Abstract
In developed countries, cardiovascular diseases are currently the first cause of death. Cardiospheres (CSs) and cardiosphere‐derived cells (CDCs) have been found to have the ability to regenerate the myocardium after myocardial infarction (MI). In recent years, much effort has been made to gain insight into the human heart repair mechanisms, in which miRNAs have been shown to play an important role. In this regard, to elucidate the involvement of miRNAs, we evaluated the miRNA expression profile across human heart biopsy, CSs and CDCs using microarray and next‐generation sequencing (NGS) technologies. We identified several miRNAs more represented in the progenitors, where some of them can be responsible for the proliferation or the maintenance of an undifferentiated state, while others have been found to be downregulated in the undifferentiated progenitors compared with the biopsies. Moreover, we also found a correlation between downregulated miRNAs in CSs/CDCs and patient age (eg miR‐490) and an inverse correlation among miRNAs upregulated in CSs/CDCs (eg miR‐31).
Keywords: cardiac repair, cardiovascular, Heart, miRNAs, myocardial infarction, NGS
1. INTRODUCTION
The leading cause of morbidity and mortality in developed countries is cardiovascular disease (CVD).1 Heart development in mammals is mainly dependent on cardiomyocyte (CM) proliferation,2 and after birth the replicative activity is reduced, with a slight renewal during adult life to repair cardiac damage in pathological conditions. Cardiomyocyte proliferation is essential for cardiac repair, but causes cardiac dysfunction when damage to cardiomyocytes occurs.
The mechanisms that regulate the self‐renewal ability in cardiomyocytes operate under high regulation. This regulation has been found to be coordinated by, among others, microRNAs (miRNAs), which have been shown in recent years to play a key role in both the development and repair processes.3 Preserving CM activity is the principal challenge in acute myocardial infarction (AMI) given that necrosis, a consequence of the ischaemic process, is difficult if not impossible to cure or prevent from causing permanent damage to CMs. The current therapy involves the administration of thrombolytic drugs, or heart transplantation, which is restricted by organ availability and donor age. In this respect, miRNAs may represent a valid therapeutic option for cardiac disease (revised by Hudson and Porrello4).
miRNAs are small noncoding RNAs of about 22 nucleotides (mature miRNAs) that regulate the post‐transcriptional expression of several genes. After the transcription, the primary miRNA transcripts are 5’ capped and 3’ polyadenylated in the nuclei and processed by Drosha‐DGCR8 complex to pre‐miRNAs hairpin loop structure (canonical pathway) or by the spliceosome to mirtrons through direct splicing of mRNA introns and refolded into a pre‐miRNA (alternative pathway). The pre‐miRNAs are exported in the cytoplasmic region by exportin 5 and cleaved by Dicer proteins, which remove the loop from the pre‐miRNAs structure, generating the miRNA duplex. Finally, this duplex is unwound, and the guide filament of the miRNA is assembled with the RNA‐induced silencing complex (RISC) and is ready to carry out its function by binding the 3’‐untranslated region (3’‐UTR) of mRNA target, repressing the translation or the deadenylation, in the case of partial complementarity with the mRNA, or degradating the mRNA when complementarity occurs, leading to the silencing of the protein synthesis.5
Each miRNA can control different genes, and each gene can be regulated by several miRNAs, generating a complex network. More than one thousand miRNAs have been described in humans to regulate about 20%–30% of the translation processes. In the healthy adult human heart and in cardiovascular pathological conditions, miRNA expression has been identified by large sequencing approaches.6 The role of various miRNAs has also been defined7, 8 during embryogenesis, in postnatal cardiac development in the adult healthy heart and in cardiac remodelling.9
As demonstrated for many tissues, the human heart is characterized by a subpopulation of adult stem cells with the properties to differentiate into cardiomyocytes, to express stem cell markers with clonogenic features, and with the ability to maintain their functionality in vitro and in vivo. These cells are the cardiosphere‐derived cells (CDCs) and cardiospheres (CSs), which represent the progenitor/stem cell‐enriched population.10
CDCs have been tested in clinical trials in which autologous CDCs are isolated from patients, and intracoronarially infused after AMI. Two major studies have demonstrated that their use decreased scar size, improving myocardial function and regeneration.11, 12 Nevertheless, these cellular treatments showed limited advantages. The cost for the manipulation and culture of the explanted cells in safe conditions calls for new approaches in AMI treatments. A characterization of the genes regulated by miRNAs could be useful in clarifying the mechanisms in the human heart, particularly in the CSs/CDCs counterpart, opening the possibility for their use in therapy. Recently, it was reported that specific miRNAs promote cardiomyocyte proliferation in rats.13 Three different groups of genes for pluripotent, transitional and mature cardiomyocyte stage were identified from gene analysis of mRNA expression during the differentiation of induced pluripotent stem cells (hiPSCs) from foetal, adult and hypertensive human heart biopsies. Moreover, a miRNA pattern controlling the stem process into the developmental stage at the transcriptional level was identified.14
The aim of our work was to characterize miRNA expression in human heart biopsies and in their CS‐ and CDC‐derived cell populations through microarrays and next‐generation sequencing (NGS) approaches. Initially, we performed the miRNA expression analysis using the Agilent microarray platform and found that a set of miRNA clusters was preferentially expressed in CSs and CDCs compared with the biopsies. To have a wider miRNA profile of the three cell populations, we used an NGS approach: while some groups of miRNAs preferentially expressed in CDCs and CSs found with microarrays were also validated with NGS analysis, other miRNAs were specifically identified as more expressed or downregulated in CDCs and CSs than the biopsies. We identified a positive miRNA expression correlation between the microarray and NGS platforms. Moreover, we identified miRNAs preferentially expressed in CSs and in CDCs, while others were downregulated. Among them, we validated some by real‐time polymerase chain reaction (PCR), and we analysed the correlation between expression and patient age. Moreover, analysis of the NGS results with Diana miRPath software revealed that in CDCs and CSs, miRNAs were involved in the Hippo pathway, the stem cell pathway, the signalling that regulates stem cell pluripotency, and in the signalling involved in arrhythmogenic right ventricular cardiomyopathy.
2. MATERIALS AND METHODS
2.1. Biopsy specimen processing and cell culture
The isolation, culturing and expansion of cardiac progenitors were carried out on fresh heart biopsies from patients who had undergone extracorporeal circulation. Human surgical auricola biopsies were obtained as part of routine surgical intervention in the Cardiac Surgery and Heart Transplantation Unit at ISMETT, in accordance with institutional guidelines and ISMETT’s Ethics Committee. Informed consent was obtained from all the voluntary participants (IRRB 35/13), as established by the declaration of Helsinki. This study was previously internally approved under protocol number ISMETT.30.09.2013.E.0020458, on 2 October 2013. About 50 biopsies were collected in this study from patients with different diseases: (AVS, aortic valve stenosis; CAD, coronary artery disease; AVRm, aortic valve regurgitation; AAA, ascending aortic aneurysm; BAV, bicuspid aortic valve) (mean age 65 years) (Table 1).
TABLE 1.
Patients’ summary: table listing the patients enrolled in the study for biological specimen isolation, age, disease and sex are indicated
| sample | diseases | Sex | age (years) |
|---|---|---|---|
| cardio 01 | AVS+CAD | M | 76 |
| cardio 02 | AVR+AAA+CAD | M | 66 |
| cardio 03 | CAD | M | 83 |
| cardio 04 | CAD | M | 57 |
| cardio 05 | CAD | M | 49 |
| cardio 06 | CAD | M | 71 |
| cardio 07 | CAD | M | 55 |
| cardio 08 | CAD | F | 76 |
| cardio 09 | MVR | F | 50 |
| cardio 10 | AVS (BAV) | M | 68 |
| cardio 11 | CAD | M | 69 |
| cardio 12 | CAD | M | 67 |
| cardio 13 | AAA (BAV)+CAD | M | 56 |
| cardio 14 | CAD | M | 69 |
| cardio 15 | AAA (BAV) | F | 63 |
| cardio 16 | AVR+AAA | M | 47 |
| cardio 17 | AVS+CAD | F | 64 |
| cardio 18 | CAD | F | 70 |
| cardio 19 | AVR | M | 64 |
| cardio 20 | CAD | F | 64 |
| cardio 21 | AVS+CAD | M | 68 |
| cardio 22 | CAD | M | 63 |
| cardio 23 | AVS | F | 82 |
| cardio 24 | CAD+AAA | M | 78 |
| cardio 25 | CAD | M | 61 |
| cardio 26 | CAD | M | 54 |
| cardio 27 | AVS | M | 73 |
| cardio 28 | AVS | F | 75 |
| cardio 30 | AVS (BAV) | M | 74 |
| cardio 31 | AVS | F | 72 |
| cardio 32 | CAD | F | 77 |
| cardio 33 | AVS (BAV) | M | 49 |
| cardio 34 | CAD | M | 70 |
| cardio 35 | CAD | M | 64 |
| cardio 36 | AVS+CAD | M | 76 |
| cardio 37 | CAD | M | 77 |
| cardio 39 | CAD | M | 71 |
| cardio 40 | AVR+AAA | M | 78 |
| cardio 41 | AVS | M | 75 |
| cardio 42 | CAD | M | 71 |
| cardio 45 | AVR (BAV)+AAA | M | 46 |
| cardio 46 | AVS | F | 71 |
| cardio 47 | AVS | F | 66 |
| cardio 48 | AVS | F | 24 |
| cardio 49 | AVR (BAV)+AAA | M | 50 |
| cardio 50 | AVS (BAV)+AAA | M | 63 |
Abbreviations: AAA, ascending aortic aneurysm; AVR, aortic valve regurgitation; AVS, aortic valve stenosis; BAV, bicuspid aortic valve; CAD, coronary artery disease; F, female; M, male.
As previously described,10 the biopsies were washed with PBS 1X without Ca++ and Mg++ (Euroclone, Milan, Italy), cleaned from the connective tissue, then mechanically reduced into small fragments and enzymatically digested (0.025%Trypsin‐EDTA, Sigma‐Aldrich, Milan, Italy). The fragments were washed with PBS and seeded on dishes coated with fibronectin (BD Biosciences, Franklin Lakes, NJ) in CDC medium. The CS‐forming cells originated from sprouting from the seeded explant grown over several days. After confluence, the fibroblastic layer cells were harvested by enzymatic digestion (0.05% Trypsin‐EDTA, Sigma‐Aldrich), and the cells were plated on Poly‐D‐Lysine‐coated dishes (Sigma) in CS medium. In these conditions, the CSs grew as floating cell clusters. For expansion, CSs were plated on fibronectin‐treated dishes and expanded as monolayer CDCs, with the following passages (5 times maximum) in CS condition. The CDC medium used was IMDM (Sigma‐Aldrich) plus 20% FCS (Lonza, Basel, Switzerland), Pen/Strep and L‐glutamine (Sigma‐Aldrich). The CS medium was IMDM/DMEM:HAM‐F12 (35%/65%, Sigma‐Aldrich), 4% B27 (Gibco, Milan, Italy), 20 ng/ml bFGF (PeproTech, London, UK), 10 ng/ml EGF (PeproTech), 40 nM Cardiotrophin‐1 (PeproTech), 40 nM L‐thrombin (Sigma‐Aldrich), 3.5% FCS (Lonza), 1% Pen/Strep and 1% L‐glutamine (Sigma‐Aldrich). The cells were also evaluated for specific markers by RT‐PCR.15, 16
2.2. RNA extraction and RT‐PCR
Total RNA was purified by miRNAeasy (Qiagen, Germantown, MD, USA) and reverse‐transcribed using TaqMan UNIVERSAL MMix II (Applied Biosystems, Waltham, MA, USA) for random priming or miRNA‐specific assay reverse transcription. Semi‐quantitative PCR was performed with TaqMan‐validated assays (Applied Biosystems). As endogenous reference gene for cDNA, we chose U6 (#001973) for miRNA. All analyses were carried out in triplicate. Real‐time data were collected using Microsoft Excel and analysed with the following formula: Expression level = 2‐ΔΔCt method.17 All experiments were done as independent triplicates and analysed using standard deviation (SD). The p value was obtained with Student's t test.
2.3. Array analysis
Total RNA was purified by miRNAeasy (Qiagen, Germantown, MD, USA) and retrotranscribed using the Agilent miRNA Labeling Reagent and Hybridization Kit small RNA Agilent—Human miRNA Microarray Kit (V3), and hybridized with the SurePrint Array HD G4470C Microarray Human miRNA Kit (V3) 8x15K containing 866 human and 89 human viral miRNAs (Release 12.0). After hybridization, the microarray was washed according to the manufacturer's protocol. The chips were scanned with an Agilent G2565BA scanner.
Processed logarithmic signal intensities were normalized by quantile normalization method. In each sample, the top‐expressed miRNA covering 90% of the whole miRNA expression was selected, and cluster analysis was performed by considering their union (n = 93). Cluster analysis was implemented with average linkage function. Microarray expression profile data, obtained from the same samples, were compared by computing Pearson's correlation.
2.4. NGS analysis
Total RNA was extracted using the miRNeasy Isolation Kit (Qiagen, Hilden, Germany). Sequencing libraries were prepared according to the Illumina Protocol for small RNA (Illumina, San Diego, CA, USA release Feb. 2014), as previously described.18 One microgram of total RNA was processed using the small RNA library kit, as described by the manufacturer (Illumina). The library was loaded in an Illumina MiSeq sequencer in a 51 bp single read mode (Illumina). The data obtained from the sequencer were filtered following several criteria. Since the sequence of the adapter is known, a Trimmomatic‐0.3319 software was used to trim, from the raw data, the adaptors. The sequence reads were then filtered for quality and clustered to unique sequences to remove redundancy, retaining their individual read count information. Unique sequences 16 nucleotides or more in length were mapped, allowing up to one mismatch on miRNA annotation according to miRBase, using Bowtie 0.12.820 software and HTSeq 0.6.021 software for quantification of the expression of each miRNA. This detects the reads corresponding to known miRNAs, giving an estimation of expression level. Identification of differentially expressed miRNAs was made with the Bioconductor DESeq222 package. Starting from the expression values, the first step was to minimize the effect of the systematic technical variations. Then, a negative binomial distribution model was used to test differential expression in deep sequencing datasets. Only miRNAs with a p value equal to or less than 0.05, and fold change equal to or less than 2.0 and equal to or greater than 2.0 were considered as differentially expressed. Given the critical roles of miRNAs in regulating gene expression and cellular functions, we predicted their putative targets, intersecting results obtained from mirPath v.3.23 MirPath provides computationally predicted miRNA gene targets.
2.5. Cluster analysis correlation
Up‐/downregulated miRNAs (+/− 2 fold change) were loaded onto DianaTools (mirPath v.3) using the option tarbase v7; we used 6 for the gene intersection analysis on the KEGG pathway. After analysis, we identified the more significant pathways (stem cell, arrhythmogenic and Hippo). The relationship between miRNAs and pathways was represented graphically with Cytoscape, while the relationship between miRNAs and genes was represented with R24 (version 3.5.1).
2.6. Immunoblotting
Cells and biopsies were lysed with a buffer containing 1% Triton X‐l00, 50 mM HEPES (pH 7.5), 150 mM NaC1, 10% glycerol, 1.5 mM MgCl2, 5 mM EGTA, protease inhibitors (4 mM phenyl methylsulfonyl fluoride and 100 mg/ml aprotinin; Sigma‐Aldrich), phosphatase inhibitors (10 mM sodium orthovanadate and 20 mM sodium pyrophosphate; Sigma‐Aldrich) and processed. For direct immunoblot analysis, we employed 20 μg of total cellular proteins, re‐suspended with 25 μl of loading buffer, boiled for 5 minutes and loaded on SDS‐PAGE for Western blot (WB). The antibodies for WB were used at the condition suggested by the suppliers: mouse anti‐Dvl3 (sc‐271295, 1/500; Santa Cruz Biotechnology, Texas, USA), mouse anti‐Yap1 (sc‐376830, 1/500; Santa Cruz Biotechnology), mouse anti‐Smad1/2/3 (sc‐7960, 1/500; Santa Cruz Biotechnology), rabbit anti‐cyclin D1 (E3P5S #55506, 1/1000; Cell Signaling) and mouse anti‐beta‐actin (sc‐81178, 1/1000; Santa Cruz Biotechnology). The WB has been acquired by the ChemiDoc MP Imaging System (Bio‐Rad Laboratories, Inc., California, USA), and the corresponding bands have been quantified by Image lab 6.1.0 (Bio‐Rad Laboratories, Inc.).
3. RESULTS
To evaluate miRNAs involved in cardiac stemness/proliferation phenotypes, we analysed miRNA expression profiles in human heart biopsies and in their derived CSs/CDCs. Cardiac biopsies (Figure 1A) were used to isolate stem/progenitor cells that originated by sprouting in appropriate culture conditions, and a part processed to extract RNA.15 The expanded progenitors were used for RNA extraction after 3–6 passages of sequential growth as CSs and CDCs, where CS growth as clusters (Figure 1B), and CDCs showed a fibroblastoid morphology (Figure 1C).
FIGURE 1.
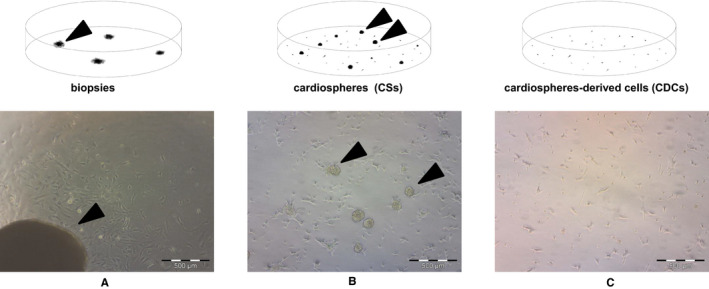
Schematic representation of CS/CDC selection from bioptic specimens. The cardiac tissue fragments were seeded on dishes(A), after cell sprouting these were trypsinized and plated on Poly‐D‐Lysine‐coated dishes to isolate floating CSs (B). CSs floating cell clusters were plated on fibronectin‐treated dishes as CDCs (C)
As a first approach, we evaluated miRNA expression using the Agilent SurePrint Array HD (8x15K) with different samples among biopsies and progenitors (CDCs and CS) (Figure 2). The analysis revealed wide miRNA variation among the diverse samples. Plot analysis displays a clusterization of miRNAs among the different samples: biopsies with biopsies and undifferentiated together, indicating that among the samples there is a conservative pattern depending on the tissue of origin. However, we observed no marked difference among CDCs and CSs, indicating that they are part of the same cell type, with any variation dependant on growth conditions, adherent vs. spheres or growth factors used. In particular, we found various miRNAs strongly downregulated in CSs and CDCs, such as miR‐30, involved in ventricular remodelling,25 and in cell proliferation,26 or miR‐133, responsible for cardiac differentiative processes27 (Figure 2).
FIGURE 2.
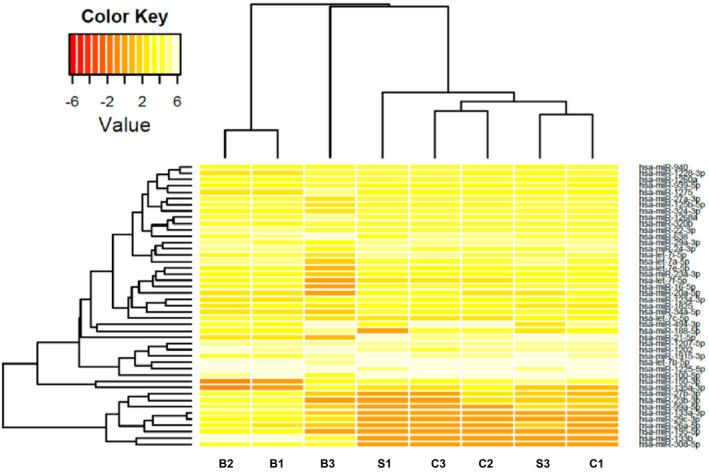
Heat map analysis of the identified miRNAs performed using human CDC “C” Cardiospheres, “S” Spheres and “B” Biopsies with different samples with Agilent microarray (orange: downregulated, white: upregulated).
To carry out a deeper evaluation of miRNA expression, we performed an NGS analysis with the Illumina platform, and to increase the variability, we used different samples from the microarray analysis.
By NGS, we found various miRNAs that are clearly differentially expressed among the two groups of CDCs/CSs and biopsies (Figure 3). A scatter plot was used to assess miRNA expression variation among sample groups. The values of x‐ and y‐axes in the scatter plot correspond to the average miRNA expression in biopsies and CDC/CS samples, respectively. This analysis clearly illustrates the miRNA expression modifications between undifferentiated and differentiated tissues (Figure 3). NGS analysis results show a correlation with microarray analysis (data not shown). NGS data were further analysed by hierarchical clustering. This approach reveals that the samples cluster based on miRNA expression. We showed through this analysis that progenitors (CDCs and CSs) cluster and that miRNA expression can clearly distinguish undifferentiated cell signature from normal bioptic tissue expression. miRNA expressions observed show a profile with clear correlations among the origin of RNA. Identified miRNA cluster among the different samples define a sharp difference between the differentiated tissue (biopsies) and the proliferating undifferentiated (CDCs/CSs) (Figures 4,5). In particular, progenitor‐overexpressed miRNAs were hierarchically clustered together, while in another analysis miRNAs were downregulated in undifferentiated cells (Figures 4,5). The most upregulated and downregulated miRNAs are summarized in the tables (Table 2: miRNAs upregulated in progenitors; Table 3: miRNAs downregulated in progenitors).
FIGURE 3.
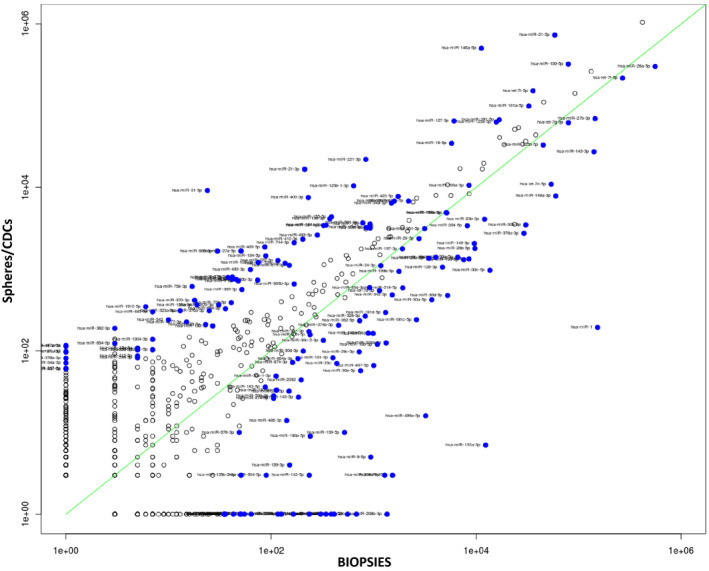
Scatter plot of total miRNA identified by NGS. On the x‐axis, the means of miRNA expression for biopsies are reported, and on the y‐axis, the miRNAs expressed by CDC/CS cells. miRNAs more expressed in progenitors are on the upper left side, and miRNAs more expressed in differentiated heart biopsies are on the lower right. Blue dots represent differentially expressed miRNAs among different samples (fold change, fc=±2; p<0.05).
FIGURE 4.
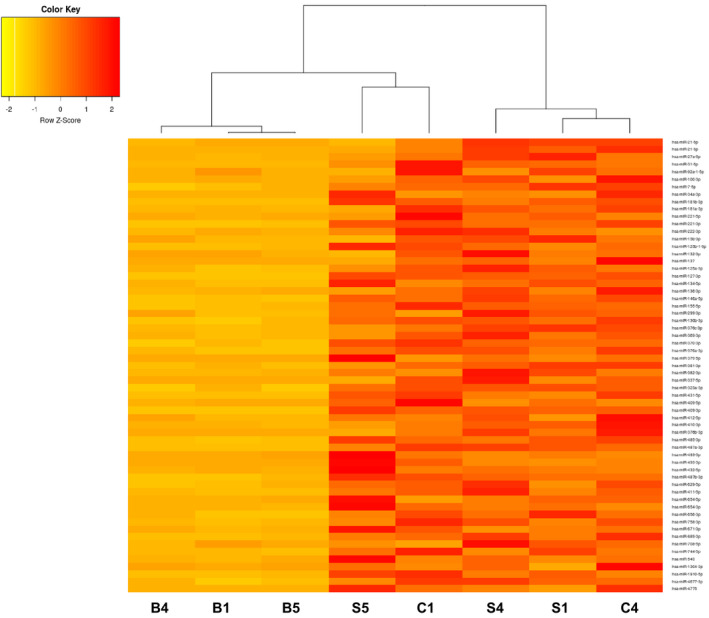
Heat map analysis of miRNAs more abundant in undifferentiated cells, showing the correlation between miRNA expressions obtained from NGS. miRNA expression was hierarchically clustered, showing the association among different cell type: CDC “C” Cardiospheres, “S” Spheres and “B” Biopsies (orange: downregulated, red: upregulated)
FIGURE 5.
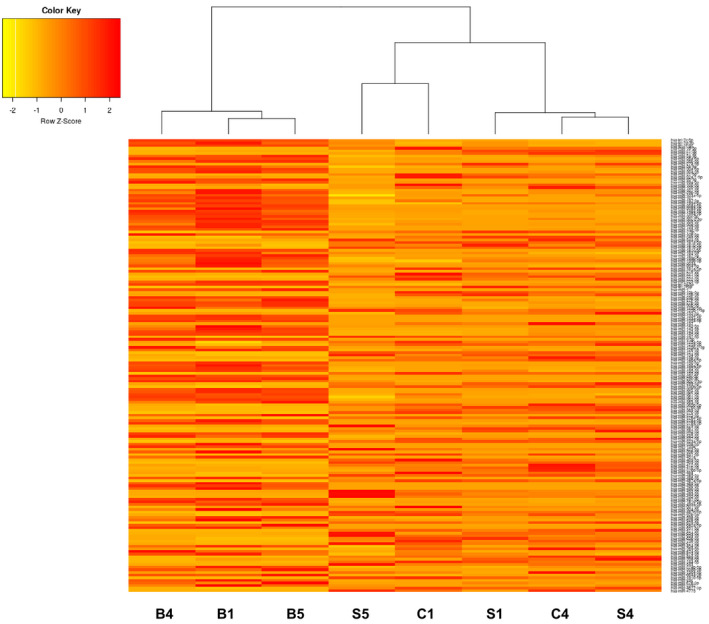
Heat map analysis of miRNAs more abundant in biopsies, showing the correlation between miRNA expressions obtained from NGS. miRNA expression was hierarchically clustered, showing the association among different cell type: CDC “C” Cardiospheres, “S” Spheres and “B” Biopsies (orange: downregulated, red: upregulated)
TABLE 2.
Upregulated in progenitors: selected miRNAs identified by NGS upregulated in progenitors vs. bioptic specimens (p value ≤0.05)
| miRNA | Fold change |
|---|---|
| hsa‐miR−21‐5p | 2.7484 |
| hsa‐miR−21‐3p | 5.3572 |
| hsa‐miR−27a−5p | 4.0918 |
| hsa‐miR−31‐5p | 7.4896 |
| hsa‐miR−92a−1‐5p | 3.7099 |
| hsa‐miR−100‐3p | 3.2196 |
| hsa‐miR−7‐5p | 2.9278 |
| hsa‐miR−34a−3p | 4.0742 |
| hsa‐miR−181b−3p | 4.4813 |
| hsa‐miR−181a−3p | 2.4548 |
| hsa‐miR−221‐5p | 3.2359 |
| hsa‐miR−221‐3p | 3.8665 |
| hsa‐miR−222‐3p | 2.6332 |
| hsa‐miR−15b−3p | 3.769 |
| hsa‐miR−125b−1‐3p | 3.2342 |
| hsa‐miR−132‐3p | 2.2501 |
| hsa‐miR−137 | 4.6336 |
| hsa‐miR−125a−3p | 3.3949 |
| hsa‐miR−127‐3p | 2.5671 |
| hsa‐miR−134‐5p | 3.1897 |
| hsa‐miR−136‐3p | 2.5875 |
| hsa‐miR−146a−5p | 4.6507 |
| hsa‐miR−155‐5p | 2.6301 |
| hsa‐miR−299‐3p | 3.3018 |
| hsa‐miR−130b−3p | 2.3135 |
| hsa‐miR−376c−3p | 2.216 |
| hsa‐miR−369‐3p | 2.5734 |
| hsa‐miR−370‐3p | 3.903 |
| hsa‐miR−376a−3p | 2.8782 |
| hsa‐miR−379‐5p | 3.5517 |
| hsa‐miR−381‐3p | 2.5755 |
| hsa‐miR−382‐3p | 4.5645 |
| hsa‐miR−337‐5p | 3.9705 |
| hsa‐miR−323a−3p | 4.4264 |
| hsa‐miR−431‐5p | 3.8686 |
| hsa‐miR−409‐5p | 3.5866 |
| hsa‐miR−409‐3p | 4.191 |
| hsa‐miR−412‐5p | 3.4294 |
| hsa‐miR−410‐3p | 2.6592 |
| hsa‐miR−376b−3p | 4.3539 |
| hsa‐miR−485‐3p | 4.4034 |
| hsa‐miR−487a−3p | 4.8424 |
| hsa‐miR−493‐5p | 2.4696 |
| hsa‐miR−493‐3p | 3.2197 |
| hsa‐miR−432‐5p | 3.6742 |
| hsa‐miR−487b−3p | 2.5732 |
| hsa‐miR−629‐5p | 2.202 |
| hsa‐miR−411‐5p | 3.1905 |
| hsa‐miR−654‐5p | 3.8602 |
| hsa‐miR−654‐3p | 2.3029 |
| hsa‐miR−656‐3p | 3.6479 |
| hsa‐miR−758‐3p | 4.2105 |
| hsa‐miR−671‐3p | 2.015 |
| hsa‐miR−889‐3p | 4.7122 |
| hsa‐miR−708‐5p | 2.5322 |
| hsa‐miR−744‐5p | 2.8278 |
| hsa‐miR−543 | 3.5297 |
| hsa‐miR−1304‐3p | 3.3442 |
| hsa‐miR−1910‐5p | 4.7108 |
| hsa‐miR−4677‐3p | 3.0669 |
| hsa‐miR−4775 | 4.7372 |
TABLE 3.
Downregulated in progenitors: selected miRNAs identified by NGS downregulated in progenitors vs. bioptic specimens (p value ≤0.05)
| miRNA | Fold change |
|---|---|
| hsa‐let‐7c‐5p | −3.0333 |
| hsa‐miR‐26b‐5p | −3.2806 |
| hsa‐miR‐30a‐5p | −3.9109 |
| hsa‐miR‐30a‐3p | −2.3937 |
| hsa‐miR‐95‐3p | −4.2943 |
| hsa‐miR‐99a‐5p | −3.1569 |
| hsa‐miR‐101‐3p | −2.9907 |
| hsa‐miR‐29b‐3p | −2.1912 |
| hsa‐miR‐107 | −3.469 |
| hsa‐miR‐208a‐5p | −7.5547 |
| hsa‐miR‐208a‐3p | −8.5667 |
| hsa‐miR‐148a‐5p | −3.2549 |
| hsa‐miR‐148a‐3p | −3.8122 |
| hsa‐miR‐30c‐5p | −4.6598 |
| hsa‐miR‐30c‐2‐3p | −2.0636 |
| hsa‐miR‐30d‐5p | −4.2635 |
| hsa‐miR‐139‐5p | −6.0925 |
| hsa‐miR‐139‐3p | −5.5364 |
| hsa‐miR‐10b‐5p | −4.1262 |
| hsa‐miR‐181c‐5p | −4.2359 |
| hsa‐miR‐183‐5p | −2.4598 |
| hsa‐miR‐187‐3p | −4.4679 |
| hsa‐miR‐203a | −5.6513 |
| hsa‐miR‐204‐5p | −2.0446 |
| hsa‐miR‐218‐5p | −2.6125 |
| hsa‐miR‐223‐3p | −5.8025 |
| hsa‐miR‐1 | −10.707 |
| hsa‐miR‐23b‐3p | −2.4521 |
| hsa‐miR‐30b‐5p | −4.0408 |
| hsa‐miR‐133a‐5p | −6.7325 |
| hsa‐miR‐133a‐3p | −11.9 |
| hsa‐miR‐135a‐5p | −7.3079 |
| hsa‐miR‐142‐5p | −6.1027 |
| hsa‐miR‐143‐5p | −3.516 |
| hsa‐miR‐143‐3p | −3.2519 |
| hsa‐miR‐145‐5p | −3.1335 |
| hsa‐miR‐9‐5p | −8.1911 |
| hsa‐miR‐125b‐2‐3p | −4.0425 |
| hsa‐miR‐126‐3p | −3.0177 |
| hsa‐miR‐150‐5p | −6.2097 |
| hsa‐miR‐190a‐5p | −5.6668 |
| hsa‐miR‐195‐5p | −3.3999 |
| hsa‐miR‐195‐3p | −2.6979 |
| hsa‐miR‐29c‐5p | −8.3012 |
| hsa‐miR‐29c‐3p | −3.761 |
| hsa‐miR‐30c‐1‐3p | −2.2355 |
| hsa‐miR‐30e‐5p | −4.4932 |
| hsa‐miR‐30e‐3p | −3.5164 |
| hsa‐miR‐362‐5p | −2.4459 |
| hsa‐miR‐363‐3p | −4.0285 |
| hsa‐miR‐372‐3p | −4.8576 |
| hsa‐miR‐378a‐5p | −4.4058 |
| hsa‐miR‐378a‐3p | −4.2366 |
| hsa‐miR‐328‐3p | −2.6698 |
| hsa‐miR‐342‐3p | −2.5186 |
| hsa‐miR‐338‐3p | −3.9172 |
| hsa‐miR‐133b | −7.1304 |
| hsa‐miR‐20b‐5p | −3.9172 |
| hsa‐miR‐451a | −8.8595 |
| hsa‐miR‐486‐3p | −5.3778 |
| hsa‐miR‐489‐3p | −4.4628 |
| hsa‐miR‐490‐5p | −7.9944 |
| hsa‐miR‐490‐3p | −7.4075 |
| hsa‐miR‐497‐5p | −4.6885 |
| hsa‐miR‐181d‐5p | −3.0345 |
| hsa‐miR‐499a‐5p | −8.9654 |
| hsa‐miR‐504‐5p | −4.6711 |
| hsa‐miR‐598‐3p | −2.7942 |
| hsa‐miR‐628‐5p | −3.3797 |
| hsa‐miR‐642a‐5p | −4.0123 |
| hsa‐miR‐651‐5p | −4.5977 |
| hsa‐miR‐874‐5p | −2.6084 |
| hsa‐miR‐208b‐3p | −9.282 |
| hsa‐miR‐664a‐3p | −2.1935 |
| hsa‐miR‐23c | −3.2984 |
| hsa‐miR‐676‐3p | −3.3675 |
| hsa‐miR‐2392 | −3.2562 |
We evaluated the reliability of our NGS results by choosing some miRNAs to check the differential expression through RT‐PCR analysis (Figure 6A). In particular, we observed that miR‐1, miR‐10b, miR‐133a and miR‐490 were downregulated in CDCs and CSs, while they were highly expressed in differentiated human heart tissues. The expression of miR‐31 was higher in CDC and CS samples (Figure 6A). Notably, the expression of the miR‐133 family, as assessed in microarray analysis, was remarkable in its role in human heart development.28 We also tested whether the differential expression was correlated with patient age. We evaluated miR‐490, miR‐31 and miR‐133a expression in different biopsies. Our analysis revealed that miR‐31, which found more expressed in undifferentiated progenitors, has an inverse correlation with patient age, while the miRNAs overexpressed in differentiated tissues, such as miR‐133a and miR‐490, increase with the patient's age (Figure 6B).
FIGURE 6.
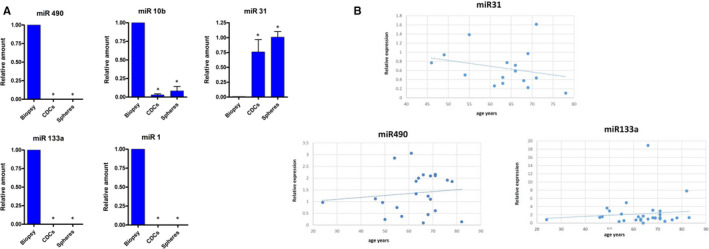
RT‐PCR analysis on selected miRNAs. (A) miR‐1, miR‐133a, miR‐10b, miR‐490 and miR‐31 expression were evaluated in human heart CDCs, CSs and biopsies (all reported experiments were performed in triplicate on three different samples *p value ≤0.05). (B) Correlation between patient age and miR‐490 (ρ=0.119543, p value=0.000188258), miR‐133a (ρ=0.112118341 p value=0.00000001) and miR‐31 (ρ=−0.2608, p value=0.005157) expressions.
To better understand the implications of miRNA expression in the repair processes and in stemness maintenance, we analysed differentially expressed miRNAs with Diana mirPath to find a possible correlation between miRNA and target genes associated with their regulation. In particular, we selected the miRNAs more than twofold upregulated and downregulated in stem cells/progenitors, with a p value <0.05 among all analysed samples. The miRNAs were used to perform a gene intersection analysis through Tarbase (v7). Many of the genes regulated by selected miRNAs are involved in proliferation supporting the reliability of our results (Figure 7A). Furthermore, miRNA gene targeting analysis software showed specific pathways on the KEGG database. Downregulated miRNAs appear to modulate many genes belonging to the Hippo pathway and the signalling pathways regulating pluripotency of stem cells (Figure 7B). The same pathways have been found by the analysis of upregulated miRNAs that also target genes involved in the arrhythmogenic right ventricular cardiomiopathy (ARVC) pathway (Figure 7C). The regulation of such specific pathways offers the idea of a precise signature in post‐transcriptional regulation that leads to a strict control of cardiac progenitor proliferation and differentiation.
FIGURE 7.
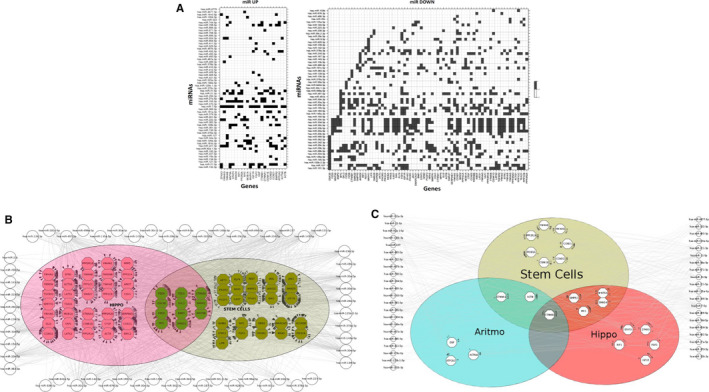
miRNA target analysis. (A) Heat map showing differentially expressed miRNAs versus target gene predicted. Using DIANA MirPath, we provided a graphical overview of selected progenitor's upregulated miRNAs (left) and progenitor's downregulated miRNAs (right). Pathway clustering analysis was also performed for the biological pathways using KEGG pathways as in the heat map. miRNAs in downregulated progenitors (B) and upregulated (C) were considered.
In order to evaluate the relationship among the differentially expressed miRNAs and the specific downstream‐targeted pathway, we analysed by WB some of the key genes belonging to the Hippo pathway. In particular, we found Yap1 clearly differentially expressed in proliferating progenitors vs. biopsies (Figure 8). As expected, other Hippo pathway members were found to be differentially expressed, such as Smad proteins (Figure 8). Likewise, dishevelled 3 (Dvl3), a scaffolding protein implicated also in Wnt and pathway regulation,29 is clearly upregulated in CDCs and CSs (Figure 8). Hippo downstream signalling, such as cyclin D1,30 which promotes proliferation appear upregulated in undifferentiated progenitors (Figure 8).
FIGURE 8.
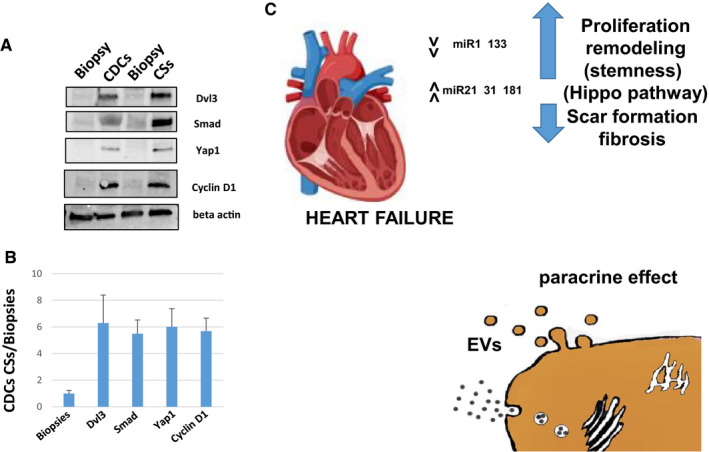
Western blot analysis on Hippo pathway proteins. (A) Cell extracts from Biopsies, CDCs and CSs were used to evaluate Dvl3, SMAD 1/2/3, Yap1 and cyclin D1, the beta‐actin as control. All proteins appear clearly overexpressed in CDCs/CSs and not in the corresponding biopsies (two out of four sample patients have been shown). (B) Intensity ratio of the indicated proteins: CDCs and CSs vs Biopsies (the analysis has been carried on four different samples in triplicate p value ≤0.05) (C) Schematic representation of miRNA’s mechanism of action as illustrated in the Discussion
4. DISCUSSION
The ability of the myocardium to regenerate after AMI was demonstrated more than twenty years ago with the observation in mice models in which delivered bone marrow (BM) progenitors can ameliorate the outcome by generating myocardial cells.31 Furthermore, mobilized BM in MI patients showed an improvement in function and survival.32 The use of human cardiac progenitors in the CADUCEUS11 and PERSEUS12 trials showed limited benefit in using cardiac progenitors (CDCs) for MI treatment. These studies found scar mass reduction, with increased heart mass and contractive activity. However, the following clinical trials were not satisfying,33 showing a low expansion efficiency of transplanted cells due to loss of cell anchorage and a short life of the transplanted cells.34 To circumvent these limitations, scaffolds embedded with cells, or even the administration of vesicles containing proteins, DNA molecules and miRNAs released by cardiovascular progenitor cells were also applied, showing that other factors could contribute to the regeneration of the infarcted heart.35 In this scenario, miRNAs may represent a promising therapeutic tool because they are easily synthesized and can be proficiently encapsulated in particles/exosomes because of their small size. For this reason, we investigated possible mechanisms from molecular signature results that could determine the proliferative ability of cardiac progenitors. In our study, through microarray and NGS analysis, we aimed to clarify the miRNA expression profile in the CDCs and CSs compared with the differentiated tissue from the same patient.
We found a different miRNA expression pattern in our CDCs/CSs compared with the biopsies, identifying several miRNAs that can be responsible for the maintenance of an undifferentiated state. Notably, miR‐1 and miR‐133 were strongly downregulated in our CDC/CS cells. From previous studies, both miRNAs have been found to play a role in the differentiation process, as well as to have a specific function in remodelling events36; moreover, they were upregulated during cardiomyocyte differentiation.14 It has been reported that miR‐133a‐deficient hearts show increased and aberrant cardiomyocyte proliferation throughout the atria and ventricles; this latter result potentially explains the development of a lethal ventricular septal defect in miR‐133 knockout mice, where miR‐133a regulates several transcription factors involved in cell cycle control such as cyclin D1, which we found overexpressed in progenitor cells.28 This cyclin is also regulated by miR‐1,37 and its overexpression promotes cardiomyocyte commitment in human cardiovascular progenitors through the suppression of WNT and FGF signalling pathways.38 The involvement of miRNAs in the FGF pathway opens an important question on the balance between the proliferative activity in cardiac repair after MI and the fibrotic processes. The FGF pathway has been described as influenced by miR‐133, which suppresses atrial remodelling.39 As here observed, miR‐133 is downregulated in undifferentiated proliferation progenitors, and its expression increases with patient age, most likely accounting for the reduced capacity to repair in aged hearts.40, 41 FGF signalling in the heart acts in a paracrine mode, influencing development, in a healthy or pathologic status. We found that undifferentiated proliferating progenitors show miR‐31 increased expression. miR‐31 has been shown to influence cell migration and proliferation by modulating different genes42; however, in mice models, miR‐31 overexpression promotes adverse cardiac remodelling and dysfunction in ischaemic heart disease.43 Cardiac remodelling is a major cause of morbidity and mortality in heart failure, where fibrosis replacement is a critical issue. For this reason, a better comprehension of the fine regulation to maintain a proliferative activity in cardiac resident cells, without an increase in fibrotic transition, is needed. It is remarkable to consider that the miRNAs also act in a paracrine way. It has been demonstrated that they can play a role when secreted in exosomes in various processes (eg in angiogenesis44). The miR‐21 (Table 2), which we found upregulated in progenitors, has been shown to have a protective effect in rats against myocardial infarction.45 Moreover, cardiomyocyte‐derived conditioned medium can exert an action, miR‐21‐dependent, in reducing infarct size in MI induced rats.45 Likewise, miR‐181 has been found to rescue deterioration of cardiac function in post‐MI models by suppressing the Aldo–MR pathway.46 This miRNA has been found to be secreted in exosomes in MSCs models.47 In a different model, it has been demonstrated that miR‐31 is secreted in plasma exosomes48; therefore, further studies could evaluate the miR‐31 or other miRNAs effects on cardiac remodelling when loaded on exosomes.
Our results underscore a regulation of Hippo pathway genes by various miRNAs identified and differentially expressed among progenitors (CDCs/CSs) vs. bioptic tissues. In particular, we found a specific involvement of the Hippo pathway in progenitors that can be responsible for a higher expression of specific proteins such as Yap1, Smad, Dvl3 and cyclin D1. It has been demonstrated that Yap1 is necessary for regeneration in neonatal injured heart mice.49, 50 Its interaction with β‐catenin remarks the connection among Hippo and Wnt signalling in the regulation of cardiomyocyte proliferation and heart size controls.51 Another association between the two pathways is represented by the interaction Yap/Dvl.29 Dvl3, which we found upregulated in progenitors, is responsible for heart formation in mice models.52 Furthermore, Robinow syndrome has been linked to a mutation on the Dvl3 gene53 (3q27.1 omim.org/entry/601368) with various heart developmental defects (ventricular septal defect, patent foramen ovale, pulmonary atresia, hypoplastic right heart, and tricuspid regurgitation) (omim.org/entry/616894, revised by Gentzel et al.54). Our data on regulation of the Hippo pathway reveal a connection among progenitor proliferation miRNA signature and the in vivo regulation of the heart development. Further analysis could open new avenues to find innovative approaches and suggest functional investigations in order to identify mechanisms responsible for the myocardial repair during the proliferative activity of undifferentiated cells. In particular, narrowed studies based on our miRNA expression can be conducted in post‐MI patients to identify the most promising for therapeutic or diagnostic purposes.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
gioacchin iannolo: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (lead); Project administration (lead); Supervision (lead); Writing‐original draft (lead); Writing‐review & editing (lead). maria rita sciuto: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Nicola Cuscino: Data curation (supporting); Formal analysis (supporting); Software (supporting). Claudia Carcione: Formal analysis (supporting); Validation (supporting). claudia coronnello: Data curation (supporting); Formal analysis (supporting); Software (supporting). cinzia maria chinnici: Investigation (supporting). giuaseppe maria raffa: Resources (supporting). michele pilato: Investigation (supporting). Pier Giulio Conaldi: Funding acquisition (lead); Investigation (supporting).
ACKNOWLEDGEMENTS
We thank G. Leone, S. Pasqua and D. Galvagno for technical help and for their support. The study was funded by IRCCS Ismett.
Iannolo G, Sciuto MR, Cuscino N, et al. miRNA expression analysis in the human heart: Undifferentiated progenitors vs. bioptic tissues—Implications for proliferation and ageing. J Cell Mol Med. 2021;25:8687–8700. 10.1111/jcmm.16824
Gioacchin Iannolo and Maria Rita Sciuto equally contributed to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics‐2020 update: a report from the american heart association. Circulation. 2020;141(9):e139‐e596. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja P, Sdek P, MacLellan WR. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev. 2007;87(2):521‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gama‐Carvalho M, Andrade J, Bras‐Rosario L. Regulation of cardiac cell fate by microRNAs: implications for heart regeneration. Cells. 2014;3(4):996‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson JE, Porrello ER. The non‐coding road towards cardiac regeneration. Cardiovasc Transl Res. 2013;6(6):909‐923. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281‐297. [DOI] [PubMed] [Google Scholar]
- 6.Thum T, Catalucci D, Bauersachs J. MicroRNAs: novel regulators in cardiac development and disease. Cardiovasc Res. 2008;79(4):562‐570. [DOI] [PubMed] [Google Scholar]
- 7.Rao PK, Toyama Y, Chiang HR, et al. Loss of cardiac microRNA‐mediated regulation leads to dilated cardiomyopathy and heart failure. Circ Res. 2009;105(6):585‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JF, Murchison EP, Tang R, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci USA. 2008;105(6):2111‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thum T, Galuppo P, Wolf C, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116(3):258‐267. [DOI] [PubMed] [Google Scholar]
- 10.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95(9):911‐921. [DOI] [PubMed] [Google Scholar]
- 11.Malliaras K, Makkar RR, Smith RR, et al. Intracoronary cardiosphere‐derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1‐year results of the CADUCEUS trial (CArdiosphere‐Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol. 2014;63(2):110‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishigami S, Ohtsuki S, Eitoku T, et al. Intracoronary cardiac progenitor cells in single ventricle physiology: The PERSEUS randomized phase 2 trial. Circ Res. 2017;120(7):1162‐1173. [DOI] [PubMed] [Google Scholar]
- 13.Eulalio A, Mano M, Dal Ferro M, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492(7429):376‐381. [DOI] [PubMed] [Google Scholar]
- 14.Babiarz JE, Ravon M, Sridhar S, et al. Determination of the human cardiomyocyte mRNA and miRNA differentiation network by fine‐scale profiling. Stem Cells Dev. 2012;21(11):1956‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iannolo G, Sciuto MR, Raffa GM, et al. MiR34 inhibition induces human heart progenitor proliferation. Cell Death Dis. 2018;9(3):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vella S, Gallo A, Lo Nigro A, et al. PIWI‐interacting RNA (piRNA) signatures in human cardiac progenitor cells. Intern J Biochem Cell Biol. 2016;76:1‐11. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods. 2001;25(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 18.Iannolo G, Sciuto MR, Cuscino N, et al. Zika virus infection induces MiR34c expression in glioblastoma stem cells: new perspectives for brain tumor treatments. Cell Death Dis. 2019;10(4):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114‐2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langmead B, Trapnell C, Pop M, et al. Ultrafast and memory‐efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high‐throughput sequencing data. Bioinformatics. 2015;31(2):166‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlachos IS, Zagganas K, Paraskevopoulou MD, et al. DIANA‐miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43(W1):W460‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dessau RB, Pipper CB. "R"–project for statistical computing. Ugeskr Laeger. 2008;170(5):328‐330. [PubMed] [Google Scholar]
- 25.Zhang X, Dong S, Jia Q, et al. The microRNA in ventricular remodeling: the miR‐30 family. Biosci Rep. 2019;39(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma YS, Yu F, Zhong XM, et al. miR‐30 Family Reduction Maintains Self‐Renewal and Promotes Tumorigenesis in NSCLC‐Initiating Cells by Targeting Oncogene TM4SF1. Mol Ther. 2018;26(12):2751‐2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchelson KR, Qin WY. Roles of the canonical myomiRs miR‐1, ‐133 and ‐206 in cell development and disease. World J Biol Chem. 2015;6(3):162‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu N, Bezprozvannaya S, Williams AH, et al. microRNA‐133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22(23):3242‐3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y, Kim NH, Cho ES, et al. Dishevelled has a YAP nuclear export function in a tumor suppressor context‐dependent manner. Nat Commun. 2018;9(1):2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Hiroi Y, Liao JK. Notch signaling as an important mediator of cardiac repair and regeneration after myocardial infarction. Trends Cardiovasc Med. 2010;20(7):228‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701‐705. [DOI] [PubMed] [Google Scholar]
- 32.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98(18):10344‐10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peruzzi M, De Falco E, Abbate A, et al. State of the art on the evidence base in cardiac regenerative therapy: overview of 41 systematic reviews. Biomed Res Int. 2015;2015:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smets FN, Chen Y, Wang LJ, et al. Loss of cell anchorage triggers apoptosis (anoikis) in primary mouse hepatocytes. Mol Genet Metab. 2002;75(4):344‐352. [DOI] [PubMed] [Google Scholar]
- 35.Kervadec A, Bellamy V, El Harane N, et al. Cardiovascular progenitor‐derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant. 2016;35(6):795‐807. [DOI] [PubMed] [Google Scholar]
- 36.Care A, Catalucci D, Felicetti F, et al. MicroRNA‐133 controls cardiac hypertrophy. Nat Med. 2007;13(5):613‐618. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D, Li X, Chen C, et al. Attenuation of p38‐mediated miR‐1/133 expression facilitates myoblast proliferation during the early stage of muscle regeneration. PLoS One. 2012;7(7):e41478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu TY, Lin B, Li Y, et al. Overexpression of microRNA‐1 promotes cardiomyocyte commitment from human cardiovascular progenitors via suppressing WNT and FGF signaling pathways. J Mol Cell Cardiol. 2013;63:146‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng WL, Kao YH, Chao TF, et al. MicroRNA‐133 suppresses ZFHX3‐dependent atrial remodelling and arrhythmia. Acta Physiol. 2019;227(3):e13322. [DOI] [PubMed] [Google Scholar]
- 40.Ballard VL. Stem cells for heart failure in the aging heart. Heart Fail Rev. 2010;15(5):447‐456. [DOI] [PubMed] [Google Scholar]
- 41.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763‐776. [DOI] [PubMed] [Google Scholar]
- 42.Stepicheva NA, Song JL. Function and regulation of microRNA‐31 in development and disease. Mol Reprod Dev. 2016;83(8):654‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez EC, Lilyanna S, Wang P, et al. MicroRNA‐31 promotes adverse cardiac remodeling and dysfunction in ischemic heart disease. J Mol Cell Cardiol. 2017;112:27‐39. [DOI] [PubMed] [Google Scholar]
- 44.Moghiman T, Barghchi B, Esmaeili SA, et al. Therapeutic angiogenesis with exosomal microRNAs: an effectual approach for the treatment of myocardial ischemia. Heart Fail Rev. 2020;26(1):205‐213. [DOI] [PubMed] [Google Scholar]
- 45.Chen CH, Hsu SY, Chiu CC, et al. MicroRNA‐21 mediates the protective effect of cardiomyocyte‐derived conditioned medium on ameliorating myocardial infarction in rats. Cells. 2019;8(8):935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garg A, Foinquinos A, Jung M, et al. MiRNA‐181a is a novel regulator of aldosterone‐mineralocorticoid receptor‐mediated cardiac remodelling. Eur J Heart Fail. 2020. [DOI] [PubMed] [Google Scholar]
- 47.Ti D, Hao H, Fu X, et al. Mesenchymal stem cells‐derived exosomal microRNAs contribute to wound inflammation. Sci China Life Sci. 2016;59(12):1305‐1312. [DOI] [PubMed] [Google Scholar]
- 48.Samsonov R, Burdakov V, Shtam T, et al. Plasma exosomal miR‐21 and miR‐181a differentiates follicular from papillary thyroid cancer. Tumour Biol. 2016;37(9):12011‐12021. [DOI] [PubMed] [Google Scholar]
- 49.Xin M, Kim Y, Sutherland LB, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA. 2013;110(34):13839‐13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moya IM, Halder G. Hippo‐YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019;20(4):211‐226. [DOI] [PubMed] [Google Scholar]
- 51.Meza AT, Rieber M. Dibutyryladenosine 3’:5'‐cyclic monophosphate‐mediated changes in rat cells involve macromolecular alterations in vinblastine‐precipitable proteins. Biochem J. 1978;174(3):1071‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etheridge SL, Ray S, Li S, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4(11):e1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C, Mazzeu JF, Eisfeldt J, et al. Novel pathogenic genomic variants leading to autosomal dominant and recessive Robinow syndrome. Am J Med Genet A. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gentzel M, Schambony A. Dishevelled paralogs in vertebrate development: redundant or distinct? Front Cell Develop Biol. 2017;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


