Abstract
The prevalence of metabolic syndrome among individuals with severe mental illness is considerably higher than in the general population, contributing to the 15–20-year shorter life expectancy of this client population. The aim of this pilot study was to evaluate the effectiveness of a novel, complex psychosocial program to reduce metabolic syndrome. Members of both the intervention (n = 78) and control (n = 31) group were psychiatric outpatients with severe/persistent mental illness struggling with one or more symptoms of metabolic syndrome. Beyond the default elements of similar programs such as diet and exercise, the intervention covered medication use, sleep hygiene, stress management, as well as addressing spiritual needs, mindfulness, addictions, and self-care. Assessment of metabolic indicators were completed at baseline, at the end of the 11-week intervention, and 6 months post-intervention. The trajectory of change over time was significantly more favorable in the treatment than in the control group in terms of waist circumference (p = 0.013, η2 = 0.093) and a positive trend emerged in relation to blood glucose level (p = 0.082, η2 = 0.057). However, no statistically reliable difference was observed between the intervention and the control group regarding the other outcome variables (body mass index, systolic and diastolic blood pressure, serum triglyceride level, serum HDL cholesterol level, overall metabolic syndrome severity). These findings suggest that to produce more robust benefits, psychosocial interventions targeting the metabolic health of individuals with complex mental health needs should be either longer in duration if resources permit or narrower in focus (diet and exercise mainly) if resources are scarce.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12144-021-02269-3.
Keywords: Metabolic syndrome, Abdominal circumference, Severe mental illness, Psychosocial intervention, Effectiveness
Introduction
Metabolic syndrome is defined as a group of conditions including abdominal obesity, dyslipidemia (increased blood cholesterol and / or decreased HDL cholesterol level), increased fasting serum glucose concentration, and increased blood pressure. When occurring together, this group of conditions significantly increases the risk of type 2 diabetes, heart disease, stroke-related morbidity, and premature mortality (Eckel et al., 2005). While diagnostic criteria have varied between expert groups in the past, the latest guidelines published by the International Diabetes Federation (presence of at least three of the following five conditions: blood pressure ≥ 130/85 mmHg, fasting blood glucose level ≥ 5.6 mmol/L, triglyceride level ≥ 1.7 mmol/L, HDL-Cholesterol level < 1.0 mmol/L, and waist circumference ≥ 102 cm) provide clear thresholds that can be applied to various geographic regions and ethnic groups (Alberti et al., 2006).
While it is estimated that approximately 25% of the world’s population struggles with metabolic syndrome (Saklayen, 2018), prevalence rates vary across countries. For instance, this prevalence rate is estimated to be 19.1% in Canada (Rao et al., 2014), 30.7% in Australia, and 39% in the United States (O'Neill & O'Driscoll, 2015). Importantly, certain groups of individuals may have an increased risk of developing metabolic syndrome compared to the general population; those diagnosed with severe mental disorders (such as schizophrenia or bipolar disorder) being one of these groups. It is estimated that those experiencing serious mental health challenges are 58% more likely to develop metabolic syndrome when compared to the general population (Vancampfort et al., 2015). Factors that can contribute to this increased risk include the use of certain psychotropic medications (Barton et al., 2020; McCloughen & Foster, 2011), unhealthy lifestyle (such as sedentary behavior, poor diet, substance abuse, smoking), and decreased motivation to seek help for physical health issues (Newcomer, 2007). The higher prevalence of metabolic syndrome in this population plays an important role in the unfortunate trend of premature mortality: individuals suffering from severe mental illness tend to die 15–20 years earlier than members of the general population (Hjorthøj et al., 2017; Laursen et al., 2012; Laursen et al., 2014).
To mitigate the effects of untreated metabolic syndrome in this vulnerable population, multiple interventions aiming to reduce risk factors have been developed in the previous decades. These interventions differ considerably, from those using pharmacologic treatment or bariatric surgery through those focusing on diet and/or exercise regiments to those that apply cognitive, behavioral or psychoeducational methods to address patients’ overall physical wellbeing. Duration and intensity of intervention programs and the length of each intervention session also vary across intervention protocols and they can be conducted in individual and/or group modalities depending on the desired level of ‘customization’ for participants (Bruins et al., 2014; Bruins et al., 2017). While pharmacological and surgical approaches have their own unique benefits (Papanastasiou, 2012), it may be preferable to attempt interventions that reduce/reverse the ‘root causes’ of metabolic syndrome (atherogenic diet, sedentary lifestyle, and overweight or obesity) prior to starting pharmacologic regimens targeting the individual symptoms of the syndrome, particularly when addressing concerns for the first time (Giugliano et al., 2008; Grundy et al., 2005). Such approaches are also more likely to promote sustained, long-term change in overall metabolic health and quality of life. Incorporating frequent objective measurements of cardiometabolic factors throughout and post-intervention can further promote success and uptake of healthy habits (Dagogo-Jack et al., 2010).
Previous studies have demonstrated the effectiveness of various psychosocial interventions in patients with severe mental illness (Bruins et al., 2014; Chacón et al., 2011). However, the majority of this research has been conducted in carefully-controlled research settings, which does not necessarily reflect the realities of preventing/treating metabolic syndrome in everyday psychiatric practice. This is an important consideration, as metabolic monitoring and treatment among those with severe mental illness is often suboptimal (Bruins et al., 2017) or nonexistent in real-world settings (Happell et al., 2016).
The non-pharmacological, psychosocial approaches in the treatment of metabolic syndrome usually include education on the syndrome itself, as well as some form of active or passive intervention on healthy eating and exercise (Dagogo-Jack et al., 2010). It is unclear though, whether a more comprehensive intervention addressing further, more distal psychological processes (e.g., stress management, self-esteem, emotion regulation, spiritual needs) and life style factors (e.g., sleep habits, certain aspects of budgeting, general medication use) would have additional benefits over the sole emphasis on more proximal factors as food choices and physical activity. Thus, the aim of the present pilot study was to investigate the effectiveness of a complex psychosocial program to reduce metabolic syndrome in a real-life setting of psychiatric outpatients at-risk for developing/worsening metabolic syndrome.
Methods
Sample and Procedure
The protocol of the present study was approved by the Research and Ethics Board of Waypoint Centre for Mental Health Care (HPRA#17.11.28). The analyses (N = 163) were based on two subsamples of psychiatric outpatients from a tertiary level psychiatric hospital specialized for the care of individuals with severe / persistent mental illness. Members of the first sample were recruited as participants of a prospective study including both an intervention (n = 32) and a control (n = 31) group, while the second (later) study was a retrospective chart review (n = 100) examining the same intervention and same outcome variables. The purpose of the addition of the second subsample was to increase the sample size of the treatment group of Study 1 and by doing so improve both the generalizability of the findings and the statistical power of the analyses.
While in the first study (controlled prospective study), informed consent was provided by participants, in case of the second subsample (retrospective chart review), the informed consent requirement was waived as the protocol met all Tricouncil Policy Guidelines for waiver of consent (Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada, 2014). In all cases, data were gathered from clinical documentation in the participants’ medical records.
All participants were clients of a specialized, tertiary level psychiatric hospital (Waypoint Centre for Mental Health Care), Canada receiving outpatient care for their severe and/or persistent mental health challenges. Beyond mental health symptoms, clients of the clinic are routinely assessed in relation to vital signs and metabolic indicators as well and those struggling with metabolic syndrome or at least one risk factor for metabolic syndrome are offered participation in the psychosocial intervention investigated in the present study.
Participants were assessed at baseline, post-intervention and at their psychiatric follow-up closest to 6 months (M = 165 days). Active participants of the present investigation were those who attended at least 60% of the intervention sessions (n = 78), while the control group consisted of clients who met eligibility criteria of the intervention but were not interested in or were not able to participate (n = 31). Clients who agreed to participate in the intervention but failed to take part for at least 60% of the sessions (or whose records were not complete enough to allow conclusions on their participation rate) – for instance due to hospitalization for mental or physical health reasons – were excluded (n = 54) from the analyses. Baseline characteristics of the study groups are displayed in Table 1. While the intervention and the treatment group differed moderately across some of the specific metabolic indicators (direction dependent on the given indicator), the data showed that – using the diagnostic standards published by the International Diabetes Federation (Alberti et al., 2009) – the prevalence of metabolic syndrome did not differ significantly (and was about 60%) between the treatment versus the intervention group (or the group of those who were excluded from the analyses due to missing data or drop-out from the intervention: χ2 = 0.24, p = 0.988, V = 0.01).
Table 1.
Baseline characteristics of the study sample stratified by participant status. Continuous variables are displayed as means (standard deviation), while sex and metabolic syndrome diagnostic status is displayed as frequency (percentage)
| Control group (n = 31) | Intervention group (n = 78) | Drop-out / insufficient information (n = 54) | Comparison of the control vs. intervention group | |
|---|---|---|---|---|
| Sex (female) | 9 (29.03) | 58 (74.36) | 38 (70.37) | χ2 = 19.2, p < 0.001, V = 0.42 |
| Age (years) | 46.71 (10.60) | 48.92 (11.74) | 49.19 (10.99) | t = −0.91, p = 0.364, d = 0.20 |
| Primary psychiatric diagnosis | χ2 = 29.67, p < 0.001, V = 0.52 | |||
| Psychotic disorder | 19 (61.29) | 21 (26.92) | 10 (20.0) | |
| Mood disorder | 5 (16.13) | 37 (47.44) | 30 (60.0) | |
| Anxiety disorder | 0 (0.00) | 9 (11.54) | 4 (8.00) | |
| Personality disorder | 1 (3.23) | 10 (12.82) | 5 (10.0) | |
| Other | 6 (19.35) | 1 (1.28) | 1 (2.0) | |
| Body mass index (kg/m2) | 32.17 (6.67) | 37.53 (9.25) | 38.56 (8.58) | t = −2.94, p = 0.004, d = 0.62 |
| Waist circumference (cm) | 107.62 (15.55) | 117.10 (15.00) | 117.11 (15.37) | t = −2.93, p = 0.004, d = 0.63 |
| Systolic blood pressure (mmHg) | 129.65 (16.12) | 123.68 (14.50) | 128.61 (14.60) | t = 1.88, p = 0.063, d = 0.40 |
| Diastolic blood pressure (mmHg) | 83.97 (9.05) | 77.23 (10.47) | 77.78 (11.74) | t = 3.14, p = 0.002, d = 0.66 |
| Serum triglyceride level (mmol/L) | 2.19 (1.56) | 2.11 (1.44) | 2.48 (1.92) | t = 0.25, p = 0.805, d = 0.05 |
| Serum high-density lipoprotein cholesterol level (mmol/L) | 1.16 (0.32) | 1.27 (0.40) | 1.32 (0.39) | t = −1.37, p = 0.175, d = 0.30 |
| Serum fasting blood glucose level (mmol/L) | 5.44 (2.18) | 6.67 (2.69) | 6.70 (3.19) | t = −2.22, p = 0.029, d = 0.48 |
| Metabolic syndrome severity index (z-score) | −0.07 (0.57) | −0.01 (0.55) | 0.09 (0.68) | t = −0.49, p = 0.623, d = 0.11 |
| Metabolic syndrome (yes) | 17 (58.62) | 41 (60.29) | 21 (60.00) | χ2 = 0.24, p = 0.878, V = 0.02 |
Intervention
As metabolic syndrome is a complex phenomenon, a unifying concept with one treatable cause may not emerge (Hjemdahl, 2002). Consequently, the aim of the developers of the present intervention was to design a program that targets a relatively large number of participant needs and factors relevant to the metabolic health of individuals with a severe/persistent mental illness. The Transtheoretical Model of Change (Prochaska & DiClemente, 1983) as an integrative, biopsychosocial model that conceptualizes the process of intentional behavioral change gave the theoretical basis for the intervention. However, as this model is limited in its awareness of the social context and factors in which change occurs (such as income or other socioeconomic variables), elements of the Health Action Process Approach (Schwarzer, 2008) was also considered to help in the identification and management of social factors that play a key role in the behavior change of individuals with severe / persistent mental illness. In addition, in the intervention design stage, regular feedback of participants and peer program facilitators was also sought and utilized to refine the program and practice client-centered service design and delivery. The resulting program was honored with the Innovation Award by the Ontario Minister of Health in 2010 for improving patient-centeredness in health care delivery (Ontario Ministry of Health, 2010).
The intervention used in the present study contained 11, weekly group sessions in the length of 2 h (Duncan, 2011a, b). Sessions were in part didactic (i.e., contained information sharing by program facilitators on the issues covered by the given module) but also allowing interactive participation in discussions regarding participants’ individual health goals and strategies in relation to managing difficulties with the implementation of the course material. Each session was facilitated by a content expert of the given field (e.g., physician, dietician, recreational therapist, pharmacist, hospital chaplain) supported by a peer support worker who provided a healthy snack to participants during the session break to demonstrate optimal food choices. A brief, weekly meeting with the metabolic nurse of the clinic was also part of the program, this way providing feedback to participants regarding their progress towards more optimal waist circumference, weight, and blood pressure.
Module 1 of the intervention provides psychoeducation on healthy and unhealthy metabolism, risk factors and symptoms of metabolic syndrome, and reversal strategies (Anderssen et al., 2007; Mujica et al., 2010). Participants are also encouraged to define their own goals to improve their metabolic health. Module two provides information on how metabolic syndrome affects body function and organ systems and how clients can avoid or reduce organ damage by engaging in primary (e.g., improving diet) or secondary (e.g., participation in annual medical check-ups, eye and foot examinations) prevention strategies. Efforts are also made to increase respect towards the body, ownership of health and health-related self-efficacy [cf. the relationship between self-esteem, internal health locus of control and health behaviors (Adolfsson et al., 2005; Cheng et al., 2016; Chuang et al., 2016)].
Module 3 and 4 focus on how to improve eating habits by covering topics such as dietary patterns proved to improve metabolic health (Pitsavos et al., 2006); optimal frequency, timing and amount of food to consume; interpretation of nutrition labels; budgeting skills in relation to food; how to choose healthier meals while eating out; and benefits of mindfulness in healthy eating (Fuentes Artiles et al., 2019). Given the dangerously low level of physical activity among individuals with severe/persistent mental illness (Nyboe & Lund, 2013), Module 5 emphasizes the importance of physical activity in optimizing metabolic health (Ostman et al., 2017; Wewege et al., 2018). The program also reviews the most frequently reported barriers in relation to a physically active life style and recommends strategies to overcome these (Firth et al., 2016). Finally, the program content also provides practical information to participants regarding local fitness amenities, subsidies, and recreational programs provided by the hospital.
Given the direct (Rosmond, 2005; Tamashiro et al., 2011) and indirect [i.e. through health behaviors (Epel et al., 2004)] effect of psychological stress on metabolic syndrome, Module 6 deals with recognizing and reducing stress via covering topics such as biochemical and metabolic aspects of stress; symptoms and effects of chronic stress; as well as strategies to reduce stress and increase resilience. In response to our growing knowledge on the beneficial effect of spirituality on physical health (Brintz et al., 2017; George et al., 2002; Powell et al., 2003), Module 7 focuses on emotional and spiritual well-being and provides some principles and practical tools for spiritual self-care (e.g., acceptance of emotions, responding to challenges, importance of finding meaning in daily life). Considering the importance of sleep quality and quantity in metabolic health (Koren et al., 2016; Lian et al., 2019), the intervention also provides psychoeducation on sleep by covering topics such as the stages and function of sleep; metabolic aspects of sleep and sleep apnea; and relationship between sleep and appetite. Module 8 also helps participants develop strategies to improve sleep habits.
Module 9 provides information on the process of behavior change (including the stages of change); how this information can be used to change addictive behaviors; parallels between our relationship with food and addictions; and the use of mindfulness to help with behavior change (Roche et al., 2019). Considering the role of psychotropic medications in the development and maintenance of the metabolic syndrome (Azevedo Da Silva et al., 2016; De Hert et al., 2009; Newcomer, 2007), a full session (Module 10) is devoted to topics such as how medications are processed in the body; benefits and risks involved with general and psychotropic medications; interactions of smoking and medications; and alternatives to commonly used general medications (e.g., non-medicinal pain management). Finally, in recognition of the importance of a positive body image in maintaining a healthy life style (Bouzas et al., 2019; Černelič-Bizjak & Jenko-Pražnikar, 2014; Khoury et al., 2014), Module 11 covers ways to improve self-confidence; recognizing and accepting body shape; and principles of dressing optimally considering body shape and budget. The full manual of the intervention can be found in the online supplementary materials to this article.
Measures
Waist circumference, height, weight, and blood pressure were measured by trained clinical staff during clinic visits, while fasting plasma lipid level (triglycerides and low density lipoprotein) as well as fasting blood glucose level were measured using the regular hospital laboratories. Waist circumference was measured to the nearest 0.1 cm using a standard, inelastic tape maintained in a horizontal plane, with the participant standing with weight distributed evenly on both feet. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (without shoes). Weight was measured to the nearest 0.1 kg using standard electronic scales (light clothing without shoes). To measure resting blood pressure, participants were seated in a chair with an occluding cuff on one arm. Following a brief rest period, readings were collected using an automatic, calibrated, oscillometric blood pressure monitor.
To quantify the overall severity of metabolic syndrome, a composite score was also calculated, which comprised of all six components of the syndrome: waist circumference, systolic as well as diastolic blood pressure, triglyceride-, HDL cholesterol-, and fasting blood glucose levels. To allow a proportionate representation of each factor regardless of original scale range, scores on each component were converted to a z-score first. In addition, HDL cholesterol level was reverse-coded to match risk direction of the other variables. The metabolic syndrome severity index was then calculated by averaging the z-scores across components (Ross et al., 2011) with higher scores indicating more severe metabolic syndrome.
Statistical Analyses
All analyses were carried out using SPSS for Windows, Version 27. Study groups at baseline were compared using independent samples t-test (continuous variables) and the Chi squared test (sex). Effect size was expressed using Cramer’s V (Chi squared test) and Cohen’s d (independent samples t-test). We considered 0.2 as a threshold for small effect, 0.5 for moderate effect, and 0.8 for large effect in case of Cohen’s d (Cohen, 1992), while the corresponding thresholds for Cramer’s V were 0.05, 0.10 and 0.15, respectively (Akoglu, 2018).
Effectiveness of the intervention was investigated using a mixed model ANOVA comparing the trends of change in the intervention versus the control group over time (time x group interaction), where effect size was expressed by eta squared. In this case, 0.01 was considered as the threshold for small effect, 0.06 for moderate effect, and 0.14 for large effect (Cohen, 1992). Post hoc analyses were also conducted to investigate if outcomes changed over time (baseline versus post-intervention and baseline versus follow-up) within the treatment or intervention groups, using Cohen’s d as the effect size indicator. As the intervention and treatment groups differed significantly at baseline across most metabolic indicators, between-group post hoc tests were not conducted at the later assessment points due to the less informative nature of such comparisons in cases when the study groups already differ at baseline.
Results
The results of those for whom data were available at least from their pre- and post-intervention assessments (ncontrol = 31, nintervention = 68) are displayed in Table 2. The data indicate a significant difference between the trajectory of scores of the intervention and the control group in terms of waist circumference (with moderate effect size): while the control group’s value did not change from baseline to post-intervention, that of the intervention group decreased significantly. For body mass index, a trend was observed toward a significant group x time interaction (with small effect size), in which case again, the values of the control group did not change over time, while those of the intervention group decreased significantly between baseline and the post-intervention assessment. No other statistically significant difference was observed in the study outcomes either in terms of group x time interactions or pre- versus post-intervention comparisons for either study group.
Table 2.
Pre- and post-intervention metabolic characteristics of study participants with data from at least 2 assessment points
| Pre-intervention M (SD) | Post-intervention M (SD) | Pre-post comparison | Time x group interaction | ||
|---|---|---|---|---|---|
| Body mass index (kg/m2) | Control group | 32.17 (6.67) | 32.20 (6.74) | p = 0.879, d < −0.01 | F = 3.50, p = 0.064, η2 = 0.032 |
| Intervention group | 37.53 (9.25) | 37.08 (9.24) | p = 0.001, d = 0.05 | ||
| Waist circumference (cm) | Control group | 107.74 (15.80) | 108.45 (16.47) | p = 0.439, d = −0.04 | F = 6.69, p = 0.011, η2 = 0.063 |
| Intervention group | 117.01 (14.98) | 114.90 (14.57) | p = 0.001, d = 0.14 | ||
| Systolic blood pressure (mmHg) | Control group | 129.65 (16.12) | 129.16 (11.27) | p = 0.843, d = 0.04 | F = 0.13, p = 0.716, η2 = 0.001 |
| Intervention group | 123.99 (14.34) | 124.56 (13.15) | p = 0.713, d = −0.04 | ||
| Diastolic blood pressure (mmHg) | Control group | 83.97 (9.05) | 84.13 (6.73) | p = 0.922, d = −0.02 | F = 0.60, p = 0.441, η2 = 0.006 |
| Intervention group | 77.45 (10.35) | 76.12 (10.39) | p = 0.200, d = 0.13 | ||
| Serum triglyceride level (mmol/L) | Control group | 2.19 (1.56) | 2.23 (1.95) | p = 0.864, d = −0.02 | F = 0.12, p = 0.732, η2 = 0.001 |
| Intervention group | 2.05 (1.49) | 2.00 (1.35) | p = 0.732, d = 0.04 | ||
| Serum high-density lipoprotein cholesterol level (mmol/L) | Control group | 1.16 (0.33) | 1.15 (0.29) | p = 0.724, d = 0.03 | F = 0.28, p = 0.599, η2 = 0.003 |
| Intervention group | 1.29 (0.44) | 1.30 (0.42) | p = 0.683, d = −0.02 | ||
| Serum fasting blood glucose level (mmol/L) | Control group | 5.44 (2.18) | 5.68 (2.26) | p = 0.421, d = −0.11 | F = 1.92, p = 0.170, η2 = 0.023 |
| Intervention group | 6.54 (2.33) | 6.26 (1.81) | p = 0.222, d = 0.13 | ||
| Metabolic syndrome severity index (z-score) | Control group | −0.07 (0.57) | 0.00 (0.66) | p = 0.355, d = −0.11 | F = 0.92, p = 0.341, η2 = 0.008 |
| Intervention group | −0.01 (0.55) | −0.03 (0.54) | p = 0.751, d = 0.04 | ||
The results of those for whom data were available for all three assessments (ncontrol = 31, nintervention = 24) are displayed in Table 3 (graphical representation of the data can be found in Supplementary Table 1). Similarly to the previous set of analyses, the data indicated a significant difference between the trajectory of scores of the intervention and the control group in relation to waist circumference (with moderate effect size): while the control group’s value did not change significantly from baseline to post-intervention, that of the intervention group decreased significantly. Within-group comparisons of the baseline versus follow-up assessments demonstrated no statistically significant difference for either the control or the intervention group. Although certain signs of positive treatment effect could be observed when comparing the patterns of change in the intervention versus the treatment group (Fig. 1), regarding no other specific variable was the time x group interaction statically significant; only a trend emerged for serum fasting blood glucose level, favoring the intervention group (small to moderate effect size).
Table 3.
Pre-, post-intervention and follow-up metabolic characteristics of study participants with data from all three assessment points
| Pre-intervention M (SD) | Post-intervention M (SD) | Follow-up M (SD) | T1 vs. T2 comparison | T1 vs. T3 comparison | Time x group interaction | ||
|---|---|---|---|---|---|---|---|
| Body mass index (kg/m2) | Control group | 32.17 (6.67) | 32.20 (6.74) | 32.26 (6.89) | p > 0.999, d < −0.01 | p > 0.999, d = −0.01 | F = 1.93, p = 0.159, η2 = 0.032 |
| Intervention group | 38.39 (9.83) | 37.67 (9.84) | 37.77 (9.63) | p = 0.007, d = 0.07 | p = 0.267, d = 0.06 | ||
| Waist circumference (cm) | Control group | 107.74 (15.80) | 108.45 (16.47) | 110.32 (17.16) | p > 0.999, d = −0.04 | p = 0.099, d = −0.16 | F = 4.95, p = 0.013, η2 = 0.093 |
| Intervention group | 115.93 (17.98) | 111.71 (19.01) | 115.26 (20.18) | p < 0.001, d = 0.23 | p > 0.999, d = 0.04 | ||
| Systolic blood pressure (mmHg) | Control group | 129.65 (16.12) | 129.16 (11.27) | 124.81 (15.22) | p > 0.999, d = 0.04 | p = 0.233, d = 0.31 | F = 0.73, p = 0.485, η2 = 0.012 |
| Intervention group | 125.03 (13.68) | 123.80 (14.29) | 123.50 (14.06) | p > 0.999, d = 0.09 | p > 0.999, d = 0.11 | ||
| Diastolic blood pressure (mmHg) | Control group | 83.97 (9.05) | 84.13 (6.73) | 83.68 (7.93) | p > 0.999, d = −0.02 | p > 0.999, d = 0.03 | F = 0.11, p = 0.899, η2 = 0.002 |
| Intervention group | 76.67 (12.16) | 75.73 (10.89) | 75.67 (9.52) | p > 0.999, d = 0.08 | p > 0.999, d = 0.09 | ||
| Serum triglyceride level (mmol/L) | Control group | 2.19 (1.56) | 2.23 (1.95) | 2.25 (1.62) | p > 0.999, d = −0.02 | p > 0.999, d = −0.04 | F = 0.63, p = 0.534, η2 = 0.013 |
| Intervention group | 2.15 (1.87) | 2.34 (1.99) | 1.96 (1.08) | p > 0.999, d = −0.10 | p > 0.999, d = 0.12 | ||
| Serum high-density lipoprotein cholesterol level (mmol/L) | Control group | 1.16 (0.33) | 1.15 (0.29) | 1.14 (0.26) | p > 0.999, d = 0.03 | p > 0.999, d = 0.07 | F = 0.18, p = 0.835, η2 = 0.004 |
| Intervention group | 1.36 (0.50) | 1.33 (0.48) | 1.36 (0.45) | p > 0.999, d = 0.06 | p > 0.999, d < 0.01 | ||
| Serum fasting blood glucose level (mmol/L) | Control group | 5.44 (2.18) | 5.68 (2.26) | 6.05 (2.37) | p = 0.787, d = −0.11 | p = 0.253, d = −0.27 | F = 2.80, p = 0.082, η2 = 0.057 |
| Intervention group | 5.97 (2.52) | 5.70 (1.61) | 5.51 (1.47) | p > 0.999, d = 0.13 | p = 0.929, d = 0.22 | ||
| Metabolic syndrome severity index (z-score) | Control group | −0.07 (0.57) | 0.00 (0.66) | 0.07 (0.66) | p = 0.940, d = −0.11 | p = 0.467, d = −0.23 | F = 0.84, p = 0.422, η2 = 0.014 |
| Intervention group | −0.04 (0.56) | −0.09 (0.60) | −0.05 (0.66) | p > 0.999, d = 0.09 | p > 0.999, d = 0.02 | ||
Fig. 1.
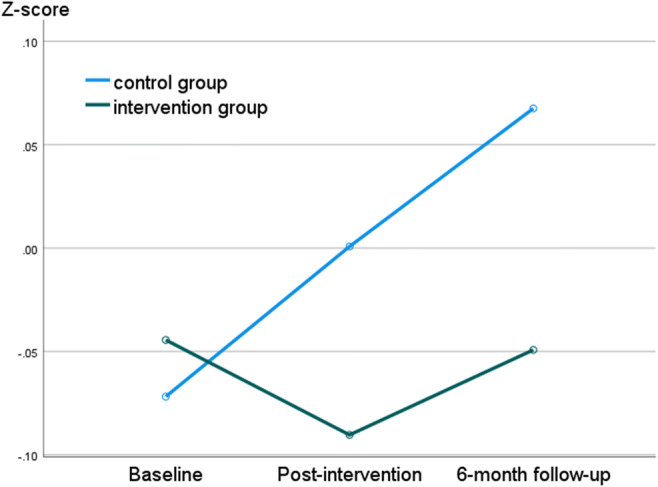
Trajectory of change of the metabolic syndrome severity index (z-score) in the intervention versus the control group
In a set of sensitivity analyses, all the above models were re-run stratifying the sample by primary psychiatric diagnosis (psychotic spectrum- versus other mental disorder). The trends were similar in both diagnostic groups to the results of the overall sample: significant improvement was observed in the treatment group in terms of body mass index and waist circumference between baseline and treatment completion, which faded away by the time of the follow-up assessment. The results of these analyses are presented in detail in the online supplementary materials to this article (Supplementary Tables 2–5).
Discussion
Metabolic syndrome is a significant, modifiable risk factor partially responsible for the high premature mortality among individuals struggling with severe mental illness (Hjorthøj et al., 2017; Laursen et al., 2012; Laursen et al., 2014). Management of this cluster of health conditions is a crucial aspect of the care of individuals with complex and long-term mental health care needs. While numerous psychosocial interventions have been developed to date to help individuals improve their metabolic health, their narrow focus on the proximal predictors of the syndrome left the question open whether a more comprehensive intervention considering more distal psychological processes in the background of metabolic health behavior would result in more robust or sustainable gains. Therefore, the aim of the present study was to preliminarily investigate the effectiveness of an 11-week, broad-focus psychosocial intervention in improving the indicators of metabolic health.
The data showed that the trajectory of change over time was moderately more favorable in the treatment group than in the control group in the case of waist circumference, which is an exceptionally common symptom of the metabolic syndrome in individuals with severe mental illness (Cohn et al., 2004) and increases not just cardiovascular risk (de Koning et al., 2007; Janssen et al., 2004; Yusuf et al., 2005) but even the chances of more adverse outcomes in the case of infectious diseases such as COVID-19 (Sattar et al., 2020). Unfortunately however, these improvements in the area of centripetal obesity were not sustained over the 6-month follow-up period in the current study. While there were certain signs of positive treatment effect in the case of body mass index, fasting blood glucose level or the metabolic syndrome severity index (cf. the consistently deteriorating trend in the control group in contrast to the improvement of the intervention group), in the case of no other specific variable was it possible to observe a reliable difference between the intervention and the control group. While these outcomes fit into the overall landscape of the effectiveness of psychosocial interventions developed to reduce metabolic syndrome in the sense that the most significant improvements occurred in relation to waist circumference and fasting blood glucose level [in contrast to for example blood pressure or serum cholesterol levels (Bruins et al., 2014)], they are closer to the lower end of the effectiveness spectrum (Gurusamy et al., 2018). Most likely, those parameters that remained unchanged as a result of the present intervention would require sustained and / or larger behavioral change to produce measurable improvements. For instance, to achieve a 1 mmHg reduction in systolic blood pressure, 1 kg of weight loss is needed (Whelton et al., 2018). Similarly, losing 5–10% of body weight is needed to improve blood sugar levels reliably (Wing et al., 1990).
The findings of the present study suggest that with a duration of 3 months, a more comprehensive psychosocial intervention is less, rather than more, effective in comparison to interventions described in the literature with a narrower focus on diet and exercise (Attux et al., 2013; Khazaal et al., 2007; Kwon et al., 2006; Vreeland et al., 2003). Consequently, when strictly aiming to improve metabolic health and when resources are not available to provide treatment with a longer duration, it may be more efficient to target proximal determinants of metabolic syndrome only such as diet and exercise. It is also possible though that with an extended duration, more complex interventions – like the present one – would have more positive or stable effects than interventions focusing on a narrow set of proximal etiological factors, which hypothesis deserves further study. In addition, it is also worthy of mentioning that while the effectiveness of the intervention in the present study was defined exclusively according to metabolic health indicators, it is possible that a more comprehensive assessment protocol including psychological variables as well (e.g., perceived stress, anxiety, perceived social support, self-efficacy etc.) could have shed light on the benefits of the more comprehensive approach offered by the intervention under study.
Further investigations into the combined effects of psychosocial and pharmacological interventions (Rask Larsen et al., 2018) to address metabolic syndrome also seems to be a promising line of research to support the maximization of the effectiveness of available treatments. Moreover, even though the present intervention was specifically designed with an eye on individuals with severe / persistent mental illness, the vast majority of program elements are similarly relevant to individuals suffering from metabolic syndrome but not mental illness. Therefore, investigating the effectiveness of the intervention after minimal alterations (e.g., putting the emphasis on general medications’ influence on metabolic health instead of those of psychiatric medications) in non-psychiatric populations might shed light on the benefits of this program for a wider range of individuals.
When interpreting the findings and the generalizability of the results, limitations of the present study also need to be considered. First, the sampling was not random and as a result, the study samples differed across numerous characteristics (e.g., primary psychiatric diagnosis) as well as outcome variables at baseline, limiting comparability of the two groups during later assessments. In addition, due to the real-world setting, the attrition / non-compliance rate was high. Further, the data were extracted from medical files on routine measures that were not originally documented for research purposes, consequently resulting in not fully standardized assessment procedures to gather, for instance, weight or blood pressure data (e.g., which arm to measure blood pressure on). In addition, the lack of compliance data in the present study regarding the behaviors targeted by the intervention (i.e., diet, exercise, sleep hygiene etc.) makes it difficult to select more or less effective elements of the complex intervention under study [cf. (Aucoin et al., 2020)]. Collecting and analyzing either qualitative or quantitative data on participant satisfaction could have provided an even richer evaluation of intervention outcomes. Finally, information gaps in the medical records also decreased our sample size reducing the statistical power of our analyses. Despite these limitations, the present study calls attention to the potential of psychosocial interventions in reducing cardio-metabolic risk, which is a vital but often neglected aspect in the health care of individuals suffering from severe / persistent mental illness.
Supplementary Information
(DOCX 125 kb)
(PDF 5.24 mb)
Acknowledgements
The authors wish to express their appreciation to Dr. Jacqueline Duncan, main developer of the intervention studied in the present study as well as staff at the Outpatient Assessment and Treatment Services, Waypoint Centre for Mental Health Care for their contributions to the development of certain intervention modules. Further, the authors are also thankful to Lauren Wright for her help in the early phases of data collection.
Data Availability Statement
The raw data of the present manuscript may not be shared publicly because the data set contains sensitive personal health information (e.g., psychiatric diagnosis) which - considering the relatively low number of participants and the very specific setting - could jeopardize participants’ privacy rights and are against the policies of the authors’ institution. Considering these, the authors have not requested permission from participants to share their data with other parties. However, the authors are happy to conduct further / alternative analyses and share the results of these along with the syntaxes with interested researchers.
Declarations
Informed Consent
Informed consent was obtained from participants of the subsample of the prospective study. In case of the subsample of the retrospective chart review, the informed consent requirement was waived through the ethical approval process as the protocol met all Tricouncil Policy Guidelines for waiver of consent (Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada, 2014).
Conflict of Interest
The authors declare not having any conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adolfsson B, Andersson I, Elofsson S, Rössner S, Undén A-L. Locus of control and weight reduction. Patient Education and Counseling. 2005;56(1):55–61. doi: 10.1016/j.pec.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Akoglu H. User's guide to correlation coefficients. Turkish Journal of Emergency Medicine. 2018;18(3):91–93. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome - A new world-wide definition. A consensus statement from the international diabetes federation. Diabetic Medicine. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC. Harmonizing the metabolic syndrome. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Anderssen SA, Carroll S, Urdal P, Holme I. Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: Results from the Oslo diet and exercise study. Scandinavian Journal of Medicine and Science in Sports. 2007;17(6):687–695. doi: 10.1111/j.1600-0838.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- Attux C, Martini LC, Elkis H, Tamai S, Freirias A, Camargo MDGM, Mateus MD, de Jesus Mari J, Reis AF, Bressan RA. A 6-month randomized controlled trial to test the efficacy of a lifestyle intervention for weight gain management in schizophrenia. BMC Psychiatry. 2013;13(1):Article # 60. doi: 10.1186/1471-244X-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aucoin M, LaChance L, Clouthier SN, Cooley K. Dietary modification in the treatment of schizophrenia spectrum disorders: A systematic review. World journal of psychiatry. 2020;10(8):187–201. doi: 10.5498/wjp.v10.i8.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo Da Silva M, Balkau B, Roussel R, Tichet J, Fumeron F, Fagherazzi G, Nabi H. Longitudinal association of antidepressant medication use with metabolic syndrome: Results of a 9-year follow-up of the D.E.S.I.R. cohort study. Psychoneuroendocrinology. 2016;74:34–45. doi: 10.1016/j.psyneuen.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Barton BB, Segger F, Fischer K, Obermeier M, Musil R. Update on weight-gain caused by antipsychotics: A systematic review and meta-analysis. Expert Opinion on Drug Safety. 2020;19(3):295–314. doi: 10.1080/14740338.2020.1713091. [DOI] [PubMed] [Google Scholar]
- Bouzas C, Bibiloni MDM, Tur JA. Relationship between body image and body weight control in overweight ≥55-year-old adults: A systematic review. International Journal of Environmental Research and Public Health. 2019;16(9):1622. doi: 10.3390/ijerph16091622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brintz CE, Birnbaum-Weitzman O, Llabre MM, Castañeda SF, Daviglus ML, Gallo LC, Giachello AL, Kim RS, Lopez L, Teng Y, Penedo FJ. Spiritual well-being, religious activity, and the metabolic syndrome: Results from the Hispanic community health study/study of Latinos sociocultural ancillary study. Journal of Behavioral Medicine. 2017;40(6):902–912. doi: 10.1007/s10865-017-9858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins J, Jörg F, Bruggeman R, Slooff C, Corpeleijn E, Pijnenborg M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: A meta-analysis. PLoS One. 2014;9(12):e112276. doi: 10.1371/journal.pone.0112276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins J, Pijnenborg GHM, van den Heuvel ER, Visser E, Corpeleijn E, Bartels-Velthuis AA, Bruggeman R, Jorg F. Persistent low rates of treatment of metabolic risk factors in people with psychotic disorders: A PHAMOUS study. Journal of Clinical Psychiatry. 2017;78(8):1117–1125. doi: 10.4088/JCP.16m10831. [DOI] [PubMed] [Google Scholar]
- Černelič-Bizjak M, Jenko-Pražnikar Z. Impact of negative cognitions about body image on inflammatory status in relation to health. Psychology & Health. 2014;29(3):264–278. doi: 10.1080/08870446.2013.844807. [DOI] [PubMed] [Google Scholar]
- Chacón F, Mora F, Gervás-Ríos A, Gilaberte I. Efficacy of lifestyle interventions in physical health management of patients with severe mental illness. Annals of General Psychiatry. 2011;10(22):1–10. doi: 10.1186/1744-859X-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Cheung MWL, Lo BCY. Relationship of health locus of control with specific health behaviours and global health appraisal: A meta-analysis and effects of moderators. Health Psychology Review. 2016;10(4):460–477. doi: 10.1080/17437199.2016.1219672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SP, Wu JYW, Wang CS, Liu CH, Pan LH. Self concepts, health locus of control and cognitive functioning associated with health-promoting lifestyles in schizophrenia. Comprehensive Psychiatry. 2016;70:82–89. doi: 10.1016/j.comppsych.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohn T, Prud'homme D, Streiner D, Kameh H, Remington G. Characterizing coronary heart disease risk in chronic schizophrenia: High prevalence of the metabolic syndrome. The Canadian Journal of Psychiatry. 2004;49(11):753–760. doi: 10.1177/070674370404901106. [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack S, Egbuonu N, Edeoga C. Principles and practice of nonpharmacological interventions to reduce cardiometabolic risk. Medical Principles and Practice. 2010;19:167–175. doi: 10.1159/000285280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Schreurs V, Vancampfort D, Van Winkel R. Metabolic syndrome in people with schizophrenia: A review. World Psychiatry. 2009;8(1):15–22. doi: 10.1002/j.2051-5545.2009.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. European Heart Journal. 2007;28(7):850–856. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- Duncan J. A clinician's guide to understanding and treating metabolic syndrome. Waypoint Centre for Mental Health Care; 2011. [Google Scholar]
- Duncan, J. (2011b, Oct 11–16). Understanding and treating metabolic syndrome in clients with a serious mental illness 61st Annual Conference of the Canadian Psychiatric Association, Vancouver.
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R. Are stress eaters at risk for the metabolic syndrome? Annals of the New York Academy of Sciences. 2004;1032:208–210. doi: 10.1196/annals.1314.022. [DOI] [PubMed] [Google Scholar]
- Firth J, Rosenbaum S, Stubbs B, Gorczynski P, Yung AR, Vancampfort D. Motivating factors and barriers towards exercise in severe mental illness: A systematic review and meta-analysis. Psychological Medicine. 2016;46(14):2869–2881. doi: 10.1017/S0033291716001732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes Artiles R, Staub K, Aldakak L, Eppenberger P, Rühli F, Bender N. Mindful eating and common diet programs lower body weight similarly: Systematic review and meta-analysis. Obesity Reviews. 2019;20(11):1619–1627. doi: 10.1111/obr.12918. [DOI] [PubMed] [Google Scholar]
- George LK, Ellison CG, Larson DB. Explaining the relationships between religious involvement and health. Psychological Inquiry. 2002;13(3):190–200. doi: 10.1207/S15327965PLI1303_04. [DOI] [Google Scholar]
- Giugliano D, Ceriello A, Esposito K. Are there specific treatments for the metabolic syndrome? American Journal of Clinical Nutrition. 2008;87(1):8–11. doi: 10.1093/ajcn/87.1.8. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. Diagnosis and Management of the Metabolic Syndrome. Circulation. 2005;112(17):2735–2752. doi: 10.1161/circulationaha.105.169404. [DOI] [PubMed] [Google Scholar]
- Gurusamy J, Gandhi S, Damodharan D, Ganesan V, Palaniappan M. Exercise, diet and educational interventions for metabolic syndrome in persons with schizophrenia: A systematic review. Asian Journal of Psychiatry. 2018;36:73–85. doi: 10.1016/j.ajp.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Happell B, Platania-Phung C, Gaskin CJ, Stanton R. Use of an electronic metabolic monitoring form in a mental health service - A retrospective file audit. BMC Psychiatry. 2016;16(109):1–8. doi: 10.1186/s12888-016-0814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjemdahl P. Stress and the metabolic syndrome. An interesting but enigmatic association. Circulation. 2002;106(21):2634–2636. doi: 10.1161/01.CIR.0000041502.43564.79. [DOI] [PubMed] [Google Scholar]
- Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. The Lancet Psychiatry. 2017;4(4):295–301. doi: 10.1016/S2215-0366(17)30078-0. [DOI] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. The American Journal of Clinical Nutrition. 2004;79(3):379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- Khazaal Y, Fresard E, Rabia S, Chatton A, Rothen S, Pomini V, Grasset F, Borgeat F, Zullino D. Cognitive behavioural therapy for weight gain associated with antipsychotic drugs. Schizophrenia Research. 2007;91(1):169–177. doi: 10.1016/j.schres.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Khoury LR, Danielsen PL, Skiveren J. Body image altered by psoriasis. A study based on individual interviews and a model for body image. Journal of Dermatological Treatment. 2014;25(1):2–7. doi: 10.3109/09546634.2012.739278. [DOI] [PubMed] [Google Scholar]
- Koren D, Dumin M, Gozal D. Role of sleep quality in the metabolic syndrome. Diabetes, metabolic syndrome and obesity : targets and therapy. 2016;9:281–310. doi: 10.2147/DMSO.S95120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, Choi J-S, Bahk W-M, Kim CY, Kim CH, Shin YC, Park B-J, Oh CG. Weight management program for treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: A 12-week randomized controlled clinical trial. The Journal of Clinical Psychiatry. 2006;67(4):547–553. doi: 10.4088/JCP.v67n0405. [DOI] [PubMed] [Google Scholar]
- Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Current Opinion in Psychiatry. 2012;25(2):83–88. doi: 10.1097/YCO.0b013e32835035ca. [DOI] [PubMed] [Google Scholar]
- Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annual Review of Clinical Psychology. 2014;10(1):425–448. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- Lian Y, Yuan Q, Wang G, Tang F. Association between sleep quality and metabolic syndrome: A systematic review and meta-analysis. Psychiatry Research. 2019;274:66–74. doi: 10.1016/j.psychres.2019.01.096. [DOI] [PubMed] [Google Scholar]
- McCloughen A, Foster K. Weight gain associated with taking psychotropic medication: An integrative review. International Journal of Mental Health Nursing. 2011;20(3):202–222. doi: 10.1111/j.1447-0349.2010.00721.x. [DOI] [PubMed] [Google Scholar]
- Mujica V, Urzúa A, Leiva E, Díaz N, Moore-Carrasco R, Vásquez M, Rojas E, Icaza G, Toro C, Orrego R, Palomo I. Intervention with education and exercise reverses the metabolic syndrome in adults. Journal of the American Society of Hypertension. 2010;4(3):148–153. doi: 10.1016/j.jash.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Metabolic syndrome and mental illness. American Journal of Managed Care. 2007;13(7):S170. [PubMed] [Google Scholar]
- Nyboe L, Lund H. Low levels of physical activity in patients with severe mental illness. Nordic Journal of Psychiatry. 2013;67(1):43–46. doi: 10.3109/08039488.2012.675588. [DOI] [PubMed] [Google Scholar]
- O'Neill S, O'Driscoll L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obesity Reviews. 2015;16(1):1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- Ontario Ministry of Health. (2010). Celebrating Innovations in Health Care Expo 2010 Winners. Retrieved 22 March 2021 from https://news.ontario.ca/en/backgrounder/15035/celebrating-innovations-in-health-care-expo-2010-winners
- Ostman C, Smart NA, Morcos D, Duller A, Ridley W, Jewiss D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovascular Diabetology. 2017;16(1):110. doi: 10.1186/s12933-017-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanastasiou E. Interventions for the metabolic syndrome in schizophrenia: A review. Therapeutic Advances in Endocrinology and Metabolism. 2012;3(5):141–162. doi: 10.1177/2042018812458697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsavos C, Panagiotakos D, Weinem M, Stefanadis C. Diet, exercise and the metabolic syndrome. The review of diabetic studies : RDS. 2006;3(3):118–126. doi: 10.1900/RDS.2006.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LH, Shahabi L, Thoresen CE. Religion and spirituality. Linkages to physical health. American Psychologist. 2003;58(1):36–52. doi: 10.1037/0003-066x.58.1.36. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51(3):390–395. doi: 10.1037/0022-006X.51.3.390. [DOI] [PubMed] [Google Scholar]
- Rao DP, Dai S, Lagacé C, Krewski D. Metabolic syndrome and chronic disease. Chronic Diseases and Injuries in Canada. 2014;34(1):36–45. doi: 10.24095/hpcdp.34.1.06. [DOI] [PubMed] [Google Scholar]
- Rask Larsen J, Dima L, Correll CU, Manu P. The pharmacological management of metabolic syndrome. Expert Review of Clinical Pharmacology. 2018;11(4):397–410. doi: 10.1080/17512433.2018.1429910. [DOI] [PubMed] [Google Scholar]
- Roche AI, Kroska EB, Denburg NL. Acceptance- and mindfulness-based interventions for health behavior change: Systematic reviews and meta-analyses. Journal of Contextual Behavioral Science. 2019;13:74–93. doi: 10.1016/j.jcbs.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30(1):1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Ross K, Martin T, Chen E, Miller GE. Social encounters in daily life and 2-year changes in metabolic risk factors in young women. Development and Psychopathology. 2011;23(3):897–906. doi: 10.1017/S0954579411000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklayen MG. The global epidemic of the metabolic syndrome. Current Hypertension Reports. 2018;20(12):1–8. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection. Circulation. 2020;142(1):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- Schwarzer R. Modeling health behavior change: How to predict and modify the adoption and maintenance of health behaviors. Applied Psychology. 2008;57(1):1–29. doi: 10.1111/j.1464-0597.2007.00325.x. [DOI] [Google Scholar]
- Tamashiro KL, Sakai RR, Shively CA, Karatsoreos IN, Reagan LP. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14(5):468–474. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, Rosenbaum S, Correll CU. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: A systematic review and meta-analysis. World Psychiatry. 2015;14(3):339–347. doi: 10.1002/wps.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeland B, Minsky S, Menza M, Radler DR, Roemheld-Hamm B, Stern R. A program for managing weight gain associated with atypical antipsychotics. Psychiatric Services. 2003;54(8):1155–1157. doi: 10.1176/appi.ps.54.8.1155. [DOI] [PubMed] [Google Scholar]
- Wewege MA, Thom JM, Rye K-A, Parmenter BJ. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis. 2018;274:162–171. doi: 10.1016/j.atherosclerosis.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Journal of the American College of Cardiology. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Wing R, Shoemaker M, Marcus M, McDermott M, Gooding W. Variables associated with weight loss and improvements in glycaemic control in type 2 diabetic patients. Archives of Internal Medicine. 1990;147:1749–1753. doi: 10.1001/archinte.1987.00370100063012. [DOI] [Google Scholar]
- Yusuf S, Hawken S, Ôunpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Razak F, Sharma AM, Anand SS. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: A case-control study. The Lancet. 2005;366(9497):1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 125 kb)
(PDF 5.24 mb)
Data Availability Statement
The raw data of the present manuscript may not be shared publicly because the data set contains sensitive personal health information (e.g., psychiatric diagnosis) which - considering the relatively low number of participants and the very specific setting - could jeopardize participants’ privacy rights and are against the policies of the authors’ institution. Considering these, the authors have not requested permission from participants to share their data with other parties. However, the authors are happy to conduct further / alternative analyses and share the results of these along with the syntaxes with interested researchers.


