Abstract
The amygdala is a collection of nuclei that support adaptive social behavior and that are implicated in disorders such as autism. The basolateral complex of the amygdala (BLA), a main subdivision of the amygdala, influences fear responses, motivated behavior, and memory of emotional events via its communication with other amygdalar nuclei and with other brain regions such as the prefrontal cortex, striatum, and hippocampus. The specific role of the BLA in responses to social stimuli is less clear. The present study of female rats investigated the role of the BLA in responding to socially-relevant information by asking how inactivation of the BLA with bilateral infusions of the GABA receptor agonist muscimol would affect spontaneous exploration of wood blocks scented either with conspecific male or female urine or with nonsocial odorants. Conspecific urine samples were used because urine conveys information about sex, health, social status, and reproductive state in rodents. The results revealed that BLA inactivation reduced female rats’ spontaneous preference for social odors over nonsocial odors, specifically for female urine. However, BLA inactivation did not generally impair rats’ ability to distinguish two odors from the same category (e.g., urine odors from two different male rats). The results indicate that the BLA is important for responding to salience of social stimuli but not for discriminating between different individuals, a result that has important implications for amygdalar modulation of downstream attention, motivation, and memory processes for social stimuli.
Keywords: amygdala, affect, social, preference, salience, urine
1. Introduction
The amygdala is a key part of the neural circuitry supporting emotion-related behavior and is implicated in autism, PTSD, and psychopathy (Mahan & Ressler, 2012). The amygdala is a heterogeneous collection of nuclei (Petrovic et al., 2001), and many lines of rodent research have shown that the basolateral complex of the amygdala (BLA) influences both fear responses and motivated behavior via its connections with other amygdalar nuclei and structures such as the striatum and medial prefrontal cortex (Janak & Tye 2015). The BLA also plays an important role in modulating memory consolidation during emotional arousal through its projections to the hippocampus and associated regions (McGaugh, 2004; Pare, 2003). Thus, the BLA is essential for processing stimuli with affective salience as well as for mediating downstream neural and physiological responses to those stimuli.
There is also accumulating evidence that the amygdala is an important node in brain networks supporting social behavior (Adolphs, 2010; Insel & Fernald, 2004; Walum & Young, 2018). For example, patients with damage to the amygdala show atypical viewing patterns of faces and consequent impairments in judging some facial expressions (Adolphs et al., 2005). In rodents, olfaction plays a key role in social behavior (Brown, 1988), and the extended medial amygdala is essential for normal responses to social odors detected by both the vomeronasal and main olfactory pathways (Brennan & Kendrick, 2006; Newman, 1999). The role of the BLA in responding to social odors is less clear. In line with the role of the BLA in modulating memory, a commonly used social task has been a delayed (minutes to hours) recognition memory task in which social odor memory is inferred at test by spontaneous preference for a novel conspecific over a previously encountered conspecific. Manipulations of BLA function have yielded inconsistent results on tasks of this type (e.g., Maaswinkel et al., 1996; Wang et al., 2014; Zinn et al., 2016), possibly because the task involves conspecific discrimination and a priori preferences in addition to memory demands.
The present study investigated the potentially separable roles of the BLA in conspecific odor discrimination and preference by asking how pharmacological inactivation of the BLA in female rats would affect spontaneous exploration of clean wood blocks scented either with conspecific male or female urine or with plant-based essential oils as nonsocial odorants. Urine was used as a social stimulus as it conveys important information about conspecifics, including sex, health, social status, and reproductive states (Brennan & Kendrick, 2006; Brown, 1988). Female subjects were used because adult female rats are less aggressive towards unfamiliar same-sex conspecifics as compared to adult male rats (Schweinfurth, 2020), thereby increasing the likelihood that same-sex urine odors would elicit affiliative rather than aggressive responses. The results revealed that BLA inactivation reduced rats’ spontaneous exploration of female urine relative to nonsocial odors but did not generally affect rats’ ability to distinguish two odors from the same category (e.g., urine odors from two different male rats). The impact of BLA inactivation on social odor preference but not individual discrimination is consistent with the broader role of the BLA in prioritizing stimuli with affective salience, with important implications for amygdalar modulation of downstream attention, motivation, and memory consolidation processes for social stimuli.
2. Materials and Methods
2.1. Subjects
Eighteen female Long Evans rats (3–5 months old; 275–350g) were group-housed by the vendor (Charles River) and then pair-housed upon arrival at Emory University’s Division of Animal Resources. Following surgery (see Section 2.2), rats were housed individually to prevent chewing on another rat’s surgical implants. Rats were kept on a 12:12 light:dark cycle, and testing occurred during the light period. Rats had ad libitum access to water and food during the experiments. A within-subjects design was used to maximize statistical power and to minimize the number of rats used. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Emory University.
2.2. Surgeries and drug infusions
Rats were anesthetized with 1–3% of isoflurane during stereotaxic surgery to implant bilateral chronic guide cannula (26 gauge; Plastics One) aimed just dorsal to the BLA (coordinates relative to bregma in mm: −3.5AP, ±5.1ML, −8.4DV). Buprenorphine (0.03 mg/kg) and meloxicam (1 mg/kg) were administered immediately before and after surgery and on subsequent days (buprenorphine for one day and meloxicam for two days). Rats recovered for at least one week before testing.
Figure 1 shows schematic diagrams of experimental procedures. Thirty minutes prior to a testing session, fluorophore-conjugated muscimol (0.5 μg/μl; ThermoFisher M23400) or saline was infused at 250 nl/min through a 33-gauge needle while rats were briefly under 1–3% isoflurane anesthesia. The needle was inserted in the guide cannula such that the tip of the needle was aimed at the BLA (−3.5AP, ±5.1ML, −8.8DV) and left in place for 2 minutes following infusion. The study was performed in two cohorts. In the first cohort of 9 rats, 1 μl of muscimol or saline was infused bilaterally in the BLA. Although postmortem histology and prior research (Allen et al., 2008) suggested that the infusion did not spread beyond the BLA, the infusion volume was reduced to 0.5 μl in the second cohort of 9 rats as a precaution. Each rat received muscimol and saline infusions in a counterbalanced order, and infusions occurred at least 24 hrs apart. Cohort 1 completed the habituation-dishabituation task first, and cohort 2 completed the preference task first (Fig. 1 and Section 2.5).
Figure 1. Schematic diagrams of task procedures.

(A) Seven to fourteen days following surgery to implant infusion cannula bilaterally in the BLA, female rats (n=9) in cohort 1 were administered the habituation-dishabituation task and then the preference task. Female rats (n=9) in cohort 2 followed a similar timeline except that they were administered the preference task prior to the habituation-dishabituation task. (B) In the habituation-dishabituation task, 30 min following infusion of saline (Sal) or muscimol (Mus) in the BLA, female rats were presented the same odor on a clean wood block repeatedly for 5 times in a row with a 15-s interval. In the sixth presentation, rats were then presented with a new odor of the same category (male urine, female urine, or nonsocial essential oil). Two habituation-dishabituation trials were administered each testing day with an intertrial interval of approximately 1 hr, one using nonsocial odorants (N) and one using conspecific urine from either male (M) or female (F) donors. New odors were used for each trial. Spontaneous exploration times were used to infer discrimination of the repeated and new odors. (C) In the preference task, 30 min following infusion of saline or muscimol in the BLA, female rats were presented on each trial with two blocks simultaneously for 120 s, each scented with a different odor. Two trials from the same condition were administered each day with an intertrial interval of approximately 1 hr. In the first test (days 1–2), one block was scented with female urine, and one block was scented with male urine. In the second test (days 3–6), one block was scented with urine (from either a female or male donor), and one block was scented with a nonsocial essential oil. New odors were used on each trial. Spontaneous exploration times were used to infer relative preference of odors.
2.3. Social and nonsocial odorants
Urine samples collected in a clean empty cage from adult healthy male and female rats that were not housed in the same room with subject rats were used as social stimuli. Household odorants such as lavender, basil, and rosemary essential oils were used as nonsocial controls. Plant-based odorants were used because these control stimuli would be unfamiliar to the subject rats, similar to the conspecific urine collected from unfamiliar donors, but would presumably offer no socially-relevant information. Immediately prior to testing, 4 μl of the odorant was pipetted onto each of five sides of a clean 3.175-cm cubic wood block (Bio-Serv), which was then taped to one corner of a clean cage (corner location was counterbalanced across sessions). To avoid cross-contamination of odors, pipette tips and wood blocks were not reused, and new gloves were used for each trial.
2.4. Estrous cycle
The estrous cycle of the female subjects was examined using a previously reported vaginal lavage approach (Perry et al., 2015), which was conducted under anesthesia (1–3% isoflurane) approximately an hour following a preference testing session. A sterile transfer pipette filled with 200 ml saline was inserted about 6 mm into the vaginal canal. The pipette was gently depressed and released several times to expel and withdraw saline from the vaginal canal. The liquid was then expelled into a microscope slide for examination under a microscope. The relative ratio of nucleated epithelial cells, cornified squamous epithelial cells, and leukocytes present in the samples were used to identify estrous stages of the rat, which was then binarized as being sexually receptive (proestrus and estrus) or unreceptive (diestrus days 1 and 2).
2.5. Behavioral procedures
Two tasks were administered in which rats’ spontaneous exploration of clean wood blocks scented with an odorant was measured. In both tasks, muscimol or saline was infused bilaterally into the BLA, and testing was conducted 30 min later in clean plastic home cages with fresh bedding. In both tasks, new odorants were used for each trial.
One test was a habituation-dishabituation task in which rats’ habituation of spontaneous exploration to repeated odors was assessed, which was followed by a presentation of a new odor to assess dishabituation and to infer discrimination between the repeated and new odorants (Fig. 1B). On each trial, the same odorant was pipetted from a single storage container onto five clean wood blocks, which were then presented one at a time to the subject rat for 60 s each with a 15-s interval between presentations. A new odorant from the same category (e.g., male urine) was pipetted onto a clean wood block (at the start of the trial) and presented 15 s after the fifth presentation of the prior odor for 60 s. Two trials were administered on each day of testing in a counterbalanced order, one using nonsocial plant-based odorants and the other using either male or female urine. The order of those testing days (muscimol male urine and nonsocial trials; muscimol female urine and nonsocial trials; saline male urine and nonsocial trials; saline female urine and nonsocial trials) was counterbalanced across rats. Across all testing days, each rat was administered two trials for the same treatment and the same social odor category (e.g., two trials using male urine in the muscimol condition).
The second test was a preference task in which rats were presented with two wood blocks simultaneously in adjacent corners, each with a different odorant, for 120 s on each trial (Fig. 1C). On each testing day, each rat was administered two trials for the same treatment and the same odor category. The preference test took place one week after the habituation-dishabituation test for the first cohort of rats and one week before the habituation-dishabituation task for the second cohort of the rats.
2.6. Data analyses
Exploration times were obtained from manual scoring of onset and offset of exploration using video recorded at 30 frames/s. Scorers were blinded to the trial type and infusion condition. For the preference task, preference scores were calculated for each trial according to the following formula in which X and Y represent exploration times of individual odors from different odor categories (e.g., female urine and male urine): (X−Y)/(X+Y) * 100. The data were analyzed in SPSS 26 using an approach similar to that described by Duricki et al. (2016). Briefly, restricted maximum likelihood estimation was used to fit a repeated-measures fixed-effects linear model to the raw trial-by-trial exploration times (habituation-dishabituation task) or preference scores (preference task), an approach that capitalized on the multiple trials’ worth of data collected for a rat in each condition and that tolerated occasional missing values. Potential differences between the two cohorts of rats (see Section 2.2) were modeled by including cohort as a nesting variable (McDonald, 2014) for the effect of infusion condition (as well as for interaction terms involving the effect of infusion condition) in all analyses. This nesting approach had the advantage that it assumed neither similar distributions of dependent variables nor similar differences between infusion conditions in the two cohorts. Means and standard errors of mean reported in the text and shown in figures were based on estimated values from these models.
3. Results
3.1. Histology and data inclusion
Fluorescent imaging of the muscimol-conjugated fluorophore for 18 rats indicated that infusion cannula were implanted bilaterally in the BLA (see Supplementary Figure S1). Due to illness or technical issues (e.g., clogged infusion needle), some data were missing. All 18 rats were included in the analysis of the preference task data, yet 6 did not have complete data for the condition in which male urine was presented alongside nonsocial odorants. Three rats became sick, and thus data from 15 rats were collected for the habituation-dishabituation task. Of those 15 rats, 3 did not have complete data for the condition involving male urine. Insufficient data regarding estrous cycle were collected for the habituation-dishabituation task. For the preference task, estrous information was collected for all sessions, with the exception that it was not collected in cohort 1 for sessions involving female urine versus nonsocial odorants.
3.2. Results from the habituation-dishabituation task
Figure 2 shows results from the habituation-dishabituation task plotted as mean exploration times across odor presentations. A repeated-measures linear model revealed a significant effect of odor type (F(2,277.75)=123.51, p<0.001), reflecting rats’ tendencies to explore male urine more than female urine or nonsocial odorants, and a significant effect of odor presentation number (1st to 6th; F(5,113.30)=39.59, p<0.001), reflecting the gradual decrease and rebound of exploration in line with detection of new odors in the habituation-dishabituation paradigm. There was not a significant effect of infusion condition (F(2,284.52)=0.56, p=0.571), and trends for male urine, female urine, and nonsocial odorants were broadly similar between muscimol and saline conditions. However, there was a significant three-way interaction between odor type, odor presentation number, and infusion condition (F(61,76.65)=2.58, p<0.001). These results were similar when the data were analyzed separately for the two cohorts (see Section 2.2). In particular, the trends were similar in both cohorts, and there was a significant three-way interaction for both cohorts (cohort 1: F(27,41,17)=5.78, p<0.001; cohort 2: F(27,29.77)=2.87, p<0.01). Thus, for all rats, BLA inactivation appeared to impact habituation-dishabituation performance in a manner that depended on odor type.
Figure 2. Results from the habituation-dishabituation task (n=15).
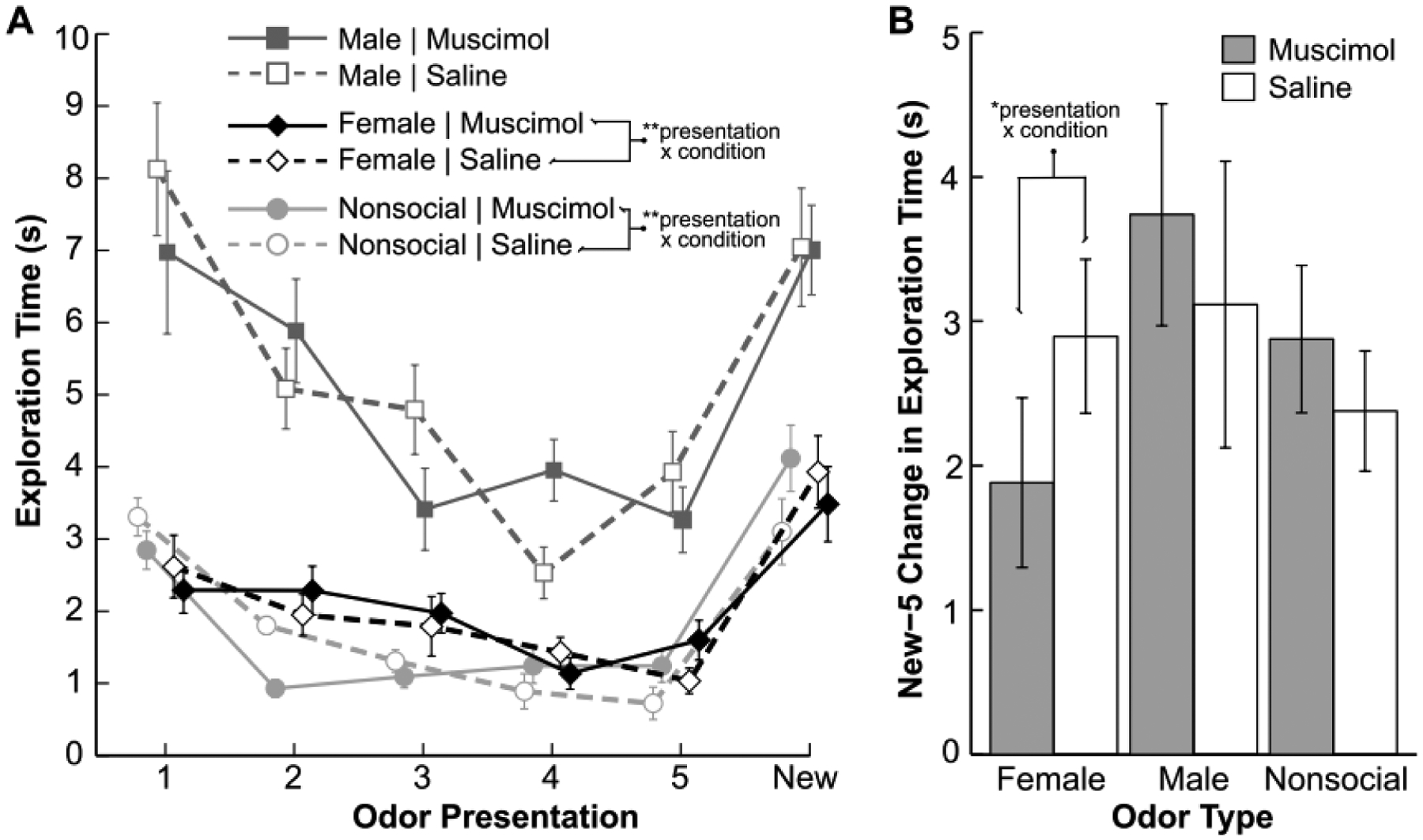
A. The results are shown as mean exploration times across five presentations of a repeated odor and one presentation of a new odor. Squares, diamonds, and circles indicate exploration times of male urine odors, female urine odors, and plant-based nonsocial odors, respectively. Filled symbols connected by solid lines indicate data from the muscimol BLA infusion condition. Open symbols connected by dashed lines indicate data from the control saline BLA infusion condition. Female rats explored male urine more than female urine or nonsocial odors, and across all odor categories, exploration rebounded when a new odor was presented following five repetitions of the initial odor. These trends were numerically similar for both the saline and muscimol BLA infusion conditions. However, there was a significant (p<0.001) three-way interaction between odor type, odor presentation number, and infusion condition. Additionally, for female urine odors and for nonsocial odorants, there was a significant (p<0.001) two-way interaction between odor presentation number and infusion condition, as noted in the legend. B. The results for the final (5th) habituation presentation and dishabituation (new) presentation are replotted as mean new-5 differences. There was a significant (p<0.05) two-way interaction between odor presentation number and infusion condition for the female urine odors. In both panels, the error bars represent standard errors of means. Three of the 18 rats became sick and thus did not complete testing on the habituation-dishabituation task. See sections 2.6 and 3.2 for details of analyses and results, respectively.
To unpack the significant three-way interaction, we repeated the above analysis of all rats separately for the exploration times of male urine, female urine, and nonsocial odorants. All three analyses identified a significant effect of odor presentation number (male: F(5,62.86)=14.37, p<.001; female: F(5,78.87)=11.16, p<.001; nonsocial: F(5,88.38)=28.22, p<.001) but not of infusion condition (male: F(2,155.43)=2.31, p=.103; female: F(2,188.01)=1.03, p=.359; nonsocial: F(2,214.45)=0.14, p=.871). Moreover, there was a significant interaction between infusion condition and odor presentation number for female urine (F(15,61.39)=2.83, p=.002) and nonsocial odorants (F(15,74.82)=2.55, p=.004) but not for male urine (F(15,47.81)=1.18, p=.320). Thus, BLA inactivation significantly impacted the pattern of habituation-dishabituation performance across odor presentations (though not overall mean exploration times) for female urine and nonsocial odorants but not male urine. A similar and final analysis of the habituation-dishabituation data focused on data from the final habituation odor presentation (5th) and the dishabituation odor presentation (6th) to ask more specifically if BLA inactivation influenced the extent to which exploration times rebounded when a new odor from the same odor type was presented. The analysis revealed a significant interaction between infusion condition and 5th-to-6th odor presentation number for female urine (F(3,43.94)=3.78, p=.017) but not for nonsocial odorants (F(3,57.92)=2.14, p=.104) or male urine (F(3,41.62)=0.40, p=.754). A caveat for this final significant interaction for female urine is that the combined above analyses included seven separate linear models (all data; data for female urine, male urine, and nonsocial odorants; and data from 5th and 6th presentations for female urine, male urine, and nonsocial odorants), and thus significance (p) values greater than 0.007 (0.05/7) would not survive strict Bonferroni correction for multiple comparisons. Nevertheless, the overall results suggested that the impact of BLA inactivation on dishabituation was most pronounced for the female urine condition
3.3. Results from the preference task
Figure 3 shows the results from the preference task plotted as preference scores that, for odors X and Y, could range from no preference (0%) to total preference for X (+100%) or total preference for Y (−100%; sign is arbitrary; see Section 2.6). The first question was whether inactivation of the BLA would impact rats’ spontaneous exploration when presented simultaneously with a block scented with female urine and a block scented with male urine (Fig. 3A). Information about estrous cycle (binarized as sexually receptive or unreceptive; see Section 2.4) was included in the model based on the possible influence of sexual receptivity on preference scores. A repeated-measures linear model indicated that the effect of infusion condition (F(2,10.96)=2.02, p=.180) and the interaction between infusion condition and estrous cycle (F(3,2.99)=0.98, p=.507) did not reach statistical significance (see Supplementary Table 1 for preference scores separated by sexual receptivity). Nevertheless, the trend was for BLA inactivation to reduce the female-vs-male preference score (mean saline-muscimol difference: −24.63%; 95% confidence interval: −54.12 to 4.87%).
Figure 3. Results from the spontaneous preference task (n=18).
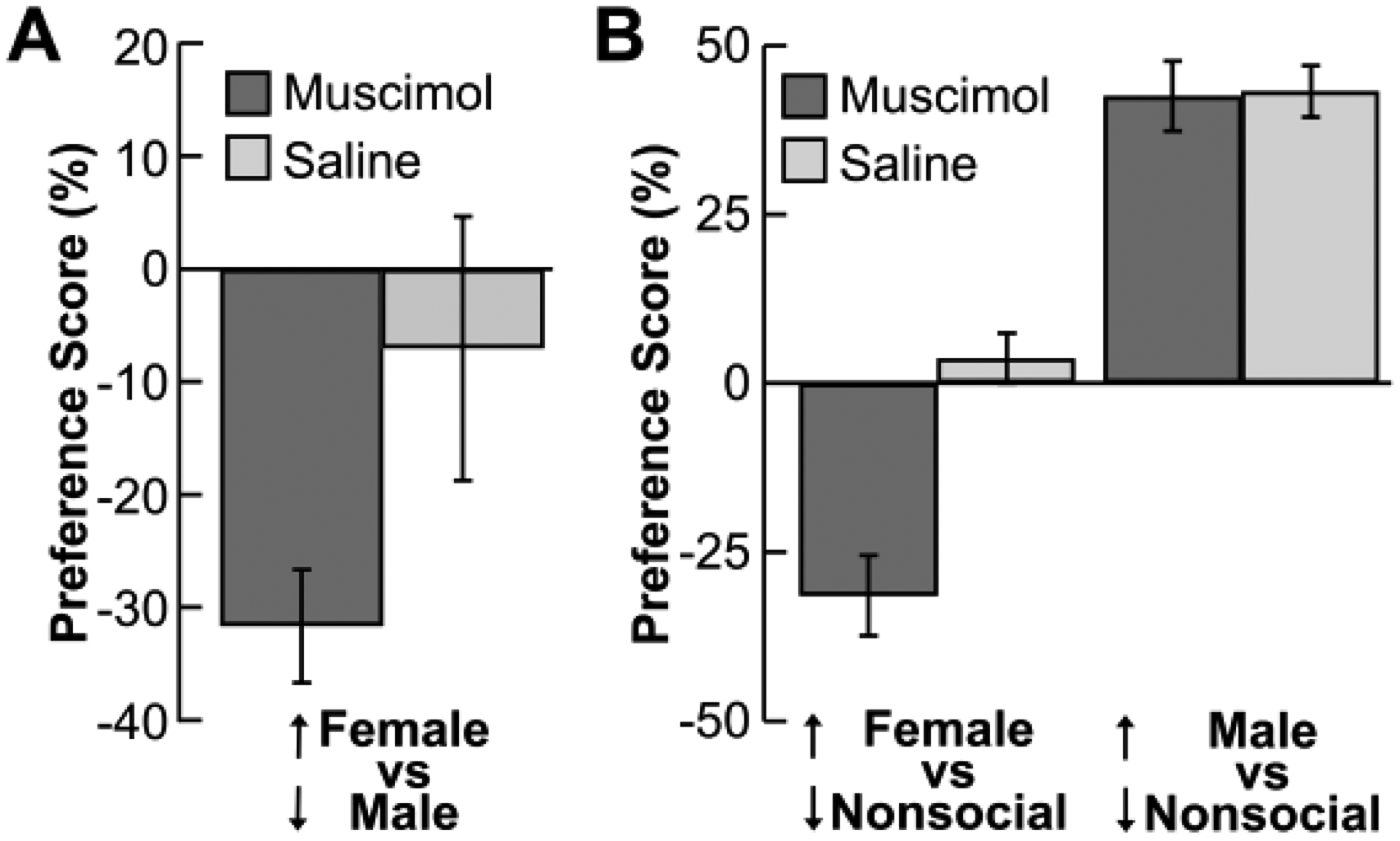
Results are shown as mean preference scores indicating relative exploration of two odor categories [(X−Y)/(X+Y) * 100], for which the sign indicating the direction of change is arbitrary. Arrows adjacent to odor categories indicate the direction of change. (A) Preference scores for a test in which a block scented with female urine and a block scented with male urine were presented simultaneously. (B) Preference scores for a test which a block scented with urine (from either a female or a male donor) was presented simultaneously with a block scented with nonsocial essential oils. Inactivation of the BLA via infusion of muscimol reduced preference scores for female urine odors relative to nonsocial odors (see Section 3.2). The error bars represent standard errors of means. See sections 2.6 and 3.3 for details of analyses and results, respectively.
One possibility was that BLA inactivation impacted rats’ tendency to explore both female and male urine, complicating a preference score that involved both. Thus, the second question was whether inactivation of the BLA would impact rats’ spontaneous exploration when presented simultaneously with blocks scented with conspecific urine (from either a male or female donor) and nonsocial odorants (Fig. 3B). A repeated-measures linear model revealed a significant effect of infusion condition (F(2,30.55)=13.40, p<.001) and odor condition (male-nonsocial vs. female-nonsocial; F(1,22.34)=163.91, p<.001) yet also a significant interaction between infusion condition and odor condition (F(2,29.45)=4.65, p=.018). Thus, inactivation of the BLA impacted preference scores particularly as a decrease in preference for female urine relative to nonsocial odorants. Indeed, the mean saline-muscimol difference in preference scores relative to nonsocial odorants was 35.19% for female urine (95% confidence interval = −49.48 to −20.89%) and 0.74% for male urine (95% confidence interval = −12.26 to 12.79%), indicating that the primary impact of BLA inactivation was a shift from roughly equal preference for female urine versus nonsocial odorants in the saline condition to a marked preference for nonsocial odorants over female urine in the muscimol condition. These trends were broadly similar for both cohorts in that the preference scores were lower in the muscimol condition versus the saline condition for female urine yet were similar between infusion conditions for male urine. Moreover, these trends were similar when the analyses were restricted to the 15 rats who were also included in the habituation-dishabituation task results (e.g., the infusion condition by odor condition interaction revealed similar results: F(2,21.24)=5.33, p=.01).
For all rats, there was not a significant overall effect of estrous cycle (F(1,25.25)=0.03, p=.865), but there was a significant three-way interaction with infusion condition and odor condition (F(5,10.63)=23.67, p<.001). Supplementary Table 1 shows preference scores separated by sexual receptivity, and the data suggest that preference scores for male urine versus nonsocial odorants varied in particular between days when female rats were sexually receptive or not. These results regarding sexual receptivity should be interpreted with caution because information about estrous cycle was not available for all testing days (see Section 3.1). Nevertheless, the results suggested that including estrous information helped account for some of the variability in the preference scores. Indeed, a direct comparison of models with estrous information (34 parameters; −2RLL = 1048.88; AIC = 1088.88) and without estrous information (16 parameters; 2RLL = 1148.60; AIC = 1164.60) indicated that the model including estrous information fit the data significantly (p<.001) better according to a likelihood ratio test that accounted for differing numbers of parameters in the models.
4. Discussion
The results demonstrated an important yet specific role for the BLA in mediating exploration of social stimuli relative to nonsocial stimuli. Specifically, BLA inactivation did not impair female rats’ ability to discriminate urine from two male donors in a habituation-dishabituation test but instead impacted how the rats responded when a repeated female urine odor was swapped out for a new one (Fig. 2). Moreover, BLA inactivation in female rats reduced preference scores for female urine relative to nonsocial odorants but not for male urine relative to nonsocial odorants (Fig. 3B). Taken together, the results indicated that the BLA in female rats is not essential for olfactory discrimination of or preference for male urine odors. Other brain structures key to olfaction-mediated reproductive behaviors such as the extended medial amygdala and medial preoptic area of the hypothalamus (McHenry et al., 2017; Newman, 1999) were likely sufficient to support normal exploration of male urine odors in these instances. In contrast, the BLA in female rats was essential for normal spontaneous exploration of female urine odors in both the habituation-dishabituation task and the preference task, indicating an important role in prioritizing social cues outside of a reproductive context. Indeed, one possible interpretation of the results is that the BLA is essential for affiliative behaviors, such as those between two females or between juveniles of either sex (Ferri et al., 2016), whereas other brain regions such as the extended medial amygdala might contribute to social interactions that also have a component of sexual or aggressive motivation.
The finding that the BLA in female rats was not essential for discrimination of or preference for male urine odors does not preclude engagement of the BLA by male urine odors in the intact female brain. For example, the BLA is reciprocally connected with the medial amygdala, which in turn projects to the medial preoptic area (Pitkänen et al., 2000)—areas that are both important for responding to conspecific odors in a reproductive context (Hosokawa & Chiba, 2007; Newman, 1999; Sakuma, 2008). Thus, the current results would be consistent with roles for the BLA in responding to social odors both inside (e.g., mate choice) and outside (e.g., same-sex affiliation) a reproductive context but that only its role outside a reproductive context would be irreplaceable following BLA dysfunction. Accordingly, the impaired habituation-dishabituation performance for female urine odors might reflect an essential role for the BLA in discrimination of female individuals specifically. Alternatively, a more parsimonious account is that it might reflect the same role of the BLA modulating spontaneous exploration of novel same-sex urine odors as shown in the preference task results—an impact on the rat’s disposition towards same-sex odors rather than discrimination of individual female conspecifics. In any case, the normal habituation-dishabituation performance for male urine odors demonstrated that the BLA was not a required brain region to discriminate conspecific individuals per se.
Information about the rats from which urine was collected was not available, other than their sex and that routine veterinary observations deemed them to be healthy and pathogen-free. Future studies would benefit from being able to document the estrous period of female donor rats and testosterone levels of male donor rats, as both could potentially explain some variability in preferences of female experimental rats (e.g., female rats prefer urine odors from healthy males over castrated males; Sakuma, 2008). Moreover, determining the sexual hormone levels of donor rats would be particularly important if the current study were repeated with adult male subject rats in addition to adult females. In particular, one prediction from the current results would be that inactivation of the BLA would influence both male and female subject rat preferences for testosterone-free male donor urine based on the idea that neither male nor female subjects would evaluate the testosterone-free urine as coming from a potential competitor or mate, that the evaluation would be outside a reproductive or aggressive context.
The view that the BLA in female rats mediated preference for social information relative to nonsocial information is consistent with the broader idea that the BLA helps to prioritize stimuli based on affective salience to regulate fear responses and appetitively-motivated behavior (Davis & Reijmers, 2018; Janak & Tye, 2015; Wassum & Izquierdo, 2015). The current results support including social odors on the list of stimuli that engage the BLA, at least for same-sex odors that presumably guide affiliation or competition rather than reproduction. The BLA projects to many regions, including the prefrontal cortex, hippocampus, and striatum (Pitkanen et al., 2000), and thus is in a position to modulate attention, memory, and motivation (Janak & Tye, 2015; Manns & Bass, 2016; McGaugh, 2004). The current results thus suggest that same-sex conspecific odors will activate these modulatory projections from the BLA to prioritize attention and memory for this type of social stimuli.
Supplementary Material
Highlights:
Basolateral amygdala (BLA) mediates responses to stimuli with affective salience
Important question is whether BLA plays key role for stimuli with social relevance
Conspecific urine provides many socially-relevant odor cues in rodents
BLA inactivation in females altered preferences for female but not male urine odors
BLA important for normal responses to stimuli with social relevance
Acknowledgements
This project is funded by NIMH R01 MH100318 to JRM and by a pilot project grant to JRM and ZS from NIH grant P50 MH100023 to Dr. Larry J. Young. We thank Love Chung, Dulin Wang, and Jeanne Powell for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Adolphs R (2010). What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences, 1191, 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, & Damasio AR (2005). A mechanism for impaired fear recognition after amygdala damage. Nature, 433, 68–72. [DOI] [PubMed] [Google Scholar]
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, & Brown TH (2008). Imaging the spread of reversible brain inactivations using fluorescent muscimol. Journal of Neuroscience Methods, 171, 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA & Kendrick KM (2006). Mammalian social odours: attraction and individual recognition. Philosophical Transactions of the Royal Society of London: B, 361, 2061–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE (1988). Individual odors of rats are discriminable independently of changes in gonadal hormone levels. Physiology and Behavior, 43, 359–363. [DOI] [PubMed] [Google Scholar]
- Davis P & Reijmers LG (2018). The dynamic nature of fear engrams in the basolateral amygdala. Brain Research Buletin, 141, 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duricki DA, Soleman S, & Moon LD (2016). Analysis of longitudinal data from animals with missing values using SPSS. Nature Protocols, 11, 1112–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR & Fernald RD (2004). How the brain processes social information: searching for the social brain. Annual Review of Neuroscience, 27, 697–722. [DOI] [PubMed] [Google Scholar]
- Janak PH & Tye KM (2015). From circuits to behaviour in the amygdala. Nature, 517, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaswinkel H, Baars A, Gispen W, & Spruijt BM (1996). Roles of the basolateral amygdala and hippocampus in social recognition in rats. Physiology and Behavior, 60, 55–63. [DOI] [PubMed] [Google Scholar]
- Mahan AL & Ressler KJ (2012). Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends in Neuroscience, 35, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR & Bass DI (2016). The Amygdala and the prioritization of declarative memories. Current Directions in Psychological Science, 25, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JH (2014). Handbook of Biological Statistics (3rd ed.). Sparky House Publishing, Baltimore, Maryland. pp. 165–172. [Google Scholar]
- McGaugh JL (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience, 27, 1–28. [DOI] [PubMed] [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, McElligott ZA, Budygin EA, Rubinow DR, & Stuber GD (2017). Hormonal gain control of a medial preoptic area social reward circuit. Nature Neuroscience, 20, 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW (1999). The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Annals of the New York Academy of Sciences, 877, 242–257. [DOI] [PubMed] [Google Scholar]
- Hosokawa N & Chiba A (2007). Effects of sexual experience on conspecific odor preference and male odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in female rats. Brain Research, 1175, 66–75. [DOI] [PubMed] [Google Scholar]
- Pare D (2003). Role of the basolateral amygdala in memory consolidation. Progress in Neurobiology, 70, 409–420. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Jagannathan L, & Becker JB (2015). The roles of dopamine and alpha1-adrenergic receptors in cocaine preferences in female and male rats. Neuropsychopharmacology, 40, 2696–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, & Swanson LW (2001). Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Reviews, 38, 247–289. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Jolkkonen E, & Kemppainen S (2000). Anatomic heterogeneity of the rat amygdaloid complex. Folia Morphology, 59, 1–23. [PubMed] [Google Scholar]
- Sakuma Y (2008). Neural substrates for sexual preference and motivation in the female and male rat. Annals of the New York Academy of Sciences, 1129, 55–60. [DOI] [PubMed] [Google Scholar]
- Schweinfurth MK (2020). The social life of Norway rats (Rattus norvegicus). eLife, 9, e54020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H & Young LJ (2018). The neural mechanisms and circuitry of the pair bond. Nature Reviews Neuroscience, 19, 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao S, Liu X, & Fu Q (2014). Effects of the medial or basolateral amygdala upon social anxiety and social recognition in mice. Turkish Journal of Medical Sciences, 44, 353–359. [DOI] [PubMed] [Google Scholar]
- Wassum KM & Izquierdo A (2015). The basolateral amygdala in reward learning and addiction. Neuroscience and Biobehavioral Reviews, 57, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn CG, Clairis N, Cavalcante LES, Furini CRG, de Carvalho Myskiw J, & Izquierdo I (2016). Major neurotransmitter systems in dorsal hippocampus and basolateral amygdala control social recognition memory. Proceedings of the National Academy of Sciences USA, 113, E4914–E4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


