Abstract
Professional society guidelines recommend semiannual screening for hepatocellular carcinoma (HCC) in patients with cirrhosis; however, studies suggest underuse of screening in clinical practice. Our study’s aim was to characterize reasons for HCC screening underuse among patients with cirrhosis. We conducted a retrospective cohort study of patients with cirrhosis diagnosed with HCC in two large health systems from 2011 to 2019. We classified screening receipt as consistent, inconsistent, or no screening in the year before HCC diagnosis. We categorized reasons for screening underuse as a potential failure at each of the following steps required for HCC screening: receipt of regular outpatient care, recognition of liver disease, recognition of cirrhosis, screening orders in patients with cirrhosis, and adherence to screening ultrasound appointments. Among 1,014 patients with cirrhosis with HCC, only 377 (37.2%) had regular outpatient care in the year before HCC presentation. Consistent screening was observed in 93 (24.7%) patients under regular outpatient care, whereas 161 (42.7%) had inconsistent screening and 123 (32.6%) no screening. We found screening underuse related to failures at each step in the screening process, although nearly half (49.6%) were due to lack of screening orders in patients with known cirrhosis. Conclusion: The most common reasons for HCC screening underuse in patients with cirrhosis are lack of regular outpatient care and lack of screening orders in those with known cirrhosis, highlighting the need for interventions targeted at these steps to increase HCC screening use.
In a retrospective cohort study of 1014 cirrhosis patients diagnosed with HCC in two large health systems, we categorized reasons for screening underuse: receipt of regular outpatient care, recognition of liver disease, recognition of cirrhosis, screening orders in patients with cirrhosis, and adherence to screening ultrasound appointments. Only 377 (37.2%) patients had regular outpatient care in the year prior to HCC presentation, of whom 93 (24.7%) had consistent screening, 161 (42.7%) had inconsistent screening, and 123 (32.6%) no screening. We found screening underuse related to failures at each step in the screening process, although nearly half (49.6%) were due to lack of screening orders in patients with known cirrhosis.

Abbreviations
- CI
confidence interval
- EMR
electronic medical record
- HCC
hepatocellular carcinoma
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OR
odds ratio
- PCP
primary care provider
- PHHS
Parkland Health and Hospital System
- UTSW
University of Texas Southwestern Medical Center
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer death worldwide and a leading cause of death in patients with cirrhosis. Overall, HCC has a dismal prognosis, with a 5‐year survival of only 18% and minimal improvement in mortality rates over time.( 1, 2 ) However, HCC prognosis varies significantly based on tumor stage at diagnosis; for patients diagnosed at an early stage, surgical resection and ablation can afford 5‐year survival rates exceeding 70%,( 3 ) whereas median survival is less than 1 year for those diagnosed at an advanced stage.( 4 )
Given the association between tumor stage and survival, professional societies recommend HCC screening among patients at highest risk. The American Association for the Study of Liver Diseases (AASLD) recommends screening with semiannual abdominal ultrasound, with or without serum alpha fetoprotein, for patients with cirrhosis or chronic hepatitis B virus (HBV) infection.( 5 ) These recommendations are supported by a large, randomized, controlled trial among patients with chronic HBV and by several cohort studies among patients with cirrhosis showing that screening is associated with early detection, curative treatment receipt, and improved survival.( 6, 7 )
For HCC screening to reduce mortality in clinical practice, it must be effectively implemented. However, a meta‐analysis of cohort studies found that less than 25% of patients with cirrhosis receive screening, with lower use among patients followed outside of gastroenterology subspecialty practices.( 8, 9 ) The screening process can be broken down into the following four discrete steps, with the potential for failure at each step: providers must 1) recognize the presence of underlying liver disease, 2) recognize cirrhosis in those with chronic liver disease, and 3) order HCC screening for patients with cirrhosis, while 4) patients must adhere to provider recommendations for screening. Granular data examining reasons for screening underuse are critical to informing interventions that can increase HCC screening, but these data are unfortunately less robust. In a study among 178 patients with HCC, we demonstrated screening failures across this continuum;( 10 ) however, that study was conducted in an older cohort at a single health system with most HCC cases related to active hepatitis C infection and few due to nonviral etiologies.
To address these limitations, we performed a retrospective cohort study to characterize screening failures among a large cohort of patients followed at two large health systems comprising a safety‐net health system and an academic tertiary care referral center.
Patients and Methods
Study Population
We identified consecutive patients diagnosed with HCC from 2011 to 2019, using a prospectively maintained database at Parkland Health and Hospital System (PHHS) and the University of Texas Southwestern Medical Center (UTSW) ‐ two sites of the North American Liver Cancer (NALC) Consortium. Detailed information regarding this cohort has been reported.( 11 ) PHHS is a publicly funded safety‐net hospital system serving a large socioeconomically disadvantaged population in Dallas County, which includes 12 community‐based primary care clinics and outpatient specialty clinics. Uninsured patients receive access to primary and specialty care at reduced cost through a sliding‐scale financial assistance program. UTSW is a large tertiary care referral center with a liver transplant program and a National Cancer Institute‐designated comprehensive cancer center; it serves as a referral center for patients with cirrhosis and HCC. Patients at both sites are cared for by the same set of clinical providers, and patients diagnosed with HCC at both sites have access to multidisciplinary management.( 12 ) All HCC diagnoses were made using either radiologic or histologic features per AASLD criteria.( 5 ) Patients were excluded if they received HCC treatment before initial presentation at one of the study sites. The study protocols were approved by the Institutional Review Board at UTSW.
Data Collection and Definition
Patient characteristics, including demographics, clinical characteristics, laboratory data, health care use, and tumor characteristics, at HCC diagnosis were collected from the electronic medical record (EMR). Demographics included patient age, sex, and race/ethnicity. Race/ethnicity was classified as non‐Hispanic white, black, Hispanic, or other.( 11 ) Clinical characteristics of interest included insurance status, liver disease etiology, and liver disease severity. Liver disease etiology was classified as hepatitis C, hepatitis B, alcohol‐related, and nonalcoholic steatohepatitis (NASH), as described.( 13 ) We captured presence of ascites and encephalopathy and relevant laboratory data to calculate Child‐Pugh score for all patients. Health care use variables included the number of outpatient, inpatient, and emergency department visits in the 2 years preceding HCC diagnosis. Among outpatient visits, we specifically recorded primary care provider (PCP) and gastroenterology (including hepatology) visits, given the strong association with screening receipt.( 9 ) Receipt of regular outpatient care was defined as having at least one PCP or gastroenterology visit more than 1 year before HCC diagnosis and another visit within the year before HCC diagnosis. Tumor burden at diagnosis was recorded, including the number of HCC lesions, maximum diameter, and presence of vascular invasion or distant metastases.
We recorded dates and indications for all abdominal ultrasound, computed tomography, and magnetic resonance imaging studies in the year before HCC diagnosis. We included images performed for nonscreening indications as they would concurrently exclude the presence of HCC and preclude the need for repeat screening imaging at that time. Outside imaging studies were captured using the Care Everywhere Network function in the EMR. Screening receipt was classified as either consistent, inconsistent, or no screening. Consistent screening was defined as having semiannual imaging in the year before diagnosis, whereas inconsistent screening was defined as having at least one imaging study during the study period but less than consistent screening. Among patients with no screening, we identified potential failures at the following steps in the screening process, as determined by review of clinic notes and imaging records: receipt of regular outpatient care (as defined above), recognition of chronic liver disease, recognition of cirrhosis among those with known liver disease, screening orders in those with known cirrhosis, and adherence to screening ultrasound appointments among those with orders. Recognition of chronic liver disease was defined as documentation of liver disease (e.g., hepatitis C, alcohol‐related liver disease, NASH, cirrhosis) in an outpatient clinic note. Recognition of cirrhosis was defined as documentation of suspected cirrhosis (including liver nodularity) or hepatic decompensation (ascites or hepatic encephalopathy) in clinic notes.
Statistics
We used the Mann‐Whitney U and Kruskal‐Wallis tests to compare continuous variables and the two‐tailed Fisher’s exact and χ2 tests to compare categorical data between patient groups. We used univariable logistic regression to identify factors associated with HCC screening receipt and potential failures in the screening process. Factors significant in univariable models were introduced into multivariable logistic regression models with forward selection based on likelihood ratios to determine the adjusted odds ratio (OR) and 95% confidence intervals (CIs). Insurance status was collinear with health system so was not included in multivariable models. A two‐tailed P < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 23 (IBM, Armonk, NY).
Results
Patient Characteristics
We identified 1,111 patients diagnosed with HCC between 2011 and 2019. Of these patients, 97 (8.7%) did not have cirrhosis and were excluded from further analyses (Supporting Table S1). Characteristics of the remaining 1,014 patients with cirrhosis are detailed in Table 1. In brief, the median age of the cohort was 59.9 years, and 76.1% of patients were men. The cohort was racially/ethnically diverse, with 34.1% non‐Hispanic white, 31.1% black, and 29.2% Hispanic white. The most common liver disease etiologies were hepatitis C (63.4%), alcohol‐related (14.0%), and NASH (10.7%). Most patients had compensated cirrhosis, the median Child‐Pugh score was 7, and 47.9% had Child‐Pugh A cirrhosis.
Table 1.
Patient Characteristics
| Characteristic | Patients With Cirrhosis (n = 1,014) |
|---|---|
| Age (median, IQR) | 59.9 (55.6‐65.2) |
| Sex (% male) | 772 (76.1%) |
| Hospital type (% public) | 668 (65.9%) |
| Race | |
| Non‐Hispanic white | 346 (34.1%) |
| Black | 315 (31.1%) |
| Hispanic white | 296 (29.2%) |
| Asian | 41 (4.0%) |
| Other/unknown | 16 (1.6%) |
| Etiology | |
| Hepatitis C | 643 (63.4%) |
| Hepatitis B | 62 (6.1%) |
| Alcohol | 142 (14.0%) |
| NASH | 108 (10.7%) |
| Other | 59 (5.8%) |
| Insurance | |
| Medicare | 298 (29.4%) |
| Medicaid | 214 (21.1%) |
| Private insurance | 163 (16.1%) |
| Other* | 234 (23.1%) |
| None | 105 (10.5%) |
| Severity of cirrhosis at HCC diagnosis | |
| Presence of hepatic encephalopathy | 212 (20.9%) |
| Presence of ascites | 475 (46.8%) |
| Platelet count (mean ± SD) | 137 ± 92 |
| Child‐Pugh score (% Child A) | 486 (47.9%) |
| Barcelona Clinic Liver Cancer stage | |
| Stage 0 | 75 (7.4%) |
| Stage A | 379 (37.4%) |
| Stage B | 120 (11.8%) |
| Stage C | 276 (27.2%) |
| Stage D | 146 (14.4%) |
Includes PHHS financial assistance.
Abbreviation: IQR, interquartile range.
Health Care Use
Patterns of health care system use are described in Fig. 1. Nearly one fourth (n = 232, 22.9%) of patients did not have any contact with a health care system before HCC diagnosis, while another 12.5% (n = 127) only had inpatient or emergency department visits without any outpatient care. Only 612 (60.4%) patients were seen by a PCP or gastroenterology provider before HCC diagnosis, and only 377 (37.2%) had regular outpatient care (Supporting Table S2). Regular outpatient care was more likely among older patients and those with thrombocytopenia but less likely among men and those with higher Child‐Pugh scores (Table 2).
FIG. 1.
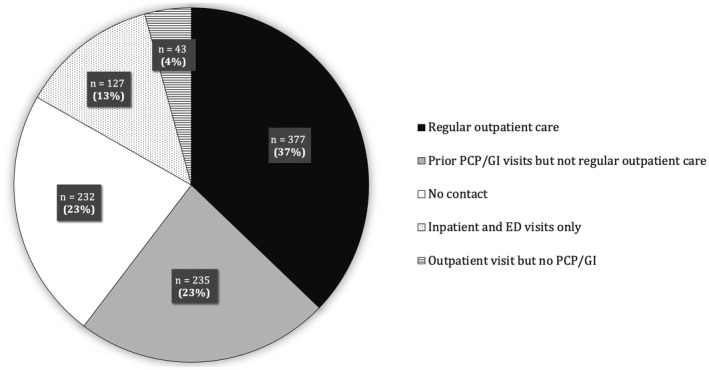
Patterns of health care use. Abbreviations: ED, emergency department; GI, gastroenterologist.
Table 2.
Correlates of regular outpatient care (n = 1,014)
| Variables | Univariable OR (95% CI) | Multivariable OR* (95% CI) |
|---|---|---|
| Age (in 10‐year increments) | 1.28 (1.10‐1.49) | 1.43 (1.21‐1.70) |
| Sex (male) | 0.57 (0.42‐0.76) | 0.63 (0.46‐0.87) |
| English | 0.68 (0.49‐0.94) | |
| Race | ||
| Non‐Hispanic white | Reference | |
| Black | 2.24 (1.63‐3.09) | 1.56 (1.09‐2.24) |
| Hispanic white | 1.61 (1.16‐2.24) | 1.05 (0.73‐1.52) |
| Asian | 1.05 (0.51‐2.14) | 0.72 (0.34‐1.53) |
| Etiology | ||
| Hepatitis C | Reference | |
| Hepatitis B | 1.12 (0.66‐1.91) | |
| Alcohol related | 0.77 (0.52‐1.13) | |
| NASH | 1.28 (0.85‐1.93) | |
| Thrombocytopenia (<150) | 1.13 (0.87‐1.49) | 1.48 (1.10‐2.00) |
| Child‐Pugh score (in increments of 1) | 0.86 (0.81‐0.93) | 0.87 (0.81‐0.94) |
Of 612 patients, 377 had regular outpatient care and 235 had prior primary or gastroenterology visits but not meeting the definition for regular outpatient care.
Adjusted for type of health care system.
Failures in the HCC Screening Process
Among the 377 patients receiving regular outpatient care, consistent semiannual screening was completed for only 93 (24.7%) patients. Inconsistent screening was conducted for 161 (42.7%) patients, and 123 (32.6%) patients had no screening in the year before HCC diagnosis (Table 3). A higher proportion of patients with consistent screening had HCC detected at an early stage compared to those with no screening (P < 0.001) or inconsistent screening (P = 0.04). In multivariable models, gastroenterology subspecialty care was the strongest correlate of consistent semiannual screening (Table 4) or receipt of any screening (Supporting Table S3). Among patients who received no screening, failures were observed at each step in the screening process, including provider recognition of liver disease (n = 22, 17.9%), provider recognition of cirrhosis (n = 30, 24.4%), provider placement of HCC screening orders (n = 61, 49.6%), and patient adherence with a screening ultrasound appointment (n = 10, 8.1%) (Fig. 2). Although provider failure to recognize cirrhosis was more common among patients with NASH than those with other etiologies, this difference did not reach statistical significance (19.1% vs. 11.7%, P = 0.26). Correlates for screening orders were consistent with those of screening use, with the strongest correlate being receipt of gastroenterology subspecialty care.
Table 3.
Characteristics of patients with consistent, inconsistent, and no screening
| Patient Characteristic | Consistent Screening (n = 93) | Inconsistent Screening (n = 161) | No Screening (n = 123) |
|---|---|---|---|
| Age (median, IQR) | 60.8 (56.0‐66.3) | 60.7 (56.4‐66.9) | 60.8 (56.9‐67.4) |
| Sex (% male) | 64 (68.8%) | 117 (72.6%) | 81 (65.9%) |
| Hospital type (% safety net) | 69 (74.2%) | 131 (81.4%) | 97 (78.9%) |
| Race (number, %) | |||
| Non‐Hispanic white | 31 (33.3%) | 40 (24.8%) | 27 (22.0%) |
| Black | 25 (26.9%) | 65 (40.4%) | 58 (47.2%) |
| Hispanic white | 33 (35.5%) | 49 (30.4%) | 33 (26.8%) |
| Asian | 4 (4.3%) | 6 (3.7%) | 2 (1.6%) |
| Etiology (number, %) | |||
| Hepatitis C | 56 (60.2%) | 113 (70.2%) | 73 (59.3%) |
| Hepatitis B | 8 (8.6%) | 10 (6.2%) | 7 (5.7%) |
| Alcohol | 14 (15.1%) | 17 (10.6%) | 14 (11.4%) |
| NASH | 11 (11.8%) | 15 (9.3%) | 21 (17.1%) |
| Other | 4 (4.3%) | 6 (3.7%) | 8 (6.5%) |
| Insurance (number, %) | |||
| Medicare | 36 (38.7%) | 65 (40.4%) | 43 (35.0%) |
| Medicaid | 20 (21.5%) | 30 (18.6%) | 28 (22.8%) |
| Private insurance | 13 (14.0%) | 11 (6.8%) | 10 (8.1%) |
| Other | 20 (21.5%) | 41 (25.5%) | 36 (29.3%) |
| None | 4 (4.3%) | 14 (8.7%) | 6 (4.9%) |
| Severity of cirrhosis (number, %) | |||
| Presence of hepatic encephalopathy | 22 (23.7%) | 37 (23.0%) | 23 (18.7%) |
| Presence of ascites | 44 (47.3%) | 61 (37.9%) | 49 (39.8%) |
| Child‐Pugh score (% Child A) | 49 (52.7%) | 91 (56.5%) | 72 (58.5%) |
| Health care use (median, IQR) | |||
| Number of any outpatient visits in year prior | 7 (4‐10) | 5 (3‐9) | 4 (2‐6) |
| Had PCP visit in year prior (%) | 67 (72.0%) | 132 (82.0%) | 105 (85.4%) |
| Number of PCP visits in year prior | 2 (0‐4) | 2 (1‐3) | 2 (1‐3) |
| Had gastroenterology visit (%) | 88 (94.6%) | 112 (69.6%) | 52 (42.3%) |
| Number of gastroenterology visits in 1 year | 2 (1‐4) | 1 (0‐2) | 1 (0‐1) |
| Barcelona Clinic Liver Cancer Stage (number, %) | |||
| Stage 0 | 15 (16.1%) | 22 (13.7%) | 7 (5.7%) |
| Stage A | 52 (55.9%) | 74 (46.0%) | 44 (35.8%) |
| Stage B | 8 (8.6%) | 19 (11.8%) | 16 (13.0%) |
| Stage C | 7 (7.5%) | 32 (19.9%) | 41 (33.3%) |
| Stage D | 9 (9.7%) | 11 (6.8%) | 15 (12.2%) |
Abbreviation: IQR, interquartile range.
Table 4.
Correlates of consistent semiannual screening within 1 year before HCC diagnosis (n = 377)
| Variables | Univariable OR (95% CI) | Multivariable OR* (95% CI) |
|---|---|---|
| Age (in 10‐year increments) | 0.89 (0.67‐1.18) | |
| Sex (male) | 0.92 (0.55‐1.53) | |
| Race | ||
| Non‐Hispanic white | Reference | |
| Black | 0.48 (0.26‐0.89) | |
| Hispanic white | 0.96 (0.53‐1.73) | |
| Asian | 1.19 (0.33‐4.26) | |
| Etiology | ||
| Hepatitis C | Reference | |
| Hepatitis B | 1.64 (0.67‐4.00) | |
| Alcohol | 1.57 (0.78‐3.17) | |
| NASH | 1.06 (0.51‐2.23) | |
| Child‐Pugh score (in increments of 1) | 1.03 (0.91‐1.17) | |
| PCP/gastroenterology visits | ||
| Only PCP visits | Reference | Reference |
| Only gastroenterology visits | 12.0 (4.74‐30.6) | 12.0 (4.74‐30.6) |
| Both PCP and gastroenterology visits | 11.8 (4.89‐28.5) | 11.8 (4.89‐28.5) |
Adjusted for type of health care system
FIG. 2.
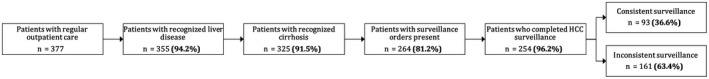
Screening process failures in patients with cirrhosis.
Discussion
We found that less than 1 in 4 patients with cirrhosis receive consistent semiannual HCC screening, which is in line with prior data.( 9 ) However, our study provides important insights into potential reasons for HCC screening underuse. The most common barrier to HCC screening was patients not having routine outpatient care before HCC presentation, with only 37% of patients having regular care by a primary care provider or gastroenterologist. Even among those under routine outpatient care, we observed failures at each subsequent step of the screening process, including at both the patient and provider level, with the most common being providers not ordering screening for patients with documented cirrhosis.
One of the most noteworthy findings from our study is that nearly two thirds of patients failed to have regular outpatient care before HCC diagnosis, with over one third not having any prior PCP or gastroenterology visits. This is particularly surprising in our patient population given that PHHS serves as a safety‐net health system and offers medical care, including HCC screening, to underinsured individuals in Dallas County. This suggests that this failure extends beyond insurance issues. Lack of health care engagement in this population may be related to issues such as mistrust of health systems, poor health literacy and awareness, or other barriers to medical care, such as lack of transportation.( 14, 15, 16 ) Some of these barriers have been reported to be prevalent in racial/ethnic minority communities and may also be common in patients with cirrhosis, who often have coexistent substance abuse and psychiatric comorbidity.( 17 ) This finding is additive to the literature on screening underuse that has primarily focused on patients who are already engaged with health care and highlights that interventions focused solely on patients within health systems (i.e., inreach) will likely have limited effectiveness.
Among those with regular outpatient care, the most common failure in subsequent steps of the process was providers not placing orders for screening in patients with known cirrhosis. This finding is consistent with the published literature,( 10 ) highlighting this as a high‐yield target for future interventions to increase HCC screening. Failure at this step was particularly common among patients who were only followed by PCPs without gastroenterology/hepatology subspecialty care. A published survey suggests primary care providers are knowledgeable about screening but reports other barriers, including limited time in clinic and competing clinical concerns.( 18 ) Although screening is consistently higher when patients are being followed by gastroenterologists, referring all patients with cirrhosis to subspecialty care is not a viable solution, particularly in areas with limited subspecialty capacity, e.g., rural communities, safety‐net health systems, and other large integrated health systems, such as the Department of Veterans Affairs (VA). Further, the number of patients with cirrhosis will likely increase in the future in parallel with an increasing prevalence of nonalcoholic fatty liver disease (NAFLD).( 19 ) Interventions, including EMR reminders and provider education, have been tested with variable success, particularly if considering semiannual screening over extended periods of time.( 20, 21 ) Therefore, novel system redesign solutions, such as mailed outreach strategies, radiology recall systems, or nurse‐driven protocols, may need to be considered to improve screening in the future.( 22, 23 )
Although failures were also observed at other steps in the HCC screening process, it was surprising that they were relatively uncommon. Failure to recognize liver disease and failure to recognize cirrhosis were both observed in less than 10% of patients without screening. This contrasts to an earlier VA study in which nearly 25% of patients had unrecognized cirrhosis at HCC diagnosis, particularly among those with NAFLD.( 24 ) We had hypothesized that this would be more of an issue in our contemporary cohort, especially given the increasing prevalence of NAFLD in the U.S. population, but this was not the case. It is possible that increased use of noninvasive markers of fibrosis and increased awareness by both PCPs and gastroenterologists may be helping to address this issue. It is also noteworthy that patient adherence to screening recommendations was high, with less than 5% of patients failing to complete screening once ordered. This is in contrast to data demonstrating patient‐reported barriers to HCC screening, including costs, difficulties with scheduling, and transportation.( 25 ) It is possible that providers may not have ordered screening in patients with these types of barriers, resulting in misclassification. While further research is needed in this area, we believe that improved patient navigation may help to increase HCC screening in the future.
As has been reported in other studies,( 6, 26, 27 ) receipt of consistent HCC screening was associated with statistical and clinically significant increases in early HCC detection compared to both inconsistent and no screening. However, nearly 40% of patients in the no‐screening group were found at an early stage, which may reflect increasing incidental detection in parallel with increasing use of cross‐sectional imaging in clinical practice.( 28 ) Overall, these data highlight the continued importance of efforts to increase and optimize HCC screening effectiveness in at‐risk patients, including efforts to increase screening as well as test performance for early HCC detection.( 29, 30, 31, 32, 33 )
Although our study has several strengths, including its large sample size, diverse patient cohort and granular data on health care use, and reasons for screening process failures, it has a few limitations. First, although we included patients from both a safety‐net health system and academic tertiary care referral system, these data may not be generalizable to all care settings. Second, there is the possibility of measurement bias and residual confounding, given the retrospective nature of the study. For example, we had limited data on comorbidity, which may impact the likelihood of having clinic visits as well as appropriateness of screening. Similarly, alcohol use may be underreported by patients and undercaptured in EMRs. Third, there is the possibility of ascertainment bias, particularly among patients who may have had imaging studies completed outside our two health systems. This was less likely to occur at the safety‐net health system given substantially higher out‐of‐pocket costs for patients receiving imaging outside of PHHS. Although this was possible at the tertiary care referral system, we recorded outside imaging available in the EMR to minimize this bias. Similarly, providers may have recognized the presence of liver disease or cirrhosis and simply not documented this clearly in their notes.
In summary, we found that HCC screening continues to be underused in patients with cirrhosis, with less than 1 in 4 patients receiving consistent semiannual screening before diagnosis. The most common failures in the screening process were patients not having routine outpatient care before presentation and providers not ordering screening imaging for patients with documented cirrhosis. These steps should serve as preferred targets for intervention strategies to increase HCC screening in the future.
Supporting information
Table S1‐S3
Supported by the National Institutes of Health (R01 MD12565 and U01 CA230694 to A.G.S.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
Potential conflict of interest: Dr. Singal consults for Bayer, Eisai, BMS, Exelixis, Genentech, AstraZeneca, Exact Sciences, Roche, Glycotest, GRAIL, Wako Diagnostics, and Target RWE. The other authors have nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol 2020;18:2650‐2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693‐699. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso MDC, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62‐67. 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 5.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018;68:723‐750. 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 6.Singal Amit G., Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta‐analysis. PLoS Med 2014;11:e1001624. 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417‐422. 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singal AG, Yopp A, S. Skinner C, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 2012;27:861‐867. 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta‐analysis. Hepatology 2021;73:713‐725. 10.1002/hep.31309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singal AG, Yopp AC, Gupta S, Skinner CS, Halm EA, Okolo E, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila) 2012;5:1124‐1130. 10.1158/1940-6207.capr-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2019;17:551‐559.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yopp AC, Mansour JC, Beg MS, Arenas J, Trimmer C, Reddick M, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol 2014;21:1287‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hester CA, Rich NE, Singal AG, Yopp AC. Comparative analysis of nonalcoholic steatohepatitis– versus viral hepatitis– and alcohol‐related liver disease–related hepatocellular carcinoma. J Natl Compr Canc Netw 2019;17:322‐329. 10.6004/jnccn.2018.7105. [DOI] [PubMed] [Google Scholar]
- 14.Wilder JM, Oloruntoba OO, Muir AJ, Moylan CA. Role of patient factors, preferences, and distrust in health care and access to liver transplantation and organ donation. Liver Transpl 2016;22:895‐905. 10.1002/lt.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen‐Mekelburg S, Waljee AK, Kenney BC, Tapper EB. Coordination of care is associated with survival and health care utilization in a population‐based study of patients with cirrhosis. Clin Gastroenterol Hepatol 2020;18:2340‐2348.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farvardin S, Patel J, Khambaty M, Yerokun OA, Mok H, Tiro JA, et al. Patient‐reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology 2017;65:875‐884. 10.1002/hep.28770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernaez R, Kramer JR, Khan A, Phillips J, McCallister K, Chaffin K, et al. Depression and anxiety are common among patients with cirrhosis. Clin Gastroenterol Hepatol 2020; 10.1016/j.cgh.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmons OL, Feng Y, Parikh ND, Singal AG. Primary care provider practice patterns and barriers to hepatocellular carcinoma surveillance. Clin Gastroenterol Hepatol 2019;17:766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beste LA, Ioannou GN, Yang Y, Chang MF, Ross D, Dominitz JA. Improved surveillance for hepatocellular carcinoma with a primary care–oriented clinical reminder. Clin Gastroenterol Hepatol 2015;13:172‐179. 10.1016/j.cgh.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Del Poggio P, Olmi S, Ciccarese F, Mazzoleni M, Jazzetti M, Jamoletti C, et al. A training program for primary care physicians improves the effectiveness of ultrasound surveillance of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2015;27:1103‐1108. [DOI] [PubMed] [Google Scholar]
- 22.Singal AG, Tiro JA, Murphy CC, Marrero JA, McCallister K, Fullington H, et al. Mailed outreach invitations significantly improve HCC surveillance rates in patients with cirrhosis: a randomized clinical trial. Hepatology 2018. 10.1002/hep.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bui HT, Rangchi A, Tran DK, Malik A, Kumar NG, Balasubramanian S. Implementing a local hepatoma surveillance program in patients with cirrhosis secondary to hepatitis C– real world experience in a community based practice. Gastroenterology 2017;152(Suppl. 1):S1191‐S1192. https://www.gastrojournal.org/article/S0016‐5085(17)33973‐2/pdf. [Google Scholar]
- 24.Walker M, El‐Serag HB, Sada Y, Mittal S, Ying J, Duan Z, et al. Cirrhosis is under‐recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment Pharmacol Ther 2016;43:621‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singal AG, Tiro JA, Murphy CC, Blackwell J‐M, Kramer JR, Khan A, et al. Patient‐reported barriers are associated with receipt of hepatocellular carcinoma surveillance in a multicenter cohort of patients with cirrhosis. Clin Gastroenterol Hepatol 2020; 10.1016/j.cgh.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi DT, Kum H‐C, Park S, Ohsfeldt RL, Shen YU, Parikh ND, et al. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol 2019;17:976‐987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costentin CE, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Compliance with hepatocellular carcinoma surveillance guidelines associated with increased lead‐time adjusted survival of patients with compensated viral cirrhosis: a multi‐center cohort study. Gastroenterology 2018;155:431‐442.e10. 10.1053/j.gastro.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Larson DB, Johnson LW, Schnell BM, Salisbury SR, Forman HP. National trends in CT use in the emergency department: 1995–2007. Radiology 2011;258:164‐173. 10.1148/radiol.10100640. [DOI] [PubMed] [Google Scholar]
- 29.Singal AG, Patibandla S, Obi J, Fullington H, Parikh ND, Yopp AC, et al. Benefits and harms of hepatocellular carcinoma surveillance in a prospective cohort of patients with cirrhosis. Clin Gastroenterol Hepatol 2021; 10.1016/j.cgh.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singal AG, Parikh ND, Hutton DW, Tapper EB. Cost effectiveness of hepatocellular carcinoma surveillance: an assessment of benefits and harms. Am J Gastroenterol 2020;115:1642‐1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singal AG, Lok AS, Feng Z, Kanwal F, Parikh ND. Conceptual model for the hepatocellular carcinoma screening continuum: current status and research agenda. Clin Gastro Hepatol 2020; 10.1016/j.cgh.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol 2020;72:250‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta‐analysis. Gastroenterology 2018;154:1706‐1718.e1. 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3


