Abstract
Background:
Obesity is a significant public health concern and clear risk factor for complications following breast reconstruction. To date, few have assessed patient reported outcomes (PROs) focused on this key determinant. Our study aimed to investigate the impact of obesity (BMI ≤ 30) on post-operative satisfaction and physical function utilizing BREAST-Q in a cohort of autologous breast reconstruction patients.
Methods:
IRB-approved prospective investigation was conducted to evaluate PROs in patients undergoing autologous breast reconstruction from 2009 – 2017 at a tertiary academic medical center. The BREAST-Q reconstruction module was used to assess outcomes between cohorts at 6 months, 1 year, 2 years, and 3 years.
Results:
Overall, 404 patients underwent autologous breast reconstruction with abdominal free-tissue transfer (244 non-obese, 160 obese) and completed the BREAST-Q. Although obese patients demonstrated lower satisfaction with breast pre-operatively (p=0.04), no significant differences were noted post-operatively (p=0.58). However, physical well-being of the abdomen was lower in the obese cohort compared to their non-obese counterparts at long-term follow-up (3 years, p=0.04).
Conclusion:
Obesity significantly impacts autologous breast reconstruction patients. Although obese patients are more likely to present with dissatisfaction with breast pre-operatively, they exhibit comparable PROs overall compared to their non-obese counterparts, despite increased complications.
Keywords: Deep inferior epigastric perforator flap, DIEP, patient reported outcome measures, PROs, BREAST-Q
Introduction
Obesity is a significant global public health concern that now impacts over one-third of the United States population.1–3 This condition is itself a risk factor for postmenopausal breast cancer, particularly in patients who have not received hormone replacement therapy.4 As such, a significant portion of patients presenting for mastectomy and subsequent reconstruction are obese, with a recent meta-analysis suggesting a 28% prevalence in reconstruction patients.5 Women who are overweight or obese are often candidates for both implant-based reconstruction and autologous reconstruction. Breast reconstruction with abdominal free tissue transfer is the most common method of autologous reconstruction and, in most cases, serves as a cost effective way to obtain excellent aesthetic results.6–8 These characteristics have contributed to an increase in the number of patients who elect this form of reconstruction.9–11
Obesity has been widely reported as an independent risk factor perioperative complications, including mastectomy skin-flap necrosis, surgical site infection, and donor-site morbidity.12–21 As such, obesity has been considered by some to be a relative contraindication for abdominal-based autologous breast reconstruction.5 However, many surgeons consider this an ideal option in the obese and morbidly obese population, with acceptable rates of abdominal bulge or hernia and flap loss when compared to non-obese counterparts, suggesting that obese patients are suitable candidates for autologous breast reconstruction with abdominal free flaps given appropriate peri-operative counseling regarding their significant increase in risk.19,20,22
Despite the availability of studies investigating obesity-related surgical outcome, few have assessed patient-reported outcomes within an obese patient population. Nelson, et al. recently suggested that obesity may significantly impact long-term physical and mental health in autologous breast reconstruction patients. Their evaluation, however, was limited by use of the general Medical Outcomes Study 36-Item Short-Form Survey (SF-36), which is not validated for assessment of breast reconstruction outcome.23 Additionally, other studies examining the topic have utilized tools not condition-specific for breast reconstruction and have demonstrated few significant differences comparing obesity cohorts.24 The introduction of the BREAST-Q instrument has allowed for validated evaluation of patient satisfaction following breast reconstruction.25–31 The use of patient-centric parameters in addition to surgical outcome has afforded the most accurate determination of reconstructive efficacy to date. As such, it has become necessary to prospectively assess patient satisfaction following breast reconstruction in varied patient populations to elucidate risk factors for dissatisfaction or impaired quality of life, given the paucity of available patient-reported outcomes data.
The purpose of our study was to investigate the association between patient satisfaction and obesity to guide clinical decision-making and peri-operative counseling for autologous breast reconstruction. We hypothesize that patient satisfaction will not differ significantly between obese and non-obese groups following autologous breast reconstruction with abdominal free-tissue transfer.
Materials and Methods
Study Population
Institutional review board (IRB)-approved examination of patients undergoing abdominal free-flap autologous breast reconstruction from 2009 to 2017 was performed at Memorial Sloan Kettering Cancer Center. Patients who underwent autologous microsurgical breast reconstruction with deep inferior epigastric perforator (DIEP), superficial inferior epigastric artery (SIEA), free transverse rectus abdominis myocutaneous (TRAM) perforator, and muscle-sparing TRAM (msTRAM) were included. Importantly, surgical technique at our institution has been standardized across flap-type, without significant difference in suture type, Scarpa’s fascia, and dermal closure. The only difference in closure would likely be the inclusion of mesh or surgical adjunct in high-risk patients, which is decided on a case by case basis by the lead surgeon. Patients were administered the BREAST-Q patient-reported outcome instrument beginning in 2009 and prospectively completed the survey as part of routine clinical care. Patients with post-operative follow-up of less than 6 months were excluded.
Data Collection
All patients who underwent autologous breast reconstruction with abdominal free flaps prospectively completed the BREAST-Q at the following time points: pre-operatively, 6 months, 1 year, and annually thereafter. Patient responses were recorded on-site, either electronically or physically. All patients with post-operative follow-up > 6 months were identified and selected for additional review. Members of the research team performed chart reviews to capture demographic data, treatment method, and post-operative outcomes. We performed cross-sectional analysis of PRO data at the following intervals: pre-operatively, 1 year, 2 years, and 3 years. Patients with follow-up from 4 – 10 years were grouped by body obesity classification in > 3-year follow-up cohorts.
Independent Variables
Patient demographics included age at surgery, body mass index (BMI), laterality (unilateral v. bilateral), timing of reconstruction (immediate v. delayed), flap type (msTRAM v. perforator), mastectomy type (nipple-sparing v. skin-sparing), adjuvant chemotherapy, pre-operative breast irradiation, history of smoking, diabetes, hypertension, hyperlipidemia, number of contralateral balancing procedures for unilateral reconstruction, and Charlson comorbidity index (CCI) were recorded for all patients. Brassiere size was used as a surrogate for breast size, as determination of mastectomy specimen mass/volume is subject to significant variability based on technique and, therefore, remains difficult to standardize. Bra sizes were stratified into small (size A – C), medium (size D – DDD), and large (size > E).
Dependent Variables
The following breast complications were identified: total flap loss, partial flap loss, free-flap necrosis, fat necrosis, mastectomy skin-flap necrosis, hematoma, seroma, surgical site infection (major infection), cellulitis (minor infection), and delayed wound healing. We also identified donor-site complications, including delayed wound healing of the donor-site, wound dehiscence, donor-site infection, donor-site seroma, and abdominal hernia or bulge. BMI was calculated as mass/meters squared (kg/m2).
The reconstruction module of BREAST-Q was used to assess patient-reported outcomes, which includes the following subscales measured at different intervals of post-operative follow-up: (1) satisfaction with breast, (2) psychosocial well-being, (3) physical well-being of the chest and upper body, (4) physical well-being of the abdomen, (5) sexual well-being, and (6) satisfaction with outcome. The BREAST-Q is a validated questionnaire that allows for assessment of patient satisfaction and quality of life following breast reconstruction at various intervals of follow-up.32 Patient responses for BREAST-Q subscales were converted to summary scores that range from zero to 100 via Q-Score software. Higher values correlate with favorable outcome, including superior satisfaction, physical function, and quality of life.33
Statistical Analysis
Data were analyzed using SPSS 24 (IBM Corp., Armonk, NY). Statistical significance was defined as p < 0.05. Obese patients (BMI ≤ 30) were compared to non-obese patients (BMI < 30). Continuous variables were compared between obese and non-obese groups using non-parametric testing when appropriate, whereas categorical variables were analyzed via Fisher’s exact test. BREAST-Q scores between obesity and non-obese patients were compared using the Mann-Whitney test. Cross-sectional analysis of BREAST-Q scores between obese and non-obese groups was conducted at 6 months, 1 year, 2 years, and 3 years. Sub-group analysis was performed to stratify those patients within the obese cohort and compare against the non-obese group in accordance with the World Health Organization (WHO) classification of obesity: (1) class I, BMI 30–34.9; (2) class II, BMI 35–39.9; and (3) class III, BMI ≥ 40. Confounding variables were controlled for via linear regression modeling, including age at surgery, timing of reconstruction, flap type, laterality, history of smoking, pre-operative breast irradiation, neoadjuvant chemotherapy, and CCI. A penalized (Firth’s) logistic regression model was constructed to determine the relationship between obesity and post-operative complication by controlling for risk factors (age at surgery, timing of reconstruction, flap type, laterality, neoadjuvant chemotherapy, history of smoking, CCI, and pre-operative irradiation therapy).34
Power analysis was conducted to determine the sample size necessary to detect clinically meaningful difference of approximately 5 points. A two-tailed t-test for difference between independent means was conducted at a significance of 0.05.
Results
Patient Characteristics
Overall, 1036 patients underwent autologous breast reconstruction with abdominal free flaps during the study period, with 404 (39.0%) patients completing the BREAST-Q with follow-up of ≥ 6 months. One hundred and sixty patients (39.6%) were obese (BMI ≤ 30), whereas the remaining 244 (60.4%) were non-obese (BMI < 30). Patient cohort characteristics are presented in table 1. Mean patient age was 50.1 ± 7.9 years. No differences were noted in timing of reconstruction, pre-operative radiation status, diabetes, hypertension, hyperlipidemia, or CCI. Flap type did vary between groups, as obese patients were less likely to undergo perforator flap reconstruction (such as DIEP flap) (51.9% v. 61.9%, p = 0.03). Obese patients were more likely to present with history of smoking (p < 0.01) and larger pre-operative breast sizes (p < 0.03).
Table 1.
Clinical Characteristics
| Variable | Total (%) | Non-Obese (%) | Obese (%) | p |
|---|---|---|---|---|
| No. Cases | 404 | 244 | 160 | |
| Timing of Reconstruction | 0.29 | |||
| Immediate | 252 (62.4) | 149 (61.1) | 103 (64.4) | |
| Delayed | 152 (37.6) | 95 (38.9) | 57 (35.6) | |
| Flap Type | 0.03* | |||
| msTRAM | 170 (42.1) | 93 (38.1) | 77 (48.1) | |
| Perforator | 234(57.9) | 151 (61.9) | 83 (51.9) | |
| Mastectomy Type† | ||||
| Skin-Sparing | 218 (54.0) | 128 (52.5) | 90 (56.3) | 0.77 |
| Nipple-Sparing | 12 (3.0) | 8 (3.3) | 4 (2.5) | 0.48 |
| Pre-operative Breast Size†† | 0.03* | |||
| Small | 175 (43.3) | 136 (55.7) | 39 (24.4) | |
| Medium | 125 (30.9) | 57 (23.3) | 68 (42.5) | |
| Large | 14(3.5) | 5 (2.0) | 9 (5.6) | |
| Mean Age ± SD (yr) | 50.1 ± 7.9 | 50.7 ± 8.0 | 49.2 ± 7.6 | 0.06 |
| Laterality | 0.14 | |||
| Unilateral | 234 (57.9) | 147 (60.2) | 87 (54.4) | |
| Bilateral | 170 (42.1) | 97 (39.8) | 73 (45.6) | |
| Adjuvant Chemotherapy | 131 (32.4) | 93 (38.1) | 38 (23.8) | 0.02* |
| Pre-operative Radiation | 124(30.7) | 77 (31.6) | 47 (29.4) | 0.36 |
| History of Smoking | 45 (11.1) | 19 (7.8) | 26 (16.3) | 0.01* |
| Diabetes | 119 (29.5) | 65 (26.6) | 54 (33.8) | 0.08 |
| Hypertension | 61 (15.1) | 38 (15.6) | 23 (14.4) | 0.43 |
| Hyperlipidemia | 61 (15.1) | 41 (16.8) | 20 (12.5) | 0.15 |
| Contralateral Balancing Procedure | 96 (23.8) | 58 (23.8) | 38 (23.8) | 0.55 |
| Charlson Comorbidity Scale | 2.9 ± 1.2 | 2.9 ±1.1 | 2.9 ±1.3 | 0.62 |
Statistically significant (p < 0.05).
Mastectomy type data unavailable for 174 patients. Percentages are reflective of available patient data (n = 230).
Pre-operative breast size data unavailable for 90 patients. Percentages are reflective of available patient data (n = 314).
Patient-Reported Outcomes
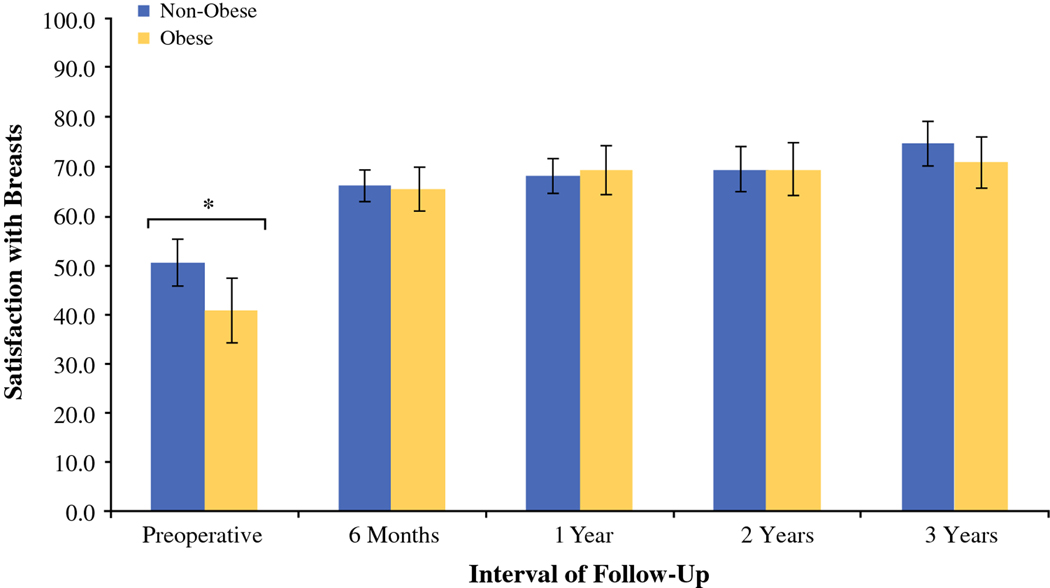
Pre-operative BREAST-Q scores were compared between obese and non-obese patient cohorts, with the obese group presenting with lower satisfaction with breast in the pre-operative period compared to non-obese patients (mean, 40.7 ± 24.0 v. 50.4 ± 22.2, respectively, p = 0.04) (Table 2). Physical well-being of the chest and upper body, physical well-being of the abdomen, and satisfaction with outcome, however, were comparable between groups.
Table 2.
BREAST-Q Scores by Obesity Status
| Pre-operative (n =142) |
Long-term follow-up (> 3 years) [n = 108] |
|||||
|---|---|---|---|---|---|---|
| BREAST-Q Dimensions |
Non-Obese ± SD | Obese ± SD | P | Non-Obese ± SD | Obese ± SD | P |
| No. Patients | 89 | 53 | 244 | 160 | ||
| Satisfaction with breasts | 50.4 ± 22.2 | 40.7 ± 24.0 | 0.04* | 72.9 ± 22.3 | 71.5 ± 19.6 | 0.58 |
| Physical well-being of the abdomen | 93.5 ± 12.7 | 91.8 ± 12.5 | 0.27 | 83.3 ± 19.1 | 83.0 ± 21.1 | 0.85 |
| Physical well-being of the chest and upper body | 72.7 ± 18.0 | 73.7 ± 116.5 | 0.81 | 79.0 ± 17.0 | 77.4 ± 12.7 | 0.36 |
| Satisfaction with outcome | - | - | - | 76.8 ± 21.1 | 77.0 ± 19.6 | 0.88 |
Statistically significant (p < 0.05).
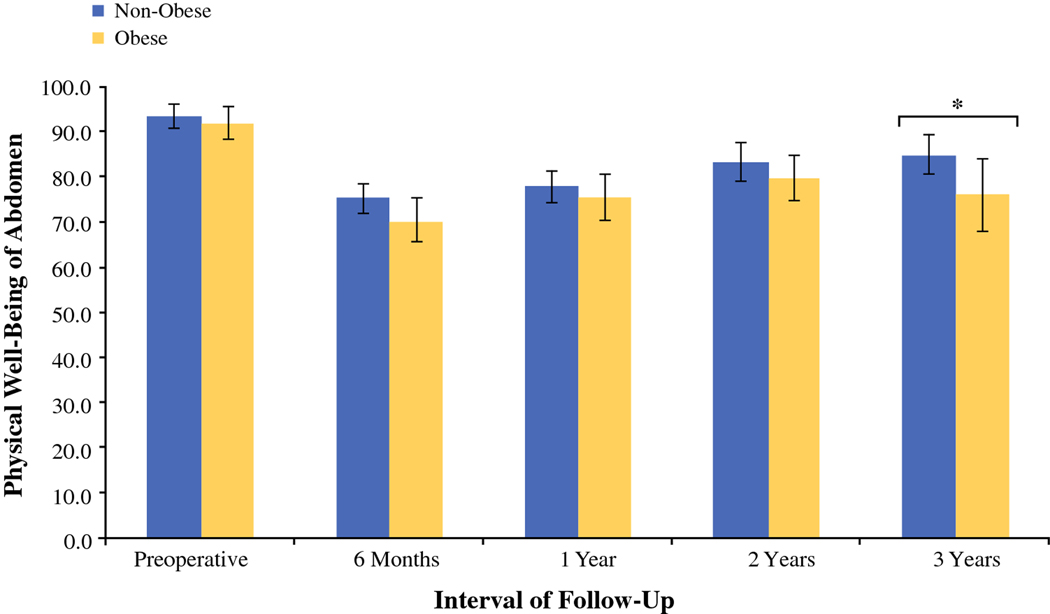
In examining long-term satisfaction with breast, no significant differences were noted between non-obese and obese cohorts with > 3-year follow-up (mean, 72.9 ± 22.3 v. 71.5 ± 19.6, respectively, p = 0.58) (Table 2). The remaining BREAST-Q subscales were comparable between non-obese and obese cohorts, including physical well-being of the abdomen (mean, 83.3 ± 19.1 v. 83.0 ± 21.1, respectively, p = 0.85), physical well-being of the chest and upper body (mean, 79.0 ± 17.0 v. 77.4 ± 12.7, respectively, p = 0.36), and satisfaction with outcome (mean, 76.8 ± 21.1 v. 77.0 ± 19.6, respectively, p = 0.88). BREAST-Q scores were stratified to allow for cross-sectional evaluation of patient-reported outcomes between obesity groups at the following intervals: 6 months, 1 year, 2 years, and 3 years post-operatively (Table 3). Physical well-being of the abdomen declined with obesity at > 3-year follow-up. No other significant differences were observed at post-operative time points. Multivariable linear regression confirmed obesity to be a risk factor for decreased pre-operative satisfaction (β = −9.63, p = 0.01) (Table 4).
Table 3.
Cross-sectional Analysis of BREAST-Q Scores at Annual Intervals, by Obesity Status
| 6 months (n = 195) |
1 year (n = 186) |
2 years (n = 147) |
3 years (n = 97) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BREAST-Q Dimensions | Non-Obese ± SD | Obese ± SD | p | Non-Obese ± SD | Obese ± SD | p | Non-Obese ± SD | Obese ± SD | p | Non-Obese ± SD | Obese ± SD | p |
| No. Patients | 120 | 75 | 117 | 69 | 83 | 64 | 64 | 33 | ||||
| Satisfaction with breasts | 66.0 ± 17.5 | 65.4 ± 19.2 | 0.78 | 68.1 ± 19.4 | 69.2 ± 20.9 | 0.56 | 69.3 ± 20.6 | 69.5 ± 21.6 | 0.86 | 74.5 ± 18.0 | 70.7 ± 14.7 | 0.35 |
| Physical well-being of the abdomen | 75.2 ± 18.9 | 70.4 ± 21.2 | 0.12 | 77.7 ± 19.5 | 75.6 ± 21.3 | 0.57 | 83.3 ± 19.1 | 79.7 ± 19.6 | 0.24 | 84.9 ± 17.3 | 75.9 ± 22.6 | 0.04* |
| Physical well-being of the chest and upper body | 72.6 ± 16.9 | 73.1 ± 20.3 | 0.73 | 73.3 ± 16.5 | 76.3 ± 18.7 | 0.24 | 73.5 ± 15.6 | 77.5 ± 17.6 | 0.09 | 78.6 ± 15.7 | 75.8 ± 13.3 | 0.16 |
| Satisfaction with outcome | 73.3 ± 20.2 | 75.3 ± 20.9 | 0.12 | 75.1 ± 21.4 | 78.0 ± 21.6 | 0.31 | 72.5 ± 22.2 | 79.4 ± 22.7 | 0.03* | 78.0 ± 18.7 | 75.9 ± 17.4 | 0.67 |
Statistically significant (p < 0.05).
Table 4.
Result of Multiple Linear Regression† Analysis of BREAST-Q Scores, by Obesity Status (Reference, Non-obese)
| 6 months |
1 year |
2 years |
3 years |
|||||
|---|---|---|---|---|---|---|---|---|
| BREAST-Q Dimensions | β | P | β | P | β | P | β | P |
| Satisfaction with breasts | −1.26 | 0.65 | 0.62 | 0.85 | 0.00 | 1.00 | −4.38 | 0.26 |
| Physical well-being of the abdomen | −4.92 | 0.11 | −1.79 | 0.57 | −1.63 | 0.62 | −7.64 | 0.08 |
| Physical well-being of the chest and upper body | −0.14 | 0.96 | 3.42 | 0.22 | 3.84 | 0.19 | −2.16 | 0.52 |
| Satisfaction with outcome | 0.85 | 0.79 | 2.27 | 0.50 | 6.39 | 0.10 | −3.28 | 0.43 |
Statistically significant (p<0.05).
Each model included the following covariates: age at surgery, timing of reconstruction (immediate v. delayed), flap type (msTRAM v. perforator), laterality (unilateral v. bilateral), neoadjuvant chemotherapy, history of smoking, Charlson comorbidity index, and history of pre-operative radiation.
The β coefficient refers to the predicted deviation in the dependent variable for each additional unit of independent variable.
We further examined outcomes according to WHO classification of obesity (Table 5). Pre-operative physical well-being of the abdomen was significantly lower in the class III obesity group when compared to other cohorts, a finding which was also mirrored at 3-year follow-up. We observed no significant difference in patient-reported outcomes among groups for satisfaction with breast, physical well-being of chest and upper body, physical well-being of the abdomen, and satisfaction with outcome subscales.
Table 5.
Cross-sectional Analysis of BREAST-Q Dimensions at Most Recent Follow-up, Stratified by WHO Obesity Classification
| BREAST-Q Dimensions | Normal (n = 86) | Overweight (n = 158) | Class I (n = 109) | Class II (n = 36) | Class III (n = 15) | p |
|---|---|---|---|---|---|---|
| Satisfaction with breast | 69.5 ± 19.3 | 67.3 ± 19.6 | 68.9 ± 20.5 | 70.7 ± 22.5 | 66.7 ± 15.3 | 0.70 |
| Physical well-being of abdomen | 81.0 ± 20.3 | 77.8 ± 19.7 | 77.1 ± 22.7 | 77.2 ± 19.0 | 69.7 ± 23.6 | 0.37 |
| Physical well-being of chest and upper body | 73.7 ± 16.5 | 73.7 ± 17.2 | 76.2 ± 18.4 | 75.6 ±20.4 | 67.4 ± 13.4 | 0.20 |
| Satisfaction with outcome | 76.5 ± 19.0 | 71.5 ± 21.3 | 78.0 ± 21.3 | 75.5 ± 23.3 | 71.1 ± 18.5 | 0.07 |
Statistically significant (p < 0.05).
Normal, BMI < 18.5–24.9; Overweight 25–29.9; Class I, BMI 30–34.9; Class II, BMI 35–39.9; Class III, BMI ≥ 40.
To evaluate longitudinal change in BREAST-Q scores, subgroup analysis was performed from pre-operative baseline. Importantly, longitudinal analysis was conducted only in those patients with successive pre-operative-to-1 year or pre-operative-to-2 year BREAST-Q scores. Our results suggest that satisfaction with breast increased significantly from baseline to 1 year (Table 6) and 2 years (Table 7) post-operatively; however, the rate of change did not vary significantly between obese and non-obese groups. We observed a similar trend with regard to physical well-being of the abdomen, with obese and non-obese patients presenting with decreased physical function at 1- and 2-year follow-up.
Table 6.
Longitudinal Analysis of Change in BREAST-Q Score after 1 Year Post-operatively
| Satisfaction with Breast |
Physical Well-Being of Abdomen |
Physical Well-Being of Chest and Upper Body |
||||
|---|---|---|---|---|---|---|
| Laterality | Δ | P | Δ | P | Δ | P |
| Non-Obese | + 19.4 | < 0.01* | −18.1 | <0.01* | +0.3 | 0.92 |
| Obese | +22.7 | < 0.01* | −15.6 | < 0.01* | −0.3 | 0.93 |
| P | 0.62 | 0.14 | 0.32 | |||
Statistically significant (p < 0.05).
The Δ statistic represents longitudinal variance in BREAST-Q score from pre-operative baseline.
Table 7.
Longitudinal Analysis of Change in BREAST-Q Score after 2 Years Post-operatively
| Satisfaction with Breast |
Physical Well-Being of Abdomen |
Physical Well-Being of Chest and Upper Body |
||||
|---|---|---|---|---|---|---|
| Laterality | Δ | P | Δ | P | Δ | P |
| Non-Obese | +23.7 | < 0.01* | −9.0 | 0.02* | +3.7 | 0.21 |
| Obese | +27.0 | < 0.01* | −7.7 | 0.06 | +4.4 | 0.19 |
| p | 0.57 | 0.84 | 0.83 | |||
Statistically significant (p < 0.05).
The Δ statistic represents longitudinal variance in BREAST-Q score from pre-operative baseline.
Post-Operative Complications
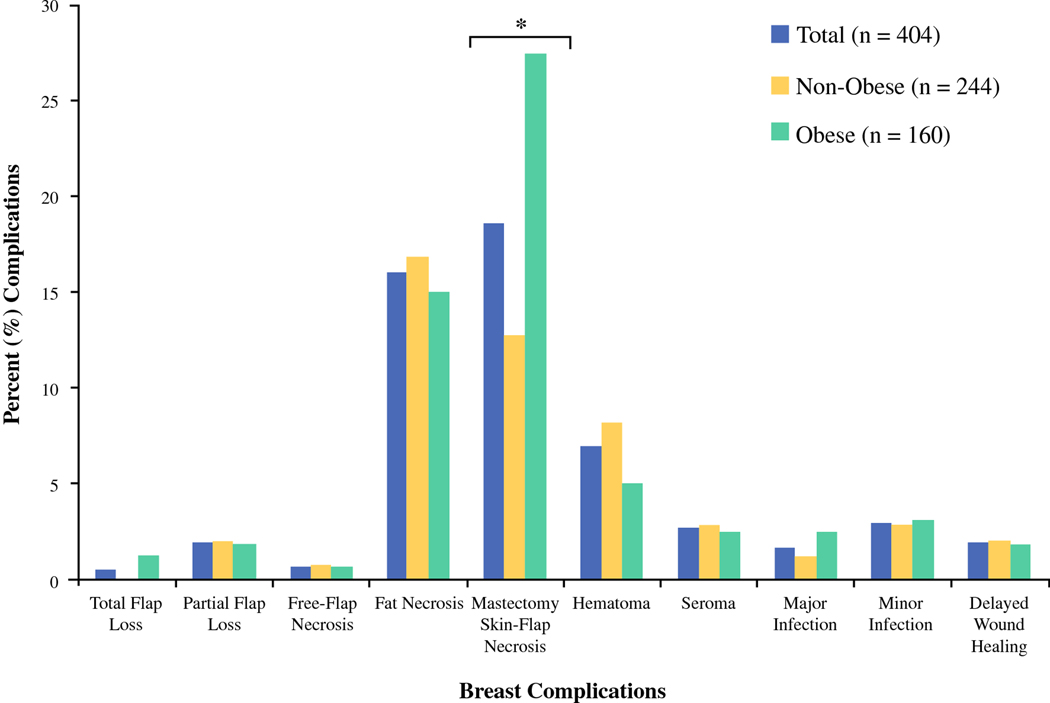
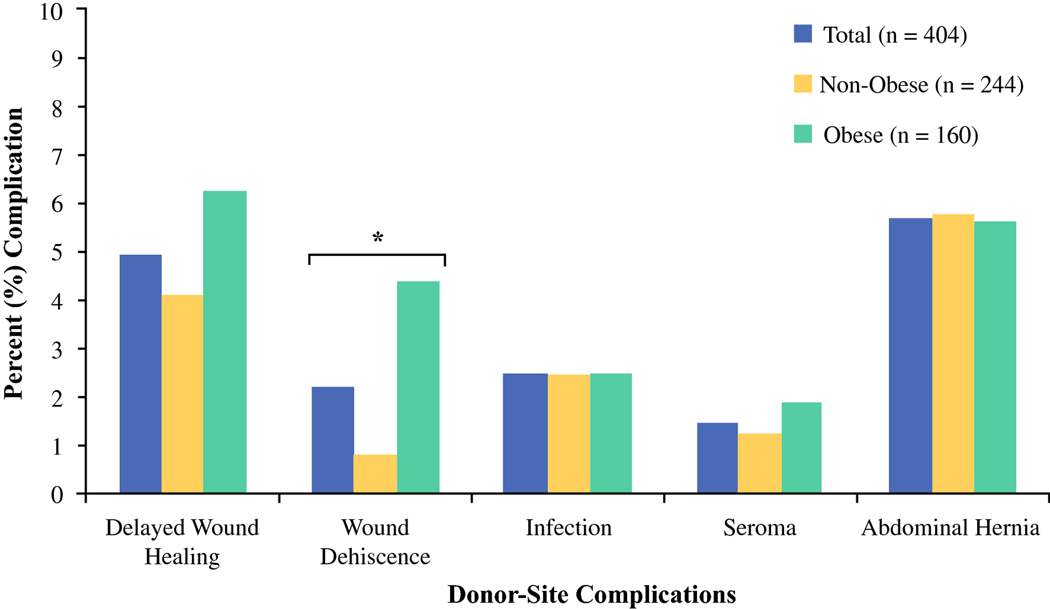
The most common breast complication overall was mastectomy skin-flap necrosis (n = 75 [18.6%]), followed by fat necrosis (n = 65 [16.1%]) and hematoma (n = 28 [6.9%]) (Table 8). We observed a greater rate of mastectomy skin-flap necrosis in obese patients when compared to non-obese patients (27.5% v. 12.7%, respectively, p < 0.01). No other significant differences in breast complications were observed between obesity groups. With regard to donor-site, abdominal hernia or bulge was most commonly observed (n = 23 [5.7%]), followed by delayed wound healing (n = 20 [4.9%]). Obese patients presented with greater rate of donor-site wound dehiscence when compared to their non-obese counterparts (4.4% v. 0.8%, respectively, p = 0.02). Logistic regression analysis (Table 9) demonstrated that obese patients were nearly 3-times more likely to develop mastectomy skin-flap necrosis and 5-times more likely to experience donor-site wound dehiscence. There were no other significant differences in outcomes between obese and non-obese groups.
Table 8.
Overview of Post-operative Complications
| Outcome | Total (%) n=404 | Non-Obese (%) n=244 | Obese (%) n=160 | P |
|---|---|---|---|---|
| Breast Complication | ||||
| Total Flap Loss | 2 (0.5) | 0 (0.0) | 2 (1.3) | 0.16 |
| Partial Flap Loss | 8 (2.0) | 5 (2.1) | 3 (1.9) | 0.60 |
| Free-Flap Necrosis | 3 (0.7) | 2 (0.8) | 1 (0.6) | 0.65 |
| Fat Necrosis | 65 (16.1) | 41 (16.8) | 24 (15.0) | 0.37 |
| Mastectomy Skin-Flap Necrosis | 75 (18.6) | 31 (12.7) | 44 (27.5) | < 0.01* |
| Hematoma | 28 (6.9) | 20 (8.2) | 8 (5.0) | 0.15 |
| Seroma | 11 (2.7) | 7 (2.9) | 4 (2.5) | 0.54 |
| Major Infection | 7 (1.7) | 3 (1.2) | 4 (2.5) | 0.28 |
| Minor Infection (Cellulitis) | 12 (3.0) | 7 (2.9) | 5 (3.1) | 0.55 |
| Delayed Wound Healing | 8 (2.0) | 5 (2.1) | 3 (1.9) | 0.60 |
| Donor-Site Complication | ||||
| Delayed Wound Healing | 20 (5.0) | 10 (4.1) | 10 (6.3) | 0.23 |
| Wound Dehiscence | 9 (2.2) | 2 (0.8) | 7 (4.4) | 0.02* |
| Infection | 10 (2.5) | 6 (2.5) | 4 (2.5) | 0.61 |
| Seroma | 6 (1.5) | 3 (1.2) | 3 (1.9) | 0.45 |
| Abdominal Hernia or Bulge | 23 (5.7) | 14 (5.7) | 9 (5.6) | 0.57 |
Statistically significant (p < 0.05).
Table 9.
Penalized Logistic Regression† Model for Odds of Post-operative Complication, by Obesity Status (Reference, Non-obese)
| Complication | OR (95 % CI) | P |
|---|---|---|
| Breast Complication | ||
| Total Flap Loss | 11.21 (0.58–2143.39) | 0.12 |
| Partial Flap Loss | 0.87 (0.20– 3.34) | 0.84 |
| Free-Flap Necrosis | 0.82 (0.05 – 7.79) | 0.87 |
| Fat Necrosis | 0.77 (0.43 – 1.35) | 0.36 |
| Mastectomy Skin-Flap Necrosis | 2.87 (1.65 – 5.06) | < 0.01* |
| Hematoma | 0.74 (0.31 – 1.67) | 0.48 |
| Seroma | 0.98 (0.27– 3.26) | 0.98 |
| Major Infection | 1.38 (0.31 – 6.61) | 0.67 |
| Minor Infection (Cellulitis) | 1.19 (0.35 – 3.83) | 0.77 |
| Delayed Wound Healing | 1.00 (0.23 – 3.87) | 0.99 |
| Donor-Site Complication | ||
| Delayed Wound Healing | 1.41 (0.55 – 3.61) | 0.47 |
| Wound Dehiscence | 5.53 (1.33 – 32.28) | 0.02* |
| Infection | 1.40 (0.36 – 5.06) | 0.61 |
| Seroma | 1.22 (0.25 – 5.94) | 0.80 |
| Abdominal Hernia or Bulge | 1.01 (0.41 – 2.36) | 0.99 |
OR, odds ratio; CI, confidence interval
Statistically significant (p < 0.05).
Each model included the following covariates: age at surgery, timing of reconstruction (immediate v. delayed), flap type (msTRAM v. perforator), laterality (unilateral v. bilateral), neoadjuvant chemotherapy, history of smoking, Charlson comorbidity index, and history of pre-operative radiation.
Discussion
Increased rates of complications in obese patients who undergo autologous breast reconstruction have been previously well characterized, including elevated rates of delayed wound healing of the donor-site, total flap loss, and mastectomy skin-flap necrosis.5,20,35–40 Yet a significant proportion of women presenting for breast reconstruction are obese, and desire reconstructive options. To date, a detailed understanding of patient reported outcomes focused on satisfaction and quality of life remains lacking within this potentially challenging and, at times, high-risk population patient population.
This study was performed to directly address this lack of understanding, with a focus on patient reported outcomes utilizing the BREAST-Q. Within, we demonstrate that postoperative patient satisfaction with reconstruction mirrors that of the non-obese patient population, but that obese individuals have significantly lower pre-operative satisfaction with their breasts. This study is also the first to closely examine physical well-being of the abdomen in obese patients in a prospective fashion, and demonstrates that, in the first two years postoperatively, no differences exist by obesity classification. However longer follow-up in obese patients suggests increased variation in physical well-being of the abdomen.
Several recent studies have added additional information to this topic, yet differ from our study methodology and focus. Kulkarni et al. demonstrated similar satisfaction scores between obese patients following autologous and prosthetic breast reconstruction, however did not specifically address obese autologous patients.41 The variability in reconstruction modality limits generalizability, as it is difficult to ascertain the changes in patient satisfaction that can be attributed to differences between autologous and prosthetic breast reconstruction. Similarly, Sinha et al. demonstrated comparable satisfaction scores between obese and non-obese patients following autologous reconstruction; however, their investigation was cross sectional with an average of 2-year follow-up, using a mailed BREAST-Q survey, which may limit the ability of the authors to draw long-term conclusions regarding patient satisfaction between obese and non-obese cohorts presenting for autologous breast reconstruction.42 Importantly, the authors submitted that generalizability may be limited by small sample size (n = 101), lack of pre-operative data, and failure to stratify by the WHO classification of obesity. The use of baseline data is necessary in a study evaluating patient-reported outcomes to control for pre-operative differences in satisfaction. The current work, therefore, contributes evidence to the available literature that evaluates patient satisfaction following autologous reconstruction in obese patient populations.
Physical well-being of the abdomen was similar in the early postoperative period, decreasing from pre-operative levels. Interestingly, variation existed late in the interval of observation, as the obese cohort demonstrated decreased physical function at 3 years post-operatively, following univariate analysis. Although this result only trended towards significance (p=0.08) on multivariate regression analysis, long-term physical well-being of the abdomen may potentially be impacted in the obese patient. It is possible that a difference in flap type could contribute to the trend towards significance late in the interval of observation. Obese patients were less likely to receive perforator flaps, and, as such, were more likely to sacrifice muscle at the donor site, which may compromise abdominal well-being. Nelson, et al recently examined long term abdominal function in abdominally based free tissue transfer, and performed a subgroup analysis on obese patients. Within that analysis, they noted long term differences in patient reported physical and mental health with the SF-36 with obese patients scoring significantly lower than non-obese patients. Interestingly, long-term cross sectional BREAST-Q physical well-being of abdomen scores did not differ, results that differ from our study. Sample size in our population or their study could certainly lead to such discrepancies – and care must be taken to understand that these long-term analyses are based on small samples of patients with potential for type 1 or 2 error. Physical well-being of the chest and upper body were comparable between groups following both univariate and multivariate analysis.
Our subgroup analysis revealed a net increase in satisfaction with breast from pre-operative baseline across cohorts, with no significant difference in rate of change after 1 or 2 years. We anticipate, however, that the change in patient satisfaction with breast for the obese population would achieve significance with a larger sample size. Conversely, physical function scores declined at post-operative intervals for both cohorts, without significant difference in rate of change between obese and non-obese groups. These results support our hypothesis that no significant difference exists in patient-reported outcomes between obesity groups when controlling for pre-operative satisfaction. Thus, although it is possible that obese patients have a less aesthetic outcome as assessed by surgeons, these patients are still highly satisfied with their reconstruction, and have significant improvement in satisfaction from their pre-operative state. Longer-term follow-up is necessary to elucidate longitudinal trends in satisfaction with breast and physical function of the abdomen for an obese patient population following autologous breast reconstruction with abdominal free flap.
Our study confirmed obesity to be an independent risk factor for mastectomy skin-flap necrosis and wound dehiscence of the donor-site. Previous investigations suggest that excess tissue along the lower pole of the breast mound in obese patients may impair perfusion and likely contribute to elevated rates of skin-flap necrosis.43 As such, use of intraoperative perfusion mapping may prove beneficial to guide patient selection for autologous reconstruction.44–46 With regard to donor-site wound dehiscence, obese patients in our study were more likely to present with history of smoking, which is an independent risk factor for impaired wound healing.47 Importantly, logistic regression, which controlled for potential confounding of smoking status, still indicated that obese patients experienced greater rates of donor-site wound dehiscence.
Although it has been speculated that harvesting flaps in obese patients may contribute to increased incidence of abdominal bulge or hernia, we did not observe significant difference between obesity groups. These results are consistent with those reported by Shaverien et al., who propose increased surveillance of the donor-site during surgery and similarity in abdominal wall strength between obese and non-obese patients to be possible explanations for comparable rates of abdominal hernia or bulge.48 There is certainly a trade-off between autologous reconstruction in obese patients and potential complications, keeping in mind improvement in breast outcomes. Such information must be included in pre-operative counseling of the obese patient, and must guide decision-making taking into account the true risk benefit profile.
The main limitations of this study include sample size and variability in patient participation in follow-up. This required both cross sectional and longitudinal analyses as a result. Our patient population was predominantly non-obese or presented with class I obesity, thereby limiting our ability to draw conclusions based on WHO classification due to small sample size. Additionally, we were unable to assess change in BMI in the post-operative period, which may provide insight regarding trends of post-operative patient satisfaction. However, in a recent study by Applebaum, et al., the authors concluded that patient weight does not change significantly over time following implant-based or autologous breast reconstruction.49 Evaluation occurred at a single institution, which could introduce potential selection bias. Additionally, variability may exist in surgeon operative technique and management of post-operative complications, which is difficult to control for through statistical manipulation.
The strength of this study is its long-term evaluation of patient-reported outcomes between non-obese and obese abdominal free-flap cohorts in a large patient population. We observed baseline satisfaction with breast to be greater in the non-obese cohort when compared to their obese counterparts. However, no significant difference in BREAST-Q dimensions was identified during the post-operative interval of observation, though physical function may decrease in obese patients in the long term. Prospective investigation with a larger patient population is warranted to overcome inherent limitations of our study. Meanwhile, these data can be utilized in pre-operative counseling, informed consent, and expectations management in obese patients considering abdominal free-flap autologous reconstruction.
Conclusion
Obesity significantly impacts the autologous breast reconstruction patient, both pre-operatively and post-operatively. Although obese patients are more likely to present with dissatisfaction with breast in the pre-operative period, they exhibit post-operative patient-reported outcomes comparable overall to their non-obese counterparts, even with increased incidence of complications.
Figure 1.
Cross-sectional analysis of BREAST-Q scores for satisfaction with breast between obesity groups throughout interval of observation (pre-operative to 3 years). Baseline satisfaction with breast was lower in obese patients scheduled to undergo autologous breast reconstruction with abdominal free flap. However, we were unable to identify differences in patient satisfaction at each post-operative time point, suggesting comparable percept of breast size, shape, and feel between obese and non-obese cohorts following reconstruction.
Figure 2.
Cross-sectional analysis of BREAST-Q scores for physical well-being of the abdomen between obesity groups at the following time intervals: pre-operative, 1 year, 2 years, and 3 years, post-operatively. We observed a significant difference in abdominal function at 3 years, suggesting that non-obese patients with autologous reconstruction report greater abdominal well-being when compared to obese patients in the long term.
Figure 3.
Percent of breast complications between obese and non-obese patient cohorts.
Figure 4.
Percent of donor-site complications between obese and non-obese patient cohorts.
Synopsis.
Obesity is a key determinant of satisfaction and physical function in patients presenting for autologous breast reconstruction to correct for mastectomy defect, with obese patients demonstrating decreased satisfaction in the pre-operative period. In the long-term, patient reported satisfaction in obese patients is comparable to their non-obese counterparts, despite increased incidence of post-operative complications, a finding which can be used to guide clinical decision making and counseling in this high-risk patient population.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflicts of Interest: Dr. Pusic is a co-developer of the BREAST-Q, which is owned by Memorial Sloan Kettering Cancer Center. She receives a portion of the licensing fees (royalty payments) when BREAST-Q is used in industry-sponsored clinical trials. Dr. Joseph Dayan is a consultant for Stryker, and owns shares in Atea Pharmaceutical. The remaining authors declare no conflicts of interest. Dr. Monica Morrow has received honoraria from Genomic Health and Roche.
Presented at:
Northeastern Society of Plastic Surgeons Annual Meeting, Boston, MA, October 26th, 2019.
American Society of Reconstructive Microsurgery Annual Meeting, Palm Desert, CA, February 5th, 2019.
American Association of Plastic Surgeons Annual Meeting, Baltimore, MD, April 9th, 2019.
Abbreviations
- PRO
patient reported outcomes
- BMI
body mass index
- SF-36
Medical Outcomes Study 36-Item Short-Form Survey
- IRB
institutional review board
- DIEP
deep inferior epigastric perforator
- SIEA
superficial inferior epigastric artery
- TRAM
transverse rectus abdominis myocutaneous perforator
- msTRAM
muscle-sparing TRAM
- CCI
Charlson comorbidity index
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Sljivic S, Gusenoff JA. The Obesity Epidemic and Bariatric Trends. Clin Plast Surg. 2019;46(1):1–7. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama. 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 3.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet (London, England). 2011;378(9793):804–814. [DOI] [PubMed] [Google Scholar]
- 4.Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer causes & control : CCC. 2002;13(8):741–751. [DOI] [PubMed] [Google Scholar]
- 5.Panayi AC, Agha RA, Sieber BA, Orgill DP. Impact of Obesity on Outcomes in Breast Reconstruction: A Systematic Review and Meta-Analysis. J Reconstr Microsurg. 2018;34(5):363–375. [DOI] [PubMed] [Google Scholar]
- 6.Kroll SS, Evans GR, Reece GP, et al. Comparison of resource costs of free and conventional TRAM flap breast reconstruction. Plast Reconstr Surg. 1996;98(1):74–77. [DOI] [PubMed] [Google Scholar]
- 7.Matros E, Albornoz CR, Razdan SN, et al. Cost-effectiveness analysis of implants versus autologous perforator flaps using the BREAST-Q. Plast Reconstr Surg. 2015;135(4):937–946. [DOI] [PubMed] [Google Scholar]
- 8.Sgarzani R, Negosanti L, Morselli PG, Vietti Michelina V, Lapalorcia LM, Cipriani R. Patient Satisfaction and Quality of Life in DIEAP Flap versus Implant Breast Reconstruction. Surg Res Pract. 2015;2015:405163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelissen AJM, Beugels J, van Kuijk SMJ, et al. Sensation of the autologous reconstructed breast improves quality of life: a pilot study. Breast Cancer Res Treat. 2018;167(3):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasari CR, Gunther S, Wisner DH, Cooke DT, Gold CK, Wong MS. Rise in microsurgical free-flap breast reconstruction in academic medical practices. Ann Plast Surg. 2015;74 Suppl 1:S62–65. [DOI] [PubMed] [Google Scholar]
- 11.Pesce CE, Liederbach E, Czechura T, Winchester DJ, Yao K. Changing surgical trends in young patients with early stage breast cancer, 2003 to 2010: a report from the National Cancer Data Base. J Am Coll Surg. 2014;219(1):19–28. [DOI] [PubMed] [Google Scholar]
- 12.Chang DW, Wang B, Robb GL, et al. Effect of obesity on flap and donor-site complications in free transverse rectus abdominis myocutaneous flap breast reconstruction. Plast Reconstr Surg. 2000;105(5):1640–1648. [DOI] [PubMed] [Google Scholar]
- 13.Fischer JP, Cleveland EC, Nelson JA, et al. Breast reconstruction in the morbidly obese patient: assessment of 30-day complications using the 2005 to 2010 National Surgical Quality Improvement Program data sets. Plast Reconstr Surg. 2013;132(4):750–761. [DOI] [PubMed] [Google Scholar]
- 14.Fischer JP, Wes AM, Kanchwala S, Kovach SJ. Effect of BMI on modality-specific outcomes in immediate breast reconstruction (IBR)--a propensity-matched analysis using the 2005–2011 ACS-NSQIP datasets. J Plast Surg Hand Surg. 2014;48(5):297–304. [DOI] [PubMed] [Google Scholar]
- 15.Gfrerer L, Mattos D, Mastroianni M, et al. Assessment of patient factors, surgeons, and surgeon teams in immediate implant-based breast reconstruction outcomes. Plast Reconstr Surg. 2015;135(2):245e–252e. [DOI] [PubMed] [Google Scholar]
- 16.Israeli R. Complications of acellular dermal matrices in breast surgery. Plast Reconstr Surg. 2012;130(5 Suppl 2):159S–172S. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen KT, Hanwright PJ, Smetona JT, Hirsch EM, Seth AK, Kim JY. Body mass index as a continuous predictor of outcomes after expander-implant breast reconstruction. Ann Plast Surg. 2014;73(1):19–24. [DOI] [PubMed] [Google Scholar]
- 18.Fischer JP, Nelson JA, Kovach SJ, Serletti JM, Wu LC, Kanchwala S. Impact of obesity on outcomes in breast reconstruction: analysis of 15,937 patients from the ACS-NSQIP datasets. J Am Coll Surg. 2013;217(4):656–664. [DOI] [PubMed] [Google Scholar]
- 19.Fischer JP, Nelson JA, Sieber B, et al. Free tissue transfer in the obese patient: an outcome and cost analysis in 1258 consecutive abdominally based reconstructions. Plast Reconstr Surg. 2013;131(5):681e–692e. [DOI] [PubMed] [Google Scholar]
- 20.Jandali S, Nelson JA, Sonnad SS, et al. Breast reconstruction with free tissue transfer from the abdomen in the morbidly obese. Plast Reconstr Surg. 2011;127(6):2206–2213. [DOI] [PubMed] [Google Scholar]
- 21.Nelson JA, Chung CU, Fischer JP, Kanchwala SK, Serletti JM, Wu LC. Wound healing complications after autologous breast reconstruction: a model to predict risk. J Plast Reconstr Aesthet Surg. 2015;68(4):531–539. [DOI] [PubMed] [Google Scholar]
- 22.Chang EI, Liu J. Prospective evaluation of obese patients undergoing autologous abdominal free flap breast reconstruction. Plast Reconstr Surg. 2018. https://www.ncbi.nlm.nih.gov/pubmed/29794640. [DOI] [PubMed] [Google Scholar]
- 23.Nelson JA, Tecci MG, Lanni MA, et al. Function and Strength after Free Abdominally Based Breast Reconstruction: A 10-year follow-up. Plast Reconstr Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson JA, Fischer JP, Yan C, et al. The impact of obesity on abdominal wall function after free autologous breast reconstruction. Microsurgery. 2014;34(5):352–360. [DOI] [PubMed] [Google Scholar]
- 25.Cano SJ, Klassen A, Pusic AL. The science behind quality-of-life measurement: a primer for plastic surgeons. Plast Reconstr Surg. 2009;123(3):98e–106e. [DOI] [PubMed] [Google Scholar]
- 26.Cohen WA, Mundy LR, Ballard TN, et al. The BREAST-Q in surgical research: A review of the literature 2009–2015. J Plast Reconstr Aesthet Surg. 2016;69(2):149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C, Sunu C, Pignone M. Patient-reported outcomes of breast reconstruction after mastectomy: a systematic review. J Am Coll Surg. 2009;209(1):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. [DOI] [PubMed] [Google Scholar]
- 29.Pusic AL, Lemaine V, Klassen AF, Scott AM, Cano SJ. Patient-reported outcome measures in plastic surgery: use and interpretation in evidence-based medicine. Plast Reconstr Surg. 2011;127(3):1361–1367. [DOI] [PubMed] [Google Scholar]
- 30.Pusic AL, Matros E, Fine N, et al. Patient-Reported Outcomes 1 Year After Immediate Breast Reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium Study. J Clin Oncol. 2017;35(22):2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugrue R, MacGregor G, Sugrue M, Curran S, Murphy L. An evaluation of patient reported outcomes following breast reconstruction utilizing Breast Q. Breast. 2013;22(2):158–161. [DOI] [PubMed] [Google Scholar]
- 32.Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL. The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg. 2012;129(2):293–302. [DOI] [PubMed] [Google Scholar]
- 33.Macadam SA, Zhong T, Weichman K, et al. Quality of Life and Patient-Reported Outcomes in Breast Cancer Survivors: A Multicenter Comparison of Four Abdominally Based Autologous Reconstruction Methods. Plast Reconstr Surg. 2016;137(3):758–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X. Firth logistic regression for rare variant association tests. Front Genet. 2014;5:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109(7):2265–2274. [DOI] [PubMed] [Google Scholar]
- 36.Chen CL, Shore AD, Johns R, Clark JM, Manahan M, Makary MA. The impact of obesity on breast surgery complications. Plast Reconstr Surg. 2011;128(5):395e–402e. [DOI] [PubMed] [Google Scholar]
- 37.Garvey PB, Buchel EW, Pockaj BA, Gray RJ, Samson TD. The deep inferior epigastric perforator flap for breast reconstruction in overweight and obese patients. Plast Reconstr Surg. 2005;115(2):447–457. [DOI] [PubMed] [Google Scholar]
- 38.Moran SL, Serletti JM. Outcome comparison between free and pedicled TRAM flap breast reconstruction in the obese patient. Plast Reconstr Surg. 2001;108(7):1954–1960; discussion 1961–1952. [DOI] [PubMed] [Google Scholar]
- 39.Spear SL, Ducic I, Cuoco F, Taylor N. Effect of obesity on flap and donor-site complications in pedicled TRAM flap breast reconstruction. Plast Reconstr Surg. 2007;119(3):788–795. [DOI] [PubMed] [Google Scholar]
- 40.Vyas RM, Dickinson BP, Fastekjian JH, Watson JP, Dalio AL, Crisera CA. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg. 2008;121(5):1519–1526. [DOI] [PubMed] [Google Scholar]
- 41.Kulkarni AR, Katz S, Hamilton AS, Graff JJ, Alderman AK. Patterns of use and patient satisfaction with breast reconstruction among obese patients: results from a population-based study. Plast Reconstr Surg. 2012;130(2):263–270. [DOI] [PubMed] [Google Scholar]
- 42.Sinha S, Ruskin O, D’Angelo A, McCombe D, Morrison WA, Webb A. Are overweight and obese patients who receive autologous free-flap breast reconstruction satisfied with their postoperative outcome? A single-centre study. J Plast Reconstr Aesthet Surg. 2016;69(1):30–36. [DOI] [PubMed] [Google Scholar]
- 43.Robertson SA, Jeevaratnam JA, Agrawal A, Cutress RI. Mastectomy skin flap necrosis: challenges and solutions. Breast Cancer (Dove Med Press). 2017;9:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg. 2012;129(5):778e–788e. [DOI] [PubMed] [Google Scholar]
- 45.Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg. 2010;125(4):1065–1073. [DOI] [PubMed] [Google Scholar]
- 46.Jeon FHK, Varghese J, Griffin M, Butler PE, Ghosh D, Mosahebi A. Systematic review of methodologies used to assess mastectomy flap viability. BJS Open. 2018;2(4):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDaniel JC, Browning KK. Smoking, chronic wound healing, and implications for evidence-based practice. J Wound Ostomy Continence Nurs. 2014;41(5):415–423; quiz E411–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaverien MV, McCulley SJ. Effect of obesity on outcomes of free autologous breast reconstruction: a meta-analysis. Microsurgery. 2014;34(6):484–497. [DOI] [PubMed] [Google Scholar]
- 49.Applebaum MA, Miller BT, Lopez J, Doren EL, Laronga C, Smith PD. Change in Body Mass Index After Breast Reconstruction and Associated Complications. Eplasty. 2015;15:e43. [PMC free article] [PubMed] [Google Scholar]