Abstract
Background:
Although cannabis may worsen nausea and vomiting for patients with gastroparesis, it may also be an effective treatment for gastroparesis-related abdominal pain. Given conflicting data and a lack of current epidemiological evidence, we aimed to investigate the association of cannabis use on relevant clinical outcomes among hospitalized patients with gastroparesis.
Methods:
Patients with a diagnosis of gastroparesis were reviewed from the National Inpatient Sample (NIS) database between 2008 and 2014. Gastroparesis was identified by International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes with patients classified based on a diagnosis of cannabis use disorder. Demographics, comorbidities, socioeconomic status, and outcomes were compared between cohorts using chi-squared and ANOVA. Logistic regression was then performed and annual trends also evaluated.
Results:
A total of 1,473,363 patients with gastroparesis were analyzed (n=33,085 [2.25%] of patients with concomitant cannabis use disorder). Patients with gastroparesis and cannabis use disorder were more likely to be younger and male gender compared to non-users [(36.7±18.8) versus 51.9±16.8;P<0.001) and (52.9% versus 33.5%;P<0.001), respectively]. Race/ethnicity was different between groups (P<0.001). Cannabis users had a lower median household income and were more likely to have Medicaid payor status (all P<0.001). Controlling for confounders, length of stay and mortality were significantly decreased for patients with gastroparesis and cannabis use (all P<0.001).
Conclusion:
While patients with gastroparesis and cannabis use disorder were younger, with a lower socioeconomic status, and disproportionately affected by psychiatric diagnoses, these patients had better hospitalization outcomes, including decreased length of stay and improved in-hospital mortality.
Keywords: Gastroparesis, Cannabis Use, Healthcare Disparities, Health Outcomes
Introduction
Gastroparesis is a neuromuscular disorder that results in delayed gastric emptying in the absence of mechanical obstruction and often presents with difficult-to-treat symptoms of nausea, vomiting, early satiety, post-prandial fullness, and chronic abdominal pain.1-4 However, correlation between symptom severity and gastric emptying remains inconsistent, and a combination of sensorimotor disturbances have been speculated to play a role in symptom pathogenesis. Given the heterogeneous underlying pathophysiology, gastroparesis can be a disabling condition for greater than 10% of patients with the diagnosis and present significant therapeutic challenges.5 As such, the economic impact of gastroparesis has escalated dramatically over the last decade, including a four-fold increase in hospitalization rate as the prevalence of diagnosis doubled within the same period.6,7
Concurrently, there has been a marked rise in the number of patients hospitalized with a history of cannabis or marijuana use– approximately 9% of all hospitalized patients are estimated to have regular, chronic use and/or cannabis use disorder.8,9 As of February 2020, 11 states plus Washington D.C. have legalized recreational use of cannabis with an additional 22 states authorizing medical use only. It is thus not surprising that the prevalence of cannabis use disorder among inpatient hospitalizations has seen a sharp increase (almost three-fold from 0.52% to 1.34%) from 2002 to 2011.10 This has resulted in a rise in hospitalizations for cannabis-related acute cardiac, psychiatric, and neurologic events as well.10,11
Large-scale national studies have demonstrated a reduction in morbidity for patients with cannabis use for a variety of gastrointestinal conditions such as acute pancreatitis, Crohn’s disease, and Hepatitis C associated liver disease.12-15 Literature among patients with irritable bowel syndrome has also demonstrated cannabis use may decrease healthcare utilization, including lower rates of upper and lower endoscopy, shorter length of hospital stay, and decreased hospital charges.16 However, there remains a paucity of data on the effect of cannabis use on patients with gastroparesis. Although cannabinoids, in physiologic conditions, inhibit gastrointestinal motility through CB1 receptors, decrease transient lower esophageal sphincter relaxation time, reduce gastric acid secretion, and delay gastric emptying, there is some literature to suggest use may dramatically improve cardinal symptoms of gastroparesis.17-21 In particular, increased cerebral CB1 receptors has been found to be a stable feature of functional dyspepsia, a condition with considerable symptom and pathophysiologic overlap with gastroparesis.22 Cannabis use may, therefore, also affect the phenotypic characteristics, symptom severity, and clinical outcomes of gastroparesis.
The primary aim of this study was to investigate comorbid conditions, demographic, socioeconomic, and health-related outcomes of patients hospitalized for gastroparesis with and without a history of cannabis use.
Methods and Materials
Data Source
Data was queried from the United States National Inpatient Sample (NIS) for admissions involving the diagnosis gastroparesis between 2008 and 2014. The NIS database, sponsored by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project (HCUP), is the largest publicly available all-payer inpatient discharge database in the United States, containing weighted data for more than 35 million annual hospitalizations nationally.23,24
Study Population
International Classification of Diseases, Ninth Revision codes (ICD9-CM) were used to identify patients with a diagnosis of gastroparesis with ICD9-CM 536.3.7,25,26 Patients were then classified according to whether they had a history of cannabis use using ICD9-CM codes (dependent use 304.3x, nondependent use 305.2x).10,12 Patients under the age of 18 years were excluded.
Statistical Methodology
The NIS is a representative 20% sample of all-payer discharges from non-federal hospitals in the U.S. and is sponsored by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project (HCUP). The NIS sampling strategy was redesigned in 2012 to use different trend weights. Correct trend weights were used for each individual year, accounting for this change.27 Patients were separated into two cohorts based on their history of cannabis use. Measured variables including age, race/ethnicity, comorbidities (including the Charlson Comorbidity Index 19), hospital type, hospital region, insurance payer type, median household income quartile, length of hospital stay, total cost (defined as total hospital charges), discharge destination, and mortality were compared between cohorts using chi-squared and t-tests.
Subgroup were performed specifically for patients with principal discharge diagnosis of gastroparesis as well as type of cannabis use (defined as dependent use 304.3x versus nondependent use 305.2x). This was done in effort to compare hospitalization outcomes between cannabis dependent users versus non-dependent users as well as those with a principal discharge diagnosis of gastroparesis. Additional sensitivity analyses were performed to assess the association of cannabis use on relevant inpatient hospitalization outcomes among patients with gastroparesis and diabetes versus non-diabetic patients with gastroparesis. Further analyses were similarly performed to determine if outcomes differed by age (comparing inpatient clinical outcomes different patients age > 65 years and older versus those younger than 65 years).
An unadjusted logistic regression followed by an adjusted logistic regression controlling for age, sex, race/ethnicity, CCI, generalized anxiety disorder, depression, opioid use disorder, alcohol use disorder, hospital type, hospital region, insurance payer, and income quartile were then used to quantify the effect of cannabis use on mortality, discharge home, and extended length of stay (defined as length of stay ≥1 standard deviation [SD] above mean length of stay). Results were displayed as odds ratios with 95% confidence intervals (CI). A time-trend linear regression was performed to depict trends in incidence of cannabis use, gastroparesis, and concomitant cannabis use and gastroparesis over the study period. Statistical analyses were performed using SPSS v25 (IBM, Armonk, NY).
Results
Patient Characteristics
In total, 1,473,363 patients with gastroparesis were identified, including 33,085 (2.25%) with a history of cannabis use and 1,440,278 (97.75%) without. Of these patients, 112,091 carried a principal discharge diagnosis of gastroparesis. Patients with co-diagnoses of gastroparesis and cannabis use were significantly younger (36.7±18.8 years versus 51.9±16.8 years; P<0.001), more likely to be male (52.9% versus 33.5%; P<0.001), and more likely to be Black (38.9% versus 25.9%; P<0.001). Additionally, patients with both conditions also had a higher proportion of alcohol use disorder (9.3% versus 2.4%; P<0.001), depression (21% versus 20.1%; P<0.001), and a history of psychoses (12.3% versus 7.6%; P<0.001). Prevalence of diabetes mellitus (9.1% versus 12.4%; P<0.001) and severe disease or complications related to diabetes (28.7% versus 39.3%; P<0.001) were lower among patients with cannabis use disorder compared to individuals without. Overall, gastroparesis patients with cannabis use had less comorbidities and lower CCI scores as compared to those without cannabis use (1.8±1.7 versus 2.9±2.2; P<0.001) –Table 1.
Table 1:
Baseline Patient and Hospital Characteristics of Patients With Gastroparesis With and Without Cannabis Use Disorder
| Gastroparesis Diagnosis | Gastroparesis Primary Diagnosis | |||||
|---|---|---|---|---|---|---|
| Variables | No Cannabis Use (n=1,440,278) |
Cannabis Use (n=33,085) |
P Value |
No Cannabis Use (n=108,376) |
Cannabis Use (n=3,715) |
P Value |
| Age in Years (SD) | 51.9 (16.8) | 36.7 (18.8) | <0.001 | 47.0 (17.1) | 35.4 (10.3) | <0.001 |
| Female (%) | 66.5 | 47.1 | <0.001 | 74.2 | 56.9 | <0.001 |
| Race (%) | <0.001 | <0.001 | ||||
| White | 59.3 | 46.7 | 66.5 | 55.9 | ||
| Black | 25.9 | 38.9 | 21.6 | 31.6 | ||
| Hispanic | 10.6 | 10.7 | 8.4 | 8.8 | ||
| Asian or Pacific Islander | 1.3 | 0.7 | 1.0 | 1.4 | ||
| Native American | 0.8 | 1.0 | 0.5 | 0.4 | ||
| Unknown | 2.1 | 2.0 | 2.0 | 1.9 | ||
| Comorbidities (%) | ||||||
| AIDS | 0.2 | 0.3 | 0.059 | 0.5 | 0.3 | 0.073 |
| Alcohol Use Disorder | 2.4 | 9.3 | <0.001 | 1.9 | 6.6 | <0.001 |
| Congestive Heart Failure | 10.6 | 2.7 | <0.001 | 6.3 | 1.9 | <0.001 |
| Connective Tissue Disease | 3.7 | 1.2 | <0.001 | 5.2 | 1.0 | <0.001 |
| Chronic Lung Disease | 18.7 | 13.2 | <0.001 | 18.8 | 13.2 | <0.001 |
| Coagulopathy | 3.3 | 1.5 | <0.001 | 2.5 | 1.0 | <0.001 |
| Depression | 20.1 | 21.0 | <0.001 | 22.0 | 19.7 | 0.001 |
| Diabetes Mellitus | 12.4 | 9.1 | <0.001 | 21.7 | 19.0 | <0.001 |
| Complicated Diabetes Mellitus | 39.3 | 28.7 | <0.001 | 8.2 | 7.0 | 0.009 |
| Hypertension | 61.1 | 51.7 | <0.001 | 50.5 | 41.0 | <0.001 |
| Mild Liver Disease | 4.2 | 4.2 | 0.689 | 4.8 | 5.2 | 0.254 |
| Severe Liver Disease | 1.0 | 0.4 | <0.001 | 0.6 | 0.6 | 0.532 |
| Obesity | 14.1 | 6.9 | <0.001 | 10.8 | 7.5 | <0.001 |
| Peptic Ulcer | 0.1 | 0.1 | 0.001 | 0.2 | 0.0 | 0.009 |
| Paralysis | 2.7 | 0.4 | <0.001 | 2.1 | 0.1 | <0.001 |
| Peripheral Vascular Disease | 8.1 | 3.0 | <0.001 | 4.0 | 3.1 | 0.003 |
| Psychoses | 7.6 | 12.3 | <0.001 | 8.2 | 12.1 | <0.001 |
| Renal Failure | 27.4 | 14.1 | <0.001 | 14.5 | 7.6 | <0.001 |
| Myocardial Infarction | 5.1 | 2.4 | <0.001 | 3.0 | 1.6 | <0.001 |
| Cerebrovascular Event | 2.2 | 0.6 | <0.001 | 0.6 | 0.1 | <0.001 |
| Dementia | 0.1 | 0.0 | <0.001 | 0.1 | 0.0 | 0.132 |
| Solid Tumor | 1.1 | 0.3 | <0.001 | 1.7 | 0.4 | <0.001 |
| Metastatic Cancer | 1.2 | 0.2 | <0.001 | 2.1 | 0.1 | <0.001 |
| Leukemia | 0.3 | 0.1 | <0.001 | 0.3 | 0.0 | <0.001 |
| Lymphoma | 0.5 | 0.1 | <0.001 | 0.6 | 0.1 | <0.001 |
| Generalized Anxiety Disorder | 0.7 | 1.1 | <0.001 | 0.9 | 1.1 | 0.219 |
| Opioid Use Disorder | 2.5 | 8.6 | <0.001 | 3.3 | 9.5 | <0.001 |
| Charlson Comorbidity Index (SD) | 2.9 (2.2) | 1.3 (1.4) | <0.001 | 2.1 (2.1) | 0.8 (1.2) | <0.001 |
Hospitalization Characteristics
In terms of hospitalization characteristics, a significantly higher proportion of patients with gastroparesis and cannabis use were hospitalized at urban teaching hospitals and in hospitals located on the West Coast (all, P<0.001). This same cohort also had a significantly higher proportion of patients paying with Medicaid (37.5% versus 18.4%; P<0.001) and patients from the lowest income quartile (43.7% versus 34.4%; P<0.001) – Table 2. Compared to patients without a history of cannabis use, those with cannabis use were significantly more likely to be discharged home or leave against medical advice and less likely to be discharged to a skilled nursing facility or home with health aid (P<0.001). Additionally, patients with a co-diagnosis of gastroparesis and cannabis use disorder had a lower in-hospital mortality (0.1% versus 0.9%; P<0.001),a shorter length of hospital stay (3.7 ±3.3 versus 5.5 ±6.5 days; P<0.001), and a lower total cost of stay ($24,548 ±28915 versus $38,708 ±58351; P<0.001) compared to those without cannabis use disorder – Table 3. Sensitivity analyses performed comparing patients with gastroparesis and diabetes versus non-diabetic patients with gastroparesis as well as by age are highlighted in Table 4.
Table 2:
Socioeconomic Status and Hospital Characteristics of Patients With Gastroparesis With and Without Cannabis Use Disorder
| Gastroparesis Diagnosis | Gastroparesis Primary Diagnosis | |||||
|---|---|---|---|---|---|---|
| Variables | No Cannabis Use (n=1,440,278) |
Cannabis Use (n=33,085) |
P Value |
No Cannabis Use (n=108,376) |
Cannabis Use (n=3,715) |
P Value |
| Hospital Type (%) | <0.001 | 0.010 | ||||
| Rural | 10.2 | 8.8 | 9.0 | 9.5 | ||
| Urban Non-Teaching | 38.1 | 33.3 | 37.7 | 35.3 | ||
| Urban Teaching | 51.7 | 57.8 | 53.3 | 55.2 | ||
| Hospital Region (%) | <0.001 | <0.001 | ||||
| Northeast | 15.6 | 15.2 | 16.9 | 15.6 | ||
| Midwest | 21.3 | 23.4 | 20.0 | 17.6 | ||
| South | 45.4 | 38.0 | 47.7 | 47.2 | ||
| West | 17.8 | 23.4 | 15.4 | 19.7 | ||
| Payer Information (%) | <0.001 | <0.001 | ||||
| Medicare | 49.9 | 24.6 | 40.4 | 15.8 | ||
| Medicaid | 18.4 | 37.5 | 17.5 | 35.1 | ||
| Private Insurance | 23.6 | 17.9 | 32.9 | 24.1 | ||
| Other | 9.0 | 20.0 | ||||
| Median Household Income (%) | <0.001 | <0.001 | ||||
| Quartile 1 | 34.4 | 43.7 | 30.5 | 40.8 | ||
| Quartile 2 | 26.8 | 25.5 | 26.0 | 27.6 | ||
| Quartile 3 | 22.8 | 20.3 | 24.6 | 20.5 | ||
| Quartile 4 | 16.0 | 10.5 | 19.0 | 11.2 | ||
Table 3:
Discharge Destination and Patient Outcomes of Patients With Gastroparesis With and Without Cannabis Use Disorder
| Gastroparesis Diagnosis | Gastroparesis Primary Diagnosis | |||||
|---|---|---|---|---|---|---|
| Variables | No Cannabis Use (n=1,440,278) |
Cannabis Use (n=33,085) |
P Value |
No Cannabis Use (n=108,376) |
Cannabis Use (n=3,715) |
P Value |
| Discharge Destination, % | <0.001 | <0.001 | ||||
| Home | 69.1 | 85.0 | 78.3 | 88.5 | ||
| Transfer to Short Term Hospital | 1.7 | 0.9 | 1.2 | 0.7 | ||
| Skilled Nursing Facility, Intermediate care, other care facility | 12.2 | 1.9 | 6.5 | 0.5 | ||
| Home Health Care | 14.1 | 4.3 | 11.8 | 3.7 | ||
| Against Medical Advice | 2.1 | 7.8 | 1.8 | 6.6 | ||
| Died in Hospital | 0.9 | 0.1 | 0.4 | 0.0 | ||
| Length of Stay (days) | 5.5 (6.4) | 3.7 (3.3) | <0.001 | 5.1 (5.2) | 3.4 (2.7) | <0.001 |
| Total Charges of Stay ($) | 38708 (58351) | 24548 (28915) | <0.001 | 31957 (41030) | 21268 (18068) | <0.001 |
| Mortality | 0.9 | 0.1 | <0.001 | 0.4 | 0.0 | <0.001 |
Table 4:
Sensitivity Analyses Comparing Mortality, Discharge, and Length of Hospital Stay
| Gastroparesis Diagnosis | Gastroparesis Primary Diagnosis | ||||
|---|---|---|---|---|---|
| Unadjusted Odds Ratio |
P Value |
Unadjusted Odds Ratio |
P Value |
||
| Diabetes | Diabetes | ||||
| Mortality | 0.34 (CI: 0.24-0.49) | <0.001 | Mortality | - | - |
| Routine Discharge | 2.35 (CI: 2.23-2.47) | <0.001 | Routine Discharge | 1.55 (CI: 1.10-2.17) | 0.012 |
| Extended LOS | 0.31 (CI: 0.28-0.35) | <0.001 | Extended LOS | - | - |
| No Diabetes | No Diabetes | ||||
| Mortality | 0.10 (CI: 0.06-0.16) | <0.001 | Mortality | - | <0.001 |
| Routine Discharge | 2.49 (CI: 2.40-2.59) | <0.001 | Routine Discharge | 1.55 (CI: 1.10-2.17) | 0.012 |
| Extended LOS | 0.27 (CI: 0.24-0.39) | <0.001 | Extended LOS | 0.21 (CI: 0.17-0.27) | <0.001 |
| Age > 65 Years | Age > 65 Years | ||||
| Mortality | - | - | Mortality | - | - |
| Routine Discharge | 2.72 (CI: 2.06-3.60) | <0.001 | Routine Discharge | - | - |
| Extended LOS | 0.50 (CI: 0.30-0.85) | 0.01 | Extended LOS | - | - |
| Age < 65 Years | Age < 65 Years | ||||
| Mortality | 0.31 (CI: 0.23-0.41) | <0.001 | Mortality | - | - |
| Routine Discharge | 1.78 (CI: 1.73-1.84) | <0.001 | Routine Discharge | 1.53 (CI: 1.38-1.69) | <0.001 |
| Extended LOS | 0.31 (CI: 0.29-0.33) | <0.001 | Extended LOS | 0.22 (CI: 0.17-0.28) | <0.001 |
Logistic Regression
When controlling for age, sex, race/ethnicity, CCI, generalized anxiety disorder, depression, opioid use disorder, alcohol use disorder, hospital type, hospital region, insurance payer, and income quartile, key hospitalization outcomes were significantly better for the cannabis use group. Routine discharge to home was much more common among gastroparesis patients with cannabis use disorder [adjusted OR 1.24 (95% CI: 1.20 to 1.28; P<0.001] with decreased length of stay [0.36 (95% CI: 0.34 to 0.39); P<0.001] and reduced in-hospital mortality [0.36 (95% CI: 0.34 to 0.39); P<0.001]. Similar results were found when stratifying by patients with a principal diagnosis of gastroparesis as well as by type of use (dependent vs non-dependent). A complete summary of logistic regression outcomes is shown in Table 5.
Table 5:
Association of Cannabis Use on Gastroparesis Outcomes
| Any Gastroparesis Diagnosis | ||||
|---|---|---|---|---|
| Variables | Unadjusted Odds Ratio |
P Value |
Adjusted Odds Ratio* |
P Value |
| Mortality | 0.17 (CI: 0.13-0.23) | <0.001 | 0.45 (0.33-0.60) | <0.001 |
| Routine Discharge (Home) | 2.53 (CI: 2.45-2.60) | <0.001 | 1.26 (1.22-1.30) | <0.001 |
| Extended LOS | 0.28 (CI: 0.26-0.29) | <0.001 | 0.36 (0.33-0.39) | <0.001 |
| Primary Gastroparesis Diagnosis | ||||
| Variables | Unadjusted Odds Ratio |
P Value |
Adjusted Odds Ratio* |
P Value |
| Mortality | - | - | - | - |
| Routine Discharge (Home) | 2.14 (1.93-2.37) | <0.001 | 1.11 (0.997-1.246) | 0.057 |
| Extended LOS | 0.20 (0.16-0.26) | <0.001 | 0.28 (0.22-0.36) | <0.001 |
| Any Gastroparesis Diagnosis | ||||
| Variables | Unadjusted Odds Ratio |
P Value |
Adjusted Odds Ratio* |
P Value |
| Mortality | ||||
| Nondependent Cannabis | 0.18 (0.14-0.24) | <0.001 | 0.48 (0.34-0.64) | <0.001 |
| Dependent Cannabis | - | - | - | - |
| Routine Discharge (Home) | ||||
| Nondependent Cannabis | 2.52 (2.44-2.60) | <0.001 | 1.25 (1.20-1.29) | <0.001 |
| Dependent Cannabis | 2.43 (2.16-2.72) | <0.001 | 1.37 (1.20-1.55) | <0.001 |
| Extended LOS | ||||
| Nondependent Cannabis | 0.27 (0.25-0.29) | <0.001 | 0.35 (0.33-0.38) | <0.001 |
| Dependent Cannabis | 0.44 (0.36-0.54) | <0.001 | 0.54 (0.43-0.67) | <0.001 |
| Primary Gastroparesis Diagnosis | ||||
| Variables | Unadjusted Odds Ratio |
P Value |
Adjusted Odds Ratio* |
P Value |
| Mortality | ||||
| Nondependent Cannabis | - | - | - | - |
| Dependent Cannabis | - | - | - | - |
| Routine Discharge (Home) | ||||
| Nondependent Cannabis | 2.19 (1.97-2.44) | <0.001 | 1.14 (1.01-1.27) | 0.030 |
| Dependent Cannabis | 1.29 (0.84-2.00) | 0.244 | 0.75 (0.46-1.22) | 0.239 |
| Extended LOS | ||||
| Nondependent Cannabis | 0.20 (0.15-0.25) | <0.001 | 0.27 (0.21-0.35) | <0.001 |
| Dependent Cannabis | 0.40 (0.16-0.97) | 0.043 | 0.58 (0.24-1.42) | 0.232 |
controls for age, sex, race/ethnicity, CCI, generalized anxiety disorder, depression, opioid use disorder, alcohol use disorder, hospital type, hospital region, insurance payer, and income quartile
Trends
Overall, the incidence of patients with gastroparesis having a diagnosis of cannabis use have increased from 2008 to 2014 (P<0.001) – Figure 1. During this same period, the incidence of cannabis and gastroparesis use alone also increased (P<0.001).
Figure 1.
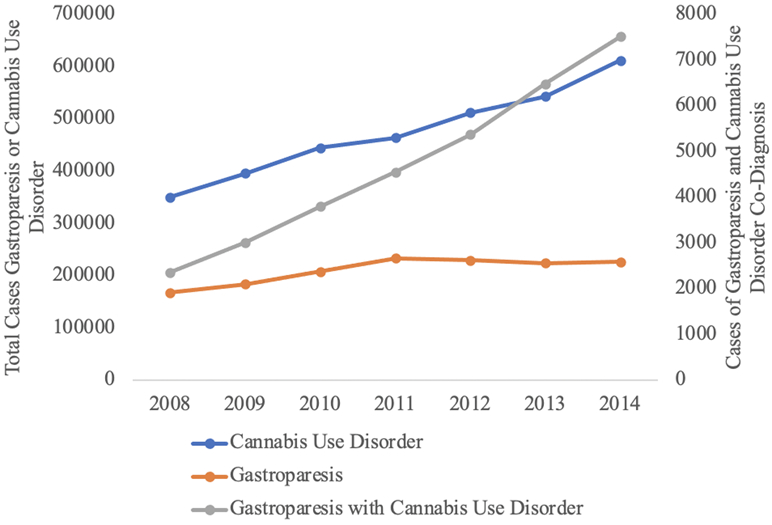
Incidence of Cannabis Use, Gastroparesis, and Co-Diagnosis
Discussion
Given the rising independent increase in incidence of both gastroparesis and cannabis use, it is not surprising that the number of patients with both diagnoses has continued to rise. In this study, we demonstrated significant demographic and clinical differences among patients with gastroparesis with and without a history of cannabis use. Specifically, patients with a history of cannabis use disorder were significantly younger and more likely to be Black, male, have Medicaid health insurance, and come from the lowest household income quartile. These patients were also less likely to have most medical comorbidities with a significantly lower CCI score. Perhaps as a result, patients with gastroparesis and cannabis use disorder had a lower in-hospital mortality, shorter length of hospital stay, lower cost of hospitalization, and higher likelihood of being discharged home.
Despite these findings, it is important to note that patients with a co-diagnosis of gastroparesis and cannabis use disorder had a higher proportion of alcohol use disorder, depression, and psychoses. While some of these findings are intuitive, such as the predilection for additional substance use disorders, our findings of a disproportionate association with depression and psychiatric illness are similar to previous studies. Literature has demonstrated that along with nicotine, cannabis is one of the most used drugs among patients with alcohol use disorder.28 Additionally, regular cannabis use is associated with an increased risk for schizophrenia and risk factors that predispose patients to major depressive disorder.29,30 These are all critical observations for clinicians and gastroenterologists to be aware of when treating patients in order to appropriately screen and refer patients to additional providers for best care practices. Alcohol use disorder and psychological distress have been associated with functional dyspepsia, a disorder of gut-brain interaction with considerable symptom and clinical overlap with gastroparesis.31,32 Therefore, patients with gastroparesis and cannabis use may also represent a subset whose symptoms are related primarily to altered brain-gut sensory input, rather than motor dysfunction.
Demographically, our results are analogous with the general scientific literature. In a French study of patients hospitalized with a direct history of cannabis use, researchers found the mean age of patients to be 28 years, of which the majority of patients were male.33 Meanwhile, in the largest cannabis use disorder study using the NIS, Charilaou et al. found that the mean age of patients hospitalized was 35.12 years with a mean CCI of 0.47.10 Additionally, lower socioeconomic status was associated with higher rates of cannabis use.34 Thus, these prior results regarding cannabis use, independent of a gastroparesis diagnosis, appear to mirror our findings among a population of patients with gastroparesis.
Current literature within the gastroenterology community has questioned the role of cannabis in the treatment of gastroparesis. The inhibitory effects of cannabis via the endocannabinoid system and the impact on gastric emptying and intestinal transit are mediated to some extent by CB1 receptors in the brain and intestinal tract.35 While this has been shown to reduce transient lower esophageal sphincter relaxation, decrease gastric acid secretion, and delay gastric emptying, cannabis has interestingly been demonstrated to reduce cardinal symptom index scores in a population with gastroparesis.17-21 There is some thought that cannabinoids in the form of dronabinol or cannabidiol may also have a positive effect on symptoms of nausea and vomiting for treatment of gastroparesis.36
These observations support the heterogeneity and complex sensorimotor dysfunctions of patients with gastroparesis. As noted in prior studies, the correlation between the degree of motor abnormality and symptom severity among patients with gastroparesis are poor. Moreover, the benefits of therapies aiming to improve gastric motility (metoclopramide and erythromycin) and emptying (pyloric myotomy) remain inconsistent. Implantable gastric electrical stimulators have been shown to improve symptoms among some patients with gastroparesis, although their mechanism of action appear to be related to more than prokinetic effects alone.37 Multiple pathways have been proposed and demonstrated to contribute to symptom of gastroparesis beyond delayed emptying alone, including gastric dysrhythmic changes, altered accommodation, autonomic dysfunction, hormonal effects, mucosal inflammation, and neurosensory modulation. Cannabis may impact one or more of these pathways through the endocannabinoid system, thereby explaining its potential effect in gastroparesis symptoms.
A previous single-center study by Barbash and colleagues demonstrated improvement in nausea and vomiting as well as abdominal pain associated with modulation of endocannabinoid system among patients with gastroparesis. In this study, the authors assessed the effect of dronabinol, medical cannabis, or both on symptoms of gastroparesis and demonstrated that patients with gastroparesis and cannabis use had lower mortality and better discharge outcomes.21 However, it remains unknown whether outcomes were better because of the effect of cannabinoids or because the population was younger with fewer comorbidities. Therefore, in this population-based analysis, we performed logistic regression to control for multiple confounders, determining cannabis use did in fact have a lower in-hospital mortality and improved discharge outcomes. As discussed previously, this may be due to a subset of individuals with functional symptoms which may benefit from tetrahydrocannabinol (THC) neuromodulators. Future studies should work to stratify patients by age and delineate the outpatient effects of cannabinoids in the patients with gastroparesis.
There are several limitations to our current study, primarily due to the nature of the NIS database and ability to only capture in-hospital data at one point in time. As a result of its retrospective, non-longitudinal manner, it was not possible to analyze information regarding longer term outcomes that may significantly influence admission patterns, socioeconomic status, or health-related outcomes. Additional limitations include the potential for ICD9-CM coding bias and inability to generalize these results to an outpatient setting. It may also be possible that lower in-hospital mortality among cannabis users was related to decreased or less severe comorbidities, not accounted for by the CCI score. Furthermore, we are unable to perform more specific analyses to accurately differentiate this heterogeneous condition of gastroparesis (i.e., functional versus motor predominate types of gastroparesis) and which patients may have benefited from treatment with cannabis. Additionally, there was no specific data on treatment course (i.e., prokinetics, antiemetics, and neuromodulators) as well as severity of gastroparesis symptoms. Additional information on alternative treatment options that may impact the natural history and clinical outcomes of gastroparesis patients including the placement of a gastric pacemaker or endoscopic treatments such as gastric peroral endosopic myotomy (G-POEM) were not recorded.
However, despite these limitations, this study possesses several strengths. Most notably, this study includes a large number of patients with gastroparesis (over 1.4 million patients) and includes a robust sample size of patients with both gastroparesis and cannabis use. Despite not having subtype data for this heterogeneous condition, we were able to perform subgroup and sensitivity analyses based on dependent vs non-dependent cannabis use, age, as well as presence or absence of diabetes mellitus. The nationwide, representative sample and heterogeneous population portends more broad generalizability and may reflect varied clinical practice throughout the United States. To these authors’ knowledge, this study utilizes the largest sample size of gastroparesis patients in the current literature to investigate differences in care. While limitations exist in the ability to form concrete conclusions, we hope this NIS analysis will provide a roadmap for future investigation and reflection of current practice patterns moving forward.
Conclusions
Given the rising incidence of patients with both gastroparesis and cannabis use disorder, it is critically important to understand the demographic characteristics of this population and the effects of cannabis use on meaningful healthcare-related outcomes. Based upon this analysis, patients with cannabis use disorder had lower income status and were younger, but had generally better clinical and health-care associated outcomes. These findings may suggest cannabis may benefit individuals with functional or sensory predominant symptoms of gastroparesis though future studies to better identify this subset group of individuals are needed. Further study into qualitative and longitudinal outcomes of patients with cannabis use disorder who are discharged following hospitalization for gastroparesis would help better shine a light on this worsening issue.
Acknowledgments
Funding: This work was funded, at least in part, by the NIH grant T32 DK007533-35.
Footnotes
Potential Conflicts of Interest:
Thomas R. McCarty has no conflicts to disclose.
Fouad Chouairi has no conflicts to disclose.
Kelly Hathorn has no conflicts to disclose.
Walter W Chan has the following disclosures: Ironwood - scientific advisory board
Christopher C Thompson has the following disclosures: Apollo Endosurgery – Consultant/Research Support (Consulting fees/Institutional Research Grants), Aspire Bariatrics – Research Support (Institutional Research Grant), BlueFlame Healthcare Venture Fund – General Partner, Boston Scientific – Consultant (Consulting fees), Covidien/Medtronic – Consultant (Consulting Fees), EnVision Endoscopy (Board Member), Fractyl – Consultant/Advisory Board Member (Consulting Fees), GI Dynamics – Consultant (Consulting Fees)/ Research Support (Institutional Research Grant), GI Windows – Ownership interest, Olympus/Spiration – Consultant (Consulting Fees)/Research Support (Equipment Loans), Spatz – Research Support (Institutional Research Grant), USGI Medical – Consultant (Consulting Fees)/Advisory Board Member (Consulting fees)/Research Support (Research Grant).
References
- 1.Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu N, Abell T. Gastroparesis Updates on Pathogenesis and Management. Gut Liver. 2017;11(5):579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L, American College of G. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108(1):18–37; quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13(4):833–844. [DOI] [PubMed] [Google Scholar]
- 5.Lacy BE, Crowell MD, Mathis C, Bauer D, Heinberg LJ. Gastroparesis: Quality of Life and Health Care Utilization. J Clin Gastroenterol. 2018;52(1):20–24. [DOI] [PubMed] [Google Scholar]
- 6.Wadhwa V, Mehta D, Jobanputra Y, Lopez R, Thota PN, Sanaka MR. Healthcare utilization and costs associated with gastroparesis. World J Gastroenterol. 2017;23(24):4428–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103(2):313–322. [DOI] [PubMed] [Google Scholar]
- 8.Ong SK, Christie PM, Windsor JA. Management of gallstone pancreatitis in Auckland: progress and compliance. ANZ J Surg. 2003;73(4):194–199. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011;115(1-2):120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charilaou P, Agnihotri K, Garcia P, Badheka A, Frenia D, Yegneswaran B. Trends of Cannabis Use Disorder in the Inpatient: 2002 to 2011. The American journal of medicine. 2017;130(6):678–687.e677. [DOI] [PubMed] [Google Scholar]
- 11.Desai R, Fong HK, Shah K, et al. Rising Trends in Hospitalizations for Cardiovascular Events among Young Cannabis Users (18-39 Years) without Other Substance Abuse. Medicina (Kaunas, Lithuania). 2019;55(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Njei B, Sharma P, McCarty TR, et al. Cannabis Use Is Associated With Increased Risk of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis: Analysis of the US Nationwide Inpatient Sample Database, 2004-2014. Pancreas. 2018;47(9):1142–1149. [DOI] [PubMed] [Google Scholar]
- 13.Simons-Linares CR, Barkin JA, Jang S, et al. The Impact of Cannabis Consumption on Mortality, Morbidity, and Cost in Acute Pancreatitis Patients in the United States: A 10-Year Analysis of the National Inpatient Sample. Pancreas. 2019;48(6):850–855. [DOI] [PubMed] [Google Scholar]
- 14.Mbachi C, Attar B, Wang Y, et al. Association Between Cannabis Use and Complications Related to Crohn's Disease: A Retrospective Cohort Study. Digestive diseases and sciences. 2019;64(10):2939–2944. [DOI] [PubMed] [Google Scholar]
- 15.Adejumo AC, Adegbala OM, Adejumo KL, Bukong TN. Reduced Incidence and Better Liver Disease Outcomes among Chronic HCV Infected Patients Who Consume Cannabis. Canadian journal of gastroenterology & hepatology. 2018;2018:9430953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai P, Mbachi C, Vohra I, et al. Association Between Cannabis Use and Healthcare Utilization in Patients With Irritable Bowel Syndrome: A Retrospective Cohort Study. Cureus. 2020;12(5):e8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bashashati M, Storr MA, Nikas SP, et al. Inhibiting fatty acid amide hydrolase normalizes endotoxin-induced enhanced gastrointestinal motility in mice. Br J Pharmacol. 2012;165(5):1556–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partosoedarso ER, Abrahams TP, Scullion RT, Moerschbaecher JM, Hornby PJ. Cannabinoid1 receptor in the dorsal vagal complex modulates lower oesophageal sphincter relaxation in ferrets. J Physiol. 2003;550(Pt 1):149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCallum RW, Soykan I, Sridhar KR, Ricci DA, Lange RC, Plankey MW. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther. 1999;13(1):77–80. [DOI] [PubMed] [Google Scholar]
- 20.Bashashati M, McCallum RW. Cannabis in Gastrointestinal Disorders. Gastrointestinal Motility and Functional Bowel Disorders Series #4. Practical Gastroenterology 2014;38(12), 36–46. [Google Scholar]
- 21.Barbash B, Mehta D, Siddiqui MT, Chawla L, Dworkin B. Impact of Cannabinoids on Symptoms of Refractory Gastroparesis: A Single-center Experience. Cureus. 2019;11(12):e6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ly HG, Ceccarini J, Weltens N, et al. Increased cerebral cannabinoid-1 receptor availability is a stable feature of functional dyspepsia: a [F]MK-9470 PET study. Psychother Psychosom. 2015;84(3):149–158. [DOI] [PubMed] [Google Scholar]
- 23.Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality (AHRQ): Advancing Excellence in Health Care. https://www.ahrq.gov/research/data/hcup/index.html. Accessed November 11, 2019.
- 24.McCarty TR, Echouffo-Tcheugui JB, Lange A, Haque L, Njei B. Impact of bariatric surgery on outcomes of patients with nonalcoholic fatty liver disease: a nationwide inpatient sample analysis, 2004-2012. Surg Obes Relat Dis. 2018;14(1):74–80. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui MT, Bilal M, Schorr-Lesnick B, Lebovics E, Dworkin B. Opioid use disorder is associated with increased mortality and morbidity in patients with gastroparesis. Annals of gastroenterology. 2019;32(4):370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bielefeldt K Factors influencing admission and outcomes in gastroparesis. Neurogastroenterol Motil. 2013;25(5):389–398, e294. [DOI] [PubMed] [Google Scholar]
- 27.Healthcare Cost and Utilization Project (HCUP). Overveiw of National (Nationwide) Inpatient Sample (NIS). Accessed December 15, 2019. [Google Scholar]
- 28.Arias AJ, Kranzler HR. Treatment of co-occurring alcohol and other drug use disorders. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2008;31(2):155–167. [PMC free article] [PubMed] [Google Scholar]
- 29.Smolkina M, Morley KI, Rijsdijk F, et al. Cannabis and Depression: A Twin Model Approach to Co-morbidity. Behavior genetics. 2017;47(4):394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World psychiatry : official journal of the World Psychiatric Association (WPA). 2008;7(2):68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halder SL, Locke GR 3rd, Schleck CD, Zinsmeister AR, Talley NJ. Influence of alcohol consumption on IBS and dyspepsia. Neurogastroenterol Motil. 2006;18(11):1001–1008. [DOI] [PubMed] [Google Scholar]
- 32.Dibaise JK, Islam RS, Dueck AC, Roarke MC, Crowell MD. Psychological distress in Rome III functional dyspepsia patients presenting for testing of gastric emptying. Neurogastroenterol Motil. 2016;28(2):196–205. [DOI] [PubMed] [Google Scholar]
- 33.Jouanjus E, Leymarie F, Tubery M, Lapeyre-Mestre M. Cannabis-related hospitalizations: unexpected serious events identified through hospital databases. British journal of clinical pharmacology. 2011;71(5):758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legleye S, Beck F, Khlat M, Peretti-Watel P, Chau N. The influence of socioeconomic status on cannabis use among French adolescents. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2012;50(4):395–402. [DOI] [PubMed] [Google Scholar]
- 35.Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48(6):859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCallum RW, Bashashati M. Cannabis for Gastroparesis: Hype or Hope? The American journal of gastroenterology. 2019;114(6):865–866. [DOI] [PubMed] [Google Scholar]
- 37.Abell TL, Kedar A, Stocker A, et al. Gastroparesis syndromes: Response to electrical stimulation. Neurogastroenterol Motil. 2019;31(3):e13534. [DOI] [PubMed] [Google Scholar]


