Abstract
Background
STK11 mutation (STK11m) in patients (pts) with stage IV non-small cell lung cancer (NSCLC) is associated with inferior survival and poor response to immune checkpoint inhibitors (ICI). The significance of STK11m in stage III NSCLC pts treated with concurrent chemoradiation (CCRT) with or without consolidation ICI is unknown.
Methods
Stage III NSCLC patients who received CCRT and had known STK11 mutational status were included in this retrospective study. The data on the STK11m pts were collected from 4 cancer institutions. A cohort of pts with wild type STK11 (STK11w) from the University of Iowa served as a comparison group. Patient demographics and clinical characteristics were collected. Cox regression models were used to explore the effect of STK11 mutation on survival.
Results
75 pts with stage III NSCLC who had known STK11 mutational status were identified. 16/75 (21%) had STK11m. 5/16 with STK11 m did not receive CCRT so they were excluded from the analysis. The clinical and demographic characteristics for the 11 STK11m and 59 STK11w pts were not statistically different (STK11m vs. STK11w): mean age: 57 vs. 64 yrs, non-squamous histology: 8/11 (73%) vs. 37/59 (63%), KRAS mutation: 3/11 (27%) vs. 11/59 (19%), TP53 mutation: 6/11 (55%) vs. 15/59 (25%), PD-L1 ≥50%: 1/8 (13%) vs. 10/32 (31%), and consolidation ICI 6/11 (55%) vs. 17/59 (29%). Regarding the 6 STK11m pts who received ICI (4 pembrolizumab, 2 durvalumab), the median number of ICI infusions was 8 (range, 3–17) vs. 6 (range, 1–25) in the 17 pts with STK11w who received ICI (durvalumab). After adjusting for performance status and cancer stage, multivariable analysis showed that progression free survival (PFS) for the STK11m pts was significantly worse than STK11w pts (HR =2.25; 95% CI, 1.03–4.88, P=0.04), whereas overall survival (OS) showed no significant difference for STK11m vs. STK11w patients (HR 1.47, 95% CI, 0.49–4.38, P=0.49).
Conclusions
In stage III NSCLC patients who received CCRT, STK11m was associated with worse PFS compared to STK11w. Larger studies are needed to further explore the prognostic implications of STK11m in stage III NSCLC and whether ICI impacts survival for this subgroup.
Keywords: Non-small cell lung cancer (NSCLC), immunotherapy, STK11, KRAS, TP53
Introduction
Unresectable stage III non-small cell lung cancer (NSCLC) in patients with good performance status is treated with concurrent platinum based chemotherapy with definitive dose radiation (CCRT) followed by the PD-L1 inhibitor, durvalumab (1). The addition of durvalumab to CCRT improved survival from 55.6% to 66.3% at 24 months (1). Although this improvement in survival is practice changing, a large number of stage III NSCLC patients still have a poor prognosis. In 2020, it is estimated there will be 229,000 new cases of lung cancer (2) of which stage III cancer is expected in approximately 30% of all NSCLC cases.
Serine/threonine kinase 11 (STK11), also known as liver kinase B1 (LKB1), is a gene found on chromosome 19p13. Germline mutation of STK11 is associated with Peutz-Jeghers Syndrome (PJS) (3). Patients with PJS can develop intestinal hamartomatous polyps and are more likely to develop malignancies such as gastrointestinal, testis, ovary, and breast cancers (3). In lung cancer, somatic mutations of STK11 are seen in up to 42% of NSCLC; however, most studies have shown a mutation frequency of approximately 20% (4). STK11 functions as a tumor suppressor gene and is involved in the activation of AMP-activated protein kinase (AMPK), which modulates cell glucose and lipid metabolism (5). More importantly, in-vivo studies indicate that STK11 is involved in the differentiation and metastases of lung cancer (6). STK11 inactivation leads to increase in pro-inflammatory cytokines such as CXCL7 and causes a shift in the microenvironment with neutrophil accumulation and decrease in T cell lymphocytes (7,8).
The effect of STK11m on outcomes of stage III NSCLC treated with curative intent is unknown. It is unclear whether STK11m affects prognosis of stage III NSCLC or predicts response to ICI consolidation after CCRT. In advanced stage NSCLC, STK11m has been associated with poor response to chemotherapy and ICI, and inferior survival outcomes (9-15). In this study, we sought to explore STK11m as a prognostic genetic alteration in stage III NSCLC patients managed with definitive chemoradiation +/− consolidative ICI. We present the following article in accordance with the REMARK reporting checklist (available at https://dx.doi.org/10.21037/tlcr-21-177).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent from the patients were waived. The study was approved by institutional Review Boards (IRBs) of University of Iowa Hospitals and Clinics, Indiana University Health, Northwestern Memorial Hospital, and the University of Illinois at Chicago. The 4 institutions provided a total of 16 patients with stage III NSCLC known to have STK11m diagnosed between 2013 and 2019. Of these, 11 received CCRT and were included in the study. A comparison group of 59 patients with STK11w stage III NSCLC who received CCRT diagnosed between 2013 and 2019 were identified at University of Iowa and served as a comparison group. Stage III NSCLC, receiving any CCRT, and having a known STK11 mutational status were requirements for inclusion in the analysis in this study.
The patients’ charts were reviewed for clinical and demographic characteristics. These included age, sex, smoking history, stage, ECOG performance status, histology, STK11, KRAS and TP53 status, PD-L1 expression, and treatment details including radiation, chemotherapy and immunotherapy. STK11m was identified by next generation sequencing of the tumor tissue using the platform of choice available in the institutions participating in this study. Specific STK11 locus alteration information was not collected. Information about other NSLCC driver mutations such as EGFR and ALK were not collected.
Statistical analysis
Chi-squared or Fisher’s exact tests were used to compare categorical variables, and Wilcoxon rank sum tests were used to compare continuous variables between STK11 wild type and mutant. Survival probabilities were estimated and plotted using the Kaplan-Meier method. Cox regression models were used to assess the effects of clinical, pathologic, and treatment variables on progression-free survival (PFS) and overall survival (OS). The time for PFS was calculated from the date of diagnosis until progression or death due to any cause. The time for OS was calculated from the date of diagnosis until death due to any cause. Estimated effects of predictors are reported as hazard ratios (HR) along with 95% confidence intervals. All statistical testing was two-sided and assessed for significance at the 5% level using SAS v9.4 (SAS Institute, Cary, NC).
Results
Patient demographics and clinical characteristics
After excluding the 5 patients with STK11m who did not receive CCRT, a total of 70 pts with stage IIIA-C NSCLC were included in the analysis; 11 patients with STK11m and 59 patients with STK11w (Table 1). When STK11m patients were compared to STK11w, there was no significant difference in the gender distribution among the two groups. Numerically, the proportion of age ≤65 years in the STK11m group compared to STK11w was higher but this was not statistically significant (82% vs. 54%, P=0.11). When all patients including the 5 with STK11m who did not receive CCRT were accounted for in the analysis, the STK11m patients were significantly younger (≤65 years) (P=0.05) (Data not shown). Majority of the patients included were current/former smokers regardless of the STK11 mutational status. Regarding performance status, most of the patients included in the study had an ECOG performance status of 0-1. Histology was divided into non-squamous and squamous NSCLC. Non-squamous histology was predominant in both the STK11w and STK11m groups comprising 62.7% and 72.7%, respectively, without noticing statistically significant difference (P=0.73). There was no significant difference in the frequency of stage IIIA versus stage IIIB/C among the groups. The frequency of KRAS or TP53 mutations were not significantly different between the STK11 groups. PD-L1 expression was <1% in 63% of the STK11m patients, but there was no significant difference in the PD-L1 expression categories among the STK11m and STK11w groups.
Table 1. Demographics and clinical data for STK11m and STK11w stage III NSCLC.
| Variable | Level | Total, N=70, n (%) | STK11, n (%) | ||
|---|---|---|---|---|---|
| STK11w, N=59 | STK11m, N=11 | P value | |||
| Sex | F | 35 (50.0) | 31 (52.5) | 4 (36.4) | 0.32 |
| M | 35 (50.0) | 28 (47.5) | 7 (63.6) | ||
| Age | ≤65 years | 41 (58.6) | 32 (54.2) | 9 (81.8) | 0.11 |
| >65 years | 29 (41.4) | 27 (45.8) | 2 (18.2) | ||
| Smoking history | Current | 65 (92.9) | 54 (91.5) | 11 (100) | 1.00 |
| Never smoker | 5 (7.1) | 5 (8.5) | 0 (0) | ||
| ECOG | 0–1 | 60 (85.7) | 49 (83.1) | 11 (100) | 0.34 |
| 2–3 | 10 (14.3) | 10 (16.9) | 0 (0) | ||
| Histology | Non-squamous | 45 (64.3) | 37 (62.7) | 8 (72.7) | 0.73 |
| Squamous | 25 (35.7) | 22 (37.3) | 3 (27.3) | ||
| Stage | IIIA | 38 (54.3) | 32 (54.2) | 6 (54.5) | 0.98 |
| IIIB/C | 32 (45.7) | 27 (45.8) | 5 (45.5) | ||
| KRAS | No | 56 (80.0) | 48 (81.4) | 8 (72.7) | 0.68 |
| Yes | 14 (20.0) | 11 (18.6) | 3 (27.3) | ||
| TP53 | No | 49 (70.0) | 44 (74.6) | 5 (45.5) | 0.07 |
| Yes | 21 (30.0) | 15 (25.4) | 6 (54.5) | ||
| PD-L1 | <1% | 21 (52.5) | 16 (50.0) | 5 (62.5) | 0.66 |
| 1–50% | 8 (20.0) | 6 (18.8) | 2 (25.0) | ||
| >50% | 11 (27.5) | 10 (31.3) | 1 (12.5) | ||
| Missing | 30 | 27 | 3 | ||
| ICI consolidation | No | 47 (67.1) | 42 (71.2) | 5 (45.5) | 0.16 |
| Yes | 23 (32.9) | 17 (28.8) | 6 (54.5) | ||
| Cycles of ICI | N | 70 | 59 | 11 | 0.10 |
| Median | 0 | 0 | 3 | ||
| Range | (0-25) | (0-25) | (0-17) | ||
NSCLC, non-small cell lung cancer.
Treatments
All patients included in the analysis received CCRT (Table 1). ICI consolidation was delivered to 17/59 STK11w and 6/11 STK11m patients. Of note, 20/59 STK11w and 4/11 STK11m patients were diagnosed with NSCLC after the publication of the PACIFIC trial (16) which was the first phase III randomized trial to show improvement in PFS when using consolidation durvalumab after CCRT in stage III NSCLC. The ICI consolidation given to the 6 STK11m patients included pembrolizumab, which was delivered on a clinical trial, for 4 patients and durvalumab for 2 patients. All ICI delivered for the STK11w group was durvalumab.
Survival outcomes
Median follow up time was 15.7 months. Univariate analysis showed a trend towards worse PFS for STK11m compared to STK11w patients; however, this was not statistically significant (HR 1.92, 95% CI, 0.91–4.06, P=0.09) (Figure 1). There was no statistically significant difference in PFS based on sex, age, ECOG performance status, histology, stage, KRAS, TP53, PD-L1 level, or ICI consolidation (Table 2). On multivariable analysis adjusting for ECOG performance status and stage (Table 3), there was a significantly worse PFS for STK11m compared to STK11w patients (HR 2.25, 95% CI, 1.03–4.88, P=0.04). OS by univariate analysis was not statistically different for the STK11m vs. STK11w (HR 1.47, 95% CI, 0.49–4.38, P=0.49) (Figure 2).
Figure 1.
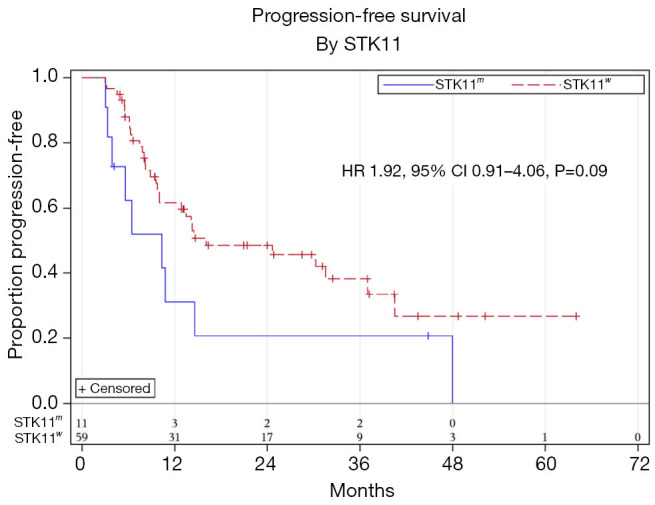
Progression free survival for STK11m and STK11w stage III NSCLC. NSCLC, non-small cell lung cancer.
Table 2. Progression free survival (PFS) by univariate analysis.
| Variable | Level | N | Progression-free survival | |||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | ||||
| Sex | M | 35 | 1.07 | 0.58 | 1.98 | 0.83 |
| F | 35 | Ref | – | – | ||
| Age | ≤65 years | 41 | 1.61 | 0.83 | 3.13 | 0.16 |
| >65 years | 29 | Ref | – | – | ||
| ECOG | 2–3 | 10 | 1.96 | 0.89 | 4.31 | 0.10 |
| 0–1 | 60 | Ref | – | – | ||
| Histology | Non-squamous | 45 | 1.31 | 0.67 | 2.58 | 0.43 |
| Squamous | 25 | Ref | – | – | ||
| Stage | IIIB/C | 32 | 1.53 | 0.82 | 2.85 | 0.18 |
| IIIA | 38 | Ref | – | – | ||
| STK11 | Yes | 11 | 1.92 | 0.91 | 4.06 | 0.09 |
| No | 59 | Ref | – | – | ||
| KRAS | Yes | 14 | 1.10 | 0.50 | 2.42 | 0.81 |
| No | 56 | Ref | – | – | ||
| TP53 | Yes | 21 | 1.02 | 0.52 | 1.99 | 0.96 |
| No | 49 | Ref | – | – | ||
| PD-L1 | <1% | 21 | 1.54 | 0.54 | 4.39 | 0.40 |
| 1-50 | 8 | 2.27 | 0.69 | 7.52 | ||
| >50% | 11 | Ref | – | – | ||
| ICI consolidation | Yes | 23 | 1.18 | 0.60 | 2.31 | 0.63 |
| No | 47 | Ref | – | – | ||
Table 3. Progression free survival (PFS) by multivariate analysis.
| Covariate | Level | N | Progression-free survival | |||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | ||||
| ECOG | 2–3 | 10 | 2.49 | 1.10 | 5.65 | 0.03 |
| 0–1 | 60 | Ref | – | – | ||
| Stage | IIIB/C | 32 | 1.65 | 0.88 | 3.08 | 0.12 |
| IIIA | 38 | Ref | – | – | ||
| STK11 | Yes | 11 | 2.25 | 1.03 | 4.88 | 0.04 |
| No | 59 | Ref | – | – | ||
Figure 2.
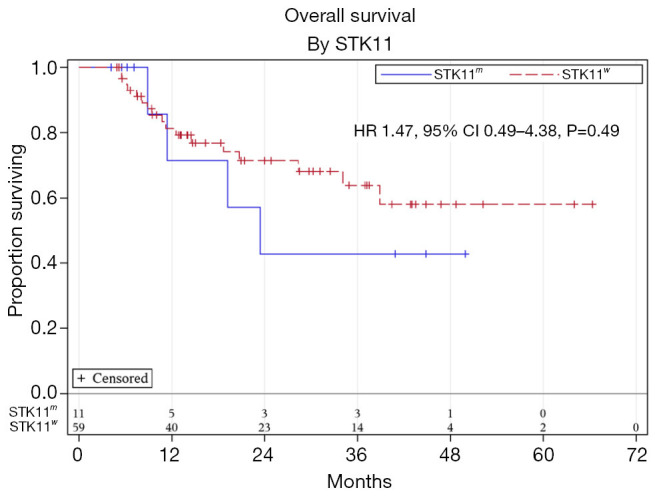
Overall survival for STK11m and STK11w stage III NSCLC. NSCLC, non-small cell lung cancer.
Discussion
To our knowledge, this is the first study to explore STK11m as a prognostic mutation in stage III NSCLC patients who received CCRT. Our results showed that PFS, after adjusting for performance status and cancer stage, was worse for stage III NSCLC patients who had STK11m compared to STK11w (HR 2.25, 95% CI, 1.03–4.88). Although data in the literature showed association of STK11m with poor outcomes in advanced stage NSCLC (9,11,15), its impact on stage III NSCLC has not been established. In a study examining the prognostic impact of STK11m in stage I-IV NSCLC (10), 21 patients had stage III disease. The median OS for all patients included was worse for the STK11m compared to STK11w patients (24 vs. 69 months, P=0.005), but there were no survival outcomes reported specifically for the stage III NSCLC patients (10). Another retrospective analysis which included 23 patients with a mix of stage IIIB and IV NSCLC also did not identify the stage III cases as a subgroup for analysis (17). Our study included only stage III NSCLC patients who received CCRT with or without ICI consolidation.
The effect of STK11m on benefit from ICI consolidation post CCRT in stage III NSCLC could not be concluded in our study due to the small sample size. In advanced stage NSCLC, STK11m was associated with low PD-L1 expression and resistance to PD-1/PD-L1 ICIs (11). However, the association of STK11m and poor response to ICI is still debatable as other reports showed good response of advanced stage STK11m NSCLC to ICI (18-20). In another driver mutation, the EGFR rather than STK11 mutation, it was questioned on retrospective studies whether there is significant benefit from durvalumab after CCRT (21,22). In a cohort of 60 patients with STK11m cancers that included NSCLC and others, STK11m correlated with poor prognosis without specifically observing inferior outcomes associated with immunotherapy (23). Potential mechanisms for the STK11m resistance to ICI include impaired activity of effector T cells, epigenetic changes of tumor cells, modification of the cytokine and/or chemokine milieu, and diminished tumor antigenicity (11). In our study, 5/11 (63%) STK11m patients had PD-L1 <1% and 6/11 (55%) received ICI consolidation. The ICI consolidation used included durvalumab or pembrolizumab (pembrolizumab received on a clinical trial). No conclusions can be made about resistance of STK11m to ICI consolidation or whether a specific PD-1/PD-L1 inhibitor would function differently in the setting of STK11m in stage III NSCLC. This will need to be further studied in future trials.
The presence of other mutations that could impact the outcomes of STK11m NSCLC were also examined. Co-mutations of STK11 with either KRAS or TP53 in NSLCC have been described in advanced stage NSCLC (12-14,24). Although KRAS mutation was associated with better response to ICI (14), the co-mutation of STK11 and KRAS was associated with inferior survival (12-14,24). A retrospective review of patients treated with first-line therapy for metastatic NSCLC found that STK11 and KRAS co-mutations were associated with worse survival compared to STK11 and TP53 co-mutation (13). In our study, only 2 patients had STK11m without co-mutation in KRAS or TP53.
Other clinical and demographic features of stage III NSCLC patients with STK11m were explored. The STK11m patients were more likely to be ≤65 years old (82%); however, there was no significant difference in age between the STK11m and STK11w patients. This is consistent with another retrospective study that examined STK11m in patients with stage I–IV non-squamous NSCLC where patients with STK11m were found to be significantly younger than STK11w patients (mean age of 58.6 vs. 61.9 years, respectively) (10). This retrospective study (10) also noted smoking to be associated with STK11w which was not seen in our study.
The authors acknowledge limitations that apply to this study. First, the retrospective nature, the small sample size, and the imbalance in the number of STK11w versus STK11m patients are recognized. Second, the majority of patients in this study were identified from stage IV NSCLC databases who were initially diagnosed and treated for stage III disease. This might inherently reflect a selection bias of stage III NSCLC with more aggressive biology and tendency for metastases. Third, it is unknown if STK11m was present at diagnosis of stage III NSCLC or was acquired subsequently upon progression to stage IV.
Conclusions
In stage III NSCLC patients who received CCRT, STK11m was associated with worse PFS compared to STK11w. Larger studies are needed to explore whether STK11m plays a role in predicting less benefit from ICI consolidation in stage III NSCLC.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: Department funding by the Division of Hematology and Oncology at the University of Iowa Hospitals and Clinics.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent from the patients were waived. The study was approved by institutional Review Boards (IRBs) of University of Iowa Hospitals and Clinics, Indiana University Health, Northwestern Memorial Hospital, and The University of Illinois at Chicago.
Footnotes
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-21-177
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tlcr-21-177
Peer Review File: Available at https://dx.doi.org/10.21037/tlcr-21-177
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-177). MF serves as an unpaid editorial board member of Translational Lung Cancer Research from Sep 2019 to Sep 2021. TK reports personal fees from AstraZeneca for consulting and advisory board, and personal fees AstraZeneca, OncLive, and Targeted Oncology for speaking. NHH’s institution received grant support from BMS, Genentech, Merck on studies that in which he is the PI, and is the medical writer for UptoDate and served on a DSMB for a study sponsored by Beyond Spring. The other authors have no conflicts of interest to declare.
References
- 1.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998;391:184-7. 10.1038/34432 [DOI] [PubMed] [Google Scholar]
- 4.Fang R, Zheng C, Sun Y, et al. Integrative genomic analysis reveals a high frequency of LKB1 genetic alteration in Chinese lung adenocarcinomas. J Thorac Oncol 2014;9:254-8. 10.1097/JTO.0000000000000056 [DOI] [PubMed] [Google Scholar]
- 5.Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A 2004;101:3329-35. 10.1073/pnas.0308061100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007;448:807-10. 10.1038/nature06030 [DOI] [PubMed] [Google Scholar]
- 7.Koyama S, Akbay EA, Li YY, et al. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res 2016;76:999-1008. 10.1158/0008-5472.CAN-15-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadara H, Choi M, Zhang J, et al. Whole-exome sequencing and immune profiling of early-stage lung adenocarcinoma with fully annotated clinical follow-up. Ann Oncol 2017;28:75-82. 10.1093/annonc/mdw436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skoulidis F, Arbour KC, Hellmann MD, et al. Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non-squamous non-small cell lung cancer. 2019. ASCO Abstract 102
- 10.Pécuchet N, Laurent-Puig P, Mansuet-Lupo A, et al. Different prognostic impact of STK11 mutations in non-squamous non-small-cell lung cancer. Oncotarget 2017;8:23831-40. 10.18632/oncotarget.6379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov 2018;8:822-35. 10.1158/2159-8290.CD-18-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aredo JV, Padda SK, Kunder CA, et al. Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung Cancer 2019;133:144-50. 10.1016/j.lungcan.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bange E, Marmarelis ME, Hwang WT, et al. Impact of KRAS and TP53 Co-Mutations on Outcomes After First-Line Systemic Therapy Among Patients With STK11-Mutated Advanced Non-Small-Cell Lung Cancer. JCO Precis Oncol 2019. doi: . 10.1200/PO.18.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torralvo J, Friedlaender A, Achard V, et al. The Activity of Immune Checkpoint Inhibition in KRAS Mutated Non-small Cell Lung Cancer: A Single Centre Experience. Cancer Genomics Proteomics 2019;16:577-82. 10.21873/cgp.20160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shire NJ, Klein AB, Golozar A, et al. STK11 (LKB1) mutations in metastatic NSCLC: Prognostic value in the real world. PLoS One 2020;15:e0238358. 10.1371/journal.pone.0238358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 17.Facchinetti F, Bluthgen MV, Tergemina-Clain G, et al. LKB1/STK11 mutations in non-small cell lung cancer patients: Descriptive analysis and prognostic value. Lung Cancer 2017;112:62-8. 10.1016/j.lungcan.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 18.Qin Y, Yu M, Zhou L, et al. Durable response to combination radiotherapy and immunotherapy in EP-resistant lung large-cell neuroendocrine carcinoma with B2M and STK11 mutations: a case report. Immunotherapy 2020;12:223-7. 10.2217/imt-2019-0166 [DOI] [PubMed] [Google Scholar]
- 19.Domingues I, Cedres S, Callejo A, et al. Long duration of immunotherapy in a STK11 mutated/KRAS wild-type non-small cell lung cancer patient. Pulmonology 2020;26:49-50. 10.1016/j.pulmoe.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 20.Nadal E, Heeke S, Benzaquen J, et al. Two Patients With Advanced-Stage Lung Adenocarcinoma With Radiologic Complete Response to Nivolumab Treatment Harboring an STK11/LKB1 Mutation. JCO Precis Oncol 2020:1239-45. 10.1200/PO.20.00174 [DOI] [PubMed] [Google Scholar]
- 21.Aredo JV, Mambetsariev I, Hellyer JA, et al. Durvalumab for Stage III EGFR-Mutated NSCLC After Definitive Chemoradiotherapy. J Thorac Oncol 2021;16:1030-41. 10.1016/j.jtho.2021.01.1628 [DOI] [PubMed] [Google Scholar]
- 22.Hellyer JA, Aredo JV, Das M, et al. Role of Consolidation Durvalumab in Patients With EGFR- and HER2-Mutant Unresectable Stage III NSCLC. J Thorac Oncol 2021;16:868-72. 10.1016/j.jtho.2020.12.020 [DOI] [PubMed] [Google Scholar]
- 23.Krishnamurthy N, Goodman AM, Barkauskas DA, et al. STK11 alterations in the pan-cancer setting: prognostic and therapeutic implications. Eur J Cancer 2021;148:215-29. 10.1016/j.ejca.2021.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Fleur L, Falk-Sörqvist E, Smeds P, et al. Mutation patterns in a population-based non-small cell lung cancer cohort and prognostic impact of concomitant mutations in KRAS and TP53 or STK11. Lung Cancer 2019;130:50-8. 10.1016/j.lungcan.2019.01.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


