Abstract
Introduction:
Despite the rise in rate of contralateral prophylactic mastectomy (CPM), few studies have utilized patient-reported outcomes to assess satisfaction between unilateral and bilateral breast reconstruction using autologous tissue. The current study aims to investigate patient satisfaction and quality of life following autologous reconstruction to determine if differences exist between unilateral and bilateral reconstructions to better guide clinical decision-making.
Methods:
The current study examined prospectively collected BREAST-Q results following abdominal free flap breast reconstruction procedures performed at a tertiary academic medical center from 2009 – 2017. The reconstruction module of the BREAST-Q was used to assess outcomes between laterality groups (unilateral versus bilateral) at 1 year, 2 year, 3 years, and > 3 years.
Results:
Overall, 405 patients who underwent autologous breast reconstruction completed the BREAST-Q. Cross-sectional analysis at 1 year, 2 years, and 3 years revealed similar satisfaction scores between groups; however, bilateral reconstruction patients demonstrated higher satisfaction scores at >3 years (p=0.04). Bilateral reconstruction patients reported lower scores of abdominal well-being at 1 year, 2 years, and > 3 years (p=0.01, p=0.03, and p=0.01, respectively).
Conclusion:
These results suggest that satisfaction with breasts does not differ with the laterality of the autologous reconstruction up to three years post-operatively, but may diverge thereafter. Bilateral reconstruction patients, however, have lower satisfaction with the abdominal donor site. These data can be utilized in preoperative counseling, informed consent, and expectations management in patients considering CPM.
Keywords: Contralateral prophylactic mastectomy, DIEP, patient-reported outcome measures, PROs, BREAST-Q, laterality
Introduction
Rates of contralateral prophylactic mastectomy (CPM) continue to rise in the setting of unilateral breast cancer.1–9 There is, however, little evidence to suggest that CPM improves patient outcome or long-term survival.10,11 As such, recent studies have focused on patient decision-making to better elucidate underlying reasons for the increase in CPM rates.12–16 A number of reports demonstrate psychological motivators to be primary drivers of patient decision to undergo CPM, including fear of second primary within the contralateral breast despite nearly comparable risk when compared to the general population.17–20 In addition, advances in surgical technique have conferred improvement in aesthetic result and breast symmetry, which may further motivate patients to elect for removal of the contralateral breast.21
The recent trend toward CPM has been met with a concurrent rise in rate of breast reconstruction, despite improvements in breast-conserving therapy.22 Prosthesis-based breast reconstruction remains a central driver toward increased utilization of CPM for unilateral breast cancer, given simplicity in technique and reliability in conferring anatomic symmetry during bilateral procedures. Autologous breast reconstruction, however, is widely considered to be the superior technique, with purported improvement in cost-effectiveness and post-operative safety profile.23–25 Additionally, data suggests that native tissue better models the aesthetic of the natural breast when compared to prosthetic devices in the long-term, thereby improving patient quality of life.26 Numerous studies utilizing the BREAST-Q patient-reported outcome (PRO) instrument have demonstrated that the use of autologous free tissue transfer leads to higher rates of patient satisfaction both in the short- and long-term.27–29 It is, therefore, unsurprising that overall rates of autologous breast reconstruction continue to rise, especially within academic medical centers.30 This rate, however, has not kept pace with alloplastic techniques.31
Importantly, breast reconstruction has been cited as an independent predictor of patient decision to undergo CPM.32 As such, patient desire for breast symmetry is likely a motivating factor for the growing prevalence of bilateral mastectomy.33 However, there exists a gap in knowledge with regard to patient satisfaction following bilateral breast reconstruction in the setting of a unilateral cancer and CPM. In fact, the majority of studies that investigate patient satisfaction between unilateral and bilateral breast reconstruction are limited by short-term follow-up or variability in reconstruction technique.34 Evaluation of the association between patient satisfaction and laterality (unilateral versus bilateral) is necessary to guide clinical decision-making, in an attempt to better reconcile the conception of improved aesthetic result with decision to undergo CPM. To date, post-operative outcomes following autologous breast reconstruction have been extensively studied.35–39 However, few studies have evaluated outcomes, such as patient satisfaction, donor-site well-being, and physical function, from the patient perspective, especially with regard to reconstruction laterality. This study aims to compare PROs using the BREAST-Q as well as post-operative complication rates between cohorts having undergone either unilateral or bilateral autologous breast reconstruction with abdominal free flaps. We hypothesize that patient satisfaction does not differ significantly between these groups.
Methods
Study Population and Data Collection
An IRB approved cohort study was performed utilizing a prospectively maintained database to identify consecutive patients who underwent autologous breast reconstruction with abdominal free flaps between January 2007-July 2017 at a tertiary academic medical center. Patients with immediate or delayed breast reconstruction using abdominal free flaps were included in the study. Exclusion criteria consisted of patients with post-operative follow-up <1 year. A retrospective chart review captured demographic data, treatment method, and post-operative outcomes.
Independent Variables
Variables recorded for each patient included: age, body mass index (BMI), obesity, history of smoking, pre-operative breast irradiation, neoadjuvant chemotherapy, diabetes, hypertension, hyperlipidemia, Charlson Comorbidity Index (CCI), reconstructive timing, and flap type. Reconstructive timing was defined as either immediate or delayed breast reconstruction. Flap type was defined by the extent of rectus muscle sacrifice or perforator only dissection. BMI was calculated as mass/meters squared ().
Dependent Variables
Breast-specific endpoints of interest included: total flap loss (requiring reoperation and total flap removal), partial flap loss (requiring partial flap surgical debridement), fat necrosis (palpable area of firm tissue within reconstructed breast >1 cm in diameter noted on physical exam or follow-up clinic note), mastectomy skin flap necrosis, hematoma, seroma, surgical site infection (major infection), cellulitis (minor infection), and delayed wound healing. The following post-operative complications of the abdominal donor-site were identified: delayed wound healing (a wound requiring dressing changes beyond 1 month postoperatively), wound dehiscence, donor-site infection, donor-site seroma, and abdominal hernia or bulge.
BREAST-Q Questionnaire
PROs were assessed via the reconstruction module of the BREAST-Q. For patients with follow-up >3 years, the most recent BREAST-Q entry on record was captured and used for analysis. Values for BREAST-Q subscales were converted to summary scores that range from zero to 100 via Q-Score software.40 The primary endpoint of interest was satisfaction with breast. Secondary endpoints included physical well-being of the chest and upper body, physical well-being of the abdomen, and satisfaction with outcome.
Statistical Analysis
Data were analyzed using SPSS (IBM Corp., Armonk, NY). A two-sided two-sample means test was conducted at a significance of 0.05 to determine this study’s ability to detect a difference of 5 – 10 points on the BREAST-Q between laterality cohorts, demonstrating a post hoc statistical power of 99%. A difference of 4 points is now understood to be clinically meaningful on the BREAST-Q satisfaction scale.41
Univariate analysis was conducted to compare patient characteristics between laterality groups (unilateral versus bilateral cohorts). Fisher’s exact test was used for categorical variables. Shapiro-Wilk test was used to evaluate for normality among continuous variables. Those variables that were non-normally distributed were analyzed via Mann-Whitney test. The remaining continuous variables were compared using independent samples t-test. BREAST-Q questionnaire scores between laterality groups were compared using non-parametric testing.
Cross-sectional analysis of BREAST-Q data was performed at the following intervals: pre-operatively, 1 year, 2 years, and 3 years. Patients with follow-up between 4–10 years were grouped in >3 year cohorts, by laterality.
Linear regression modeling was conducted to control for confounders such as age, reconstruction timing, flap type, BMI, history of pre-operative breast irradiation, and hypertension, as well as to identify independent variables associated with patient satisfaction. Unadjusted logistic regression was used to analyze outcome data by patient. A penalized (Firth) logistic regression model was constructed to determine the relationship between laterality and post-operative complication by controlling for confounding variables (age, BMI, flap type, pre-operative radiation treatment, history of smoking, diabetes, and reconstruction timing). Only those covariates that are known predictors of complication or were unmatched between patient groups were included in the regression models.42 For each outcome, an odds ratio, 95% confidence interval, and p-value were calculated. Statistical significance was defined as p<0.05.
Results
Patient Characteristics
Overall, 1036 patients underwent autologous breast reconstruction with abdominal free flaps during the study period. Four hundred and five of these patients completed the BREAST-Q with >6 month follow up and were included in the final analysis. Two hundred and thirty-four patients (57.8%) underwent unilateral autologous breast reconstruction, whereas the remaining 171 (42.2%) underwent bilateral reconstruction. Table 1 presents patient characteristics by laterality group. Timing of reconstruction varied between groups, as bilateral cases were more likely to be immediate when compared to unilateral procedures (71.3% v. 56.0%, respectively, p<0.01). Clinical variables were well-matched between cohorts. However, bilateral patients were slightly younger compared to unilateral patients (48.0±8.0 years v. 51.0±8.0, respectively, p<0.01). Additionally, pre-operative radiation therapy and elevated CCI score were more commonly observed in the unilateral group. Of the 234 patients who underwent unilateral breast reconstruction, 96 (41%) received balancing procedures to the contralateral breast, the majority of which occurred after breast reconstruction, with relatively few performed simultaneously with the index operation. Satisfaction with breast was comparable between patients with and without contralateral balancing procedures.
Table 1.
Clinical Characteristics.
| Variable | Total (%) | Unilateral (%) | Bilateral (%) | p |
|---|---|---|---|---|
|
| ||||
| No. Cases | 405 | 234 | 171 | |
| Timing of Reconstruction | < 0.01* | |||
| Immediate | 253 (62.5) | 131 (56.0) | 122 (71.3) | |
| Delayed | 152 (37.5) | 103 (44.0) | 49 (28.7) | |
| Flap Type | 0.48 | |||
| msfTRAM/TRAM | 171 (42.2) | 98 (41.9) | 73 (42.7) | |
| perforator flap | 234 (57.8) | 136 (58.1) | 98 (57.3) | |
| Mean Age ± SD (yr) | 50.1 ± 7.9 | 51.0 ± 8.0 | 48.0 ± 8.0 | < 0.01* |
| Mean BMI ± SD (kg/m2) | 28.9 ± 5.1 | 28.8 ± 4.9 | 29.8 ± 5.0 | 0.12 |
| No. Obese† | 160 (39.5) | 87 (37.2) | 73 (42.7) | 0.15 |
| Adjuvant Chemotherapy | 131 (32.3) | 81 (34.6) | 50 (29.2) | 0.16 |
| Pre-operative Radiation | 124 (30.6) | 81 (34.6) | 43 (25.1) | 0.03* |
| History of Smoking | 45 (11.1) | 31 (13.2) | 14 (8.2) | 0.08 |
| Diabetes | 119 (29.4) | 73 (31.2) | 46 (26.9) | 0.22 |
| Hypertension | 61 (15.1) | 37 (15.8) | 24 (14.0) | 0.37 |
| Hyperlipidemia | 61 (15.1) | 35 (15.0) | 26 (15.2) | 0.52 |
| Charlson Comorbidity Scale | 2.9 ± 1.2 | 3.1 ± 1.1 | 2.6 ± 1.3 | < 0.01* |
BMI, body mass index
Statistically significant (p < 0.05).
BMI > 30.
Post-Operative Complications
Mastectomy skin flap necrosis was the most common breast-specific post-operative complication [n=75(18.52%)], followed by fat necrosis [n=65(16.05%)] and hematoma [n=28(6.91%)] (Table 2). We observed no differences in breast complication rates between laterality groups on univariate analysis, although regression analysis suggested a difference in mastectomy skin flap necrosis, with greater likelihood in the unilateral cohort (OR, 0.55; 95% CI, 0.30–0.98, p=0.04). With regard to donor site morbidity, abdominal hernia or bulge was most commonly observed [n=23(5.68%)], followed by delayed wound healing [n=20(4.94%)] and donor-site infection (n=10(2.47%)]. Bilateral reconstruction patients were more likely to experience delayed wound healing of the abdominal donor-site when compared to unilateral reconstructions (8.77% v. 2.14%, p<0.01). Additionally, bilateral reconstructions were nearly 5 times more likely to result donor-site infection (n=7(4.1%) v. n=3(1.3%), OR, 5.20; 95%CI, 1.38–24.30; p=0.02)(Table 3).
Table 2.
Overview of post-operative complications.
| Outcome | Total (%) | Unilateral (%) | Bilateral (%) | p |
|---|---|---|---|---|
|
| ||||
| Breast Complication | ||||
| Total Flap Loss | 2 (0.5) | 0 (0.0) | 2 (1.8) | 0.18 |
| Partial Flap Loss | 8 (2.0) | 6 (2.6) | 2 (1.8) | 0.27 |
| Fat Necrosis | 65 (16.1) | 39 (16.7) | 26 (15.2) | 0.40 |
| Mastectomy Skin-Flap Necrosis | 75 (18.5) | 47 (20.1) | 28 (16.4) | 0.21 |
| Hematoma | 28 (6.9) | 18 (7.7) | 10 (5.9) | 0.30 |
| Seroma | 11 (2.7) | 5 (2.1) | 6 (3.5) | 0.29 |
| Major Infection | 7 (1.7) | 3 (1.3) | 4 (2.3) | 0.33 |
| Minor Infection (Cellulitis) | 12 (3.0) | 6 (2.6) | 6 (3.5) | 0.39 |
| Delayed Wound Healing | 8 (2.0) | 5 (2.1) | 3 (1.8) | 0.33 |
| Donor-Site Complication | ||||
| Delayed Wound Healing | 20 (4.9) | 5 (2.1) | 15 (8.8) | < 0.01* |
| Wound Dehiscence | 9 (2.2) | 3 (1.3) | 6 (3.5) | 0.12 |
| Infection | 10 (2.5) | 3 (1.3) | 7 (4.1) | 0.07 |
| Seroma | 6 (1.5) | 3 (1.3) | 3 (1.8) | 0.50 |
| Abdominal Hernia or Bulge | 23 (5.7) | 12 (5.1) | 11 (6.4) | 0.36 |
Statistically significant (p < 0.05).
Table 3.
Penalized logistic regression† model for odds of post-operative complication, by laterality (reference, unilateral).
| Complication | OR (95% CI) | p |
|---|---|---|
|
| ||
| Breast Complication | ||
| Total Flap Loss | 9.54 (0.53–1655.08) | 0.14 |
| Partial Flap Loss | 0.52 (0.09–2.21) | 0.39 |
| Free Flap Necrosis | 0.90 (0.07–7.96) | 0.92 |
| Fat Necrosis | 0.98 (0.56–1.71) | 0.95 |
| Mastectomy Skin Flap Necrosis | 0.55 (0.30–0.98) | 0.04* |
| Hematoma | 0.74 (0.32–1.62) | 0.45 |
| Seroma | 1.78 (0.53–6.22) | 0.35 |
| Major Infection | 1.84 (0.42–8.51) | 0.41 |
| Minor Infection (Cellulitis) | 1.58 (0.50–5.06) | 0.43 |
| Delayed Wound Healing | 0.56 (0.12–2.25) | 0.42 |
| Donor-Site Complication | ||
| Delayed Wound Healing | 4.19 (1.57–12.90) | < 0.01* |
| Wound Dehiscence | 3.43 (0.88–15.77) | 0.08 |
| Infection | 5.20 (1.38–24.30) | 0.02* |
| Seroma | 1.43 (0.27–7.44) | 0.66 |
| Abdominal Hernia or Bulge | 1.20 (0.51–2.82) | 0.67 |
OR, odds ratio; CI, confidence interval
Statistically significant (p < 0.05).
Each model included the following covariates: age at surgery, body mass index, timing of reconstruction (immediate v. delayed), flap type (muscle v. non-muscle), diabetes, Charlson Comorbidity Index, and history of pre-operative radiation.
Cross-sectional Analysis of PROs
BREAST-Q scores were stratified by time interval from reconstruction and compared between laterality groups. Satisfaction with breast did not vary between unilateral and bilateral cohorts at 1 year, 2 years, or 3 years post-operatively (Table 4). Follow-up in years 4–10 was limited by small sample size (Figure 1). No differences were noted between groups for patients with 4 – 10 year follow-up at individual yearly time-points. In order to overcome small sample bias at yearly intervals beyond 3 years, we defined a >3 year cohort, for which the most recent BREAST-Q entry on record was captured and used for analysis. We observed that patients having undergone bilateral reconstruction with abdominal free flap reported greater satisfaction with breast after 3 years when compared to their unilateral counterparts (mean, 77.1±18.9 v. 69.2±22.0, respectively, p=0.04)(Figure 2), with no significant difference in average follow-up time for the > 3 year cohort (mean, 5.6±1.9 years v. 5.4±1.9 years, respectively, p=0.72).
Table 4.
Cross-sectional analysis of BREAST-Q scores at annual intervals, by laterality.
| 1 year (n=186) |
2 years (n=147) |
3 years (n=97) |
> 3 years (n=111) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BREAST-Q Dimensions | Unilateral ± SD (n=106) | Bilateral ± SD (n=80) | p | Unilateral ± SD (n=81) | Bilateral ± SD (n=66) | p | Unilateral ± SD (n=54) | Bilateral ± SD (n=43) | p | Unilateral ± SD (n=68) | Bilateral ± SD (n=43) | p |
|
| ||||||||||||
| Satisfaction with breasts | 66.9 ± 21.1 | 70.7 ± 18.2 | 0.22 | 68.8 ± 21.3 | 70.1 ± 20.7 | 0.52 | 72.1 ± 16.3 | 74.6 ± 17.8 | 0.64 | 69.2 ± 22.0 | 77.1 ± 18.9 | 0.04* |
| Physical well-being of the abdomen | 80.1 ± 20.1 | 72.7 ± 19.6 | < 0.01 | 85.0 ± 17.8 | 77.7 ± 20.6 | 0.03* | 84.7 ± 18.1 | 78.2 ± 21.1 | 0.10 | 86.6 ± 19.2 | 77.9 ± 20.1 | 0.01* |
| Physical well-being of the chest and upper body | 75.7 ± 17.5 | 72.8 ± 17.1 | 0.28 | 74.8 ± 15.2 | 75.9 ± 18.1 | 0.22 | 76.0 ± 16.9 | 79.7 ± 11.8 | 0.36 | 76.2 ± 15.5 | 81.6 ± 14.5 | 0.06 |
| Satisfaction with outcome | 75.4 ± 21.1 | 77.2 ± 22.0 | 0.50 | 74.2 ± 22.9 | 77.1 ± 22.3 | 0.50 | 79.1 ± 18.4 | 75.2 ± 18.0 | 0.24 | 74.9 ± 22.0 | 80.0 ± 17.2 | 0.32 |
Statistically significant (p < 0.05).
Figure 1.
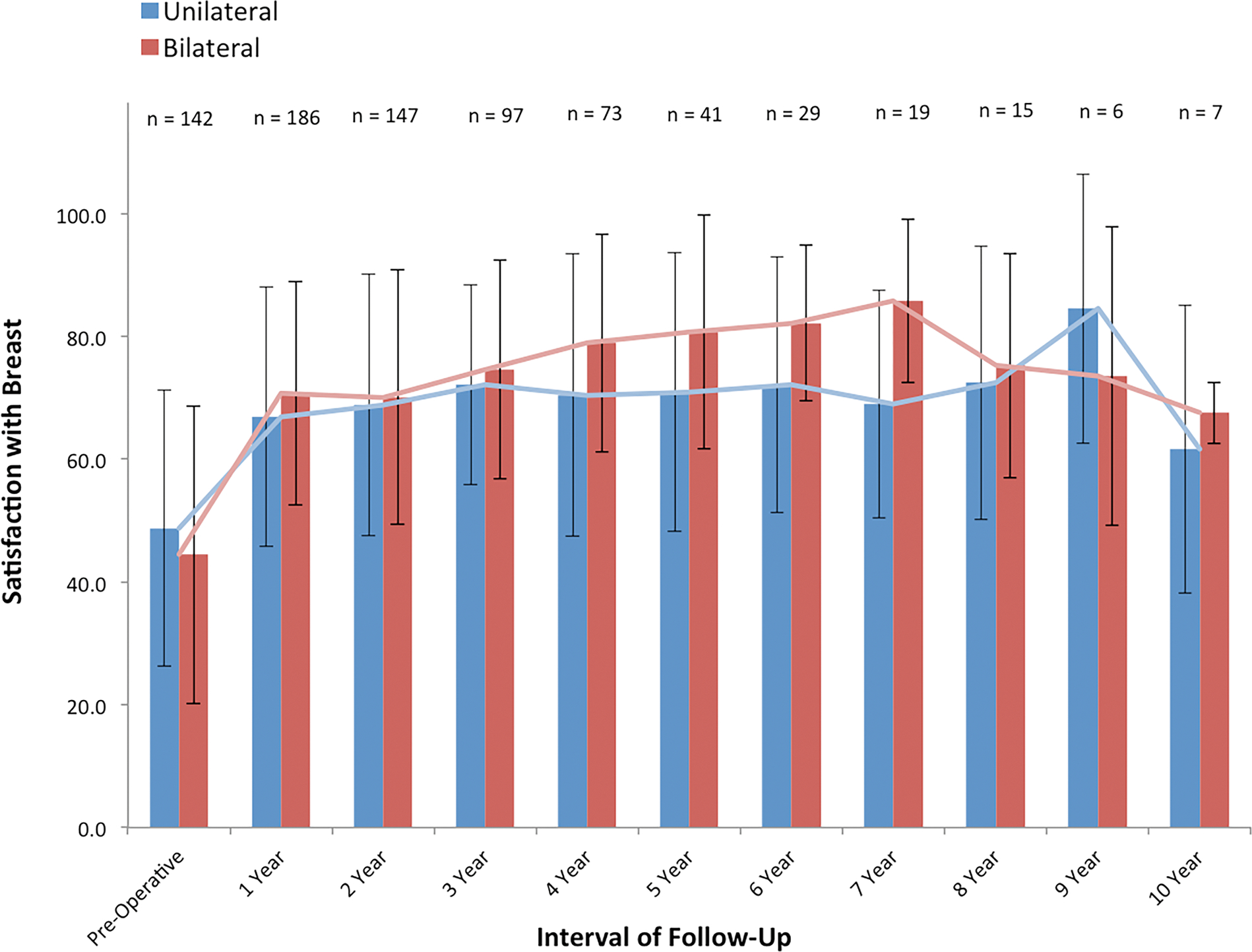
Cross-sectional analysis of BREAST-Q scores for satisfaction with breast between laterality groups throughout interval of observation (years 1 – 10). We were unable to identify differences in patient satisfaction at each time point, suggesting comparable percept of breast size, shape, and feel between unilateral and bilateral autologous reconstruction cohorts.
Figure 2.
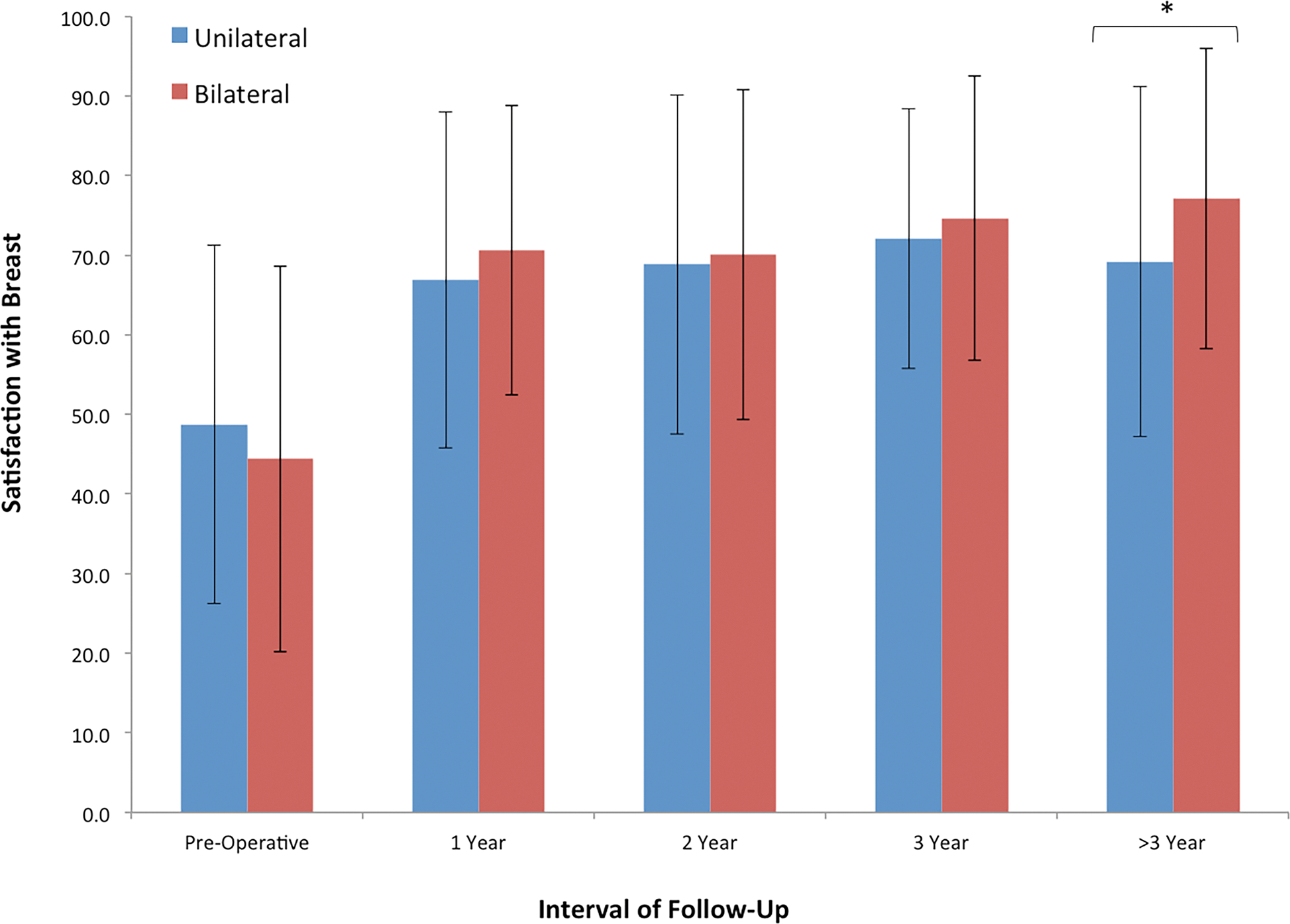
Cross-sectional analysis of BREAST-Q scores for satisfaction with breast between laterality groups at the following time intervals: pre-operative, 1 year, 2 years, 3 years, and > 3 years, post-operatively. No difference in satisfaction was observed early in the interval of observation; however, bilateral autologous reconstruction was associated with greater satisfaction with breast when compared to unilateral reconstruction at > 3 year follow-up.
Physical well-being of the abdomen differed between groups, as unilateral reconstruction was associated with more favorable abdominal function than bilateral reconstruction at 1 year, 2 years, and >3 years (Figure 3).
Figure 3.
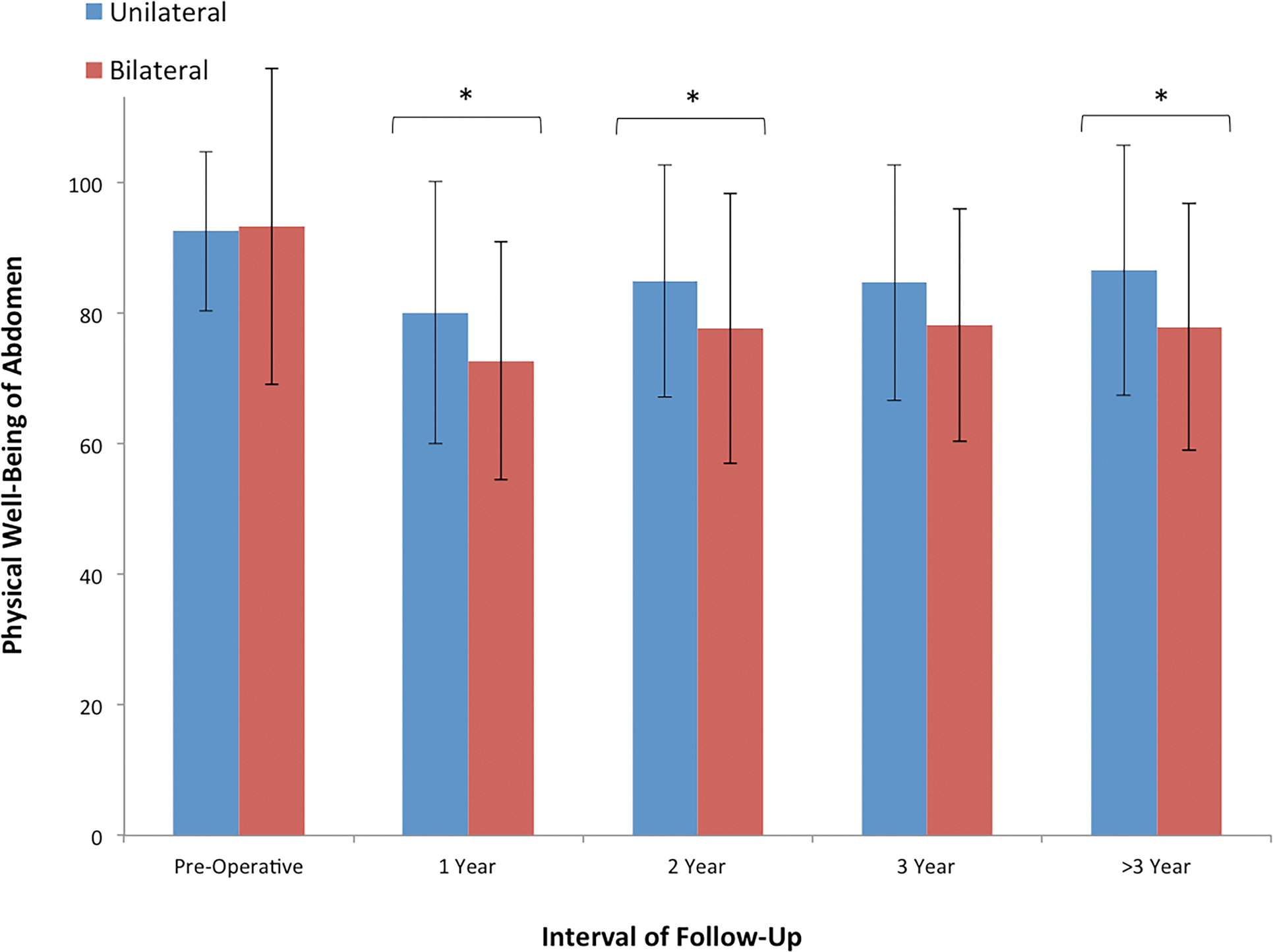
Cross-sectional analysis of BREAST-Q scores for physical well-being of the abdomen between laterality groups at the following time intervals: pre-operative, 1 year, 2 years, 3 years, and > 3 years, post-operatively. We observed a significant difference in abdominal function at 1 year, 2 years, and > 3 years, suggesting that patients with unilateral autologous reconstruction report greater abdominal well-being when compared to patients with bilateral reconstruction.
In order to control for potential confounders, we performed a multivariable linear regression to examine satisfaction with breasts and physical well-being of the abdomen. Regression analysis revealed bilaterality to be associated with greater satisfaction with breast at follow-up >3 years (β=9.13, p=0.03). However, linear regression indicated that physical well-being of abdomen decreased with bilateral reconstruction at 1 year follow-up, but the difference was no longer significant at >2 years (Table 5).
Table 5.
Result of multiple linear regression† analysis of BREAST-Q scores, by laterality (reference, unilateral).
| 1 year |
2 years |
3 years |
> 3 years |
|||||
|---|---|---|---|---|---|---|---|---|
| BREAST-Q Dimensions | β | p | β | p | β | p | β | p |
|
| ||||||||
| Satisfaction with breasts | 3.11 | 0.32 | 2.23 | 0.56 | 2.29 | 0.58 | 9.57 | 0.03* |
| Physical well-being of the abdomen | −8.33 | 0.01* | −5.88 | 0.08 | −2.77 | 0.53 | −7.25 | 0.08 |
| Physical well-being of the chest and upper body | −2.85 | 0.30 | 0.79 | 0.79 | 3.19 | 0.38 | 5.16 | 0.09 |
| Satisfaction with outcome | 2.13 | 0.53 | 4.01 | 0.32 | −2.45 | 0.58 | 9.13 | 0.03* |
Statistically significant (p < 0.05).
Each model included the following covariates: age at surgery, body mass index, timing of reconstruction (immediate v. delayed), flap type (muscle v. non-muscle), hypertension, Charlson Comorbidity Index, and history of pre-operative radiation.
The β coefficient refers to the predicted deviation in the dependent variable for each additional unit of independent variable.
Evaluation of Long-Term PROs
Scores did not differ between groups at pre-operative baseline (Table 6). Sub-group analysis was performed to evaluate longitudinal changes in BREAST-Q scores from pre-operative baseline. Although we observed a net increase in satisfaction with breast at 1 year, post-operatively, the rate of change did not differ significantly between unilateral and bilateral groups (+17.2 v. +25.1, respectively, p=0.22) (Table 7). Interestingly, rate of change for satisfaction with breast appeared to equalize between groups after two years (+25.9 v. +24.4, respectively, p=0.79)(Table 8), both demonstrating clinically meaningful improvement from baseline.
Table 6.
BREAST-Q scores at pre-operative consult and most recent follow-up, by laterality.
| Pre-operative (n=142) |
Most recent follow-up (n=405) |
|||||
|---|---|---|---|---|---|---|
| BREAST-Q Dimensions | Unilateral ± SD (n=78) | Bilateral ± SD (n=64) | p | Unilateral ± SD (n=234) | Bilateral ± SD (n=171) | p |
|
| ||||||
| Satisfaction with breasts | 48.7 ± 22.5 | 44.4 ± 24.2 | 0.21 | 67.0 ± 20.1 | 70.5 ± 19.3 | 0.06 |
| Physical well-being of the abdomen | 92.6 ± 12.2 | 93.3 ± 13.3 | 0.48 | 81.3 ± 20.3 | 73.1 ± 20.6 | < 0.01* |
| Physical well-being of the chest and upper body | 72.7 ± 16.1 | 73.6 ± 19.0 | 0.34 | 74.1 ± 16.8 | 74.7 ± 18.6 | 0.36 |
| Satisfaction with outcome | - | - | - | 73.5 ± 21.3 | 75.9 ± 20.8 | 0.31 |
Statistically significant (p < 0.05).
Table 7.
Longitudinal analysis of change in BREAST-Q score after 1 year, post-operatively.
| Satisfaction with Breast |
Physical Well-Being of Abdomen |
Physical Well-Being of Chest and Upper Body |
||||
|---|---|---|---|---|---|---|
| Laterality | Δ | p | Δ | p | Δ | p |
|
| ||||||
| Unilateral | +17.2 | < 0.01* | −12.5 | < 0.01* | +4.2 | 0.09 |
| Bilateral | +25.1 | < 0.01* | −23.7 | < 0.01* | −5.4 | 0.16 |
| p | 0.22 | 0.43 | 0.32 | |||
Statistically significant (p < 0.05).
The Δ statistic represents longitudinal variance in BREAST-Q score from pre-operative baseline and represents the subgroup of patients with scores at both timepoints.
Table 8.
Longitudinal analysis of change in BREAST-Q score after 2 years, post-operatively.
| Satisfaction with Breast |
Physical Well-Being of Abdomen |
Physical Well-Being of Chest and Upper Body |
||||
|---|---|---|---|---|---|---|
| Laterality | Δ | p | Δ | p | Δ | p |
|
| ||||||
| Unilateral | +25.9 | < 0.01* | −6.5 | 0.04* | +5.4 | 0.09 |
| Bilateral | +24.4 | < 0.01* | −11.0 | 0.03* | +2.2 | 0.44 |
| p | 0.79 | 0.38 | 0.48 | |||
Statistically significant (p < 0.05).
The Δ statistic represents longitudinal variance in BREAST-Q score from pre-operative baseline.
Discussion
CPM rates have continued to rise despite low risk of contralateral breast cancer. Recent studies have demonstrated that a number of factors influence this decision, including fear of contralateral cancer, avoidance of surveillance imaging, desire for aesthetic symmetry, and peace of mind.43–46 To arrive at this decision, many patients seek and value surgeon counseling with regard to comparative advantages and disadvantages of CPM.32,47,48 To date, claims regarding improved satisfaction following bilateral reconstruction remain unsubstantiated.33 The BREAST-Q was designed to assess patient satisfaction following breast reconstruction and has since become the gold standard in the field of reconstructive breast surgery. PROs obtained with the BREAST-Q allow for novel evaluation of patient satisfaction to contextualize reconstructive preference, aesthetic result, and post-operative donor-site physical function between unilateral and bilateral autologous reconstruction.46,49,50 Importantly, a recent study by Matros, et al. demonstrated superior patient satisfaction with aesthetic result following unilateral autologous reconstruction compared to unilateral implant-based reconstruction. As such, it has become necessary to evaluate laterality in autologous reconstruction patients to further elucidate factors that contribute to patient satisfaction.32 This current study compares long-term PROs between patients who underwent unilateral or bilateral abdominal free flap breast reconstruction to assess satisfaction and physical well-being in an effort to more clearly understand whether differences in PROs exist.
In the autologous reconstruction population examined, satisfaction with breasts did not differ significantly between the unilateral and bilateral cohorts up to three years post reconstruction, suggesting that bilateral autologous reconstructions do not offer significant improvement in breast aesthetic compared to unilateral reconstructions early after reconstruction. Beyond 3 years, a divergence may occur, suggesting that bilateral reconstruction may be associated with more favorable aesthetic outcome in the long-term, though this portion of analysis was limited by sample size. Physical well-being of the abdomen, however, varied early in the interval of observation, as bilateral reconstruction was associated with inferior physical function at 1 and 2 years post-operatively. Satisfaction with outcome and physical well-being of the chest and upper body were comparable across groups. Taken together, this data suggest that patients undergoing bilateral breast reconstruction with abdominal free flaps have decreased physical well-being of the abdomen without a significant improvement in satisfaction with their reconstructed breasts compared to patients undergoing unilateral reconstructions in the initial years after reconstruction.
Subgroup analysis revealed no significant difference in rate of change from pre-operative baseline for satisfaction with breast after 1 year, though both unilateral and bilateral cohorts experienced net clinically meaningful improvement in patient satisfaction during the interval of observation. Interestingly, the increase in patient satisfaction appeared to equalize at 2-year follow-up, suggesting that the increase in patient satisfaction following breast reconstruction does not differ with laterality of procedure when controlling for pre-operative satisfaction. Conversely, physical well-being of the abdomen decreased at a greater rate following bilateral reconstruction than unilateral reconstruction, although this difference did not achieve significance. Similar trends were previously noted in abdominal wall strength examinations of unilateral and bilateral reconstructions, though differences were more focused on flap types rather than comparison of unilateral compared to bilateral outcomes.51,52 To date, the only other long-term prospective follow-up of abdominal wall morbidity suggests that overall function returns to excellent levels in both unilateral and bilateral patients, though small sample size limits interpretation.53 Additionally, Hunsinger, et al. have demonstrated good physical function through cross sectional analysis in a majority unilateral cohort at 5 years using the SF-36 instrument.54
In patients undergoing unilateral mastectomy, contralateral balancing procedures are often performed to achieve symmetric reconstruction. PROs following balancing procedures were beyond the scope of the current study; however, subgroup analysis found no difference in satisfaction with breast between patients that underwent only unilateral reconstruction and those that underwent unilateral reconstruction with contralateral symmetrizing procedure. Most of these procedures were performed in secondary revision operations and not at the time of the initial autologous reconstruction.
Several recent studies have evaluated PROs between laterality cohorts following abdominal free flap-based breast reconstruction, often with conflicting results. Kuykendall, et al. compared BREAST-Q outcomes based on reconstruction laterality and detected higher satisfaction with outcome following unilateral autologous reconstruction compared to bilateral reconstruction at most recent follow-up.55 Their investigation, however, was limited to the use of deep inferior epigastric perforator (DIEP) flaps in a small number of patients. Similarly, Sinno, et al. reported that patient satisfaction was greater following bilateral reconstruction, likely due to enhanced symmetry and superior aesthetic without clothing.56 Although these results provide valuable insight into patient preference, the authors failed to use a validated PRO instrument, instead opting to develop a questionnaire to capture satisfaction data from a small patient cohort.
Although satisfaction in our study did not differ at 1, 2, or 3 years postoperatively, differences were noted in the period cohort of >3 years. Due to insufficient sample size, individual time-point results were unable to achieve significance. However, when examining longest follow-up in this period for each patient, a significant difference was noted favoring bilateral reconstructions. This may be due to differences in the aging of a reconstructed breast compared to a native breast and is certainly an area for future examination. Further, it is also possible that decision regret for not having a prophylactic mastectomy could factor into this timepoint (or vice versa), however the stability of psychosocial wellbeing points away from this hypothesis.
In this study, bilaterality was found to be an independent risk factor for donor-site complication whereas unilaterality was correlated with an unexpected finding of elevated mastectomy skin-flap necrosis. It is possible that prolonged operative time associated with bilateral reconstruction contributed to the donor-site morbidity observed in our patient population.57–59 Additionally elevated age at surgery in the unilateral group could have contributed to the increased rate of mastectomy skin-flap necrosis. Previous reports suggest that advanced age may serve as a predictor for mastectomy skin-flap necrosis, though recent results are mixed.60–63 Importantly, multivariate linear regression, which controlled for potential confounding of age, still indicated that patients with unilateral breast reconstruction experienced higher rates of mastectomy skin-flap necrosis, whereas univariate analysis showed a non-significant difference. As such, this finding may demonstrate statistical significance without true clinical relevance.
The main limitations of this study are sample size and variability in follow-up for each patient across the interval of observation, limiting longitudinal evaluation of change in satisfaction. Responder bias is certainly possible given the portion of patients, which completed postoperative BREAST-Q assessments, including the possibility of healthy responder bias. Observation occurred within a single academic medical center. Furthermore, differences exist in the degree of muscle sacrifice in DIEP or msTRAM flaps, which may impact hernia and bulge rates at later timepoints beyond the study period in both study groups. Such differences would not necessarily be captured in the surgical techniques portion of our study. Additionally, given the institutional preference during the study period to performed delayed autologous reconstruction following radiation, we were unable to examine the impact of radiation and subsequently the effects of radiation timing on PROs. Preoperative radiation was however included as a variable in the regression models, so the results presented adjust for this important factor. From our larger BREAST-Q dataset, we understand that radiation significant impacts outcomes, but recent multicenter data indicates that timing may not play as critical of a role. Further research is certainly needed on this topic.64–65
The strength of this study is its comparison of PROs between unilateral and bilateral abdominal free flap reconstruction cohorts within a large patient population with a validated PRO instrument. This study presents the longest follow-up of a prospective examination on this topic to date. The data presented herein can be used to help patients understand that satisfaction may be comparable in the long-term regardless of whether they elect to undergo CPM. This may help alter patient preconception regarding the necessity of CPM within the setting of unilateral breast cancer. Alternatively, similarity in patient satisfaction and abdominal function following unilateral and bilateral breast reconstruction may actually contribute to patient decision to undergo CPM, given the opportunity to avoid future cancer, prophylactic chemotherapy, or ongoing surveillance with comparable post-operative PROs. Therefore, shared-decision making remains paramount in the medical and oncologic management of breast cancer from both a clinical and patient-centered perspective.
Conclusion
Utilizing 8 years of BREAST-Q data, the current study suggests that satisfaction with autologous breast reconstruction does not differ with laterality of reconstructive procedure up to three years post-operatively. Conversely, abdominal well-being is significantly lower in bilateral reconstruction patients, likely due to inherent donor site morbidity associated with harvesting of multiple abdominal flaps. Longer-term data suggest possible divergence in breast satisfaction beyond three years.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Financial Disclosures: Joseph Dayan is a consultant for Stryker. The remaining authors have no conflicts of interest to disclose.
References
- 1.Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16(10):2697–2704. [DOI] [PubMed] [Google Scholar]
- 2.Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32(9):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones NB, Wilson J, Kotur L, Stephens J, Farrar WB, Agnese DM. Contralateral prophylactic mastectomy for unilateral breast cancer: an increasing trend at a single institution. Ann Surg Oncol. 2009;16(10):2691–2696. [DOI] [PubMed] [Google Scholar]
- 4.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27(25):4082–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16(10):2682–2690. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin CC, Lillquist PP, Edge SB. Surveillance of prophylactic mastectomy: trends in use from 1995 through 2005. Cancer. 2009;115(23):5404–5412. [DOI] [PubMed] [Google Scholar]
- 7.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–5209. [DOI] [PubMed] [Google Scholar]
- 8.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–1367. [DOI] [PubMed] [Google Scholar]
- 9.Yi M, Hunt KK, Arun BK, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev Res (Phila). 2010;3(8):1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158–2164. [DOI] [PubMed] [Google Scholar]
- 11.Portschy PR, Kuntz KM, Tuttle TM. Survival outcomes after contralateral prophylactic mastectomy: a decision analysis. J Natl Cancer Inst. 2014;106(8). [DOI] [PubMed] [Google Scholar]
- 12.Fisher CS, Martin-Dunlap T, Ruppel MB, Gao F, Atkins J, Margenthaler JA. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol. 2012;19(10):3246–3250. [DOI] [PubMed] [Google Scholar]
- 13.Hawley ST, Jagsi R, Morrow M, et al. Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA Surg. 2014;149(6):582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jagsi R, Hawley ST, Griffith KA, et al. Contralateral Prophylactic Mastectomy Decisions in a Population-Based Sample of Patients With Early-Stage Breast Cancer. JAMA Surg. 2017;152(3):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nekhlyudov L, Bower M, Herrinton LJ, et al. Women’s decision-making roles regarding contralateral prophylactic mastectomy. J Natl Cancer Inst Monogr. 2005(35):55–60. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med. 2013;159(6):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewster AM, Parker PA. Current knowledge on contralateral prophylactic mastectomy among women with sporadic breast cancer. Oncologist. 2011;16(7):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz SJ, Morrow M. The challenge of individualizing treatments for patients with breast cancer. JAMA. 2012;307(13):1379–1380. [DOI] [PubMed] [Google Scholar]
- 19.Abbott A, Rueth N, Pappas-Varco S, Kuntz K, Kerr E, Tuttle T. Perceptions of contralateral breast cancer: an overestimation of risk. Ann Surg Oncol. 2011;18(11):3129–3136. [DOI] [PubMed] [Google Scholar]
- 20.Hegde JV, Wang X, Attai DJ, et al. Assessing the Effect of Lifetime Contralateral Breast Cancer Risk on the Selection of Contralateral Prophylactic Mastectomy for Unilateral Breast Cancer. Clin Breast Cancer. 2018;18(2):e205–e218. [DOI] [PubMed] [Google Scholar]
- 21.Fu Y, Zhuang Z, Dewing M, Apple S, Chang H. Predictors for contralateral prophylactic mastectomy in breast cancer patients. Int J Clin Exp Pathol. 2015;8(4):3748–3764. [PMC free article] [PubMed] [Google Scholar]
- 22.Gurunluoglu R, Gurunluoglu A, Williams SA, Tebockhorst S. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg. 2013;70(1):103–110. [DOI] [PubMed] [Google Scholar]
- 23.Kroll SS, Evans GR, Reece GP, et al. Comparison of resource costs of free and conventional TRAM flap breast reconstruction. Plast Reconstr Surg. 1996;98(1):74–77. [DOI] [PubMed] [Google Scholar]
- 24.Matros E, Albornoz CR, Razdan SN, et al. Cost-effectiveness analysis of implants versus autologous perforator flaps using the BREAST-Q. Plast Reconstr Surg. 2015;135(4):937–946. [DOI] [PubMed] [Google Scholar]
- 25.Sgarzani R, Negosanti L, Morselli PG, Vietti Michelina V, Lapalorcia LM, Cipriani R. Patient Satisfaction and Quality of Life in DIEAP Flap versus Implant Breast Reconstruction. Surg Res Pract. 2015;2015:405163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu W, Nelson CM. Adipose and mammary epithelial tissue engineering. Biomatter. 2013;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pusic AL, Matros E, Fine N, et al. Patient-Reported Outcomes 1 Year After Immediate Breast Reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium Study. J Clin Oncol. 2017;35(22):2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirro O, Mestak O, Vindigni V, et al. Comparison of Patient-reported Outcomes after Implant Versus Autologous Tissue Breast Reconstruction Using the BREAST-Q. Plast Reconstr Surg Glob Open. 2017;5(1):e1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santosa KB, Qi J, Kim HM, Hamill JB, Wilkins EG, Pusic AL. Long-term Patient-Reported Outcomes in Postmastectomy Breast Reconstruction. JAMA Surg. 2018;153(10):891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson GW. Trends in autologous breast reconstruction. Semin Plast Surg. 2004;18(2):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15–23. [DOI] [PubMed] [Google Scholar]
- 32.Momoh AO, Cohen WA, Kidwell KM, et al. Tradeoffs Associated With Contralateral Prophylactic Mastectomy in Women Choosing Breast Reconstruction: Results of a Prospective Multicenter Cohort. Ann Surg. 2017;266(1):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchanan PJ, Abdulghani M, Waljee JF, et al. An Analysis of the Decisions Made for Contralateral Prophylactic Mastectomy and Breast Reconstruction. Plast Reconstr Surg. 2016;138(1):29–40. [DOI] [PubMed] [Google Scholar]
- 34.Taylor EM, Wilkins EG, Pusic AL, et al. Impact of Unilateral versus Bilateral Breast Reconstruction on Procedure Choices and Outcomes. Plast Reconstr Surg. 2019;143(6):1159e–1168e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer JP, Nelson JA, Cleveland E, et al. Breast reconstruction modality outcome study: a comparison of expander/implants and free flaps in select patients. Plast Reconstr Surg. 2013;131(5):928–934. [DOI] [PubMed] [Google Scholar]
- 36.Fischer JP, Wes AM, Nelson JA, et al. Propensity-matched, longitudinal outcomes analysis of complications and cost: comparing abdominal free flaps and implant-based breast reconstruction. J Am Coll Surg. 2014;219(2):303–312. [DOI] [PubMed] [Google Scholar]
- 37.Gardani M, Bertozzi N, Grieco MP, et al. Breast reconstruction with anatomical implants: A review of indications and techniques based on current literature. Ann Med Surg (Lond). 2017;21:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hershman DL, Richards CA, Kalinsky K, et al. Influence of health insurance, hospital factors and physician volume on receipt of immediate post-mastectomy reconstruction in women with invasive and non-invasive breast cancer. Breast Cancer Res Treat. 2012;136(2):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagares-Borrego A, Gacto-Sanchez P, Infante-Cossio P, Barrera-Pulido F, Sicilia-Castro D, Gomez-Cia T. A comparison of long-term cost and clinical outcomes between the two-stage sequence expander/prosthesis and autologous deep inferior epigastric flap methods for breast reconstruction in a public hospital. J Plast Reconstr Aesthet Surg. 2016;69(2):196–205. [DOI] [PubMed] [Google Scholar]
- 40.Cano SJ, Klassen AF, Scott AM, Pusic AL. A closer look at the BREAST-Q((c)). Clin Plast Surg. 2013;40(2):287–296. [DOI] [PubMed] [Google Scholar]
- 41.Voineskos SH, Klassen AF, Cano SJ, et al. Giving Meaning to Differences in BREAST-Q Scores: Minimal Important Difference for Breast Reconstruction. Plast Reconstr Surg. 2019;In Press. [DOI] [PubMed] [Google Scholar]
- 42.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurian AW, Lichtensztajn DY, Keegan TH, Nelson DO, Clarke CA, Gomez SL. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998–2011. JAMA. 2014;312(9):902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steiner CA, Weiss AJ, Barrett ML, Fingar KR, Davis PH. Trends in Bilateral and Unilateral Mastectomies in Hospital Inpatient and Ambulatory Settings, 2005–2013: Statistical Brief #201. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD)2006. [PubMed] [Google Scholar]
- 45.Yi M, Meric-Bernstam F, Middleton LP, et al. Predictors of contralateral breast cancer in patients with unilateral breast cancer undergoing contralateral prophylactic mastectomy. Cancer. 2009;115(5):962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrow M, Pusic AL. Time for a new era in outcomes reporting for breast reconstruction. J Natl Cancer Inst. 2011;103(1):5–7. [DOI] [PubMed] [Google Scholar]
- 47.Katz SJ, Hawley ST, Hamilton AS, et al. Surgeon Influence on Variation in Receipt of Contralateral Prophylactic Mastectomy for Women With Breast Cancer. JAMA Surg. 2018;153(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katz SJ, Janz NK, Abrahamse P, et al. Patient Reactions to Surgeon Recommendations About Contralateral Prophylactic Mastectomy for Treatment of Breast Cancer. JAMA Surg. 2017;152(7):658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett KG, Qi J, Kim HM, et al. Association of Fat Grafting With Patient-Reported Outcomes in Postmastectomy Breast Reconstruction. JAMA Surg. 2017;152(10):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu ES, Pusic AL, Waljee JF, et al. Patient-reported aesthetic satisfaction with breast reconstruction during the long-term survivorship Period. Plast Reconstr Surg. 2009;124(1):1–8. [DOI] [PubMed] [Google Scholar]
- 51.Selber JC, Fosnot J, Nelson J, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: Part II. Bilateral reconstruction. Plast Reconstr Surg. 2010;126(5):1438–1453. [DOI] [PubMed] [Google Scholar]
- 52.Selber JC, Nelson J, Fosnot J, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: part I. unilateral reconstruction. Plast Reconstr Surg. 2010;126(4):1142–1153. [DOI] [PubMed] [Google Scholar]
- 53.Nelson JA, Tecci MG, Lanni MA, et al. Function and Strength after Free Abdominally Based Breast Reconstruction: A 10-Year Follow-Up. Plast Reconstr Surg. 2019;143(1):22e–31e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunsinger V, Hivelin M, Derder M, Klein D, Velten M, Lantieri L. Long-Term Follow-Up of Quality of Life following DIEP Flap Breast Reconstruction. Plast Reconstr Surg. 2016;137(5):1361–1371. [DOI] [PubMed] [Google Scholar]
- 55.Kuykendall LV, Tugertimur B, Agoris C, Bijan S, Kumar A, Dayicioglu D. Unilateral Versus Bilateral Breast Reconstruction: Is Less Really More? Ann Plast Surg. 2017;78(6S Suppl 5):S275–S278. [DOI] [PubMed] [Google Scholar]
- 56.Sinno S, Salvino MJ, Vandevender D. Comparing patient satisfaction in bilateral and unilateral breast reconstruction. Plast Surg Nurs. 2014;34(3):141–145; quiz 146–147. [DOI] [PubMed] [Google Scholar]
- 57.Daley BJ, Cecil W, Clarke PC, Cofer JB, Guillamondegui OD. How slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Am Coll Surg. 2015;220(4):550–558. [DOI] [PubMed] [Google Scholar]
- 58.Nelson JA, Chung CU, Fischer JP, Kanchwala SK, Serletti JM, Wu LC. Wound healing complications after autologous breast reconstruction: a model to predict risk. J Plast Reconstr Aesthet Surg. 2015;68(4):531–539. [DOI] [PubMed] [Google Scholar]
- 59.Lin IC, Nelson JA, Wu LC, Kovach SJ 3rd, Serletti JM. Assessing Surgical and Medical Complications in Bilateral Abdomen-Based Free Flap Breast Reconstructions Compared With Unilateral Free Flap Breast Reconstructions. Ann Plast Surg. 2016;77(1):61–66. [DOI] [PubMed] [Google Scholar]
- 60.McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121(6):1886–1892. [DOI] [PubMed] [Google Scholar]
- 61.Mlodinow AS, Fine NA, Khavanin N, Kim JY. Risk factors for mastectomy flap necrosis following immediate tissue expander breast reconstruction. J Plast Surg Hand Surg. 2014;48(5):322–326. [DOI] [PubMed] [Google Scholar]
- 62.Woerdeman LA, Hage JJ, Hofland MM, Rutgers EJ. A prospective assessment of surgical risk factors in 400 cases of skin-sparing mastectomy and immediate breast reconstruction with implants to establish selection criteria. Plast Reconstr Surg. 2007;119(2):455–463. [DOI] [PubMed] [Google Scholar]
- 63.Chang EI, Vaca L, DaLio AL, Festekjian JH, Crisera CA. Assessment of advanced age as a risk factor in microvascular breast reconstruction. Ann Plast Surg. 2011;67(3):255–259. [DOI] [PubMed] [Google Scholar]
- 64.Nelson JA, Allen RJ Jr, Polanco T, Shamsunder M, Patel AR, McCarthy CM, Matros E, Dayan JH, Disa JJ, Cordeiro PG, Mehrara BJ, Pusic AL. Long-term Patient-reported Outcomes Following Postmastectomy Breast Reconstruction: An 8-year Examination of 3268 Patients. Ann Surg. 2019September;270(3):473–483. [DOI] [PubMed] [Google Scholar]
- 65.Billig J, Jagsi R, Qi J, Hamill JB, Kim HM, Pusic AL, Buchel E, Wilkins EG,Momoh AO. Should Immediate Autologous Breast Reconstruction Be Considered in Women Who Require Postmastectomy Radiation Therapy? A Prospective Analysis of Outcomes. Plast Reconstr Surg. 2017June;139(6):1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]


