immunostaining for μ-δ heteromer opioid receptor and NF200 from inflamed and non-inflamed rats, respectively. A1 shows immunostaining for μ-δ heteromer, A2 is NF200 staining and A3 is merged image showing colocalization of μ-δ heteromer and NF200 expression in DRGs from inflamed rats. Similarly, B1, B2 and B3 represent μ-δ heteromer, NF200 and co-expression of μ-δ heteromer and NF200, respectively in non-inflamed rats. Panels C and D illustrate immunostaining for μ-δ heteromer opioid receptor and TRPV1-positive cell bodies from inflamed and non-inflamed bladder, respectively. C1 represents μ-δ heteromer, C2 is TRPV1 staining and C3 is merged image showing colocalization of μ-δ heteromer and TRPV1 expression in inflamed rats. Similarly, D1, D2 and D3 represent μ-δ heteromer, TRPV1 and co-expression of μ-δ heteromer and TRPV1, respectively in non-inflamed rats. The white arrow heads indicate μ-δ heteromer positive cells, the open arrow heads represent NF200/TRPV1 positive cells and the white arrows represent neurons expressing μ-δ heteromer and NF200/TRPV1. The scale bar is 100μm. E: Shows the quantitative data analysis of μ-δ heteromer receptor positive cells in non-inflamed and inflamed groups, *p < 0.05.
3.4. Effect of BOM and OXM on distension-sensitive bladder afferent fibers in L6 dorsal roots
The effects of BOM and OXM were tested on mechanotransduction properties of UBD-sensitive afferent fibers during graded distension. The effect of BOM was tested on bladder afferent fibers from inflamed rats and compared that with responses of fibers from non-inflamed rats. OXM was tested on UBD-sensitive afferent fibers from inflamed rats. The effect of BOM or OXM was tested on a separate set of afferent fibers from separate set of rats. The SRFs to graded UBD were constructed before and after the injection of the drug. Figure 5 illustrates examples of responses of one distension-sensitive bladder afferent fibers from inflamed bladder before and after the injection of BOM (10mg/Kg, i.v.). The fiber exhibited intensity-dependent increase in firing frequency to incrementing bladder pressure (10 thru 60mmHg) (fig 5i). The mechanosensitivity of the fiber was inhibited about 10 minutes after the injection of BOM (fig 5ii). The responses of the fibers were completely inhibited when the SRF was repeated after 45 minutes (fig 5iii). The recovery of these afferent fibers varied from fibers to fibers ranging from 15 to 60 minutes. Responses of some fibers (n=6) did not recover within one hour. In contrast to afferent fibers from inflamed bladder, responses of fibers from non-inflamed bladder were not inhibited by BOM. We also tested the effect of OXM on responses of UBD-sensitive afferent fibers (n=5). Figure 6 illustrates examples of responses of one afferent fiber from inflamed bladder to graded UBD before and after the injection of OXM (10mg/Kg, i.v.). The drug produced inhibition of responses of the fiber to graded bladder distension (fig 6, second column).
Figure 5:
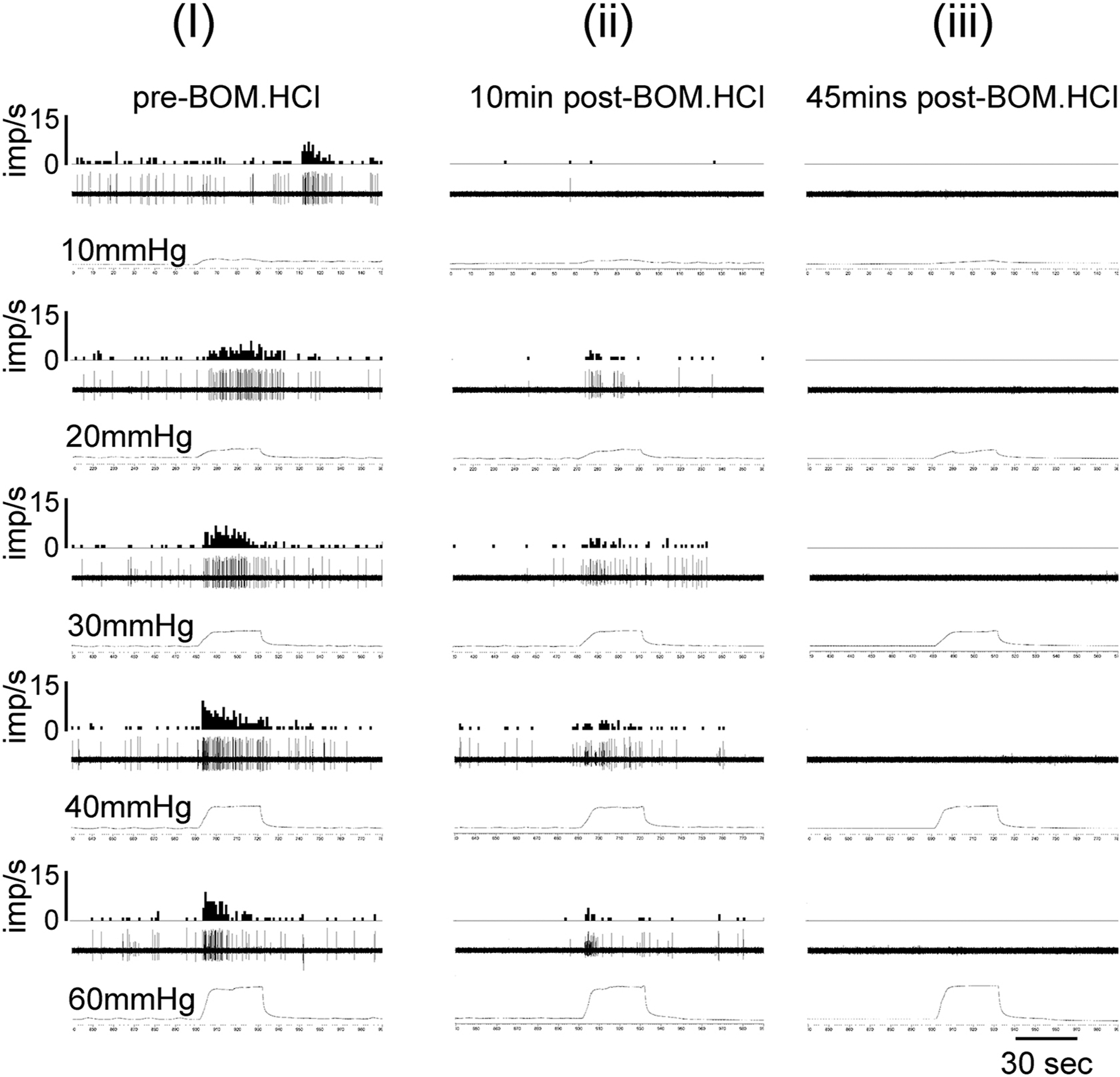
Illustrates the examples of responses of a UBD-sensitive afferent fiber in L6 spinal dorsal root before and after injection of BOM (10mg/Kg, i.v.). In each panel, the top trace represents frequency histogram (1s binwidth) of nerve action potentials. The middle trace shows evoked nerve action potentials, and the bottom trace is intravesical pressure. Column (i) shows increase in firing frequency of the nerve fiber to incremental changes of intravesical pressure (10 thru 60mmHg). Column (ii) shows partial inhibition of firing frequency of the nerve after the injection of BOM. The fiber did not exhibit any firing when the bladder was distended after 45 minutes (column iii).
Figure 6:
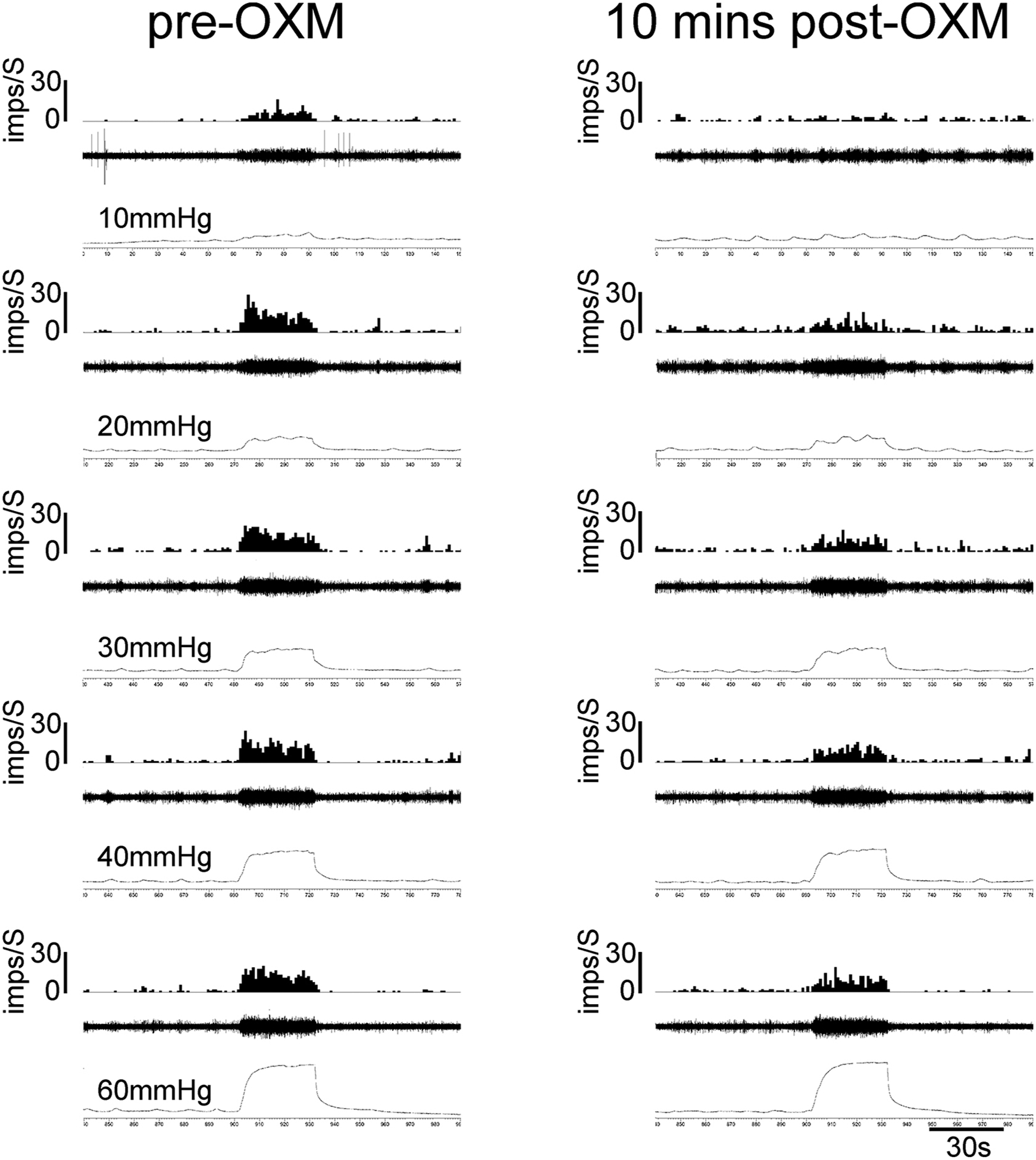
Illustrates the examples of responses of a UBD-sensitive afferent fiber in L6 spinal dorsal root before and after injection of OXM (10mg/Kg, i.v.). In each panel, the top trace represents frequency histogram (1s binwidth) of nerve action potentials. The middle trace shows evoked nerve action potentials, and the bottom trace is intravesical pressure. The first column shows the increase in firing frequency of the nerve fiber to incremental changes of intravesical pressure (10 thru 60mmHg) prior to injection of OXM. The second column shows partial inhibition of firing frequency of the about 10 mins after the injection of OXM.
Figures 7A and 7B illustrate the analytical data of dose-dependent effect of BOM on responses of bladder afferent fibers from non-inflamed and inflamed bladder. Figure 7A shows the mean SRFs of fibers from non-inflamed rats before and after i.v. injections of 5mg/Kg (n=5) and 10mg/Kg (n=16) of BOM indicating that the drug did not produce inhibition of responses of these fibers. Subsequently, three doses (1mg, 5mg and 10mg/Kg, i.v.) of BOM were tested on responses of afferent fibers from inflamed bladder to standardize an optimum dose of BOM for subsequent experiments. Figure 7B illustrates the mean SRFs of bladder afferent fibers before and after the i.v. injection of 1mg/Kg (n=11), 5mg/Kg (n=7) and 10mg/Kg (n=18) of BOM. Our results show that BOM dose-dependently inhibited responses of UBD-sensitive afferent fibers from inflamed bladder (Finteraction (25, 396) = 2.064, Fdistension (5, 396) = 125.7, Ftreatment (3, 396) = 13.85, *p < 0.01, **p < 0.001, ***p < 0.0001; fig. 7B). The results showed that among three doses 10mg/Kg of BOM produced maximum inhibition of responses of these afferent fibers. Therefore, in rest of the experiments we used this dose of the drug. To determine whether the anticociceptive effects produced by BOM were μ opioid receptor-dependent, we also tested whether NLX can reverse the inhibitory effect of BOM. Therefore, in another set of experiments NLX (10mg/Kg, i.v.) was injected after recording the effect of BOM on responses of the fibers and the SRF was repeated. NLX not only reversed the firing frequency of these fibers, but also significantly enhanced the responses (Finteraction (10, 90) = 14.36, Fdistension (5, 90) = 141.4, Ftreatment (2, 90) = 158.2, *p < 0.01, **p < 0.001, #p < 0.01; ##p < 0.001, ###p < 0.0001; fig. 7C). Similarly, the inhibitory effect of OXM (10mg/Kg, i.v.) was also reverted by NLX (F interaction (10,72) = 1.768, Fdistension (5, 72) = 39.89, Ftreatment (2, 72) = 17.26, *p <0.01, **p < 0.001, ***p < 0.001; #p < 0.01; ##p < 0.001, ###p < 0.0001, fig. 7D).
Figure 7:
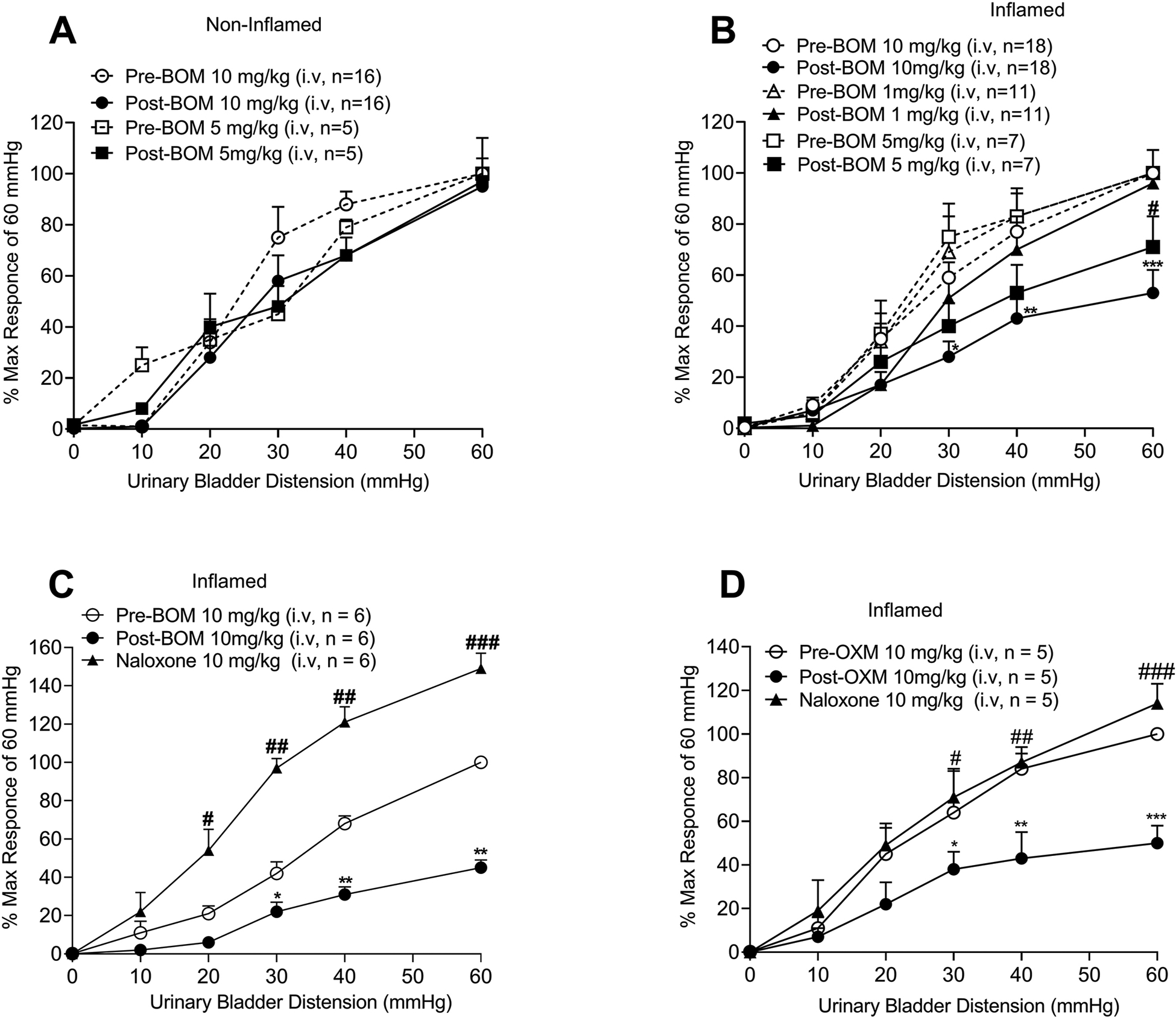
Shows the mean stimulus-response functions (SRFs) of UBD-sensitive afferent fibers before and after the injection of BOM or OXM and the reversal of inhibitory effects of these compounds by NLX (10mg/Kg, i.v.). BOM was tested on fibers from both non-inflamed and inflamed bladder. 7A: BOM (5mg/Kg, n=5 and 10mg/Kg, i.v., n=16) did not produce inhibition of firing of UBD-sensitive afferent fibers innervated non-inflamed bladder. 7B: shows the mean SRFs of UBD-sensitive afferent fibers from inflamed bladder. BOM produced dose-dependent (5mg/Kg, n=7, open and filled triangles and 10mg/Kg, i.v. n=18, open and filled circles) inhibition of firing of these fibers (5mg/Kg: #p < 0.01 vs pre-BOM; 10mg/Kg: *p < 0.01; **p < 0.001 vs pre-BOM). The low dose (1mg/Kg, i.v., n=11, open and filled squares) of the drug did not produce inhibition of responses of these afferent fibers. 7C: shows the mean SRFs of fibers before and after the injection of BOM (10mg/Kg, i.v., n=6) followed by injection of naloxone (10mg/Kg, i.v.). BOM (10mg/Kg) significantly inhibited the responses of these fibers (*p < 0.01; **p < 0.001 vs pre-BOM, filled circles), which was reversed and significantly enhanced by NLX (#p < 0.01, ##p < 0.01 ###p < 0.01 post-BOM, filled triangles). 7D: shows the mean SRFs of UBD-sensitive afferent fibers before and after the injection of OXM (10mg/Kg, i.v., n=5) followed by NLX (10mg/Kg, i.v.). OXM significantly inhibited the responses of these fibers (*p < 0.01; **p < 0.001 vs pre-BOM, filled circles), which was reversed by naloxone; (#p < 0.01, ##p < 0.001 ###p < 0.0001 post-BOM, filled triangles).
3.5. The inhibition of VMRs to bladder distension following injection of BOM and OXM in awake rats.
The main objective of testing these compounds in a behavioral model of bladder pain was to validate the electrophysiology results that suggested that the attenuation of mechanotransduction of UBD-sensitive bladder afferent fibers by BOM can alleviate painful distension-evoked bladder pain. Effects of BOM and OXM were tested by injecting the drugs via two routes; (a) intravenously (i.e., systemically), and (b) intravesical (i.e., topically on the urothelium) to test whether the drug can block VMRs by inhibiting the impulse transmission from the nerve terminals. In these experiments, BOM (10mg/Kg, i.v.) attenuated VMR to graded UBD only in bladder inflamed rats, but not in non-inflamed rats. Figures 8A and 8B illustrate EMG responses of external oblique muscles of lower abdomen during graded UBD and slow filling (analytical data are not shown) of the bladder, respectively, before and after BOM (10mg/Kg, i.v.) injection. BOM markedly attenuated VMRs during graded UBD (middle panel of 8A) and to slow filling (middle panel of 8B). This inhibitory effect of BOM was fully reversed after injection of NLX (10mg/Kg, i.v.) shown in bottom two panels of figs 8A and 8B. The mean SRFs clearly show that BOM significantly attenuated VMRs to graded UBD of bladder inflamed rats (Finteraction (5, 264) = 5.716, Fdistension (5, 264) =35.44, Ftreatment (2, 264) = 36.27, *p < 0.01; *p < 0.001; fig. 9B) but failed to produce inhibition in non-inflamed rats (fig. 9A). The inhibitory effect of BOM in bladder inflamed rats was significantly reversed by NLX (10mg/kg, i.v.) (Finteraction (10, 288) = 3.702, Fdistension (5, 288) = 34.71, Ftreatment (2, 288) = 26.81, #p < 0.01; ##p < 0.001; 10mg/Kg, i.v., fig. 9B), suggesting that the antinociceptive effect of BOM is μ opioid receptor-dependent. In another set of experiments, the effect of OXM on VMRs was tested. Our results show that OXM (10mg/Kg, i.v.) also significantly inhibited VMRs to bladder distension in bladder inflamed rats and this effect, which was significantly reversed by NLX (10mg/kg, i.v.) (Finteraction (10, 54) = 4.557, Fdistension (5, 54) =61.61, F treatment (2, 54) = 70.06, * p < 0.01; * p < 0.001; #p < 0.01; ##p < 0.001, n=4, fig. 9C).
Figure 8:
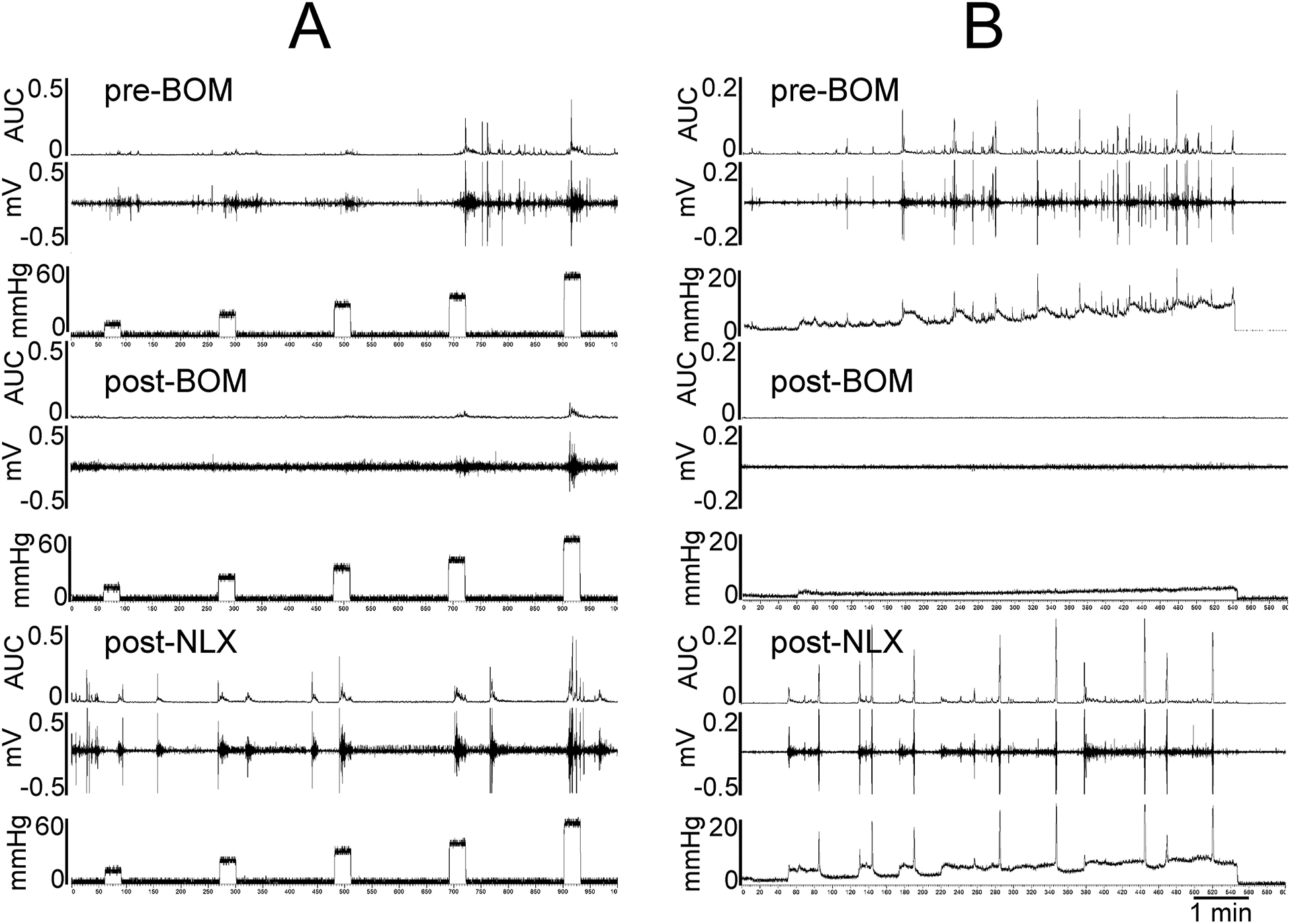
Illustrates viscero-motor responses (VMRs) represented as EMG activities during bladder distension of one awake rat. In each panel, the top trace show the integrated EMG activities represented as are under the curve (AUC). The middle trace is distension-evoked electrical activities (EMG) during muscle contractions and the bottom trace is bladder pressure recorded by external pressure transducer. 8A: shows the VMRs to graded (10–60mmHg, 30s) before (top panel) and after (middle panel) the injection of BOM (10mg/Kg, i.v.) followed by NLX (10mg/Kg, i.v.). The rat exhibited distension intensity-dependent increase in EMG activities prior to BOM injection (top panel). After BOM injection the EMG activities were markedly decreased (middle panel). The bottom panel shows that following NLX injection the rat exhibited increasing EMG activities to graded distension. 8B: shows the EMG responses during the slow filling (0–0.8ml @ 0.1ml/min) of the bladder. The EMG of the abdominal muscle progressively increased with increasing bladder filling pressure (top panel). Following the injection of BOM (10mg/Kg, i.v.) EMG activities completely abolished (middle panel). The drug also inhibited spontaneous contractions of bladder. However, after the injection of naloxone BOM-induced inhibition of EMG not only reversed, but also exhibited an increase in EMG activities (lower panel).
Figure 9:
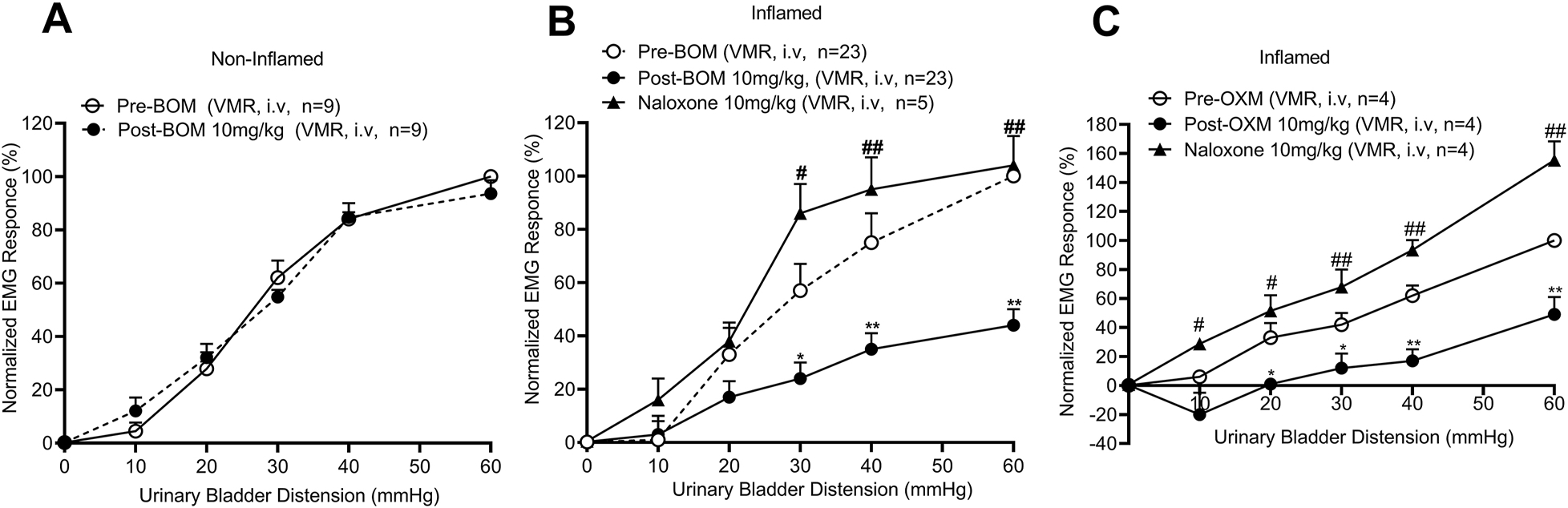
Illustrates the mean SRFs of VMRs before and after the injection of BOM or OXM (10mg/kg, i.v.) injection in non-inflamed and inflamed rats and the effect of NLX (10mg/kg, i.v.). In bladder non-inflamed rats, BOM did not attenuate VMR (9A), whereas in bladder inflamed rats BOM significantly inhibited VMRs to graded UBD (*p < 0.01; **p < 0.001 vs pre-BOM, filled circles, 9B). The injection of NLX reversed and significantly enhanced the VMRs (#p < 0.01; ##p < 0.001 vs post-BOM, filled triangles, 9B). Similarly, OXM (10mg/Kg, i.v.) produced significant inhibition of VMRs (*p < 0.01, **p < 0.001, ***p < 0.0001 vs pre-drug, filled circles, 9C) and naloxone completely reversed the inhibition and significantly enhanced the EMG responses (#p < 0.01, ##p < 0.001, ###p < 0.0001 vs pre-drug, filled triangles, 9C).
3.6. Effect of intravesical injection of BOM or OXM on VMRs to UBD in awake rats
Since the electrophysiology recordings from bladder afferent fibers have shown that both BOM and OXM inhibit mechanosensitivity of these fibers to bladder distension, it is conceivable that these compounds can inhibit the impulse transmission by blocking the generator potential at the nerve endings or by blocking the propagation of nerve action potentials. To test whether these compounds act locally at the nerve terminals, we instilled BOM or OXM into the inflamed bladder and measured the VMR to graded UBD. Intravesical application of BOM (0.1ml of 10mg/ml) significantly attenuated VMR to graded UBD in inflamed rats (Finteraction (10, 54) = 2.366, Fdistension (5, 54) =36.40, Ftreatment (2, 54) = 28.71, *p < 0.01; **p < 0.001, ***p < 0.0001; fig. 10A), suggesting a local effect of BOM. This inhibition of VMR fully reverted after instillation of NLX (0.1ml of 10mg/ml, #p < 0.01; ##p < 0.001, ###p < 0.0001 vs post-BOM). Similarly, intravesical application of OXM (0.1ml of 10mg/ml) significantly attenuated VMR to graded UBD, which was reversed by NLX (10mg/kg, i.v., Finteraction (10, 54) = 1.846, Fdistension (5, 54) = 36.18, Ftreatment (2, 54) = 42.30, *p < 0.01; #p < 0.01; ##p < 0.001, ###p < 0.001, fig. 10B).
Figure 10:
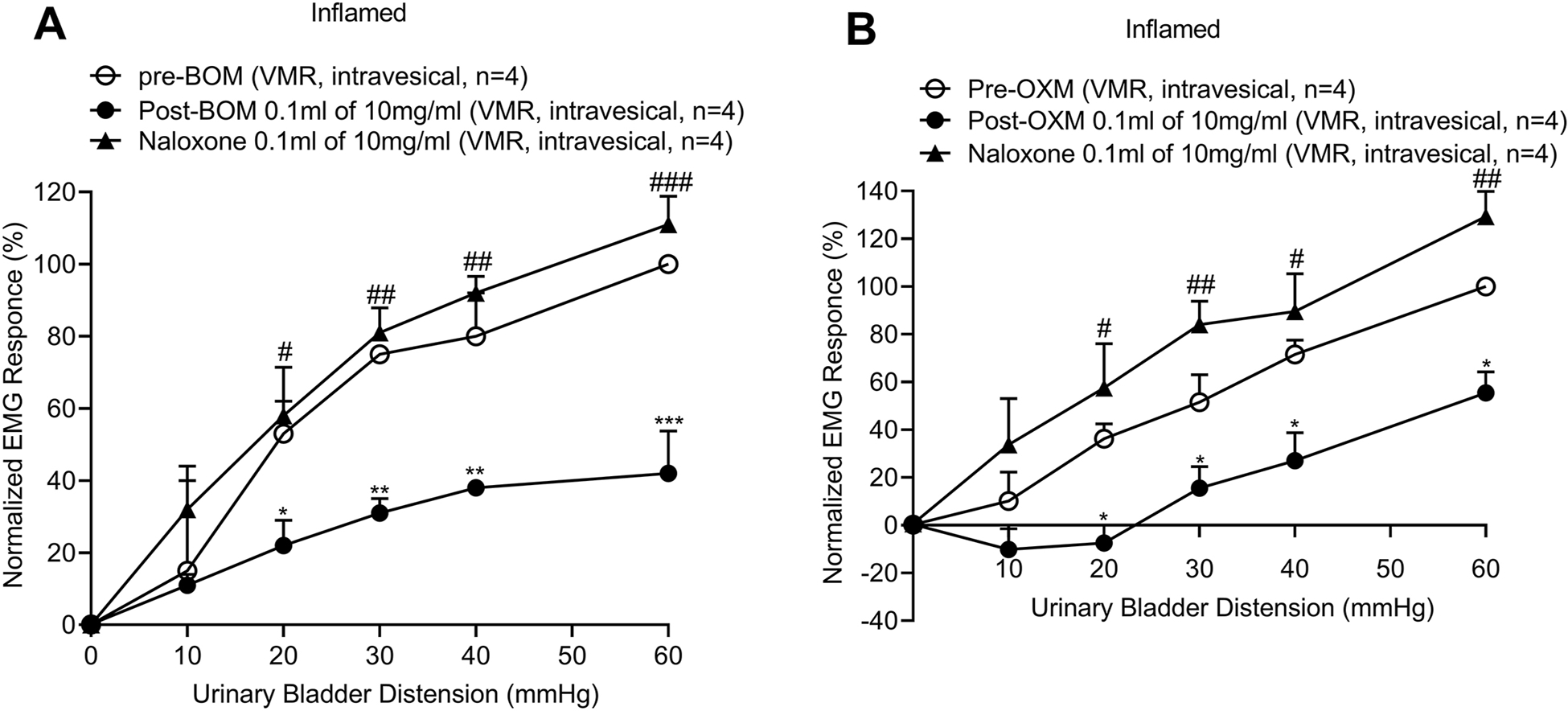
The mean VMRs to graded (10–60mmHg, 30s) UBD in awake rats before and after intravesicular instillation of BOM or OXM and reversal of effects of these drugs’ by NLX. 10A: shows the mean SRFs of VMRs to UBD before and after the instillation of BOM (0.1ml of 10mg/ml) into the urinary bladder. About 5 minutes after BOM instillation the mean VMR was significantly inhibited (*p < 0.01, **p < 0.001, ***p < 0.0001 vs pre-BOM, n=4, filled circles). This inhibition was significantly reversed after the instillation of NLX (0.1ml of 10mg/ml) into the bladder (#p < 0.01, ##p < 0.001, ###p < 0.0001 vs post-BOM, n=4, filled triangles). 10B: shows the mean SRFs to graded UBD before and after the instillation of OXM (0.1ml of 10mg/ml) into the bladder. OXM significantly inhibited the mean VMRs (*p < 0.001 vs pre-OXM, n=4, filled circles). This inhibition of VMRs was significantly reverted after the intravesical instillation of NLX (0.1ml of 10mg/ml, #p < 0.001, ##p < 0.0001 vs post-BOM, n=4).
4. Discussion
IC/PBS is one of the major factors that causes chronic pelvic pain (CPP). The prevalence of CPP is significantly higher in women than in men (5:1) (Berry et al., 2011). The exact etiology of PBS/IC is not known, but there could be multiple contributing factors including underlying chronic bladder inflammation, subclinical infection, urothelial dysfunction, autoimmunity, genetics, and hyperexcitability of peripheral and central neurons (Grover et al., 2011; Jhang and Kuo, 2016; Sant, 2002). Currently, there is no safe and highly potent drug that can manage chronic visceral pain including IC/PBS. The use of opioid in severe bladder pain is controversial and often it is associated with multiple CNS effects. For example, MOR agonists morphine, fentanyl and codeine can cause euphoria, sedation, respiratory depression, antidiuresis, and urinary retention (Quirion et al., 2020). Similarly, κ opioid receptor agonists like U50,488 produce diuresis, dysphoria, hallucinations, nausea, vomiting, bradycardia, and vasodilation (Katzung, (2009); Khademi et al., 2016). It has been documented that κ opioid receptor agonists like U50,488, fedotozine and EMD 61 753 can produce visceral antinociception by modulating responses of mechanosensitive pelvic nerve afferent fibers innervating colon or bladder and vagal afferent fibers innervating the stomach (Gebhart et al., 2000; Ozaki et al., 2000; Sengupta et al., 1999; Sengupta et al., 1996; Su et al., 1997a, b). However, the inhibitory effect of κ opioid receptor agonists on responses of mechanosensitive afferent fibers is basically via Na+-channel block, not via the activation of opioid receptors (Joshi and Gebhart, 2003; Su et al., 2002).
Compared to μ and κ opioid receptor agonists, the analgesic effects of δ opioid receptor agonists in visceral pain have not been studied in detail, especially their influence on the primary sensory afferents innervating the viscera. The presence of δ opioid receptors in the somatosensory afferent fibers has been documented and the receptors are present in both large and small diameter unmyelinated C-fibers (Ji et al., 1995; Mennicken et al., 2003; Scherrer et al., 2009). The expressions of μ, κ, and δ-like immunoreactivities in rat DRGs have been shown after carrageenan-induced paw inflammation (Bardoni et al., 2014; Wang and Wessendorf, 2001). δ opioid receptors are present both in IB4 and P2X3 expressing non-peptidergic (Francois and Scherrer, 2018) and substance P and CGRP containing peptidergic unmyelinated C-fibers (Guan et al., 2005; He et al., 2010; Wang et al., 2010). The presence of δ opioid receptors on both types of somatosensory afferents supports that these receptors are possibly involved both in thermal and mechanical pain modulation (Dubois and Gendron, 2010; Normandin et al., 2013). In normal non-inflamed skin, opioid agonists including δ opioid receptor agonists do not produce any analgesic effect; however, following Freund’s adjuvant-induced skin inflammation, morphine (μ opioid receptor agonist) and deltorphin (δ opioid receptor agonist) produce analgesic effects. IHC examination has revealed higher expressions of functional μ and δ opioid receptors in cutaneous sensory afferents (Brederson and Honda, 2015).
Due to widespread misuse and addiction of opioids, the U.S. Department of Health Services (USDHS) declared the opioid crisis in 2017 (Iwanicki et al., 2018; Kirson et al., 2017), which led NIH to initiate the HEAL (Helping to End Addiction Long-term; https://heal.nih.gov/) initiative research plan. Goals of the HEAL initiative include: (1) to design safe, effective, and non-addictive analgesics to manage chronic pain; (2) to identify new, innovative medications and therapeutic approaches to treat opioid use disorders; and (3) to implement the strategy of overdose prevention and reversal interventions to save lives and support recovery. The growing availability of bifunctional ligands acting at multiple opioid receptor targets provides unique opportunities to discover safe, effective medications to manage chronic pain conditions (Morphy et al., 2004; Morphy and Rankovic, 2005, 2009; Mosberg et al., 2014).
Therefore, the objective of this study was to test the analgesic effect of BOM in cystitis-induced bladder pain. The first strategy of the study was to investigate whether BOM can peripherally attenuate excitation of distension-sensitive bladder afferent fibers to noxious bladder distension and if the effect is via multiple opioid receptors present in the sensory neurons. The second strategy was to validate the electrophysiology results in behavioral model of bladder pain both in non-inflamed bladder and protamine sulfate and zymosan-induced inflamed bladder. Since our results exhibit that BOM can modulate bladder pain by inhibiting the functions of bladder afferent fibers from cystitis rats, our third strategy was to investigate if inflammation influences the expressions of μ and δ opioid receptors and μ-δ heteromers in primary sensory afferents. BOM is a bifunctional μ opioid receptor partial agonist and δ opioid receptor antagonist. The compound is typically a partial agonist for μ- and δ-opioid receptor antagonist. The compound has been structurally modified from OXM, a MOR agonist, to enhance the affinity for δ opioid receptor and lower the efficacy for μ opioid receptor. Therefore, in the present study we also tested the effect of OXM to identify any major differences of these drugs in modulation of functions of bladder sensory afferents and bladder pain.
The electrophysiology recordings have shown that BOM dose-dependently attenuates responses of UBD-sensitive afferent fibers projecting to lumbar spinal cord via L6 dorsal root. Interestingly, the drug can only inhibit responses of afferent fibers from inflamed bladder, but not the fibers from non-inflamed bladder. Similarly, OXM produced inhibition of responses of bladder afferent fibers from cystitis rats. NLX completely reversed the effect of BOM and OXM, suggesting that the inhibition of fibers’ responses by BOM and OXM is via μ opioid receptors and not a non-specific effect. The role of δ opioid receptor antagonism on modulating the effects on bladder pain is currently unclear: since our main objective with this initial study was to test the peripheral effect of a bifunctional μ opioid receptor agonist in modulation of bladder pain, we did not explore whether δ agonists are similarly effective in this model, or whether BOM would be able to reverse the effects of δ opioid receptor agonists. Since δ opioid receptor agonists produce proconvulsive effects in vivo, their development as analgesics and antidepressants has been limited (Comer et al., 1993; Hong EJ, 1998). Although these results clearly document that BOM inhibits impulse transmission through the spinal bladder afferent fibers, it does not exclude the effect of the drug at the spinal level. It has been shown previously that BOM produces antinociception in the hot plate and tail-flick latency tests following systemic administration (Healy et al., 2017). Subsequently, drug discrimination tests determined that BOM has central availability and produces central μ opioid receptor-mediated effects in rats (Mada et al., 2020). Our current study documents for the first time that apart from its central effect, the drug also exerts peripheral effects by modulating the responses of primary sensory afferents.
The painful bladder distension produces contraction of external oblique muscle of the abdomen in fully awake rats, a pseudaffective reflex action via the spinal pathway (Woodworth and Sherrington, 1904). This phenomenon is known as viscero-motor response (VMR) when the muscular rigidity develops during noxious visceral distension. The VMR is a well standardized test to study the antinociceptive effects of various agents. To validate our electrophysiology results that the attenuation of responses of bladder afferents by BOM can produce visceral antinociception, our next strategy was to test the effect of this drug in a behavioral model of bladder pain. The results clearly demonstrate that BOM (10mg/Kg, i.v.) significantly inhibited VMRs during graded (10–60mmHg) UBD and to slow filling of the bladder in cystitis rats, but not in non-inflamed rats. A striking difference that occurred in this study is that the dose magnitude and potency of the drug to attenuate bladder pain is much less compared to previously observed effects in thermal pain where 20mg/Kg, s.c. of BOM virtually produced indistinguishable effect in hot plate and tail flick tests (Healy et al., 2017; Mada et al., 2020). We believe that the inflammation of the organ plays a critical role on the effective dose of opioids, since the expressions of opioid receptors and functional character of opioid receptors markedly change after inflammation. Multiple studies have shown that inflammation and nerve injured neuropathic pain enhance expressions of μ and δ opioid receptors and μ-δ heteromers in DRGs (Brederson and Honda, 2015; Gomes et al., 2004; Pol et al., 2001; Stein, 2018; Stein and Zollner, 2009; Tiwari et al., 2020; Wenk et al., 2006). Our IHC results clearly show that following bladder inflammation there was a significant increase of μ and δ opioid receptors and μ-δ heteromers in L6 DRGs. Our IHC experiments focused on these three receptor types because BOM binds to μ and δ opioid receptors with highest affinity. Based on previous reports and our present IHC results it is conceivable that BOM is efficacious due to overexpression of functional opioid receptors in DRG cell somas. Our present study complements a previous report that documented that peripherally-restricted opioids attenuate behavioral hyperalgesia in models of inflammatory pain, with little or no effect in normal non-inflamed animals (Stein and Zollner, 2009), suggesting that the activation of opioid receptors on sensory axons decreases afferent excitability. It is also known that the inflammation of peripheral tissues that results in overexpression of opioid receptors leads to increased axonal transport of opioid receptors from the DRG cell somas (Puehler et al., 2004), resulting in enhanced G-protein coupling at sensory nerve terminals. In this context, even a small increase in receptor expression as observed in our present study may not be the only cause of effectiveness of BOM in inflamed animals, but it could also involve other factors including inflammation-induced altered allosteric modulation, binding affinity, intrinsic efficacy, or phosphorylation and other post-translation modifications of the receptor molecules. We have documented in bladder pain measurement experiments that topical application of BOM by injecting the drug into the bladder can also produce antinociception, suggesting that drug binding with the available opioid receptors at the nerve endings can decrease afferent excitability.
In summary, the use of high efficacy μ opioid receptor agonists in treating IC/PBS remains controversial due to the severe central adverse effect profile of this class of receptors. Bifunctional μ and δ opioid receptor ligands offer promise as analgesics with a low propensity to produce tolerance and dependence on repeated administration (Ananthan, 2006). Moreover, studies indicating that the δ-opioid receptor antagonist naltrindole reversed alfentanil-induced respiratory depression (Freye et al., 1992) and enhanced colonic propulsion (Foxx-Orenstein et al., 1998) suggest that a bifunctional opioid ligand may cause lesser respiratory depression and gastrointestinal side effects.
Conclusion
The high efficacy we observed of BOM in blocking visceral pain suggests that bifunctional μ opioid receptor partial agonists also have significant potential in treating inflammatory visceral pain while producing blunted adverse effects. In the context of previous studies indicating low abuse liability, our current results suggest that BOM may provide a powerful therapeutic action for safe, effective pain relief for patients with IC/PBS. The results reinforce our VMR results that BOM possibly produces analgesic effect partly by attenuating functions of bladder afferent fibers.
Highlights.
Bladder inflammation (cystitis) enhances the expressions of μ-, δ-, and μ-δ-heteromer receptors in L6 DRGs.
A bifunctional opioid ligand compound benzylideneoxymorphone (BOM), a partial μ-opioid receptor agonist and δ-opioid receptor antagonist, attenuates mechanotransduction properties of bladder afferent fibers in the lumbar 6 (L6) root.
In objective bladder pain measurement model, BOM significantly inhibits bladder distension-evoked pain.
Results clearly indicate that BOM produce analgesic effect in cystitis.
Results show that BOM produces antinociception by modulating the functions primary sensory neurons innervating the urinary bladder.
Acknowledgements
This study was supported by 2R01DK099201-05-NIDDK grant awarded to Drs. JN Sengupta, B Banerjee and P Sanvanson. The work was partly supported by the funding received from Digestive Disease Center of Medical College of Wisconsin awarded to JN Sengupta in 2018. Dr. CW. Cunningham was supported by the Department of Pharmaceutical and Administrative Sciences at Concordia University Wisconsin School of Pharmacy. We acknowledge Dr. Pradeep Kannampalli who assisted during the initial part of the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest of authors:
None of the listed authors have any conflict of interests.
References
- Ananthan S, 2006. Opioid ligands with mixed mu/delta opioid receptor interactions: an emerging approach to novel analgesics. AAPS J 8, E118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson VR, Perry CM, 2006. Pentosan polysulfate: a review of its use in the relief of bladder pain or discomfort in interstitial cystitis. Drugs 66, 821–835. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Tawfik VL, Wang D, Francois A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, de Nooij JC, Mennicken F, O’Donnell D, Kieffer BL, Woodbury CJ, Basbaum AI, MacDermott AB, Scherrer G, 2014. Delta Opioid Receptors Presynaptically Regulate Cutaneous Mechanosensory Neuron Input to the Spinal Cord Dorsal Horn. Neuron 81, 1443. [DOI] [PubMed] [Google Scholar]
- Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ, 2011. Prevalence of Symptoms of Bladder Pain Syndrome/Interstitial Cystitis Among Adult Females in the United States. Journal of Urology 186, 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brederson JD, Honda CN, 2015. Primary afferent neurons express functional delta opioid receptors in inflamed skin. Brain Res 1614, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin HJ, Diamond CJ, Kavash RW, Cai C, Dyatkin AB, Miskowski TA, Zhang SP, Wade PR, Hornby PJ, He W, 2012. Identification of a dual delta OR antagonist/mu OR agonist as a potential therapeutic for diarrhea-predominant Irritable Bowel Syndrome (IBS-d). Bioorg Med Chem Lett 22, 4869–4872. [DOI] [PubMed] [Google Scholar]
- Burns SM, Cunningham CW, Mercer SL, 2018. DARK Classics in Chemical Neuroscience: Fentanyl. ACS Chem Neurosci 9, 2428–2437. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Chancellor MB, Seki S, Yoshimura N, Tyagi P, Huang L, Lavelle JP, De Groat WC, Fraser MO, 2003. Intravesical protamine sulfate and potassium chloride as a model for bladder hyperactivity. Urology 61, 664–670. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang KJ, De Costa BR, Mosberg HI, Woods JH, 1993. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther 267, 888–895. [PubMed] [Google Scholar]
- Cunningham CW, Elballa WM, Vold SU, 2019. Bifunctional opioid receptor ligands as novel analgesics. Neuropharmacology 151, 195–207. [DOI] [PubMed] [Google Scholar]
- Dahan A, 2006. Opioid-induced respiratory effects: new data on buprenorphine. Palliat Med 20 Suppl 1, s3–8. [PubMed] [Google Scholar]
- Devereaux AL, Mercer SL, Cunningham CW, 2018. DARK Classics in Chemical Neuroscience: Morphine. ACS Chem Neurosci 9, 2395–2407. [DOI] [PubMed] [Google Scholar]
- Dubois D, Gendron L, 2010. Delta opioid receptor-mediated analgesia is not altered in preprotachykinin A knockout mice. Eur J Neurosci 32, 1921–1929. [DOI] [PubMed] [Google Scholar]
- Foley KM, 1993. Opioid analgesics in clinical pain management. In: Akil H, Herz A, Simon EJ, (Eds), Opioids II. Springer-Verlag, New York, pp. 697–744. [Google Scholar]
- Foster HE Jr., Hanno PM, Nickel JC, Payne CK, Mayer RD, Burks DA, Yang CC, Chai TC, Kreder KJ, Peters KM, Lukacz ES, FitzGerald MP, Cen L, Landis JR, Propert KJ, Yang W, Kusek JW, Nyberg LM, Interstitial Cystitis Collaborative Research, N., 2010. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol 183, 1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxx-Orenstein AE, Jin JG, Grider JR, 1998. 5-HT4 receptor agonists and delta-opioid receptor antagonists act synergistically to stimulate colonic propulsion. Am J Physiol 275, G979–983. [DOI] [PubMed] [Google Scholar]
- Francois A, Scherrer G, 2018. Delta Opioid Receptor Expression and Function in Primary Afferent Somatosensory Neurons. Handb Exp Pharmacol 247, 87–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freye E, Latasch L, Portoghese PS, 1992. The delta receptor is involved in sufentanil-induced respiratory depression--opioid subreceptors mediate different effects. Eur J Anaesthesiol 9, 457–462. [PubMed] [Google Scholar]
- Gebhart GF, Su X, Joshi S, Ozaki N, Sengupta JN, 2000. Peripheral opioid modulation of visceral pain. Ann N Y Acad Sci 909, 41–50. [DOI] [PubMed] [Google Scholar]
- Generali JA, Cada DJ, 2014. Amitriptyline: interstitial cystitis (painful bladder syndrome). Hosp Pharm 49, 809–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusto LL, Zahner PM, Shoskes DA, 2018. An evaluation of the pharmacotherapy for interstitial cystitis. Expert Opin Pharmacother 19, 1097–1108. [DOI] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA, 2004. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A 101, 5135–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S, Srivastava A, Lee R, Tewari AK, Te AE, 2011. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 3, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, Elde R, Zimmer A, He C, Pei G, Bao L, Zhang X, 2005. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell 122, 619–631. [DOI] [PubMed] [Google Scholar]
- Hanno PM, 1997. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology 49, 93–99. [DOI] [PubMed] [Google Scholar]
- He BJ, Zempel JM, Snyder AZ, Raichle ME, 2010. The temporal structures and functional significance of scale-free brain activity. Neuron 66, 353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy JR, Bezawada P, Griggs NW, Devereaux AL, Matsumoto RR, Traynor JR, Coop A, Cunningham CW, 2017. Benzylideneoxymorphone: A new lead for development of bifunctional mu/delta opioid receptor ligands. Bioorg Med Chem Lett 27, 666–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, R. K, Calderon SN, Woods JH and Traynor JR, 1998. Convulsive behavior of nonpeptide d-opioid ligands: Comparison of SNC80 and BW373U86 in mice. Analgesia 3, 269–276. [Google Scholar]
- Iwanicki JL, Severtson SG, Margolin Z, Dasgupta N, Green JL, Dart RC, 2018. Consistency Between Opioid-Related Mortality Trends Derived From Poison Center and National Vital Statistics System, United States, 2006–2016. Am J Public Health 108, 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhang JF, Kuo HC, 2016. Pathomechanism of Interstitial Cystitis/Bladder Pain Syndrome and Mapping the Heterogeneity of Disease. Int Neurourol J 20, S95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T, 1995. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci 15, 8156–8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SK, Gebhart GF, 2003. Nonopioid actions of U50,488 enantiomers contribute to their peripheral cutaneous antinociceptive effects. J Pharmacol Exp Ther 305, 919–924. [DOI] [PubMed] [Google Scholar]
- Kannampalli P, Babygirija R, Zhang J, Poe MM, Li G, Cook JM, Shaker R, Banerjee B, Sengupta JN, 2017. Neonatal bladder inflammation induces long-term visceral pain and altered responses of spinal neurons in adult rats. Neuroscience 346, 349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzung BG, Masters SB and Trevor AJ, (2009) Basic and Clinical Pharmacology. 11th Edition,. [Google Scholar]
- Khademi H, Kamangar F, Brennan P, Malekzadeh R, 2016. Opioid Therapy and its Side Effects: A Review. Arch Iran Med 19, 870–876. [DOI] [PubMed] [Google Scholar]
- Kirson NY, Scarpati LM, Enloe CJ, Dincer AP, Birnbaum HG, Mayne TJ, 2017. The Economic Burden of Opioid Abuse: Updated Findings. J Manag Care Spec Pharm 23, 427–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusty A, Kavaler E, Zakariasen K, Tolls V, Nickel JC, 2018. Treatment effectiveness in interstitial cystitis/bladder pain syndrome: Do patient perceptions align with efficacy-based guidelines? Can Urol Assoc J 12, E1–E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Cowan A, 2004. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol 2, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mada S, Gerak LR, Soyer A, Maguire DR, Hu Z, Minervini V, Cunningham CW, France CP, 2020. Behavioral effects of benzylideneoxymorphone (BOM), a low efficacy micro opioid receptor agonist and a delta opioid receptor antagonist. Psychopharmacology (Berl) 237, 3591–3602. [DOI] [PubMed] [Google Scholar]
- Madras BK, 2018. The President’s Commission on Combating Drug Addiction and the Opioid Crisis: Origins and Recommendations. Clin Pharmacol Ther 103, 943–945. [DOI] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O’Donnell D, 2003. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol 465, 349–360. [DOI] [PubMed] [Google Scholar]
- Miranda A, Mickle A, Schmidt J, Zhang Z, Shaker R, Banerjee B, Sengupta JN, 2011. Neonatal cystitis-induced colonic hypersensitivity in adult rats: a model of viscero-visceral convergence. Neurogastroenterol Motil 23, 683–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphy R, Kay C, Rankovic Z, 2004. From magic bullets to designed multiple ligands. Drug Discov Today 9, 641–651. [DOI] [PubMed] [Google Scholar]
- Morphy R, Rankovic Z, 2005. Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem 48, 6523–6543. [DOI] [PubMed] [Google Scholar]
- Morphy R, Rankovic Z, 2009. Designing multiple ligands - medicinal chemistry strategies and challenges. Curr Pharm Des 15, 587–600. [DOI] [PubMed] [Google Scholar]
- Mosberg HI, Yeomans L, Anand JP, Porter V, Sobczyk-Kojiro K, Traynor JR, Jutkiewicz EM, 2014. Development of a bioavailable mu opioid receptor (MOPr) agonist, delta opioid receptor (DOPr) antagonist peptide that evokes antinociception without development of acute tolerance. J Med Chem 57, 3148–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland SG, Hanno P, Parsons CL, Sant GR, Staskin DR, 1990. Pentosan polysulfate sodium for therapy of interstitial cystitis. A double-blind placebo-controlled clinical study. Urology 35, 552–558. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Randich A, 2010. Neonatal bladder inflammation alters activity of adult rat spinal visceral nociceptive neurons. Neurosci Lett 472, 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niku SD, Stein PC, Scherz HC, Parsons CL, 1994. A new method for cytodestruction of bladder epithelium using protamine sulfate and urea. J Urol 152, 1025–1028. [DOI] [PubMed] [Google Scholar]
- Normandin A, Luccarini P, Molat JL, Gendron L, Dallel R, 2013. Spinal mu and delta opioids inhibit both thermal and mechanical pain in rats. J Neurosci 33, 11703–11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Sengupta JN, Gebhart GF, 2000. Differential effects of mu-, delta-, and kappa-opioid receptor agonists on mechanosensitive gastric vagal afferent fibers in the rat. J Neurophysiol 83, 2209–2216. [DOI] [PubMed] [Google Scholar]
- Pape J, Falconi G, De Mattos Lourenco TR, Doumouchtsis SK, Betschart C, 2019. Variations in bladder pain syndrome/interstitial cystitis (IC) definitions, pathogenesis, diagnostics and treatment: a systematic review and evaluation of national and international guidelines. Int Urogynecol J. [DOI] [PubMed] [Google Scholar]
- Parsons CL, Benson G, Childs SJ, Hanno P, Sant GR, Webster G, 1993. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosanpolysulfate. J Urol 150, 845–848. [DOI] [PubMed] [Google Scholar]
- Pol O, Alameda F, Puig MM, 2001. Inflammation enhances mu-opioid receptor transcription and expression in mice intestine. Mol Pharmacol 60, 894–899. [DOI] [PubMed] [Google Scholar]
- Puehler W, Zollner C, Brack A, Shaqura MA, Krause H, Schafer M, Stein C, 2004. Rapid upregulation of mu opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience 129, 473–479. [DOI] [PubMed] [Google Scholar]
- Quirion B, Bergeron F, Blais V, Gendron L, 2020. The Delta-Opioid Receptor; a Target for the Treatment of Pain. Front Mol Neurosci 13, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, 2018. The Staining of Mast Cells: A Historical Overview. Int Arch Allergy Immunol 176, 55–60. [DOI] [PubMed] [Google Scholar]
- Sant GR, 2002. Etiology, pathogenesis, and diagnosis of interstitial cystitis. Rev Urol 4 Suppl 1, S9–S15. [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI, 2009. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137, 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF, 1994. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 72, 2420–2430. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Medda BK, Shaker R, 2002. Effect of GABA(B) receptor agonist on distension-sensitive pelvic nerve afferent fibers innervating rat colon. Am J Physiol Gastrointest Liver Physiol 283, G1343–1351. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Pochiraju S, Kannampalli P, Bruckert M, Addya S, Yadav P, Miranda A, Shaker R, Banerjee B, 2013. MicroRNA-mediated GABA Aalpha-1 receptor subunit down-regulation in adult spinal cord following neonatal cystitis-induced chronic visceral pain in rats. Pain 154, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Snider A, Su X, Gebhart GF, 1999. Effects of kappa opioids in the inflamed rat colon. Pain 79, 175–185. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Su X, Gebhart GF, 1996. Kappa, but not mu or delta, opioids attenuate responses to distention of afferent fibers innervating the rat colon. Gastroenterology 111, 968–980. [DOI] [PubMed] [Google Scholar]
- Stein C, 2013. Targeting pain and inflammation by peripherally acting opioids. Front Pharmacol 4, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, 2018. New concepts in opioid analgesia. Expert Opin Investig Drugs 27, 765–775. [DOI] [PubMed] [Google Scholar]
- Stein C, Zollner C, 2009. Opioids and sensory nerves. Handb Exp Pharmacol, 495–518. [DOI] [PubMed] [Google Scholar]
- Su X, Joshi SK, Kardos S, Gebhart GF, 2002. Sodium channel blocking actions of the kappa-opioid receptor agonist U50,488 contribute to its visceral antinociceptive effects. J Neurophysiol 87, 1271–1279. [DOI] [PubMed] [Google Scholar]
- Su X, Sengupta JN, Gebhart GF, 1997a. Effects of kappa opioid receptor-selective agonists on responses of pelvic nerve afferents to noxious colorectal distension. J Neurophysiol 78, 1003–1012. [DOI] [PubMed] [Google Scholar]
- Su X, Sengupta JN, Gebhart GF, 1997b. Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 77, 1566–1580. [DOI] [PubMed] [Google Scholar]
- Tiwari V, He SQ, Huang Q, Liang L, Yang F, Chen Z, Tiwari V, Fujita W, Devi LA, Dong X, Guan Y, Raja SN, 2020. Activation of micro-delta opioid receptor heteromers inhibits neuropathic pain behavior in rodents. Pain 161, 842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzan CJ, Berg J, Lewis SA, 1993. Effect of protamine sulfate on the permeability properties of the mammalian urinary bladder. J Membr Biol 133, 227–242. [DOI] [PubMed] [Google Scholar]
- van Ophoven A, Hertle L, 2005. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol 174, 1837–1840. [DOI] [PubMed] [Google Scholar]
- Wade PR, Palmer JM, McKenney S, Kenigs V, Chevalier K, Moore BA, Mabus JR, Saunders PR, Wallace NH, Schneider CR, Kimball ES, Breslin HJ, He W, Hornby PJ, 2012. Modulation of gastrointestinal function by MuDelta, a mixed micro opioid receptor agonist/ micro opioid receptor antagonist. Br J Pharmacol 167, 1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wessendorf MW, 2001. Equal proportions of small and large DRG neurons express opioid receptor mRNAs. J Comp Neurol 429, 590–600. [DOI] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X, 2010. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A 107, 13117–13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way EL, 1993. Opioid tolerance and physical dependence and their relationship. In: Akil H, Herz A, Simon EJ, (Eds), Opioids II. Springer-Verlag, New York, pp. 573–596. [Google Scholar]
- Wenk HN, Brederson JD, Honda CN, 2006. Morphine directly inhibits nociceptors in inflamed skin. J Neurophysiol 95, 2083–2097. [DOI] [PubMed] [Google Scholar]
- Woodworth RS, Sherrington CS, 1904. A pseudaffective reflex and its spinal path. J Physiol 31, 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillioux J, Clements M, Pike CW, Rapp D, 2020. Opioid prescription use in patients with interstitial cystitis. International Urogynecology Journal. [DOI] [PubMed] [Google Scholar]


