Abstract
In this review, we examine the role of microRNAs in the development of the prefrontal cortex (PFC) in adolescence and in individual differences in vulnerability to mental illness. We describe results from clinical and preclinical research indicating that adolescence coincides with drastic changes in local microRNA expression, including microRNAs that control gene networks involved in PFC and cognitive refinement. We highlight that altered levels of microRNAs in the PFC are associated with psychopathologies of adolescent onset, notably depression and schizophrenia. We show that microRNAs can be measured non-invasively in peripheral samples and could serve as longitudinal physiological readouts of brain expression and psychiatric risk in youth.
Keywords: Biomarkers, Vulnerability, Resilience, MiR-218, DCC receptors, Depression, Schizophrenia
1. Introduction
Adolescence is a developmental period marked by sexual maturation, novel-experience seeking, and transition in cognitive skills. This malleable window is sensitive to perturbations including drugs of abuse and stress, which can render individuals at risk for mental illness. Enhanced psychiatric vulnerability in adolescence has been attributed to substantial changes occurring in the maturing prefrontal cortex (PFC) during this time. Dysfunction of the PFC is linked to changes in personality, emotional response, memory, attention, and social behavior [1–7], and is observed in psychopathologies that emerge in adolescence [8–11]. The molecular mechanisms and the timeline underlying susceptibility or resilience to PFC dysfunction remain elusive. MicroRNAs are essential coordinators of developmental programing, and mediators between environmental factors and changes in gene expression. In this review we propose that microRNA-mediated shaping of PFC circuitry maturation in adolescence may be an important determinant of lifetime mental health.
1.1. MicroRNAs: functional complexity
Microribonucleic acids, or microRNAs, are small RNA molecules that serve as key regulators of large-scale gene expression. MicroRNAs are non-coding RNA 19–25 nucleotides long and are single-stranded. They can match and bind seed regions of several different messenger RNAs (mRNAs). This property allows a single microRNA to control and coordinate activity of entire gene networks and cellular pathways [12]. MicroRNA biogenesis is a multi-step process. Briefly, the more common canonical biogenesis starts with the transcription of primary microRNAs, which are double-stranded long hairpin-like structures with a polyA tail. The polyA tail is then cleaved by the microprocessor complex (Drosha/DGCR8), generating a double-stranded precursor microRNA, which is exported from the nucleus to the cytoplasm by the Exportin-5 protein. Once in the cytoplasm, Dicer endoribonuclease cleaves the precursor microRNA, to produce a single-stranded and functional mature microRNA, either the forward 5’ (5p) or the reverse 3’ direction (3p) of the original strand. The mature microRNA is then loaded into an Argonaute family protein (AGO 1–4 in humans) to form a microRNA-induced silencing complex (miRISC) that interacts with the 3’-untranslated region (UTR) of the mRNA target (for detailed reviews see [13,14]).
The binding of microRNAs to perfectly complementary mRNA sequences (2–7 nucleotides) of target genes usually induces transcript degradation and/or prevention of mRNA translation [13]. However, increased mRNA translation upon microRNA binding to mRNAs targets has also been described [15,16]. Mature functional microRNAs can be found in the cell’s cytoplasm and in the nucleus, and their distribution can change in response to environmental factors [17,18]. MicroRNAs localized to the cytoplasm and those in the nucleus seem to be involved in different processes. Nuclear microRNAs can trigger gene transcription by binding and activating enhancer regions [19] or by directly binding to promoter regions [20]. The variety of mechanisms involved in microRNA control over gene expression indicates the complexity of the system and that much remains to be uncovered.
1.2. Contribution of microRNAs to early cortical development
The majority of microRNAs are evolutionary conserved and play analogous biological functions across species, including in neurodevelopment. In this review we focus on the role of microRNAs in the development of the cerebral cortex, the most superficial sheet of the mammalian brain, which is involved in higher-order cognitive function and sensory processing. MicroRNAs are proving to be essential regulators of cortical development [21], playing critical roles in the production of progenitor cells [22–25] and in the survival, differentiation and spatiotemporal organization of cortical neurons [26–29]. A microRNA network appears to have evolved to specifically shape the developmental fate of corticospinal and callosal projection neurons, which are at the root of mammalian (more specifically eutherian) brain anatomy and function [30]. Obstruction of microRNA function or of enzymes involved in microRNA synthesis (e.g. Dicer, DGCR8) induces dramatic disruption of cortical development, from cell viability and premature progenitor differentiation during corticogenesis to cortical malformations [29,31,32]. MicroRNA control over cortical development extends to adolescence [33], when the prefrontal portion of the cortex continues to undergo substantial maturation [34–42], remaining particularly vulnerable to environmental factors.
2. Adolescence is a critical period for PFC development, microRNA expression, and psychiatric vulnerability
The PFC is often characterized as the “executive” center where information is processed and integrated to carry out complex functions, including planning, decision-making and goal-directed behavior. One third of the cerebral cortex in primates is the anterior region of the frontal lobe [43], although in rodents the PFC takes smaller percentage of the total cortical area. As reviewed elsewhere, differences in PFC cytoarchitecture between primates and rodents are important limitations to the translation of findings across species [8,44,45]. However, both anatomically and functionally, the PFC is believed to be homologous between primates and rodents, with some disagreement remaining as to the specific correspondences between PFC subregions between primates and rodents [45].
A common feature of the PFC in rodents and primates is that it continues to sustain significant structural and functional maturation across the adolescent period and into early adulthood [34–38,42]. This protracted development is accompanied by corresponding transitions in behaviors and cognitive function, including the gradual stabilization of emotional reactivity, novelty seeking, cognitive control, and decision-making [36,46]. During adolescence, the PFC undergoes gray matter volume reduction, white matter content increase, and refinement in circuitry cytoarchitecture, including axonal myelinization, dendritic morphology and synaptic density [34,38,47]. The pari passu unfolding of structural and behavioral correlates indicates that proper PFC development is critical for the maturation of cognition and behavior. Adolescence is in fact a sensitive developmental time that sets the stage for lifelong mental health (see [48]).
Here we review evidence implicating microRNAs in adolescent maturation of the PFC and in the increased vulnerability to psychiatric disorders during this age. Nevertheless, changes in microRNA expression in the cortex at any point of the developmental continuum can affect the function of gene networks controlling ongoing homeostatic processes [49]. Emerging results from rodent studies supports the notion that microRNAs are key epigenetic mediators of early environmental programing on neurocircuitry, cognitive development and function throughout the lifespan [33,50,51], even lasting across generations [52, 53].
Human postmortem brain studies have shown that microRNA expression in the PFC is highly influenced by age with some microRNAs being differentially expressed between infancy, early and late childhood, and adolescence [54]. Remarkably, the adolescent period coincides with a shift in the pattern of global microRNA expression. A study analyzing average expression of microRNAs in the PFC from neonates to older adults found that precisely in adolescence the pattern of microRNA expression splits into two diverging directions: while some microRNAs begin to be upregulated, others are downregulated, with the new pattern of expression maintained for the rest of life [55]. To probe possible biological implications of the age-associated microRNA expression split, the authors performed gene target, pathway and function analyses. They found that gene targets of the “split” microRNAs are involved in nervous system development and function, cell-to-cell signaling and interactions (e.g. synaptic transmission and axon and neurite morphology) and have been associated with schizophrenia, bipolar and other mood disorders. This study also revealed that the expression of genes involved in microRNA biogenesis, notably EXPORTIN-5 and DICER, also switches from teenagehood onward [55]. These findings suggest that abnormalities in the adolescent pattern of microRNA expression trajectories in the PFC could lead to predisposition to mental illness.
Dysfunction of the PFC has been strongly implicated in the etiology of psychiatric disorders of adolescent onset, including schizophrenia, major depressive disorder (MDD) and substance use disorder (see: [8, 56–58]). An increasing number of studies, including those by our group, have identified alterations in microRNA expression in postmortem PFC samples of psychiatric patients. A summary of results from studies in schizophrenia, bipolar disorder, depression, and substance use disorder, are presented in Table 1. Some microRNAs have been found to be dysregulated in multiple disorders, suggesting that they may play a role in core neurodevelopmental processes. miR-29, for example, is involved in cortical maturation [59], neuronal differentiation [60], neuron survival [61], and synapse formation [62], and appears to be altered in both schizophrenia and bipolar disorder. In fact, a recent study in rodents links miR-29 to neurobehavioral deficits through the regulation of postnatal cortical maturation [63]. Studies are needed to assess the role and timing of specific microRNAs in the pathogenesis of these disorders.
Table 1.
Evidence showing alterations in microRNA expression in postmortem prefrontal cortex samples of psychiatric patients. The methods for measuring microRNA expression vary across studies, most likely explaining the variability of findings.
| microRNAs | Cortex region | Human condition | Reference |
|---|---|---|---|
| miR-26b, miR-30b, miR-29b, miR-195, miR-92, miR-30a-5p, miR-30d, miR-20b, miR-29c, miR-29a, miR-212, miR-106b, miR-7, miR-24, miR-30e, miR-9-3p | PFC BA-9 | Schizophrenia vs. non-psychiatric control subjects | Perkins et al., 2007 [149] |
| miR-346 | BA46 | Zhu et al., 2009 [150] | |
| miR-34a, miR-132, miR-212, miR-544, miR-7, miR-154, | Dorsolateral PFC BA-46 | Schizophrenia vs. control individuals | Kim et al., 2010 [151] |
| miR-504, miR-454, miR-29a, miR-520c-3p, miR-140-3p, miR-145, miR-767-5p, miR-22, miR-145, miR-874, miR-133b, miR-154, miR-32, miR-573, miR-889 | Bipolar vs. control individuals | ||
| let-7d, miR-128a, miR-16, miR-181a, b, miR-20a, miR-219, miR-27a, miR-29c, miR-7 | Dorsolateral PFC BA-9 | Schizophrenia vs. control individuals | Beveridge et al., 2010 [152] |
| miR-553, miR-369-3p, miR-18a, miR-339-5p, miR-1, miR-7, miR-196a, miR-301a, miR-144, let-7g, miR-153, let-7f, miR-203, miR-34c-5p, miR-101, miR-376c, miR-665, miR-152, miR-194, miR-423-5p, miR-515-3p, miR-374b, miR-140, miR-519b-3p, miR-586, miR_135b, miR-92a, miR-15b, miR-580, miR-146a, miR-454-3p, miR-380, miR-652, miR-802, miR-196b | PFC | Alcohol user vs. control | Lewohl et al., 2011 [153] |
| miR-328, miR-17-5p, miR-134, miR-652, miR-382, and miR-107 | Dorsolateral PFC BA-46 | Schizophrenia/schizoaffective disorder vs. control individuals | Santarelli et al., 2011 [154] |
| miR-330, miR-33, miR-193b, miR-545, miR-138, miR-151, miR-210, miR-324-3p, miR-22, miR-425, miR-181a, miR-106b, miR-193a, miR-192, miR-301, miR-27b, miR-148b, miR-338, miR-639, miR-15a, miR-186, miR-99a, miR-190, miR-339 | BA-9 | Schizophrenia vs. control individuals AND Bipolar vs. control individuals | Moreau et al., 2011 [155] |
| miR-132 | Dorsolateral BA-46 | Schizophrenia vs. control individuals | Miller et al., 2012 [156] |
| miR-383, miR-32, miR-490-5p, miR-165b, miR-513-5p, miR-876-3p, miR-449b, miR-297, miR-188-5p, miR-187 |
miR-142-5p, miR-137, miR-489, miR-148b, miR-101, miR-324-5p, miR-301a, miR-146a, miR-335, miR-494, miR-20b, miR-376a, miR-190, miR-155, miR-660, miR-130a, miR-27a, miR-497, miR-10a, miR-20a, miR-142-3p |
Bipolar vs. control individuals PFC BA-9 |
Depressed suicide vs. control individuals |
| Smalheiser et al., 2012 [157] | |||
| miR-185 | Anterior PFC BA-10 | Depression vs. control individuals | Maussion et al., 2012 [158] |
| hsa-miR-375, hsa-miR-3065-5p, hsa-miR-488-star, hsa-miR-299-3p, hsa-miR-377, hsa-mir-516a-2, hsa-miR-767-5p, hsa-miR-493, hsa-miR-379, hsa-miR-105, hsa-miR-29b, hsa-miR-149 | Frontal BA-9 | Alcohol user vs. control | Manzardo et al., 2013 [159] |
| miR-31, miR-33, miR-96, miR-28, miR-30e-5p, miR-199a, miR-501, miR-504, miR-15b, miR-29c, miR-455, miR-380-3p, miR-323, miR-527, miR-93, miR-32, miR-20b, miR-516-5p, miR-92, miR-30a-3p, miR-497 etc. | PFC BA-9 | Differential expression for schizophrenia, bipolar and control groups | Banigan et al., 2013 [160] |
| miR-17-5p, miR-331-5p, miR-16-5p, miR-187-3p, miR-106b-5p, miR-485-5p, miR-129-2-3p, miR-454-3p, miR-185-5p, miR-429-3p, miR-511, miR-18a-5p, miR-590-5p, miR-106a-5p, miR-145-5p, miR-642a-5p, miR-625-5p, miR-508-3p, miR-219-2-3p | PFC BA-10 | Schizophrenia vs. control individuals | Smalheiser et al., 2014 [161] |
| miR-17-5p, miR-145-5p, miR-579, miR-106b-5p, miR-485-5p, miR-370, miR-500a-5p, miR-34a-5p, miR-29c-3p | Bipolar vs. control individuals | ||
| miR-508-3p, miR-152-3p | Depression vs. control individuals | ||
| miR-34c-5p, miR-139-5p, miR-195, miR-320c | Ventrolateral PFC BA-44 | Depression vs. control individuals | Lopez et al., 2014 [162] |
| miR-1202 | Ventrolateral PFC BA-44 | Depression vs. control individuals | Lopez et al., 2014 [163] |
| miR-218 | Ventrolateral PFC BA-44 | Depression vs. control individuals | Torres-Berrio et al., 2017 [79] |
| miR-124 | Dorsolateral PFC BA-46 | Depression vs. control individuals | Roy et al., 2017 [164] |
| miR-146a-5p, miR-146b-5p, miR-24-3p, miR-425-3p | Ventrolateral PFC BA-44 | Depression vs. control individuals | Lopez et al., 2017 [165] |
| miR-19a-13p | Dorsolateral BA-10 | Depression vs. control individuals | Wang et al., 2018 [166] |
| miR-3162, miR-936 | BA-46 | Schizophrenia vs. control individuals | Hu et al., 2019 [167] |
| miR-30e | Dorsolateral PFC BA-9 | Depression vs. control individuals | Gorinski et al., 2019 [168] |
2.1. Role of miR-218 in psychiatric disorders of PFC dysfunction and adolescent onset
Major depression is one of the most widespread psychiatric disorders, ranked by the World Health Organization as the leading cause of disability worldwide [64], and as many as 40% of patients diagnosed with MDD do not respond to common antidepressant therapies [65–67]. The incidence of depression is high between the ages 12–25 [68–71] and an alarming 25% of adolescents meet the criteria for depression [72–75]. Experiencing a depressive episode in adolescence increases the risk of depression and of higher severity in adulthood up to 3-fold [76–78].
In our studies investigating microRNAs involved in PFC maturation and MDD vulnerability, we identified the microRNA miR-218-5p (whose matured sequence originates from the 5’ strand of its miR-218-1 or miR-218-2 stem loop precursors), as a repressor of the DCC gene. DCC is a receptor for the guidance cue Netrin-1 [79] and is intimately involved in the formation of neuronal networks during early neurodevelopment. Notably, DCC-mediated Netrin-1 signaling controls the protracted maturation of the PFC circuitry specifically in adolescence [80–82]. Using postmortem brain samples, we showed that DCC expression in the PFC of adult individuals who were diagnosed with MDD and died by suicide is significantly upregulated compared to control individuals who died by sudden death [79]. We replicated this finding in two independent cohorts [79,80]. To probe the role of miR-218–5p (henceforth referred to as miR-218) in the DCC expression changes observed in MDD, we assessed its expression in the same postmortem PFC samples and found that miR-218 is downregulated in MDD by approximately 50% compared to controls. Reduced levels of miR-218 correlate with elevated expression of DCC in the PFC of individuals with depression, suggesting a causal link between miR-218 and DCC alterations in MDD pathogenesis [79].
A rapidly increasing number of studies show that changes in DCC are linked to vulnerability to psychopathologies of adolescent onset and involving PFC dysfunction, most prominently MDD (reviews from [83, 84]). Furthermore, we have shown that variation in the DCC gene co-expression network within the PFC is associated with total brain volume across childhood, highlighting the role of this system in broad postnatal neurodevelopment [85]. Whether genetic variants (single nucleotide polymorphisms) within DCC (see Ref. [86]) affect the binding of microRNAs, or whether changes in expression of the DCC network are linked to altered microRNA function, remains to be shown.
Postmortem studies cannot reveal whether alterations in microRNA expression in MDD mediate atypical development of the PFC and/or play a causal role in disorder symptomatology. MicroRNAs are highly conserved, and miR-218-5p is homologous between humans, macaques, and rodents (UCSC genome browser [87]), offering a link between preclinical and translational studies. The social defeat stress paradigm is a well-established model used in rodents to study stress-induced behavioral abnormalities that resemble depression-like traits [88,89]. Using this model, we assessed the effects of reducing the levels of miR-218 directly in the PFC of adult male rodents via anti-sense oligonucleotides (“antagomirs”). Consistent with the reduced expression of miR-218 in the PFC of MDD patients, we found that downregulating miR-218 in the adult mouse PFC increases Dcc expression and elicits vulnerability to stress-induced depression-like behavioral abnormalities [90]. Furthermore, in mice that show resilience to stress, intranasal administration of miR-218 antagomir induces a similar vulnerability. Upregulating miR-218 in the PFC, via viral-mediated gene transfer, instead reduces Dcc expression and protects against stress-induced depression-like behavioral traits, pointing at miR-218 as a potential therapeutic target. Altered miR-218 levels in the PFC have also been reported in adult stress-exposed rats (either through the chronic stress paradigm or corticosterone injections) [91], and in adult mice exposed to chronic unpredictable mild stress [92].
Given that DCC receptors control the adolescent maturation of the PFC [80–82], we investigated miR-218 expression in the mouse PFC across postnatal life. Consistent with the adolescent shift in global microRNA expression observed in the PFC in humans [54,55], miR-218 levels in mice increase from early adolescence to adulthood. Postnatal PFC miR-218 and Dcc expression in mice correlate negatively [33]. Disrupting this developmental pattern has enduring behavioral consequences: downregulation of miR-218 in the PFC of adolescent mice via antagomir microinfusion, induces resilience to detrimental effects of chronic social defeat stress in adulthood. This finding is opposite to the effects seen following miR-218 downregulation in the adult matured PFC, yet in line with the idea that changes in the adolescent pattern of microRNA expression in the PFC are associated with vulnerability to developing psychiatric traits. Interestingly, results from a preliminary experiment show that intranasal administration of miR-218 antagomir in adolescent male mice leads to reduced anxiety-like behavior in adulthood, without affecting motor abilities [93–95], indicating that targeting microRNA expression in adolescence may have enduring preventative and/or treatment benefits.
The opposite effects that adolescent versus adult antagomir-218 microinfusions in the PFC have on stress vulnerability are intriguing [33,79,90], particularly within the context of miR-218 regulation of Dcc expression across postnatal life [33]. The Netrin-1/DCC guidance cue pathway organizes neural connectivity in the developing and matured brain via age-specific molecular and cellular processes. In adolescence, DCC-mediated Netrin-1 signaling is involved in axonal targeting and growth, whereas in the adult brain this signaling pathway controls the refinement of already established circuitries, by modifying neuronal structure, including dendritic spine morphology [83,84]. The role of miR-218 in shaping psychiatric vulnerability or resilience is likely to be dictated by the function of the Netrin-1/DCC pathway at that particular developmental period. Downregulating miR-218 in the adolescent PFC, when its levels are significantly lower than in adulthood, would prolong high expression of Dcc and likely extend the postnatal window of axonal targeting and growth and synapse formation. In contrast, reducing miR-218 in the adult PFC, would bring Dcc expression to levels observed in the immature brain, eliciting aberrant changes in the organization of synaptic circuitry. RNA sequencing based strategies assessing alterations in PFC gene expression under antagomir-218 treatment in adolescence versus adulthood would aid in elucidating gene networks and pathways involved in the distinct behavioral outcomes.
2.2. microRNAs may coordinate the organization of synaptic circuits in the developing adolescent PFC
The maturation of the PFC requires tight regulation of gene expression to drive proper neuronal connectivity and plasticity [42,96]. In cultured cortical neurons, microRNAs have been shown to modulate synaptogenesis [97] and axon extension [97–100]. In rodent studies with a focus on early postnatal life, microRNAs have been found to regulate cortical pyramidal neuron dendritic structure [101] and spine morphology [102], which are key determinants of intercellular communication and circuitry organization. The influence of microRNAs over PFC development in adolescence likely involves coordinating expression of gene networks that control the establishment of synaptic connections, including those influencing dendritic spine morphology and plasticity. Consistent with this idea, postnatal deficiency in microRNA production in pyramidal neurons, due to conditional downregulation or deletion of the DGCR8 microprocessor, results in altered dendritic morphology and synaptic transmission in the PFC [103,104]. miR-218 is highly expressed in dendritic spine compartments of PFC pyramidal neurons [102], and changes in its expression are associated with modification of spine morphogenesis in these cells [90]. Changes in somatodendritic properties of PFC pyramidal neurons are well documented in psychiatric disorders of adolescent onset [105,106]. Abnormal neuronal size [107], dendritic outgrowth [108], reduced basal dendrite [109] and spine density [110] have all been reported in schizophrenia. In MDD, modifications in dendritic spine morphology, density of PFC pyramidal neurons, and loss of synapses in PFC circuitry have also been consistently documented [106,111]. Altered microRNA expression in the PFC in adolescence may disrupt ongoing synaptic pruning and the balance of excitatory and inhibitory signaling, inducing psychiatric vulnerability [61,105].
3. Circulating microRNAs as biomarkers of psychiatric risk in adolescence
The neurobiological processes underlying the elevated risk for psychiatric disorders in adolescence has not been representatively studied and there is a pressing need to identify biological factors that would aid in early identification and prevention. Depressive symptoms and rates in adolescents have been on a rapid rise with the ongoing global viral pandemic [112–116]. The abrupt change in psychosocial interaction and elevated stress stemming from home confinement already appears to be disproportionately exacerbating depressive symptoms in youth [112,115,116]. Unfortunately, the number of studies in psychiatry aimed at discovering biological markers of risk in adolescence is scant. Using keywords “biomarker” and “psychiatry” in the Scopus® search engine yields a track record from the year 1989 with ~ 500 articles added per year in the last two decades (Fig. 1A). Adding the keyword “adolescence” to the search shows a track record from the year 2000 with only ~80 articles published annually in the last 6 years (Fig. 1B). More studies aimed at discovering biomarkers to detect psychiatric vulnerability in adolescence are urgently needed.
Fig. 1.
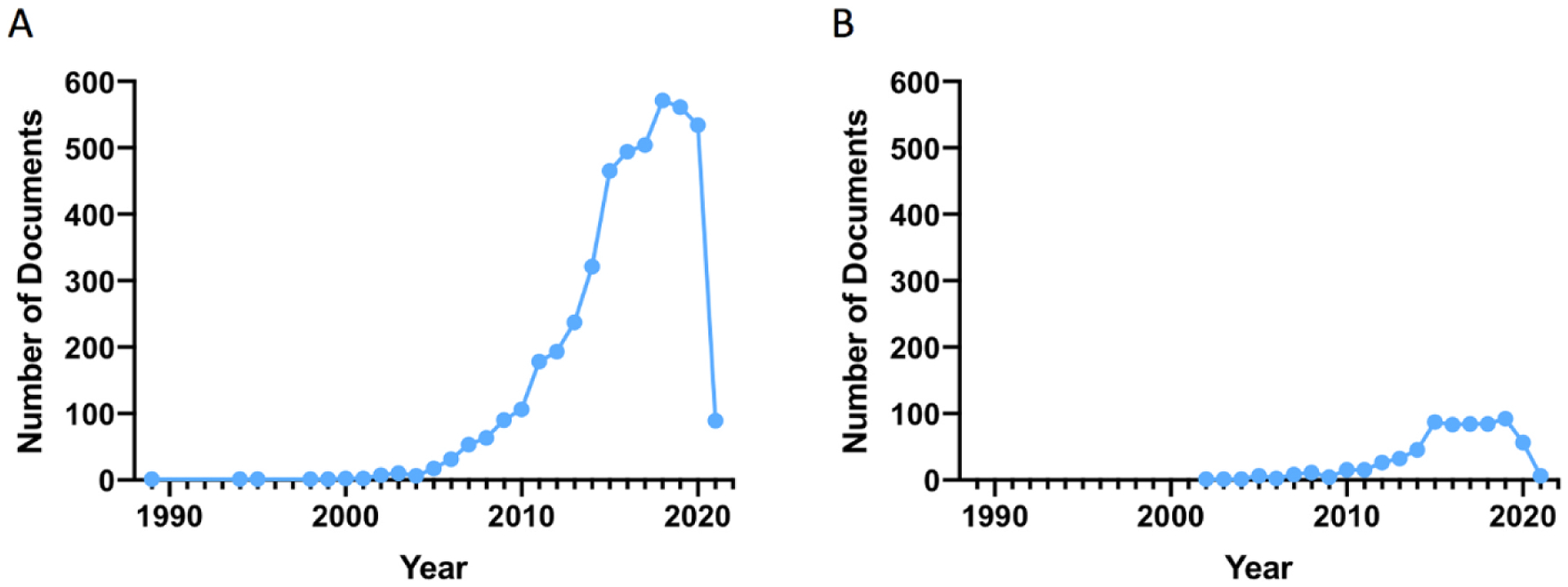
Number of articles published in the topic of biomarkers for psychiatric risk according to searches using Scopus ® Elsevier B.V. (A) Using keywords “biomarker” and “psychiatry” yields a track record, as of February 2021, from the year 1989 with ~ 500 articles added per year in the last two decades. (B) Using the keywords “biomarker”, “psychiatry” and “adolescence” to the search shows a track record from the year 2000 with only ~80 articles published annually in the last 6 years.
MicroRNAs are emerging as promising diagnostic and therapeutic tools in human disease [117]. They are very stable, do not degrade due to heat [118] or after prolonged storage [119,120], and are abundant and readily detectable in a variety of peripheral fluids, such as blood, saliva, and urine [121,122]. MicroRNAs are secreted into peripheral fluids and transported out of cells via exosomes, microvesicles, or by binding to proteins, achieving post-transcriptional regulation of gene expression at far away targets. Parallel changes in microRNA expression have been observed between brain and peripheral samples [33,79, 123–126]. In our miR-218 studies in mice, we have found that the dynamic pattern of miR-218 expression in the postnatal PFC is also observed in blood and that stress-induced reduction of miR-218 levels in the PFC is also detected in blood samples [33]. In humans, miR-218 levels in blood also appear to be downregulated in MDD, as observed in a study in aging individuals with MDD and cognitive impairment [127]. Remarkably, direct upregulation and downregulation of miR-218 in the PFC of adult mice, including specifically within pyramidal neurons, lead to corresponding changes in peripheral blood [79]. MicroRNAs in peripheral fluids may serve as readout of brain expression and function, and be used for early prediction of risk severity for mental illness [128].
To address whether peripheral microRNAs in adolescence could serve as biomarkers of psychiatric vulnerability, we collected blood samples from adolescent male mice that were subjected to chronic social defeat stress in adulthood. We found that circulating miR-218 in adolescence predictsvulnerability to stress-induced depression-like behavioral abnormalities in adulthood. In comparison to control and resilient groups, adult mice that showed susceptibility to stress had elevated blood levels of miR-218 in adolescence [33]. Previous studies assessing the role of microRNAs as early biomarkers of psychiatric risk in humans have provided an exciting direction for the field. However these studies have focused on associating participant-reported adverse childhood events with later outcomes or diagnosis [50], or comparing a child cohort with a separate adult group [129], rather than longitudinally following up on adolescent cohorts. We propose that future longitudinal studies be designed to identify microRNA profiles in peripheral samples from adolescent boys and girls to determine whether they can serve as identification markers of risk and guide preventative and intervention measures specifically during this age (Fig. 2). Analysis of global microRNA expression through unbiased small RNA sequencing of peripheral sampling is a promising strategy we are currently using to investigate changes associated with the development of maladaptive behaviors in longitudinal adolescent cohorts. These studies may identify microRNAs not previously linked to psychiatric traits as well as provide more information regarding the potential role of microRNAs differentially expressed in psychiatric disorders (e.g. Table 1) in adolescent neurodevelopment. Finding associations between circulating microRNAs and changes in cognitive function and behavior during adolescence may also shed light into whether specific microRNAs play a role in shaping developmental trajectories in the human brain.
Fig. 2.
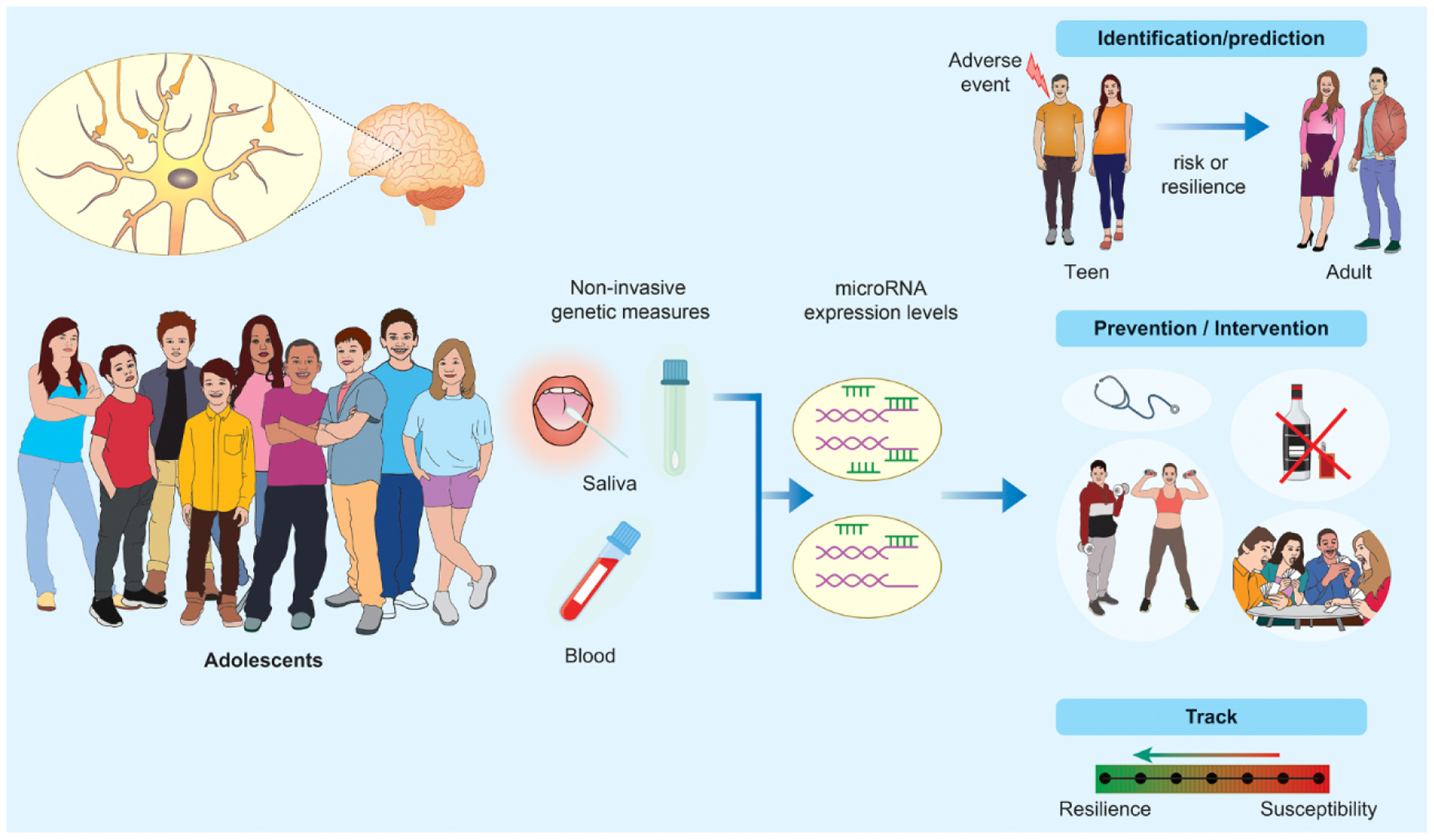
Peripheral microRNAs in adolescence as indicators of neurodevelopmental state and psychiatric risk. Assessment of microRNA profiles in peripheral samples from adolescent boys and girls in longitudinal studies could (i) help identifying markers of risk and resilience, (ii) guide prevention and intervention programs specifically for this age, and (ii) allow monitoring of disease severity.
The transition from adolescence to adulthood does not have a clearly defined differentiating boundary [130–132]. Given our studies in rodents showing that circulating miR-218 levels in adolescence and in adulthood predict opposite outcomes regarding stress susceptibility, diagnosis prediction using peripheral microRNAs in late adolescence may be limited and would need to be considered with caution. Assessing microRNA biomarkers in combination with clinical observations and markers of developmental stages, including the Tanner pubertal staging and hypothalamic-pituitary-adrenal axis responses [133], is an important next step.
4. Conclusions and future directions
This review shows that microRNA control over gene networks that organize PFC circuitry during adolescence may be a key mechanism in the development of vulnerability or resilience to mental illness. MicroRNAs measured in saliva or blood in adolescence may serve as indicators of PFC maturational stage and function and be used to predict behavioral outcomes to stress later in life. In biofluids, microRNAs are enriched in exosomes [134] and the approach of isolating circulating brain-specific exosomes [135–137] during adolescence could serve as a more objective measure of brain function and maturational state, enhancing the specificity and sensitivity of microRNAs as diagnostic tools. In this review we focused on microRNA expression in the PFC. However, microRNAs in non-cortical regions are also likely to contribute to PFC development and to the etiology of MDD, schizophrenia and substance use disorders. Indeed, miR-218 levels have been shown to be also elevated in the postmortem lateral amygdala of individuals with neuroticism and anxiety [138]. In rodents, the disruption of PFC dopamine development by exposure to recreational-like doses of stimulant drugs of abuse in adolescence requires miR-218 upregulation in the ventral tegmental area [139]. Manipulations of brain-wide microRNA expression via systemic administration of antagomir will aid in prevention, and in the discovery of therapeutic treatments [117].
The incidence rate and onset of PFC-related psychiatric disorders that emerge during adolescence are sex specific [140,141]. This poses an important limitation to the results derived from studies assessing the role of microRNAs on PFC development and adolescent risk, which have largely been conducted exclusively in males. As the prevalence of mental illness in adolescent boys and girls is on a rise, there is a pressing need for basic and clinical research in both males and females. In our own unpublished studies we are finding sexual dimorphisms in the pattern of microRNA expression in the postnatal PFC and in our translational studies we are prioritizing cohorts with representative male and female ratios.
How experiences in adolescence impact microRNA systems in the developing brain needs to be assessed in more detail. Particularly, whether they are regulated by adaptive and coping mechanisms, including biological, psychological, or social factors, that result in resilience despite adversity-related risk [142]. Individuals with the highest risk for mental illness are often the ones who benefit the most from early positive interventions [143]. A few recent studies have begun to address the involvement of microRNAs in this regard [123,144–148], but the developmental adolescent perspective in this line of research is missing. Efforts to understand microRNA function in PFC development prior to the closing of the formative adolescent window may ultimately help improving mental health outcomes for youth.
Acknowledgments
We thank Dr. Daniel Hoops for providing comments and suggestions on this manuscript. CF is supported by the National Institute on Drug Abuse (R01DA037911), USA, the Canadian Institutes for Health Research (MOP-74709; FRN 170130), Canada, and the Natural Science and Engineering Research Council of Canada (2982226), Canada. AM is supported by the Healthy Brains Healthy Lives initiative of the Canada First Research Excellence Fund at McGill University, Canada.
Footnotes
Declarations of interest
None.
References
- [1].Harlow JM, Recovery from the passage of an iron bar through the head, Publ. Mass Med. Soc 2 (1868) 327–347. [Google Scholar]
- [2].Choroschko WK, Die Stirnlappen des Gehirns in funktioneller Beziehung, Z. für die Gesamt Neurol. Psychiatr 83 (1) (1923) 291–302. [Google Scholar]
- [3].Feuchtwanger E, Die Funktionen des Stirnhirns, ihre Pathologie und Psychologie, in: Foerster O, Williams K (Eds.), Monogr. aus Gesget. Neurol. Psychiat, Heft, 38, Springer-Verlag OHG, Berlin, 1923, pp. 4–194. [Google Scholar]
- [4].Brickner RM, An interpretation of frontal lobe function based upon the study of a case of partial bilateral frontal lobectomy, Res. Publ. Assoc. Res. Nerv. Ment. Dis 13 (1934) 259–351. [Google Scholar]
- [5].Ackerly SS, Instinctive, emotional and mental changes following pre-frontal lobe extirpation, Am. J. Psychiatry 92 (1935) 717–729. [Google Scholar]
- [6].Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y, Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents, J. Neurosci 30 (2010) 15007–15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fuster J, The Prefrontal Cortex, Academic Press, 2015. [Google Scholar]
- [8].Gao WJ, Wang HX, Snyder MA, Li YC, The Unique Properties of the Prefrontal Cortex and Mental Illness. When Things Go Wrong-Diseases and Disorders of the Human Brain, IntechOpen, 2012. [Google Scholar]
- [9].Goldstein RZ, Volkow ND, Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications, Nat. Rev. Neurosci 12 (2011) 652–669, 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tan H-Y, Callicott JH, Weinberger DR, Prefrontal cognitive systems in schizophrenia: towards human genetic brain mechanisms, Cogn. Neuropsychiatry 14 (2009) 277–298. [DOI] [PubMed] [Google Scholar]
- [11].Rice F, Riglin L, Thapar AK, Heron J, Anney R, O’Donovan MC, Thapar A, Characterizing developmental trajectories and the role of neuropsychiatric genetic risk variants in early-onset depression, JAMA Psychiatry 76 (2019) 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rajman M, Schratt G, MicroRNAs in neural development: from master regulators to fine-tuners, Development 144 (13) (2017) 2310–2322. [DOI] [PubMed] [Google Scholar]
- [13].Ha M, Kim VN, Regulation of microRNA biogenesis, Nat. Rev. Mol. Cell Biol 15 (8) (2014) 509–524. [DOI] [PubMed] [Google Scholar]
- [14].O’Brien J, Hayder H, Zayed Y, Peng C, Overview of microRNA biogenesis, mechanisms of actions, and circulation, Front. Endocrinol 9 (2018) 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vasudevan S, Tong Y, Steitz JA, Switching from repression to activation: microRNAs can up-regulate translation, Science 318 (5858) (2007) 1931–1934. [DOI] [PubMed] [Google Scholar]
- [16].Rocchi A, Moretti D, Lignani G, Colombo E, Scholz-Starke J, Baldelli P, Benfenati F, Neurite-enriched microRNA-18 stimulates translation of the GluA2 subunit and increases excitatory synaptic strength, Mol. Neurobiol 56 (8) (2019) 5701–5714. [DOI] [PubMed] [Google Scholar]
- [17].Li ZF, Liang YM, Lau PN, Shen W, Wang DK, Cheung WT, Lam YW, Dynamic localisation of mature microRNAs in Human nucleoli is influenced by exogenous genetic materials, PLoS One 8 (8) (2013) 70869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Turunen TA, Roberts TC, Laitinen P, Väänänen MA, Korhonen P, Malm T, Turunen MP, Changes in nuclear and cytoplasmic microRNA distribution in response to hypoxic stress, Sci. Rep 9 (1) (2019) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xiao M, Li J, Li W, Wang Y, Wu F, Xi Y, Yu W, MicroRNAs activate gene transcription epigenetically as an enhancer trigger, RNA Biol. 14 (10) (2017) 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Matsui M, Chu Y, Zhang H, Gagnon KT, Shaikh S, Kuchimanchi S, Janowski BA, Promoter RNA links transcriptional regulation of inflammatory pathway genes, Nucleic Acids Res. 41 (22) (2013) 10086–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Volvert ML, Rogister F, Moonen G, Malgrange B, Nguyen L, MicroRNAs tune cerebral cortical neurogenesis, Cell Death Differ. 19 (10) (2012) 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Desai AR, McConnell SK, Progressive restriction in fate potential by neural progenitors during cerebral cortical development, Development 127 (2000) 2863–2872, 10.1073/pnas.1215707110. [DOI] [PubMed] [Google Scholar]
- [23].Bian S, Hong J, Li Q, Schebelle L, Pollock A, Knauss JL, Sun T, MicroRNA cluster miR-17–92 regulates neural stem cell expansion and transition to intermediate progenitors in the developing mouse neocortex, Cell Rep. 3 (5) (2013) 1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen Y, Bian S, Zhang J, Zhang H, Tang B, Sun T, The silencing effect of microRNA miR-17 on p21 maintains the neural progenitor pool in the developing cerebral cortex, Front. Neurol 5 (2014) 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abdullah AI, Zhang H, Nie Y, Tang W, Sun T, CDK7 and miR-210 co-regulate cell-cycle progression of neural progenitors in the developing neocortex, Stem Cell Rep. 7 (1) (2016) 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tonelli DDP, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB, miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex, Development 135 (23) (2008) 3911–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schwambom JC, Berezikov E, Knoblich JA, The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors, Cell 136 (5) (2009) 913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cremisi F, MicroRNAs and cell fate in cortical and retinal development, Front. Cell. Neurosci 7 (2013) 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barca-Mayo O, Tonelli DDP, Convergent microRNA actions coordinate neocortical development, Cell. Mol. Life Sci 71 (16) (2014) 2975–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Diaz JL, Siththanandan VB, Lu V, Gonzalez-Nava N, Pasquina L, MacDonald JL, Tharin S, An evolutionarily acquired microRNA shapes development of mammalian cortical projections, Proc. Natl. Acad. Sci. USA 117 (46)(2020) 29113–29122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Marinaro F, Marzi MJ, Hoffmann N, Amin H, R Pelizzoli F Niola, D. De Pietri Tonelli, MicroRNA-independent functions of DGCR8 are essential for neocortical development and TBR1 expression, EMBO Rep. 18 (4) (2017) 603–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R, DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal, Nat. Genet 39 (3) (2007) 380–385, 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Torres-Berrío A, Morgunova A, Giroux M, Cuesta S, Nestler EJ, Flores C, miR-218 in adolescence predicts and mediates vulnerability to stress, Biol. Psychiatry (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM, Dynamic mapping of human cortical development during childhood through early adulthood, Proc. Natl. Acad. Sci. USA 101 (21) (2004) 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Luna B, Developmental changes in cognitive control through adolescence, Adv. Child Dev. Behav 37 (2009) 233–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sturman DA, Moghaddam B, The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies, Neurosci. Biobehav. Rev 35 (8) (2011) 1704–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ordaz SJ, Foran W, Velanova K, Luna B, Longitudinal growth curves of brain function underlying inhibitory control through adolescence, J. Neurosci 33 (46) (2013) 18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Larsen B, Luna B, Adolescence as a neurobiological critical period for the development of higher-order cognition, Neurosci. Biobehav. Rev 94 (2018) 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB, Development of the dopaminergic innervation in the prefrontal cortex of the rat, J. Comp. Neurol 269 (1) (1988) 58–72. [DOI] [PubMed] [Google Scholar]
- [40].Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E, Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence, J. Neurosci 32 (46) (2012) 16223–16232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Willing J, Cortes LR, Brodsky JM, Kim T, Juraska JM, Innervation of the medial prefrontal cortex by tyrosine hydroxylase immunoreactive fibers during adolescence in male and female rats, Dev. Psychobiol 59 (5) (2017) 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hoops D, Flores C, Making dopamine connections in adolescence, Trends Neurosci. 40 (12) (2017) 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fuster JM, Frontal lobe syndrome, in: Fogel BS, Scheffer RB, Rao SM (Eds.), Neuropsychiatry, Williams and Wilkins, London, 1996, pp. 407–413. [Google Scholar]
- [44].Carlén M, What constitutes the prefrontal cortex? Science 358 (6362) (2017) 478–482. [DOI] [PubMed] [Google Scholar]
- [45].Laubach M, Amarante LM, Swanson K, White SR, What, if anything, is rodent prefrontal cortex? eneuro 5 (5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Spear L, The Behavioral Neuroscience of Adolescence, W.W. Norton, New York, 2010. [Google Scholar]
- [47].Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Mapping cortical change across the human life span, Nat. Neurosci 6 (2003) 309–315. [DOI] [PubMed] [Google Scholar]
- [48].Marín O, Developmental timing and critical windows for the treatment of psychiatric disorders, Nat. Med 22 (11) (2016) 1229–1238. [DOI] [PubMed] [Google Scholar]
- [49].Somel M, Guo S, Fu N, Yan Z, Hu HY, Xu Y, Khaitovich P, MicroRNA, mRNA, and protein expression link development and aging in human and macaque brain, Genome Res. 20 (9) (2010) 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Allen L, Dwivedi Y, MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior, Mol. Psychiatry 25 (2) (2020) 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cattaneo A, Suderman M, Cattane N, Mazzelli M, Begni V, Maj C, Riva MA, Long-term effects of stress early in life on microRNA-30a and its network: preventive effects of lurasidone and potential implications for depression vulnerability, Neurobiol. Stress 13 (2020), 100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL, Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation, J. Neurosci 33 (2013) 9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zucchi FC, Yao Y, Ward ID, Ilnytskyy Y, Olson DM, Benzies K, A Metz G, Maternal stress induces epigenetic signatures of psychiatric and neurological diseases in the offspring, PLoS One 8 (2) (2013) 56967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ziats MN, Rennert OM, Identification of differentially expressed microRNAs across the developing human brain, Mol. Psychiatry 19 (7) (2014) 848–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Beveridge NJ, Santarelli DM, Wang X, Tooney PA, Webster MJ, Weickert CS, Cairns MJ, Maturation of the human dorsolateral prefrontal cortex coincides with a dynamic shift in microRNA expression, Schizophr. Bull 40 (2) (2014) 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].O’Donnell P, Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models, Schizophr. Bull 37 (2011) 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Goldstein RZ, Volkow ND, Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications, Nat. Rev. Neurosci 12 (2011) 652–669, 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Myers-Schulz B, Koenigs M, Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders, Mol. Psychiatry 17 (2) (2012) 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kole AJ, Swahari V, Hammond SM, Deshmukh M, miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis, Genes Dev. 25 (2) (2011) 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang Y, Shen B, Zhang D, Wang Y, Tang Z, Ni N, Gu P, miR-29a regulates the proliferation and differentiation of retinal progenitors by targeting Rbm8a, Oncotarget 8 (19) (2017) 31993–32008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Roshan R, Shridhar S, Sarangdhar MA, Banik A, Chawla M, Garg M, Pillai B, Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice, RNA 20 (8) (2014) 1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lippi G, Steinert JR, Marczylo EL, D’Oro S, Fiore R, Forsythe ID, Young KW, Targeting of the Arpc3 actin nucleation factor by miR-29a/b regulates dendritic spine morphology, J. Cell Biol 194 (6) (2011) 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Swahari V et al. MicroRNA-29 is an essential regulator of brain maturation through regulation of CH methylation. Cell Reports, Volume 35, Issue 1,108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Whiteford HA, Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010, PLoS Med 10 (11) (2013), 1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M, Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report, Am. J. Psychiatry 163 (2006) 1905–1917. [DOI] [PubMed] [Google Scholar]
- [66].Zhou X, Michael KD, Liu Y, Del Giovane C, Qin B, Cohen D, Xie P, Systematic review of management for treatment-resistant depression in adolescents, BMC Psychiatry 14 (1) (2014) 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cuijpers P, Stringaris A, Wolpert M, Treatment outcomes for depression: challenges and opportunities, Lancet Psychiatry 7 (2020) 925–927. [DOI] [PubMed] [Google Scholar]
- [68].Wittchen HU, Nelson CB, Lachner G, Prevalence of mental disorders and psychosocial impairments in adolescents and young adults, Psychol. Med 28 (1) (1998) 109–126. [DOI] [PubMed] [Google Scholar]
- [69].Lewinsohn PM, Hops H, Roberts RE, Seeley JR, Andrews JA, Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III—R disorders in high school students, J. Abnorm. Psychol 102 (1) (1993) 133–144. [DOI] [PubMed] [Google Scholar]
- [70].Beesdo K, Höfler M, Leibenluft E, Lieb R, Bauer M, Pfennig A, Mood episodes and mood disorders: patterns of incidence and conversion in the first three decades of life, Bipolar Disord. 11 (6) (2009) 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Keyes KM, Gary D, O’Malley PM, Hamilton A, Schulenberg J, Recent increases in depressive symptoms among US adolescents: trends from 1991 to 2018, Soc. Psychiatry Psychiatr. Epidemiol 54 (8) (2019) 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Saluja G, Iachan R, Scheidt PC, Overpeck MD, Sun W, Giedd JN, Prevalence of and risk factors for depressive symptoms among young adolescents, Arch. Pediatr. Adolesc. Med 158 (8) (2004) 760–765. [DOI] [PubMed] [Google Scholar]
- [73].Avenevoli S, Knight E, Kessler RC, & Merikangas KR (2008). Epidemiology of depression in children and adolescents.
- [74].Merikangas KR, Nakamura EF, Kessler RC, Epidemiology of mental disorders in children and adolescents, Dialog. Clin. Neurosci 11 (1) (2009) 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lee FS, Heimer H, Giedd JN, Lein ES, Šestan N, Weinberger DR, Casey BJ, Adolescent mental health—opportunity and obligation, Science 346 (6209) (2014) 547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pine DS, Cohen P, Gurley D, Brook J, Ma Y, The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders, Arch. Gen. Psychiatry 55 (1) (1998) 56–64. [DOI] [PubMed] [Google Scholar]
- [77].Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, Wickramaratne P, Depressed adolescents grown up, JAMA 281 (18) (1999) 1707–1713. [DOI] [PubMed] [Google Scholar]
- [78].Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication, Arch. Gen. Psychiatry 62 (2005) 593–602. [DOI] [PubMed] [Google Scholar]
- [79].Torres-Berrío A, Lopez JP, Bagot RC, Nouel D, Dal Bo G, Cuesta S, Flores C, DCC confers susceptibility to depression-like behaviors in humans and mice and is regulated by miR-218, Biol. Psychiatry 81 (4) (2017) 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Manitt C, Eng C, Pokinko M, Ryan RT, Torres-Berrío A, Lopez JP, Yogendran SV, Daubaras MJ, Grant A, Schmidt ER, Tronche F, Krimpenfort P, Cooper HM, Pasterkamp RJ, Kolb B, Turecki G, Wong TP, Nestler EJ, Giros B, Flores C, dcc orchestrates the development of the prefrontal cortex during adolescence and is altered in psychiatric patients, Transl. Psychiatry 3 (2013) 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Reynolds LM, Pokinko M, Torres-Berrío A, Cuesta S, Lambert LC, Pellitero EDC, Flores C, DCC receptors drive prefrontal cortex maturation by determining dopamine axon targeting in adolescence, Biol. Psychiatry 83 (2) (2018) 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hoops D, Reynolds LM, Restrepo-Lozano JM, Flores C, Dopamine development in the mouse orbital prefrontal cortex is protracted and sensitive to amphetamine in adolescence, ENeuro 5 (1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Vosberg DE, Leyton M, Flores., (2019) The Netrin-1/DCC Guidance System: Dopamine Pathway Maturation and Psychiatric Disorders Emerging in Adolescence. [DOI] [PMC free article] [PubMed]
- [84].Torres-Berrío A, Hernandez G, Nestler EJ, Flores C, The Netrin-1/DCC guidance cue pathway as a molecular target in depression: translational evidence, Biol. Psychiatry 88 (2020) 611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Morgunova A, Pathik Wadhwa MD, Gilmore J, Sassi RB, Silveira PP, CCNP innovations in neuropsychopharmacology award: DCC gene network in the prefrontal cortex is associated with total brain volume in childhood, J. Psychiatry Neurosci. JPN 46 (1) (2021) E154–E163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Grant A, Fathalli F, Rouleau G, Joober R, Flores C, Association between schizophrenia and genetic variation in DCC: a case–control study, Schizophr. Res 137 (1) (2012) 26–31. [DOI] [PubMed] [Google Scholar]
- [87].Kuhn RM, Haussler D, Kent WJ, The UCSC genome browser and associated tools, Brief. Bioinforma 14 (2) (2013) 144–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].A Golden S, Covington HE, Berton O, Russo SJ, A standardized protocol for repeated social defeat stress in mice, Nat. Protoc 6 (2011) 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Nestler EJ, Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress, Science 311 (5762) (2006) 864–868. [DOI] [PubMed] [Google Scholar]
- [90].Torres-Berrío A, Nouel D, Cuesta S, Parise EM, Restrepo-Lozano JM, Larochelle P, Flores C, MiR-218: a molecular switch and potential biomarker of susceptibility to stress, Mol. Psychiatry 25 (5) (2020) 951–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dwivedi Y, Roy B, Lugli G, Rizavi H, Zhang H, Smalheiser NR, Chronic corticosterone-mediated dysregulation of microRNA network in prefrontal cortex of rats: relevance to depression pathophysiology, Transl. Psychiatry 5 (2015) 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ma K, Guo L, Xu A, Cui S, Wang JH, Molecular mechanism for stress-induced depression assessed by sequencing miRNA and mRNA in medial prefrontal cortex, PLoS One 11 (7) (2016), 0159093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Amin ND, Bai G, R Klug J, Bonanomi D, Pankratz MT, Gifford WD, Lee KF, Loss of motoneuron-specific microRNA-218 causes systemic neuromuscular failure, Science 350 (6267) (2015) 1525–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hoye ML, Koval ED, Wegener AJ, Hyman TS, Yang C, O’Brien DR, Miller TM, MicroRNA profiling reveals marker of motor neuron disease in ALS models, J. Neurosci 37 (22) (2017) 5574–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cerro-Herreros E, Sabater-Arcis M, Fernandez-Costa JM, Moreno N, Perez-Alonso M, Llamusi B, Artero R, miR-23b and miR-218 silencing increase Muscleblind-like expression and alleviate myotonic dystrophy phenotypes in mammalian models, Nat. Commun 9 (1) (2018) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Caballero A, Granberg R, Tseng KY, Mechanisms contributing to prefrontal cortex maturation during adolescence, Neurosci. Biobehav. Rev 70 (2016) 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kos A, Loohuis NO, Meinhardt J, van Bokhoven H, Kaplan BB, Martens GJ, Aschrafi A, MicroRNA-181 promotes synaptogenesis and attenuates axonal outgrowth in cortical neurons, Cell. Mol. Life Sci 73 (18) (2016) 3555–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, Zhang ZG, The microRNA-17–92 cluster enhances axonal outgrowth in embryonic cortical neurons, J. Neurosci 33 (16) (2013) 6885–6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Li H, Mao S, Wang H, Zen K, Zhang C, Li L, MicroRNA-29a modulates axon branching by targeting doublecortin in primary neurons, Protein Cell 5 (2) (2014) 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kos A, Klein-Gunnewiek T, Meinhardt J, Loohuis NFO, van Bokhoven H, Kaplan BB, Aschrafi A, MicroRNA-338 attenuates cortical neuronal outgrowth by modulating the expression of axon guidance genes, Mol. Neurobiol 54 (5) (2017) 3439–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Christensen M, Larsen LA, Kauppinen S, Schratt G, Recombinant adeno-associated virus-mediated microRNA delivery into the postnatal mouse brain reveals a role for miR-134 in dendritogenesis in vivo, Front. Neural Circuits 3 (2010) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, Khudayberdiev S, Leuschner PF, Busch CJ, Kane C, Hübel K, Dekker F, Hedberg C, Rengarajan B, Drepper C, Waldmann H, Kauppinen S, Greenberg ME, A Draguhn M Rehmsmeier, J. Martinez, G.M. Schratt, A functional screen implicates microRNA- 138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis, Nat. Cell Biol 11 (2009) 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hsu R, Schofield CM, Cruz CGD, Jones-Davis DM, Blelloch R, Ullian EM, Loss of microRNAs in pyramidal neurons leads to specific changes in inhibitory synaptic transmission in the prefrontal cortex, Mol. Cell. Neurosci 50 (3–4) (2012) 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Schofield CM, Hsu R, Barker AJ, Gertz CC, Blelloch R, Ullian EM, Monoallelic deletion of the microRNA biogenesis gene Dgcr8 produces deficits in the development of excitatory synaptic transmission in the prefrontal cortex, Neural Dev. 6 (1) (2011) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lewis DA, Hashimoto T, Volk DW, Cortical inhibitory neurons and schizophrenia, Nat. Rev. Neurosci 6 (4) (2005) 312–324. [DOI] [PubMed] [Google Scholar]
- [106].Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Son H, Decreased expression of synapse-related genes and loss of synapses in major depressive disorder, Nat. Med 18 (9) (2012) 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rajkowska G, Halaris A, Selemon LD, Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder, Biol. Psychiatry 49 (9) (2001) 741–752. [DOI] [PubMed] [Google Scholar]
- [108].Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT, Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia, Am. J. Psychiatry 161 (2004) 742–744. [DOI] [PubMed] [Google Scholar]
- [109].Glantz LA, Lewis DA, Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia, Arch. Gen. Psychiatry 57 (2000) 65–73. [DOI] [PubMed] [Google Scholar]
- [110].Broadbelt K, Byne W, Jones LB, Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex, Schizophr. Res 58 (2002) 75–81. [DOI] [PubMed] [Google Scholar]
- [111].Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM, Dendritic spines in depression: what we learned from animal models, Neural Plast. 2016 (2016), 8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Esterwood E, Saeed SA, Past epidemics, natural disasters, COVID19, and mental health: learning from history as we Deal with the present and prepare for the future, Psychiatr. Q 91 (2020) 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].de Figueiredo CS, Sandre PC, Portugal LCL, Mázala-de-Oliveira T, da Silva Chagas L, Raony Í, Bomfim POS, COVID-19 pandemic impact on children and adolescents’ mental health: biological, environmental, and social factors, Prog. Neuro Psychopharmacol. Biol. Psychiatry 106 (2021), 110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Fegert JM, Vitiello B, Plener PL, Clemens V, Challenges and burden of the coronavirus 2019 (COVID-19) pandemic for child and adolescent mental health: a narrative review to highlight clinical and research needs in the acute phase and the long return to normality, Child Adolesc. Psychiatry Ment. Health 14 (2020) 20, 10.1186/sl3034-020-00329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Nwachukwu I, Nkire N, Shalaby R, Hrabok M, Vuong W, Gusnowski A, Agyapong VI, COVID-19 pandemic: age-related differences in measures of stress, Int. J. Environ. Res. Public Health 17 (17) (2020) 6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ravens-Sieberer U, Kaman A, Erhart M, Devine J, Schlack R, Otto C, Impact of the COVID-19 pandemic on quality of life and mental health in children and adolescents in Germany, Eur. Child Adolesc. Psychiatry (2021) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Atri C, Guerfali FZ, Laouini D, MicroRNAs in diagnosis and therapeutics. AGO-Driven Non-Coding RNAs, Academic Press, 2019, pp. 137–177. [Google Scholar]
- [118].Jung M, Schaefer A, Steiner I, Kempkensteffen C, Stephan C, Erbersdobler A, Jung K, Robust microRNA stability in degraded RNA preparations from human tissue and cell samples, Clin. Chem 56 (6) (2010) 998–1006. [DOI] [PubMed] [Google Scholar]
- [119].Nelson PT, Baldwin DA, Kloosterman WP, Kauppinen S, Plasterk RH, Mourelatos Z, RAKE and LNA-ISH reveal microRNA expression and localization in archival human brain, RNA 12 (2) (2006) 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Tewari M, Circulating microRNAs as stable blood-based markers for cancer detection, Proc. Natl. Acad. Sci. USA 105 (30) (2008) 10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Layne TR, Green RA, Lewis CA, Nogales F, Dawson Cruz TC, Zehner ZE, Seashols-Williams SJ, micro RNA detection in blood, urine, semen, and saliva stains after compromising treatments, J. Forensic Sci 64 (6) (2019) 1831–1837. [DOI] [PubMed] [Google Scholar]
- [122].Fujimoto S, Manabe S, Morimoto C, Ozeki M, Hamano Y, Hirai E, Tamaki K, Distinct spectrum of microRNA expression in forensically relevant body fluids and probabilistic discriminant approach, Sci. Rep 9 (1) (2019) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, Chen A, MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity, Neuron 83 (2) (2014) 344–360. [DOI] [PubMed] [Google Scholar]
- [124].An T, Fan T, Zhang XQ, Liu YF, Huang J, Liang C, Jiang GJ, Comparison of Alterations in miRNA expression in matched tissue and blood samples during spinal Cord Glioma progression, Sci. Rep 9 (1) (2019) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zeng L, Liu J, Wang Y, Wang L, Weng S, Tang Y, Yang GY, MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia, Front Biosci. (Elite Ed.) 3 (3) (2011) 1265–1272. [DOI] [PubMed] [Google Scholar]
- [126].Wang Q, Roy B, Turecki G, Shelton RC, Dwivedi Y, Role of complex epigenetic switching in tumor necrosis factor-α up-regulation in the prefrontal cortex of suicide subjects, Am. J. Psychiatry 175 (3) (2018) 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mendes-Silva AP, Diniz BS, Tolentino Araújo GT, de Souza Nicolau E, Pereira KS, Silva Ferreira CM, Barroso LS, [P3–213]: mirnas and their role in the correlation between major depressive disorder, mild cognitive impairment and alzheimer’s disease, Alzheimer’s Dement. 13 (7S_Part_21) (2017) P1017–P1018. [Google Scholar]
- [128].Roy B, Yoshino Y, Allen L, Prall K, Schell G, Dwivedi Y, Exploiting circulating microRNAs as biomarkers in psychiatric disorders, Mol. Diagn. Ther 24 (3) (2020) 279–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Jawaid A, Kunzi M, Mansoor M, Khan ZY, Abid A, Taha M,. & Mansuy I (2020). Distinct microRNA signature in human serum and germline after childhood trauma. medRxiv. [Google Scholar]
- [130].Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sharma S, Maturation of the adolescent brain, Neuropsychiatr. Dis. Treat 9 (2013) 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Schneider M, Adolescence as a vulnerable period to alter rodent behavior, Cell Tissue Res. 354 (1) (2013) 99–106. [DOI] [PubMed] [Google Scholar]
- [132].Somerville LH, Searching for signatures of brain maturity: what are we searching for? Neuron 92 (6) (2016) 1164–1167. [DOI] [PubMed] [Google Scholar]
- [133].Halligan SL, Herbert J, Goodyer I, Murray L, Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents, Biol. Psychiatry 62 (2007) 40–46. [DOI] [PubMed] [Google Scholar]
- [134].Gallo A, Tandon M, Alevizos I, Illei GG, The majority of microRNAs detectable in serum and saliva is concentrated in exosomes, PLoS One 7 (3) (2012) 30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kanninen KM, Bister N, Koistinaho J, Malm T, Exosomes as new diagnostic tools in CNS diseases, Biochim. Et. Biophys. Acta (BBA) Mol. Basis Dis 1862 (3) (2016) 403–410. [DOI] [PubMed] [Google Scholar]
- [136].Tavakolizadeh J, Roshanaei K, Salmaninejad A, Yari R, Nahand JS, Sarkarizi HK, Mousavi SM, Salarinia R, Rahmati M, Mousavi SF, Mokhtari R, Mirzaei H, MicroRNAs and exosomes in depression: potential diagnostic biomarkers, J. Cell Biochem 119 (2018) 3783–3797. [DOI] [PubMed] [Google Scholar]
- [137].Saeedi S, Israel S, Nagy C, Turecki G, The emerging role of exosomes in mental disorders, Transl. Psychiatry 9 (1) (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Jurkiewicz M, Moser D, Koller A, Yu L, Chen EI, Bennett DA, Canli T, Integration of postmortem amygdala expression profiling, GWAS, and functional cell culture assays: neuroticism-associated synaptic vesicle glycoprotein 2A (SV2A) gene is regulated by miR-133a and miR-218, Transl. Psychiatry 10 (1) (2020) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Cuesta S, Restrepo-Lozano JM, Silvestrin S, Nouel D, Torres-Berrío A, Reynolds LM, Flores C, Non-contingent exposure to amphetamine in adolescence recruits miR-218 to regulate Dcc expression in the VTA, Neuropsychopharmacology 43 (4) (2018) 900–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Zahn-Waxler C, Shirtcliff EA, Marceau K, Disorders of childhood and adolescence: gender and psychopathology, Annu. Rev. Clin. Psychol 4 (2008) 275–303. [DOI] [PubMed] [Google Scholar]
- [141].Shaw GA, Dupree JL, Neigh GN, Adolescent maturation of the prefrontal cortex: role of stress and sex in shaping adult risk for compromise, Genes Brain Behav. 19 (2020) 12626. [DOI] [PubMed] [Google Scholar]
- [142].Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, Yehuda R, Resilience definitions, theory, and challenges: interdisciplinary perspectives, Eur. J. Psychotraumatol 5 (2014) 5, 10.3402/ejpt.v5.25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Boyce WT, The Orchid and the Dandelion: Why Sensitive People Struggle and How all Can Thrive, Pan MacMillan, 2019. [Google Scholar]
- [144].Dias C, Feng J, Sun H, yi Shao N, Mazei-Robison MS, Damez-Wemo D, Nestler EJ, β-catenin mediates stress resilience through Dicer1/microRNA regulation, Nature 516 (7529) (2014) 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chen RJ, Kelly G, Sengupta A, Heydendael W, Nicholas B, Beltrami S, Bhatnagar S, MicroRNAs as biomarkers of resilience or vulnerability to stress, Neuroscience 305 (2015) 36–48. [DOI] [PubMed] [Google Scholar]
- [146].Higuchi F, Uchida S, Yamagata H, Abe-Higuchi N, Hobara T, Hara K, Watanabe Y, Hippocampal microRNA-124 enhances chronic stress resilience in mice, J. Neurosci 36 (27) (2016) 7253–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lopizzo N, Zonca V, Cattane N, Pariante CM, Cattaneo A, miRNAs in depression vulnerability and resilience: novel targets for preventive strategies, J. Neural Transm 126 (9) (2019) 1241–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Yang J, Sun J, Lu Y, An T, Lu W, Wang JH, mRNA and microRNA profiles are associated with stress susceptibility and resilience induced by psychological stress in the prefrontal cortex, Psychopharmacology 237 (10) (2020) 3067–3093. [DOI] [PubMed] [Google Scholar]
- [149].Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Hammond SM, microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder, Genome Biol. 8 (2) (2007) 27, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zhu Y, Kalbfleisch T, Brennan MD, Li Y, A MicroRNA gene is hosted in an intron of a schizophrenia-susceptibility gene, Schizophr. Res 109 (1–3) (2009) 86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kim AH, Reimers M, Maher B, Williamson V, McMichael O, McClay JL, Vladimirov VI, MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders, Schizophr. Res 124 (1–3) (2010) 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ, Schizophrenia is associated with an increase in cortical microRNA biogenesis, Mol. Psychiatry 15 (2010)1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD, Up-regulation of microRNAs in brain of human alcoholics, Alcohol. Clin. Exp. Res 35 (11) (2011) 1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ, Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia, Biol. Psychiatry 69 (2) (2011) 180–187. [DOI] [PubMed] [Google Scholar]
- [155].Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM, Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder, Biol. Psychiatry 69 (2) (2011) 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Miller BH, Zeier Z, Xi L, Lanz TA, Deng S, Strathmann J, Wahlestedt C, MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function, Proc. Natl. Acad. Sci. USA 109 (8) (2012) 3125–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Smalheiser NR, Lugli G, Rizavi HS, Torvik VI, Turecki G, Dwivedi Y, MicroRNA expression is downregulated and reorganized in prefrontal cortex of depressed suicide subjects, PLoS One 7 (2012) 33201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Maussion G, Yang J, Yerko V, Barker P, Mechawar N, Ernst C, Turecki G, Regulation of a truncated form of tropomyosin-related kinase B (TrkB) by Hsa-miR-185* in frontal cortex of suicide completers, PLoS One 7 (2012) 39301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Manzardo AM, Gunewardena S, Butler MG, Over-expression of the miRNA cluster at chromosome 14q32 in the alcoholic brain correlates with suppression of predicted target mRNA required for oligodendrocyte proliferation, Gene 526 (2) (2013) 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Banigan MG, Kao PF, Kozubek JA, Winslow AR, Medina J, Costa J, Vanderburg CR, Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients, PLoS One 8 (1) (2013) 48814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Smalheiser NR, Lugli G, Zhang H, Rizavi H, Cook EH, Dwivedi Y, Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects, PLoS One 9 (1) (2014) 86469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Lopez JP, Fiori LM, Gross JA, Labonte B, Yerko V, Mechawar N, Turecki G, Regulatory role of miRNAs in polyamine gene expression in the prefrontal cortex of depressed suicide completers, Int J. Neuropsychopharmacol 17 (2014) 23–32 ([a]). [DOI] [PubMed] [Google Scholar]
- [163].Lopez JP, Lim R, Cruceanu C, Crapper L, Fasano C, Labonte B, Maussion G, Yang JP, Yerko V, Vigneault E, El Mestikawy S, Mechawar N, Pavlidis P, Turecki G, miR-1202 is a primate-specific and brain enriched microRNA involved in major depression and antidepressant treatment, Nat. Med 20 (2014) 764–768 ([b]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Roy B, Dunbar M, Shelton RC, Dwivedi Y, Identification of microRNA-124–3p as a putative epigenetic signature of major depressive disorder, Neuropsychopharmacology 42 (2017) 864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Lopez JP, Fiori LM, Cruceanu C, Lin R, Labonte B, Cates HM, Heller EA, Vialou V, Ku SM, Gerald C, Han MH, Foster J, Frey BN, Soares CN, Müller DJ, Farzan F, Leri F, MacQueen GM, Feilotter H, Tyryshkin K, Evans KR, Giacobbe P, Blier P, Lam RW, Milev R, Parikh SV, Rotzinger S, Strother SC, Lewis CM, Aitchison KJ, Wittenberg GM, Mechawar N, Nestler EJ, Uher R, Kennedy SH, Turecki G, MicroRNAs 146a/b-5 and 425–3p and 24–3p are markers of antidepressant response and regulate MAPK/Wntsystem genes, Nat. Commun 8 (2017) 15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Wang Q, Roy B, Turecki G, Shelton RC, Dwivedi Y, Role of complex epigenetic switching in tumor necrosis factor-α up-regulation in the prefrontal cortex of suicide subjects, Am. J. Psychiatry 175 (3) (2018) 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Hu Z, Gao S, Lindberg D, Panja D, Wakabayashi Y, Li K, Kleinman JE, Zhu J, Li Z, Temporal dynamics of miRNAs in human DLPFC and its association with miRNA dysregulation in schizophrenia, Transl. Psychiatry 9 (1) (2019) 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Gorinski N, Bijata M, Prasad S, Wirth A, Abdel Galil D, Zeug A, Bazovkina D, Kondaurova E, Kulikova E, Ilchibaeva T, Zareba-Koziol M, Papaleo F, Scheggia D, Kochlamazashvili G, Dityatev A, Smyth I, Krzystyniak A, Wlodarczyk J, Richter DW, Strekalova T, Sigrist S, Bang C, Hobuß L, Fiedler J, Thum T, Naumenko VS, Pandey G, Ponimaskin E, Attenuated palmitoylation of serotonin receptor 5-HT1a affects receptor function and contributes to depression-like behaviors, Nat. Commun 10 (1) (2019) 3924. [DOI] [PMC free article] [PubMed] [Google Scholar]


