Abstract
Background and aims
Incomplete microscopic colitis (MCi) is a subtype of microscopic colitis (MC). Budesonide is recommended as a first‐line treatment for MC. However, randomised trials on efficacy of treatment in MCi are missing. We therefore performed a randomised, placebo‐controlled trial to evaluate budesonide as induction therapy for MCi.
Methods
Patients with active MCi were randomly assigned to either budesonide 9 mg once daily or placebo for 8 weeks in a double‐blind, double‐dummy design. The primary endpoint was clinical remission, defined as a mean of <3 stools/day and a mean of <1 watery stool/day in the 7 days before week 8.
Results
Due to insufficient patient recruitment, the trial was discontinued prematurely. The intention‐to‐treat analysis included 44 patients (21 budesonide and 23 placebo). The primary endpoint of clinical remission at week 8 was obtained by 71.4% on budesonide and 43.5% on placebo (p = 0.0582). All clinical secondary endpoints were in favour of budesonide. Budesonide decreased the number of soft or watery stools (16.3 vs. 7.7, p = 0.0186) and improved health‐related quality of life for all four dimensions of the short health scale. Adverse events with a suspected relation to study drug were reported in one patient in the budesonide group and two patients in the placebo group. Neither serious nor severe adverse events occurred during the double‐blind phase.
Conclusions
Budesonide decreased the frequency of soft or watery stools and improved the patients' quality of life significantly in MCi, but the primary endpoint was not met due to the low sample size (type 2 error). Budesonide was safe and well tolerated during the 8‐weeks treatment course.
Keywords: budesonide, drug, incomplete microscopic colitis, induction therapy, MCi, microscopic colitis, QoL, quality of life, randomised clinical trial, watery diarrhoea
INTRODUCTION
The term microscopic colitis (MC) was first used in 1980 to describe a series of incidental findings in a subset of patients with chronic watery diarrhoea of unknown origin.1 Since then, MC has been acknowledged as a separate disease entity and is known to be a leading cause of chronic non‐bloody diarrhoea, particularly in elderly women,2, 3, 4, 5, 6 with a profound negative impact on the quality of life.7
Key summary.
Summarise the established knowledge on this subject
-
•
Incomplete microscopic colitis (MCi) is a subgroup falling short to fulfilling the classical histological criteria for microscopic colitis.
-
•
These patients have also chronic watery diarrhoea.
-
•
No treatment has been tested in MCi.
What are the significant and/or new findings of this study?
-
•
Budesonide seems to reduce loose/watery stools.
-
•
Budesonide improves the quality of life in patients with MCi.
-
•
Budesonide is safe and well tolerated during an 8‐weeks course.
Patients with MC present with chronic or recurrent watery diarrhoea, the colonic mucosa is macroscopically normal or near normal and histology demonstrates specific histopathological findings,8 usually throughout the colon.9, 10
Historically, two main subtypes of MC are distinguished, collagenous colitis (CC) and lymphocytic colitis (LC). Collagenous colitis is characterised by a thickened subepithelial collagen band, that is, >10 μm in well‐oriented biopsies cut perpendicularly to the surface. Lymphocytic colitis is characterised by an increased number of intraepithelial lymphocytes (IELs), that is, ≥20 IEL per 100 epithelial cells counted on hematoxylin and eosin (HE) stained biopsies.8 However, it has become increasingly obvious that these two entities fail to encompass all histological presentations of MC.
Thus, in 2011, the term ‘incomplete microscopic colitis’ (MCi) was proposed by Bjørnbak et al.11 The term was chosen, as in this entity lymphocytic and collagenous changes are present, yet they fall short of the criteria defined for either LC or CC.12, 13, 14 Patient characteristics and the subjective response to treatment with budesonide was, however, similar to those of patients with MC.11 Accordingly, the histological criteria for MCi have been defined as an increased lymphoplasmacellular infiltrate in the lamina propria, an abnormally thickened collagenous band (>5 and <10 μm) and/or abnormal IELs (>5 and <20 per 100 epithelial cells) in HE stained slides.15
According to an epidemiological study in the United States, of the 8745 MC patients, LC was diagnosed in 51%, CC in 43% and MCi in 6%.16 The average age was 63.3 years in LC, 66.4 years in CC and 67.3 years in MCi. There was a striking female predominance in all the three subtypes (72%–82%).16 To gather similar epidemiological data, a European registry for all patients diagnosed with MC has been established in 2015 (PRO‐MC registry; http://www.emcg‐ibd.eu/european‐registry‐promc.html). In the current incidental database of the PRO‐MC registry, the rate of MCi among all the MC patients is 14% (data not yet published), thus considerably higher than what has been reported by Sonnenberg and Genta16 but smaller than the 19% reported by Bjørnbak et al.11
The discrimination between MCi and MC is subject to inter‐observer variability, and a revision of the histological criteria of the MC subtypes has been proposed.17, 18 Currently, the diagnosis of MC and MCi rests on abnormal microscopic findings and the presence of chronic diarrhoea for at least 1 month.8 In addition to watery diarrhoea, common symptoms include imperious defecation, abdominal pain and weight loss.11 The non‐specific clinical presentation and differential diagnoses stress the importance of colonoscopy and histological assessment for correct diagnosis.8 This is particularly relevant as there is considerable overlap with the symptoms of irritable bowel syndrome, which presents with recurrent abdominal pain or discomfort as well as changes in stool frequency in the presence of an endoscopically normal intestinal mucosa.19
In a retrospective cohort study by Bjørnbak et al., clinical characteristics were largely indistinguishable between MC subtypes, and the highest rates of subjective effectiveness (84%–88%) across all the three entities were shown for budesonide.11
Most controlled interventional trials on the induction of remission in patients with MC have been performed with budesonide.8 However, most trials were restricted to either CC or LC. Thus, efficacy data in patients with MCi are largely missing. The objective of this randomised trial was therefore to fill this data gap and to demonstrate the superiority of treatment with budesonide (9‐mg budesonide/day) versus placebo for induction of remission in active MCi after 8 weeks of treatment.
In most trials, the primary efficacy parameter, clinical remission, has been determined after 4–8 weeks of double‐blind treatment. However, several studies indicate that the effect is evident already after 2 weeks.20, 21 To allow for direct comparison with previous studies, the primary efficacy parameter was therefore determined after 8 weeks of treatment. In addition, early efficacy was a secondary efficacy parameter, anticipating that shorter treatment periods would be potentially feasible in future studies.
METHODS
Study design and conduct
This was a double‐blind, randomised, placebo‐controlled, multi‐centre, phase 3 study comparing the efficacy and tolerability of 8‐weeks treatment with budesonide (9‐mg gastro‐resistant granules once daily) versus placebo in patients with MCi. Patients in clinical remission at the end of the 8‐weeks double‐blind phase entered a 24‐weeks treatment‐free follow‐up phase. The double‐blind phase took place between August 2014 and July 2019, and the follow‐up phase of the last patient was completed in January 2020. The study was performed at 17 gastroenterology centres in Sweden, Germany, Austria, Denmark, Hungary, Netherlands, Spain and Lithuania.
The study was registered in the US National Library of Medicine (ClinicalTrials.gov number, NCT02142634) and the EU Clinical Trials Register (EudraCT number, 2013‐001912‐31). The study protocol was in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice and was approved by the National Ethics Committees of all participating countries. All patients provided written informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
Patients
Key inclusion criteria were a history of chronic non‐bloody, watery diarrhoea for at least 4 weeks and a clinically active disease, defined as a mean of ≥3 stools/day, thereof a mean of ≥1 watery stool/day during the week prior to randomisation in patients with histologically established diagnosis of MCi.22 The key exclusion criteria were evidence of other significant abnormalities in colonoscopy that may have been the cause of diarrhoea, except for colonic diverticulosis and non‐dysplastic polyps <2 cm; infectious diarrhoea or history of infectious diarrhoea within the last 3 months prior to inclusion or local intestinal infection; clinical suspicion of drug‐induced diarrhoea; prior and present MC; abnormal hepatic function; treatment with immunomodulators within 3 months prior to baseline; treatment with budesonide or other steroids or antibiotics within 4 weeks prior to baseline; and treatment with anti‐diarrheal drugs, cholestyramine, bulking agents, spasmolytics, bismuth and probiotics within 2 weeks prior to baseline.
Randomisation and interventions
After a screening phase lasting up to 2 weeks, eligible patients were randomly assigned to one of the two treatment groups at a 1:1 ratio. For allocation of the patients, a computer‐generated list of random numbers was prepared using a block size of 4. Group assignment was blinded for all patients and investigators as well as all other persons involved in the conduct of the study, by the use of identical packaging of the different investigational medicinal products. The consecutively numbered study medication was dispensed by the investigators according to the randomisation schedule.
Patients administered either budesonide 9 mg once daily (1 sachet of Budenofalk® 9‐mg gastro‐resistant granules) or placebo (1 sachet of placebo Budenofalk® 9‐mg granules), to be taken in the morning, for 8 weeks. Adherence to the study treatment was monitored by the sachet count at each study visit.
During the entire study period, the use of other anti‐inflammatory drugs, Boswellia serrata extract, immunosuppressants, oral or intravenous antibiotics, anti‐diarrheals, spasmolytics, bismuth and probiotics, was not permitted.
Sample size
The study protocol pre‐specified a two‐stage group‐sequential adaptive design with possible sample size adjustment or early stopping of the study for efficacy or futility after the interim analysis.
Assuming the rates of clinical remission of 80% in the budesonide group and of 50% in the placebo group, the statistical power of the test procedure was 90% with 35 patients per group in the first stage and an additional 18 patients per group in the second stage, resulting in a total sample size of 106 patients (2 × 53 patients) in the intention‐to‐treat (ITT) analysis.
Evaluation schedule and study endpoints
Post‐baseline study visits took place at weeks 2 (V2), 4 (V3) and 8 (V4) during the double‐blind treatment phase and telephone follow‐up visits at weeks 20 and 32 post‐randomisation.
The primary endpoint was clinical remission at the week‐8 visit (applying the last observation carried forward [LOCF] method). Clinical remission was defined according to the Hjortswang criteria,22 that is, a mean of <3 stools/day and a mean of <1 watery stool/day in the preceding 7 days.23 Secondary efficacy endpoints included time to clinical remission, change in the number of watery stools per week, change in the number of soft or watery stools per week (endpoint was defined post hoc), change in the number of days without abdominal pain, change in the quality of life (short health scale [SHS]) and overall safety and tolerability.
Endoscopy and histology
A complete colonoscopy was performed at screening visit (or within 12 weeks before the screening visit) and at the end of the 8‐weeks double‐blind treatment. At each colonoscopy, two biopsy samples were obtained from each of the following colon segments: rectum, sigmoid, descending, transverse, ascending/cecum and terminal ileum, as an alternative sigmoidoscopy was allowed.
Biopsy specimens were fixed in 4% formalin and embedded in paraffin. Sections (2–3 μm) were stained with haematoxylin and eosin. CD3 immunohistochemistry staining was performed if the number of IELs could not be determined otherwise. Masson‐Goldner trichrome staining was used to assess the subepithelial collagen layer. Well‐oriented sections in which at least three adjacent crypts were cut in their vertical plane were suitable for evaluation. Sections from each colon section were assessed for the (i) inflammation of the lamina propria with lymphocytes and plasma cells (semiquantitative score: 0–3); (ii) inflammation of the lamina propria with neutrophilic and eosinophilic granulocytes (semiquantitative score: 0–3); (iii) thickness of the subepithelial collagen band (μm); (iv) total number of IELs/100 surface epithelium cells; and (v) degeneration of the surface epithelium (present/absent). All biopsies were analysed in a blinded fashion by a central pathologist (Daniela Aust, Dresden, Germany). Histological remission was defined as presence of no/mild lamina propria inflammation (score < 2 for lymphocytes/plasma cells as well as for neutrophilic/eosinophilic granulocytes infiltration) and normal subepithelial collagen band (≤5 μm) and physiological numbers of IEL (≤5 IEL/100 epithelial cells).
Safety and tolerability
Patients underwent physical examination at the randomisation and final visit, vital signs and adverse events were recorded at all visits and general laboratory tests and urinalysis were performed.
Statistical analyses
Efficacy was analysed for the ITT population with a sensitivity analysis for the per‐protocol (PP) population. Patients with lack of compliance, intake of forbidden concomitant medication, violation of eligibility criteria or early discontinuation due to reasons not related with the study drug were excluded from the PP population. Safety analyses were performed for the safety population, which included all patients who took the study drug at least once. As enrolment of patients was terminated prior to the planned interim analysis due to an insufficient patient recruitment rate, a two‐stage group‐sequential adaptive design was no longer appropriate and the test‐statistical procedure was adjusted (Fisher's exact test). To test the hypothesis of the primary endpoint, the type I error rate was defined as α = 0.025 (one‐sided). All other statistical tests were performed two‐sided with a significance level of α = 0.05 on an exploratory basis. All statistical analyses were conducted using the SAS statistical package for Windows (SAS Institute, Cary, NC, USA), version 9.4.
RESULTS
Study population
The sponsor terminated patient recruitment prior to the interim analysis due to an insufficient patient recruitment rate. A total of 63 patients were screened for enrolment. Of these, 44 patients were included in the ITT and safety populations (21 budesonide and 23 placebo). Twelve patients discontinued the study prematurely (six patients in each of both treatment groups). One patient in each of both treatment groups discontinued the study prematurely due to adverse events, five patients (2 budesonide and 3 placebo) due to lack of efficacy and another five patients (3 budesonide and 2 placebo) for other reasons (Figure S1). The primary analysis was performed for the full analysis set, defined according to the ITT principle. Five patients (3 of budesonide and 2 of placebo arms) were included despite being in clinical remission using the Hjortswang criteria and one patient of the budesonide arm had not histological confirmation of MCi. These six patients (besides other patients) were excluded for the PP analysis which comprised 28 patients.
Baseline demographic and clinical characteristics were similar in both treatment groups. However, in the placebo group, there was higher proportions of males, current smokers, of patients fulfilling the Rome III criteria for diarrhoea‐predominant irritable bowel syndrome (IBS‐D) and of patients with a longer duration of diarrhoea (Table 1). Five patients (3 budesonide and 2 placebo) met one or both Hjortswang criteria for remission at inclusion by mistake and were thus incorrectly included. Three patients (1 budesonide and 2 placebo) had received budesonide for treatment of the previous disease. Treatment for the current episode had been given to six patients, most frequently anti‐diarrheals (n = 3). As stipulated by the protocol, no patient had been medicated with budesonide or antibiotics in the 4 weeks prior to baseline.
TABLE 1.
Baseline demographic and clinical characteristics (ITT population)
| Budesonide (n = 21) | Placebo (n = 23) | |
|---|---|---|
| Female gender, number of patients (n) (%) | 16 (76.2) | 14 (60.9) |
| Age (years), mean (SD) | 52.2 (19.0) | 46.5 (15.5) |
| Body mass index (kg/m2), mean (SD) | 25.7 (5.0) | 27.2 (5.9) |
| Smoking habit, n (%) | ||
| Current | 3 (14.3) | 6 (26.1) |
| Former | 7 (33.3) | 4 (17.4) |
| Never | 11 (52.4) | 13 (56.5) |
| Caffeine intake, n (%) | 18 (85.7) | 16 (69.6) |
| Duration of diarrhoea (years), median (interquartile range) | 1.0 (0.4, 3.9) | 2.1 (0.7, 4.8) |
| Acute onset of symptoms, n (%) | 15 (71.4) | 13 (56.5) |
| Rome III criteria for IBS‐Da fulfilled, n (%) | 13 (61.9) | 20 (87.0) |
| Number of stools/day in the last 7 days, mean (SD) | 4.4 (2.1) | 4.3 (1.4) |
| Number of watery stools/day in the last 7 days, mean (SD) | 2.2 (1.7) | 2.5 (1.7) |
| Number of soft stools/day in the last 7 days, mean (SD) | 1.9 (1.9) | 1.4 (1.0) |
Abbreviations: ITT, intention‐to‐treat; SD, standard deviation.
Diarrhoea‐predominant irritable bowel syndrome.
Clinical efficacy
The primary endpoint of clinical remission at week 8 (LOCF) was met more frequently in the budesonide group than in the placebo group in the ITT population (71.4% [15/21] vs. 43.5% [10/23]), however failed statistical significance (p = 0.0582) (Figure 1a). Similar results were observed in the PP population (budesonide 75.0% [9/12] vs. placebo 43.8% [7/16], p = 0.1019) (Figure 1b).
FIGURE 1.
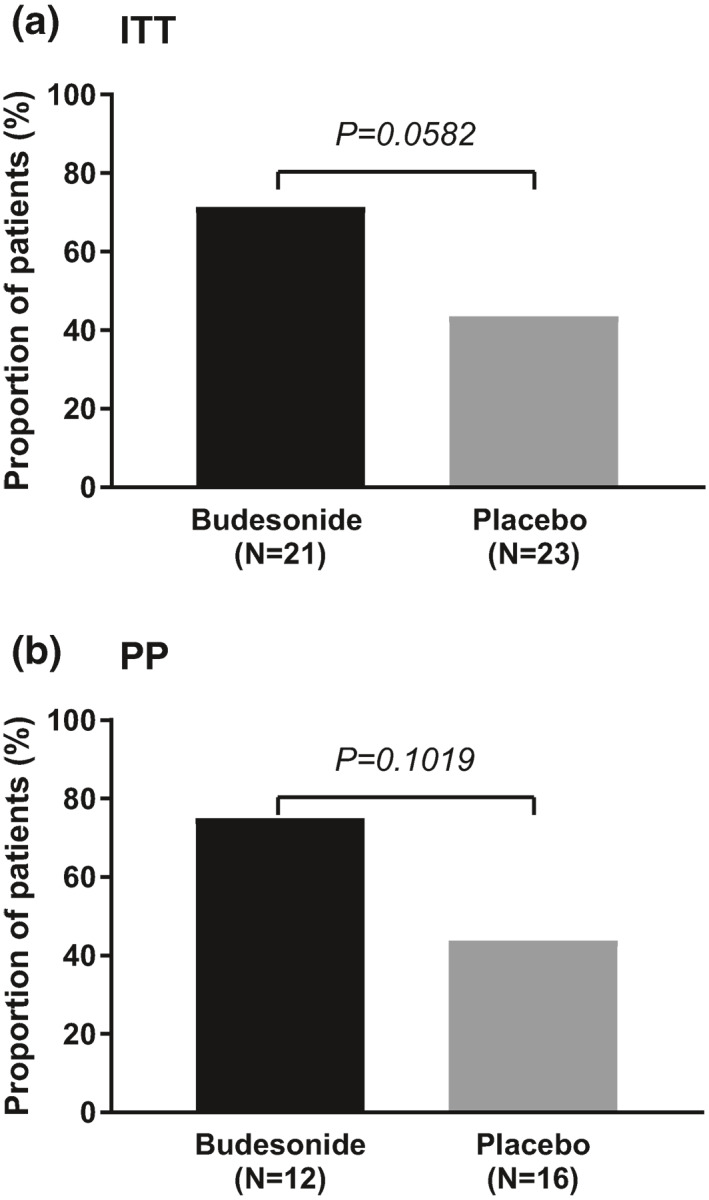
Proportion of patients in clinical remission for (a) intention‐to‐treat (ITT) population and (b) per‐protocol (PP) population during the double‐blind phase of the study. Clinical remission was defined as a mean of <3 stools/day and a mean of <1 watery stools/day in the 7 days prior to week 8 or withdrawal visit (last observation carried forward method)
The Kaplan–Meier analysis revealed that the median time to clinical remission was shorter in the budesonide group than the placebo group (7 vs. 33 days, p = 0.0915) (Figure 2). The mean number of watery stools during the preceding 7 days decreased from 15.3 (95% confidence interval (CI) [9.86, 20.81]) at baseline to 2.8 (95% CI [0.54, 5.08]) at week 8 (LOCF) for the budesonide group. From baseline to week 8 (LOCF), the mean reduction in the number of watery stools in the budesonide group was 12.5 (95% CI [7.22, 17.83]) and in the placebo group 5.4 (95% CI [0.03, 10.69]). The difference between both the treatment groups of the mean reduction in the number of watery stools was 7.16 (95% CI [−0.14, 14.46]; p = 0.0542) and evident within 1 week of treatment (Figure 3). The mean reduction in the number of soft or watery stools in the budesonide group was 16.3 (95% CI [10.60, 21.97]) and in the placebo group 7.7 (95% CI [3.14, 12.31]). The difference between both the treatment groups in the mean reduction in the number of soft or watery stools was 8.56 (95% CI [1.51, 15.61]; p = 0.0186).
FIGURE 2.
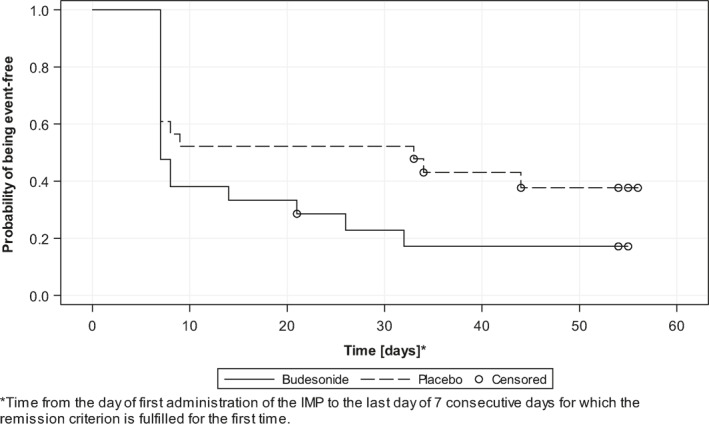
Time to clinical remission, defined as the time from the day of first administration of the study drug to the last of ≥7 consecutive days each with on average <3 stools/day thereof <1 watery stool/day (intention‐to‐treat population)
FIGURE 3.
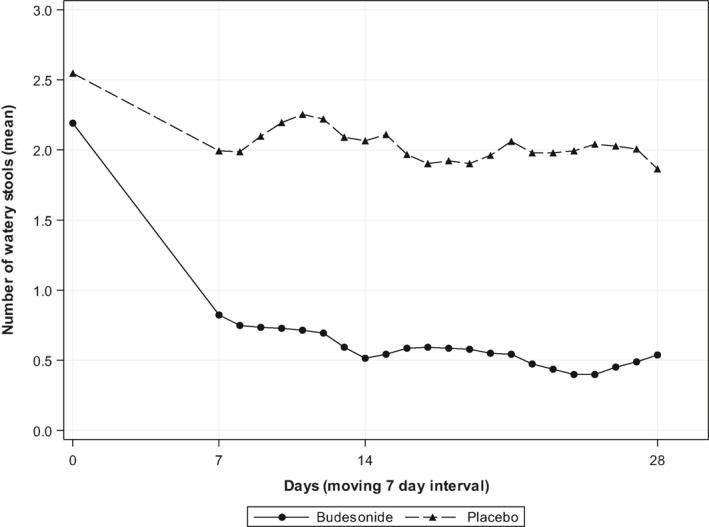
Mean number of watery stools/day during the first 28 days (intention‐to‐treat population). A data point reflects the mean number of watery stools per day during the preceding 7 days
The mean number of days without abdominal pain during the last 7 days before the visit increased by 1.1 (95% CI [0.10, 2.00]) from baseline to week 8 (LOCF) in the budesonide group and decreased by 0.6 (95% CI [−0.49, 1.76]) in the placebo group. The difference in the mean number of days without abdominal pain between both the treatment groups was 1.69 (95% CI [0.24, 3.13]; p = 0.0233).
The mean SHS values decreased (signifying improvement) from baseline to week 8 (LOCF) in all four health dimensions (symptom burden, social function, disease‐related worry and general well‐being). The improvements were greater in the budesonide group versus the placebo group, and all between‐group differences were statistically significant: symptom burden 18.95 (95% CI [4.35, 33.54]), p = 0.0122; social function 19.18 (95% CI [1.68, 36.68]), p = 0.0325; disease‐related worry 20.73 (95% CI [3.87, 37.59]), p = 0.0172; general well‐being 28.93 (95% CI [12.50, 45.36]), p = 0.0010 (Figure 4a–d).
FIGURE 4.
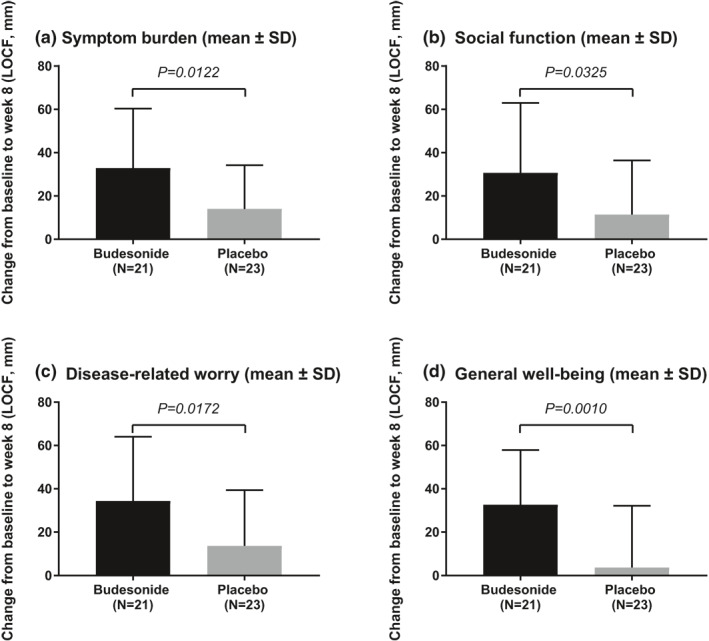
Short health scale change from baseline to week 8 (last observation carried forward [LOCF]) (intention‐to‐treat population)
Histology
Histological examination at baseline confirmed the diagnosis of MCi in all patients except for one patient in the budesonide group. In this study, the criterion (iii) – thickened subepithelial collagenous band (>5 μm and <10 μm) on H&E – was fulfilled in five patients, and the criterion (iv) – abnormal IELs (>5 and <20 per 100 epithelial cells) on H&E – was fulfilled in all patients showing an overlap of both criteria in some patients.
Biopsies were available at week 8 in 27 patients, allowing a comparison between pre‐ and post‐treatment histology. Histological remission was achieved in 3/14 and 1/13 patients in the budesonide and placebo groups, respectively, representing remission rates of 21.4% with budesonide and 7.7% with placebo (p = 0.5956).
Safety
The incidence of adverse events after baseline was slightly higher in the budesonide (42.9%) versus the placebo group (34.8%) (Table 2). Adverse events with a suspected relation to study drug were reported in one patient in the budesonide group (gastroesophageal reflux disease) and two patients in the placebo group (dyspepsia, nausea and rash). Neither serious nor severe (with respect to intensity) adverse events occurred during the double‐blind phase. Changes of laboratory parameters attributed to the study drug or study disease were not clinically relevant in any treatment group.
TABLE 2.
Adverse events during the double‐blind phase (safety population), number of patients (n) (%)
| Budesonide (n = 21) | Placebo (n = 23) | |
|---|---|---|
| Any adverse event | 9 (42.9) | 8 (34.8) |
| Serious adverse event | – | – |
| Adverse events with suspected relation to study drug | 1 (4.8) | 2 (8.7) |
| Adverse events leading to discontinuation of study drug | 1 (4.8) | 2 (8.7) |
| Adverse events occurring in ≥2 patients overall | ||
| Gastrointestinal disorders | 5 (23.8) | 4 (17.4) |
| Abdominal distension | 2 (9.5) | – |
| Gastroesophageal reflux disease | 1 (4.8) | 1 (4.3) |
| Nausea | 1 (4.8) | 1 (4.3) |
| Nervous system disorders | 3 (14.3) | 1 (4.3) |
| Headache | 3 (14.3) | – |
| Infections and infestations | 2 (9.5) | 1 (4.3) |
| Musculoskeletal and connective tissue disorders | 2 (9.5) | 1 (4.3) |
| Investigations | – | 2 (8.7) |
| Skin and subcutaneous tissue disorders | 1 (4.8) | 1 (4.3) |
| Rash | 1 (4.8) | 1 (4.3) |
DISCUSSION
Despite insufficient patient recruitment and a resulting low statistical power, results provided supportive evidence that budesonide is effective in the treatment of acute MCi. Clinical remission was achieved in 71.4% of patients (43.5% in the placebo group) after an 8‐weeks course of oral budesonide at a dose of 9 mg/day. Most likely, due to the low sample size, the difference to placebo was not statistically significant (type 2 error). In addition, patients reached clinical remission markedly faster with budesonide (after 1 week, according to the Hjortswang criteria) than with placebo (after 4–5 weeks). In accordance, a marked improvement in clinical symptoms, most notably a reduction in the number of watery or loose stools and days with abdominal pain, was observed.
The decrease in the combined number of soft or watery stools in the budesonide group was more than twofold higher than in the placebo group, implicating a considerable improvement of patient's quality of life as reflected in the significant improvement in the SHS as compared to the placebo group.
The low recruitment rate was attributable to several causes, the most obvious cause being low prevalence and lack of fulfilling the inclusion criteria (especially amount of watery stools). As pointed out above, the prevalence of MCi among MC patients appears to range between 6%16 and 14% (PRO‐MC, unpublished data). An important obstacle was in many cases that the histological diagnostic criteria as applied in this trial were not met. The histologic criteria are based on the assessment of haematoxylin and eosin stained samples. However, the more extensive use of CD3 staining highlights the IELs and thus changes the diagnosis from incomplete MC to LC.18, 24 In fact, the incidence of MCi declined dramatically in parallel with the more extensive use of CD3 staining in routine practice.25 Langner and colleagues recommend >10 IEL per 100 epithelial cells as the lower threshold for the diagnosis of MCi,26 which was recently confirmed by the recommendations of the United European Gastroenterology and European Microscopic Colitis Group (EMCG).8 Another obstacle for correct diagnosis is the inter‐observer variability when discriminating MCi from CC and LC17, 27 and the fact that there is not yet a consensus on the diagnosis of MCi among pathologists. In summary, the risk of both over‐ and under‐diagnosis exists and should be addressed by improved criteria in the future.26, 28, 29
Efficacy outcomes were largely consistent with the previous results of various randomised trials as well as observational studies on the induction of remission in the treatment of the other subtypes of MC with budesonide.30, 31 The EMCG has recently reiterated the recommendation of budesonide 9 mg/day for 6–8 weeks as first‐line therapy for active MC, however, without extending the treatment recommendation specifically to MCi.8 As budesonide is currently the only drug approved for the treatment of MC, the presented data suggest that the use of budesonide is also beneficial in the treatment of active MCi.
The safety results in this study were largely in line with data from prior trials on treatment of MC with budesonide.30, 32, 33 No new safety signal was detected. Budesonide has in general low systemic effects due to a high first‐pass metabolism in the liver.34, 35, 36 As a result, systemic effects on bone density and adrenal function are less common with budesonide than with conventional glucocorticoids such as prednisolone.37, 38 Consistently, the recent EMCG guideline clearly recommends against the use for any corticosteroid other than budesonide.8
Unfortunately, as only a smaller subset of patients had a follow‐up endoscopy at the end of treatment, the effect of budesonide on the histological abnormalities cannot be reliably interpreted.
The main mechanism responsible for budesonide's effect on diarrhoea appears to be its ability to improve the water‐absorption capacity of the large bowel.39 Recently, a specific mechanism for diarrhoea has come into focus which can be called ‘malabsorptive'. In CC, impaired water resorption may be attributable to down‐regulation of Aquaporin 8 (AQP8) water channels. In fact, reduced AQP8 expression may be a common pathomechanism in several diarrheal disorders as it has also been observed in other inflammatory bowel diseases, infectious colitis and IBS‐D.40, 41, 42 Interestingly, budesonide has been shown to revert the loss of AQP8 expression, which provides a plausible explanation for its strong anti‐diarrheal effect.43
Another potentially beneficial effect of budesonide might be the partial restoration of impaired bile salt absorption. It has been seen that bile acid malabsorption is common in MC.44, 45 Budesonide is able to induce an increase in ileal bile acid transporter expression in healthy volunteers46 and to normalise the SeHCAT test results and reduce bile acid production in patients with CC.45
In this study, 75% of the MCi patients fulfilled the ROME III criteria for IBS‐D. These results underline the importance to take biopsies in patients with chronic watery diarrhoea to identify patients with MC and allow these a rational, evidence‐based treatment.
Limitations
Sample size was lower than intended due to insufficient patient recruitment. Therefore, statistical power was too low to prove superiority of budesonide over placebo for the primary endpoint. In addition, the small sample size hinders a comprehensive picture on the range of adverse drug reactions. As pointed out, strict histological criteria were applied. Additional criteria excluded other groups of patients, for example, those suspected to suffer from drug‐induced disease. However, efficacy was similar to that reported in real‐world experience.11 Even so, the results cannot be extrapolated to all individuals with MCi. In this trial, budesonide was administered using pH‐modified release oral granules (Budenofalk®) which release the active ingredient only at pH 6.4 or higher,47 that is, specifically in the target areas terminal ileum and colon. Thus, the current results may not be applicable to other budesonide formulations with a different release profile.
CONCLUSION
In summary, 8‐weeks budesonide treatment was effective and safe for the induction of clinical remission in MCi. More than 71.4% of the patients achieved clinical remission with a profound improvement seen already after 1 week of treatment. These results on the efficacy of budesonide for the treatment of acute MCi are in line with existing recommendations on other subtypes of MC for induction of remission.8
CONFLICT OF INTEREST
Daniela Aust, Gerd Bouma, Juozas Kupčinskas, Ahmed Madisch, Stephan Miehlke and Andreas Münch have received consultancy honoraria or speaker's fees from Dr Falk Pharma GmbH, Freiburg, Germany. Ralf Mohrbacher and Roland Greinwald are employees of Dr Falk Pharma GmbH, Freiburg, Germany. All other authors have nothing to disclose.
AUTHORSHIP STATEMENT
Guarantor of the article: Prof. Andreas Münch had full access to all of the data in the study and takes responsibility for its integrity and the accuracy of the data analysis.
AUTHOR CONTRIBUTIONS
All authors contributed to study design, analysis, drafting and finalisation of the manuscript, in particular: Study concept and design: Roland Greinwald, Stephan Stephan Miehlke, Ralf Mohrbacher, Andreas Münch and Lars Kristian Munck; Recruitment of study patients: Blanca Belloc, Johan Bohr, Gerd Bouma, Jordi Guardiola, Juozas Kupčinskas, Ahmed Madisch, Stephan Miehlke, Emese Mihaly, Andreas Münch, Lars Kristian Munck, Ferenc Nagy and Chunliang Shi; Central Pathology: Daniela Aust; Generation, collection assembly, analysis and interpretation of data: Roland Greinwald, Ralf Mohrbacher, Andreas Münch and Lars Kristian Munck; Drafting of the manuscript: Dr Christoph Engler and Dr Karl Scheithe (see Acknowledgement); Critical revision of the manuscript for important intellectual content: Roland Greinwald, Ralf Mohrbacher, Andreas Münch and Lars Kristian Munck; Statistical analysis: Dr Karl Scheithe (see Acknowledgement); Approval of the final version of the manuscript: all authors.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank all patients and investigators for their participation in the study and are grateful to Dr Karl Scheithe for his statistical expertise, Dr Claudia Ederer for her assistance in conducting the clinical trial and Dr Christoph Engler and Dr Karl Scheithe for medical writing support (all GKM Gesellschaft für Therapieforschung mbH, Munich, Germany). This study was funded in full by Dr Falk Pharma GmbH, Freiburg, Germany. The writing of this study was funded by Dr Falk Pharma GmbH, Freiburg, Germany. Initial data analyses were undertaken by Dr Karl Scheithe of GKM Gesellschaft für Therapieforschung mbH, Munich, Germany and received funding from Dr Falk Pharma GmbH, Freiburg, Germany. Writing support was provided by Dr Christoph Engler and Dr Karl Scheithe of GKM Gesellschaft für Therapieforschung mbH, Munich, Germany and funded by Dr. Falk Pharma GmbH, Freiburg, Germany.
Münch A, Mihaly E, Nagy F, Madisch A, Kupčinskas J, Miehlke S, et al. Budesonide as induction therapy for incomplete microscopic colitis: a randomised, placebo‐controlled multicentre trial. United European Gastroenterol J. 2021;9(7):837–847. 10.1002/ueg2.12131
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Read NW, Krejs GJ, Read MG, Santa Ana CA, Morawski SG, Fordtran JS. Chronic diarrhea of unknown origin. Gastroenterology. 1980;78:264–71. [PubMed] [Google Scholar]
- 2.Jaskiewicz K, Rzepko R, Adrych K, Smoczyński M. Microscopic colitis in routine colonoscopies. Dig Dis Sci. 2006;51:241–4. [DOI] [PubMed] [Google Scholar]
- 3.Pardi DS, Loftus EV Jr., Smyrk TC, Kammer PP, Tremaine WJ, Schleck CD, et al. The epidemiology of microscopic colitis: a population based study in Olmsted County, Minnesota. Gut. 2007;56:504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyhlin N, Bohr J, Eriksson S, Tysk C. Microscopic colitis: a common and an easily overlooked cause of chronic diarrhoea. Eur J Intern Med. 2008;19:181–6. [DOI] [PubMed] [Google Scholar]
- 5.Williams JJ, Kaplan GG, Makhija S, Urbanski SJ, Dupre M, Panaccione R, et al. Microscopic colitis‐defining incidence rates and risk factors: a population‐based study. Clin Gastroenterol Hepatol. 2008;6:35–40. [DOI] [PubMed] [Google Scholar]
- 6.Weimers P, Ankersen DV, Lophaven S, Bonderup OK, Münch A, Løkkegaard ECL, et al. Incidence and prevalence of microscopic colitis between 2001 and 2016: a Danish nationwide cohort study. J Crohns Colitis. 2020;14:1717–23. [DOI] [PubMed] [Google Scholar]
- 7.Hjortswang H, Tysk C, Bohr J, Benoni C, Vigren L, Kilander A, et al. Health‐related quality of life is impaired in active collagenous colitis. Dig Liver Dis. 2011;43:102–9. [DOI] [PubMed] [Google Scholar]
- 8.Miehlke S, Guagnozzi D, Zabana Y, Tontini GE, Kanstrup Fiehn AM, Wildt S, et al. European guidelines on microscopic colitis: United European Gastroenterology (UEG) and European Microscopic Colitis Group (EMCG) statements and recommendations. United European Gastroenterol J. 2021;9(1):13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiehn AK, Miehlke S, Aust D, Vieth M, Bonderup O, Fernandez‐Banares F, et al. Distribution of histopathological features along the colon in microscopic colitis. Int J Colorectal Dis. 2021;36(1):151–9. [DOI] [PubMed] [Google Scholar]
- 10.Kanstrup Fiehn AM, Heiberg Engel PJ, Lanzarotto F, Goudkade D, Landolfi S, Munck LK, et al. Topographical distribution of microscopic colitis and the importance of orientation of paraffin‐embedded biopsies. Hum Pathol. 2020;103:63–71. [DOI] [PubMed] [Google Scholar]
- 11.Bjørnbak C, Engel PJH, Nielsen PL, Munck LK. Microscopic colitis: clinical findings, topography and persistence of histopathological subgroups. Aliment Pharmacol Ther. 2011;34(10):1225–34. 10.1111/j.1365-2036.2011.04865.x [DOI] [PubMed] [Google Scholar]
- 12.Fernandez‐Banares F, Casalots J, Salas A, Esteve M, Rosinach M, Forné M, et al. Paucicellular lymphocytic colitis: is it a minor form of lymphocytic colitis? A clinical pathological and immunological study. Am J Gastroenterol. 2009;104:1189–98. [DOI] [PubMed] [Google Scholar]
- 13.Kitchen PA, Levi AJ, Domizio P, Talbot IC, Forbes A, Price AB. Microscopic colitis: the tip of the iceberg? Eur J Gastroenterol Hepatol. 2002;14:1199–204. [DOI] [PubMed] [Google Scholar]
- 14.Fraser AG, Warren BF, Chandrapala R, Jewell DP. Microscopic colitis: a clinical and pathological review. Scand J Gastroenterol. 2002;37:1241–5. [DOI] [PubMed] [Google Scholar]
- 15.Langner C, Aust D, Ensari A, Villanacci V, Becheanu G, Miehlke S, et al. Histology of microscopic colitis‐review with a practical approach for pathologists. Histopathology. 2015;66:613–26. [DOI] [PubMed] [Google Scholar]
- 16.Sonnenberg A, Genta RM. Lymphocytic and collagenous colitis: epidemiologic differences and similarities. Dig Dis Sci. 2013;58:2970–5. [DOI] [PubMed] [Google Scholar]
- 17.Fiehn AM, Bjornbak C, Warnecke M, Engel PJH, Munck LK. Observer variability in the histopathologic diagnosis of microscopic colitis and subgroups. Hum Pathol. 2013;44:2461–6. [DOI] [PubMed] [Google Scholar]
- 18.Fiehn AK, Clausen LN, Engel U, Wildt S, Munck LK, Kristensson M, et al. Is revision of cutoff values needed when using CD3 immunohistochemical staining in histopathologic diagnosis of lymphocytic colitis? Hum Pathol. 2019;84:115–23. [DOI] [PubMed] [Google Scholar]
- 19.Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421–31. [DOI] [PubMed] [Google Scholar]
- 20.Baert F, Schmit A, D'Haens G, Dedeurwaerdere F, Louis E, Cabooter M, et al. Budesonide in collagenous colitis: a double‐blind placebo‐controlled trial with histologic follow‐up. Gastroenterology. 2002;122:20–5. [DOI] [PubMed] [Google Scholar]
- 21.Miehlke S, Heymer P, Bethke B, Bästlein E, Meier E, Bartram H‐P, et al. Budesonide treatment for collagenous colitis: a randomized, double‐blind, placebo‐controlled, multicenter trial. Gastroenterology. 2002;123:978–84. [DOI] [PubMed] [Google Scholar]
- 22.Hjortswang H, Tysk C, Bohr J, Benoni C, Kilander A, Larsson L, et al. Defining clinical criteria for clinical remission and disease activity in collagenous colitis. Inflamm Bowel Dis. 2009;15:1875–81. [DOI] [PubMed] [Google Scholar]
- 23.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 24.Fiehn AM, Engel U, Holck S, Munck LK, Engel PJH. CD3 immunohistochemical staining in diagnosis of lymphocytic colitis. Hum Pathol. 2016;48:25–31. [DOI] [PubMed] [Google Scholar]
- 25.Davidson S, Sjoberg K, Engel PJH, Lo Rinc E, Fiehn A‐MK, Vigren L, et al. Microscopic colitis in Denmark and Sweden: incidence, putative risk factors, histological assessment and endoscopic activity. Scand J Gastroenterol. 2018;53:818–24. [DOI] [PubMed] [Google Scholar]
- 26.Langner C, Aust D, Ensari A, Villanacci V, Becheanu G, Miehlke S, et al. Histology of microscopic colitis‐review with a practical approach for pathologists. Histopathology. 2015;66:613–26. [DOI] [PubMed] [Google Scholar]
- 27.Goudkade D, Fiehn AK, Landolfi S, Villanacci V, Munck LK, Engel PJH . An investigation of European pathologists' approach to diagnose microscopic colitis. Ann Diagn Pathol. 2020;46:151520. [DOI] [PubMed] [Google Scholar]
- 28.Munch A, Sanders DS, Molloy‐Bland M, Hungin APS. Undiagnosed microscopic colitis: a hidden cause of chronic diarrhoea and a frequently missed treatment opportunity. Frontline Gastroenterol. 2020;11:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiehn AM, Kristensson M, Engel U, Munck L, Holck S, Engel P. Automated image analysis in the study of collagenous colitis. Clin Exp Gastroenterol. 2016;9:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miehlke S, Aust D, Mihaly E, Armerding P, Böhm G, Bonderup O, et al. Efficacy and safety of budesonide, vs mesalazine or placebo, as induction therapy for lymphocytic colitis. Gastroenterology. 2018;155:1795–804. [DOI] [PubMed] [Google Scholar]
- 31.Chande N, Al Yatama N, Bhanji T, Nguyen TM, McDonald JWD, MacDonald JK. Interventions for treating lymphocytic colitis. Cochrane Database Syst Rev. 2017. 10.1002/14651858.cd006096.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart MJ, Seow CH, Storr MA. Prednisolone and budesonide for short‐ and long‐term treatment of microscopic colitis: systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2011;9:881–90. [DOI] [PubMed] [Google Scholar]
- 33.Munch A, Bohr J, Miehlke S, Benoni C, Olesen M, Öst Å, et al. Low‐dose budesonide for maintenance of clinical remission in collagenous colitis: a randomised, placebo‐controlled, 12‐month trial. Gut. 2016;65:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunes T, Barreiro‐de Acosta M, Marin‐Jimenez I, Nos P, Sans M. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis. 2013;7:183–91. [DOI] [PubMed] [Google Scholar]
- 35.Edsbäcker S, Andersson T. Pharmacokinetics of budesonide (Entocort™ EC) capsules for Crohn's disease. Clin Pharmacokinet. 2004;43:803–21. [DOI] [PubMed] [Google Scholar]
- 36.Abdalla MI, Herfarth H. Budesonide for the treatment of ulcerative colitis. Expet Opin Pharmacother. 2016;17:1549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iborra M, Alvarez‐Sotomayor D, Nos P. Long‐term safety and efficacy of budesonide in the treatment of ulcerative colitis. Clin Exp Gastroenterol. 2014;7:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wildt S, Munck LK, Becker S, Brockstedt H, Bonderup OK, Hitz MF. Risk of osteoporosis in microscopic colitis. Postgrad Med. 2018;130:348–54. [DOI] [PubMed] [Google Scholar]
- 39.Ecker KW, Stallmach A, Seitz G, Gierend M, Greinwald R, Achenbach U. Oral budesonide significantly improves water absorption in patients with ileostomy for Crohn disease. Scand J Gastroenterol. 2003;38:288–93. [DOI] [PubMed] [Google Scholar]
- 40.Ricanek P, Lunde LK, Frye SA, Morth J, Rydning A, Vatn M, et al. Reduced expression of aquaporins in human intestinal mucosa in early stage inflammatory bowel disease. Clin Exp Gastroenterol. 2015;8:49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardin JA, Wallace LE, Wong JFK, O'Loughlin EV, Urbanski SJ, Gall DG, et al. Aquaporin expression is downregulated in a murine model of colitis and in patients with ulcerative colitis, Crohn's disease and infectious colitis. Cell Tissue Res. 2004;318:313–23. [DOI] [PubMed] [Google Scholar]
- 42.Wang JP, Hou XH. Expression of aquaporin 8 in colonic epithelium with diarrhoea‐predominant irritable bowel syndrome. Chin Med J (Engl). 2007;120:313–6. [PubMed] [Google Scholar]
- 43.Escudero‐Hernandez C, Munch A, Ostvik AE, van Beelen Granlund A, Koch S. The water channel aquaporin 8 is a critical regulator of intestinal fluid homeostasis in collagenous colitis. J Crohns Colitis. 2020;14:962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandez‐Banares F, Esteve M, Salas A, Forné M, Espinós JC, Martín‐Comín J, et al. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci. 2001;46:2231–8. [DOI] [PubMed] [Google Scholar]
- 45.Ung KA, Gillberg R, Kilander A, Abrahamsson H. Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut. 2000;46:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung D, Fantin AC, Scheurer U, Fried M, Kullak‐Ublick GA. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut. 2004;53:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miehlke S, Acosta MB, Bouma G, Carpio D, Magro F, Moreels T, et al. Oral budesonide in gastrointestinal and liver disease: a practical guide for the clinician. J Gastroenterol Hepatol. 2018;33:1574–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


