Key message
This case portrays a rare overlap of statin-induced Anti-HMG-CoA necrotizing autoimmune myositis and dermatomyositis.
Dear Editor, A 73-year-old woman with a history of hyperlipidaemia had been treated with a statin for approximately 2 years. She suddenly developed persistent bilateral leg and arm weakness as well as occasional morning stiffness. Her initial blood work showed a markedly elevated creatine kinase (CK) level of 13 300 U/L and she had persistent liver enzyme elevation. Her ANA was positive and elevated at 1:1280 in a speckled pattern. MRI of her lower extremities showed a small amount of oedema and enhancement in her hamstrings. Subsequent muscle biopsy of the lower hamstrings showed multiple degenerating myofibres; multifocal perivascular and endomysial infiltrates; CD3, CD4 and CD8 lymphocytes and CD68 macrophages. Her serology was positive for anti-3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase (HMGCR) antibodies >200 and weakly positive for Ku antibodies, which were detected using ELISA. Her statin was discontinued and she was initially started on intermittent corticosteroid bursts and tapers, as well as IVIG infusions. She was also started on weekly rituximab and MTX. Due to persistent symptoms, prior medications were stopped and she was switched to MMF. During the course of this treatment she developed erythematous, pruritic plaques with overlying excoriation and superficial scaling on her upper chest (Supplementary Fig. S1, available at Rheumatology Advances in Practice online). She also had bilateral upper extremity muscle weakness. She was evaluated by dermatology and an incisional biopsy was performed that was consistent with DM, including overexpression of MHC class 1 within the muscle fibres (Fig. 1). She was prescribed topical steroids and was also started on a steroid taper. After a few weeks of treatment the muscle weakness in her lower extremities improved significantly and her rash began to resolve. Her CK levels also normalized and she was started on maintenance AZA.
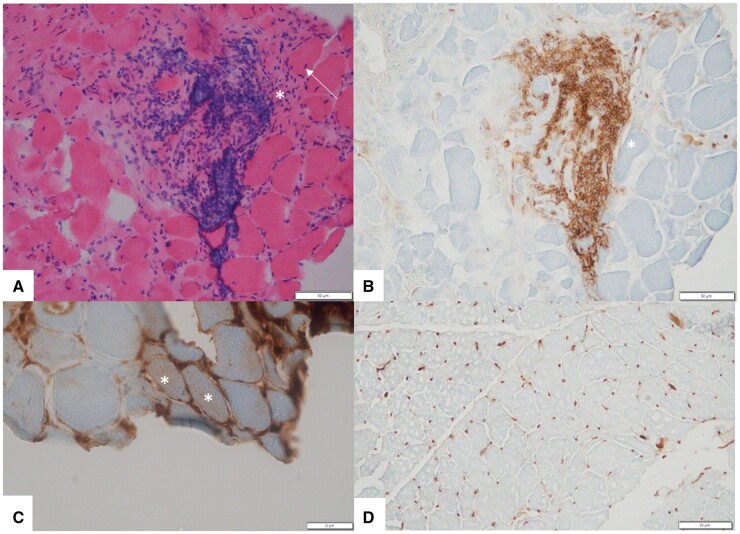
Fig. 1.
Myopathologic features in this patient
(A) Haematoxylin and eosin–stained section shows variation in fibre size, few internal nuclei (arrow) and a large perimysial inflammatory infiltrate (asterisk). (B) CD45-stained section highlights CD45-positive perimysial inflammatory cells (asterisk). (C) MHC-1-stained section shows two fibres with sarcolemmal staining (asterisks). (D) MHC-1-stained control section shows normal capillary staining and no sarcolemmal upregulation.
Idiopathic inflammatory myopathies, including DM, are a group of autoimmune diseases characterized by proximal muscle weakness, elevated muscle enzymes, autoantibodies and responsiveness to immunosuppressive medications [1]. DM is a unique type of inflammatory myopathy in that cutaneous manifestations often precede or accompany weakness, which is found at presentation in only 50–60% of patients [2]. Necrotizing autoimmune myositis (NAM) is a disabling type of immune-mediated myopathy also characterized by severe muscle weakness and markedly elevated CK, similar to DM. However, unlike DM, NAM only rarely presents itself with prominent extramuscular involvement such as rash, arthritis or interstitial lung disease.
It is imperative to discuss the unique serologies of DM and NAM in order to differentiate the two from each other. DM is classically associated with Mi-2 antibodies; however, more recent subtypes have been associated with anti-MDA5, anti-TIF1-γ, anti-NXP2 and anti-SAE antibodies [3]. There are three serologically defined subtypes of NAM: anti-SRP myopathy, anti-HMGCR myopathy and antibody-negative NAM. The cause of NAM is currently unknown, however, immunogenetics analysis has shown an association with HLA-DRB1*11:01 and HLA-DRB1*07:01 alleles, which have been reported particularly in patients with anti-HMGCR myopathy. Although all variants of NAM have myofibre necrosis as the predominant histological feature on muscle biopsy, they vary in their environmental risk factors, one of which is statin exposure [4]. Our patient’s serology was positive for anti-HMGCR antibodies as well as Ku antibodies, which has been shown to manifest in conditions that have overlapping characteristics of DM and NAM [5].
Our patient had multiple findings indicative of an overlap of DM and NAM. While she did exhibit erythematous plaques on her chest, which is classic of DM, these lesions occurred almost a full year after she began to experience muscle weakness. Cutaneous manifestations of DM often precede weakness, however, a minority of patients can have skin disease with no appreciable muscle symptoms or vice versa. Thus the temporal presentation of her symptoms alone cannot confirm an overlap of DM and NAM, and it becomes essential to investigate serologic and histologic findings. While our patient had high levels of anti-HMGCR antibodies associated with a history of statin use and suggestive of NAM, her muscle biopsy did not exhibit classic features of NAM. Her histology indicated no necrosis and instead she had significant inflammation suggesting an autoimmune inflammatory myopathy. Subsequently she was referred to dermatology and received an incisional biopsy that was very typical of DM, including overexpression of MHC class I and inflammatory cells around fascicles and atrophy of muscle fibres near the border of fascicles. Thus these results are strongly suggestive of a rare overlap of DM and NAM, as the patient’s serology pointed towards NAM but her biopsy was indicative of DM.
There are no formal recommendations or specific guidelines on the treatment of NAM. However, based on prior reports, first-line treatment remains oral high-dose corticosteroids or pulse i.v. methylprednisolone. Some experts recommend IVIG as a first-line therapy, especially in anti-HMGCR myopathy [6]. AZA is the preferred agent in patients where steroids are contraindicated.
Overall, idiopathic inflammatory myopathy is a group of autoimmune disorders that typically has various distinctions in presentation, serology and histology. However, an overlapping of these disorders can exist, as outlined in this case. Thus it becomes essential to fully evaluate patients for all forms of autoimmune myopathies when they present with progressive muscle weakness and rash, as there could be a rare overlapping as evidenced in our patient. Early diagnosis can lead to fewer complications and decreased mortality.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have nothing to disclose.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Supplementary data
Supplementary data are available at Rheumatology Advances in Practice online.
Supplementary Material
Contributor Information
Priya Parikh, Department of Internal Medicine.
Nneoma Onuorah, Department of Neurology & Rehabilitation Medicine and Department of Pathology & Laboratory Medicine, University of Cincinnati, Cincinnati, Ohio.
Hani Kushlaf, Department of Neurology & Rehabilitation Medicine and Department of Pathology & Laboratory Medicine, University of Cincinnati, Cincinnati, Ohio.
Priyanka Vashisht, Department of Rheumatology, University of Cincinnati Medical Center, Cincinnati, OH, USA.
References
- 1. Mammen A. Autoimmune myopathies. Continuum (Minneap Minn) 2016;22:1852–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bohan A, Peter JB, Bowman RL, Pearson CM.. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977;56:255–86. [DOI] [PubMed] [Google Scholar]
- 3. Mimori T, Imura Y, Nakashima R, Yoshifuji H.. Autoantibodies in idiopathic inflammatory myopathy: an update on clinical and pathophysiological significance. Curr Opin Rheumatol 2007;19:523–9. [DOI] [PubMed] [Google Scholar]
- 4. Rider LG, Miller FW.. Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA 2011;305:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koenig M, Fritzler MJ, Targoff IN. et al. Heterogeneity of autoantibodies in 100 patients with autoimmune myositis: insights into clinical features and outcomes. Arthritis Res Ther 2007;9:R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mammen A, Tiniakou E.. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015;373:1680–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.