Abstract
Background
A major clinical feature of severe coronavirus diease 2019 (COVID‐19) is microvascular thrombosis linked to endothelial cell activation. Consistent with this, a number of studies have shown that patients with severe COVID‐19 have highly elevated plasma levels of von Willebrand Factor (VWF) that may contribute to the prothrombotic phenotype. In the current study, we investigated the extent of endothelial activation in patients receiving hemodialysis who had either mild or severe COVID‐19.
Methods
Plasma VWF, ADAMTS‐13, angiopoietin‐2 (Ang2), and syndecan‐1 levels were determined by ELISA. The sialic acid content of VWF was investigated using a modified ELISA to measure elderberry bark lectin, specific for sialic acid residues, binding to VWF.
Results
Patients receiving hemodialysis with severe COVID‐19 had significantly higher plasma levels of VWF and lower ADAMTS‐13. VWF levels peaked and were sustained during the first 10 days after positive confirmation of infection. While Ang2 trended toward being higher in severely ill patients, this did not reach significance; however, severely ill patients had significantly higher soluble syndecan‐1 levels, with high levels related to risk of death. Finally, higher VWF levels in severely ill patients were correlated with lower VWF sialic acid content.
Conclusions
Severe COVID‐19 in patients undergoing hemodialysis is associated with both acute and sustained activation of the endothelium, leading to alteration of the VWF/ADAMTS‐13 axis. Lower VWF sialic acid content represents altered VWF processing and further confirms the disturbance caused to the endothelium in COVID‐19.
Keywords: ADAMTS‐13, COVID‐19, endothelium, glycosylation, von Willebrand factor
Essentials.
Severe coronavirus disease 2019 (COVID‐19) is associated with a risk of thrombosis.
We investigated markers of endothelial activation in patients with COVID‐19 undergoing hemodialysis.
Severe COVID‐19 was associated with elevated von Willebrand factor (VWF) and soluble syndecan‐1 and decreased ADAMTS‐13.
VWF sialic acid content was significantly decreased in severe COVID‐19.
1. INTRODUCTION
Since its emergence in late 2019, coronavirus disease 2019 (COVID‐19) has become a worldwide pandemic with over 117 million cases worldwide and an overall mortality rate of 2% as of March 2021. The causative agent, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infects cells via interaction of its Spike protein with the angiotensin‐converting enzyme 2 (ACE2) receptor.1 Although many infected individuals remain asymptomatic or only experience mild symptoms, ≈15% of infected patients develop severe disease characterized by respiratory distress and a cytokine storm.2 Another emerging feature of severe COVID‐19 is excessive endothelial activation leading to profound alterations in blood coagulation that result in venous and arterial thrombosis in the lungs and other organs.3, 4, 5, 6 Recent work has demonstrated that SARS‐Cov‐2 is unlikely to directly infect the endothelium due to lack of ACE2 expression on endothelial cells7 and thus the endothelial damage and activation is likely caused by the associated cytokine storm and circulating platelet, immune cells, and complement activation.
Von Willebrand factor (VWF) is ubiquitously expressed throughout the endothelium and plays a major role in hemostasis by capturing platelets to sites of vessel damage and is characterized by its large multimeric structure that is essential to its hemostatic function.8 Within endothelial cells VWF is stored in Webiel‐Palade bodies along with a series of proteins involved in angiogenesis, inflammation, and thrombosis.9 The multimeric size of VWF is controlled by the enzyme ADAMTS‐13 that cleaves VWF in its A2 domain, reducing its multimeric size and platelet capture potential.10 A growing body of evidence has implicated the VWF–ADAMTS‐13 axis as playing a major role in the pathogenesis of severe COVID‐19.11, 12, 13, 14, 15, 16, 17 There is now clear evidence that in severe COVID‐19 infection plasma VWF levels can be highly elevated with a corresponding decrease in ADAMT‐13 levels and activity. In this report, we describe highly elevated VWF and lower ADAMTS‐13 levels in patients receiving in‐center hemodialysis with severe COVID‐19 compared to hemodialysis patients without COVID‐19, and show that extreme VWF levels are associated with an alteration in its glycosylation pattern.
2. STUDY DESIGN AND METHODS
Citrate anticoagulated plasma was obtained from patients receiving hemodialysis (HDx) recruited from the Imperial College Renal and Transplant Centre, London. The study was approved by the Health Research Authority, Research Ethics Committee (reference: 20/WA/0123–The Impact of COVID‐19 on Patients With Renal Disease and Immunosuppressed Patients). Patients were defined as being COVID positive based on a positive polymerase chain reaction (PCR) test result. Non–COVID‐19 control patients receiving hemodialysis were obtained from the same patient group and were asymptomatic with no clinical evidence of COVID and negative for the presence of IgM and IgG antibodies against the receptor‐binding domain of SARS‐CoV‐2 using a lateral flow immunoassay.
COVID‐positive patients were separated into mild (remained as an outpatient for the duration of infection) or severe hospitalized cases, classified according to the World Health Organization criteria for severe disease (respiratory rate ≥30/min, blood oxygen saturation ≤90%, arterial oxygen partial pressure:fractional inspired oxygen ratio <300, or infiltrates affecting 50% of the lung field within 24‐48 hours).
Von Willebrand factor levels were determined by an “in‐house” ELISA as previously described.18 ADAMTS‐13, angiopoitein‐2 (Ang2) and syndecan‐1 levels were determined by ELISAs all purchased from R&D Systems (Minneapolis, MN, USA) and all carried out according to the manufacturer’s protocol. Binding of elderberry bark lectin (EBL), specific for terminal sialic acid residues, to VWF was performed using a modified VWF ELISA essentially as previously described.19 Statistical analysis was performed using Prism version 9 (GraphPad Software, La Jolla, CA, USA).
3. RESULTS AND DISCUSSION
Thirty‐nine patients receiving HDx with confirmed symptomatic COVID‐19 were recruited. Fifteen patients were classified as having mild COVID, 24 had severe COVID, and 10 patients were COVID negative. Patient demographics and characteristics are shown in Table 1. There were no notable differences between the demographics and clinical status of the COVID‐19–positive and –negative groups. Serial samples were obtained for patients with mild and severe disease. Twenty‐five percent of the patients (n = 6) with severe COVID died. D‐dimer and hemoglobin levels and platelet counts were obtained for the mild and severe cases. In keeping with previous reports, hemoglobin levels were significantly lower in severe COVID cases compared to mild disease (Figure 1A).20 Platelet counts did not differ between severe and mild cases (Figure 1B), and although no significant difference in D‐dimer levels was seen between disease severities (Figure 1C), both groups of mild and severe patients had D‐dimer levels above the upper limit of normal (250 ng/mL). However, this may be attributed to the chronic kidney disease status of these patients rather COVID‐19, especially with the mild patients.
TABLE 1.
Characteristics and demographics of patients included in this study
| Patient characteristics | All SARS‐CoV‐2 positive (n = 39) | SARS‐CoV‐2 negative (n = 10) |
|---|---|---|
| Age, y, median (range) | 70.5 (38‐86) | 67.5 (23‐85) |
| Male/Female, n/n | 29/10 | 7/3 |
| Ethnicity, n (%) | ||
| Black | 9 (23.7) | 1 (10) |
| Caucasian | 18 (44.7) | 1 (10) |
| Indoasian | 10 (26.3) | 7 (70) |
| Other/not known | 2 (5.3) | 1 (10) |
| Antiplatelet therapy, n (%) | ||
| Yes | 25 (63.2) | 8 (80) |
| No | 14 (36.8) | 2 (20) |
| Months on hemodialysis, median (range) | 31 (11‐72) | 36 (0.5‐192) |
| Outcome, n (%) | ||
| Death | 6 (15.8) | N/A |
| Recovered | 33 (84.2) | N/A |
Abbreviations: N/A, not applicable; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
FIGURE 1.
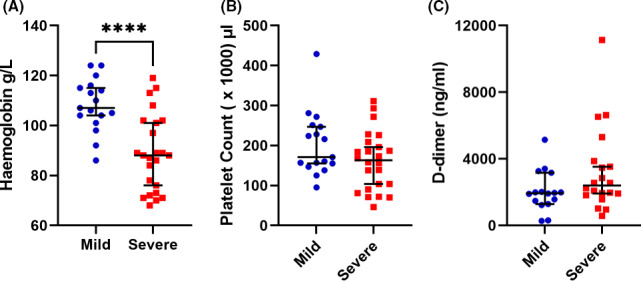
Blood parameters in mild and severe coronavirus disease 2019 (COVID‐19). Plasma samples were obtained from patients receiving in‐center hemodialysis with either mild or severe COVID‐19. Hemoglobin (A), platelet count (B), and D‐dimer levels (C) were obtained from clinical records. Data are presented as the median and interquartile range. (****<.0001, Student’s t test)
Since VWF is elevated in severe COVID‐19, we compared peak plasma VWF levels between groups. No significant differences in VWF levels were observed between control patients median (interquartile range [IQR]) 156 (114‐210) IU/dL and patients with mild disease 196 (145–232) IU/dL. However, in patients with severe COVID‐19, VWF was substantially higher than both negative and mild cases 417 (294‐651) IU/dL (Figure 2A), with some patients demonstrating extreme levels, almost 5 times the average plasma range. In conjunction with raised VWF, plasma ADAMTS‐13 was significantly lower in patients with severe disease median (IQR) 133 (93‐185) ng/mL compared to control patients, 212 (192‐256) ng/mL (Figure 2B), and moreover, the VWF/ADAMTS‐13 ratio was significantly higher in severe cases compared to both controls and patients with mild COVID (Figure 2C). Next, we examined the variation in VWF levels during the time course of the disease. For 5 patients with mild disease and 12 patients with severe disease, four or more samples were obtained over a 182‐day period. In comparison to mild cases where VWF levels remained relatively stable over the sampling period (Figure 2D), VWF levels in patients with severe disease peaked during the first 10 days after positive PCR test, with most patients having sustained levels above the upper limit of normal (200 IU/dL). This demonstrates not only an acute release of VWF, but also sustained activation of the endothelium. Therefore, in severe COVID‐19 a significant imbalance can exist between VWF and ADAMTS‐13 contributing to the prothrombotic phenotype of the disease. Although not measured in this study, there have been reports of elevated interleukins 6 and 8 and tumor necrosis factor in severe COVID‐19.21 Previous work has demonstrated that these cytokines not only promote the release of VWF from the endothelium but also inhibit ADAMTS‐13 synthesis and activity and can therefore perpetuate the imbalance.22, 23 It should be noted, however, that despite the overwhelming evidence of sustained endothelial cell activation in severe COVID‐19, there is also clear evidence of platelet activation. Thus, VWF stored in α‐granules will be released, contributing to the elevated levels. Despite these observations, none of the patients with severe COVID‐19 experienced a recorded thrombotic event. All the patients in this study were receiving anticoagulation before dialysis, and 24 patients (63.25%) were also taking antiplatelet agents and therefore may have had a degree of protection against thrombotic events. Several reports have suggested the clinical benefit of anticoagulation in the treatment of COVID‐19, and a recent study demonstrated that prophylactic anticoagulation reduced the risk of death in severe COVID‐19 cases.24
FIGURE 2.
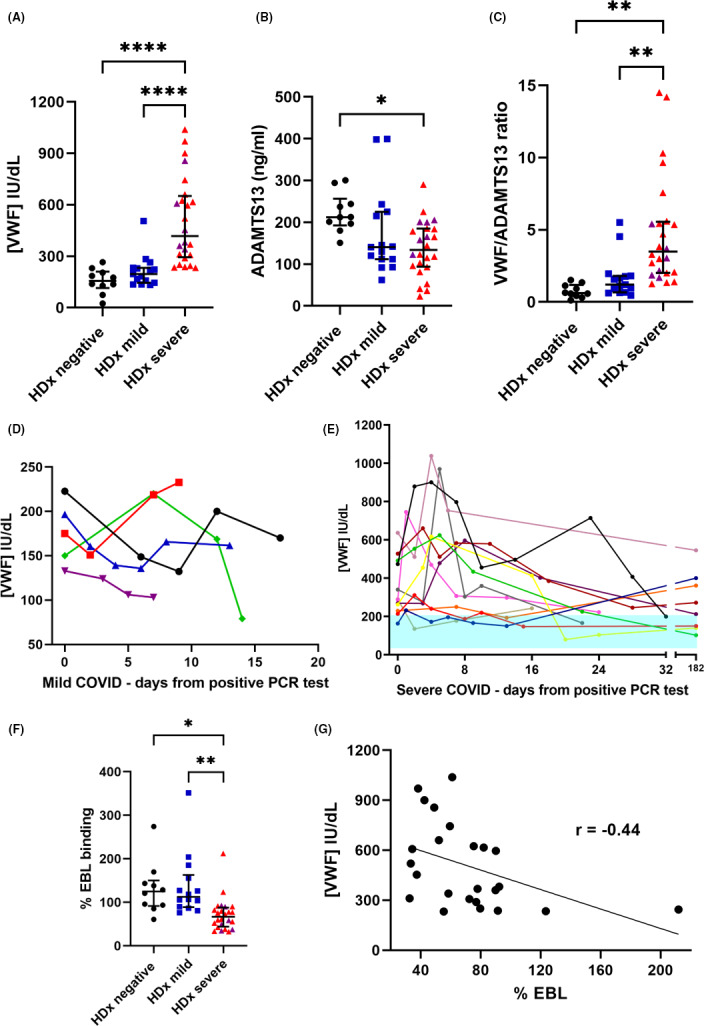
Analysis of von Willebrand factor (VWF) and ADAMTS‐13 levels and VWF sialic acid status in patients receiving hemodialysis with coronavirus disease 2019 (COVID‐19). (A) Plasma VWF levels were determined by “in‐house” ELISA and were significantly higher in severe COVID‐19 cases compared to non–COVID‐19 controls and mild COVID‐19 cases. (B) ADAMTS‐13 levels were determined by ELISA and were significantly lower in severe COVID‐19 compared to controls. (C) Patients with severe COVID‐19 had a disrupted VWF:ADAMTS‐13 ratio. (D) VWF levels in patients with mild COVID‐19; and (E) VWF levels in severe COVID‐19 cases for patients with more than four serial samples collected over 182 days. Shaded area represents the lower and upper normal range of the normal range. (F) Silaic acid on VWF was determined using a modified ELISA measuring binding of elderberry bark lectin (EBL) to VWF. Patients with severe COVID‐19 had a significantly decreased VWF sialic acid content. (G) Correlation between plasma VWF concentration and sialic acid content. (*<.05, **<.005, ****<.0001. One‐way ANOVA, Tukey’s multiple comparisons). Purple symbols represent patients who died
In light of the high and in some cases extreme levels of VWF observed in this study, we hypothesized that biosynthesis of VWF may be altered. VWF undergoes extensive glycosylation with many of its N‐ and O‐linked glycan chains capped with terminal sialic acid residues.25 A modified ELISA measuring the binding of EBL to VWF was used to measure the sialic acid status of VWF. The control and mild COVID‐19 groups both demonstrated similar EBL binding: 124% (91‐150) and 112% (88‐162), respectively. Interestingly, patients with severe COVID‐19 had significantly less sialic acid present on their VWF (66%; 44‐87) (Figure 2F). There was a significant inverse correlation between VWF levels and EBL binding, demonstrating that high VWF levels were associated with lower sialic acid content (Figure 2G). The significance of this is unknown; however, we have previously shown that VWF lacking sialic acid is less susceptible to ADAMTS‐13 proteolysis, and previous reports also demonstrate that VWF devoid of sialic acid has increased reactivity toward platelets.26, 27 Thus, in severe COVID‐19, the elevation of VWF and associated decrease in ADAMTS‐13 may be further compounded by the VWF molecules themselves being more prothrombotic. Further work is now needed to establish if other patient groups have reduced sialic acid content on their VWF; however, since in this cohort patients with mild disease have comparable EBL binding to the control patients, this is unlikely to be a phenomenon linked to dialysis. A previous study demonstrated reduced VWF sialic acid in patients with precapillary pulmonary hypertension alongside a twofold increase in plasma VWF levels.28 The cause of the decrease in sialylation also warrants investigation. It may be directly linked to enhanced synthesis of VWF increasing the rate of protein turnover, resulting in faster transit time through the cell. Since addition of sialic acid occurs in the Golgi and is likely to be a final step of VWF biosynthesis, this final stage in the “production line” of VWF may be missed. Moreover, stimulation of the endothelium with proinflammatory cytokines may alter glycosyltransferase gene expression resulting in altered glycosylation. Alternatively, platelet VWF has been shown to have an altered glycosylation profile, with the presence of less terminal sialic acid, and this could in part account for our observations of highly elevated VWF but lower VWF sialic acid in severe disease. Further work is now needed to establish the contribution of platelet VWF to severe COVID‐19 (Figure 3).
FIGURE 3.
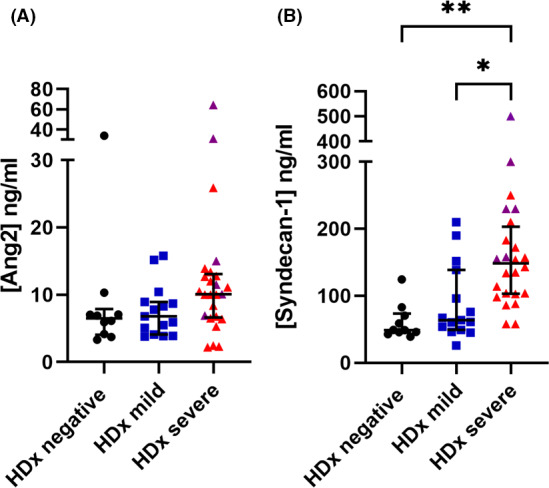
Analysis of plasma Angiopoietin‐2 (Ang2) and syndecan‐1 levels in patients receiving hemodialysis with coronavirus disease 2019 (COVID‐19). Plasma levels of Ang2 and syndecan‐1 were determined by ELISA. (A) No significant difference in Ang2 levels were observed between the patient groups. (B) Syndecan‐1 levels were significantly higher in patients with severe COVID compared to controls and mild cases. (*<.05, **<.005. One‐way ANOVA, Tukey’s multiple comparisons). Purple symbols represent patients who died
We next investigated if the elevation in VWF levels was associated with an increase of other Webiel‐Palade body components. Ang2 is stored alongside VWF and has been shown to be a marker of severe COVID‐19.29 In this study, plasma Ang2 levels were not statistically different between the controls and mild and severe COVID‐19 cases, although there was a trend toward higher Ang2 levels in patients with severe COVID. Finally, we measured plasma levels of syndecan‐1, which has been previously shown to be a marker of endothelial damage.30 Plasma syndecan‐1 levels were higher in severe COVID: 148.5 (103.3‐203.3) ng/mL compared to controls and mild cases 48 (44.9‐73.30 ng/mL and 63.8 (49‐138.6) ng/mL, respectively. Furthermore, elevated sydecan‐1 was higher in those who later died: 230 ng/mL compared to 124 ng/mL in survivors (P = .01, Fisher’s exact test).
4. CONCLUSION
This study allowed direct comparison between mild and severe COVID‐19 cases. The findings from this study confirm that during severe COVID‐19 infection the endothelium is severely disrupted, resulting in release of VWF and subsequent consumption of ADAMTS‐13, which has the potential to lead to a thrombotic phenotype, although the prophylactic anticoagulation given to our patient cohort may have prevented this and adds support to the use of anticoagulants in the treatment of COVID‐19. Our observations that the sialic acid content of VWF in severe COVID‐19 is altered, raises a new avenue for investigation, and will be of interest to see if this phenomenon is observed in other patient cohorts.
AUTHOR CONTRIBUTIONS
GM performed experiments, analyzed data, and wrote the paper. AD collected data and wrote the paper. CC, MP, and SM collected samples, analyzed data, and wrote the paper. RK performed experiments, T Malik collected samples, and MW analyzed data and wrote the paper. T McKinnon performed experiments, analyzed data, and wrote the paper.
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest.
Mobayen G, Dhutia A, Clarke C, et al. Severe COVID‐19 is associated with endothelial activation and abnormal glycosylation of von Willebrand factor in patients undergoing hemodialysis. Res Pract Thromb Haemost. 2021;5:e12582. 10.1002/rth2.12582
Handling Editor: Alisa Wolberg
Funding information
Funding for this study was provided by Imperial College London
Contributor Information
Amrita Dhutia, @amritajd.
Candice Clarke, @clclarke227.
Maria Prendecki, @Maria_Prend.
Stephen McAdoo, @stephenmcadoo.
Michelle Willicombe, @mkwillicombe.
Thomas McKinnon, Email: t.mckinnon03@imperial.ac.uk.
REFERENCES
- 1.Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoglu U. Severe covid‐19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372:n436. [DOI] [PubMed] [Google Scholar]
- 3.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Micco P, Russo V, Carannante N, Imparato M, Cardillo G, Lodigiani C. Prognostic value of fibrinogen among COVID‐19 patients admitted to an emergency department: an Italian cohort study. J Clin Med. 2020;9(12):4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Micco P, Russo V, Lodigiani C. Venous thromboembolism and its association with COVID‐19: still an open debate. Medicina (Kaunas). 2020;56(10):506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCracken IR, Saginc G, He L, et al. Lack of evidence of angiotensin‐converting enzyme 2 expression and replicative infection by SARS‐CoV‐2 in human endothelial cells. Circulation. 2021;143(8):865‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395‐424. [DOI] [PubMed] [Google Scholar]
- 9.Valentijn KM, Sadler JE, Valentijn JA, Voorberg J, Eikenboom J. Functional architecture of Weibel‐Palade bodies. Blood. 2011;117(19):5033‐5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furlan M, Robles R, Lammle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87(10):4223‐4234. [PubMed] [Google Scholar]
- 11.Bazzan M, Montaruli B, Sciascia S, Cosseddu D, Norbiato C, Roccatello D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID‐19 patients. Intern Emerg Med. 2020;15(5):861‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huisman A, Beun R, Sikma M, Westerink J, Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS‐CoV‐2. Int J Lab Hematol. 2020;42:e211–e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latimer G, Corriveau C, DeBiasi RL, et al. Cardiac dysfunction and thrombocytopenia‐associated multiple organ failure inflammation phenotype in a severe paediatric case of COVID‐19. Lancet Child Adolesc Health. 2020;4(7):552‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiscia GL, Favuzzi G, De Laurenzo A, et al. Reduction of ADAMTS13 levels predicts mortality in SARS‐CoV‐2 patients. TH Open. 2020;4(3):e203‐e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancini I, Baronciani L, Artoni A, et al. The ADAMTS13‐von Willebrand factor axis in COVID‐19 patients. J Thromb Haemost. 2021;19(2):513‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward SE, Curley GF, Lavin M, et al. Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID‐19): evidence of acute and sustained endothelial cell activation. Br J Haematol. 2021;192(4):714‐719. [DOI] [PubMed] [Google Scholar]
- 17.von Meijenfeldt FA, Havervall S, Adelmeijer J, et al. Prothrombotic changes in patients with COVID‐19 are associated with disease severity and mortality. Res Pract Thromb Haemost. 2021;5(1):132‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinnon TA, Chion AC, Millington AJ, Lane DA, Laffan MA. N‐linked glycosylation of VWF modulates its interaction with ADAMTS13. Blood. 2008;111(6):3042‐3049. [DOI] [PubMed] [Google Scholar]
- 19.Aguila S, Lavin M, Dalton N, et al. Increased galactose expression and enhanced clearance in patients with low von Willebrand factor. Blood. 2019;133(14):1585‐1596. [DOI] [PubMed] [Google Scholar]
- 20.Anai M, Akaike K, Iwagoe H, et al. Decrease in hemoglobin level predicts increased risk for severe respiratory failure in COVID‐19 patients with pneumonia. Respir Investig. 2021;59(2):187‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Valle DM, Kim‐Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID‐19 severity and survival. Nat Med. 2020;26(10):1636‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao WJ, Niiya M, Zheng XW, Shang DZ, Zheng XL. Inflammatory cytokines inhibit ADAMTS13 synthesis in hepatic stellate cells and endothelial cells. J Thromb Haemost. 2008;6(7):1233‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell‐derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104(1):100‐106. [DOI] [PubMed] [Google Scholar]
- 24.Lopes RD, de Barros ESPGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID‐19 and elevated D‐dimer concentration (ACTION): an open‐label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253‐2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Titani K, Kumar S, Takio K, et al. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986;25(11):3171‐3184. [DOI] [PubMed] [Google Scholar]
- 26.McGrath RT, McKinnon TA, Byrne B, et al. Expression of terminal alpha2‐6‐linked sialic acid on von Willebrand factor specifically enhances proteolysis by ADAMTS13. Blood. 2010;115(13):2666‐2673. [DOI] [PubMed] [Google Scholar]
- 27.Nowak AA, Canis K, Riddell A, Laffan MA, McKinnon TA. O‐linked glycosylation of von Willebrand factor modulates the interaction with platelet receptor glycoprotein Ib under static and shear stress conditions. Blood. 2012;120(1):214‐222. [DOI] [PubMed] [Google Scholar]
- 28.Lopes AA, Ferraz de Souza B, Maeda NY. Decreased sialic acid content of plasma von Willebrand factor in precapillary pulmonary hypertension. Thromb Haemost. 2000;83(5):683‐687. [PubMed] [Google Scholar]
- 29.Villa E, Critelli R, Lasagni S, et al. Dynamic angiopoietin‐2 assessment predicts survival and chronic course in hospitalized patients with COVID‐19. Blood Adv. 2021;5(3):662‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki K, Okada H, Tomita H, et al. Possible involvement of syndecan‐1 in the state of COVID‐19 related to endothelial injury. Thromb J. 2021;19(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]


