Abstract
Drug‐induced liver injury (DILI) should be considered in all patients with recent elevation of liver tests without obvious etiology and normal hepatobiliary imaging. There is currently no biomarker that is helpful in diagnosis which relies on clinical and laboratory findings. Diagnosis is dependent on temporal relationship with a recently started drug or herbal and dietary supplement and elevated liver tests with exclusion of competing etiologies. The implicated agent should be discontinued and the patient should be observed closely. This is particularly important in patients with jaundice who have approximately 10% risk of liver related mortality and/or need for liver transplantation. There is no specific therapy for DILI which is only symptomatic such as for itching. Patients with jaundice and coagulopathy usually require hospitalization.
Keywords: clinical management, dietary supplements, DILI, drug‐induced liver injury, elevated liver enzymes, hepatitis, herbal, herb‐induced liver injury, HILI, supplement
INTRODUCTION
Idiosyncratic drug‐induced liver injury (DILI) is a relatively rare adverse reaction and an important differential diagnosis among patients who present with elevated liver tests of unknown etiology.1 In the vast majority of cases, the liver injury is of acute nature.2 The reason for development of DILI in a small proportion of patients taking the same drug is largely unknown. Risk in susceptible individuals have mostly been associated with specific human leucocyte antigen (HLA) types associated with reactive metabolites which can serve as haptens.3, 4 Thus, immunologic pathogenesis has been suggested. Liver injury due to TNF‐alpha and check point inhibitors has been named indirect injury postulated to be due to immune dysregulation.5, 6
Idiosyncratic DILI and direct liver injury due to paracetamol are the most common causes of acute liver failure in both Europe and the United States7, 8 and accounted for 3%–5% of patients hospitalized for jaundice in the United States9 and 11% among patients with ALT > 500 IU in Iceland similar to the proportion of patients with viral hepatitis.10 Incidence of DILI has been found to be 14–19 cases per 100,000 inhabitants.1, 11 The proportion of patients treated with amoxicillin‐clavulanate (AC), a common cause of DILI was found to occur in 1:2350 patients treated but in only 1:133 patients taking azathioprine and 1:148 patients receiving infliximab.1 Understanding of the natural history, phenotypic presentation and pathogenesis has increased considerably during the last two decades.3, 4 However, there is a medical need to improve the diagnostic accuracy of DILI and there is still no specific therapy that can alter the natural history of DILI. Thus, there are large areas of uncertainty in DILI. The aim of the current review was to present an update on the diagnostic approach, natural history, and current management of DILI. Two case reports are presented to illustrate the morbidity that can be associated with DILI and challenges in management.
Case Report 1
A sixty‐year‐old woman presented with nausea and jaundice with three times upper limit of normal (ULN) in ALP and ALT 5xULN, bilirubin peaked after 3 weeks at 208 (normal <25). Hepatobiliary imaging was normal. DILI due to AC and azithromycin was suspected. Viral serologies negative. The itching was very severe making it difficult to wear clothes, bathing, being touched. She was treated with cholestyramine for itching with a partial response. She recovered clinically and biochemically after approximately 3 months.
HOW TO DIAGNOSE DILI?
Major advances in the diagnosis in most liver diseases have taken place during the last 2–3 decades, such as in viral hepatitis with PCR technology, routine use of different autoantibodies, identification of hepatitis E, phosphatidylethanol (B‐Peth) for suspicion of alcoholic liver disease and gene mutations in patients with iron overload to name a few. Unfortunately, progress in the diagnosis of DILI has only been indirect by improving exclusion of other potential causes of elevated liver tests. Currently, there is no biological marker for DILI although novel biomarkers are tested in International multicenter studies. The most important in the diagnostic process is to include DILI among the differential diagnoses. The types and phenotypes and DILI have been a subject of a recent review showing a large variation in biochemical, immunological, functional, and even structural changes in the liver and the bilary system.3
The question is in which patients with elevated liver tests DILI should be expected? This relies heavily on the clinical and biochemical context. The vast majority of patients present acutely.3, 5, 9, 10 Chronic hepatitis has though been reported to occur after long term treatment of nitrofurantoin and minocycline.12, 13
The diagnosis is sometimes obvious as is illustrated in case report 1. The patient presents with acutely with symptoms of liver disease such as itching and nausea and has recently been treated with a drug well recognized for leading to DILI such as AC but has also been treated with another drug azithromycin which also has well documented potential causing liver injury.14 Other differential diagnoses quickly ruled out, DILI by clinical judgment seems obvious but it is impossible to state which drugs is responsible. Both drugs should be contraindicated in the future. However, if a patient is suspected of DILI and only one drug is known to cause DILI, other drugs should not be discontinued as illustrated in case report 2. Apart from viral hepatitis that needs to be ruled out in patients with acute hepatocellular liver injury, autoimmune hepatitis is the main differential diagnosis in a patient with a clear ALT predominance over ALP. If liver injury is accompanied by moderate and severe abdominal pain this argues against DILI and it should be remembered that choledocholithiasis can lead to acute hepatocellular injury at the first presentation and MRCP is often needed to illustrate this.10 When the patient presents subacutely, the diagnosis of DILI is more difficult as demonstrated in Case report 2.
Case Report 2
A 76‐year‐old woman referred for painless jaundice with normal hepatobiliary CT and MRCP. Her ALP was 444 (normal 105), ALT 197 (<45), bilirubin 62 (<25). Liver tests were normal 3 months earlier when she was put on aspirin 75 mg, clopidogrel 75 mg, and atorvastatin 40 mg. Atorvastatin was discontinued but other drugs continued. Liver tests normalized after a few weeks and she recovered clinically. Atorvastatin induced liver injury was considered a very likely cause of jaundice.
Case 2 illustrates the importance of the documentation of liver injury associated with different agents. Atorvastatin has been associated with more than 50 published cases with causality assessment according to categorization of drugs included in LiverTox (https://www.ncbi.nlm.nih.gov/books/NBK547852/), therefore belonging to Category A, with drugs with established potential of causing liver injury.15 However, only 14 cases of clopidogrel induced liver injury have been published, although it has been in use for almost 25 years (marketed in the United States 1997). Atorvastatin has been mostly associated with cholestatic type of injury and in a study from Sweden the median duration of atorvastatin prior to diagnosis of liver injury was 4 months.16 Atorvastatin induced liver injury is rare, occurring in approximately one out of 3700 users according to one study.5 The latency and the biochemical signature can be helpful to judge which drug is the most likely culprit as some important drugs should not be discontinued, such as clopidogrel in patients with coronary stents to avoid stent occlusion.
Currently, the most important elements in the diagnostic process are latency of onset, results of dechallenge, exclusion of other causes and information about the previous hepatotoxicity of the drug. The most commonly used causality assessment method, Roussel Uclaf Causality Assessment Method (RUCAM)17 is rarely used in clinical practice but has been widely used in published papers on patients with DILI.1, 4, 18, 19, 20 RUCAM is a good checklist for the most important elements that needs to be taken into consideration when confronted with a suspected DILI. However, RUCAM has until now not been updated since it was published in 1993 and it has probably not stood the test of time.17 RUCAM has recently been validated by the use of clinical and biochemical data from the Spanish18 and DILIN21 prospective registries. RUCAM has been updated with the aims to clarify operating instructions and increase reproducibility and a computerized version or RECAM (revised electronic (version) of causality assessment method) has been created.22 The so‐called Expert opinion method has been used by the DILIN investigators, who score the likelihood of DILI in patient suspected of that diagnosis.21 The problem is if the opinion of the “experts” differ and when a consensus is reached as has been pointed out, this carries the risk of overruling an insightful minority opinion.20 The most important things that need to be taken into consideration in terms of the diagnosis of DILI is illustrated in Table 1.
TABLE 1.
Diagnostic algorithm that can be useful in the diagnostic evaluation and management of DILI
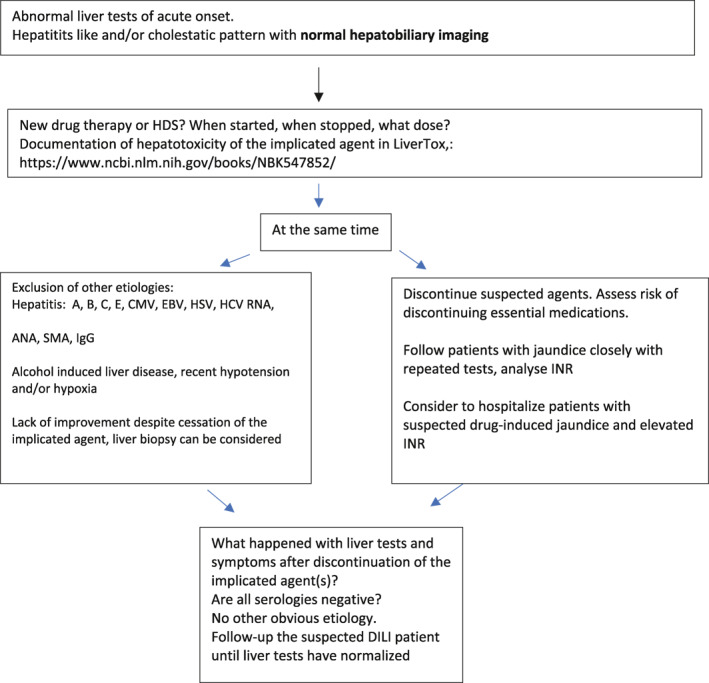
|
Abbreviation: DILI, drug‐induced liver injury.
In Table 2, the most commonly implicated agents in three prospective studies are demonstrated.
TABLE 2.
The most common implicated agents causing DILI in three prospective studies on DILI
| Spanish registry (n = 843), Reference 24 | DILIN (n = 899), Reference 21 | Icelandic study (n = 96), Reference 1 |
|---|---|---|
| Amoxicillin‐clavulanate (22%) | Amoxicillin‐clavulanate (10%) | Amoxicillin‐clavulanate (22%) |
| Anti‐tuberculosis (4.5%) | Isoniazid (5.3%) | Diclofenac (6.3%) |
| Ibuprofen (3%) | Nitrofurantoin (4.7%) | Nitrofurantoin (4%) |
| Flutamide (2.6%) | Sulfam‐trimeth (3.4%) | Azathioprine (4%) |
| Atorvastatin (1.9%) | Minocycline (3.1%) | Infliximab (4%) |
| Diclofenac (1.8%) | Cefazolin (2.2%) | Isotretinoin (3%) |
| Ticopidine (1.4%) | Azithromycin (2%) | Atorvastatin (2%) |
| Azathioprine (1.3%) | Ciprofloxacin (1.8%) | Doxycyline (2%) |
| Fluvastatin (1.3%) | Levofloxacin (1.4%) | Imatinib (1%) |
| Simvastatin (1.3%) | Diclofenac (1.3%) | Isoniazid (1%) |
| HDS (3.4%) | HDS (16.1%) | HDS (16%) |
Abbreviations: DILI, drug‐induced liver injury; HDS, herbal and dietary supplements; Sulfam‐trimeth, sulfamethoxazole‐trimethoprim.
NATURAL HISTORY, INCLUDING OUTCOMES OF DILI
Once DILI is diagnosed on good clinical grounds and the implicated agent is discontinued, the vast majority of patients recover clinically and biochemically. This is true also for patients who present with drug‐induced jaundice but as could be expected their prognosis is worse than patients who do not have jaundice. The late Hyman Zimmerman found that drug‐induced jaundice was associated with 10% mortality, called the Hy's rule2, 3 which has been validated.18, 19, 21 If jaundice is accompanied by coagulopathy the condition is more severe as in other types of liver disease. The worst‐case scenario, is the development of acute liver injury, worsening jaundice and coagulopathy, and then encephalopathy.8 In contrast with paracetamol induced liver failure that develops very acutely, the development of liver failure due to idiosyncratic DILI is mostly subacute, that is, develops slower and has a poor transplant free survival.7, 8 In a study from the United States, Reuben et al. reported that among patients with acute liver failure due to DILI, transplant free survival was only 23% and 40% underwent a liver transplant. Remarkably similar results were reported from Sweden.7
From large cohorts of DILI patients mortality of 5%–10% has been reported mostly from acute liver failure in those who presented with jaundice and coagulopathy.18, 19, 20 In a recent update of more than 800 patients recruited in the Spanish hepatotoxicity registry, liver related mortality was more frequent in hepatocellular damage, aged >65 years and in patients with underlying liver disease.23 Most data on prognosis from the DILI registries are from within 6 months.18, 19, 21 Hayashi et al. reviewed all fatalities that occurred within 2 years of the DILI.24 Among 1089 DILIN patients, 7.6% of fatalities were due to liver injury, partially or primarily within 2 years of follow‐up and 9.5% died among those with jaundice.24 Overall 68/86 (82%) died or underwent liver transplantation as a direct consequence of liver injury, majority 59/68 (87%) within 6 months whereas the remaining 13% developed a more chronic injury and died of liver failure within the 2 years observation period.24 DILI was associated with a late mortality, directly and/or indirectly in approximately 1% of the total cohort.24 This is in line with long‐term follow‐up of patients who presented with drug‐induced jaundice in Sweden, where 1.5% developed chronic DILI.25 Thus, chronic liver injury can occur and can in rare instances be associated with liver related morbidity and mortality.24, 25
CURRENT MANAGEMENT
The most important step in management is to discontinue the implicated agent to decrease risk of further liver damage. Patients with DILI and comitant jaundice should be followed closely with repeated measurements of liver tests and those with significant coagulopathy (INR > 1.5) often need hospitalization.
There is limited data to suggest that the natural course of DILI can be changed by available therapeutic agents. However, N‐Acetylcysteine (NAC) should be used in acute liver failure due to idiosyncratic DILI, particularly in early stages. This is based on a NAC‐trial in patients with non‐paracetamol induced acute liver failure that induced by DILI in a large proportion of patients.26 Transplant free survival was found to be 52% of those randomized to NAC versus only 27% for those who were on placebo. Given the lack of other therapies and the safety of NAC it is reasonable to treat drug‐induced ALF with significant coagulopathy (INR > 1.5) with NAC before they develop overt hepatic encephalopathy. Patients who present with autoimmune features who have been on drugs with well documented autoimmune like biochemical picture, that is, with hepatocellular pattern, positive autoantibodies and/or elevated IgG and do not recover clinically and biochemically after withdrawal of the offending drug, should be treated with corticosteroids as patients with genuine autoimmune hepatitis (AIH).5, 13, 27 Long standing increase in ALT after four infusions of Infliximab in 40 year old woman is demonstrated in Figure 1, prompt response to corticosteroids and liver histology prior to steroid therapy.
FIGURE 1.
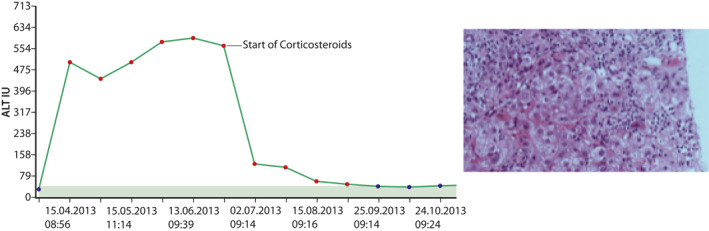
Rapid increase in ALT observed in 40‐year‐old woman after four infusions of infliximab and rapid decrease in ALT, approximately 2 months after liver injury was first detected. On the right liver histology from this patient is shown, with dense inflammatory infiltration of lymphocytes and plasma cells as well as ballooning and apoptotic hepatocytes
Drugs that typically induce AIH‐like picture are nitrofurantoin, minocycline, methyl‐dopa, hydralazine, and infliximab. Patients show prompt resolution of liver tests within days of corticosteroid therapy as shown in Figure 1.5, 12, 13 Similarly, patients with check point inhibitors, can develop liver injury requiring corticosteroids.27
A meta‐analysis of randomized clinical trials for prevention and treatment of idiosyncratic DILI has recently been published.28 Overall, 22 RCTs were included: 12 on prevention and 10 in management. Silymarin (8 studies), bicyclol (n = 4), isoglycyrrhizinate (n = 3), N‐acetylcysteine (n = 3), traditional Chinese medicines (n = 2), tiopronin and L‐carnitine were used in the treatment arm, but the control arm received placebo or standard medical care. A large heterogeneity was observed in terms of inclusion criteria and methodologic quality. The interventional studies performed demonstrated limited efficacy of specific interventions. It was concluded that International research collaboration is needed to design a trial in DILI with proper therapeutic endpoints. However, it is not clear which therapeutic intervention should be tried but there is a medical need to try to improve the prognosis of patients with DILI. DILI patients often develop severe morbidity, with jaundice, severe lethargy and itching for weeks or months and at the current time, symptomatic treatment and cholestyramine for itching are the only options to help these patients.
AREAS OF UNCERTINTY AND FUTURE PROSPECTS
The diagnosis of DILI has been called the acchiles heel of the study of DILI. Although computerized version or RECAM is under way and will be available in the public domain soon, there is still a lack of biological marker that can be helpful to distinguish DILI from other liver diseases. International research projects are ongoing to validate novel biomarkers for diagnosis and also biomarkers that can be valuable to predict prognosis. Other problematic clinical aspect in DILI is the lack of specific intervention that can affect the natural history of the liver injury and randomized controlled trials are needed to establish efficacy on clinical outcomes. Given the increase in immunomodulatory treatment of cancer with accompanying indirect DILI, controlled trials are needed to compare different doses of corticosteroids as the ultra‐high doses recommended by the oncological societies are not without side effects.
CONCLUSIONS
In recent years, the understanding of DILI has increased considerably. However, further research is needed to improve the diagnostic strategies. At the current time the most important first step in the diagnostic process is to suspect the diagnosis, follow the patient closely, concomitantly exclude competing etiologies, recognize typical signatures of the most common agents leading to DILI and to find out how well documented hepatotoxicity of the implicated agent is. This will enable us to assess a patient with suspected DILI and help us make up our mind about how likely it is that the patient suffers from DILI.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare
Björnsson ES. Clinical management of patients with drug‐induced liver injury (DILI). United European Gastroenterol J. 2021;9(7):781–786. 10.1002/ueg2.12113
DATA AVAILABILITY STATEMENT
Data is available on request.
REFERENCES
- 1.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug‐induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [DOI] [PubMed] [Google Scholar]
- 2.Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug‐induced liver injury. Clin Pharmacol Ther. 2011;89:806–15. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH, Bjornsson ES. Drug induced liver injury: types and phenotypes. New Eng J Med. 2019;381:264–73. [DOI] [PubMed] [Google Scholar]
- 4.Andrade RJ, Björnsson ES, Kaplowitz N, Kaplowitz N, Kullak‐Ublick GA, Larrey D, et al. EASL Clinical Practice Guidelines: drug‐induced liver injury. J Hepatol. 2019;70:1222–61. [DOI] [PubMed] [Google Scholar]
- 5.Björnsson E, Bergmann O, Jonasson JG, Grondal G, Gudbjornsson B, Olafsson S. Drug‐induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol. 2017;15:1635–6. [DOI] [PubMed] [Google Scholar]
- 6.Regev A, Avigan MI, Kiazand A, Vierling JM, Lewis JH, Omokaro SO, et al. Best practices for detection, assessment and management of suspected immune‐mediated liver injury caused by immune checkpoint inhibitors during drug development. J Autoimmun. 2020;114:102514. 10.1016/j.jaut.2020.102514 [DOI] [PubMed] [Google Scholar]
- 7.Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S, Almer S, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262:393–401. [DOI] [PubMed] [Google Scholar]
- 8.Reuben A, Koch DG, Lee WM. Drug‐ induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuppalanchi R, Liangpunsakul S, Chalasani N. Etiology of new‐onset jaun‐dice: how often is it caused by idiosyncratic drug‐induced liver injury in the United States? Am J Gastroenterol. 2007;102:558–62. [DOI] [PubMed] [Google Scholar]
- 10.Bjornsson HK, Bergmann OM, Olafsson S, Bjornsson ES. A prospective study on the causes of notably raised alanine aminotransferase. Scand J Gastroenterol. 2016;51:594–600. [DOI] [PubMed] [Google Scholar]
- 11.Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug‐induced hepatic injuries: a French population‐based study. Hepatology. 2002;36:451–5. [DOI] [PubMed] [Google Scholar]
- 12.Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug‐induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–8. [DOI] [PubMed] [Google Scholar]
- 13.de Boer YS, Kosinski AS, Urban TJ, Zhao Z, Long N, Chalasani N, et al. Features of autoimmune hepatitis in patients with drug‐induced liver injury. Clin Gastroenterol Hepatol. 2017;15:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, et al. Clinical and histologic features of azithromycin‐induced liver injury. Clin Gastroenterol Hepatol. 2015;13:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: critical assessment based upon published case reports. Hepatology. 2016;63:590–603. [DOI] [PubMed] [Google Scholar]
- 16.Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post‐marketing. J Hepatol. 2012;56:374–80. [DOI] [PubMed] [Google Scholar]
- 17.Danan G, Benichou C. Causality assessment of adverse reactions to drugs‐I. A novel method based on the conclusions of international consensus meetings: application to drug‐induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. [DOI] [PubMed] [Google Scholar]
- 18.Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, Garciaruiz E, et al. Drug‐induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10‐year period. Spanish Group for the Study of Drug‐Induced Liver Disease. Gastroenterology. 2005;129:512–21. [DOI] [PubMed] [Google Scholar]
- 19.Björnsson E, Olsson R. Outcome and prognostic markers in severe drug‐induced liver disease. Hepatology. 2005;42:481–9. [DOI] [PubMed] [Google Scholar]
- 20.Kullak‐Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, et al. Drug‐induced liver injury: recent advances in diagnosis and risk assessment. Gut. 2017;66:1154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalasani N, Fontana RJ, Bonkovsky HL, Fontana R, Lee W, Stolz A, et al. Features and outcomes of 899 patients with drug‐induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi PH, Lucena MI, Fontana RJ, Bjornsson ES, Aithal GP, Barnhart H, et al. A revised electronic version of RUCAM for the diagnosis of drug induced liver injury. Hepatology. 2020;72(51):abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephens C, Roblez‐Diaz M, Medina‐Caliz I, Garcia‐Cortes M, Ortega‐Alonso A, Sanabria‐Cabrera J, et al. Comprehensive analysis and insight gained from long‐term experience of the Spanish DILI registry. J Hepatology. 2021;75(1):86–97. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi PH, Rockey DC, Fontana RJ, Tillmann HL, Kaplowitz N, Barnhart HX, et al. Death and liver transplantation within 2 years of onset of drug‐induced liver injury. Hepatology. 2017;66:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Björnsson E, Davidsdottir L. The long‐term follow‐up after idiosyncratic drug‐induced liver injury with jaundice. J Hepatology. 2009;50:511–7. [DOI] [PubMed] [Google Scholar]
- 26.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N‐acetylcysteine improves transplant free‐survival in early stage non‐acetaminophen acute liver failure. Gastroenterology. 2009;137:856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah P, Sundaram V, Bjornsson ES. Biologic and checkpoint inhibitor‐induced liver injury: a systematic literature review. Hepatol Commun. 2020;4:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niu H, Sanabria‐Cabrera J, Alvarez‐Alvarez I, Robles‐Diaz M, Stankevičiūtė S, Aithal GP, et al. Prevention and management of idiosyncratic drug‐induced liver injury: systematic review and meta‐analysis of randomised clinical trials. Pharmacol Res. 2020;164:105404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available on request.


