Abstract
Interactions between plants and leaf herbivores have long been implicated as the major driver of plant secondary metabolite diversity. However, other plant-animal interactions, such as those between fruits and frugivores, may also be involved in phytochemical diversification. Using 12 species of Piper, we conducted untargeted metabolomics and molecular networking with extracts of fruits and leaves. We evaluated organ-specific secondary metabolite composition and compared multiple dimensions of phytochemical diversity across organs, including richness, structural complexity, and variability across samples at multiple scales within and across species. Plant organ identity, species identity, and the interaction between the two all significantly influenced secondary metabolite composition. Leaves and fruit shared a majority of compounds, but fruits contained more unique compounds and had higher total estimated chemical richness. While the relative levels of chemical richness and structural complexity across organs varied substantially across species, fruit diversity exceeded leaf diversity in more species than the reverse. Furthermore, the variance in chemical composition across samples was higher for fruits than leaves. By documenting a broad pattern of high phytochemical diversity in fruits relative to leaves, this study lays groundwork for incorporating fruit into a comprehensive and integrative understanding of the ecological and evolutionary factors shaping secondary metabolite composition at the whole-plant level.
Keywords: chemical diversity, molecular networking, metabolomics, fruit, seed, leaf, Piper
Introduction
Phytochemistry plays a key role in mediating the ecological and evolutionary dynamics of plant interactions (Kessler and Baldwin, 2002; Wittstock and Gershenzon, 2002; Hartmann, 2007). Secondary metabolites can significantly affect plant fitness by defending plants against antagonists, altering the competitive ability of neighboring plants, protecting plants from harsh environmental conditions, and attracting and rewarding mutualists, both above and below ground (Iason et al., 2012). However, research on secondary metabolites and their role in the ecology and evolution of plants has been disproportionately focused on vegetative organs, specifically the leaf (e.g., Kursar et al., 2009; Richards et al., 2015; Salazar et al., 2018; Volf et al., 2018). While secondary metabolites have numerous demonstrated functions mediating plant-animal interactions surrounding leaves, they also likely perform a crucial and complex set of functions in reproductive organs.
Plant reproductive organs have been a nexus of plant-animal interactions since before the emergence of angiosperms. However, the ecological role that secondary metabolites play in the biology of these plant organs has received less attention than the role played in leaves. Fruits, and the seeds they contain, provide a direct link to plant fitness and are therefore likely to be under intense selection pressure to attract mutualists and deter antagonists. These complex and contrasting selective pressures are distinct from those acting on leaves, and may lead to the occurrence of secondary metabolites not found in other organs. Indeed, given the complex and often contrasting nature of selective pressures to which fruits and seeds are exposed, fruits and seeds may serve as evolutionary incubators of novel secondary metabolites, and disproportionately contribute to the diversity of phytochemical traits (Whitehead et al., 2021). This is especially likely in systems involving animal-mediated seed dispersal (zoochory), in which plants face the ecological and physiological challenge of attracting and offering a nutritional reward to dispersal vectors while also repelling seed predators, pathogens, and non-target frugivores (Herrera, 1982; Tewksbury, 2002; Whitehead et al., 2016).
Secondary metabolites endemic to fruits, and with demonstrated functional significance in seed dispersal and/or fruit defense, have been shown in several systems, including iridoid glycosides in honeysuckles (Whitehead and Bowers, 2013a,b), capsaicinoids in Capsicum (Suzuki and Iwai, 1984; Tewksbury and Nabhan, 2001; Tewksbury et al., 2008), and amides and alkenylphenols in Piper (Whitehead et al., 2013, 2016; Whitehead and Bowers, 2014; Maynard et al., 2020). For capsaicinoids in Capsicum and alkenylphenols in Piper, the entire class of compounds is synthesized only in the fruits (Suzuki and Iwai, 1984; Maynard et al., 2020). Overall, these studies suggest that unique and potentially contrasting selective pressures on fruits may be an important factor shaping phytochemical diversification in plants. However, our understanding of the relative importance of interactions across plant organs in shaping phytochemical diversity is limited by a paucity of studies that compare chemical composition and metabolomic diversity across plant organs in an ecological context.
Comparative metabolomic studies across plant organs have the potential to greatly expand our understanding of secondary metabolite function and evolution. Given that metabolites may be organ-specific, the location in which they are expressed in the plant (and consequently, the ecological interactions in which they are involved) can provide valuable insight into both the evolutionary origins and ecological consequences of the vast diversity of undescribed plant secondary metabolites.
Despite the likelihood of distinct selective pressures promoting divergent evolution of secondary metabolites across plant organs, in numerous cases the phytochemical composition in one organ may be constrained by physiological or genetic linkages with the phytochemistry of other organs (Adler et al., 2006, 2012; Kessler and Halitschke, 2009; Keith and Mitchell-Olds, 2019). Physiological constraints may result when a majority of the steps in a secondary metabolite pathway are localized to a particular part of the plant, yielding complete or nearly complete end products that are then transported to the organs in which they are utilized, e.g., glucosinolates in the Brassicaceae (Keith and Mitchell-Olds, 2019). Such a pathway has a limited capacity to generate organ-specific modifications of its end products prior to transport, and the sink organs may lack the metabolic machinery required for such modifications. Other secondary metabolites are locally synthesized, but in this case organ-specific metabolites derived from a shared metabolic pathway may be limited by genetic linkage, through co-localization of genes responsible for modifications within a metabolic pathway, e.g., terpene synthase clusters (Falara et al., 2011; Chen et al., 2020; Xu et al., 2020). Certainly, evolutionary processes may overcome these constraints when there are conflicting selection pressures among organs, as evidenced by the examples above of compounds occurring only in specific organs. Furthermore, even when fruits and leaves do share compounds, these compounds may be quantitatively uncorrelated (Cipollini et al., 2004; Whitehead and Bowers, 2013a; Berardi et al., 2016). Thus, while all plant species are biochemically circumscribed to some extent by the biosynthetic pathways acquired through their evolutionary history, broad evolutionary patterns of such constraints across plant organs have yet to be elucidated. Comparative metabolomics provide us with the tools to define and characterize these patterns of constraint in conjunction with patterns of phytochemical innovation.
In this study, we use comparative untargeted metabolomics to explore whether and how differential selective pressures and constraints across reproductive and vegetative organs have shaped the diversity and distribution of secondary metabolites in Piper, a pantropical species-rich genus. Piper are diverse and dominant members of neotropical lowland forest understories and are known to contain a rich array of secondary metabolites (Kato and Furlan, 2007; Richards et al., 2015). Their well-studied chemical composition and a long history of ecological research have made them a model system for understanding phytochemical diversification and its role in shaping plant interactions and community structure (Dyer and Palmer, 2004; Richards et al., 2015; Salazar et al., 2016).
Our overall objective in this study is to test the hypothesis that fruits can act as incubators of phytochemical diversification in plants (Whitehead et al., 2021). First, we describe the occurrence patterns of secondary metabolites across leaves, fruit pulp, and seeds in 12 Piper species, providing baseline data for understanding Piper secondary metabolite function. We use untargeted mass spectrometry-based metabolomics, molecular networking, and in-silico fragmentation modeling to characterize undescribed metabolites at the class level, followed by machine learning and distance-based methods to compare composition across organs and species. Second, we use these data to test predictions of high relative diversity in fruits derived from our hypothesis of fruit-driven phytochemical diversification. We compare multiple dimensions of phytochemical diversity across leaves and fruits, including the richness at multiple scales (alpha and gamma diversity), variability (beta diversity), and structural complexity of secondary metabolites.
Materials and Methods
Study System
Encompassing over 1,000 species across the Neotropics (Quijano-Abril et al., 2006), the genus Piper is diverse and abundant in forest understories, clearings, and edges (Gentry, 1990; Dyer and Palmer, 2004). Fruits of Neotropical Piper are borne on distinct spike-shaped infructescences that are dispersed primarily by bats of the genus Carollia (Phyllostomidae). While sharing this general infructescence morphology, Piper species vary in growth form, from herbs and vines to shrubs and small trees (Gentry, 1990; Dyer and Palmer, 2004), as well as in shade tolerance, fruit size, seed number, and reproductive phenology (Greig, 1993a,b). Fruit antagonists of Piper include insect seed predators, which have been found to consume up to 87% of seeds (Greig, 1993a), and a largely uncharacterized suite of pathogens, which rapidly attack fruit upon ripening (Thies and Kalko, 2004; Whitehead and Bowers, 2014; Maynard et al., 2020). Leaves of Piper are subject to herbivory from a broad array of arthropods, including a genus of specialist geometrid moths, Eois, estimated to include over 1,000 species in the Neotropics (Brehm et al., 2016), as well as other geometrid moths, coleopterans, and orthopterans (Dyer and Palmer, 2004).
Field Collections
All field collections took place between 2009 and 2012 at La Selva Biological Station, Heredia Province, Costa Rica. Samples were collected during a phenology census across 28 species of Piper during 2009–2010 and opportunistically from 2010–2012 when ripe fruits were available. Ripe fruits were distinguished by a distinct softening and swelling of the fruit along an infructescence combined with a partial senescence of the infructescence from the branch (presumably to allow bats to easily remove the entire infructescence in flight). In most Piper species included in this study, one or a few infructescences ripen per day per plant during the fruiting period, and the vast majority of these are removed on the same night of ripening by bats (Thies and Kalko, 2004; Maynard et al., 2020). Those that are not removed rapidly decompose; therefore, we always took care to collect freshly-ripened infructescences. We chose 12 species for inclusion in this study for which we were able to obtain collections from at least three individual plants. These include shade-tolerant and shade-intolerant shrubs (P. auritum, P. generalense, P. glabrescens, P. peltatum, P. sancti-felicis, and P. umbricola), shade-tolerant trees (P. aduncum, P. biolleyi, P. colonense, and P. reticulatum), and mid-successional shade-tolerant vines (P. multiplinervium and P. silvivagum) (Greig, 1993b). For each individual, we collected 1–2 ripe infructescences and the unripe infructescences that were immediately distal to the ripe ones on the same branch. Fruits on a Piper branch mature sequentially from the proximal to the distal end of the branch; thus, these adjacent unripe infructescences were the next closest to maturity on that branch. Leaves were collected from the same branch. We chose the youngest fully expanded leaf that did not have extensive herbivore damage. All samples were transported immediately to the laboratory (within 2 h) and frozen at −80°C prior to analysis. Subsequent analyses involved four sample types: complete leaves, pulp from unripe and ripe infructescences, and seeds from ripe infructescences.
Chemical Extractions
The frozen plant material was freeze-dried (−20°C/−55°C, shelf/condenser), then ground to a fine powder using a FastPrep-24 homogenizer. Seeds and pericarp were separated prior to grinding by gently rubbing the dried fruit over fine mesh; the lignified central rachis of the infructescence was discarded. In unripe fruit, seeds that were not sufficiently developed to be separated from the pericarp by this method were homogenized with the pericarp. For each sample, 50 mg of homogenized powder was weighed into a 2 mL Eppendorf tube using a microbalance. To isolate the broadest possible range of phytochemicals while excluding the broadest possible range of primary metabolites, extracts were prepared using buffered acetonitrile and acetone in series. The acetonitrile and acetone extraction solutions were prepared with an aqueous acetate buffer adjusted to pH 4.8 with acetic acid (44.3 mmol/L ammonium acetate), both at 70:30 solvent: buffer, v/v. The solutions were prepared with Nanopure® water, Fisher HPLC-grade acetic acid, and Fisher Optima®-grade ammonium acetate, acetonitrile, and acetone. All containers and instruments coming into contact with the extracts were rinsed with Fisher Optima®-grade methanol. Each 50 mg sample was extracted twice with 1.5 mL buffered acetonitrile, then twice more with 1.5 mL buffered acetone (6.0 mL total extraction solution). During each of these four extractions, the sample was mixed with the extraction solvent for 5 min in a vortexer and then centrifuged for 5 min at 15,870 rcf, after which the supernatant was removed and added to a 20 mL glass scintillation vial. The supernatant from each of the four extractions was combined in the same 20 mL vial. The combined extract was dried at 30°C using a nitrogen evaporator until no solvent was visible, then further dried in a lyophilizer for 12 h (−20°C/−55°C, shelf/condenser) before being transferred to storage at −80°C until analysis.
Untargeted Metabolomics
LC-MS data were collected using an Acquity I-class UPLC coupled to a Waters Synapt G2-S quadrupole time-of-flight mass spectrometer (Waters). For analysis, dried extracts were resuspended at 10 mg/mL in 75:25 water: acetonitrile + 0.1% formic acid, with 1.0 μg/mL N-oleoylglycine as an internal standard. The extract was then sonicated for 10 min, after which a 20 μL aliquot was taken and diluted 10-fold with 75:25 water: acetonitrile + 0.1% formic acid. The diluted aliquot was then vortexed and centrifuged (10 min, 13,000 ×g) and an aliquot (180 μL) was transferred to an LC-MS vial for analysis. Solvent blanks and combined, quality-control samples were injected at regular intervals during data collection. The autosampler temperature was 10°C and the injection volume was 1.5 μL. The column employed was a reverse-phase Acquity BEH C18 (2.1 mm ID × 150 mm, 1.7 μm particle size, Waters) maintained at 35°C at a flow rate of 0.2 mL/min. Solvent A was water with 0.1% formic acid and solvent B was acetonitrile with 0.1% formic acid (LCMS grade, Fisher Chemical). Solvent gradient: 0–0.5 min, 90% A; 0.5–1.0 min, 75% A; 1.0–8.0 min, 5% A; 8.0–10.0 min, held at 5% A; 10.0–11.0 min, 90% A; 11.0–15.0 min, held at 90% A. Mass spectra and fragmentation spectra were collected simultaneously using Waters’ MSE in positive-ion mode, with the following parameters: peak data recorded in centroid mode; 0.185 s MS scan time; 20–35 V collision energy ramp; argon collision gas; 125°C source temperature; 3 V capillary voltage; 30 V sample cone voltage; 350°C desolvation temperature; nitrogen desolvation at 500 L/h; 10 μL/min lockspray flow rate; 0.1 s lockspray scan time; 20 s lockspray scan frequency; 3 lockspray scans to average; 0.5 Da lockspray mass window; 3 V lockspray capillary voltage. The lockspray solution was 1 ng/ μL leucine enkephalin, and sodium formate was used to calibrate the mass spectrometer.
Alignment, deconvolution, and annotation of molecular and adduct ions were conducted using the XCMS and CAMERA packages in R statistical software (Smith et al., 2006; Tautenhahn et al., 2008; Benton et al., 2010; Kuhl et al., 2012).
Molecular Networking
Molecular networking was used to quantify and visualize the dimensions of the chemical structural trait space occupied by the secondary metabolites in our study (Aron et al., 2020). This technique employs tandem mass spectrometry to generate fragmentation spectra for each putative compound. These fragmentation spectra are diagnostic of molecular structure, and through pairwise comparison they are used to generate a network linking putative compounds to one another based on structural similarity.
In our study, fragmentation spectra data files were aligned, deconvoluted, and converted to mgf using MS-DIAL software (v4.10) and were then uploaded to the Global Natural Products Social Molecular Networking (GNPS) online workflow for molecular networking and library-based annotation (Wang et al., 2016). The following parameters were used for the GNPS workflow METABOLOMICS-SNETS-V2 (v14): 0.02 Da precursor ion mass tolerance; 0.02 Da fragment ion mass tolerance; minimum matched fragment peaks = 6; minimum cluster size = 3; minimum cosine score for network pairs = 0.7; network TopK = 1,000; maximum connected component size = 0. All mass spectral libraries available through GNPS which contained data collected in positive ion mode were used for annotation. Library search parameters were: minimum matched peaks = 6; cosine score threshold = 0.6; maximum analog mass difference = 100. Workflow options for advanced filtering, advanced GNPS repository search, and advanced output were not used.
For further annotation via in-silico modeling, results of the METABOLOMICS-SNETS-V2 workflow were passed to a second GNPS workflow, Network Annotation Propagation (NAP_CCMS v1.2.5). The parameters used for NAP_CCMS were as follows: all clusters selected; subselection cosine value = 0.7; first candidates for consensus score = 10; fusion results used for consensus; accuracy for exact mass candidate search = 15 ppm; acquisition mode = positive; adduct ion types = [M+H]+ and [M+Na]+; all structure databases selected; no custom database or parameter file; compound class not specified; parent mass selection enabled; maximum number of graphed candidate structures = 10; standard workflow type.
Finally, the outputs from METABOLOMICS-SNETS-V2 and NAP_CCMS were combined and exported for visualization using the GNPS workflow MolNetEnhancer (v15). Network visualization and curation was conducted using Cytoscape software (v3.7.2). Parent masses of features in the molecular network were curated based on the XCMS-CAMERA output described above, with primary metabolites and artifactual or pseudoreplicated features removed from the network and subsequent analyses. Features in the molecular network were annotated to the level of chemical class, e.g., flavonoid or prenol lipid, based on ClassyFire chemical taxonomy as applied by MolNetEnhancer. The overall process of molecular networking, library matching, and annotation propagation yields level 3 identification of molecular features with respect to the Metabolomics Standards Initiative (Sumner et al., 2007). The list of annotated molecular features returned by XCMS-CAMERA processing was used to compare overall phytochemical composition across organs and species.
Unfragmented ions collected during single-mass-spectrometry and subsequently aligned, deconvoluted, and annotated, as described above, were used to compare overall phytochemical composition across organs and species. Ion abundance data were transformed to presence/absence data using the peak recognition parameters in XCMS (R code repository). Ion presence/absence was used for analyses rather than relative ion abundance for two reasons: (1) our sample size affords limited capacity to account for variation in abundance within a given organ of a given species, and (2) the relationship between signal intensity and analyte concentration is likely to differ widely across the structurally diverse compounds in Piper due to variation in ionization efficiency (Cech and Enke, 2001).
Comparisons of Phytochemical Composition Across Organs and Species
To compare metabolome-level patterns of phytochemical composition across organs and species, we conducted two separate analyses of the multivariate sample composition, focused first on compound occurrences (presence/absence data) and second on the structural composition of samples. First, to visualize differences in patterns of compound occurrence across samples, we used non-metric multidimensional scaling (NMDS) based on the Sørensen dissimilarity index (binary Bray-Curtis). We then tested for effects of organ, species, and their interaction on compound composition using PERMANOVA, implemented with the “adonis2” function in the R package “vegan.” The individual plant identity was included in these analyses as a “strata” (i.e., random effect), and we used 999 permutations (note that this means the minimum possible P-value is P = 0.001, indicating that the observed differences in sample composition could not be replicated in any of the 999 permutations). To further understand specific differences among the four organ types, we followed this analysis with post-hoc pairwise PERMANOVAs for all possible combinations of organ types, correcting for multiple comparisons using the “pairwise.adonis2” function (Martinez Arbizu, 2020). In addition, based on strongly supported interactions between organ and species (see “Results”), we also divided the data by species and tested for the effects of organ on compound composition for each species individually. All analyses were conducted using the “vegan” package in R (Oksanen et al., 2019).
In addition to our analysis of compound occurrence, we also examined how the structural composition of samples was affected by organ, species, and their interaction. To account for structural features, we generated a multivariate structural dissimilarity index that was a modification of Sedio et al.’s (2017) Chemical Structural and Compositional Similarity (CSCS) index, which quantifies the pairwise similarity of samples by calculating the maximum cosine similarity of the aligned MS-MS ion fragmentation spectra for each inter-sample pair of molecular features. We modified this index by representing ion abundance as a binary term and expressing the index in terms of dissimilarity (1-CSCS). The structural dissimilarity matrix was then used as the basis for NMDS and PERMANOVAs as above that examined the effects of organ, species, and their interaction on structural composition.
Machine Learning
To identify molecular features that distinguished different organs, we used random forest analysis via the “randomForest” and “Boruta” packages for R statistical software (Liaw and Wiener, 2002; Kursa and Rudnicki, 2010). All molecular features distinguished in XCMS-CAMERA processing were used as variables in these analyses. The random forest analysis used a decision tree model to assign samples to our four organ groups (Breiman, 2001, 2002). In the process, the analysis ranked molecular feature variables according to their importance in the model’s group assignments. Boruta analysis complemented the random forest analysis by applying a search for molecular features that were important in informing group assignments. This is accomplished by comparing the features’ importance with importance achievable at random, using “shadow” variables which are generated by permuting the original variables (Kursa and Rudnicki, 2010).
Comparisons of Chemical Diversity Across Organs
Phytochemical diversity is a multifarious concept that includes the number of compounds (richness), their relative abundances (evenness), their structural complexity, and their variation in space and time (Wetzel and Whitehead, 2020). Considering the challenges associated with estimating abundances in untargeted LC-MS-MS data, we focus here on richness and structural complexity, both of which were examined at multiple scales within and across species. For each organ type, we define gamma diversity as the total diversity observed across all samples for that organ, alpha diversity as the average diversity within a single sample from one organ from one Piper individual, and beta-diversity as the variation (both intra- and inter-specific) across samples.
Gamma Diversity
To compare the gamma diversity (total number of compounds detected across all species) of different organs, we used a rarefaction analysis analogous to those commonly used to assess species diversity (Gotelli and Colwell, 2011) with compounds as “species” as in Wetzel and Whitehead (2020). This allowed us to: (1) explicitly visualize the relationship between chemical diversity and sampling scale across different organs (i.e., alpha, beta, and gamma diversity), and (2) estimate the total compound richness in each organ type. Because our individual samples were not independent (we collected three samples per species for 12 species), we used a constrained rarefaction that is similar conceptually to spatially constrained rarefaction (Chiarucci et al., 2009). Briefly, samples were added to bootstrapped accumulation curves in a semi-random manner in which samples from the same species were grouped. For each iteration, a random sample was chosen as a starting point, then other samples from that species were added in random order prior to choosing another sample at random, following with all other samples from that species, and so on until all species were included. We estimated total species richness from these curves using the “fitspecaccum” function in “vegan” based on an asymptotic regression model. Accumulation curves and fits were averaged across 5,000 bootstrapped samples with random starting points.
Alpha Diversity
To compare the average compound richness in a sample (i.e., alpha diversity) across organs, we used a linear mixed model with organ, species, and their interaction as fixed effects and plant identity as a random effect. For hypothesis testing, we compared the full model to simplified versions with fixed effects terms deleted using likelihood ratio tests. Based on a strong interaction between organ and species (see section “Results”), we further divided the data by species and examined differences in richness among organs for each species separately.
Structural Complexity
To compare structural complexity across organ types, we first calculated an index similar to the structural dissimilarity index described above, but in this case focused on within-sample complexity. This within-sample CSCS represents the mean pairwise similarity among all individual molecular features detected in a sample. We used the inverse of this similarity index (1-CSCS) as a measure of overall structural complexity present in a sample. To examine how structural complexity varied across organs and species, we used a linear mixed model with species, organ, and their interaction as fixed effects and plant identity as a random effect. Hypothesis testing was conducted as described above using likelihood ratio tests. Based on strong interactions between organ and species (see section “Results”), we examined differences among organs separately for each Piper species.
Beta Diversity
We examined differences in beta-diversity (i.e., sample-to-sample variance in composition) across organs in two ways, focusing first on variation in compound occurrences (presence/absence) and second on structural features. These analyses were based on the same distance matrices described above that we used to assess overall differences in composition across samples, but instead focused on variance (i.e., dispersion) among samples. This was assessed using the function “betadisper” in the R package “vegan” to compare the dispersion around the group centroid across the four organ types. The “betadisper” function calculated the distances from each sample to the group centroid, and statistical support for differences in dispersion across organs was assessed using a permutation test (N = 999 permutations) followed by a post-hoc Tukey HSD test to assess pairwise differences among individual organs. Because this analysis focused on sample-to-sample variance and our dataset included multi-level sampling (multiple species and multiple individuals within species), significant differences in beta diversity across organs could be due to both intraspecific and interspecific variance among samples. Thus, we followed this analysis with a set of PERMANOVAs, conducted separately for each organ type, with Piper species as an explanatory factor. This analysis allowed us to test if Piper species explained a significant portion of the variation in composition within an organ, and partitioned sample-to-sample variance within an organ type according to the percent of variance explained by species and the percent explained by differences among individual samples within species (i.e., the residual variance).
Results
Untargeted Metabolomics and Molecular Networking Reveal High Chemical Diversity and Many Compounds Unique to Fruits
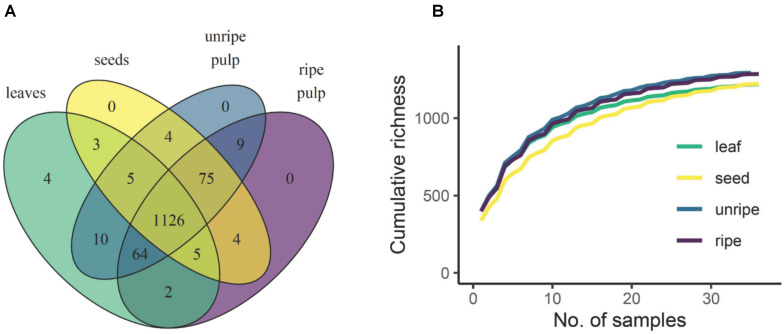
Alignment, deconvolution, and annotation of molecular and adduct ions via XCMS and CAMERA yielded 1,311 unique molecular features across all species and organs. Most of these compounds (1,126) occurred in all organ types, 92 of these were unique to fruits (unripe pulp, ripe pulp, and/or seeds) and 4 were unique to leaves (Figure 1A). It is important to note that, like all other metabolomic approaches, our analytical approach is likely to overestimate the true number of individual chemical compounds present in our samples. The combination of XCMS-CAMERA followed by manual curation unfortunately cannot condense all features (m/z and retention time pairs) into individual compounds. In-source fragmentation, ion clusters, centroid peak splitting of highly abundant ions, and centroid merging of ions near the noise level can all contribute to expanding the dataset beyond individual compounds. The 1,311 features described in this work thus overestimates the number of individual molecular species, though to a lesser extent than in uncurated datasets. Nevertheless, this overestimation is likely to represent a small fraction of the total chemical diversity captured in our analysis. Furthermore, this overestimation is also likely to be of equal magnitude across all species and organs and therefore, will not have a significant impact on the general conclusions of our study. Regarding terminology, these 1,311 features meet or exceed the level of curation beyond which features have, for clarity, been described as “compounds” in the chemical ecology literature (e.g., Sedio et al., 2017; Schneider et al., 2019; Christian et al., 2020; Ricigliano et al., 2020). Thus, for the sake of consistency and clarity, we refer to our curated features as compounds.
FIGURE 1.
Chemical gamma diversity parsed by organ type. A Venn diagram (A) shows the total number of compounds detected across all samples that were unique and shared across organ types. The rarefaction curve (B) shows how compound richness accumulates with sampling scale in each organ type. Curves represent an average across 5,000 bootstrapped accumulation curves with random starting points. Because samples from the same species were not independent, the rarefaction was constrained by species such that samples from the same species were always added in sequence.
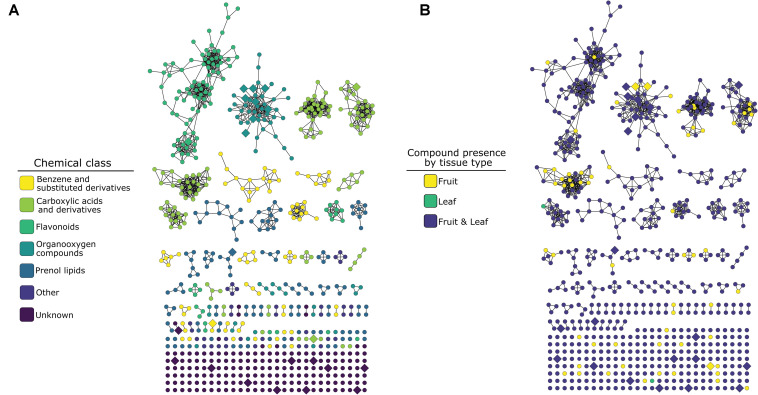
Tandem mass spectrometry yielded fragmentation spectra for 706 of these compounds (Table 1 and Figure 2). Library- and in silico-based classification of fragmentation spectra and parent ions via GNPS resulted in annotation at the level of “class” sensu ClassyFire chemical taxonomy for 527 compounds in 23 classes and 179 were unknowns (Table 1 and Figure 2). Of the 706 compounds that yielded fragmentation spectra, 62 were unique to fruits and 2 were unique to leaves (Table 1). Fruit-specific richness was higher than leaf-specific richness across all chemical classes; these differences were statistically supported for carboxylic acids and derivatives and prenol lipids (Table 1 and Figure 2).
TABLE 1.
Summary of GNPS molecular network annotations and class-level compound richness.
| Chemical classa | Examples of class known from Piper spp. | Total compound richnessb | Fruit-specific compound richnessb,d,e | Leaf-specific compound richnessb,d,e |
| Benzene and substituted derivatives | Cyanogenic benzoates, non-prenylated benzoic acids | 75 | 5 | 0 |
| Carboxylic acids and derivatives | Amides, chromenes, kavalactones | 122 | 25* | 1 |
| Flavonoids | Flavonoids | 104 | 3 | 0 |
| Organo-oxygen compounds | Oxygenated or glycosidic derivatives of other classes | 65 | 5 | 0 |
| Otherc | Amides, chalcones, chromenes, imides | 37 | 4 | 0 |
| Prenol lipids | Chalcones, prenylated benzoic acids, neryl catechol diols, terpenes | 124 | 6* | 0 |
| Unknown | 179 | 14* | 1 | |
| Totalf | 706 | 62* | 2 |
aChemical classes are per ClassyFire chemical taxonomy.
bCompound richness indicates the number of putative compounds, for which fragmentation spectra were obtained, that fall under the given category.
cChemical classes which represented ≤ 1% of all annotated compounds were categorized as “Other.”
dA compound was labeled as fruit or leaf-specific if it was detected only in that organ within the 12 focal Piper species.
eAsterisks indicate statistical support (P < 0.05) for differences between fruit-specific and leaf-specific richness (binomial test with probability = 0.5).
fRichness data shown represent the set of compounds for which fragmentation spectra were obtained in tandem MS analysis, a subset of the total number of compounds detected (Figure 1).
FIGURE 2.
Molecular network of 706 compounds from 12 Piper species color-coded by ClassyFire chemical classification annotation (A) or by organ-specific occurrence across the 12 species (B). Nodes represent compounds, and edges represent the structural similarity between different compounds (see section “Molecular Networking” methods for detail). Enlarged, diamond-shaped nodes represent compounds identified by the Boruta analysis as important for distinguishing among organs. In (B), compounds are coded as occurring in “fruit” if they occur in one or more of the three sample types (unripe pulp, ripe pulp, or seeds).
Phytochemical Composition Differs Across Organs and Species
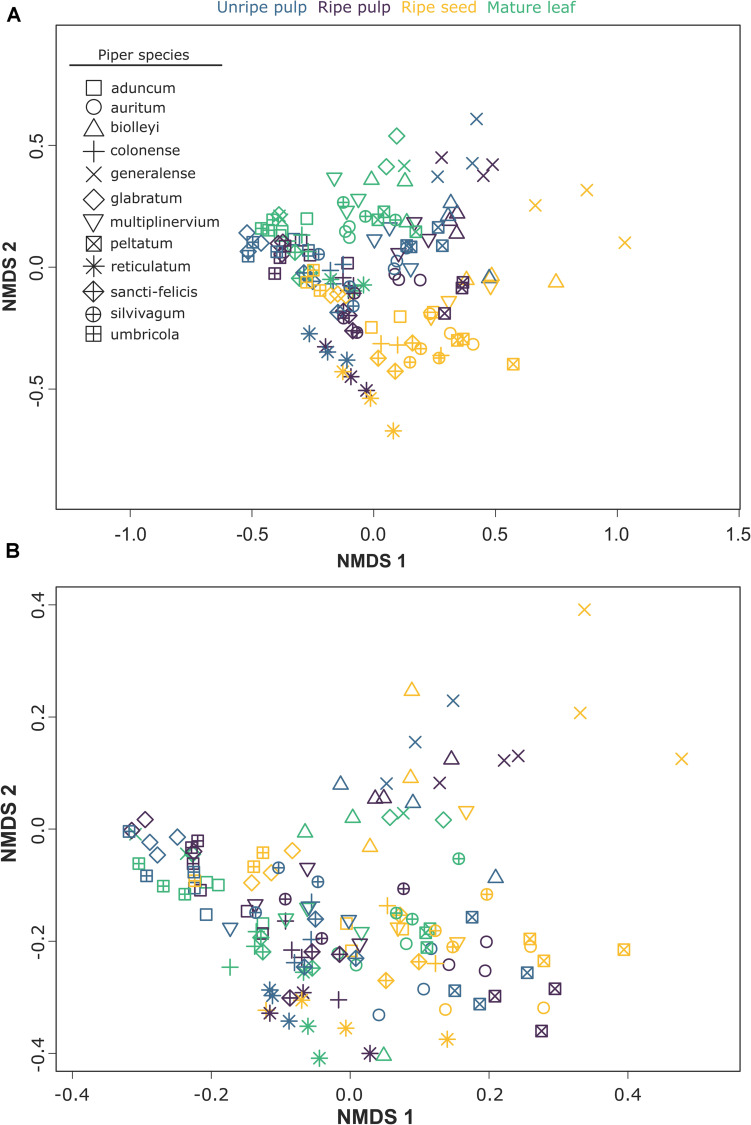
The multivariate patterns of phytochemical occurrence were strongly affected by organ, species, and their interaction [organ: F(3, 95) = 24.19, P = 0.001; species: F(11, 95) = 27.65, P = 0.001; organ × species: F(33, 95) = 1.99, P = 0.001; Figure 3A]. Pairwise comparisons among organs indicated strong differences among all organs (P = 0.001 for all comparisons). Further examination of differences among organs for each of the 12 Piper species individually also revealed strong effects of organ in all cases (Table 2). Similarly, when we assessed factors influencing the multivariate patterns of structural composition across samples, we found a strong effect of organ, species, and their interaction [organ: F(3, 95) = 17.34, P = 0.001; species: F(11, 96) = 21.28, P = 0.001; organ × species: F(33, 96) = 2.31, P = 0.001; Figure 3B], significant differences among organs in all pairwise comparisons (P = 0.001 for all comparisons), and differences among organs for each individual species (Table 2).
FIGURE 3.
NMDS plots showing the effects of organ and species on two aspects of multivariate chemical composition across samples: (A) compound occurrences (presence/absence) and (B) structural composition.
TABLE 2.
Results from PERMANOVAs, conducted separately for each species, testing the effects of organ type (leaves, seed, unripe pulp, or ripe pulp) on two aspects of phytochemical composition: compound occurrences and structural composition.
|
Compound occurrence
|
Structural composition
|
|||
| Piper species | F(3, 11) | P | F(3, 11) | P |
| aduncum | 3.06 | 0.001 | 3.06 | 0.001 |
| auritum | 3.52 | 0.002 | 3.52 | 0.001 |
| biolleyi | 2.19 | 0.009 | 2.19 | 0.012 |
| colonense | 4.40 | 0.001 | 4.40 | 0.001 |
| generalense | 4.12 | 0.001 | 4.12 | 0.001 |
| glabrescens | 4.18 | 0.001 | 4.18 | 0.001 |
| multiplinervum | 3.46 | 0.001 | 3.46 | 0.001 |
| peltatum | 4.51 | 0.002 | 4.51 | 0.001 |
| reticulatum | 5.38 | 0.001 | 5.38 | 0.001 |
| sancti-felicis | 3.67 | 0.001 | 3.67 | 0.001 |
| silvivagum | 5.20 | 0.001 | 5.20 | 0.001 |
| umbricola | 2.89 | 0.002 | 2.89 | 0.001 |
Machine Learning, Informed by Numerous Compounds From Diverse Chemical Classes in Each Organ, Accurately Distinguishes Between Reproductive and Vegetative Organs
The random forest decision tree model used 2,000 trees with 36 variables at each split. Our analysis showed an overall out-of-bag (OOB) mean error rate of 11.72% across the four organ groups. In other words, using secondary metabolites alone, the algorithm was able to predict if a sample was from a leaf, ripe fruit, unripe fruit, or seed approximately 9 times out of every 10 samples. Examining the error rate of each organ group, it was apparent that correctly assigning pulp samples to the correct ripeness stage was the main source of OOB error, with error rates of 27.78 and 18.92% for unripe and ripe pulp, respectively. Leaves and ripe seeds both exhibited zero OOB error. Boruta analysis, designed to both identify important classification features and assess their relative contribution to the final classification performance, identified 23 features exhibiting a significantly higher variable importance score (VIS) than shadow variables. These 23 features are detailed in Supplementary Table 1.
Comparisons of Chemical Diversity Across Organs
Gamma Diversity
Of the 1,311 compounds detected across all organ types, 1,126 were shared across all organs, 92 were found only in fruit (unripe pulp, ripe pulp, and/or seeds), and four were found only in leaves. Rarefaction analysis indicated that the observed richness of secondary metabolites was approaching the asymptote for all organ types (Figure 1B). Further, this analysis showed that the estimated total gamma diversity (total number of compounds across all 12 species of Piper) was highest in unripe fruit pulp, followed by ripe pulp, seeds, and leaves (Figure 1B and Table 3).
TABLE 3.
Rarefaction results showing total estimated richness for each organ across all 12 Piper species sampled.
| Organ | Observed richness | Estimated richness | SE | 95% CI high | 95% CI low |
| Leaf | 1,219 | 1,225 | 0.48 | 1226.1 | 1224.2 |
| Seed | 1,222 | 1,272 | 0.95 | 1273.9 | 1270.2 |
| Unripe pulp | 1,293 | 1,314 | 0.66 | 1315.0 | 1312.5 |
| Ripe pulp | 1,285 | 1,309 | 0.72 | 1310.0 | 1307.2 |
Alpha Diversity
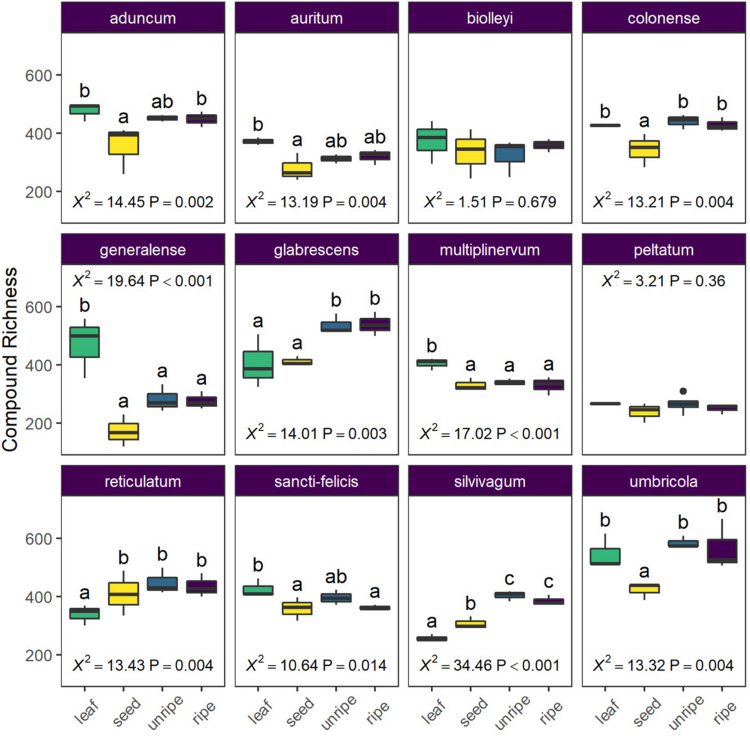
In our analysis of average differences in compound richness across organs and species, we found a strong interaction between organ and species (X2 = 128.99, P < 0.0001) and further examined differences among organs for each species separately. Organs often showed clear differences in average richness, but the patterns were highly variable across species (Figure 4). In three species (P. glabrescens, P. reticulatum, and P. slivivagum), pulp and/or seeds had higher compound richness than leaves. However, in two species (P. multiplinervum and P. generalense) leaves had higher compound richness than all other fruit organs.
FIGURE 4.
Average chemical richness differs across species and organ type (leaf, seed, unripe pulp, and ripe pulp). Letters indicate results of pairwise Tukey post-hoc comparisons of organs within each species, with non-shared letters indicating a significant difference at P < 0.05. Each species plot includes χ2 and P-values from species-level LMMs.
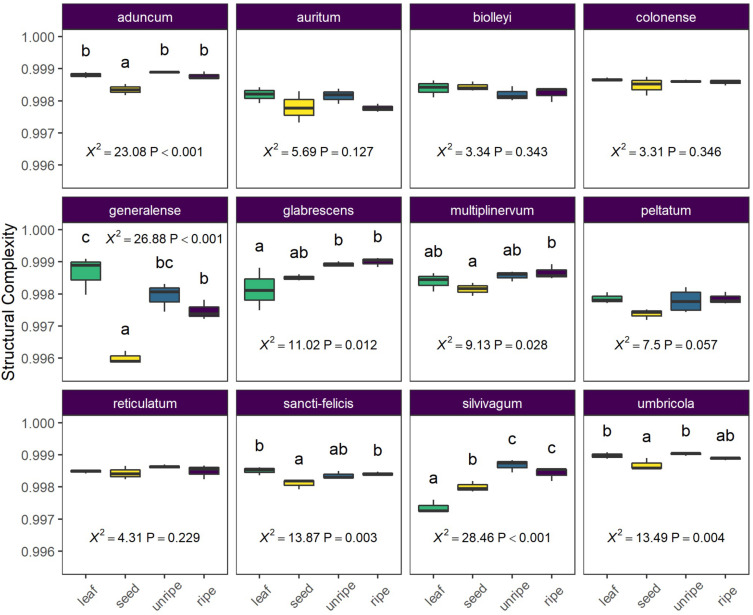
Structural Complexity
In our analysis of average differences in structural complexity across organs and species, we found a strong interaction between organ and species (X2 = 131.13, P < 0.0001) and further examined differences among organs for each species separately. For seven of 12 species, organs showed differences in average structural complexity, but the patterns were variable across species (Figure 5). In two species (P. glabrescens and P. slivivagum), one or more fruit organs had higher complexity than leaves. In another species (P. generalense), leaves had higher complexity than all other fruit organs. Often, seeds had the lowest structural complexity, or at least lower structural complexity than unripe or ripe fruit pulp (Figure 5).
FIGURE 5.
Average structural complexity differs across species and organ type (leaf, seed, unripe pulp, and ripe pulp). Letters above each box plot column indicate results of pairwise Tukey post-hoc comparisons of organs within each species, with non-shared letters indicating a significant difference at P < 0.05. Each species plot includes χ2 and P-values from species-level LMMs.
Beta Diversity
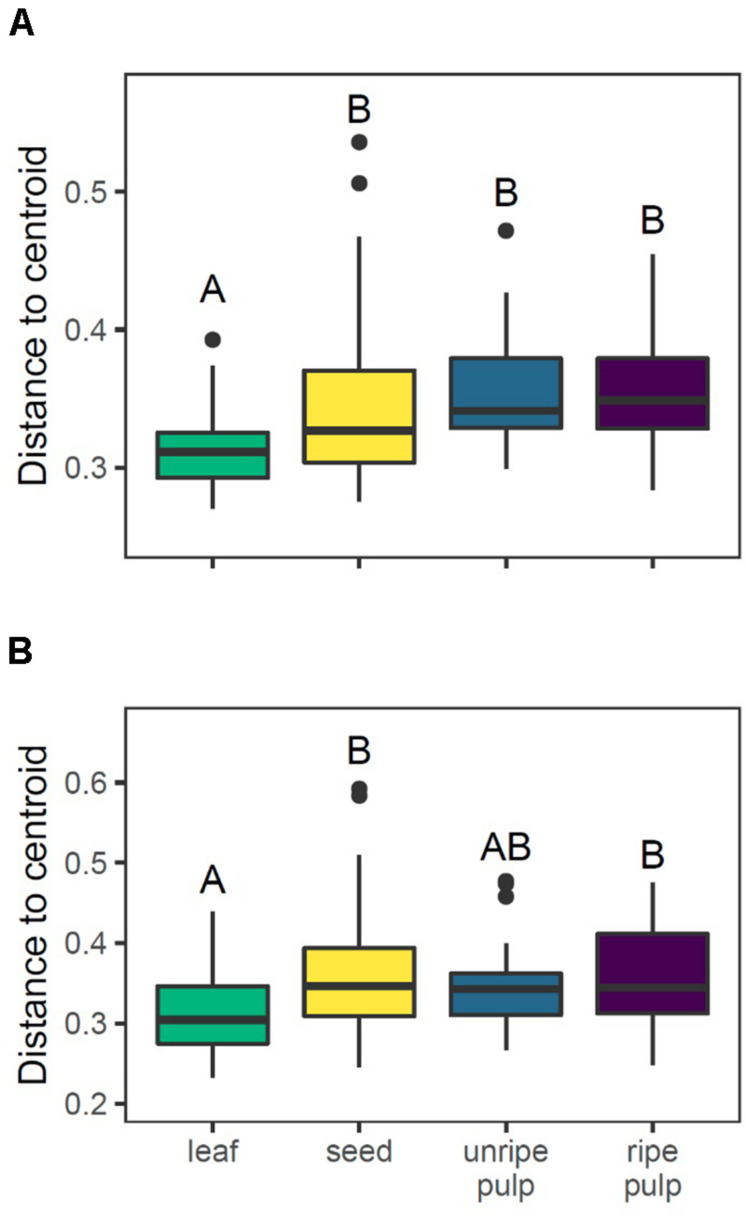
We found that beta-diversity in chemical composition was higher for fruits than leaves when considering only compound occurrences as well as structural composition. First, for compound occurrences, there was strong support for overall differences in beta diversity across organ types [F(3, 139) = 7.56, P = 0.001], with higher average distances to the group centroid for seeds, unripe pulp, and ripe pulp relative to leaves (Figure 6A). Next, for structural composition, there was also strong support for overall differences in beta diversity across organ types [F(3, 139) = 4.10, P = 0.009]. In this case, leaves had lower beta diversity than seeds or ripe pulp, and unripe pulp was intermediate (Figure 6B). Further analyses conducted separately for each organ type showed that the differences in beta-diversity among organ types was due to variation both at the interspecific and intraspecific level (Table 4). A large proportion of the sample-to-sample variation within organ types (67–86%) was explained by differences among species relative to that explained by variation within species (14–33%), and this was especially true for unripe and ripe fruit pulp (Table 4).
FIGURE 6.
Beta diversity in chemical composition is higher for reproductive organs than leaves when considering variance in compound occurrences (A) or structural composition (B). Letters indicate results of pairwise Tukey post-hoc comparisons of organs, with non-shared letters indicating a significant difference at P < 0.05.
TABLE 4.
Results from PERMANOVAs showing a large percentage of sample-to-sample variance in composition within organ types (i.e., beta diversity) is explained by species.
| F11,35b | Pb | η2 (Species)c | η2 (Residual)d | |
| Compound occurrencesa | ||||
| Leaf | 6.29 | 0.001 | 0.74 | 0.26 |
| Seed | 5.17 | 0.001 | 0.70 | 0.30 |
| Unripe pulp | 12.46 | 0.001 | 0.86 | 0.14 |
| Ripe pulp | 13.18 | 0.001 | 0.86 | 0.14 |
| Structural compositiona | ||||
| Leaf | 4.51 | 0.001 | 0.67 | 0.33 |
| Seed | 6.58 | 0.001 | 0.75 | 0.25 |
| Unripe pulp | 7.90 | 0.001 | 0.79 | 0.21 |
| Ripe pulp | 10.70 | 0.001 | 0.83 | 0.17 |
aSeparate sets of PERMANOVAs were conducted for each aspect of compound composition: compound occurrences (presence/absence) and structural composition.
bStatistical results from permutation tests showing strong support for an effect of Piper species on composition.
cProportion of sample-to-sample variance explained by species (i.e., interspecific variation).
dProportion of sample-to-sample variance explained by individual and within-individual (residual) variance.
Discussion
Secondary metabolites occur in all plant parts, but theory in chemical ecology has largely focused on interactions between leaves and leaf herbivores. In this study, we surveyed and compared the compositional and structural diversity of secondary metabolites across vegetative and reproductive organs in 12 species of the genus Piper. Most metabolites were shared across organs, but fruits contained many more unique compounds than leaves. Furthermore, relative to leaves, fruits contained a higher total number of compounds (gamma diversity) and a higher sample-to-sample variance in composition (beta diversity). While Piper species varied in terms of whether fruits or leaves had a higher average compound richness per sample (alpha diversity), richness was more often higher in fruits than in leaves. Taken together, these patterns reveal that, in neotropical Piper, fruits are overall more chemically diverse than leaves and point to a key role for mutualistic and antagonistic fruit-frugivore interactions in shaping phytochemical evolution and diversity.
Our untargeted metabolomic survey of phytochemical occurrence patterns revealed that fruit organs harbor fruit-specific metabolites from a variety of chemical classes (Table 1 and Figure 2), in each class equal to or greater in number than those that were leaf-specific (Table 1). This included classes of compounds that have previously been found to be more numerous and abundant in fruit organs (e.g., amides; Whitehead et al., 2013), as well as numerous chemical classes previously described in studies of Piper spp. leaf chemistry (Parmar et al., 1997; Baldoqui et al., 1999; Kato and Furlan, 2007; Richards et al., 2015). The occurrence of numerous fruit-specific secondary metabolites from a variety of unlinked biosynthetic pathways suggests a pattern of fruit-specific secondary metabolite trait evolution, likely a result of fruit-specific selective pressures.
The evolution of organ-specific phytochemical traits across our target plant species is also made evident by the results of our machine learning analysis. Here, our random forest model was very successful at distinguishing among organ types based solely on their secondary metabolite composition. Most notably, the exceptional performance of the classification algorithm to distinguish between vegetative and reproductive organs can only be explained by the presence of strong association between chemical composition and organ type. Despite the fact that our species set included vines, understory shrubs, and pioneering taxa, all adapted to very different local habitats, these associations are consistent across all 12 focal species.
The clustering patterns found by our NMDS analysis (Figure 3) show clustering at two different levels. First, the samples from different organs from the same species cluster together. This pattern strongly suggests the presence of physiological or genetic linkage constraints in the organ specific evolution of phytochemicals. The strong chemical similarity across organs within a species suggests that changes in the expression or composition of secondary compounds in one plant organ could influence the expression or composition of other organs. Although our data do not allow us to disentangle the precise mechanisms that give rise to these patterns, it is clear that chemical changes in one plant organ are likely to be mirrored, to some extent, by changes in the chemical architecture of the whole plant. Second, as expected, and despite the strong chemical similarity exhibited by organs within a Piper species, samples also show a clear pattern of clustering by organ type (Figure 3). This pattern reinforces the expectation that the distinctive regimes of selective pressures imposed upon the different plant organs are sufficiently strong to create convergent organ-specific patterns of the chemical composition, and that these selective regimes are likely to be consistent across species and habitats. The Boruta variable importance model, a widely used machine learning algorithm designed to identify statistically important classification variables from large datasets, revealed specific compounds from at least six different chemical classes as key features that distinguish vegetative and reproductive organs (Supplementary Table 1).
While the majority of significant Boruta variables, like the overall majority of secondary metabolites cataloged in our study, exhibited some overlap in occurrence across leaf and fruit organs when the 12 Piper species were evaluated as a group (Figure 1A), there was substantially less overlap at the level of individual species (Supplementary Figure 1). In many cases, these patterns of variance were the result of numerous compounds occurring in only one organ type in a certain species or subset of species, but occurring more widely in another species or subset of species.
The broad overlap across organs in compound occurrence at the genus level provides a degree of insight into the extent of constraints on organ-specific chemical trait evolution at this taxonomic scale. However, to a degree this overlap can also be attributed to the shared demand for defensive compounds across vegetative and reproductive organ types. While phylogenetic data will be required in order to infer the ancestral organ localizations of phytochemical traits of Piper, the widespread variation in organ localizations that we observed across species suggests that genetic constraints have not bound these traits to a certain organ type over the course of Piper speciation. Further, the apparent mobility of secondary metabolite traits across organ types within the genus suggests a bidirectional exchange of these traits, which, when vegetative and reproductive organs are each threatened by separate assemblages of consumers, may allow more rapid defense trait adaptation than can arise from novel mutations.
Our untargeted metabolomic survey has shown that fruits are at the very least a reservoir of phytochemical richness. While the alpha diversity of organs at the species level was highly variable (Figure 4), rarefaction analysis of gamma diversity showed a small but clear trend toward higher richness of secondary metabolites in reproductive organs (Figure 1B and Table 3). Similarly, while chemical structural complexity of organs at the species level was highly variable (Figure 5), chemical structural variance (beta-diversity) across species was significantly higher for reproductive organs than for leaves (Figure 6). In summary, these trends indicate not only that reproductive organs accumulate a higher number of secondary metabolite traits than do leaves, but also that these traits are more divergent from one another across species than those of leaves. These trends are consistent with higher overall evolutionary diversification of phytochemical traits in reproductive organs, suggesting that fruits may be an important, but underappreciated, force in shaping chemical trait evolution at the whole plant level.
Data Availability Statement
The UPLC-MS data, chromatograms, peak lists, molecular network and associated library and in silico classification results, and associated programming scripts are available at 10.5281/zenodo.5142600 and 10.5281/zenodo.5171397. The UPLC-fragmentation data, peak lists, and associated programming scripts are available at 10.25345/C5C80P.
Author Contributions
GS, SW, and DS designed the research. SW collected field samples. GS, SH, and RH conducted chemical analyses. GS conducted molecular networking and data curation. GS and SW conducted the statistical analysis with contributions from DS. GS wrote the first draft of the manuscript with contributions from SW. All authors contributed substantially to revisions and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Organization for Tropical Studies for logistical support at La Selva Biological Station. Marisol Luna Martinez assisted with sample collection and Orlando Vargas Ramírez assisted with plant identification. Natalie Rodeman and Katherine Berg assisted with sample processing for chemical analyses. José Juan Ordaz-Ortiz and Zhentian Lei provided helpful comments on the manuscript.
Footnotes
Funding. This research was supported by the National Science Foundation (Grants Nos. DEB-1210884 and DEB-1856776 to SW) and start-up funds to SW from the Virginia Tech Department of Biological Sciences. The mass spectrometry resources used in this work were maintained with funds from the Fralin Life Science Institute as well as the Virginia Agricultural Experiment Station Hatch Program (Grant No. VA-160085). Open-access publication was supported by the Open Access Subvention Fund at Virginia Tech and the Open Access Funding Initiative at Utah State University.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.693739/full#supplementary-material
References
- Adler L. S., Seifert M. G., Wink M., Morse G. E., Turlings T. (2012). Reliance on pollinators predicts defensive chemistry across tobacco species. Ecol. Lett. 15 1140–1148. 10.1111/j.1461-0248.2012.01838.x [DOI] [PubMed] [Google Scholar]
- Adler L. S., Wink M., Distl M., Lentz A. J. (2006). Leaf herbivory and nutrients increase nectar alkaloids. Ecol. Lett. 9 960–967. 10.1111/j.1461-0248.2006.00944.x [DOI] [PubMed] [Google Scholar]
- Aron A. T., Gentry E. C., McPhail K. L., Nothias L. F., Nothias-Esposito M., Bouslimani A., et al. (2020). Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat. Protoc. 15 1954–1991. [DOI] [PubMed] [Google Scholar]
- Baldoqui D. C., Kato M. J., Cavalheiro A. J., Bolzani V. D. S., Young M. C. M., Furlan M. (1999). A chromene and prenylated benzoic acid from Piper aduncum. Phytochemistry 51 899–902. 10.1016/s0031-9422(99)00132-6 [DOI] [PubMed] [Google Scholar]
- Benton H. P., Want E. J., Ebbels T. M. D. (2010). Correction of mass calibration gaps in liquid chromatography-mass spectrometry metabolomics data. Bioinformatics 26:2488. 10.1093/bioinformatics/btq441 [DOI] [PubMed] [Google Scholar]
- Berardi A. E., Hildreth S. B., Helm R. F., Winkel B. S. J., Smith S. D. (2016). Evolutionary correlations in flavonoid production across flowers and leaves in the Iochrominae (Solanaceae). Phytochemistry 130 119–127. 10.1016/j.phytochem.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Brehm G., Hebert P. D. N., Colwell R. K., Adams M. O., Bodner F., Friedemann K., et al. (2016). Turning up the heat at a hotspot: DNA barcodes reveal 80% more species of geometrid moths along an Andean elevational gradient. PloS One 11:e0150327. 10.1371/journal.pone.0150327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. (2001). Random forests. Mach. Learn. 45 5–32. [Google Scholar]
- Breiman L. (2002). Manual on Setting up, Using, and Understanding Random Forests v3. 1. Available online at: www.stat.berkeley.edu/~breiman/Using_random_forests_V3.1.pdf (Accessed January 20, 2021) [Google Scholar]
- Cech N. B., Enke C. G. (2001). Practical implications of some recent studies in electrospray ionization fundamentals. Mass Spectrom. Rev. 20 362–387. 10.1002/mas.10008 [DOI] [PubMed] [Google Scholar]
- Chen H., Köllner T. G., Li G., Wei G., Chen X., Zeng D., et al. (2020). Combinatorial evolution of a terpene synthase gene cluster explains terpene variations in Oryza. Plant Physiol. 182 480–492. 10.1104/pp.19.00948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarucci A., Bacaro G., Rocchini D., Ricotta C., Palmer M., Scheiner S. (2009). Spatially constrained rarefaction: incorporating the autocorrelated structure of biological communities into sample-based rarefaction. Commun. Ecol. 10 209–214. 10.1556/comec.10.2009.2.11 [DOI] [Google Scholar]
- Christian N., Sedio B. E., Florez-Buitrago X., Ramírez-Camejo L. A., Rojas E. I., Mejía L. C., et al. (2020). Host affinity of endophytic fungi and the potential for reciprocal interactions involving host secondary chemistry. Am. J. Bot. 107 219–228. 10.1002/ajb2.1436 [DOI] [PubMed] [Google Scholar]
- Cipollini M. L., Paulk E., Mink K., Vaughn K., Fischer T. (2004). Defense tradeoffs in fleshy fruits: effects of resource variation on growth, reproduction, and fruit secondary chemistry in Solanum carolinense. J. Chem. Ecol. 30, 1––17. 10.1023/B:JOEC.0000013179.45661.68 [DOI] [PubMed] [Google Scholar]
- Dyer L. A., Palmer A. D. N. (2004). Piper: a Model Genus for Studies of Phytochemistry, Ecology, and Evolution. Amsterdam: Kluwer Academic/ Plenum Publishers. [Google Scholar]
- Falara V., Akhtar T. A., Nguyen T. T. H., Spyropoulou E. A., Bleeker P. M., Schauvinhold I., et al. (2011). The tomato terpene synthase gene family. Plant Physiol. 157 770–789. 10.1104/pp.111.179648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry A. H. (1990). Four Neotropical Rainforests. New Haven, CT: Yale University Press. [Google Scholar]
- Gotelli N. J., Colwell R. K. (2011). “Estimating species richness,” in Biological Diversity: Frontiers in Measurement and Assessment, eds Magurran A. E., McGill B. J. (Oxford, UK: Oxford University Press; ), 39–54. [Google Scholar]
- Greig N. (1993a). Predispersal seed predation on five Piper species in tropical rain-forest. Oecologia 93 412–420. 10.1007/bf00317886 [DOI] [PubMed] [Google Scholar]
- Greig N. (1993b). Regeneration mode in neotropical Piper: habitat and species comparisons. Ecology 74 2125–2135. 10.2307/1940857 [DOI] [Google Scholar]
- Hartmann T. (2007). From waste products to ecochemicals: fifty years research of plant secondary metabolism. Phytochemistry 68 2831–2846. 10.1016/j.phytochem.2007.09.017 [DOI] [PubMed] [Google Scholar]
- Herrera C. M. (1982). Defense of ripe fruit from pests: its significance in relation to plant-disperser interactions. Am. Nat. 120 218–241. 10.1086/283984 [DOI] [Google Scholar]
- Iason G. R., Dicke M., Hartley S. E. (2012). The Ecology of Plant Secondary Metabolites: from Genes to Global Processes. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Kato M. J., Furlan M. (2007). Chemistry and evolution of the Piperaceae. Pure Appl. Chem. 79 529–538. 10.1351/pac200779040529 [DOI] [Google Scholar]
- Keith R. A., Mitchell-Olds T. (2019). Antagonistic selection and pleiotropy constrain the evolution of plant chemical defenses. Evolution 73 947–960. 10.1111/evo.13728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A., Baldwin I. T. (2002). Plant responses to insect herbivory: the emerging molecular analysis. Annu. Rev. Plant Biol. 53 299–328. [DOI] [PubMed] [Google Scholar]
- Kessler A., Halitschke R. (2009). Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: predictions and case study. Funct. Ecol. 23 901–912. 10.1111/j.1365-2435.2009.01639.x [DOI] [Google Scholar]
- Kuhl C., Tautenhahn R., Boettcher C., Larson T. R., Neumann S. (2012). CAMERA: an integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Anal. Chem. 84 283–289. 10.1021/ac202450g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursa M. B., Rudnicki W. R. (2010). Feature selection with the Boruta package. J. Stat. Softw. 36 1–13. [Google Scholar]
- Kursar T. A., Dexter K. G., Lokvam J., Pennington R. T., Richardson J. E., Weber M. G., et al. (2009). The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proc. Natl. Acad. Sci. U.S.A. 106 18073–18078. 10.1073/pnas.0904786106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw A., Wiener M. (2002). Classification and regression by randomForest. R News 2 18–22. [Google Scholar]
- Martinez Arbizu P. (2020). pairwiseAdonis: Pairwise Multilevel Comparison Using Adonis. R Package Version 0.4. Available online at: https://github.com/pmartinezarbizu/pairwiseAdonis. (Accessed January 9, 2021). [Google Scholar]
- Maynard L. D., Slinn H. L., Glassmire A. E., Matarrita-Carranza B., Dodson C. D., Nguyen T. T., et al. (2020). Secondary metabolites in a neotropical shrub: spatiotemporal allocation and role in fruit defense and dispersal. Ecology 101:e03192. [DOI] [PubMed] [Google Scholar]
- Oksanen J., Blanchet F. G., Friendly M., Kindt R., Legendre P., McGlinn D., et al. (2019). vegan: Community Ecology Package. R Package Version 2.5-6. Available online at: https://CRAN.R-project.org/package=vegan. (Accessed January 9, 2021). [Google Scholar]
- Parmar V. S., Jain S. C., Bisht K. S., Jain R., Taneja P., Jha A., et al. (1997). Phytochemistry of the genus Piper. Phytochemistry 46 597–673. [Google Scholar]
- Quijano-Abril M. A., Callejas-Posada R., Miranda-Esquivel D. R. (2006). Areas of endemism and distribution patterns for Neotropical Piper species (Piperaceae). J. Biogeogr. 33, 1266––1278. 10.1111/j.1365-2699.2006.01501.x [DOI] [Google Scholar]
- Richards L. A., Dyer L. A., Forister M. L., Smilanich A. M., Dodson C. D., Leonard M. D., et al. (2015). Phytochemical diversity drives plant–insect community diversity. Proc. Natl. Acad. Sci. U.S.A. 112 10973–10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricigliano V. A., Sica V. P., Knowles S. L., Diette N., Howarth D. G., Oberlies N. H. (2020). Bioactive diterpenoid metabolism and cytotoxic activities of genetically transformed Euphorbia lathyris roots. Phytochemistry 179:112504. 10.1016/j.phytochem.2020.112504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar D., Jaramillo M. A., Marquis R. J. (2016). Chemical similarity and local community assembly in the species rich tropical genus Piper. Ecology 97 3176–3183. 10.1002/ecy.1536 [DOI] [PubMed] [Google Scholar]
- Salazar D., Lokvam J., Mesones I., Pilco M. V., Milagros J., Zuñiga A., et al. (2018). Origin and maintenance of chemical diversity in a species-rich tropical tree lineage. Nat. Ecol. Evol. 2 983–990. 10.1038/s41559-018-0552-0 [DOI] [PubMed] [Google Scholar]
- Schneider G. F., Coley P. D., Younkin G. C., Forrister D. L., Mills A. G., Kursar T. A. (2019). Phenolics lie at the centre of functional versatility in the responses of two phytochemically diverse tropical trees to canopy thinning. J. Exp. Bot. 70 5853–5864. 10.1093/jxb/erz308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedio B. E., Echeverri J. C. R., Boya C. A., Wright S. J. (2017). Sources of variation in foliar secondary chemistry in a tropical forest tree community. Ecology 98 616–623. 10.1002/ecy.1689 [DOI] [PubMed] [Google Scholar]
- Smith C. A., Want E. J., O’Maille G., Abagyan R., Siuzdak G. (2006). XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching and identification. Anal. Chem. 78 779–787. [DOI] [PubMed] [Google Scholar]
- Sumner L. W., Amberg A., Barrett D., Beale M. H., Beger R., Daykin C. A., et al. (2007). Proposed minimum reporting standards for chemical analysis. Metabolomics 3 211–221. 10.1007/s11306-007-0082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Iwai K. (1984). “Constituents of red Pepper species: chemistry, biochemistry, pharmacology, and food science of the pungent principle of Capsicum species,” in The Alkaloids, Vol. 23 ed. Brossi A. (Cambridge, MA: Academic Press; ), 227–299. 10.1016/s0099-9598(08)60072-3 [DOI] [Google Scholar]
- Tautenhahn R., Boettcher C., Neumann S. (2008). Highly sensitive feature detection for high resolution LC/MS. BMC Bioinformatics 9:504. 10.1186/1471-2105-9-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewksbury J. J. (2002). Fruits, frugivores and the evolutionary arms race. New Phytol. 156 137–139. 10.1046/j.1469-8137.2002.00522.x [DOI] [PubMed] [Google Scholar]
- Tewksbury J. J., Nabhan G. P. (2001). Seed dispersal: directed deterrence by capsaicin in chillies. Nature 412 403–404. 10.1038/35086653 [DOI] [PubMed] [Google Scholar]
- Tewksbury J. J., Reagan K. M., Machnicki N. J., Carlo T. A., Haak D. C., Peñaloza A. L. C., et al. (2008). Evolutionary ecology of pungency in wild chilies. Proc. Natl. Acad. Sci. U.S.A. 105 11808–11811. 10.1073/pnas.0802691105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies W., Kalko E. K. V. (2004). Phenology of Neotropical Pepper plants (Piperaceae) and their association with their main dispersers, two short-tailed fruit bats, Carollia perspicillata and C. castanea (Phyllostomidae). Oikos 104 362–376. 10.1111/j.0030-1299.2004.12747.x [DOI] [Google Scholar]
- Volf M., Segar S. T., Miller S. E., Isua B., Sisol M., Aubona G., et al. (2018). Community structure of insect herbivores is driven by conservatism, escalation and divergence of defensive traits in Ficus. Ecol. Lett. 21 83–92. 10.1111/ele.12875 [DOI] [PubMed] [Google Scholar]
- Wang M., Carver J. J., Phelan V. V., Sanchez L. M., Garg N., Peng Y., et al. (2016). Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 34 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel W. C., Whitehead S. R. (2020). The many dimensions of phytochemical diversity: linking theory to practice. Ecol. Lett. 23 16–32. 10.1111/ele.13422 [DOI] [PubMed] [Google Scholar]
- Whitehead S. R., Bowers M. D. (2013a). Evidence for the adaptive significance of secondary compounds in vertebrate-dispersed fruits. Am. Nat. 182 563–577. 10.1086/673258 [DOI] [PubMed] [Google Scholar]
- Whitehead S. R., Bowers M. D. (2013b). Iridoid and secoiridoid glycosides in a hybrid complex of bush honeysuckles (Lonicera spp., Caprifolicaceae): implications for evolutionary ecology and invasion biology. Phytochemistry 86 57–63. 10.1016/j.phytochem.2012.10.012 [DOI] [PubMed] [Google Scholar]
- Whitehead S. R., Bowers M. D. (2014). Chemical ecology of fruit defence: synergistic and antagonistic interactions among amides from Piper. Funct. Ecol. 28 1094–1106. 10.1111/1365-2435.12250 [DOI] [Google Scholar]
- Whitehead S. R., Jeffrey C. S., Leonard M. D., Dodson C. D., Dyer L. A., Bowers M. D. (2013). Patterns of secondary metabolite allocation to fruits and seeds in Piper reticulatum. J. Chem. Ecol. 39 1373–1384. 10.1007/s10886-013-0362-4 [DOI] [PubMed] [Google Scholar]
- Whitehead S. R., Quesada M. F. O., Bowers M. D. (2016). Chemical tradeoffs in seed dispersal: defensive metabolites in fruits deter consumption by mutualist bats. Oikos 125 927–937. 10.1111/oik.02210 [DOI] [Google Scholar]
- Whitehead S. R., Schneider G. F., Dybzinski R., Nelson A. S., Gelambi M., Jos E., et al. (2021). Fruits, frugivores, and the evolution of phytochemical diversity. Oikos 10.1111/oik.08332 [DOI] [Google Scholar]
- Wittstock U., Gershenzon J. (2002). Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 5 300–307. 10.1016/s1369-5266(02)00264-9 [DOI] [PubMed] [Google Scholar]
- Xu S., Kreitzer C., McGale E., Lackus N. D., Guo H., Köllner T. G., et al. (2020). Allelic differences of clustered terpene synthases contribute to correlated intraspecific variation of floral and herbivory-induced volatiles in a wild tobacco. New Phytol. 228 1083–1096. 10.1111/nph.16739 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The UPLC-MS data, chromatograms, peak lists, molecular network and associated library and in silico classification results, and associated programming scripts are available at 10.5281/zenodo.5142600 and 10.5281/zenodo.5171397. The UPLC-fragmentation data, peak lists, and associated programming scripts are available at 10.25345/C5C80P.