Abstract
Background
A novel variant of SARS-CoV-2, the Delta variant of concern (VOC, also known as lineage B.1.617.2), is fast becoming the dominant strain globally. We reported the epidemiological, viral, and clinical characteristics of hospitalized patients infected with the Delta VOC during the local outbreak in Guangzhou, China.
Methods
We extracted the epidemiological and clinical information pertaining to the 159 cases infected with the Delta VOC across seven transmission generations between May 21 and June 18, 2021. The whole chain of the Delta VOC transmission was described. Kinetics of viral load and clinical characteristics were compared with a cohort of wild-type infection in 2020 admitted to the Guangzhou Eighth People's Hospital.
Findings
There were four transmission generations within the first ten days. The Delta VOC yielded a significantly shorter incubation period (4.0 vs. 6.0 days), higher viral load (20.6 vs. 34.0, cycle threshold of the ORF1a/b gene), and a longer duration of viral shedding in pharyngeal swab samples (14.0 vs. 8.0 days) compared with the wild-type strain. In cases with critical illness, the proportion of patients over the age of 60 was higher in the Delta VOC group than in the wild-type strain (100.0% vs. 69.2%, p = 0.03). The Delta VOC had a higher risk than wild-type infection in deterioration to critical status (hazards ratio 2.98 [95%CI 1.29-6.86]; p = 0.01).
Interpretation
Infection with the Delta VOC is characterized by markedly increased transmissibility, viral loads and risk of disease progression compared with the wild-type strain, calling for more intensive prevention and control measures to contain future outbreaks.
Funding
National Grand Program, National Natural Science Foundation of China, Guangdong Provincial Department of Science and Technology, Guangzhou Laboratory
Keywords: COVID-19, Delta variant, Transmission, Risk factor
Research in context.
Evidence before this study
SARS-CoV-2 Delta lineages (B.1.617.2) is one of four types of lineages identified as variants of concern (VOC) by WHO, and is fast becoming the dominant strain in many countries and continuing to evolve and mutate. We searched PubMed without language restriction for studies published from databases inception until June 18, 2021, using keywords “COVID-19”, “SARS-CoV-2”, “variants of concern” AND “outbreak” OR “viral load” OR “B.1.617.2” OR “Delta VOC”. Although there are reports on emergent Delta VOC, no data are available for characteristics of transmission chain and viral kinetics. The clinical characteristics of Delta VOC in comparison with wild-type SARS-CoV-2 are not fully understood.
Added value of this study
We identified a clear chain of transmission comprised 159 cases with the Delta VOC across seven transmission generations in Guangzhou, China between May 21 and June 18, 2021. A concurrent comparison is conducted which includes 260 cases confirmed with wild-type SARS-CoV-2 admitted in earlier 2020. We found the Delta VOC had a shorter incubation period (4.0 vs. 6.0 days), higher viral load (20.6 vs. 34.0 of cycle threshold), and a more extended period of viral presence in upper respiratory swab samples (14.0 vs. 8.0 days) than the wild-type SARS-CoV-2. The Delta VOC had a higher risk than wild-type SARS-CoV-2 in deterioration to critical status among the elder people (HR, 2.98; 95% CI, 1.29–6.86).
Implications of all the available evidence
Our study revealed a higher transmissibility, viral load and increased risk of disease prognosis of Delta VOC, compared to the SARS-CoV-2 wild-type lineage. These data provided insights to understanding of characteristics of the Delta VOC, which is helpful for achieving effective containment of this emerging wide-spread variant.
Alt-text: Unlabelled box
1. Introduction
Since the coronavirus disease 2019 (COVID-19) outbreak associated with the wild-type strain of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in December 2019, there have been several globally circulating SARS-CoV-2 variant strains that have resulted in a considerable socioeconomic burden. Concerns have been raised regarding their transmissibility and virulence (assessed by the disease severity). To date, there have been four main lineages that are identified as the variants of concern (VOC) by the World Health Organization (WHO), including Alpha (known as B.1.1.7 strain), Beta (known as B.1.351 strain), Gamma (known as P.1 strain) and Delta (known as B.1.617.2 strain) [1]. The Delta VOC was initially detected in October 2020 and has spread to at least 98 countries [2]. According to the latest data from GISAID database, as of July 28, the Delta VOC accounted for 100%, 99.5%, 99.1%, 94% and 99.5% of SARS-CoV-2 infections in the India, United Kingdom, Singapore, Indonesia and Russia in the past 4 weeks [3]. The Delta VOC is fast becoming the dominant strain in many countries and continuing to evolve and mutate [2]. The transmissibility of the Delta VOC was reported to have increased by 97% compared with the wild-type strain [4]. A study of the clinical characteristics of patients with the Delta VOC in Scotland indicates that children aged 5–9 years were more susceptible to viral infection and those with comorbidities were at high risk of hospital admission [5]. However, the epidemiological, viral load and clinical features of patients infected with Delta VOC remain unclear. Furthermore, the impact of Delta VOC on the disease severity and the difference with other SARS-CoV-2 strains also need further investigation.
Recently, the Delta VOC had led to a new wave of outbreak in Guangdong Province. Since the identification of the first case in May 2021 in Guangzhou city (the epicenter), this outbreak had resulted in the infection of 167 confirmed cases throughout Guangdong. Based on the preliminary data of 47 cases, the peak effective reproduction number was estimated to be 3.5 [6]. According to the epidemiological tracing and citywide viral nucleic acid screening assays, the infections were identified as a cluster having a clear chain of transmission and sharing the same nucleic acid sequence as the Delta VOC. Therefore, this represented a unique opportunity for a better understanding of the transmission and clinical characteristics of Delta VOC.
Meanwhile, the cases infected with SARS-CoV-2 wild-type strain during the first wave of COVID-19 in 2020 and the Delta VOC of the current outbreak in Guangzhou were compared to explore their difference in epidemiological, viral kinetics and clinical characteristics.
2. Methods
2.1. Study design and participants
A total of 159 individuals infected with SARS-CoV-2 Delta VOC admitted in the Guangzhou Eighth People's Hospital from May 21 (the first case was officially confirmed) to Jun 18, 2021 (the last case related to the first case) was included as the Delta VOC cohort. Due to the rapid action of community isolation and repeated nucleic acid real-time polymerase chain reaction (RT-PCR) assays organized by the local government, the transmission of Delta VOC was confined to only several communities. All patients were the local residents and epidemiologically confirmed to be linked to a single Delta VOC origin only. The epidemiologic characteristics, viral genomic sequences, kinetics of viral load, clinical characteristics and laboratory finding were collected. Meanwhile, a wild-type SARS-CoV-2 cohort from the same hospital was also included for comparison. The wild-type cohort consisted of all the 260 cases with complete medical records from January to February 2020, the first wave of the pandemic.
All patients were confirmed by the local Centers for Disease Control and transferred to Guangzhou Eighth People's Hospital, Guangzhou Medical University, the only official designated hospital to manage the SARS-CoV-2 patients in Guangzhou. Referring to guidelines issued by Chinese National Health Commission [7] and WHO [8], the severity of the disease was described in the Supplementary Appendix Methods. The study was approved by Guangzhou Eighth People's Hospital Ethics Committee (No. 202001134 and 202115202). Written informed consents were obtained from all patients.
2.2. Epidemiological and clinical data collection
Demographic, clinical characteristic, laboratory findings were collected in both cohorts. All diagnoses were made based on the Guidelines for the Diagnosis and Treatment of Novel Coronavirus Infection produced by the Chinese National Health Commission (Trial Version 8) [7]. Epidemiological data were collected including the exposure histories directly to the confirmed cases or travel to where the confirmed individuals have stayed, such as restaurants, shopping malls, convenience stores, and schools. The incubation period was defined as the days from exposure to the onset of illness, estimated among patients who could provide the exact date of close contact with confirmed or suspected cases.
Laboratory and radiologic findings were derived from the electronic medical charts. The laboratory findings on admission were collected. Chest CT was the primary source of radiologic assessment and was performed within three days of admission. The data were extracted by two independent clinicians into the electronic database. Any major dispute was resolved by consultation with a third reviewer.
2.3. Viral RNA detection with RT-PCR
The collection of nasopharyngeal swabs was performed by well-trained medical staff in the same hospital, and the standardized procedures were strictly followed. The samples were stored in virus medium. Viral RNA was extracted within 2 h using the Nucleic Acid Isolation Kit (Da'an Gene Co. Ltd, Cat: DA0630, China) according to the manufacturer's instructions. RT-PCR was performed by using the RNA Detection Kit for SARS-CoV-2 (Da'an Gene Co. Ltd, Cat: DA0930, China) subsequently [9,10]. RT-PCR was conducted with primers and probes targeting at the N, ORF1a/b genes and a positive reference gene. Reaction system and amplification conditions were performed according to the manufacturer's specification (Da'An Gene Co., Ltd. Of Sun Yat-sen University). The detection limit of cycle threshold (Ct) was set to be 40 (500 copies/ml). Samples with Ct of less than 40 were considered positive. The cut-off Ct value of 40 was determined via the receiver operating characteristics (ROC) curve method. All tests were performed under strict biosafety conditions and the standard operating procedures.
2.4. Viral RNA sequencing and analysis
The sequencing library was prepared using an amplicon-based enrichment method as described previously [9,11]. The sequencing library of all samples was prepared using the hybrid capture-based enrichment method according to the instructions (BGI Co. Ltd, China, Cat no: 1000023555). All samples were sequenced on the MGISEQ-2000 platform. Genomic assembly was conducted using the nCoV Finder pipeline (https://github.com/BGI-IORI/nCoV_Meta). Assessment of variation was carried out using the nCoV Variant detection pipeline (BGI, Co. Ltd, China) for hybrid capture-based sequencing data (https://github.com/BGI-IORI/nCoV_Variants) and SARS-CoV-2 Multi-PCR v1.0 (MGI Tech Ltd., Co.) for amplicon-based sequencing data (https://github.com/MGI-tech-bioinformatics/SARS-CoV-2_Multi-PCR_v1.0). To guarantee reliable variation detection in positive retest samples, only mutation sites with a sequencing depth greater than 100 × were reported.
2.5. Phylogenetic tree construction
Representative SARS-CoV-2 genome sequences were downloaded as the reference sequences from the GISAID database (http://gisaid.org). The full-length SARS-CoV-2 genome sequence was aligned with the above reference sequences and manually checked using BioEdit software (Version 7.0.5). The neighbour-joining (NJ) phylogenetic tree was constructed by the program MEGA 6.0.6 through the Kimura two-parameter model [12]. Bootstrap support values were calculated from 1000 pseudo-replicate trees.
2.6. Statistical analysis
Continuous variables are expressed as median and interquartile range (IQR). Categorical variables are described as number (percentage). The Shapiro-Wilk test was performed to determine whether the distribution of the continuous data was Gaussian or not. The continuous data is considered to be Gaussian if the p-value is above 0.05. Univariate comparisons of continuous variables were performed with an independent Student t-test for normally distributed data; otherwise, with Mann-Whitney U test. Categorical variables were compared using χ2 test or Fisher's exact tests. Survival curves were plotted by using the Kaplan-Meier method and compared by using the log-rank test. To estimate the association between linages and disease severity, the Cox proportional hazards model was performed with variables including virus lineages, demographics, comorbidities, symptom with 3 days of admission. A final model was performed via a backward selection process that adhered to the Akaike information criteria. Estimates of adjusted hazard ratios (HRs), 95% confidence interval (CI) and p value were displayed. All statistical analyses were conducted using R software, version 4.1.0 (http://CRAN.R-project.org, R Foundation, Vienna, Austria). All p values less than 0.05 were considered statistically significant.
2.7. Role of funding source
Funders had no role in the study design, study participant selection and recruitment, data collection and analysis, data interpretation, decision to publish, or preparation of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Local transmission of SARS-COV-2 Delta VOC in Guangzhou
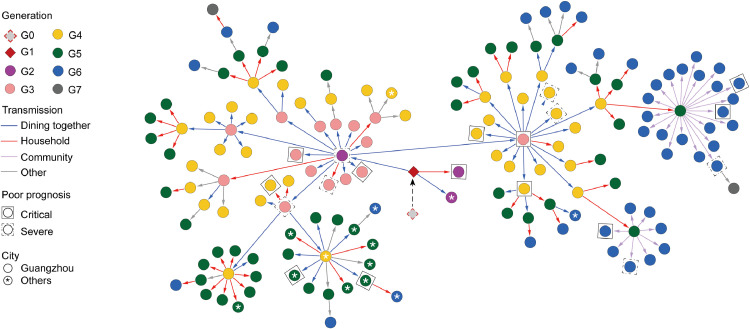
The cluster of Delta VOC infections comprised 159 cases, and the chain of Delta VOC transmission could be traced (Fig. 1). The index case, a 75-year-old female, is a resident in Guangzhou. She had no overseas or domestic travel history in the past 4 weeks and contracted the disease due to accidental exposure to an imported case. She was identified as the first confirmed local case on May 21, 2021 (G1 in Fig. 1; gz4925 in Fig. 2). According to SARS-CoV-2 whole-genome sequencing, the Delta VOC (B.1.617.2 variant) were detected and confirmed in her nasopharyngeal swabs (Appendix p 1). Both of the epidemiological investigation and phylogenetic tree analysis confirmed the women as the first local case (G1) and led to the subsequent transmission. Three cases (G2) were infected by G1 mainly through close familial contact or dining gathering. Among them, the close familial contactor (gz5002 in Fig. 2) progressed to critical illness after admission to the hospital. Another patient (gz5087 in Fig. 2), who progressed into severe illness, was the key transmitter in the third generation and has infected five generations in the cluster. In addition, the third case, the restaurant's waitress with a short period of exposure to G1, was confirmed outside Guangzhou later.
Fig. 1.
Epidemiological transmission network of SARS-CoV-2 Delta VOC transmission in Guangzhou.
One hundred and fifty-seven infected patients with a clear transmission chain were shown in the network. Each transmission generation is shown in rhombus or circles with different colors. The first-generation patient (rhombus with black solid line, G1) is in the middle. G1 was phylogenetically linked to an imported case (rhombus with red dotted line, G0). Colored arrows indicate different transmission routes. The transmission includes dining together, household, community (chat, encounter, taking the elevator together) and others (work and social contacts). Severe (dotted line) and critical (solid line) patients were labelled with squared shapes. Asterisks indicate patients in or to other cities (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
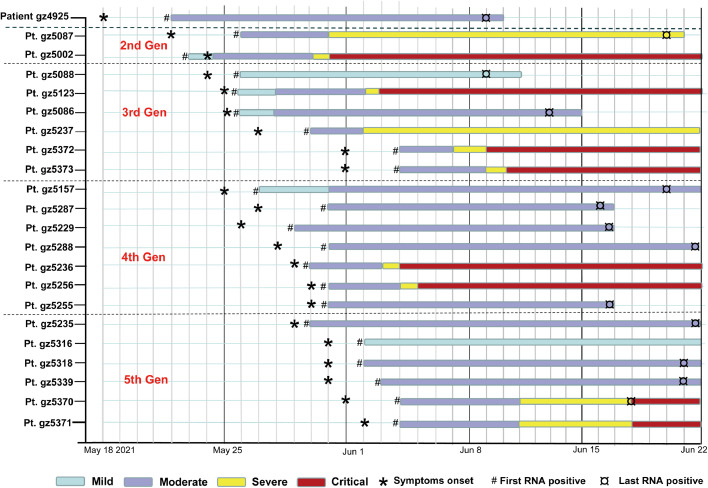
Fig. 2.
Representative individuals from first to fifth generation.
Patient (Pt.) numbers were list to the left. Generations (1st,2nd,3rd 4th,5th Gen) were shown. Timeline of patient clinical symptoms (Mild, Moderate, Severe and Critical) was shown. First symptom (‘*’) indicates the first time point the individual reported illness. First positive (‘#’) and last positive (‘¤’) represent for the first and last time points of viral RNA positive, respectively.
The epidemic was successfully contained within the seven generations. The routes of viral transmission were mainly through short-distance contact directly and indirectly, such as dining, familial close-contact and community connection (Fig. 1). We observed transmission via dining (30.8%) most frequently, followed by household contact (29.6%) and community transmission (18.2%). Severe or critical patients could be found in each generation. In each generation the phylogenetically identical viral strain compared with the second-generation viral strain could be consistently detected (Appendix p 2).
3.2. Rapid transmission of SARS-CoV-2 Delta VOC in Guangzhou
To investigate the contagiousness of Delta VOC, the clinical course of the representative patients from the first to fifth generation is shown in Fig. 2. In this cluster, the first case (Pt. gz4925) presented with symptoms on May 18, 2021, and positive SARS-CoV-2 nucleic acid was initially confirmed on 3 days after symptom onset. Patient gz4925 transmitted the virus to the second-generation case (gz5087) on May 19, 2021, who served as the crucial case resulting in the third-generation infection. Patient gz5088 was the first to develop symptoms in the third generation and was associated with the infection of the fourth-generation case (gz5157). The earliest date of symptom onset in the fifth-generation occurred on May 29, 2021. Overall, SARS-CoV-2 Delta VOC spread four generations within only ten days.
3.3. Clinical characteristics of patients infected with Delta VOC
A total of 159 confirmed cases infected with Delta VOC in the Guangzhou Eighth People's Hospital from May 21 to June 18, 2021, were included, of whom 25.8% (41/159) were mild, 61.6% (98/159) were moderate, 5.0% (8/159) were severe, and 7.5% (12/159) were critical. None had been living or traveled abroad in the previous 14 days (Table 1). 33% (52/159) of patients were aged 60 years or greater, 60% (95/159) were female, and 30.8% (49/159) had at least one comorbidity. Ten cases completed full vaccination. Among them, 30% (3/10) were mild cases, 70% (7/10) were moderate cases. The most common symptoms within three days on admission was cough (65%), followed by fever (63%) and expectoration (53%). Gastrointestinal symptoms including diarrhea (5%) and vomiting (4%) were infrequent.
Table 1.
Demographics and baseline characteristics of COVID-19 patients with different strains.
| Total (n = 419) |
Mild (n = 62) |
Moderate (n = 294) |
Severe (n = 38) |
Critical (n = 25) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT strain (n = 260) | Delta strain (n = 159) | WT strain (n = 21) | Delta strain† (n = 41) | WT strain (n = 196) | Delta strain (n = 98) | WT strain (n = 30) | Delta strain (n = 8) | WT strain (n = 13) | Delta strain (n = 12) | |
| Demographics | ||||||||||
| Sex, n (%) | ||||||||||
| Male | 119 (46) | 64 (40) | 8 (38) | 21 (51) | 86 (44) | 33 (34) | 15 (50) | 2 (25) | 10 (77) | 8 (67) |
| Female | 141 (54) | 95 (60) | 13 (62) | 20 (49) | 110 (56) | 65 (66) | 15 (50) | 6 (75) | 3 (23) | 4 (33) |
| Age (years) | ||||||||||
| Median (Min, Max) | 49.0 (1, 90) | 47.0 (1, 92) | 32.0 (3, 62) | 21.0 (1, 38) | 46.5 (1, 82) | 52.0 (4, 84) | 59.5 (33, 90) | 65.5 (50, 84) | 65.0 (49, 84) | 74.0 (63, 92) |
| < 18, n/N (%) | 7/260 (3) | 26/159 (16)** | 2/21 (10) | 18/41 (44) | 5/196 (3) | 8/98 (8) | 0/30 (0) | 0/8 (0) | 0/13 (0) | 0/12 (0) |
| 18–59$, n/N (%) | 170/260 (65) | 81/159 (51) | 17/21 (81) | 20/41 (49) | 134/196 (68) | 58/98 (59) | 15/30 (50) | 3/8 (37) | 4/13 (31) | 0/12 (0) |
| ≥ 60, n/N (%) | 83/260 (32) | 52/159 (33) | 2/21 (10) | 3/41 (7) | 57/196 (29) | 32/98 (33) | 15/30 (50) | 5/8 (63) | 9/13 (69) | 12/12 (100)* |
| Atopic, n/N (%) | 22/260 (8) | 5/159 (3) | 3/21 (14) | 2/41 (5) | 18/196 (9) | 3/98 (3) | 0/30 (0) | 0/8 (0) | 1/13 (8) | 0/12 (0) |
| Smoking history, n/N (%) | 21/260 (8) | 12/159 (8) | 3/21 (14) | 1/41 (2) | 17/196 (9) | 10/98 (10) | 1/30 (3) | 0/8 (0) | 0/13 (0) | 1/12 (8) |
| Drinking history, n/N (%) | 11/260 (4) | 1/159 (1) | 0/21 (0) | 0/41 (0) | 9/196 (5) | 0/98 (0) | 2/30 (7) | 0/8 (0) | 0/13 (0) | 1/12 (8) |
| Comorbidities, n/N (%) | ||||||||||
| Hypertension | 59/260 (23) | 31/159 (19) | 6/21 (29) | 2/41 (5)* | 35/196 (18) | 18/98 (18) | 13/30 (43) | 4/8 (50) | 5/13 (38) | 7/12 (58) |
| Chronic heart disease | 18/260 (7) | 6/159 (4) | 0/21 (0) | 0/41 (0) | 13/196 (7) | 4/98 (4) | 4/30 (13) | 1/8 (12) | 1/13 (8) | 1/12 (8) |
| Diabetes | 22/260 (8) | 12/159 (8) | 3/21 (14) | 0/41 (0) | 12/196 (6) | 9/98 (9) | 4/30 (13) | 0/8 (0) | 3/13 (23) | 3/12 (25) |
| Chronic respiratory disease | 20/260 (8) | 8/159 (5) | 1/21 (5) | 1/41 (2) | 17/196 (9) | 5/98 (5) | 0/30 (0) | 0/8 (0) | 2/13 (15) | 2/12 (17) |
| Hepatic disease | 16/260 (6) | 2/159 (1)* | 0/21 (0) | 0/41 (0) | 13/196 (7) | 2/98 (2) | 1/30 (3) | 0/8 (0) | 2/13 (15) | 0/12 (0) |
| Kidney disease | 8/260 (3) | 3/159 (2) | 1/21 (5) | 0/41 (0) | 4/196 (2) | 2/98 (2) | 2/30 (7) | 1/8 (12) | 1/13 (8) | 0/12 (0) |
| Psychiatric disorder | 1/260 (0) | 2/159 (1) | 0/21 (0) | 1/41 (2) | 1/196 (1) | 1/98 (1) | 0/30 (0) | 0/8 (0) | 0/13 (0) | 0/12 (0) |
| Thyroid disease | 10/260 (4) | 9/159 (6) | 1/21 (5) | 0/41 (0) | 8/196 (4) | 8/98 (8) | 1/30 (3) | 0/8 (0) | 0/13 (0) | 1/12 (8) |
| Symptoms#, n/N (%) | ||||||||||
| Fever | 196/260 (75) | 100/159 (63)** | 11/21 (52) | 25/41 (61) | 146/196 (74) | 58/98 (59)* | 27/30 (90) | 6/8 (75) | 12/13 (92) | 11/12 (92) |
| Cough | 193/260 (74) | 104/159 (65) | 13/21 (62) | 22/41 (54) | 142/196 (72) | 65/98 (66) | 28/30 (93) | 6/8 (75) | 10/13 (77) | 11/12 (92) |
| Sputum | 112/260 (43) | 84/159 (53) | 3/21 (14) | 18/41 (44)* | 81/196 (41) | 51/98 (53) | 21/30 (70) | 5/8 (62) | 7/13 (54) | 10/12 (83) |
| Sore throat | 49/260 (19) | 39/159 (25) | 7/21 (33) | 7/41 (17) | 32/196 (16) | 23/98 (23) | 7/30 (23) | 4/8 (50) | 3/13 (23) | 5/12 (42) |
| Dyspnea | 60/260 (23) | 18/159 (11)** | 4/21 (19) | 2/41 (5) | 33/196 (17) | 9/98 (9) | 16/30 (53) | 3/8 (38) | 7/13 (54) | 4/12 (33) |
| Vomit | 39/260 (15) | 7/159 (4)** | 1/21 (5) | 1/41 (2) | 33/196 (17) | 5/98 (5)** | 5/30 (17) | 1/8 (12) | 0/13 (0) | 0/12 (0) |
| Headache | 49/260 (19) | 34/159 (21) | 2/21 (10) | 11/41 (27) | 39/196 (20) | 21/98 (21) | 7/30 (23) | 1/8 (12) | 1/13 (8) | 1/12 (8) |
| Diarrhea | 30/260 (12) | 8/159 (5)* | 2/21 (10) | 1/41 (2) | 19/196 (10) | 5/98 (5) | 6/30 (20) | 0/8 (0) | 3/13 (23) | 2/12 (17) |
| Fatigue | 65/260 (25) | 25/159 (16)* | 4/21 (19) | 5/41 (12) | 41/196 (21) | 16/98 (16) | 14/30 (47) | 1/8 (12) | 6/13 (46) | 3/12 (25) |
| PCR cycle threshold values | ||||||||||
| N gene (peak) Median (IQR) | 31.2 (28.0,36.0) | 19.0 (16.1,22.3)** | 28.0 (25.0,36.0) | 18.3 (15.8,22.8)** | 32 .0 (29.0,36.0) | 19.2 (16.5,22.3)** | 32.0 (29.0,36.0) | 19.0 (17.3,20.5)** | 27.0 (25.2,30.4) | 19.6 (16.0,21.2)** |
| ORF1a/b gene (peak) Median (IQR) | 34.0 (30.0,38.0) | 20.6 (17.7,23.6)** | 32 .0 (28.0,38.0) | 19.5 (17.6,23.6)** | 34.0 (31.0,38.0) | 20.7 (17.8,24.1)** | 35.0 (32.2,37.7) | 20.3 (18.7,22.3)** | 28.0 (26.5,30.8) | 21.0 (17.8,23.1)** |
1. Data are median (IQR), median (min, max), n (%) or n/N (%). N is the total number of patients with available data.
2. *p < 0.05, **p < 0.01 compared with the wild type strain. p values were calculated by Mann-Whitney U test, χ² test or Fisher‘s exact test, as appropriate.
3. †: mild cases in Delta group consisted of 12 asymptomatics.
4. Symptoms#, symptoms within 3 days of admission.
5. Abbreviations: WT strain, wild-type strain.
6. $: In the Delta cohort, 10 cases completed full doses of the inactivated vaccines. Among them, 3 cases were mild and 7 cases were moderate.
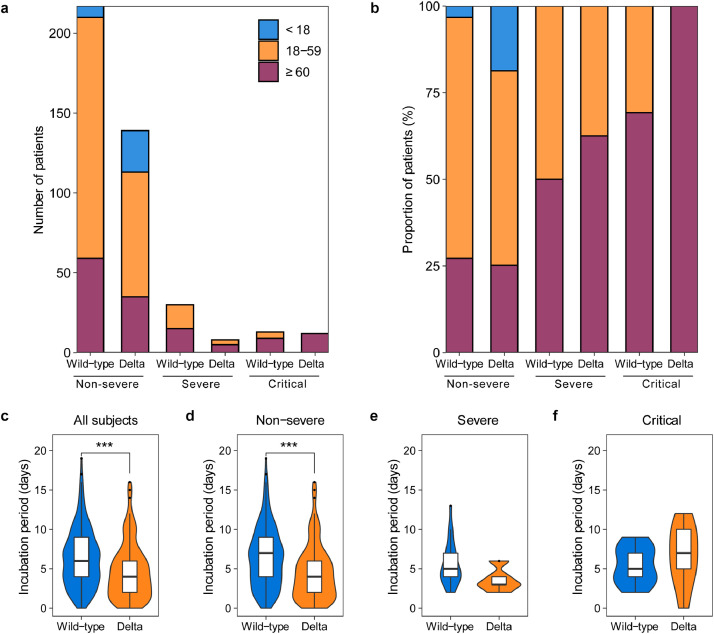
Patients infected with the Delta VOC and those infected with the wild-type strain shared similar distribution of gender, atopy and smoking history. A larger proportion of COVID-19 patients aged < 18 years was found in the Delta VOC group (16% vs 3%; p < 0.01). In patients younger than 18 years old, there were no severe or critical cases regardless of infection with the wild-type strain or Delta VOC. No differences in the proportion of patients aged ≥ 60 years or between 18 and 59 years were found between the two cohorts. In subgroup analysis, patients infected with the Delta VOC (100%) showed a higher proportion of the elderly (≥ 60 years) among the critical cases when compared with 69% of patients infected with the wild-type strain (Fig. 3a,b). There were 79.6% (207/260) of patients in the wild-type cohort and 79.9% (127/159) patients in the Delta VOC cohort with exact exposure times. No significant differences in demographic and clinical characteristics (including gender, age distribution, disease severity distribution, viral load, etc) were found between patients with and without clear incubation periods in each cohort (Appendix pp 9,10). The median incubation period in the Delta VOC cohort was significantly shorter than that in the wild-type cohort (4.0 vs 6.0 days, P < 0.001; Fig. 3c). Further analysis revealed that non-severe patients infected with Delta VOC had a significantly shorter median incubation period than those in the wild-type cohort (4.0 day vs. 7.0 days; P < 0.001; Fig. 3d). Lower frequencies of fever (63% vs 75%), dyspnea (11% vs 23%), diarrhea (5% vs 12%), vomit (4% vs 15%) were found in the Delta VOC cohort compared with the wide-type cohort (all P < 0.01; Table 1). The key laboratory findings are shown in table S1 (Appendix pp 5–8), Infection with the Delta VOC was associated with increased peripheral blood leukocytes, neutrophils and neutrophil-to-lymphocyte ratio and decreased lymphocytes, compared with the wild-type (all P < 0.01).
Fig. 3.
Age groups and incubation period across severity of illness of patients infected with SARS-CoV-2 wild-type or Delta VOC.
Top panel shows absolute counts (a) and proportion (b) of patients in three age groups, including < 18 years, 18–59 years and ≥ 60 years. Bottom panel of the box/violin plot shows the difference of the incubation period between wild-type (Dark blue) and Delta VOC (Earth yellow) across all subjects (c), non-severe (d), severe (e) and critical (f) patients, calculated by the Wilcoxon Test, ***: p < 0.001. In the box plot, the boundary of the box closest to zero indicates the 25th percentile, a black line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers below and above the box indicate the smallest and largest value no further than 1.5 * IQR (inter-quartile range) from 25th percentile or 75th percentile, respectively (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
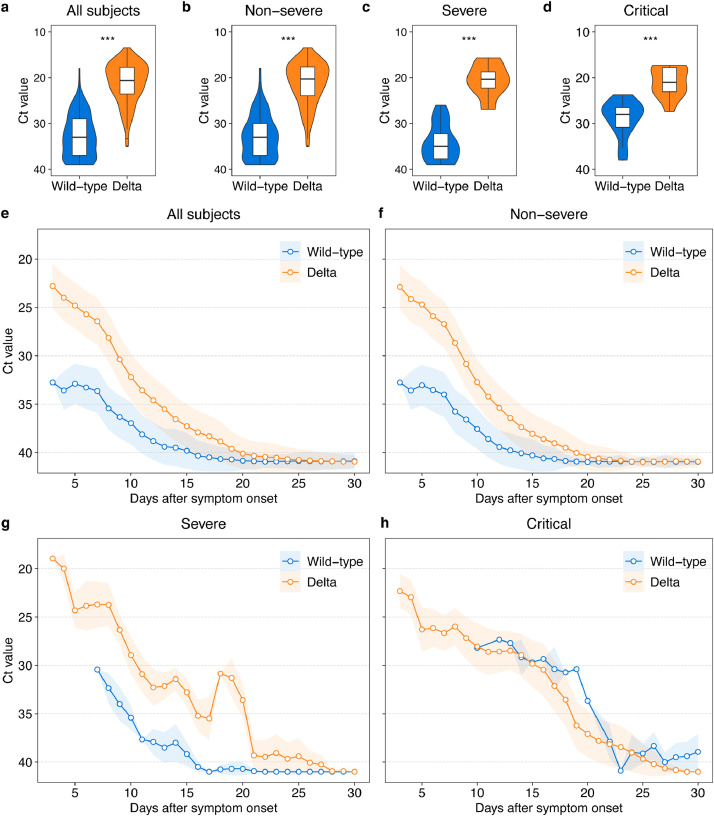
3.4. Higher viral load and prolonged viral shedding in Delta VOC infection
The peak viral load (lowest Ct value) and dynamic changes of ORF1a/b gene in the Delta VOC cohort and wild-type cohort are presented in Fig. 4. Compared with the wild-type cohort, the Delta VOC cohort was characterized by significant higher peak virus loads (median Ct: 20.6 vs 34.0; p < 0.001; Fig. 4a). The same trend of changes was consistently observed in patients with non-severe, severe and critical illness (Fig. 4b–d). The viral load in both groups presented a gradually decreased trend over time. The Delta VOC cohort showed prolonged viral shedding, compared with wild-type group (Fig. 4e). As shown in the supplementary appendix (p 4), the duration of nucleic acid conversion (Ct > 40) in patients infected with the Delta VOC was also longer. Similar changes in the viral loads also applied in patients with different categories of clinical severity (Fig. 4f–h; Appendix p 4). In addition, the results of the N gene shown in appendix (p 3) was similar to those of the ORF1a/b gene.
Fig. 4.
The highest and dynamics of viral load among hospitalized COVID-19 patients infected with SARS-CoV-2 wild-type or Delta VOC.
Box/violin plot shows Ct values at highest viral load during hospitalization with their significance compared between wild-type or Delta VOC-infected patients in total (a), non-severe (b), severe (c) and critical (d) patients. In the box plot, the boundary of the box closest to zero indicates the 25th percentile, a black line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers below and above the box indicate the largest and smallest value no further than 1.5 * IQR (inter-quartile range) from 75th percentile or 25th percentile, respectively. To show the dynamics of Ct values of patients infected with wild-type or Delta VOC, arithmetic mean (circle point) and standard error (colored range) is firstly calculated for each individual day, then a moving average of three consecutive days is computed for all subjects (e) and patients with different severity of illness (f–h). The viral load was presented by Ct Value of ORF1a/b gene. P values less than 0.001 is calculated by the Wilcoxon Test and shown as three asterisks (***). Refer to appendix (p3) for patterns of Ct values of N gene (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
3.5. The risk factors for disease progression and outcomes
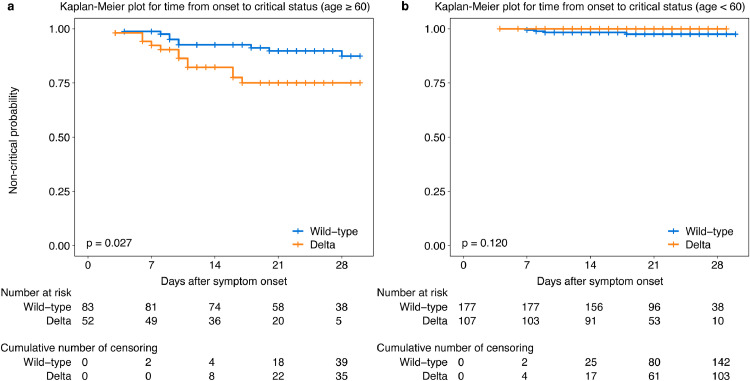
The univariate analysis indicated that the age, sex, comorbidity (including chronic respiratory disease, hypertension, diabetes) and symptoms within three days of admission (including fever, dyspnea) were associated with the deterioration to critical illness (p < 0.05). Multivariate Cox regression analysis revealed that Delta VOC infection (HR 2.98 [95%CI 1.29–6.86]), age > 60 years (HR 11.13 [95%CI 3.78–32.82]), male (HR 3.49 [95%CI 1.45–8.41]), dyspnea (HR 2.60 [95%CI 1.14–5.93]) and fever within 3 days of admission (HR 4.77 [95%CI 1.10–20.64]) were the independent risk factors associated with the deterioration to critical illness (Table 2). The Kaplan-Meier survival plots for the time from symptom onset to critical status categorized by virus lineage (Delta VOC vs wild-type strain) in patients ≥ 60 years and < 60 years were shown in Fig. 5a and b.
Table 2.
Univariate and Multivariate Cox regression analysis of the risk factors for deterioration to critical status in patients with COVID-19.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Lineage | ||||
| Wide-type | Ref. | - | Ref. | - |
| Delta | 1.65 (0.75–3.64) | 0.210 | 2.98 (1.29–6.86) | 0.010 |
| Age | ||||
| <60 | Ref. | - | Ref. | - |
| ≥ 60 | 11.13 (3.82–32.44) | <0.001 | 11.13 (3.78–32.82) | < 0.001 |
| Sex | ||||
| Female | Ref. | - | Ref. | - |
| Male | 3.49 (1.45–8.41) | 0.005 | 3.49 (1.45–8.41) | 0.005 |
| Smoking history | ||||
| No | Ref. | - | - | - |
| Yes | 0.49 (0.07–3.59) | 0.480 | ||
| Comorbidities | ||||
| Chronic respiratory disease | ||||
| No | Ref. | - | - | - |
| Yes | 3.21 (1.10–9.37) | 0.033 | ||
| Chronic heart disease | ||||
| No | Ref. | - | - | - |
| Yes | 1.35 (0.32–5.74) | 0.680 | ||
| Hypertension | ||||
| No | Ref. | - | - | - |
| Yes | 3.49 (1.59–7.67) | 0.002 | ||
| Diabetes | ||||
| No | Ref. | - | - | - |
| Yes | 3.85 (1.53–9.64) | 0.004 | ||
| Symptoms# | ||||
| Fever | ||||
| No | Ref. | - | Ref. | - |
| Yes | 4.42 (1.04–18.75) | 0.044 | 4.77 (1.10–20.65) | 0.037 |
| Cough | ||||
| No | Ref. | - | - | - |
| Yes | 2.01 (0.69–-5.86) | 0.200 | ||
| Dyspnea | ||||
| No | Ref. | - | Ref. | - |
| Yes | 3.34 (1.51–7.37) | 0.003 | 2.60 (1.14–5.93) | 0.023 |
| Diarrhea | ||||
| No | Ref. | - | - | - |
| Yes | 2.50 (0.94–6.67) | 0.067 | ||
HR, hazard ratio; CI, confidence interval; Symptoms#, symptoms within 3 days of admission.
Fig. 5.
Kaplan-Meier survival plots for the prognostic factors.
The figure displays the Kaplan-Meier plot of time from symptom onset to critical status categorized by virus lineage (Delta VOC vs wild-type) in patients ≥ 60 (a) and < 60 years (b).
4. Discussion
To our knowledge, this is the first study which has thoroughly described the whole chain of the Delta VOC transmission, the kinetics of viral load and clinical characteristics in a local outbreak. Compared with a wild-type cohort admitted to the same hospital, infection with the Delta VOC was associated with a shorter incubation period, higher viral loads and was proven as an independent risk factor of greater disease severity.
Delta VOC was first identified in Oct 2020, and has become the dominant variant globally since April 2021 [1]. Current global reports have announced Delta VOC with a feature of rapid transmissibility around different countries, such as India, Britain [13]. According to the report by Public Health England, the B.1.617.2 strain displayed an increased growth rate compared with the B.1.1.7 strain under investigation from 1 January 2021 to 18 May 2021. By using the sequencing data from GISAID, Campbell et al reported a 97% (95%CI: 76–117%) increase in the effective reproductive number of B.1.617.2 strain by 3 June, 2021 [4]. However, due to the wide spread of this variant, it is difficult to identify the characteristics of the transmission accurately.
The local outbreak in Guangzhou comprised 159 locally transmitted cases during the study period. All patients were confirmed infected with the same strain of Delta VOC by next-generation sequencing. Moreover, the local government implemented a thorough epidemiological tracing and follow-up, quarantined highly suspected persons in hotels, and initiated community and citywide viral RNA screening, thus offering a clear and precise overall picture of virus transmission. Our study provided a unique opportunity to understand the transmission and clinical characteristics of Delta VOC.
Our data demonstrated a rapid transmission of Delta VOC. First, Delta VOC accomplished four consecutive generations of transmissions within ten days, with an average of 2.5 days per generation. Transmission was mainly achieved through short-distance contacts, including dining together, community and household. In some individuals, the duration from acquisition of infection to further spreading spanned only a single day. Second, compared with the wide-type cohort, the incubation period was shorter in the Delta VOC cohort, which implied that the Delta VOC was more virulent. Third, an outstanding finding was that most viral genomes from different epidemiological generations were almost identical with less than three mutations. Since the SARS-CoV-2 virus keeps mutating quickly in vivo [14,15], the absence of new mutation in the fifth and sixth generations compared with the second generation corroborated the rapid virus replication and transmission.
The higher viral load was a key feature of Delta VOC. Several crucial mutations in the receptor-binding domain have dramatically altered the direct contact of spike protein with the ACE2 receptor and resulted in compromised immune protection elicited by natural infection or vaccination [5]. High levels of Delta VOC virus was detected in patients in vivo [16]. We observed that Delta VOC group had about over 12 Ct values lower than the wild-type group, equaling in theory > 1,000 times more virus in patients infected with Delta VOC. We also observed that the Delta VOC cohort yielded a slower viral clearance, compared with the wild-type cohort.
A recent preprint report by Indian National Organization has investigated the association between the viral load and infectivity among patients infected with the lineage B.1.617. The data showed the Ct kept falling from March to April 2021, with an increasing proportion of B.1.617 stain, compared to the baseline between Dec 2020 to Feb 2021 in Delhi, India [16]. The growth of B.1.617 was paralleled by a significant increase in the seropositivity rate. Consistently, our data also showed the peak viral load of Delta VOC was more than a 1000-fold increase than in wild-type SARS-CoV-2 strains, suggesting that Delta VOC infection was associated with higher viral loads. The higher transmissibility and viral load were considered to be related to the mutations of Delta VOC. Molecular profiling studies have revealed that the Delta strain was characterized by several salient spike protein changes such as T19R, Δ157,158, L452R, T478K, D614G, P681R, and D950N. As reported recently, the spike L452R mutation conferred enhanced transmissibility and infectivity [17,18]. Via analysis of construction computational model, B.1.617 variant with L452R–E484Q mutations was indicated performing a stronger binding affinity for the host ACE2, likely enhancing virus infectivity [19,20]. Furthermore, P681R mutation can accelerate viral fusion, resulting in enhanced infectivity of Delta VOC as well as a higher viral load [21,22].
A larger proportion of infected cases aged < 18 years was found in the Delta VOC cohort. This was in line with the recent study in Scotland which found a larger proportion of the S gene-positive cases aged 5–9 years compared with the S gene-negative cases [5]. The similar trend was also reported for the lineage B.1.1.7 [23]. No difference in the proportion of COVID-19 patients aged ≥ 60 years was found between the Delta VOC cohort and the wide-type cohort. However, in subgroup analysis, all critical cases in Delta VOC cohort were the elderly individuals aged > 60 years, compared with 69% in the wide-type cohort. The difference indicated that the elderly patients were more susceptible to the infection with the Delta VOC. The susceptibility to Delta VOC infection in the elderly has been associated with immunosenescence, particularly the increased production of inflammatory cytokines [24]. In addition, the clinical outcomes of elderly patients were significantly different in the infection of Delta VOC and wild-type strain. Patients aged greater than 60 years in the Delta VOC cohort have an increased risk in deteriorating to critical illness, compared with those in the wild-type cohort.
The Cox regression analysis showed that the virus lineage was related to disease progression. We found the infection with the lineage of Delta VOC had a higher risk than the wide-type cohort in deteriorating to critical illness A recent study conducted in Scotland demonstrated that the risk of COVID-19 hospital admission approximately doubled in those infected with the Delta VOC when compared with the Alpha VOC. Together, infection with Delta VOC indicated a possibility of more severe clinical status and poorer outcome, which called for a more intensive surveillance program.
Consistent with our previous findings [25], fever and cough were two salient symptoms on hospital admission among patients infected with the Delta strain, suggesting the need to integrate molecular testing and symptom screening in identifying the cases in community settings. Gastrointestinal symptoms including vomiting, nausea and diarrhea were less frequent compared with the wide-type strain infection. Moreover, our data showed that the Delta VOC cohort had a decreased lymphocytes and increased neutrophil counts, compared with the wide-type cohort. Lymphopenia and high neutrophil-lymphocyte ratio were considered to be the predictors for deterioration whereas higher lymphocyte counts predicted a better clinical outcome [26,27]. It is worth noting that the differences of lymphocytes and the neutrophil-lymphocyte ratio were obvious in mild to moderate cases in the Delta VOC cohort compared with wild-type cohort. This might be associated with a higher viral load in patients with Delta VOC, which warrants a further investigation of the immune characteristic.
The prevention and control measures against the wild-type SARS-CoV-2 outbreaks in China have been proven to be effective [28]. These measures including wearing facial masks, social distancing, restrictions on social activities and regional lockdowns of communities are also contributed to contain the current outbreak of the Delta VOC. However, the novel features of Delta VOC pose new challenges to Delta VOC prevention and control. In response to the significantly greater transmissibility in community settings, new measures are taken against the Delta VOC during the Delta VOC outbreak in Guangzhou. Firstly, tens of millions of massive citywide viral RNA screening tests have been launched, targeting at both the general and high-risk population for screening of any residual unidentified cases. Second, a detailed epidemiology investigation and a big data-based QR code system helped identify, classify and manage close contacts of confirmed cases. With these efforts, the outbreak was quickly contained and resulted in only 167 cases within one month. These experiences could be valuable for the emerging wide-spread variant.
Some limitations should be noted. First, comparison of the viral-specific antibody generation was missing because two different antibody measurement kits were used for the wide-type virus in 2020 and Delta VOC in 2021. Given the significantly higher peak viral titers and the more extended shedding of the Delta VOC, the Delta VOC might elicit a higher antibody concentration. Comparing the humoral immune responses of wild-type strain and the Delta VOC would be valuable. Second, data related to infection-induced immune response was limited which has been suggested to be vital in driving the progression of COVID-19. Third, given that the cohort infected with the Delta VOC was included from a local outbreak, which was quickly contained within about one month, the sample size remained relatively small. Forth, compared with the early phase of the pandemic, the current public health prevention and control measurements are more systematic and effective. There were 12 asymptomatic cases in the Delta VOC group, while the wild-type cohort had no asymptomatic subjects. It was difficult to make a subgroup comparison of asymptomatic cases between two cohorts.
Our study revealed a higher transmissibility, viral load and increased risk of disease prognosis of Delta VOC, compared with the SARS-CoV-2 wild-type lineage. These data provide insights to the understanding of characteristics of the Delta VOC, which is helpful for combating this rapidly spreading global pandemic. Intensive prevention and control measures including rapidly tracing, quarantine, restrictions on social settings and citywide nucleic acid screening are valuable to contain the outbreak of emergent high transmissible variants.
Contributors
XT, NZ, FL, YW, RC and FH had the idea for and designed the study. All authors had full access to the data and had final responsibility for the decision to submit for publication. XT, NZ, FL, YW, RC, FH, YL, ZY, CZ, SZ, WG drafted the paper. JS, XD, MJ, SZ, BL, KD, Y.M.L, SL did the analysis and all authors critically revised the manuscript for important intellectual content and gave final approval for the version to be published. JT, LG, M.L.J, QF, ML, JL, YS, XD, XX, MK, YL collected the data. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data sharing
The data that support the findings of this study are available from the corresponding author (FL, NZ, XT) upon reasonable request and with permission of Guangzhou Eighth People's Hospital, Guangzhou Medical University, Guangzhou, China.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank all the physicians and nurses who collected the samples and cared for these patients in the isolation ward. We also thank Weichun Zhu, Guofang Tang, Jiaojiao Li, Jianping Cui, Sisi Chen, Kun Zeng in Guangzhou Eighth People's Hospital and Xiaolong Ji, Bizhou Li, Haopeng Zhi, Liman Fang in Guangzhou Medical University for their assistance in data preparation.
Funding
This work was financially supported by Emergency Key Program of Guangzhou Laboratory (EKPG21-29), National Natural Science Foundation of China (81770593), National Grand Program on Key Infectious Disease Control (2018ZX10301404-003-002), Guangdong Provincial Department of Science and Technology Fund (No. 2020B1111330002).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101129.
Contributor Information
Feng Li, Email: gz8h_lifeng@126.com.
Nanshan Zhong, Email: nanshan@vip.163.com.
Xiaoping Tang, Email: tangxp@gzhmu.edu.cn.
Appendix. Supplementary materials
References
- 1.Update on SARS-CoV-2 variant nomenclature, https://www.who.int/publications/m/item/update-60-sars-cov-2-nomenclature-variants
- 2.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid- 19-2-july- 2021.
- 3.https://www.gisaid.org/hcov19-variants/. Accessed on July 28, 2021.
- 4.Campbell F., Archer B., Laurenson-Schafer H. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheikh A., McMenamin J., Taylor B., Robertson C. SARS-CoV-2 delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.M. Zhang, J. Xiao, A.P. Deng, et al. Transmission dynamics of an outbreak of the COVID-19 delta variant B.1.617.2-Guangdong Province, China, May–June 2021; 2021. doi: 10.46234/ccdcw2021.148. [DOI] [PMC free article] [PubMed]
- 7.National Health Commission & State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 8). August 19, 2020. Available at: https://http://www.nhc.gov.cn/cms-search/downFiles/a449a3e2e2c94d9a856d5faea2ff0f94.pdf
- 8.World Health Organization. COVID-19 Clinical management: living guidance, 2021. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
- 9.Hu F., Chen F., Ou Z. A compromised specific humoral immune response against the SARS-CoV-2 receptor-binding domain is related to viral persistence and periodic shedding in the gastrointestinal tract. Cell Mol Immunol. 2020;17:1119–1125. doi: 10.1038/s41423-020-00550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAndrews K.M., Dowlatshahi D.P., Dai J. Heterogeneous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications for COVID-19 immunity. JCI Insight. 2020;5 doi: 10.1172/jci.insight.142386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao M., Liu X., Ji J. Multiple approaches for massively parallel sequencing of SARS-CoV-2 genomes directly from clinical samples. Genome Med. 2020;12:57. doi: 10.1186/s13073-020-00751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabibzadeh A., Zamani F., Laali A. SARS-CoV-2 Molecular and Phylogenetic analysis in COVID-19 patients: a preliminary report from Iran. Infect Genet Evol. 2020;84 doi: 10.1016/j.meegid.2020.104387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://www.ecdc.europa.eu/sites/default/files/documents/Emergence-of-SARS-CoV-2-B.1.617-variants-in-India-and-situation-in-the-EUEEA_0.pdf
- 14.Gao R., Zu W., Liu Y. Quasispecies of SARS-CoV-2 revealed by single nucleotide polymorphisms (SNPs) analysis. Virulence. 2021;12:1209–1226. doi: 10.1080/21505594.2021.1911477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghorbani A., Samarfard S., Ramezani A. Quasi-species nature and differential gene expression of severe acute respiratory syndrome coronavirus 2 and phylogenetic analysis of a novel Iranian strain. Infect Genet Evol. 2020;85 doi: 10.1016/j.meegid.2020.104556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.M.S. Dhar, R. Marwal, V. Radhakrishnan, et al. Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. medRxiv 2021. doi; 10.1101/2021.06.02.21258076. [DOI] [PMC free article] [PubMed]
- 17.Deng X., Garcia-Knight M.A., Khalid M.M. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell. 2021;184:3426–3437. doi: 10.1016/j.cell.2021.04.025. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.X. Deng, M.A. Garcia-Knight, M.M. Khalid, et al. Transmission, infectivity, and antibody neutralization of an emerging SARS-CoV-2 variant in California carrying a L452R spike protein mutation. medRxiv the preprint server for health sciences 2021. doi:10.1101/2021.03.07.21252647.
- 19.Khan A., Wei D.Q., Kousar K. Preliminary structural data revealed that the SARS-CoV-2 B.1.617 variant's RBD binds to ACE2 receptor stronger than the wild type to enhance the infectivity. Chembiochem. 2021 doi: 10.1002/cbic.202100191. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinonez E., Vahed M., Hashemi Shahraki A., Mirsaeidi M. Structural analysis of the novel variants of SARS-CoV-2 and forecasting in North America. Viruses. 2021;13:930. doi: 10.3390/v13050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh J., Rahman S.A., Ehtesham N.Z., Hira S., Hasnain SE. SARS-CoV-2 variants of concern are emerging in India. Nat Med. 2021 doi: 10.1038/s41591-021-01397-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.A. Saito, H. Nasser, K. Uriu, et al. SARS-CoV-2 spike P681R mutation enhances and accelerates viral fusion. bioRxiv 2021. doi:10.1101/2021.06.17.448820.
- 23.Frampton D., Rampling T., Cross A. Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietrobon A.J., Teixeira F.M.E., Sato M.N. I mmunosenescence and Inflammaging: risk Factors of severe COVID-19 in older people. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.579220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang W., Liang H., Ou L. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180:1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long L., Zeng X., Zhang X. Short-term outcomes of COVID-19 and risk factors for progression. Eur Respir J. 2020;55 doi: 10.1183/13993003.00990-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z., Li J. Letter from China: response after the first wave of COVID-19. Respirology. 2021;26:273–274. doi: 10.1111/resp.13998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.