Abstract
Background
Pancreatic ductal adenocarcinoma is the deadliest cancer worldwide with a 98% loss‐of‐life expectancy and a 30% increase in the disability‐adjusted life years during the last decade in Europe. The disease cannot be effectively prevented nor being early detected. When diagnosed, 80% of patients have tumors that are in incurable stages, while for those who undergo surgery, 80% of patients will present with local or distant metastasis. Importantly, chemotherapies are far from being effective.
Objective
Pancreatic cancer represents a great challenge and, at the same time, a huge opportunity for advancing our understanding on the basis of the disease, the molecular profiles, that would lead to develop tools for early detection and effective treatments, thus, boosting patient survival.
Results
Research on pancreatic cancer has being receiving little or minimal funds from European funding bodies. UEG is calling for public‐private partnerships that would effectively fund research on pancreatic cancer.
Conclusion
This would increase our understanding of this disease and better treatment, through pan‐European efforts that take advantage of the strong academic European research landscape on pancreatic cancer, and the contribution by the industry of all sizes.
Keywords: chronic pancreatitis, familial pancreatic cancer, funding, Horizon Europe, intraductal papillary mucinous neoplasm, pancreatic cancer, pancreatic ductal adenocarcinoma
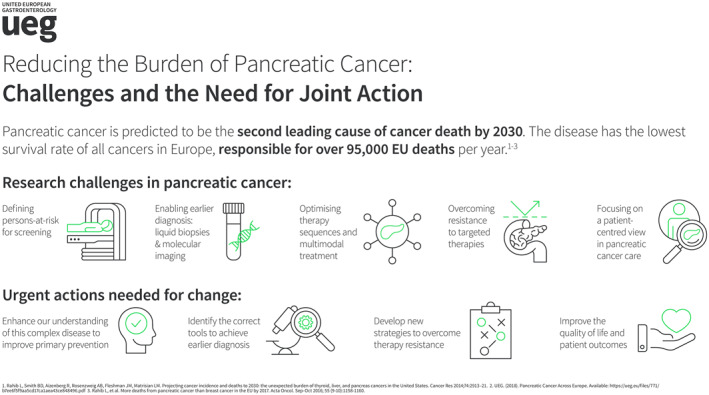
INTRODUCTION
Pancreatic cancer has become an increasing health problem in the Westernized world, which is currently out of control. Pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer, could be considered an orphan disease, as it has not received the necessary attention from the pharmaceutical industry to date. The U.S. Congress has defined PDAC as a “recalcitrant cancer,” leading to the implementation of a 5‐year effective “Pancreatic Cancer Research and Education Act” involving research for biomarkers of early detection. Similarly, the European Commission‐supported “Innovative Partnership for Action Against Cancer” called PDAC a “neglected cancer” due to its biological aggressiveness, late diagnosis, and lack of effective treatments and therefore prioritized all actions to control this disease.1 Remarkably, public and private funding for PDAC research over time has been significantly less than for other cancers for which a large impact in their survival has been achieved.2
Facing this situation, the following position paper initiated by members of the UEG Public Affairs and Research committees and co‐authored by scientists from six European countries aims to increase awareness for pancreatic cancer among European stakeholders from politics, industry, and academia by identifying areas with immediate need for joint actions.
EPIDEMIOLOGY: CURRENT SITUATION AND PERSPECTIVES FOR 2030 AND BEYOND
Incidence & mortality
Despite its relatively low population‐wide incidence, PDAC remains the deadliest cancer worldwide. In 2020, the estimated number of new PDAC cases in Europe is 140,116, with a crude incidence rate of 18.7 per 100,000 men and women per year. The corresponding mortality figures are 132,134 and 17.6, respectively, almost paralleling the incidence numbers.3 PDAC is a deadly tumor still harboring a 98% loss‐of‐life expectancy and associated with a 30% increase in the disability‐adjusted life years during the last decade in Europe.4, 5 Due to its aggressiveness, it has risen from the seventh position in the cancer incidence ranking to the fourth leading cause of cancer‐related deaths in Europe3 and the third leading cause in USA.6 PDAC incidence and mortality increases with age; therefore, this tumor will become an increasing health problem in the aging European countries. Recent publications have raised awareness of the dramatically increasing incidence of PDAC in the Western world unless urgent action is taken. In Europe, PDAC death rates are steadily rising while rates for all other cancers continue to fall.7, 8 US data suggest that PDAC will become the second‐leading cause of cancer‐related deaths by 2030.9 The high mortality from PDAC is mainly due to the difficulties in early diagnosis, in particular the absence of specific symptoms and biomarkers in early stages, as well as the aggressive nature of the disease and frequent resistance to systemic therapies, which makes early detection and treatment highly challenging.
Clinical Scenarios and survival
PDAC is the only cancer entity for which there has been only marginal improvement of its appalling prognosis over the last decades despite considerable efforts in tertiary prevention and novel treatment strategies. Overall, PDAC still has a 5‐year survival of <10%.3, 10 At the time of diagnosis, 80% of patients have tumors that are in incurable stages (locally advanced or metastatic). In the remaining 20% (localized resectable disease), surgery is the only potentially curative treatment. However, even after complete surgical resection, 80% of patients will present with local or distant relapses4 due to the existence of micrometastatic disease at the time of diagnosis. Adjuvant chemotherapy (Gemcitabine‐fluoropyrimidine/mFOLFIRINOX) is commonly used to improve this scenario.11 The impact of this strategy on the survival of PDAC is significant, but still 60%–70% of patients relapse at 3 years.12 Unfortunately, new personalized treatment strategies, through improved multimodal approaches including surgical resection, in combination with neoadjuvant and targeted chemotherapy, have yet to prove a significant benefit for the majority of patients.
ETIOLOGY, PREVENTION, & SCREENING: CHALLENGES TO DEFINE THE POPULATION‐AT‐RISK
Given the rising incidence of PDAC over the past 2 decades, special efforts must be made to better understand the risk factors contributing to disease development in order to promote prevention strategies and define the population at risk which could benefit from a protocolized screening.13 Currently, the known risk factors do not fully explain the rising incidence and its dramatic projections for the future. To date, no screening protocol exists that has demonstrated a medical and socio‐economic benefit for patients at high‐risk or the general population.
Etiology
PDAC is a paradigm of complex disease. Known environmental risk factors explain only about 40% of the disease. Family history (population attributable fraction, PAF = 3%–10%), tobacco (PAF = 11%–32%), type‐2 diabetes mellitus (T2DM) (PAF = 1%–16%), obesity (PAF = 3%–16%), chronic pancreatitis (PAF ≤ 3%), heavy alcohol intake (PAF ≤ 3%), and A/B blood group (PAF = 13%–19%) are the only firmly established environmental risk factors for PDAC, while allergy/asthma (PAF = 3%–7%) decrease the risk.14, 15, 16, 17, 18, 19
The growing prevalence of diabetes and obesity, mainly in Westernized countries (www.who.int/diabetes/global‐report), may partly account for the increased incidence of PDAC. Many meta‐analyses, carried out collecting datasets from studies including >3000 PDAC patients assessed that diabetic patients showed a 1.8‐fold increased risk of PC (95% CI 1.5–2.1).14 Similarly, obesity and overweight at any age are associated with increased incidence of PDAC, with hazard ratio ranging from 1.15 to 1.53. In this context, a longer duration of BMI > 25 kg/m2 is significantly associated with PDAC risk (HR per 10 years increment of duration: 1.06 95% CI: 1.05–1.32).20
Chronic inflammation is well‐known to play an important role in pancreatic carcinogenesis. For chronic pancreatitis, the pooled relative risk estimates for PDAC varies from 2.7 to 13.3. The PDAC risk is especially high in hereditary pancreatitis due to PRSS1 mutations. Among all risk factors associated with PDAC, pancreatitis has the highest relative risk of greater than 2, however, the prevalence of PDAC in sporadic chronic pancreatitis is not high enough to justify screening or surveillance, except for hereditary pancreatitis.21 In addition, recent data also suggests a contribution of the microbiome22 which warrants further clinical validation and exploitation. Despite these accumulating data, there are still important knowledge gaps on the association between all these factors and the resulting PDAC risk. In particular, knowledge is limited on the cumulative risk of cancer in case of the presence of multiple parallel risk factors.
Epidemiological studies have classically approached the analysis of risk factors on a “one‐by‐one basis.” However, this strategy is too simplistic when considering the reality that genetic and non‐genetic exposures concur within a given individual. In most cases, a context of interactions between multiple (epi)genomic, and environmental risk factors is involved. This fact supports the use of multifactorial strategies as a must to mimic biology. The technological omics revolution provides a wealth of information that can be exploited under this concept to generate knowledge that can be applied at the population level. As the complexity of the biological mechanisms underlying disease increases, so does the need to adopt an interdisciplinary or trans‐disciplinary approach in order to disentangle the intricate factors involved in disease development and progression.
A better understanding of the modifiable risk factors, the major component involved in pancreas carcinogenesis, and their interactions with (epi)genetic host profiles would allow designing appropriate primary prevention interventions with the ultimate goal of decreasing PDAC incidence and mortality in Europe. Large‐scale international collaboration is essential to accelerate and strengthen discovery in PDAC etiology, as well as to speed‐up translation of results into the public health domains. Primary prevention is aimed at reducing the effects of contributory risk factors of which tobacco smoking is a moderate, and obesity, excess alcohol consumption (>30 g [3.8 units] per day) and red meat consumption are low risk relative factors.14 Thus, the top 6 of the 12 recommendations of the European Code against Cancer to prevent cancer are all directly relevant to pancreatic cancer prevention.23 The difficulties in defining a high‐risk population explain, in part, the lack of success of the screening interventions showing a large proportion of false positive findings. More multi‐national large‐scale studies are needed to elucidate the impact of lifestyle habits, diets and dietary constituents, physical activity, microbiota, hydrocarbon compounds, pesticides, fine particles, heavy metals, and other yet unidentified triggers of carcinogenesis.
Genetic susceptibility
PDAC, like many other complex diseases, has genetic and environmental components to its etiology. Up to 40 common genetic variants with modest effects on PDAC risk have been identified playing a role in genetic susceptibility to sporadic forms of the disease.24 The relatively high frequency of such variants means that they could potentially explain a substantial portion of disease risk. Several attempts to build polygenetic scores have not been successful in their translation into the prevention field.25 More comprehensive approaches are needed to make progress, including global analyses of biologically relevant pathways and genome‐wide association studies. In this regard, a recent multi‐step GWAS strategy conducted solely in European population, combined with an in‐depth in silico functional analysis, allowed to identify further low‐penetrance loci.26 A number of consortia comprising pre‐existing studies have already been formed to facilitate the identification of further low‐penetrance variants and gene‐environment interaction through meta‐GWAS strategies. However, these approaches do not substitute for the design of novel, sufficiently powered studies that apply uniform criteria to case selection, the acquisition of environmental exposure information, and to biological sample collection.27
Screening of individuals‐at‐risk (IAR)
For now, a specific screening program is proposed to individuals with a strong family history of pancreatic cancer and/or genetic susceptibility of developing PDAC, among them individuals with suspected familial pancreatic cancer (FPC). FPC is defined by occurrence of PDAC in at least two first‐degree relatives and could account for up to 10% of all PDAC cases.28 The risk increases with the number of first‐degree relatives with PDAC, with a standardized incidence ratio between 17 and 32 for individuals with ≥3 first‐degree relatives with PDAC. In 2020, the international CAPS consortium proposed updated recommendations for surveillance. The main objectives of this screening are to detect high‐grade dysplastic precancerous lesions (including intraductal papillary mucinous neoplasm [IPMN] and PanIN) and T1N0M0 pancreatic cancer. This surveillance relies on a morphological evaluation using endoscopic ultrasound (EUS) and/or Magnetic Resonance Imaging (MRI)/magnetic retrograde cholangiopancreatography (MRCP) as preferred procedures. However, no consensus was found regarding the preferred modality. An annual surveillance was proposed with no robust data to support this recommendation. Moreover, no consensus was reached for surveillance in case of chronic pancreatitis due to genetic defects. The difficulties to reach consensus by this consortium (which combined the main experts of this field) reflects the lack of robust data in the literature. It also highlights the lack of biological tools to detect early precancerous lesions.28
EARLY DIAGNOSIS: CHALLENGES TO FIND INVISIBLE PRECURSORS
Due to its location deep in the body, the pancreas is not easily accessible for screening procedures such as the breast, colon, or prostrate. When suspected, only macroscopic pancreatic cancer lesions can be detected using imaging techniques, often revealing advanced or metastatic stages. Therefore, early diagnosis has to rely on imaging and blood tests rather than direct inspection.
Given the absence of early clinical symptoms or currently available reliable clinical indicators for preneoplastic stages or early PDAC in the general population, early diagnosis is most likely to succeed in high‐risk individuals described above. In this population, potential novel markers can be searched that eventually can be used for screening after clinical validation.
In this context, two groups of individuals‐at‐risk (IAR) stand out: (1) members from families with heredity for PDAC development, particularly in case of suspected familial pancreatic cancer (FPC) and (2) patients with preneoplastic lesions such as cystic pancreatic tumors, so‐called “intraductal papillary mucinous neoplasm” (IPMN). IPMN are further divided in main‐duct IPMN that represent an obligatory precancerous lesion and are generally subjected to surgery, and side‐branch IPMN which represent cystic lesions with a lower potential for malignant transformation. Current recommendations and guidelines for both IAR28 and IPMN29 describe how to conduct surveillance but are frequently limited by lack of evidence.
State‐of‐the‐art imaging
Modern imaging technologies, preferably with MRI and MRCP given the need for repetitive investigations in these patients groups,30 are able to detect millimeter‐size lesions. Current guidelines define “worrisome features” indicating a progress towards malignancy in IPMN.29 All suspicious lesions IAR need to be further studied.28 In such patients, more invasive diagnostic methods could be discussed in a multidisciplinary team31: EUS with fine‐needle biopsy32 being standard of care, and ERCP with direct pancreatoscopy (Spyglass),33 eventually with confocal laser microscopy (pCLE)34 representing appealing additional tools. While there is a definitive diagnostic gain, all these invasive methods carry a risk for complications. They should only be performed in patients in which the diagnosis is doubtful and a therapeutic consequence (e.g., surgery) is considered. While current imaging is able to detect millimeter‐size lesions, the vast majority of these cystic lesions will never progress to malignancies. To date, imaging tools to reliably predict the malignant potential are lacking. In this context, PET imaging modalities have attracted increasing attention which needs to be further explored.35 To achieve a higher diagnostic accuracy, molecular imaging, radiomics, digital pathology approaches, and refined cross‐sectional and EUS‐based techniques have to be developed and evaluated. In addition, blood‐based liquid biopsies may be suitable to identify high‐risk lesions more reliably.
Liquid biopsies
Identifying biomarkers in body fluids (liquid biopsies) for early PDAC or preinvasive precursors with potential for malignant transformation is the holy grail in pancreatic cancer research. Although the tumor marker CA 19‐9 has a low sensitivity, it is the best available marker for PDAC to date.36 Increase in CA 19‐9, even if still in the normal range (<35 u/ml), is indicative of a progress in IPMN37 and indication for surgery.38 In contrast to other tumors, the development of liquid biopsies in PDAC is lacking behind. Circulating tumor cells are a rare event in PDAC.39 Some reports described the presence of circulating epithelial cells in premalignant lesions such as IPMN.40, 41 Free circulating DNA (ctDNA)42, 43 and exosomes have been studied in invasive PDAC,44 less so in premalignant conditions. Currently, extracellular vesicles (exosomes) are among the most promising candidates for early diagnosis of PDAC.45 In these vesicles, several candidate markers for IPMN in exosomes such as KRAS mutations and glycipan have been proposed.46 Apart from serum, several publications have proposed pancreatic juice as another promising source for molecular analysis for diagnosis of early lesions.47 In addition to individual markers, panels of different markers are more likely to predict malignancy with sufficient diagnostic accuracy. Stemming from a study in serum protein profiling,48 a panel of immunoregulatory marker proteins has been tested in PDAC patients.49 A study in IAR testing this panel has finished recruiting (PANFAM, NCT03693378). Given the fact that many initially promising markers or combinations thereof have failed validation in large trials, it is evident that all of these approaches need confirmation in independent multi‐center cross‐national prospective trials requiring a pan‐European network that utilizes all state‐of‐the‐art methodologies including multi‐omics platforms and artificial intelligence algorithms developed by academic centers and industry.
CURATIVE THERAPY: CHALLENGES TO IMPROVE SURGICAL OUTCOMES – THERAPY SEQUENCE AND MULTIMODAL THERAPY
Systemic chemotherapy is the mainstay for improving progression free and overall survival (OS) in patients with metastatic disease and/or locally advanced PDAC.50, 51, 52, 53, 54 Using additional chemoradiotherapy increases toxicity without improving OS, either upfront to systemic chemotherapy or following chemotherapy without progression for locally advanced disease.55, 56, 57
Adjuvant therapy
In patients with locally resectable tumors but without metastatic disease, advances in surgical techniques and post‐operative management combined with multimodality therapy in the form of adjuvant systemic chemotherapy has led to a dramatic improvement in outcomes (Table S1).55, 56, 57 There has been a remarkable reduction in post‐operative mortality from 30% in general surgical units to less than 5% in specialist centralized units.58, 59 The use of adjuvant systematic chemotherapy has resulted in an amazing increase in a 5‐year survival from 8% with resection alone to 30%–50% when this was followed by 6 months combination chemotherapy.11, 12, 60, 61, 62, 63 The longest OS achieved with adjuvant chemotherapy is using mFOLFIRINOX (modified folinic acid, 5‐fluorouracil [5‐FU], irinotecan, and oxaliplatin), but this comes with added toxicity and frequent hospitalizations compared to adjuvant gemcitabine and capecitabine.11, 12, 60, 61, 62, 63 Although mFOLFIRINOX is recommended as the preferred treatment it should be borne in mind that the PRODIGE24 trial used highly selected patients with a more favorable prognosis than those in the ESPAC‐4 trial and there may be differences between the two regimens in survival responses in different subgroups.11, 12, 60, 61, 62, 63
The combination of gemcitabine and nab‐paclitaxel was found to be effective in the metastatic setting, although less so than FOLFIRINOX in this same setting. In the adjuvant setting gemcitabine and nab‐paclitaxel was not superior to gemcitabine for the primary endpoint of disease‐free survival and is not approved for this purpose by the FDA.64 Nab‐paclitaxel was developed to target the stroma of the primary cancer and may explain the failure of the combination in this setting.65 As in advanced PDAC the use of chemoradiotherapy in addition to chemotherapy does not improve survival whilst increasing toxicity and may even be deleterious.11, 12, 60, 61, 62, 63, 64, 66, 67, 68, 69, 70, 71
Neoadjuvant therapy
The use of neoadjuvant therapy appears to increase resectability and hence survival in patients with otherwise unresectable local disease due to local vessel encasement, even in the presence of resectable oligometastatic disease (Table S2).72, 73 An argument has also been made to extend the use of neoadjuvant therapy to patients with resectable disease because the total chemotherapy delivered might be reduced to post‐operative complications but in reality, this is not achieved.74, 75, 76 A recent trial of perioperative chemotherapy comprising 12 weeks preoperative chemotherapy, followed by surgery, and then 12 weeks postoperative chemotherapy randomized patients to either mFOLFIRINOX or gemcitabine plus nab‐paclitaxel.74, 75, 76 The findings showed that using neoadjuvant chemotherapy, compared with adjuvant trials did not demonstrate an improved OS.74, 75, 76
Randomized studies using a mixture of resectable, borderline resectable, and locally advanced disease have tended to produce a confused picture.77, 78, 79 Two recent trials focusing only on borderline resectable disease, however, have produced more consistent results.80, 81 The ESPAC‐5FT trial has shown that using neoadjuvant therapy is superior to upfront surgery with little difference in OS between mFOLFIRINOX and gemcitabine/capecitabine.80, 81 Both ESPAC‐5FT and the Alliance A021501 randomized trials showed that chemoradiotherapy (as in the advanced and adjuvant settings) was inferior to chemotherapy alone.80, 81 However, further evidence from other ongoing trials is awaited.
Despite marked progress achieved through the advent of potent adjuvant and neoadjuvant chemotherapy regimens, many questions on optimized treatment sequences depending on defined molecular subtypes or on the role of targeted therapies in the (neo)adjuvant setting remain to be addressed and require a concerted pan‐European trial network.
OVERCOMING RESISTANCE: CHALLENGES TO TARGETED THERAPIES
Genetically, PDAC is an extremely heterogenous disease, characterized by an abundant stroma reaction and a highly immune‐evasive phenotype, contributing to the primary or secondary resistance to systemic therapies frequently observed in this tumor.
In patients with metastatic PDAC, systemic chemotherapy represents the only treatment option. During the last decade clinically relevant survival benefits could be achieved by using combination regimens such as FOLFIRINOX52 and gemcitabine + Nab‐paclitaxel.82 However, only a fraction of patients responds to these cytotoxic drugs and current targeted options have proven largely futile.83
Genetic Heterogeneity
Following genomic and transcriptomic profiling results, two major transcriptomic subtypes of PDAC (“classical” and “basal‐like”) have been proposed,84, 85, 86 with basal‐like PDACs frequently responding poorly to conventional chemotherapy protocols.83 Moffitt et al. further refined the transcriptional subtypes by proposing “normal” and “activated” stromal subtypes, the latter being associated with poor survival.85 Given the fact that currently systemic therapies of PDAC still rely on conventional chemotherapy, increasing interest is focused on developing transcriptomic signatures predictive of treatment response to different agents.87 So far, however, subtype‐specific treatment strategies have not been shown to be clinically relevant.88
Similarly, targeting single driver mutations has proven difficult in the clinical setting, given the low frequency of individual druggable mutations.83 Exceptions affect only small fractions of PDAC patients and include microsatellite instable tumors amenable to checkpoint inhibition,89 tumors with germline BRCA1/2 mutations benefiting from platinum‐based chemotherapies and PARP‐inhibitors90 and the rare KRAS wild‐type tumors with a higher frequency of mutations such as NRG1 or NTRK fusions.83, 91, 92 In contrast to other tumor entities such as melanoma, lung cancer, and breast cancer, prospective evidence for genetic testing strategies in sporadic PDAC patients is still missing and molecular characterization of PDAC in patients with advanced disease has not yet entered routine clinical practice.83 Due to the low frequency of individual genetic alterations, clinical evaluation requires innovative trial concepts (umbrella or basket trials) involving large patient cohorts that are evaluated through a common genetic testing platform and subsequently enrolled in mutation‐specific trials arm.
Complex stromal reaction
PDAC is characterized by an extensive stromal reaction with massive extracellular matrix deposition accounting for up to 90% of the tumor volume, rendering this tumor entity one of the most stroma‐rich solid tumor types.83, 93 The pancreatic tumor microenvironment has attracted much interest in the past decade, particularly its role as a determinant of therapy resistance. The PDAC stroma contains a variety of cellular components, including inflammatory cells, cancer‐associated fibroblasts, endothelial and nerve cells as well as numerous acellular components including collagens, fibronection and hyaluronic acid.93 The initial enthusiasm to target the dense, hypovascular stroma was belied by the failure of all previous large trials targeting cellular or acellular stroma components as well as stromal signaling pathways,93 indicating that the highly complex, time‐ and space‐dependent interactions between different stromal components and tumor cells represent an ongoing challenge for therapeutic intervention.
Immune‐evasive phenotype
In contrast to numerous cancer types, most prominently malignant melanoma, PDAC is largely immune‐evasive and refractory to conventional immunotherapy by checkpoint inhibitors, probably reflecting the immunosuppressive nature of the its microenvironment,93 Mechanisms by which PDAC suppresses the immune response are currently being extensively studied, including activation of regulatory T cells (Treg cells),94 or myeloid‐derived suppressor cells (MDSCs),95 inhibition of cytotoxic T cells, or modulation of macrophage populations within the tumor.93, 96 Numerous therapeutic combination strategies addressing various components of the adaptive and innate immune response are currently being evaluated to enhance the immunogenicity of PDAC. However, none of these strategies so far has proven to be improve patients outcome so far.
A PATIENT‐CENTERED VIEW OF PANCREATIC CANCER CARE
While measurement of “value of healthcare,” engagement of patients and evaluation of patient‐reported outcomes (PROs) are becoming essential parts of modern medicine,97 this is particularly relevant for a disease such as PDAC, where life expectancy is often modest, quality of life (QoL) is heavily impaired and care requires specific skills and organization. However, specific performance indicators for the care of PDAC patients that take into account their view and preferences are rarely employed in clinical practice.
PROs are defined as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”98 Accordingly, PROs should be measured by structured instruments, defined patient‐reported outcome measures (PROMs) that should be developed and validated in conjunction with patients and caregivers and patients’ advocacy groups. A recent systematic review on PROs and PDAC identified only 170 studies employing PROMs in PDAC patients.99
The more frequently employed tools to evaluate patient‐reported outcome measure (PROMs) in PDAC are the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ‐C30) and the EORTC Quality of Life Questionnaire Pancreatic Cancer Module (QLQ‐PAN26).100
QLQ‐C30 has been used more diffusely in PDAC patients with different disease stage, typically as secondary outcome in randomized controlled trials investigating treatment strategies.11 Overall, the available data suggest that PDAC patients report a lower quality of life with more challenges for themselves and caregivers compared to other tumor types.100
The QLQ‐PAN26, which is more specific and should possibly be used together with the QLQ‐C30 to gain a global view of different aspects of QoL, has been developed taking into consideration the view of patients and patients’ advocacy groups.101 This instrument, however, needs further validation in the different disease stages and has not been translated in many languages yet. Besides PAN26, another specific instrument developed with PDAC patients, and employed in a more limited number of studies being the Functional Assessment of Cancer Therapy–Hepatobiliary.102
Notably, the number of studies investigating PROs in advanced disease, especially beyond 12 months of disease is extremely limited, urging more investigation in this field, as the introduction of more effective intensified chemotherapy regimens is likely to guarantee a longer life expectancy for many patients.99
Over the past few years, there have been other attempts to develop specific sets of PROs with the collaboration of patients’ representatives, but they have not been validated yet.103, 104
Another important aspect of patient‐centered care is the need to engage the patient in the treatment process to obtain optimal compliance and better QoL and clinical results. Patient‐engagement can be measured by specific validate scales, such as the PHE, that is currently being explored in PDAC patients.105
Finally, the experience and perception of patients during each step of the care process should be measured with questionnaires reporting patient‐reported experience measures (PREMs).106 There are no PREMs investigating PDAC patients trajectories during the different specific steps of care, such as endoscopic procedures, surgery, chemotherapy, or other clinical episodes.
Integration of PROMs and PREMs into the whole process of cure of PDAC patients is likely to improve not only the engagement of patients and their QoL, increasing the awareness of the treating medical team toward unmet needs, but eventually to result in improved survival as recently demonstrated.107 The development of specific user‐friendly mobile Applications (App) may result particularly useful in this setting as suggested by preliminary findings with an App developed for the management of PROs after pancreaticoduodenectomy.108 The development of such integrated, digitalized systems to monitor the whole health care process, requires joint public‐private efforts with technical know‐how from the industry and input from academic sites and patients’ advocacy groups.
THE URGENT NEED: A PAN‐EUROPEAN CONCERTED ACTION ON PANCREATIC CANCER WITH STRONG PUBLIC‐PRIVATE PARTNERSHIPS
Pancreatic cancer represents a huge challenge for researchers across Europe, spanning from understanding the fundamental basis of the disease, its peculiar molecular profiles, lack of existing tools for early detection, and effective treatments that would enhance patient survival and quality of life.
Despite its staggering growing epidemiology, research on pancreatic cancer is receiving fractions of the budgeted funds for cancer research established by European funding bodies. In order to effectively slow down the epidemiological trend and beat the disease, it is mandatory to establish quick and concerted actions to accelerate advances and reduce the high mortality burden that will need to see multiple stakeholders involved throughout the continent. Thus, coordinated efforts are absolutely vital in order to generate advancements that will inevitably lead to better prevention and treatment.
The new framework program, Horizon Europe, will aim at providing significant opportunities to improve European citizens’ health. Within Horizon Europe, the new Innovative Health Initiative will create a European multi‐sector partnership for health innovation, fostering in particular PPP, with the overall goal to “accelerate the development of safer and more effective healthcare interventions that respond to unmet public health needs, and that can be taken up by healthcare systems” (https://www.euhealthppp.org). We believe that research on pancreatic cancer fits completely within the agenda for Innovation in Healthcare that falls in the framework of the proposed PPP.
While policy makers must produce a targeted EU funding policy for pancreatic cancer, PPP are crucial to tackle the barriers that are hampering the advancement for understanding and treating this disease, boosting cross‐national, pan‐European efforts. This will require co‐operation across sectors, that will lead to meeting the priorities set by the Europe’s beating cancer plan and the Mission on Cancer. The partnerships will take advantage of the strong academic European landscape, which consists of existing centers and researchers of excellence devoted to research on pancreatic cancer.
The contribution by the industry of all sizes will be crucial for the development of new programs that would develop new technological tools (including AI), platforms for high‐quality data sharing and new pharmacological strategies by taking advantage of a more synergic efforts through the sharing of big data from relevant cohorts, registries, and by federating biobanks across Europe.
While boosting the competitiveness of the industry across the continent, such partnership will lead to a more precise prevention, early diagnosis, effective treatment and personalized, patient‐centered care, for a disease that up to now has not reached any of these goals.
Thus, we strongly support such partnership and the inclusion of the challenges of pancreatic cancer in the Mission on Cancer and Europe´s Beating Cancer Plan. We believe that these initiatives represent a unique opportunity for beating pancreatic cancer in Europe, with the ultimate goal of contributing to better health outcomes across the EU.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Supplementary Information Material
ACKNOWLEDGEMENTS
John P. Neoptolemos has received research funding from the Dietmar Hopp Stiftung GmbH, the Stiftung Deutsche Krebshilfe, and the Heidelberger Stiftung Chirurgie, all of which were not related to the submitted work.
Patrick Michl is supported by the German Research Foundation (DFG) and Eli Lilly & Co., all of which are not related to the submitted work.
Matthias Löhr is supported by the Signe och Olof Wallenius Stiftelsen, Cancerfonden, Radiumhemmets Forskningsfonder, Familjen Kamprad Stiftelsen, and the EU (PRECODE, PANCAIM). The work on liquid biopsy is supported by EU project CanDO, CIMED, and Vetenskapsrådet.
Luigi Ricciardiello is Chair of the UEG Research committee (RC), Patrick Michl is member of the UEG Public Affairs Committee, MO is Research Policy Officer at the UEG Secretariat, Matthias Löhr serves as secretary and Vinciane Rebours as council member to the European Pancreatic Club (EPC) & member of UEG RC and Nuria Malats serves as member of the Pancreatic Cancer Europe (PCE) consortium.
No Relevant Funding for this paper.
Michl P, Löhr M, Neoptolemos JP, Capurso G, Rebours V, Malats N, et al. UEG position paper on pancreatic cancer. Bringing pancreatic cancer to the 21st century: prevent, detect, and treat the disease earlier and better. United European Gastroenterol J. 2021;9(7):860–871. 10.1002/ueg2.12123
Contributor Information
Patrick Michl, Email: patrick.michl@uk-halle.de.
Luigi Ricciardiello, Email: luigi.ricciardiello@unibo.it.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1.Prades J, Arnold D, Brunner T, Cardone A, Carrato A, Coll‐Ortega C, et al. Bratislava Statement: consensus recommendations for improving pancreatic cancer care. ESMO Open. 2020;5:e001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall BR, Cannon A, Atri P, Wichman CS, Smith LM, Ganti AK, et al. Advanced pancreatic cancer: a meta‐analysis of clinical trials over thirty years. Oncotarget. 2018;9:19396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca ‐ Cancer J Clin. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- 5.Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd‐Allah F, Abdel‐Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. Ca – Cancer J Clin. 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 7.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25:1650–6. [DOI] [PubMed] [Google Scholar]
- 8.Carioli G, Malvezzi M, Bertuccio P, Boffetta P, Levi F, La Vecchia C, et al. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann Oncol. 2021;32:478–87. [DOI] [PubMed] [Google Scholar]
- 9.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Canc Res. 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- 10.Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D, et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999‐2007: results of EUROCARE‐5. Eur J Canc. 2015;51:2169–78. [DOI] [PubMed] [Google Scholar]
- 11.Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC‐4): a multicentre, open‐label, randomised, phase 3 trial. Lancet. 2017;389:1011–24. [DOI] [PubMed] [Google Scholar]
- 12.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–406. [DOI] [PubMed] [Google Scholar]
- 13.Barone E, Corrado A, Gemignani F, Landi S. Environmental risk factors for pancreatic cancer: an update. Arch Toxicol. 2016;90:2617–42. [DOI] [PubMed] [Google Scholar]
- 14.Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta‐analytical studies. Int J Epidemiol. 2015;44:186–98. [DOI] [PubMed] [Google Scholar]
- 15.Rosato V, Polesel J, Bosetti C, Serraino D, Negri E, La Vecchia C. Population attributable risk for pancreatic cancer in Northern Italy. Pancreas. 2015;44:216–20. [DOI] [PubMed] [Google Scholar]
- 16.Gomez‐Rubio P, Pinero J, Molina‐Montes E, Gutierrez‐Sacristan A, Marquez M, Rava M, et al. Pancreatic cancer and autoimmune diseases: an association sustained by computational and epidemiological case‐control approaches. Int J Canc. 2019;144:1540–9. [DOI] [PubMed] [Google Scholar]
- 17.Gomez‐Rubio P, Rosato V, Marquez M, Bosetti C, Molina‐Montes E, Rava M, et al. A systems approach identifies time‐dependent associations of multimorbidities with pancreatic cancer risk. Ann Oncol. 2017;28:1618–24. [DOI] [PubMed] [Google Scholar]
- 18.Molina‐Montes E, Gomez‐Rubio P, Marquez M, Rava M, Lohr M, Michalski CW, et al. Risk of pancreatic cancer associated with family history of cancer and other medical conditions by accounting for smoking among relatives. Int J Epidemiol. 2018;47:473–83. [DOI] [PubMed] [Google Scholar]
- 19.Molina‐Montes E, Coscia C, Gómez‐Rubio P, Fernández A, Boenink R, Rava M, et al. Deciphering the complex interplay between pancreatic cancer, diabetes mellitus subtypes and obesity/BMI through causal inference and mediation analyses. Gut. 2021;70:319–29. [DOI] [PubMed] [Google Scholar]
- 20.Stolzenberg‐Solomon RZ, Schairer C, Moore S, Hollenbeck A, Silverman DT. Lifetime adiposity and risk of pancreatic cancer in the NIH‐AARP Diet and Health Study cohort. Am J Clin Nutr. 2013;98:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenhalf W, Lévy P, Gress T, Rebours V, Brand RE, Pandol S, et al. International consensus guidelines on surveillance for pancreatic cancer in chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and European Pancreatic Club. Pancreatology. 2020;20:910–8. [DOI] [PubMed] [Google Scholar]
- 22.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 2019;178:795‐806.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schüz J, Espina C, Villain P, Herrero R, Leon ME, Minozzi S, et al. European Code against Cancer 4th Edition: 12 ways to reduce your cancer risk. Cancer Epidemiol. 2015;39(Suppl 1):S1–10. [DOI] [PubMed] [Google Scholar]
- 24.Amundadottir LT. Pancreatic cancer genetics. Int J Biol Sci. 2016;12:314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galeotti AA, Gentiluomo M, Rizzato C, Obazee O, Neoptolemos JP, Pasquali C, et al. Polygenic and multifactorial scores for pancreatic ductal adenocarcinoma risk prediction. J Med Genet. 2020. [DOI] [PubMed] [Google Scholar]
- 26.López de Maturana E, Rodríguez JA, Alonso L, Lao O, Molina‐Montes E, Martín‐Antoniano IA, et al. A multilayered post‐GWAS assessment on genetic susceptibility to pancreatic cancer. Genome Med. 2021;13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milne RL, Greenhalf W, Murta‐Nascimento C, Real FX, Malats N. The inherited genetic component of sporadic pancreatic adenocarcinoma. Pancreatology. 2009;9:206–14. [DOI] [PubMed] [Google Scholar]
- 28.Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020;69:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Study Group on Cystic Tumours of the P . European evidence‐based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Chiaro M, Verbeke CS, Kartalis N, Pozzi Mucelli R, Gustafsson P, Hansson J, et al. Short‐term results of a magnetic resonance imaging‐based Swedish screening program for individuals at risk for pancreatic cancer. JAMA Surg. 2015. [DOI] [PubMed] [Google Scholar]
- 31.Del Chiaro M, Segersvard R, Pozzi Mucelli R, Rangelova E, Kartalis N, Ansorge C, et al. Comparison of preoperative conference‐based diagnosis with histology of cystic tumors of the pancreas. Ann Surg Oncol. 2014;21:1539–44. [DOI] [PubMed] [Google Scholar]
- 32.Chen YI, Chatterjee A, Berger R, Kanber Y, Wyse JM, Lam E, et al. EUS‐guided fine needle biopsy alone vs. EUS‐guided fine needle aspiration with rapid on‐site evaluation of cytopathology in pancreatic lesions: a multicenter randomized trial. Endoscopy. 2021. [DOI] [PubMed] [Google Scholar]
- 33.Arnelo U, Siiki A, Swahn F, Enochsson L, Segersvärd R, Del Chiaro M, et al. Single‐operator pancreatoscopy is helpful in the evaluation of suspected intraductal papillary mucinous neoplasms (IPMN). Pancreatology. 2014;14:510–4. [DOI] [PubMed] [Google Scholar]
- 34.Löhr JM, Lonnebro R, Stigliano S, Haas SL, Swahn F, Enochsson L, et al. Outcome of probe‐based confocal laser endomicroscopy (pCLE) during endoscopic retrograde cholangiopancreatography: a single‐center prospective study in 45 patients. United European Gastroenterol J. 2015;3:551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghaneh P, Hanson R, Titman A, Lancaster G, Plumpton C, Lloyd‐Williams H, et al. PET‐PANC: multicentre prospective diagnostic accuracy and health economic analysis study of the impact of combined modality 18fluorine‐2‐fluoro‐2‐deoxy‐d‐glucose positron emission tomography with computed tomography scanning in the diagnosis and management of pancreatic cancer. Health Technol Assess. 2018;22:1–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee T, Teng TZJ, Shelat VG. Carbohydrate antigen 19‐9 – tumor marker: past, present, and future. World J Gastrointest Surg. 2020;12:468–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciprani D, Morales‐Oyarvide V, Qadan M, Hank T, Weniger M, Harrison JM, et al. An elevated CA 19‐9 is associated with invasive cancer and worse survival in IPMN. Pancreatology. 2020;20:729–35. [DOI] [PubMed] [Google Scholar]
- 38.Jan IS, Chang MC, Yang CY, Tien YW, Jeng YM, Wu CH, et al. Validation of indications for surgery of European evidence‐based guidelines for patients with pancreatic intraductal papillary mucinous neoplasms. J Gastrointest Surg. 2020;24:2536–43. [DOI] [PubMed] [Google Scholar]
- 39.Luchini C, Veronese N, Nottegar A, Cappelletti V, Daidone MG, Smith L, et al. Liquid biopsy as surrogate for tissue for molecular profiling in pancreatic cancer: a meta‐analysis towards precision medicine. Cancers; 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhim AD, Thege FI, Santana SM, Lannin TB, Saha TN, Tsai S, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146:647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenbaum MW, Cauley CE, Kulemann B, Liss AS, Castillo CF, Warshaw AL, et al. Cytologic characteristics of circulating epithelioid cells in pancreatic disease. Cancer. 2017;125:332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strijker M, Soer EC, de Pastena M, Creemers A, Balduzzi A, Beagan JJ, et al. Circulating tumor DNA quantity is related to tumor volume and both predict survival in metastatic pancreatic ductal adenocarcinoma. Int J Canc. 2020;146:1445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metzenmacher M, Varaljai R, Hegedus B, Cima I, Forster J, Schramm A, et al. Plasma next generation sequencing and droplet digital‐qPCR‐based quantification of circulating cell‐free RNA for noninvasive early detection of cancer. Cancers. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican‐1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu S, Li Y, Liao Z, Wang Z, Wang Z, Li Y, et al. Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for the detection of pancreatic ductal adenocarcinoma. Gut. 2020;69:540–50. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka M, Ishikawa S, Ushiku T, Morikawa T, Isagawa T, Yamagishi M, et al. EVI1 modulates oncogenic role of GPC1 in pancreatic carcinogenesis. Oncotarget. 2017;8:99552–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levink IJM, Nesteruk K, Visser DI, Sieuwerts AM, Fernandes CJC, Jansen MPHM, et al. Optimization of pancreatic juice collection: a first step toward biomarker discovery and early detection of pancreatic cancer. Am J Gastroenterol. 2020;115:2103–8. [DOI] [PubMed] [Google Scholar]
- 48.Wingren C, Sandstrom A, Segersvard R, Carlsson A, Andersson R, Löhr M, et al. Identification of serum biomarker signatures associated with pancreatic cancer. Canc Res. 2012;72:2481–90. [DOI] [PubMed] [Google Scholar]
- 49.Mellby LD, Nyberg AP, Johansen JS, Wingren C, Nordestgaard BG, Bojesen SE, et al. Serum biomarker signature‐based liquid biopsy for diagnosis of early‐stage pancreatic cancer. J Clin Oncol. 2018;36:2887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.BurrisHA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. [DOI] [PubMed] [Google Scholar]
- 51.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- 53.Wang‐Gillam A, Li C‐P, Bodoky G, Dean A, Shan Y‐S, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine‐based therapy (NAPOLI‐1): a global, randomised, open‐label, phase 3 trial. Lancet. 2016;387:545–57. [DOI] [PubMed] [Google Scholar]
- 54.Gill S, Ko YJ, Cripps C, Beaudoin A, Dhesy‐Thind S, Zulfiqar M, et al. PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second‐line advanced pancreatic cancer in patients who have received gemcitabine‐based chemotherapy. J Clin Oncol. 2016;34:3914–20. [DOI] [PubMed] [Google Scholar]
- 55.Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta‐analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007;25:2607–15. [DOI] [PubMed] [Google Scholar]
- 56.Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 Months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. J Am Med Assoc. 2016;315:1844–53. [DOI] [PubMed] [Google Scholar]
- 57.Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11–26. [DOI] [PubMed] [Google Scholar]
- 58.Hata T, Motoi F, Ishida M, Naitoh T, Katayose Y, Egawa S, et al. Effect of hospital volume on surgical outcomes after pancreaticoduodenectomy: a systematic review and meta‐analysis. Ann Surg. 2016;263:664–72. [DOI] [PubMed] [Google Scholar]
- 59.Mackay TM, Wellner UF, van Rijssen LB, Stoop TF, Busch OR, Groot Koerkamp B, et al. Variation in pancreatoduodenectomy as delivered in two national audits. Br J Surg. 2019;106:747–55. [DOI] [PubMed] [Google Scholar]
- 60.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. [DOI] [PubMed] [Google Scholar]
- 61.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative‐intent resection of pancreatic cancer: a randomized controlled trial. J Am Med Assoc. 2007;297:267–77. [DOI] [PubMed] [Google Scholar]
- 62.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. J Am Med Assoc. 2010;304:1073–81. [DOI] [PubMed] [Google Scholar]
- 63.Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S‐1 versus gemcitabine for resected pancreatic cancer: a phase 3, open‐label, randomised, non‐inferiority trial (JASPAC 01). Lancet. 2016;388:248–57. [DOI] [PubMed] [Google Scholar]
- 64.Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. 2019;17:603–5. [DOI] [PubMed] [Google Scholar]
- 65.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab‐paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. Adjuvant radiotherapy and 5‐fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–82. discussion 82‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smeenk HG, van Eijck CH, Hop WC, Erdmann J, Tran KC, Debois M, et al. Long‐term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long‐term results of EORTC trial 40891. Ann Surg. 2007;246:734–40. [DOI] [PubMed] [Google Scholar]
- 68.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–85. [DOI] [PubMed] [Google Scholar]
- 69.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long‐term outcomes among patients with resected pancreatic cancer: the CONKO‐001 randomized trial. J Am Med Assoc. 2013;310:1473–81. [DOI] [PubMed] [Google Scholar]
- 70.Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, et al. CONKO‐005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: a multicenter randomized phase III trial. J Clin Oncol. 2017;35:3330–7. [DOI] [PubMed] [Google Scholar]
- 71.Baschnagel A, Shah C, Margolis J, Nadeau L, Stein J, Jury R, et al. Survival after chemoradiation in resected pancreatic cancer: the impact of adjuvant gemcitabine. Int J Radiat Oncol Biol Phys. 2012. [DOI] [PubMed] [Google Scholar]
- 72.Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016;264:457–63. [DOI] [PubMed] [Google Scholar]
- 73.Diener MK, Mihaljevic AL, Strobel O, Loos M, Schmidt T, Schneider M, et al. Periarterial divestment in pancreatic cancer surgery. Surgery. 2020. [DOI] [PubMed]
- 74.Golcher H, Brunner TB, Witzigmann H, Marti L, Bechstein WO, Bruns C, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ettrich TJ, Berger AW, Perkhofer L, Daum S, König A, Dickhut A, et al. Neoadjuvant plus adjuvant or only adjuvant nab‐paclitaxel plus gemcitabine for resectable pancreatic cancer ‐ the NEONAX trial (AIO‐PAK‐0313), a prospective, randomized, controlled, phase II study of the AIO pancreatic cancer group. BMC Canc. 2018;18:1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sohal DPS, Duong M, Ahmad SA, Gandhi NS, Beg MS, Wang‐Gillam A, et al. Efficacy of perioperative chemotherapy for resectable pancreatic adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2021;7:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Versteijne E, Suker M, Groothuis K, Akkermans‐Vogelaar JM, Besselink MG, Bonsing BA, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38:1763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open‐label, multicenter phase 2/3 trial. Ann Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 79.Reni M, Balzano G, Zanon S, Zerbi A, Rimassa L, Castoldi R, et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT‐15): a randomised, open‐label, phase 2‐3 trial. Lancet Gastroenterol Hepatol. 2018;3:413–23. [DOI] [PubMed] [Google Scholar]
- 80.Ghaneh P, Palmer DH, Cicconi S, Halloran C, Psarelli EE, Rawcliffe CL, et al. Four‐arm, prospective, multicenter, international randomized phase II trial of immediate surgery compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or FOLFIRINOX or chemoradiotherapy (CRT) in patients with borderline resectable pancreatic cancer. J Clin Oncol. 2020;38:suppl_4505. [Google Scholar]
- 81.Katz MHG, Shi Q, Meyers JP, Herman JM, Choung M, Wolpin BM, et al. A021501: preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J Clin Oncol. 2021;39:abstr 377. [Google Scholar]
- 82.Von Hoff DD, Goldstein D, Renschler MF. Albumin‐bound paclitaxel plus gemcitabine in pancreatic cancer. N Engl J Med. 2014;370:479–80. [DOI] [PubMed] [Google Scholar]
- 83.Huber M, Brehm CU, Gress TM, Buchholz M, Alashkar Alhamwe B, von Strandmann EP, et al. The immune microenvironment in pancreatic cancer. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aguirre AJ, Collisson EA. Advances in the genetics and biology of pancreatic cancer. Canc J. 2017;23:315–20. [DOI] [PubMed] [Google Scholar]
- 85.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor‐ and stroma‐specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. [DOI] [PubMed] [Google Scholar]
- 87.Tiriac H, Belleau P, Engle DD, Plenker D, Deschenes A, Somerville T, et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Canc Discov; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pishvaian MJ, Bender RJ, Halverson D, Rahib L, Hendifar AE, Mikhail S, et al. Molecular profiling of pancreatic cancer patients: initial results from the know your tumor initiative. Clin Canc Res. 2018. [DOI] [PubMed] [Google Scholar]
- 89.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science. 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline. N Engl J Med. 2019;381:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O'Reilly EM, Hechtman JF. Tumour response to TRK inhibition in a patient with pancreatic adenocarcinoma harbouring an NTRK gene fusion. Ann Oncol. 2019;30(Suppl 8):viii36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heining C, Horak P, Uhrig S, Codo PL, Klink B, Hutter B, et al. NRG1 fusions in KRAS wild‐type pancreatic cancer. Cancer Discov; 2018. [DOI] [PubMed] [Google Scholar]
- 93.Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333–48. [DOI] [PubMed] [Google Scholar]
- 94.Tang H, Qiao J, Fu YX. Immunotherapy and tumor microenvironment. Canc Lett. 2016;370:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Markowitz J, Brooks TR, Duggan MC, Paul BK, Pan X, Wei L, et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid‐derived suppressor cells upon progression of disease. Cancer Immunol Immunother. 2015;64:149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borgoni S, Iannello A, Cutrupi S, Allavena P, D'Incalci M, Novelli F, et al. Depletion of tumor‐associated macrophages switches the epigenetic profile of pancreatic cancer infiltrating T cells and restores their anti‐tumor phenotype. OncoImmunology. 2018;7: e1393596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kotronoulas G, Kearney N, Maguire R, Harrow A, Di Domenico D, Croy S, et al. What is the value of the routine use of patient‐reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480–501. [DOI] [PubMed] [Google Scholar]
- 98.U.S. Department of Health and Human Services Food and Drug Administration (FDA), Maryland . Guidance for industry: patient‐reported outcome measures: use in medical product development to support labeling claims; 2009. [Google Scholar]
- 99.Maharaj AD, Samoborec S, Evans SM, Zalcberg J, Neale RE, Goldstein D, et al. Patient‐reported outcome measures (PROMs) in pancreatic cancer: a systematic review. HPB. 2020;22:187–203. [DOI] [PubMed] [Google Scholar]
- 100.Macarulla T, Hendifar AE, Li CP, Reni M, Riess H, Tempero MA, et al. Landscape of health‐related quality of life in patients with early‐stage pancreatic cancer receiving adjuvant or neoadjuvant chemotherapy: a systematic literature review. Pancreas. 2020;49:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fitzsimmons D, Johnson CD, George S, Payne S, Sandberg AA, Bassi C, et al. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ‐C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Canc. 1999;35:939–41. [DOI] [PubMed] [Google Scholar]
- 102.Yount S, Cella D, Webster K, Heffernan N, Chang C, Odom L, et al. Assessment of patient‐reported clinical outcome in pancreatic and other hepatobiliary cancers: the FACT Hepatobiliary Symptom Index. J Pain Symptom Manag. 2002;24:32–44. [DOI] [PubMed] [Google Scholar]
- 103.van Rijssen LB, Gerritsen A, Henselmans I, Sprangers MA, Jacobs M, Bassi C, et al. Core set of patient‐reported outcomes in pancreatic cancer (COPRAC): an international delphi study among patients and health care providers. Ann Surg. 2019;270:158–64. [DOI] [PubMed] [Google Scholar]
- 104.Cherkaoui Z, González C, Wakabayashi T, Delattre B, Léost E, Serra S, et al. A standard set of value‐based patient‐centered outcomes for pancreatic carcinoma: an international delphi survey. Ann Surg Oncol. 2021;28:1069–78. [DOI] [PubMed] [Google Scholar]
- 105.Consolandi M, Martini C, Reni M, Arcidiacono PG, Falconi M, Graffigna G, et al. COMMUNI.CARE (COMMUNIcation and patient engagement at diagnosis of PAncreatic CAncer): study protocol. COMMUNI. Front Med (Lausanne). 2020;7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Apadula L, Capurso G, Arcidiacono PG. Patient‐reported experience measure in pancreatobiliary endoscopy: a systematic review to highlight areas for improvement. Eur J Gastroenterol Hepatol. 2020. [DOI] [PubMed] [Google Scholar]
- 107.Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gustavell T, Sundberg K, Segersvärd R, Wengström Y, Langius‐Eklöf A. Decreased symptom burden following surgery due to support from an interactive app for symptom management for patients with pancreatic and periampullary cancer. Acta Oncol. 2019;58:1307–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Material
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


