Abstract
Memories of emotionally arousing events tend to endure longer than other memories. This review compiles findings from several decades of research investigating the role of the amygdala in modulating memories of emotional experiences. Episodic memory is a kind of declarative memory that depends upon the hippocampus, and studies suggest that the basolateral complex of the amygdala (BLA) modulates episodic memory consolidation through interactions with the hippocampus. Although many studies in rodents and imaging studies in humans indicate that the amygdala modulates memory consolidation and plasticity processes in the hippocampus, the anatomical pathways through which the amygdala affects hippocampal regions that are important for episodic memories were unresolved until recent optogenetic advances made it possible to visualize and manipulate specific BLA efferent pathways during memory consolidation. Findings indicate that the BLA influences hippocampal-dependent memories, as well as synaptic plasticity, histone modifications, gene expression, and translation of synaptic plasticity associated proteins in the hippocampus. More recent findings from optogenetic studies suggest that the BLA modulates spatial memory via projections to the medial entorhinal cortex, and that the frequency of activity in this pathway is a critical element of this modulation.
Keywords: Amygdala, Hippocampus, Synaptic plasticity, Memory systems, Memory consolidation
1. Introduction
On a fundamental level, memory creates who we are. Indeed, without memories, it is difficult to imagine how we would conceive of ourselves and our relationship with the world. Not surprisingly then, how memories are formed and retained has been an enduring question in the field of psychology and neuroscience for over a century, beginning with early psychologists like William James and Hermann Ebbinghaus. Across this time, considerable work has created a framework for how some of this process unfolds. In particular, for declarative-style memories, evidence indicates a critical role for the hippocampus and associated medial temporal lobe structures in the initial formation and consolidation of such memories. Because these memories are the cornerstone for our own sense of self and our explicit connections to the world around us, the systems underlying these memories have received significant attention.
However, not all experiences are successfully transformed into long-lasting memories and, in almost everyone, it appears that the vast majority of one’s daily experiences is not stored in long-term memory form. Rather, evidence suggests that experiences involving emotionally significant events are far more likely to be successfully consolidated into a long-lasting memory than are relatively less emotional experiences. The ability of emotional arousal to influence memory consolidation appears to depend heavily on the amygdala and, in the case of declarative-like memories, its interactions with the hippocampal system. This review will describe how the amygdala and hippocampus act synergistically to create and strengthen the consolidation of these memories.
1.1. Hippocampus and memory consolidation
Beginning with early work by Brenda Milner with patients such as Henry Molaison (H.M.) (Scoville & Milner, 1957), considerable evidence has pointed to the hippocampus and associated medial temporal lobe structures including the rhinal and parahippocampal cortices as critical loci in the formation and consolidation of declarative-style memories. The ability to form new declarative memories is profoundly impaired in patients with significant lesions of the hippocampal formation, such as the famous patient H.M. In contrast, the ability to form non-declarative memories, such as procedural motor skills, appears to be largely intact in these patients (Squire, 2009). These patients with medial temporal lobe lesions also appear to have temporally graded retrograde amnesia for declarative memories of experiences that occurred before the lesion, with more distant events remembered better than those closer in time to the lesion. Thus, it appears that the hippocampus plays a role in the initial formation of such memories as well as the early stages of consolidation of these memories.
The role of the hippocampal system in memory consolidation has been investigated in both human and non-human animals. Declarative memory consists of episodic and semantic memories, and animals demonstrate memory for relationships among experiences, indicating that they, too, have episodic, declarative memories (Bunsey & Eichenbaum, 1996). Evidence suggests that declarative memories eventually undergo a “systems consolidation” in which the memory trace needed for recall shifts from the hippocampus to neocortical structures (Frankland & Bontempi, 2005; Hardt & Nadel, 2018; Kitamura et al., 2017; Klinzing, Niethard, & Born, 2019; Takehara-Nishiuchi, 2020). Although beyond the scope of the current review, some theories of systems consolidation argue that this process is actually more complex than a simple trace transfer. Rather, these theories argue that the hippocampus is always involved in the recall of episodic and autobiographical memories and that memories that no longer require the hippocampus for recall have been transformed into schematic maps stored in neocortical regions. Without the hippocampus, these memories are more “gist-like” or semantic in style, rather than the contextually and temporally rich episodic memories (Corkin, 2002; Moscovitch, Cabeza, Winocur, & Nadel, 2016; Winocur & Moscovitch, 2011; Winocur, Moscovitch, & Bontempi, 2010). Regardless of whether a particular memory depends on the hippocampus for recall for a lifetime, it is clear that the initial formation and consolidation of declarative memories depends heavily on an intact and functioning hippocampus as well as associated medial temporal lobe structures. It is likely that the hippocampus mediates spatial and contextual components of memories that involve associations between contextual and emotionally arousing information (Roesler et al., 1998; 2000b, 2003).
Studies indicate that the information regarding various declarative memories enters the hippocampus through associated nearby cortices including the entorhinal, perirhinal, postrhinal, and medial prefrontal cortices (Dickerson & Eichenbaum, 2010; Eichenbaum, 2017; Squire & Zola-Morgan, 1991). Indeed, evidence suggests that these regions play important roles in the processing and consolidation of recently acquired information. Lesions of the hippocampus alone produce considerably milder dysfunction in memory-based tasks than do lesions that include and extend beyond the hippocampus to include nearby medial temporal lobe cortices (Squire, 2009). Likewise, it is believed that H.M. displayed such profound anterograde amnesia due to his surgical resection encompassing hippocampus-adjacent areas including the anterior parahippocampal cortex (Corkin, Amaral, Gonzalez, Johnson, & Hyman, 1997; Squire, 2009).
Further evidence supporting the crucial role for these associated brain regions in working with the hippocampus proper in memory formation comes from numerous studies examining how these brain systems map spatial and temporal characteristics of the environment. For example, evidence indicates that neurons in the hippocampus, especially those of the dorsal hippocampus, serve as “place cells” that fire in a highly selective manner when the animal is in a specific location in the environment (O’Keefe, 1976; Wilson & McNaughton, 1993). In contrast, recordings from the medial entorhinal cortex have observed “grid cells” that fire in a selective manner in a hexagonal or triangular grid-like fashion across space (Fyhn, Hafting, Treves, Moser, & Moser, 2007; Hafting, Fyhn, Molden, Moser, & Moser, 2005). Though the mechanisms are not fully understood, it is believed that grid cells of the entorhinal cortex then contribute to the place cell encoding in the hippocampus (Moser, Rowland, & Moser, 2015). Although much work has focused on place and grid cells due to the relative ease of identifying such cells, studies have found that hippocampal cells also encode other aspects of the world, such as odor, time, and time-space relationships that are the building blocks for episodic memories (Eichenbaum, Kuperstein, Fagan, & Nagode, 1987; Hampson, Heyser, & Deadwyler, 1993; Leutgeb, Leutgeb, Barnes, Moser, McNaughton, & Moser, 2005; Moser, Kropff, & Moser, 2008). Thus, this encoding across the hippocampus and associated cortices would be expected to form the fundamental basis of declarative memories. Multiple neuronal processes at the molecular level have been uncovered and investigated extensively over the past four decades. The molecular basis of memory formation in the hippocampus, entorhinal cortex, and associated brain structures is beyond the scope of this review, and we direct the reader to recent review articles that focus on the molecular basis of memory formation and storage (Alberini & Kandel, 2014; Asok, Leroy, Rayman, & Kandel, 2018; Josselyn & Tonegawa, 2020; Leighton et al., 2018; Rao-Ruiz, Visser, Mitrić, Smit, and van den Oever, 2021). The complexity of this system with multiple regions and sub-regions provides several avenues by which other regions, such as the amygdala, can influence the different elements of declarative memory processing.
1.2. Amygdala and memory consolidation
As noted above, considerable evidence indicates that emotional arousal at the time of learning events enhances the consolidation of memories for these events. Emotional arousal increases levels of the stress hormones cortisol (corticosterone in rodents) and epinephrine. Previous findings indicate that systemic administration of these hormones after training enhances the consolidation of memories in rodents (Gold & van Buskirk, 1976a; 1976b; Roozendaal & McGaugh, 1996). Importantly, amygdala lesions prevent the memory-modulating effects of peripheral stress hormones (Roozendaal & McGaugh, 1996), highlighting the critical role of the amygdala in mediating the effects of emotional arousal on memory consolidation. More specifically, it appears that the basolateral amygdala (BLA; also termed basolateral complex), composed of the lateral, basal (also termed basolateral), and accessory basal nuclei (also termed basomedial nucleus; Pitkänen, Savander, & LeDoux, 1997; Price, Russchen, & Amaral, 1987) is the critical amygdala region responsible for this memory modulatory ability (Parent & McGaugh, 1994; Roozendaal & McGaugh, 1997b). Studies suggest that BLA manipulations immediately after training alter the consolidation of memories, including declarative-style ones (Hatfield & McGaugh, 1999; Packard, Cahill, & McGaugh, 1994). For example, posttraining intra-BLA infusions of a range of compounds that either stimulate or inhibit specific receptors for neurotransmitters, including acetylcholine, dopamine, noradrenaline, glutamate, γ-aminobutyric acid (GABA), opioids, endogenous cannabinoids, and serotonin, alter memory consolidation (Campolongo et al., 2009a; 2009b; Dickinson-Anson & McGaugh, 1997; Ferry & McGaugh, 2008; Introini-Collison, Miyazaki, & McGaugh, 1991; Introini-Collison, Nagahara, & McGaugh, 1989; Khakpoor, Nasehi, Vahdati, Hoseyni, & Zarrindast, 2016; LaLumiere, Nguyen, & McGaugh, 2004; Nasehi, Davoudi, Ebrahimi-Ghiri, & Zarrindas, 2016; Power, McIntyre, Litmanovich, & McGaugh, 2003; Roesler et al., 2000a; 2003). Evidence points to an especially important role for noradrenergic inputs to the BLA during emotionally influenced memory consolidation (Gallagher, Kapp, Musty, & Driscoll, 1977). For example, posttraining intra-BLA infusions of norepinephrine enhance consolidation (LaLumiere, Buen, & McGaugh, 2003), whereas β-adrenergic receptor blockade in the BLA prevents the memory-enhancing effects of systemic administration of epinephrine, glucocorticoid agonists, the opioid antagonist naltrexone, ketamine, and the endogenous lipid mediator oleoylethanolamide, among other agents (Campolongo et al., 2009a; Liang, Juler, & McGaugh, 1986; McGaugh, Introini-Collison, & Nagahara, 1988; Morena et al., 2021; Quirarte, Roozendaal, & McGaugh, 1997). Moreover, the amount of norepinephrine released in the amygdala immediately following inhibitory avoidance training correlates with the degree of retention two days later (McIntyre, Hatfield, & McGaugh, 2002). This finding suggests that the degree of BLA activation after an emotionally arousing event determines the strength of memory modulation such that greater BLA activation leads to better retention for that event. Indeed, evidence from functional imaging studies in humans supports this, as the degree of amygdala activity during encoding predicts the likelihood of remembering visual images at a later surprise retention test but only for emotionally arousing visual images (Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000). Such work further supports the idea that the amygdala is critical for modulating the strength of different kinds of memories.
Together, the research reviewed above points to a likely complex interaction between the BLA and hippocampal-dependent declarative memory. In particular, it appears that a hippocampus-based system plays an important role in processing and encoding a variety of components of an experience, including time and space, to create a memory. In contrast, the BLA seems to mediate the emotional significance of the event by modulating memory consolidation processes and thereby altering the strength of the resulting memory. The findings of Packard et al. (1994) are particularly seminal in illustrating these interactions. In their work, they found that posttraining amphetamine infusions into the hippocampus, but not caudate, enhanced retention of the spatial version of the water maze, whereas these infusions into the caudate, but not hippocampus, enhanced retention of the cued version of the water maze. In contrast to this double dissociation, posttraining intra-amygdala infusions of amphetamine enhanced the retention of both spatial and cued versions of the water mask task. Moreover, pharmacological inactivation of the amygdala before the retention test for either version did not reverse the enhancement produced by posttraining intra-amygdala infusions of amphetamines. Thus, these findings indicate that a) the amygdala modulates the consolidation of multiple forms of memories that are selectively mediated by other brain regions (i.e., hippocampus for spatial learning and caudate for cued learning) and b) the amygdala is not a critical site of long-term storage of either type of memory. Although there is evidence that the BLA may modulate hippocampal function related to spatial memory retrieval (Roozendaal, Griffith, Buranday, De Quervain, & McGaugh, 2003; Saha, Kriebel, Volkmer, Richter-Levin, & Albrecht, 2018), it is particularly clear that a critical interaction between the BLA and hippocampus occurs during the consolidation period of contextual and spatial memory formation. This review, therefore, will focus on the interactions between the amygdala and hippocampus during the consolidation of declarative-style memories.
2. Anatomical interactions between the hippocampus and amygdala
The following section will review the anatomical connections between the BLA and hippocampal formation, demonstrating that the BLA is poised anatomically to influence hippocampal function through an extensive array of amygdalo-hippocampal efferent projections. Although the amygdala and hippocampus communicate bidirectionally (McDonald & Mott, 2017; Pitkänen, Pikkarainen, Nurminen, & Ylinen, 2000), only amygdala projections to the hippocampus will be described given the focus of this article on amygdala modulation of hippocampal-dependent function. Although human studies also show evidence for coordinated activity in the amygdala and hippocampus during consolidation of emotional memories, this section will focus on rodent neuroanatomy and use rodent-based nomenclature because the majority of experimental evidence implicating the amygdala in modulation of hippocampal mnemonic function has been obtained in rats and mice.
The hippocampal formation consists of the entorhinal cortex, dentate gyrus (DG), hippocampus proper (CA1, CA2, and CA3 subfields), subiculum, presubiculum and parasubiculum (Amaral & Witter, 1989). Along the septo-temporal plane, the hippocampus proper is divided into dorsal and ventral regions (Fanselow & Dong, 2010; Moser & Moser, 1998; Strange, Witter, Lein, & Moser, 2014). Dorsal and ventral hippocampus have different patterns of afferent and efferent connections; generally, dorsal hippocampus is necessary for spatial and episodic/declarative-like memory, whereas ventral hippocampus is essential for affective and motivational processes and emotional memory (Fanselow & Dong, 2010, Strange et al., 2014).
In terms of information processing, sensory information from all modalities enters the amygdala primarily via the lateral nucleus, which in turn projects to the basal and accessory basal nuclei, and to other amygdala regions, including the central nucleus and periamygdaloid cortex (Pitkänen et al. 1997; Sah, Faber, Lopez De Armentia, & Power, 2003). The basal and accessory basal nuclei send reciprocal connections to the lateral nucleus (Savander, LeDoux, & Pitkänen, 1996; Savander, Miettinen, Ledoux, & Pitkänen, 1997). The central nucleus receives inputs from all other amygdala nuclei, including all basolateral nuclei, but does not reciprocate these connections. It is the major source of amygdalar outputs, although none of these appear to target the hippocampal formation (McDonald & Mott, 2017; McDonald & Zaric, 2015a; 2015b; Petrovich, Canteras, & Swanson, 2001).
Basolateral efferents to the hippocampal formation arise primarily from principal glutamatergic neurons, although there are inhibitory GABAergic projections to entorhinal cortex as well (McDonald & Zaric 2015a; 2015b). The majority of projections are ipsilateral, the few contralateral projections that have been observed are not substantial and monosynaptic projections to the presubiculum and DG have not been identified (Pikkarainen, Rönkkö, Savander, Insausti, & Pitkänen, 1999; Pitkänen et al., 2000). The three basolateral nuclei have distinct topographical projection patterns to the hippocampal formation that do not overlap, with the basal nucleus having the most widespread projections and serving as the major source of inputs to the hippocampus proper (Pikkarainen et al., 1999).
The amygdala does not project directly to the dorsal hippocampus but innervates the ventral hippocampus (McDonald & Mott, 2017; Pitkänen, et al., 2000). Thus, inputs to the hippocampus are heavily concentrated in the ventral pole (McDonald & Mott, 2017; Pikkarainen et al., 1999; Pitkänen et al., 2000). Ventral CA2 and CA3 receive significant projections from lateral and basal amygdala and lighter projections from the accessory basal nucleus (Petrovich et al., 2001; Pikkarainen et al., 1999). In comparison, ventral subiculum and ventral CA1 receive more amygdalar projections than do ventral CA2 and CA3, and these arise from all basolateral nuclei (McDonald & Mott, 2017; Petrovich et al., 2001; Pikkarainen et al., 1999; Pitkänen et al., 2000). Given that the ventral hippocampus is not critical for spatial memory, including in the spatial water maze (Moser, Moser, & Andersen, 1993; Moser, Moser, Forrest, Andersen, & Morris, 1995), one possibility is that amygdala-induced increases in ventral hippocampal activity could, in turn, impact memory consolidation in dorsal hippocampus via projections from ventral hippocampus to dorsal hippocampus. This is anatomically possible because dorsal and ventral hippocampus are connected via longitudinal projections (Amaral & Witter, 1989; Tamamaki, Abe, & Nojyo, 1988; Tamamaki, Watanabe, & Nojyo, 1984; Yang et al., 2014). However, the possibility that ventral hippocampus impacts dorsal hippocampal memory consolidation is not supported by evidence suggesting that dorsal hippocampal neurons can influence ventral hippocampal neural activity, but not vice versa (Takata et al., 2015). Perhaps amygdala modulation of spatial memory need not involve dorsal hippocampus because the long-held theory that the ventral hippocampus is not involved in spatial memory is based on lesion studies, but more recent findings suggest that place cells are present in the ventral hippocampus (Kjestrup et al., 2008), stimulation of monosynaptic glutamatergic projection from the basal amygdala to the ventral hippocampus enhances spatial memory (Yang et al., 2016) and ventral hippocampal projections to prefrontal cortex are critical for encoding spatial cues during a spatial working memory task (Spellman et al., 2015).
All three basolateral nuclei also innervate the parasubiculum and entorhinal cortex (Petrovich et al., 2001; Pikkarainen et al., 1999; Pitkänen et al., 2000). It may be noteworthy that the densest projections from the amygdala to the hippocampus are in latter portions of the hippocampal trisynaptic circuit (i.e., ventral CA1, ventral subiculum, and deep layers of the lateral entorhinal cortex). These amygdala targets receive information that has been significantly processed by hippocampal neurons and they are the major sources of hippocampal projections, including cortical efferents, and thus may serve as a pathway for systems consolidation (McDonald & Mott, 2017). The amygdala may also facilitate hippocampal memory via synchronous oscillations involving reciprocal connections between the lateral nucleus and perirhinal cortex and indirect connections between the lateral nucleus and dorsal CA1, likely via lateral entorhinal cortex (McDonald & Mott, 2017; Paré, Collins, & Pelletier, 2002; Pelletier & Paré, 2002). Although the BLA does not project to the DG, it alters DG neural activity (Ikegaya, Saito, & Abe, 1994; 1995; 1996; Nakao, Matsuyama, Matsuki, & Ikegaya, 2004). The polysynaptic pathway through which BLA influences DG function likely involves projections from the entorhinal cortex, parasubiculum, and/or amygdalopirifrom transition area (McDonald & Mott, 2017; Narayanan et al., 2007; Pikkarainen et al., 1999). Indeed, recent findings support this contention. Optogenetic stimulation and inhibition of the pathway from the BLA to the medial entorhinal cortex enhances and impairs, respectively, spatial memories known to be dependent on the hippocampus (Wahlstrom, Alvarez-Dieppa, McIntyre, & LaLumiere, 2021; Wahlstrom et al., 2018). Intriguingly, the same manipulations have the opposite effect on dorsal striatum-dependent cued-response memory, suggesting that this pathway is specific for modulating spatial memory and does so in competition with dorsal striatum-dependent memories.
3. Amygdala-hippocampal interactions in memory consolidation
As noted in the introductory section, critical work by Packard et al. (1994) pointed to interactions between the amygdala and hippocampus during the consolidation of spatial memories. Since then, much work has further investigated the nature of these interactions during memory consolidation. Indeed, evidence suggests that amygdala manipulations affect learning-related neurochemical and molecular changes in the hippocampus and that BLA activity is required for hippocampal manipulations to modulate memory consolidation. Selective excitotoxic lesions of the BLA, but not of the central amygdala (CeA), prevent the memory-enhancing effect of a glucocorticoid receptor agonist infused into the dorsal hippocampus shortly after inhibitory avoidance training, as well as the impairing effect of a pretraining intrahippocampal infusion of a glucocorticoid receptor antagonist in a spatial water maze task (Roozendaal & McGaugh, 1997a). Importantly, BLA lesions alone do not affect memory retention of either task. The effect of BLA lesions is mimicked by intra-BLA infusion of the β-adrenoceptor antagonist atenolol given prior to IA training, which prevents the enhancing effect of an intrahippocampal GR agonist. Together, these findings indicate that an intact BLA is necessary for glucocorticoid effects on memory consolidation via the dorsal hippocampus (Nathan, Griffith, McReynolds, Hahn, & Roozendaal, 2004; Roozendaal, Nguyen, Power, & McGaugh, 1999).
However, this necessity of the BLA for manipulations of the hippocampus to alter consolidation is not limited to stress-based glucocorticoid effects. Evidence suggests that blockade of either D1 or NMDA receptors in the BLA prevents the memory-modulatory effects of cannabinoid agonist infusions into the hippocampus (Rezayof, Habibi, & Zarrindast, 2011). Moreover, findings indicate that intra-BLA infusions of norepinephrine promote the persistence of hippocampus-dependent context discrimination, maintaining this discrimination ability even when tested long after training, an effect not observed in control animals. The enhanced maintenance of hippocampus-dependent context discrimination memory suggests that BLA norepinephrine affects systems consolidation. Intra-BLA infusions of norepinephrine were also associated with DNA methylation and transcription of memory-related genes in the hippocampus (Atucha et al., 2017). The crucial role of the BLA in allowing memory formation in the hippocampus appears to extend to influences by epigenetic drugs. Pharmacological BLA inactivation via administration of the GABA receptor type A (GABAA) agonist muscimol prevents the memory-enhancing effects of posttraining intra-dorsal hippocampal infusions of a histone deacetylase (HDAC) inhibitor (Blank et al., 2014). Taken together, these findings indicate that the facilitation of memory consolidation produced by relaxation of chromatin structure and resulting increase in gene expression in the hippocampus depends on BLA activity (Fig. 1).
Fig. 1.
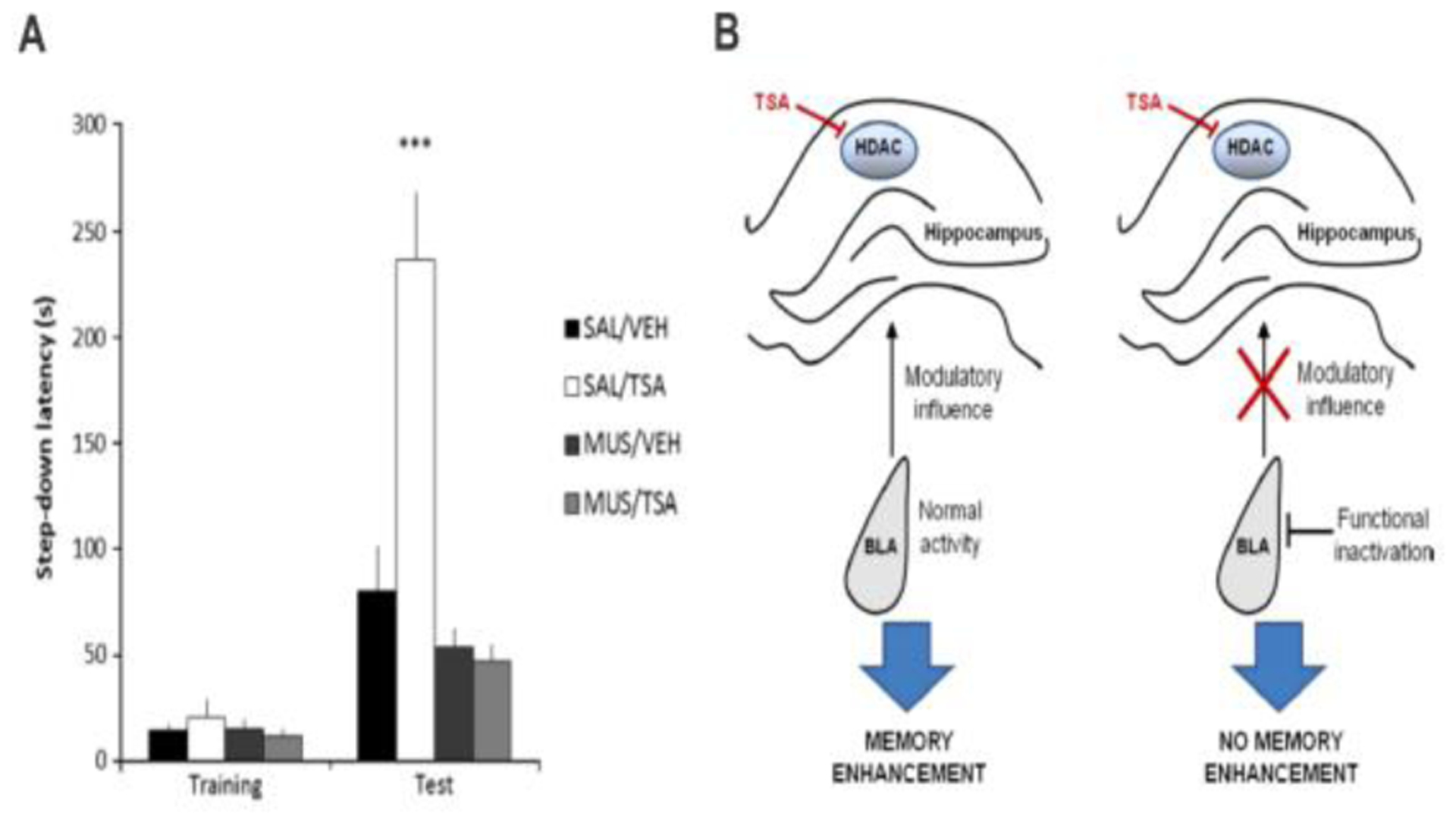
BLA activity is required for a chromatin-modifying epigenetic agent to enhance memory in the dorsal hippocampus. (A) Male rats were given a unilateral infusion of saline or muscimol (0.5 μg) into the left BLA before IA training, followed by a unilateral infusion of drug vehicle or the HDAC inhibitor TSA (22 mM) into the CA1 area of the dorsal hippocampus immediately after training (N = 11–15 rats per group). Memory retention was assessed 24 h after training. Data are mean + S.E.M. retention test latencies to step-down (s); ***p < 0.001 compared to controls given saline followed by vehicle. (B) Schematic model of BLA requirement for memory enhancement induced by HDAC inhibition in the hippocampus. Normal BLA activity may be necessary to enable the influence of drugs acting on HDACs on memory consolidation. Muscimol-induced functional inactivation of the BLA prevents the memory-enhancing effect of posttraining intrahippocampal administration of TSA. Adapted from Blank et al. (2014). See main article text for abbreviation definitions.
As noted above, the ventral and dorsal hippocampal subregions likely have different roles in memory with the ventral subregion involved in emotionally related information and the dorsal subregion involved in spatial information. Indeed, using a contextual fear conditioning task in which the footshock and spatial components are temporally separated from one another, Malin and McGaugh (2006) found that posttraining dorsal hippocampal manipulations influence the consolidation of the context learning, but not of the footshock learning. In contrast, BLA manipulations alter the consolidation of both, indicating that the BLA may interact with specific subregions of the hippocampus to influence distinct aspects or types of memories. In support of this, evidence indicates that optogenetic manipulation of the BLA pathway to the ventral hippocampus alters the consolidation of the memory of the footshock, but not of the context (Huff, Emmons, Narayanan, & LaLumiere, 2016). In contrast, the BLA likely influences the memory of the contextual component of an experience via projections to the medial entorhinal cortex, which in turn, influences dorsal hippocampal activity (Wahlstrom et al., 2018). These findings strongly indicate that the BLA-medial entorhinal cortex pathway modulates contextual and spatial memory formation by influencing other areas, including the hippocampus, and mediating effects on ARC-based synaptic plasticity. Selected findings showing BLA modulation of memory formation in the dorsal hippocampus are summarized in Table 1.
Table 1.
Summary of evidence from selected rat studies showing BLA influences on memory formation in the dorsal hippocampus.
| BLA intervention | Hippocampal intervention | Main findings | References |
|---|---|---|---|
| Excitotoxic lesions | Posttraining infusion of the GR agonist RU 28362 or the GR antagonist RU 38486 | BLA lesions but not CeA lesions prevent the enhancing effect of the GR agonist in IA and the impairing effect of the GR antagonist in water maze | Roozendaal & McGaugh, 1997 |
| Infusion of the β-adrenoceptor antagonist atenolol before IA training | Posttraining infusion of the GR agonist RU 28362 | Intra-BLA atenolol prevented the enhancing effect of RU 28362 | Roozendaal et al., 1999 |
| Infusion of the D1 dopamine receptor antagonist SCH23390 or the NMDA receptor antagonist AP5 after IA training | Posttraining infusion of the CB1/CB2 cannabinoid receptor agonist WIN55,212–2 | Either SCH23390 or AP5 into the BLA attenuated the impairing effect of WIN55,212–2 | Rezayof et al., 2011 |
| Muscimol infusion before IA training | Posttraining infusion of the HDAC inhibitor TSA | Muscimol inactivation of the BLA prevents the enhancing effect of TSA | Blank et al., 2014 |
See main article text for abbreviation definitions.
3.1. Cellular and molecular mechanisms
Extensive evidence suggests that BLA activity regulates memory consolidation, in part, via effects on activity-regulated cytoskeletal associated protein (ARC, also termed Arg 3.1) levels in the dorsal hippocampus. Arc is an immediate-early gene implicated in hippocampal synaptic plasticity that is necessary for memory consolidation (Guzowski et al., 2000). Intra-BLA infusions of the β-adrenoreceptor agonist clenbuterol increase ARC levels in the dorsal hippocampus of rats trained on either inhibitory avoidance or a heightened arousal version of the object recognition task (McIntyre et al., 2005; McReynolds, Anderson, Donowho, & McIntyre, 2014). In contrast, intra-BLA infusions of a memory-impairing dose of lidocaine reduces hippocampal ARC protein levels. Recent evidence suggests that the projection from the BLA to entorhinal cortex is critical for this effect, as posttraining optogenetic stimulation of BLA axons in the entorhinal pathway increases ARC expression in the dorsal hippocampus, although this was observed only in male rats (Wahlstrom et al., 2021). This stimulation decreased ARC in the dorsal striatum of female rats, a finding consistent with the idea that there is competition between hippocampal and striatum-based learning mechanisms. Increases in ARC protein are not accompanied by increases in Arc mRNA, suggesting that amygdala modulation of Arc occurs at the posttranscriptional level (McIntyre et al., 2005). However, Huff and colleagues (2006) found that muscimol inactivation of the BLA attenuates increases in hippocampal Arc and c-fos mRNA associated with contextual fear conditioning, suggesting that the BLA may exert both a transcriptional and posttranscriptional influence on hippocampal Arc expression.
Both Arc and c-fos serve as targets for cAMP-responsive element-binding protein (CREB), which in turn can be phosphorylated and activated by protein kinase signaling pathways to act as a transcription factor contributing to memory formation. Social memory formation is accompanied by increases in Arc, c-fos and CREB activation in brain areas including the BLA and dorsal hippocampus (Tanimizu, Kenney, Okano, Kadoma, Frankland, & Kida, 2017). The BLA regulates the expression of hippocampal extracellular signal-regulated kinase (ERK), which is an upstream regulator of CREB and several immediate early genes (Jeon et al., 2012). Neutrotrophin signaling is another cellular system involved in mediating BLA influences on the hippocampus. For example, intra-BLA administration of an HDAC inhibitor enhances inhibitory avoidance memory and increases the levels of brain-derived neurotrophic factor (BDNF) in the dorsal hippocampus but not in the BLA itself (Valiati et al., 2017). Multiple studies have investigated the interaction between the hippocampus and BLA at the molecular level over the past four decades, and details on the molecular basis of BLA modulation of hippocampus-dependent memory consolidation have been reviewed elsewhere (McReynolds & McIntyre, 2012). Table 2 summarizes cellular and molecular alterations in the hippocampus associated with BLA influences on memory formation.
Table 2.
Summary of selected cellular and molecular changes related to memory formation in the dorsal hippocampus after experimental interventions in the BLA in rodents.
| BLA intervention | Hippocampal changes | References |
|---|---|---|
| Infusion of the β-adrenoreceptor agonist clenbuterol | Increased hippocampal ARC in rats trained on IA or high-arousal OR |
McIntyre et al., 2005
McReynolds et al., 2014 |
| Optogenetic stimulation of the BLA-entorhinal cortex pathway after spatial training | Increased Arc expression in the hippocampus in male rats | Wahlstrom et al., 2021 |
| Infusion of muscimol into the BLA before contextual fear conditioning | Muscimol inactivation of the BLA attenuates increases in hippocampal Arc and c-fos mRNA associated with contextual fear conditioning | Huff et al., 2006 |
| Infusion of the HDAC inhibitor TSA into the BLA at different time intervals after IA training | Increases in BDNF levels in the dorsal hippocampus, accompanied by memory enhancement with infusions given between 1.5 and 6 h posttraining | Valiati et al., 2017 |
| Infusion of midazolam before contextual fear conditioning | Blockade of dendritic spine remodeling in the hippocampus accompanied by impairment in contextual fear conditioning | Giachero et al., 2015 |
| Infusion of the GABAA receptor antagonist bicuculine before contextual fear conditioning | Facilitation of both hippocampal synaptic structural remodeling and contextual fear conditioning | Giachero et al., 2015 |
| Inhibition through viral vector-mediated overexpression of an outwardly rectifying potassium | Prevention of immature newborn neuron activation in response to a fear-conditioning task | Kirby et al., 2012 |
See main article text for abbreviation definitions.
Beyond intracellular signaling and molecular mechanisms, the BLA also influences the hippocampus at the structural level, affecting memory-related spine remodeling in hippocampal neurons as well as the number of hippocampal neurons. Intra-BLA infusions of a benzodiazepine impairs both contextual fear conditioning and hippocampal dendritic spine remodeling, whereas inhibition of GABAA receptors within the BLA facilitated both contextual fear conditioning and structural remodeling in the hippocampus. These findings suggest that GABAergic transmission in the BLA regulates structural changes related to fear memory in hippocampal neurons (Giachero, Calfa, & Molina, 2015). BLA lesions and BLA inhibition induced by overexpression of an outwardly rectifying potassium channel both suppress adult hippocampal neurogenesis, while lesions of the amygdala central nucleus do not. In addition, BLA lesions prevent activation of newly born neurons in response to contextual fear conditioning (Kirby et al., 2012).
3.2. Human studies
Interactions between the amygdala and the hippocampus in memory formation are much less understood in humans than in rodents. Nevertheless, human studies have indicated that increased amygdala-hippocampal communication during learning is associated with enhanced memory encoding. A study using functional magnetic resonance imaging (fMRI) found that the strength of the connection from the amygdala to the hippocampus increases rapidly during encoding of emotionally positive or negative pictures (Fastenrath et al., 2014). Another fMRI study in patients with left hippocampal and amygdala pathology who performed a verbal encoding task found that encoding-related hippocampal activity for successfully remembered items correlates with the degree of left amygdala pathology, whereas amygdala-evoked activity related to remembered emotional items correlated with the degree of left hippocampal pathology (Richardson, Strange, & Dolan, 2004). These data suggest a reciprocal interaction between the amygdala and hippocampus during emotional memory encoding. Evidence also indicates that amygdala connectivity with the hippocampus measured by fMRI increases progressively after fear conditioning acquisition and predicts the strength of fear memory retention in human subjects (Hermans et al., 2017). Other fMRI experiments found that age- or stress-related individual variability in memory performance are associated with the functioning of amygdala-hippocampal circuits (de Voogd, Klumpers, Fernández, & Hermans, 2017; Leal, Noche, Murray, & Yassa, 2017). Thus, functional imaging studies in humans support the extensive evidence obtained in rodents demonstrating a relationship between the amygdala and hippocampus in memory processing.
4. Amygdala-hippocampal interactions in neural activity and synaptic plasticity: electrophysiological evidence
Consistent with the idea that the BLA modulates hippocampus-dependent memory consolidation, accumulating evidence suggests the BLA has a strong capacity to alter synaptic strength in hippocampal structures. Studies from over 20 years ago indicate that amygdala stimulation modulates hippocampal long-term potentiation (LTP) (Richter-Levin & Akirav, 2000). Priming (i.e., stimulation that facilitates responses to a subsequent stimulus) of the BLA before application of high-frequency stimulation (HFS) to the perforant path (PP) enhances LTP in the hippocampal dentate gyrus (DG) (Akirav and Richter-Levin, 1999a; 1999b). In contrast, higher-intensity stimulation of the BLA with spaced intervals impairs DG LTP (Li & Richter-Levin, 2012). Intriguingly, the modulatory effect of the BLA on DG LTP is absent in aged rats (Almaguer, Estupiñán, Uwe Frey, & Bergado, 2002). Other work observed a bidirectional shift toward LTP and long-term depression (LTD) depending on the degree and timing of neural activity upon BLA stimulation (Nakao et al., 2004). Evidence also suggests that HFS of the BLA induces DG LTP in vivo in anesthetized rats (Abe, Niikura, & Misawa, 2003), indicating that alterations in amygdala activity are sufficient to induce hippocampal synaptic plasticity on its own. In contrast, both low- and high-intensity BLA priming appear to impair LTP in the CA1 hippocampal area (Li & Richter-Levin, 2012; Vouimba & Richter-Levin, 2005; 2013). Priming studies have also revealed differential roles for distinct subnuclei of the BLA. For example, priming stimulation of the lateral subnucleus does not affect DG LTP, whereas priming of the basal subnucleus enhances LTP with weaker stimulation and impairs it with stronger stimulation (Li & Richter-Levin, 2013).
Neural activity in amygdala neurons seems to be necessary for emotional modulation of DG LTP. Lesions of the amygdala reverse the impairing effect of stress on hippocampal LTP (Kim, Lee, Han, & Packard, 2001). BLA inactivation by lidocaine infusions and permanent electrolytic BLA lesions block the enhancing effect of a motivational stimulus (drinking after 24-h deprivation) on DG LTP (Almaguer-Melian, Martínez-Martí, Frey JU, & Bergado, 2003). Reversible amygdala inactivation with infusions of muscimol prevents stress-induced impairment of LTP in hippocampal CA1 (Kim, Koo, Lee, & Han, 2005). Glutamate-induced activation of the BLA impairs LTP in the hippocampal-prefrontal cortex pathway and BLA inactivation with muscimol has the opposite effect. In addition, alpha- and beta-adrenoceptors have opposing effects on LTP in this pathway (Lim, Dawe, & Jay, 2017).
Many BLA manipulations that modulate hippocampal-dependent memory have a parallel effect on hippocampal synaptic strength. For example, both the enhancing and the impairing effects of amygdala stimulation on DG LTP require norepinephrine and corticosterone (CORT), as shown by pharmacologically-induced depletion of these neurotransmitters (Akirav and Richter-Levin, 2002). BLA infusions of the selective β-adrenergic receptor agonist clenbuterol enhance short-term plasticity and LTP induction in the DG (Noorani, Hojati, Ardeshiri, Akbari, & Ehsani, 2020). The combination of inhibitory avoidance (IA) training with intra-BLA administration of clenbuterol increases the excitability of hippocampal CA1 pyramidal neurons (Lovitz & Thompson, 2015). Neither norepinephrine nor glucocorticoid (GR) receptors in the BLA are required for LTP induction in the DG or CA1 areas or in mediating the impairing effect of BLA activation on CA1 LTP. However, blocking noradrenergic receptors or GRs in the BLA suppresses the enhancing effect of BLA stimulation on DG LTP (Vouimba, Yaniv, & Richter-Levin, 2007). Intra-BLA infusions of the noradrenergic antagonist propranolol given 10 min before tetanus impairs DG LTP induction, whereas intra-BLA infusions of the muscarinic cholinergic receptor antagonist scopolamine has no effect on LTP (Ikegaya, Nakanishi, Saito, & Abe,1997). Electrical stimulation of the BLA enhances DG LTP in freely moving rats when the stimulation occurs within a time window of 30 min before or after perforant path tetanization. The effect of BLA stimulation is blocked by intracereboventricular administration of either muscarinic or β-adrenergic receptor antagonists, or the protein synthesis inhibitor anisomycin (Frey, Bergado-Rosado, Seidenbecher, Pape, & Frey, 2001).
Additional studies support the idea that the BLA is capable of altering neural activity and synaptic plasticity within the hippocampus, providing a candidate mechanism through which the BLA influences hippocampal-dependent memories. Infusions of selective antagonists that block receptors for the neuropeptide orexin, namely SB-334867-A (orexin 1 receptor antagonist) and TCS-OX2-29 (orexin receptor 2 antagonist) into the BLA before tetanic stimulation attenuates DG LTP (Ardeshiri, Hosseinmardi, & Akbari, 2018). Furthermore, blocking of signaling by intracellular intracellular Zn2+ in the BLA attenuates DG LTP (Fujise, Kubota, Suzuki, Tamano, & Takeda, & 2017). BLA inactivation via muscimol mimics the effects of electrical BLA priming, enhancing DG LTP while inhibiting CA1 LTP, indicating a modulatory role for GABAA-mediated inhibitory transmission in BLA-induced effects on hippocampal LTP (Vouimba, Anunu, & Richter-Levin, 2020). Reduction of GABAergic inhibition in principal neurons within the BLA by lentiviral knockdown of neurofascin prevents priming-induced impairment of DG LTP maintenance in vivo (Saha et al., 2018). GABA release by interneurons onto principal neurons within the BLA, precisely regulated in a time-, domain-, and sensory-specific manner, promotes neuronal synchrony with coordinated theta-frequency oscillations between the BLA and the hippocampus, which likely contributes to contextual fear memory (Bienvenu, Busti, Magill, Ferraguti, & Capogna, 2012).
4.1. The entorhinal cortex as a mediator of BLA-hippocampus coherence
As discussed above, the entorhinal cortex (EC) is a likely bridge between the BLA and the dorsal hippocampus. Recent findings indicate that the medial EC (mEC) may participate in synchronizing activity in the BLA and dorsal hippocampus. Priming studies have also revealed differential roles for distinct subnuclei of the BLA. For example, priming stimulation of the lateral subnucleus does not affect DG LTP, whereas priming of the basal subnucleus enhances LTP with weaker stimulation and impairs it with stronger stimulation (Li & Richter-Levin, 2013). Both the BLA and the hippocampus receive connections from the entorhinal cortex (EC), and EC stimulation induces LTP simultaneously in the BLA and dorsal hippocampus (Yaniv, Vouimba, Diamond, & Richter-Levin, 2003). Brief electrical stimulation of the BLA elicits CA3-CA1 synchrony in the hippocampus in the low gamma frequency range, which is associated with memory enhancement (Bass & Manns, 2015). More recently, Ahlgrim & Manns (2019) found that optogenetic stimulation of the BLA enhances theta-modulated gamma oscillations in the hippocampus. Additionally, recent work using machine learning approaches suggests that amygdala stimulation modulates hippocampal network activity during encoding, increasing hippocampal connectivity in a manner that is consistent with enhanced synaptic plasticity and memory (Sendi et al., 2020).
Based on evidence that the BLA modulates spatial memories and affects synaptic plasticity in the dorsal hippocampus, Wahlstrom and colleagues investigated the role of the mEC in BLA modulation of hippocampus-dependent memory consolidation and plasticity. They found that posttraining optogenetic stimulation of glutamatergic BLA axons in the mEC at 8Hz, but not 40 Hz, enhanced long-term spatial memory and ARC expression in the dorsal hippocampus of male rats (Wahlstrom et al., 2021). In another study, Wahlstrom et al. found that memory-enhancing 8 Hz optogenetic stimulation of BLA axons in the mEC increased local field potential power in the same frequency range in the mEC and in the dorsal hippocampus, while 40 Hz stimulation was not as effective in changing local field potentials in the dorsal hippocampus (Fig. 2). These findings are consistent with evidence that theta rhythm (~8 Hz) activity in the mEC and hippocampus is critical for spatial memory consolidation and suggests that coherence in the theta range involves the BLA-mEC pathway (Wahlstrom et al., 2018; 2021).
Fig. 2.
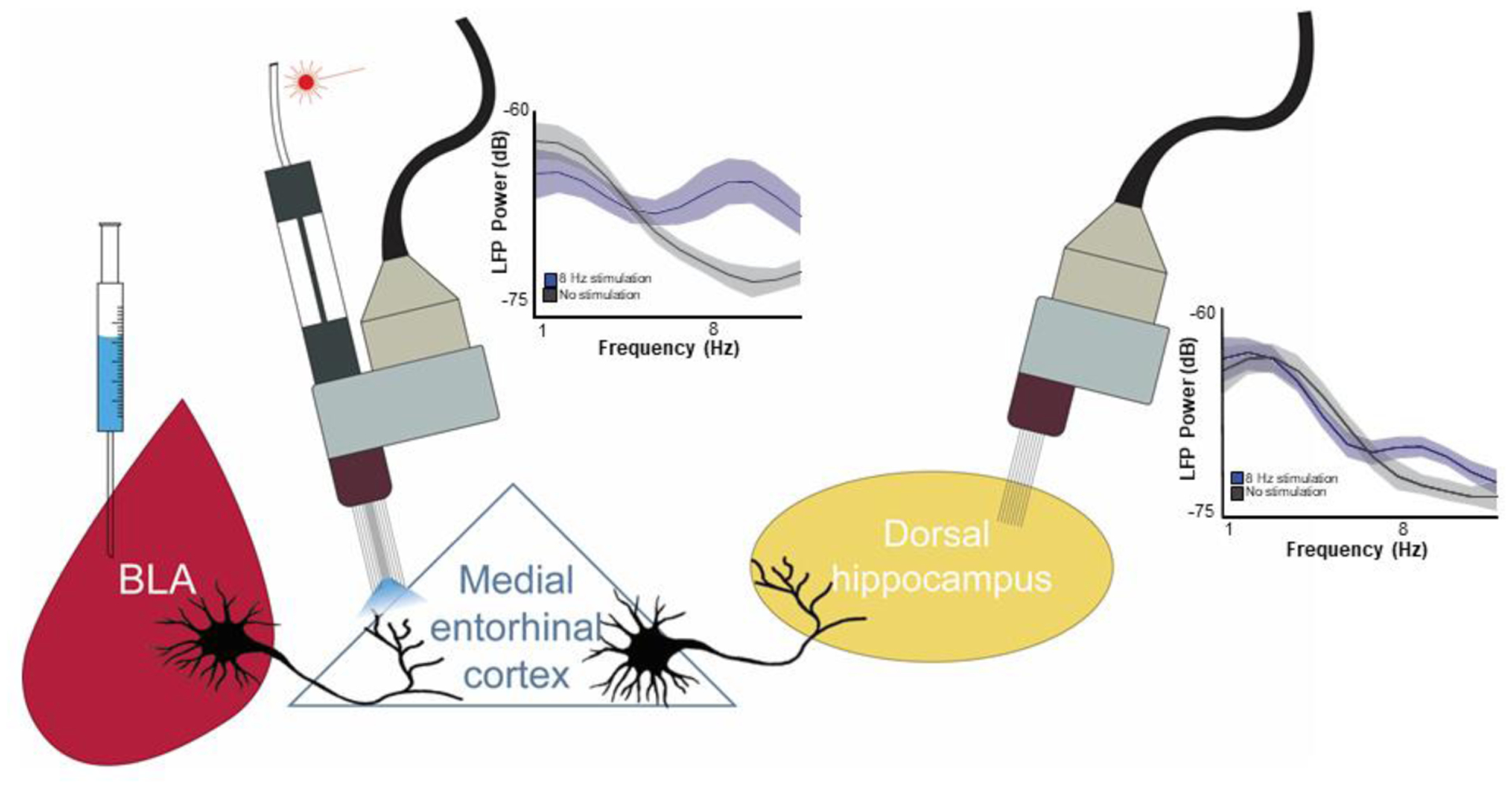
Schematic diagram showing a circuit by which the BLA influences dorsal hippocampus functioning. Work highlighted in this review indicates that optogenetically stimulating the BLA inputs to the medial entorhinal cortex with bursts of 8 Hz pulses alters the consolidation of spatial memories known to depend on the dorsal hippocampus. Such stimulation given with bursts of 8 Hz pulses also alters dorsal hippocampal levels of the plasticity-associated protein ARC in males while decreasing ARC levels in the dorsal striatum of females. Finally, as depicted in this figure, electrophysiological recordings reveal that 8 Hz bursts of stimulation to BLA axons in the medial entorhinal cortex increase local-field potential (LFP) power in the theta (6–10 Hz) range both in the medial entorhinal and downstream in the dorsal hippocampus (adapted from Wahlstrom et al., 2018, and from presentation slides kindly provided by Dr. Krista Wahlstrom with illustration assistance from Dr. Sean Farley). Together, these findings point to this circuit as a strong candidate for how the BLA influences dorsal hippocampal memory-related activity. See main article text for abbreviation definitions.
5. Conclusions and future directions
The many decades of work discussed in this review indicate that emotional arousal influences memory consolidation by activating the BLA which, in turn, modulates activity and plasticity in a variety of downstream brain regions, including the hippocampus (McGaugh, 2000; 2002; Roesler & McGaugh, 2010). Almost 20 years ago, Jim McGaugh wrote a review in a special issue of this journal where he described amygdala modulation of multiple memory systems (McGaugh, McIntyre, & Power, 2002). He wrote that “Findings of many studies indicate that the amygdala modulates the consolidation of long-term explicit memories of emotionally arousing experiences by influencing other brain regions involved in memory consolidation” (p. 540). In the two decades that have passed since this statement was made, laboratories from around the world have reported evidence for amygdala interactions with other brain regions during memory consolidation in humans and rodents, and many have identified candidate pathways and cellular mechanisms.
Pioneering discoveries made in the McGaugh laboratory, from the observation of enhancement of long-term memory with posttraining administration of epinephrine in rats (Gold & van Buskirk, 1976a) and humans (Cahill & Alkire, 2003) to the discovery that the amygdala plays a critical role in stress hormone enhancement of memory (Liang et al., 1986) and a view of the amygdala as a modulator of multiple kinds of memories (Packard et al., 1994), rather than the locus of memory storage (Parent, West, & McGaugh, 1994; Vazdarjanova & McGaugh, 1998), laid the foundation for a better understanding of how emotional arousal influences memory consolidation. Other studies carried out in the McGaugh laboratory identified neuromodulators that signal within the BLA to influence memory consolidation, including glucocorticoids (Roozendaal & McGaugh, 1996), norepinephrine (McIntyre et al., 2002; Quirarte, Galvez, Roozendaal, & McGaugh, 1998), dopamine (LaLumiere et al., 2004), acetylcholine (Power & McGaugh, 2002), and endocannabinoids (Campolongo et al., 2009b). Still others investigated the interactions of the BLA with the structures it projects to directly, such as the nucleus accumbens (Setlow, Roozendaal, & McGaugh, 2000), anterior cingulate cortex (Malin & McGaugh, 2006) and the entorhinal cortex (Roesler, Roozendaal, & McGaugh, 2002), or indirectly, such as the dorsal caudate (Packard et al., 1994) and dorsal hippocampus (Roozendaal & McGaugh, 1997a). Memory-enhancing manipulations of the BLA increase expression of the synaptic plasticity-associated protein ARC in the dorsal hippocampus, supporting the hypothesis that the BLA modulates the consolidation of different kinds of memory by affecting synaptic plasticity in downstream brain regions such as the dorsal hippocampus (McIntyre et al., 2005), but the mechanisms and pathways remained unresolved.
Today, technological advances have offered new opportunities for testing hypotheses that were conceived by these findings. The advent of human connectome projects has provided opportunities for investigation and comparison of functional connectivity between the BLA and hippocampus in humans during rest, learning, and remembering. Results indicate that emotional arousal modulates amygdala-hippocampal connectivity (Fastenrath et al., 2014), and functional connectivity between the amygdala and hippocampus at rest predicts memory performance under stress (de Voogd et al., 2017). Furthermore, long-lasting fear memories are associated with persistent amygdala-hippocampal connectivity after learning (Hermans et al., 2017).
The findings of recent animal studies indicate that BLA NE does more than modulate hippocampal plasticity and initial memory consolidation. It also alters systems consolidation timing by maintaining hippocampal-dependency of a contextual memory while altering DNA methylation and transcription of memory-related genes in the hippocampus (Atucha et al., 2017). Epigenetic mechanisms within the amygdala have also been reported. For example, HDAC inhibition in the BLA enhances consolidation for a hippocampus-dependent inhibitory avoidance task and increases hippocampal expression of the neurotrophic factor BDNF (Valiati et al., 2017). Inhibitors of HDACs have broad effects, including increasing expression of the norepinephrine transporter gene (Bayles, Baker, Eikelis, El-Osta, & Lambert, 2010) as well as increased biosynthesis of monoamines (Balasubramanian, Deng, Doudney, Hampton, & Kennedy, 2015), so it is possible that chromatin modifications affect norepinephrine levels in the BLA, which in turn, increases DNA methylation in the hippocampus, increasing transcription of BDNF, which can influence the translation of ARC.
Electrophysiological studies have indicated a role for the entorhinal cortex in mediating BLA effects on the dorsal hippocampus, demonstrating facilitation of transmission from the perirhinal to entorhinal cortex that was associated with BLA activity, and induction of LTP in the dorsal hippocampus following entorhinal cortex stimulation (Paz, Pelletier, Bauer, & Paré, 2006; Yaniv et al., 2003). To directly test the hypothesis that the BLA modulation of contextual memory and synaptic plasticity in the dorsal hippocampus is mediated by the entorhinal cortex, Wahlstrom and colleagues optogenetically stimulated BLA axons in the medial entorhinal cortex immediately after training, and found that optogenetic stimulation at a frequency of 8 Hz, but not 40 Hz, enhanced consolidation of spatial and contextual tasks (Wahlstrom et al., 2018), and increased expression of ARC protein in synaptic tissue taken from the dorsal hippocampus (Wahlstrom et al., 2021). Optogenetic stimulation of BLA axons in the medial entorhinal cortex at 8 Hz also increased local field activity in the theta-frequency range in the dorsal hippocampus (Wahlstrom et al., 2018). The effective stimulation frequency is informative, and consistent with other findings indicating that BLA-hippocampus interactions are rooted in synchronous oscillations in the theta range (Ahlgrim & Manns, 2019; Bienvenu et al., 2012). Theta oscillations are associated with spatial memory (Buzsaki & Moser, 2013), and BLA-hippocampus theta synchrony increases during fear memory retrieval (Seidenbecher, Laxmi, Stork, & Pape, 2003). These findings suggest that the BLA could entrain activity in the dorsal hippocampus through its actions on the medial entorhinal cortex to enhance the consolidation of emotionally arousing episodic memories.
Epidemiological studies reveal sex differences in incidence of anxiety and trauma-related disorders, yet the majority of the animal studies described in this review were carried out in male rodents. In recent years, the National Institutes of Health has mandated inclusion of both sexes in animal research, and recent findings suggest that the circuitry that underlies BLA modulation of hippocampus-dependent and caudate-dependent memory may not function precisely the same in male and female rats (Wahlstrom et al., 2021). Optogenetic stimulation of BLA axons in the medial entorhinal cortex enhances spatial memory in male and female rats, yet effects of optogenetic stimulation on ARC protein expression indicate a shift in the balance of plasticity in the dorsal hippocampus and dorsolateral striatum that is driven by increased ARC expression in the dorsal hippocampus in male rats and decreased ARC expression in the dorsolateral striatum in female rats. These are not the only findings revealing sex differences in physiological mechanism despite similar behavioral effects (Gruene, Roberts, Thomas, Ronzio, & Shansky, 2015; McCarthy, Arnold, Ball, Blaustein, & De Vries, 2012).
Future work should closely investigate learning-associated changes in oscillations and coherence between the BLA and the hippocampus while probing the potential pathways and direction of entrainment. Such studies could be carried out during learning, post-training consolidation, and retrieval. Simultaneously recording in the dorsal hippocampus and BLA during sleep may provide further information about the interaction between these two areas during consolidation of declarative memories (Wilson and McNaughton, 1993). Combining molecular investigations with electrophysiological studies may identify activity patterns that lead to chromatin modifications, gene expression, or translation of synaptic plasticity-associated proteins. Finally, future studies must carefully consider how the BLA modulates other memory systems in both male and female subjects.
Highlights.
We review research investigating how the amygdala modulates memories of emotional experiences
The amygdala influences memory consolidation and related molecular changes in the hippocampus
The amygdala modulates hippocampal memory via projections to the medial entorhinal cortex
Acknowledgements
The writing of this article was supported by the National Council for Scientific and Technological Development (CNPq, MCTI, Brazil) grant 305647/2019-9 (R.R.); Rio Grande do Sul State Research Foundation (FAPERGS, RS, Brazil) grant 17/2551-0001 071-0 (R.R.); National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) grant R01DK114700 (M.B.P.); and National Institutes of Mental Health (NIMH), NIH grant R01MH104384 (R.L. and C.K.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding institutions. Authors thank Dr. Krista L. Wahlstrom, Dr. Sean Farley, and Dr. Marialva Sinigaglia for assistance in preparing the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Niikura Y, & Misawa M (2003). The induction of long-term potentiation at amygdalo-hippocampal synapses in vivo. Biological & Pharmaceutical Bulletin, 26(11), 1560–1562. doi: 10.1248/bpb.26.1560. [DOI] [PubMed] [Google Scholar]
- Ahlgrim NS, & Manns JR (2019). Optogenetic stimulation of the basolateral amygdala increased theta-modulated gamma oscillations in the hippocampus. Frontiers in Behavioral Neuroscience, 13, 87. doi: 10.3389/fnbeh.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, & Richter-Levin G (1999a). Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. Journal of Neuroscience, 19(23), 10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, & Richter-Levin G (2002). Mechanisms of amygdala modulation of hippocampal plasticity. Journal of Neuroscience, 22, 9912–9921. doi: 10.1523/JNEUROSCI.22-22-09912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, & Richter-Levin G (1999b). Priming stimulation in the basolateral amygdala modulates synaptic plasticity in the rat dentate gyrus. Neuroscience Letters, 270(2), 83–86. doi: 10.1016/s0304-3940(99)00488-7. [DOI] [PubMed] [Google Scholar]
- Alberini CM, & Kandel ER (2014). The regulation of transcription in memory consolidation. Cold Spring Harbor Perspectives in Biology, 7(1), a021741. doi: 10.1101/cshperspect.a021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaguer-Melian W, Martínez-Martí L, Frey JU, & Bergado JA (2003). The amygdala is part of the behavioural reinforcement system modulating long-term potentiation in rat hippocampus. Neuroscience, 119(2), 319–322. doi: 10.1016/s0306-4522(02)00867-9. [DOI] [PubMed] [Google Scholar]
- Almaguer W, Estupiñán B, Uwe Frey J, & Bergado JA (2002). Aging impairs amygdala-hippocampus interactions involved in hippocampal LTP. Neurobiology of Aging, 23(2), 319–324. doi: 10.1016/s0197-4580(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Amaral DG, & Witter MP (1989). The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience, 31(3), 571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Ardeshiri MR, Hosseinmardi N, & Akbari E (2018). Orexin 1 and orexin 2 receptor antagonism in the basolateral amygdala modulate long-term potentiation of the population spike in the perforant path-dentate gyrus-evoked field potential in rats. Neurobiology of Learning and Memory, 149, 98–106. doi: 10.1016/j.nlm.2018.02.024. [DOI] [PubMed] [Google Scholar]
- Asok A, Leroy F, Rayman JB, & Kandel ER (2019). Molecular mechanisms of the memory trace. Trends in Neurosciences, 42(1), 14–22. doi: 10.1016/j.tins.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atucha E, Vukojevic V, Fornari RV, Ronzoni G, Demougin P, Peter F, Atsak P, Coolen MW, Papassotiropoulos A, McGaugh JL, de Quervain DJ, & Roozendaal B (2017). Noradrenergic activation of the basolateral amygdala maintains hippocampus-dependent accuracy of remote memory. Proceedings of the National Academy of Sciences United States of America, 114(34), 9176–9181. doi: 10.1073/pnas.1710819114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian D, Deng AX, Doudney K, Hampton MB, & Kennedy MA (2015). Valproic acid exposure leads to upregulation and increased promoter histone acetylation of sepiapterin reductase in a serotonergic cell line. Neuropharmacology 99, 79–88. doi: 10.1016/j.neuropharm.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Bass DI, & Manns JR (2015). Memory-enhancing amygdala stimulation elicits gamma synchrony in the hippocampus. Behavioral Neuroscience, 129(3), 244–256. doi: 10.1037/bne0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayles R, Baker E, Eikelis N, El-Osta A, & Lambert G (2010). Histone modifications regulate the norepinephrine transporter gene. Cell Cycle, 9(22), 4600–4601. doi: 10.4161/cc.9.22.13888. [DOI] [PubMed] [Google Scholar]
- Bienvenu TC, Busti D, Magill PJ, Ferraguti F, & Capogna M (2012). Cell-type-specific recruitment of amygdala interneurons to hippocampal theta rhythm and noxious stimuli in vivo. Neuron, 74(6), 1059–1074. doi: 10.1016/j.neuron.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M, Dornelles AS, Werenicz A, Velho LA, Pinto DF, Fedi AC, Schröder N, & Roesler R (2014). Basolateral amygdala activity is required for enhancement of memory consolidation produced by histone deacetylase inhibition in the hippocampus. Neurobiology of Learning and Memory, 111, 1–8. doi: 10.1016/j.nlm.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Bunsey M, & Eichenbaum H (1996). Conservation of hippocampal memory function in rats and humans. Nature, 379(6562), 255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, & Moser EI (2013). Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience 16(2), 130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, & Alkire MT (2003). Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiology of Learning and Memory, 79(2), 194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Cuomo V, Astarita G, Fu J, McGaugh JL, & Piomelli D (2009a). Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proceedings of the National Academy of Sciences United States of America, 106(19), 8027–8031. doi: 10.1073/pnas.0903038106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, & Cuomo V (2009b). Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proceedings of the National Academy of Sciences United States of America, 106(12), 4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, & Cahill L (2000). Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience, 20(19), RC99. 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S (2002). What’s new with the amnesic patient H.M.? Nature Reviews Neuroscience, 3(2), 153–160. 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- Corkin S, Amaral DG, Gonzalez RG, Johnson KA, & Hyman BT (1997). H. M.’s medial temporal lobe lesion: findings from magnetic resonance imaging. Journal of Neuroscience, 17(10), 3964–3979. 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Voogd LD, Klumpers F, Fernández G, & Hermans EJ (2017). Intrinsic functional connectivity between amygdala and hippocampus during rest predicts enhanced memory under stress. Psychoneuroendocrinology 75, 192–202. doi: 10.1016/j.psyneuen.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, & Eichenbaum H (2010). The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology, 35(1), 86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson-Anson H, & McGaugh JL (1997). Bicuculline administered into the amygdala after training blocks benzodiazepine-induced amnesia. Brain Research, 752(1–2), 197–202. doi: 10.1016/s0006-8993(96)01449-7. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2017). On the integration of space, time, and memory. Neuron, 95(5), 1007–1018. doi: 10.1016/j.neuron.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Kuperstein M, Fagan A, & Nagode J (1987). Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. Journal of Neuroscience, 7(3), 716–732. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, & Dong HW (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron, 65(1), 7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fastenrath M, Coynel D, Spalek K, Milnik A, Gschwind L, Roozendaal B, Papassotiropoulos A, & de Quervain DJ (2014). Dynamic modulation of amygdala-hippocampal connectivity by emotional arousal. Journal of Neuroscience, 34(42), 13935–13947. doi: 10.1523/JNEUROSCI.0786-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, & McGaugh JL (2008). Involvement of basolateral amygdala alpha2-adrenoceptors in modulating consolidation of inhibitory avoidance memory. Learning & Memory, 15(4), 238–243. doi: 10.1101/lm.760908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, & Bontempi B (2005). The organization of recent and remote memories. Nature Reviews Neuroscience, 6(2), 119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, & Frey JU (2001). Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP. Journal of Neuroscience, 21(10), 3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujise Y, Kubota M, Suzuki M, Tamano H, & Takeda A (2017). Blockade of intracellular Zn2+ signaling in the basolateral amygdala affects object recognition memory via attenuation of dentate gyrus LTP. Neurochemistry International, 108, 1–6. doi: 10.1016/j.neuint.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Hafting T, Treves A, Moser MB, & Moser EI (2007). Hippocampal remapping and grid realignment in entorhinal cortex. Nature, 446(7132), 190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Kapp BS, Musty RE, & Driscoll PA (1977). Memory formation: evidence for a specific neurochemical system in the amygdala. Science, 198(4315), 423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- Giachero M, Calfa GD, & Molina VA (2015). Hippocampal dendritic spines remodeling and fear memory are modulated by GABAergic signaling within the basolateral amygdala complex. Hippocampus 25(5), 545–55. doi: 10.1002/hipo.22409. [DOI] [PubMed] [Google Scholar]
- Gold PE, & van Buskirk R (1976a). Effects of posttrial hormone injections on memory processes. Hormones and Behavior, 7(4), 509–517. doi: 10.1016/0018-506x(76)90021-0. [DOI] [PubMed] [Google Scholar]
- Gold PE, & Van Buskirk R (1976b). Enhancement and impairment of memory processes with post-trial injections of adrenocorticotrophic hormone. Behavioral Biology, 16(4), 387–400. doi: 10.1016/s0091-6773(76)91539-x. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, & Shansky RM (2015). Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biological Psychiatry, 78(3), 186–93. doi: 10.1016/j.biopsych.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, & Barnes CA (2000). Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. Journal of Neuroscience, 20(11), 3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, & Moser EI (2005). Microstructure of a spatial map in the entorhinal cortex. Nature, 436 (7052), 801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Heyser CJ, & Deadwyler SA (1993). Hippocampal cell firing correlates of delayed-match-to-sample performance in the rat. Behavioral Neuroscience, 107(5), 715–739. doi: 10.1037//0735-7044.107.5.715. [DOI] [PubMed] [Google Scholar]
- Hardt O, & Nadel L (2018). Systems consolidation revisited, but not revised: The promise and limits of optogenetics in the study of memory. Neuroscience Letters, 680, 54–59. doi: 10.1016/j.neulet.2017.11.062. [DOI] [PubMed] [Google Scholar]
- Hatfield T, & McGaugh JL (1999). Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiology of Learning and Memory, 71(2), 232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Kanen JW, Tambini A, Fernández G, Davachi L, & Phelps EA (2017). Persistence of amygdala-hippocampal connectivity and multi-voxel correlation structures during awake rest after fear learning predicts long-term expression of fear. Cerebral Cortex, 27(5), 3028–3041. doi: 10.1093/cercor/bhw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, Emmons EB, Narayanan NS, & LaLumiere RT (2016). Basolateral amygdala projections to ventral hippocampus modulate the consolidation of footshock, but not contextual, learning in rats. Learning and Memory, 23(2), 51–60. doi: 10.1101/lm.039909.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, & Rudy JW (2006). Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. Journal of Neuroscience, 26(5), 1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Nakanishi K, Saito H, & Abe K (1997). Amygdala beta-noradrenergic 3146. doi: 10.1097/00001756-199709290-00027. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, & Abe K (1994). Attenuated hippocampal long-term potentiation in basolateral amygdala-lesioned rats. Brain Research, 656(1), 157–164. doi: 10.1016/0006-8993(94)91377-3. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, & Abe K (1995). Requirement of basolateral amygdala neuron activity for the induction of long-term potentiation in the dentate gyrus in vivo. Brain Research, 671(2), 351–354. doi: 10.1016/0006-8993(94)01403-5. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, & Abe K (1996). The basomedial and basolateral amygdaloid nuclei contribute to the induction of long-term potentiation in the dentate gyrus in vivo. European Journal of Neuroscience, 8(9), 1833–1839. doi: 10.1111/j.1460-9568.1996.tb01327.x. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Miyazaki B, & McGaugh JL (1991). Involvement of the amygdala in the memory-enhancing effects of clenbuterol. Psychopharmacology (Berl), 104(4), 541–544. doi: 10.1007/BF02245663. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Nagahara AH, & McGaugh JL (1989). Memory enhancement with intra-amygdala post-training naloxone is blocked by concurrent administration of propranolol. Brain Research, 476(1), 94–101. doi: 10.1016/0006-8993(89)91540-0. [DOI] [PubMed] [Google Scholar]
- Jeon B, Hwang YK, Lee SY, Kim D, Chung C, & Han JS (2012). The role of basolateral amygdala in the regulation of stress-induced phosphorylated extracellular signal-regulated kinase expression in the hippocampus. Neuroscience, 224, 191–201. doi: 10.1016/j.neuroscience.2012.08.035. [DOI] [PubMed] [Google Scholar]
- Josselyn SA & Tonegawa S (2020). Memory engrams: Recalling the past and imagining the future. Science, 367(6473), eaaw4325. doi: 10.1126/science.aaw4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpoor M, Nasehi M, Vahdati A, Hoseyni SE, & Zarrindast MR (2016). Additive effect of BLA GABAA receptor mechanism and (+)-MK-801 on memory retention deficit, an isobologram analysis. Pharmacology, Biochemistry, and Behavior, 143, 57–64. doi: 10.1016/j.pbb.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Koo JW, Lee HJ, & Han JS (2005). Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. Journal of Neuroscience, 25(6), 1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han JS, & Packard MG (2001). Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. Journal of Neuroscience, 21(14), 5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ED, Friedman AR, Covarrubias D, Ying C, Sun WG, Goosens KA, Sapolsky RM, & Kaufer, (2012). Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Molecular Psychiatry, 17(5), 527–536. doi: 10.1038/mp.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Redondo RL, & Tonegawa S (2017). Engrams and circuits crucial for systems consolidation of a memory. Science, 356(6333), 73–78. doi: 10.1126/science.aam6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, & Moser MB (2008). Finite scale of spatial representation in the hippocampus. Science, 321(5885), 140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Klinzing JG, Niethard N, & Born J (2019). Mechanisms of systems memory consolidation during sleep. Nature Neuroscience, 22, 1598–1610. doi: 10.1038/s41593-019-0467-3. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, & McGaugh JL (2003). Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. Journal of Neuroscience, 23(17), 6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Nguyen LT, & McGaugh JL (2004). Post-training intrabasolateral amygdala infusions of dopamine modulate consolidation of inhibitory avoidance memory: involvement of noradrenergic and cholinergic systems. European Journal of Neuroscience, 20(10), 2804–2810. doi: 10.1111/j.1460-9568.2004.03744.x. [DOI] [PubMed] [Google Scholar]
- Leal SL, Noche JA, Murray EA, & Yassa MA (2017). Age-related individual variability in memory performance is associated with amygdala-hippocampal circuit function and emotional pattern separation. Neurobiology of Aging, 49, 9–19. doi: 10.1016/j.neurobiolaging.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton LJ, Ke K, Zajaczkowski EL, Edmunds J, Spitale RC, & Bredy TW (2018). Experience-dependent neural plasticity, learning, and memory in the era of epitranscriptomics. Genes, Brain, & Behavior, 17(3), e12426. doi: 10.1111/gbb.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, & Moser MB (2005). Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science, 309(5734), 619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Li Z, & Richter-Levin G (2012). Stimulus intensity-dependent modulations of hippocampal long-term potentiation by basolateral amygdala priming. Frontiers in Cellular Neuroscience, 6, 21. doi: 10.3389/fncel.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, & Richter-Levin G (2013). Priming stimulation of basal but not lateral amygdala affects long-term potentiation in the rat dentate gyrus in vivo. Neuroscience, 246, 13–21. doi: 10.1016/j.neuroscience.2013.03.059. [DOI] [PubMed] [Google Scholar]
- Liang KC, Juler RG, & McGaugh JL (1986). Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Research, 368(1), 125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Lim EP, Dawe GS, & Jay TM (2017). Activation of beta- and alpha-2-adrenoceptors in the basolateral amygdala has opposing effects on hippocampal-prefrontal long-term potentiation. Neurobiology of Learning and Memory, 137, 163–170. doi: 10.1016/j.nlm.2016.11.020. [DOI] [PubMed] [Google Scholar]
- Lovitz ES, & Thompson LT (2015). Memory-enhancing intra-basolateral amygdala clenbuterol infusion reduces post-burst afterhyperpolarizations in hippocampal CA1 pyramidal neurons following inhibitory avoidance learning. Neurobiology of Learning and Memory, 119, 34–41. doi: 10.1016/j.nlm.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Malin EL, & McGaugh JL (2006). Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proceedings of the National Academy of Sciences United States of America, 103(6), 1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, & De Vries GJ (2012). Sex differences in the brain: the not so inconvenient truth. Journal of Neuroscience, 32(7), 2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, & Mott DD (2017). Functional neuroanatomy of amygdalohippocampal interconnections and their role in learning and memory. Journal of Neuroscience Research, 95(3), 797–820. doi: 10.1002/jnr.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ,& Zaric V (2015a). Extrinsic origins of the somatostatin and neuropeptide Y innervation of the rat basolateral amygdala. Neuroscience, 294, 82–100. doi: 10.1016/j.neuroscience.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, & Zaric V (2015a). GABAergic somatostatin-immunoreactive neurons in the amygdala project to the entorhinal cortex. Neuroscience, 290, 227–242. doi: 10.1016/j.neuroscience.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL (2000). Memory--a century of consolidation. Science, 287(5451), 248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2002). Memory consolidation and the amygdala: a systems perspective. Trends in Neurosciences, 25(9), 456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Introini-Collison IB, & Nagahara AH (1988). Memory-enhancing effects of posttraining naloxone: involvement of beta-noradrenergic influences in the amygdaloid complex. Brain Research, 446(1), 37–49. doi: 10.1016/0006-8993(88)91294-2. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, & Power AE (2002). Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiology of Learning and Memory, 78(3), 539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, & McGaugh JL (2002). Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. European Journal of Neuroscience 16(7), 1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, & McGaugh JL (2005). Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proceedings of the National Academy of Sciences United States of America, 102(30), 10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Anderson KM, Donowho KM, & McIntyre CK (2014). Noradrenergic actions in the basolateral complex of the amygdala modulate Arc expression in hippocampal synapses and consolidation of aversive and non-aversive memory. Neurobiology of Learning and Memory, 115, 49–57. doi: 10.1016/j.nlm.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, & McIntyre CK (2012). Emotional modulation of the synapse. Reviews in Neuroscience, 23(5–6), 449–461. doi: 10.1515/revneuro-2012-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Colucci P, Mancini GF, De Castro V, Peloso A, Schelling G, & Campolongo P (2021). Ketamine anesthesia enhances fear memory consolidation via noradrenergic activation in the basolateral amygdala. Neurobiology of Learning and Memory, 178, 107362. doi: 10.1016/j.nlm.2020.107362. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, & Nadel L (2016). Episodic memory and beyond: The hippocampus and neocortex in transformation. Annual Review of Psychology, 67, 105–134. doi: 10.1146/annurev-psych-113011-143733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E, & Moser MB (2008). Place cells, grid cells, and the brain’s spatial representation system. Annual Review of Neuroscience, 31, 69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Moser MB, & Moser EI (1998). Functional differentiation in the hippocampus. Hippocampus, 8(6), 608–619. doi: . [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, & Andersen P (1993). Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. Journal of Neuroscience, 13(9), 3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, & Morris RG (1995). Spatial learning with a minislab in the dorsal hippocampus. Proceedings of the National Academy of Sciences United States of America, 92(21), 9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Rowland DC, & Moser EI (2015). Place cells, grid cells, and memory. Cold Spring Harbor Perspectives in Biology, 7(2), a021808. doi: 10.1101/cshperspect.a021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Matsuyama K, Matsuki N, & Ikegaya Y (2004). Amygdala stimulation modulates hippocampal synaptic plasticity. Proceedings of the National Academy of Sciences United States of America, 101(39), 14270–14275. doi: 10.1073/pnas.0405709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, & Pape HC (2007). Dissociated theta phase synchronization in amygdalo- hippocampal circuits during various stages of fear memory. European Journal of Neuroscience, 25(6), 1823–1831. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- Nasehi M, Davoudi K, Ebrahimi-Ghiri M, & Zarrindas MR (2016). Interplay between serotonin and cannabinoid function in the amygdala in fear conditioning. Brain Research, 1636, 142–151. doi: 10.1016/j.brainres.2016.01.034. [DOI] [PubMed] [Google Scholar]
- Nathan SV, Griffith QK, McReynolds JR, Hahn EL, & Roozendaal B (2004). Basolateral amygdala interacts with other brain regions in regulating glucocorticoid effects on different memory functions. Annals of the New York Academy of Sciences, 1032, 179–182. doi: 10.1196/annals.1314.015. [DOI] [PubMed] [Google Scholar]
- Noorani SK, Hojati V, Ardeshiri MR, Akbari E, & Ehsani S (2020). Modulation of long-term and short-term plasticity in the dentate gyrus granule cells by activating the β-adrenergic receptors of the basolateral amygdala. Neuroscience Letters 725, 134878. doi: 10.1016/j.neulet.2020.134878. [DOI] [PubMed] [Google Scholar]
- O’Keefe J (1976). Place units in the hippocampus of the freely moving rat. Experimental Neurology, 51(1), 78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L, & McGaugh JL (1994). Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proceedings of the National Academy of Sciences United States of America, 91(18), 8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Collins DR, & Pelletier JG (2002). Amygdala oscillations and the consolidation of emotional memories. Trends in Cognitive Sciences, 6(7), 306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Parent MB, & McGaugh JL (1994). Posttraining infusion of lidocaine into the amygdala basolateral complex impairs retention of inhibitory avoidance training. Brain Research, 661(1–2), 97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- Parent MB, West M, & McGaugh JL (1994). Memory of rats with amygdala lesions induced 30 days after footshock-motivated escape training reflects degree of original training. Behavioral Neuroscience, 108(6), 1080–1087. doi: 10.1037//0735-7044.108.6.1080. [DOI] [PubMed] [Google Scholar]
- Paz R, Pelletier JG, Bauer EP, & Paré D (2006). Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nature Neuroscience, 9(10), 1321–1329. doi: 10.1038/nn1771. Epub 2006 Sep 10. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, & Paré D (2002). Uniform range of conduction times from the lateral amygdala to distributed perirhinal sites. Journal of Neurophysiology, 87(3), 1213–1221. doi: 10.1152/jn.00623.2001. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, & Swanson LW (2001). Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research. Brain Research Reviews, 38(1–2), 247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Rönkkö S, Savander V, Insausti R, & Pitkänen A (1999). Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. Journal of Comparative Neurology, 403(2), 229–260. [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, & Ylinen A (2000). Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Academy of Sciences, 911, 369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, & LeDoux JE (1997). Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in Neurosciences, 20(11), 517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Power AE, McIntyre CK, Litmanovich A, & McGaugh JL (2003). Cholinergic modulation of memory in the basolateral amygdala involves activation of both m1 and m2 receptors. Behavioural Pharmacology, 14(3), 207–213. doi: 10.1097/00008877-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Power AE, & McGaugh JL (2002). Phthalic acid amygdalopetal lesion of the nucleus basalis magnocellularis induces reversible memory deficits in rats. Neurobiology of Learning and Memory, 77(3), 372–388. doi: 10.1006/nlme.2001.4030. [DOI] [PubMed] [Google Scholar]
- Price JL, Russchen FT, & Amaral DG (1987). The limbic region. II: The amygdaloid complex. In: Björklund A, Hökfelt T, Swanson LW, editors. Handbook of Chemical Neuroanatomy, vol. 5, integrated systems of the NS, part I. Amsterdam: Elsevier. P. 279–388. [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, & McGaugh JL (1998). Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Research, 808(2), 134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, & McGaugh JL (1997). Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences United States of America, 94(25), 14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Ruiz P, Visser E, Mitrić M, Smit AB, & van den Oever MC (2021). A synaptic framework for the persistence of memory engrams. Frontiers in Synaptic Neuroscience, 13, 661476. doi: 10.3389/fnsyn.2021.661476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezayof A, Habibi P, & Zarrindast MR (2011). Involvement of dopaminergic and glutamatergic systems of the basolateral amygdala in amnesia induced by the stimulation of dorsal hippocampal cannabinoid receptors. Neuroscience, 175, 118–126. doi: 10.1016/j.neuroscience.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, & Dolan RJ (2004). Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neuroscience, 7(3), 278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, & Akirav I (2000). Amygdala-hippocampus dynamic interaction in relation to memory. Molecular Neurobiology, 22(1–3), 11–20. doi: 10.1385/MN:22:1-3:011. [DOI] [PubMed] [Google Scholar]
- Roesler R, & McGaugh JL (2010). Memory consolidation. In Koob GF, Le Moal M, & Thompson RF (Eds.). Encyclopedia of Behavioral Neuroscience (Vol. 2, pp. 206–214). Oxford: Academic Press. [Google Scholar]
- Roesler R, Roozendaal B, & McGaugh JL (2002). Basolateral amygdala lesions block the memory-enhancing effect of 8-Br-cAMP infused into the entorhinal cortex of rats after training. European Journal of Neuroscience, 15(5), 905–910. doi: 10.1046/j.1460-9568.2002.01924.x. [DOI] [PubMed] [Google Scholar]
- Roesler R, Schröder N, Vianna MR, Quevedo J, Bromberg E, Kapczinski F, & Ferreira MB (2003). Differential involvement of hippocampal and amygdalar NMDA receptors in contextual and aversive aspects of inhibitory avoidance memory in rats. Brain Research, 975(1–2), 207–213. doi: 10.1016/s0006-8993(03)02656-8. [DOI] [PubMed] [Google Scholar]
- Roesler R, Vianna MR, de Paris F, Quevedo J, Walz R, & Bianchin M (2000a). Infusions of AP5 into the basolateral amygdala impair the formation, but not the expression, of step-down inhibitory avoidance. Brazilian Journal of Medical and Biological Research, 33, 829–834. doi: . [DOI] [PubMed] [Google Scholar]
- Roesler R, Vianna MR, de Paris F, Rodrigues C, Sant’Anna MK, Quevedo J, & Ferreira MB (2000b). NMDA receptor antagonism in the basolateral amygdala blocks enhancement of inhibitory avoidance learning in previously trained rats. Behavioural Brain Research, 112(1–2), 99–105. doi: 10.1016/s0166-4328(00)00169-8. [DOI] [PubMed] [Google Scholar]
- Roesler R, Vianna M, Sant’Anna MK, Kuyven CR, Kruel AV, Quevedo J, & Ferreira MB (1998). Intrahippocampal infusion of the NMDA receptor antagonist AP5 impairs retention of an inhibitory avoidance task: protection from impairment by pretraining or preexposure to the task apparatus. Neurobiology of Learning and Memory, 69(2), 87–91. doi: 10.1006/nlme.1997.3810. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Griffith QK, Buranday J, De Quervain DJ, & McGaugh JL (2003). The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: dependence on the basolateral amygdala. Proceedings of the National Academy of Sciences United States of America, 100(3), 1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, & McGaugh JL (1996). Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiology of Learning and Memory, 65(1), 1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, & McGaugh JL (1997a). Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. European Journal of Neuroscience, 9(1), 76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, & McGaugh JL (1997b). Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiology of Learning and Memory, 67(2), 176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. (1999). Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proceedings of the National Academy of Sciences United States of America, 96(20), 11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, & Power J (2003). The amygdaloid complex: anatomy and physiology. Physiological Reviews, 83(3), 803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Saha R, Kriebel M, Volkmer H, Richter-Levin G, & Albrecht A (2018). Neurofascin knock down in the basolateral amygdala mediates resilience of memory and plasticity in the dorsal dentate gyrus under stress. Molecular Neurobiology, 55(9), 7317–7326. doi: 10.1007/s12035-018-0930-2. [DOI] [PubMed] [Google Scholar]
- Savander V, LeDoux JE, & Pitkänen A (1996). Topographic projections from the periamygdaloid cortex to select subregions of the lateral nucleus of the amygdala in the rat. Neuroscience Letters, 211(3), 167–170. doi: 10.1016/0304-3940(96)12750-6. [DOI] [PubMed] [Google Scholar]
- Savander V, Miettinen R, Ledoux JE, & Pitkänen A (1997). Lateral nucleus of the rat amygdala is reciprocally connected with basal and accessory basal nuclei: a light and electron microscopic study. Neuroscience, 77(3), 767–781. doi: 10.1016/s0306-4522(96)00513-1. [DOI] [PubMed] [Google Scholar]
- Scoville WB, & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20(1), 11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, & Pape HC (2003). Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science, 301(5634), 846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Sendi MSE, Kanta V, Inman CS, Manns JR, Hamann S, Gross RE, Willie JT, & Mahmoudi B (2020). Amygdala stimulation leads to functional network connectivity state transitions in the hippocampus. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2020, 3625–3628. doi: 10.1109/EMBC44109.2020.9176742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Roozendaal B, & McGaugh JL (2000). Involvement of a basolateral amygdala complex-nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. European Journal of Neuroscience, 12(1), 367–375. doi: 10.1046/j.1460-9568.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, & Gordon JA (2015). Hippocampal-prefrontal input supports spatial encoding in working memory. Nature, 522(7556), 309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR (2009). The legacy of patient H.M. for neuroscience. Neuron, 61(1), 6–9. doi: 10.1016/j.neuron.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, & Zola-Morgan S (1991). The medial temporal lobe memory system. Science, 253(5026), 1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, & Moser EI (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience, 15(10), 655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Takata N, Yoshida K, Komaki Y, Xu M, Sakai Y, Hikishima K, Mimura M, Okano H, & Tanaka KF (2015). Optogenetic activation of CA1 pyramidal neurons at the dorsal and ventral hippocampus evokes distinct brain-wide responses revealed by mouse fMRI. PLoS One, 10(3), e0121417. doi: 10.1371/journal.pone.0121417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K (2020). Neurobiology of systems memory consolidation. European Journal of Neuroscience. doi: 10.1111/ejn.14694. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Abe K, & Nojyo Y (1988). Three-dimensional analysis of the whole axonal arbors originating from single CA2 pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique. Brain Research, 452(1–2), 255–272. doi: 10.1016/0006-8993(88)90030-3. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Watanabe K, & Nojyo Y (1984). A whole image of the hippocampal pyramidal neuron revealed by intracellular pressure-injection of horseradish peroxidase. Brain Research, 307(1–2), 336–340. doi: 10.1016/0006-8993(84)90489-x. [DOI] [PubMed] [Google Scholar]
- Tanimizu T, Kenney JW, Okano E, Kadoma K, Frankland PW, & Kida S (2017). Functional connectivity of multiple brain regions required for the consolidation of social recognition memory. Journal of Neuroscience, 37(15), 4103–4116. doi: 10.1523/JNEUROSCI.3451-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiati FE, Vasconcelos M, Lichtenfels M, Petry FS, de Almeida RMM, Schwartsmann G, Schröder N, de Farias CB, & Roesler R (2017). Administration of a histone deacetylase inhibitor into the basolateral amygdala enhances memory consolidation, delays extinction, and increases hippocampal BDNF levels. Frontier in Pharmacology, 8, 415. doi: 10.3389/fphar.2017.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, & McGaugh JL (1998). Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proceedings of the National Academy of Sciences United States of America, 95(25), 15003–15007. doi: 10.1073/pnas.95.25.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouimba RM, Anunu R, & Richter-Levin G (2020). GABAergic transmission in the basolateral amygdala differentially modulates plasticity in the dentate gyrus and the CA1 areas. International Journal of Molecular Sciences, 21(11), 3786. doi: 10.3390/ijms21113786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouimba RM, Yaniv D, & Richter-Levin G (2007). Glucocorticoid receptors and beta-adrenoceptors in basolateral amygdala modulate synaptic plasticity in hippocampal dentate gyrus, but not in area CA1. Neuropharmacology, 52(1), 244–252. doi: 10.1016/j.neuropharm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, & Richter-Levin G (2005). Physiological dissociation in hippocampal subregions in response to amygdala stimulation. Cerebral Cortex 15(11), 1815–1821. doi: 10.1093/cercor/bhi058. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, & Richter-Levin G (2013). Different patterns of amygdala priming differentially affect dentate gyrus plasticity and corticosterone, but not CA1 plasticity. Frontiers in Neural Circuits, 7:80. doi: 10.3389/fncir.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom KL, Alvarez-Dieppa AC, McIntyre CK, & LaLumiere RT (2021). The medial entorhinal cortex mediates basolateral amygdala effects on spatial memory and downstream activity-regulated cytoskeletal-associated protein expression. Neuropsychopharmacology, 46(6), 1172–1182. doi: 10.1038/s41386-020-00875-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom KL, Huff ML, Emmons EB, Freeman JH, Narayanan NS, McIntyre CK, & LaLumiere RT (2018). Basolateral amygdala inputs to the medial entorhinal cortex selectively modulate the consolidation of spatial and contextual learning. Journal of Neuroscience, 38(11), 2698–2712. doi: 10.1523/JNEUROSCI.2848-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, & McNaughton BL (1994). Reactivation of hippocampal ensemble memories during sleep. Science, 29, 265(5172):676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Winocur G, & Moscovitch M (2011). Memory transformation and systems consolidation. Journal of the International Neuropsychological Society: JINS, 17(5), 766–780. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, & Bontempi B (2010). Memory formation and long-term retention in humans and animals: convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia, 48(8), 2339–2356. doi: 10.1016/j.neuropsychologia.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Wilson MA, & McNaughton BL (1993). Dynamics of the hippocampal ensemble code for space. Science, 261(5124), 1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang ZH, Jin S, Gao D, Liu N, Chen SP, Zhang S, Liu Q, Liu E, Wang X, Liang X, Wei P, Li X, Li Y, Yue C, Li HL, Wang YL, Wang Q, Ke D, Xie Q, Xu F, Wang L, & Wang JZ (2016). Opposite monosynaptic scaling of BLP-vCA1 inputs governs hopefulness- and helplessness-modulated spatial learning and memory. Nature Communications, 7, 11935. doi: 10.1038/ncomms11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yang S, Moreira T, Hoffman G, Carlson GC, Bender KJ, Alger BE, & Tang CM (2014). Interlamellar CA1 network in the hippocampus. Proceedings National Academy of Sciences United States of America, 111(35), 12919–12924. doi: 10.1073/pnas.1405468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv D, Vouimba RM, Diamond DM, Richter-Levin G (2003). Simultaneous induction of long-term potentiation in the hippocampus and the amygdala by entorhinal cortex activation: mechanistic and temporal profiles. Neuroscience, 120(4), 1125–1135. doi: 10.1016/s0306-4522(03)00386-5. [DOI] [PubMed] [Google Scholar]


