Abstract
Mitogen-activated protein (MAP) kinases phosphorylate the estrogen receptor and activate transcription from estrogen receptor-regulated genes. Here we examine potential interactions between the MAP kinase cascade and androgen receptor-mediated gene regulation. Specifically, we have studied the biological effects of mitogen-activated protein kinase kinase kinase 1 (MEKK1) expression in prostate cancer cells. Our findings demonstrate that expression of constitutively active MEKK1 induces apoptosis in androgen receptor-positive but not in androgen receptor-negative prostate cancer cells. Reconstitution of the androgen receptor signaling pathway in androgen receptor-negative prostate cancer cells restores MEKK1-induced apoptosis. MEKK1 also stimulates the transcriptional activity of the androgen receptor in the presence or absence of ligand, whereas a dominant negative mutant of MEKK1 impairs activation of the androgen receptor by androgen. These studies demonstrate an unanticipated link between MEKK1 and hormone receptor signaling and have implications for the molecular basis of hormone-independent prostate cancer growth.
Steroid hormones play a critical role in the development and maintenance of multiple organs, including mammary glands (estrogens), the uterine lining (progesterone), and the adrenal medulla (glucocorticoids) (19, 26). In addition to responding to their ligands, steroid hormone receptors are modified by kinase signaling pathways which directly or indirectly alter the biological response to hormones (27). In the case of the androgen receptor, one model system for functional studies is the prostate gland. Prostate development is dependent on androgen, and normal prostate secretory epithelial cells undergo apoptosis in response to androgen withdrawal (9). Prostate cancer cells are also dependent on androgen for growth but eventually acquire the ability to proliferate in the absence of androgen in patients after prolonged anti-androgen drug therapy (2, 35). Although androgen independent, these cells continue to express androgen-responsive genes, indicating ligand-independent activation of the androgen receptor signaling pathway. Defining the mechanism for this conversion to androgen independence will have important implications in prostate cancer therapy (47).
A number of protein kinase signaling pathways have been implicated in androgen receptor signaling. Protein kinase A can activate androgen receptor-mediated gene transcription in the absence of androgen (23, 38). The protein kinase C activator and tumor promoter 12-O-tetradecanoylphorbol-13-acetate negatively regulates androgen receptor-mediated gene transcription through a presumed interaction of c-jun and androgen receptor (43). Epidermal growth factor (EGF), keratinocyte growth factor (KGF), or insulin-like growth factor 1 (IGF-1) can activate transcription from androgen receptor-regulated genes in prostate cancer cells (11, 42). Transgenic mice expressing KGF under the control of the hormone-responsive mouse mammary tumor virus promoter develop prostatic hyperplasia, suggesting that tonic exposure to certain growth factors results in dysregulated prostate growth in vivo (28). These reports establish that interactions between androgen receptor and non-steroid receptor signaling pathways exist, but the molecular details are unclear.
Because many of these growth factors activate the mitogen-activated protein (MAP) kinase pathway, we hypothesized that isolated activation of this pathway may affect androgen receptor-mediated gene regulation and the prostate cancer cell phenotype. In particular, we examined the effect of MAP kinase kinase kinase 1 (MEKK1) signaling in prostate cancer cells. Activation of MEKK1 results in the downstream activation of MKK4 (SEK1) and subsequently JNK (36), as well as phosphorylation of IκB kinase leading to the release of NF-κB (30, 37, 56). JNK activation is associated with diverse outcomes which vary in different cell types and in the presence of concurrent signals from other pathways. JNK activation is necessary for cellular transformation by the Bcr-Abl oncogene (14, 41) but is also associated with apoptosis in response to growth factor deprivation or withdrawal of extracellular matrix (anoikis) (5, 53). Constitutively active alleles of MEKK1 induce apoptosis in diverse cell types (25, 51). A model for MEKK1-mediated apoptosis has emerged in which genotoxic stress leads to phosphorylation and activation of MEKK1 followed by MEKK1-initiated cleavage of DEVD-directed caspases. MEKK1 is itself a target for cleavage by caspases, which leads to further activation of MEKK1 by removal of a negative regulatory domain (5, 50). Thus, MEKK1 participates in a caspase activation loop which requires both the kinase activity of MEKK1 as well as the caspase recognition site, permitting its cleavage by caspases.
Here we address the role of the MEKK pathway in prostate cancer. Our findings demonstrate that expression of constitutively active MEKK1 leads to apoptosis of androgen receptor-positive but not of androgen receptor-negative prostate cancer cells. Reconstitution of the androgen receptor pathway sensitizes prostate cancer cells to MEKK1-induced apoptosis. MEKK1 also activates androgen-regulated gene expression in an androgen receptor-dependent fashion. These data demonstrate cross-talk between the androgen receptor signaling pathway and MEKK1 that results in transcriptional regulation of androgen receptor-regulated genes and apoptosis.
MATERIALS AND METHODS
Cell culture and reagents.
LNCaP, PC3, and DU145 human prostate cells were obtained from the American Type Culture Collection and maintained in phenol red-free RPMI with 10% fetal calf serum (FCS) or 10% charcoal-stripped FCS (Gemini, Thousand Oaks, Calif.). LAPC4 cells were derived from a human prostate cancer xenograft implanted in SCID mice and express wild-type androgen receptor (exons 2 to 8) (29). LAPC4 cells were grown in Iscove’s medium with 10% FCS. R1881 was used as a synthetic androgen (DuPont-NEN), and Casodex was used as an androgen receptor antagonist (ICI, Cheshire, United Kingdom).
Plasmids, transfections, and retroviral infections.
The cDNAs for MEKKΔ (MEKK-dominant active or MEKKΔ-DA) and MEKKΔ(K432M) (MEKK-dominant negative or MEKKΔ-DN) (a kind gift of Michael Karin) were subcloned into pCDNA3 and the retroviral pSRαMSV-tkNeo vector (36). MEKKΔ is a truncated form of MEKK1 in which amino acids 1 to 351 have been deleted and MEKKΔ(K432M) contains a mutation in the ATP-binding site rendering it catalytically inactive. Cells were infected with amphotropically packaged retrovirus and selected in G418. Transient transfection of cells was performed by lipid-mediated gene transfer with Lipofectamine (Gibco-BRL) or TFX-50 (Promega, Madison, Wis.). Successful gene transfer was confirmed by cotransfection with a vector encoding enhanced green fluorescent protein (GFP; Clontech). 2X-TRE-luciferase was used to measure activator protein 1 activity. For androgen receptor-regulated gene transcription, a 600-bp fragment of the prostate-specific antigen (PSA) promoter with an additional 2.4-kb enhancer sequence cloned upstream of luciferase (PSA P/E-luc) was used (39). Additionally, an androgen-regulated reporter vector was created by multimerizing four consensus androgen receptor response elements from the PSA promoter (ARE-I) cloned upstream of the chloramphenicol acetyltransferase (CAT) gene in the pBXG0 vector and referred to as 4X-ARE/E4-CAT (a gift from Michael Carey). For the ZEBRA reporter experiments, pZRE-5/E4-CAT was used with pZEBRA driven by a simian virus 40 enhancer (31). Full-length wild-type androgen receptor was expressed by using a cytomegalovirus-driven plasmid expression vector (a gift of Marco Marcelli) (34). The plasmid pCDNA3-JBD was used to inhibit JNK1 activity. This construct contains the domain of JIP-1 that binds JNK-1 (JBD) cloned into pCDNA3 (14).
The protocol used for transfection of cell lines was as follows. Cells were plated at a density of 5 × 105 cells in a 60-mm-diameter dish on the day prior to transfection. In all cases, the total amount of transfected DNA was kept constant with control vector. For LNCaP and LAPC4 cells, TFX-50 (Promega) was used to transfect cells. A total of 4.4 μg of DNA and 20 μl of lipid reagent was added to the cells in Optimem (Gibco). After a 1-h incubation, medium containing 10% charcoal-stripped serum was added to the cells. For DU145, PC3 and 293T cells, Lipofectamine (Gibco) was used to transfect cells. A total of 4.4 μg of DNA and 18 μl of lipofectamine was added to the cells in Optimem. After a 5-h incubation, medium containing 10% charcoal-stripped serum was added to the cells. In some cases, androgen (R1881) or Casodex was added with the medium containing 10% charcoal-stripped serum. Luciferase assays, CAT assays, and apoptosis measurements were performed 48 h after transfection unless otherwise stated.
Reporter assays.
Luciferase activity was measured with a Luciferase Assay Kit (Promega). Cells were lysed in 100 μl of 1× lysis buffer, and 20 μl was used to react with luciferase substrate. Light units were measured with a luminometer. CAT activity was measured with a CAT enzyme-linked immunosorbent assay (ELISA) kit (Boehringer-Mannheim) or by conventional CAT assay as previously described (41). Samples were analyzed by thin-layer chromatography and exposed to a Storm phosphorimager screen. Radioactivity was quantitated by using ImageQuant software.
Kinase assays and Western blots.
JNK, ERK, and p38 kinase activity were measured as previously described (41). Briefly, equal numbers of cells were lysed in radioimmunoprecipitation assay buffer and JNK1 (sc474; Santa Cruz), ERK1/2 (Zymed), or p38 (sc535-G; Santa Cruz) was immunoprecipitated with antibodies as indicated. Immunoprecipitates were reacted with the substrates glutathione S-transferase (GST)-c-jun (1-79), myelin basic protein, or GST-ATF-2, respectively, in the presence of [γ-32P]ATP and analyzed by sodium dodecyl sulfate (SDS)–10% polyacrylamide gel electrophoresis (PAGE). In indicated cases, FLAG-tagged JNK1 was immunoprecipitated with anti-FLAG-conjugated beads (Sigma) and reacted with GST-jun as described above. For MEKK Western blots, anti-MEKK1 was used at 0.5 μg/ml (sc252; Santa Cruz) with anti-rabbit horseradish peroxidase secondary (Jackson Laboratories). For androgen receptor Western blots, whole-cell lysates were analyzed by 8% PAGE and reacted with rabbit anti-human androgen receptor antibody (N-20, sc816; Santa Cruz) used at a 1:500 dilution.
Apoptosis assays.
Apoptosis was detected morphologically by using acridine orange or transfected GFP. A fluorescent microscope was used to count 200 fluorescent cells per condition, and the percentage of blebbing cells was calculated. Cells were scored by an investigator blinded to the experimental condition. DNA staining of cells was performed with Hoechst 33258. At 48 h after transient transfection, cells were rinsed with phosphate-buffered saline, fixed with paraformaldehyde 4% for 15 min, permeabilized with Triton X-100 0.5%, and then stained in the dark with Hoechst dye at 2.5 μg/ml (53). Chromatin condensation was used as an additional morphologic marker of apoptosis in cells cotransfected with GFP.
Statistical analysis.
Statistical analysis was performed by parametric analysis using the paired Student t test and Microsoft Excel.
RESULTS
Expression of activated MEKK1 induces apoptosis in androgen receptor-positive but not in androgen receptor-negative prostate cancer cells.
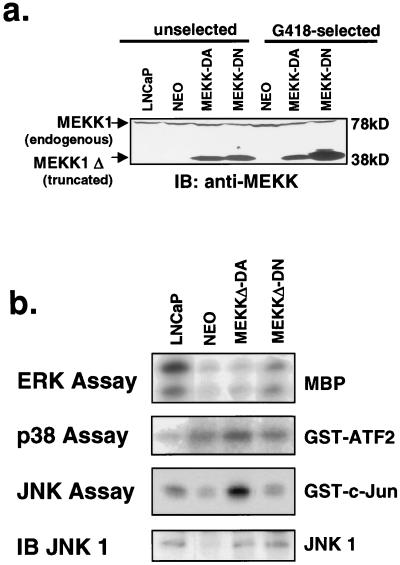
The androgen receptor-positive prostate cancer cell line LNCaP is a well-characterized model for the study of androgen receptor-mediated growth and signal transduction (32, 48). We examined the role of the stress-activated MAP kinase signaling pathway in LNCaP cells by utilizing retroviruses expressing a truncated, constitutively active form of MEKK1 (MEKKΔ-DA) and a catalytically inactive mutant containing a point mutation in the ATP binding site (MEKKΔ-DN) (36). At 48 h after infection with retrovirus, MEKKΔ-DA- and MEKKΔ-DN-infected cells expressed similar levels of the truncated MEKK1 protein (Fig. 1a). Biochemical characterization of LNCaP cells stably expressing MEKKΔ-DA demonstrated selective activation of the JNK pathway (sixfold) over parental cells and minimal p38 (twofold) or ERK activation (Fig. 1b). After antibiotic selection, populations of cells stably expressing MEKKΔ-DA were derived. In five independent experiments, these cells consistently demonstrated reduced MEKKΔ-DA protein expression compared with MEKKΔ-DN (Fig. 1a). These data suggest that high-level expression of MEKKΔ-DA is not well tolerated in LNCaP cells, as reported previously in fibroblasts (25, 51). To look directly for effects on growth, the LNCaP sublines were plated at equal densities, and cells were counted after 5 days in culture. In five independent experiments in which mass populations of cells were selected, LNCaP cells expressing MEKKΔ-DA were difficult to expand compared with Neo control cells or MEKKΔ-DN-expressing cells (Fig. 2b). These findings demonstrate that the expression of MEKKΔ-DA impairs the expansion of LNCaP cells in vitro.
FIG. 1.
Biochemical characterization of LNCaP cells stably expressing mutant MEKK1. (a) MEKK1 immunoblot blot of LNCaP cells before and after G418 selection. LNCaP cells were infected with retrovirus pSRαMEKKΔ-DA or pSRαMEKKΔ-DN or Neo control virus. Whole-cell lysates were prepared from cells on the day after retroviral infection prior to G418 selection (unselected) or 2 weeks after G418 selection (G418-selected). The expression of truncated MEKK1 protein is similar in cells infected with pSRαMEKKΔ-DA or pSRαMEKK-DN immediately after infection, suggesting similar viral titers. After G418 selection, however, surviving cells express lower amounts of MEKKΔ-DA. Full-length MEKK1 is a 190-kDa protein not shown on this blot. This C-terminal-directed antibody recognizes a cleaved form of endogenous MEKK1 which runs at approximately 78-kDa and is the same in all lanes (5, 56). Equal protein loading was confirmed by protein assay and Ponceau S staining. (b) In vitro kinase assays of LNCaP sublines stably expressing MEKK isoforms as indicated. Cells expressing MEKKΔ-DA show approximately sixfold activation of JNK activity compared with control cells but only twofold activation of p38 kinase activity. A JNK1 immunoblot demonstrates the relative amounts of immunoprecipitated JNK1 in the different sublines.
FIG. 2.
Effect of stable expression of MEKKΔ-DA in prostate cancer cell lines. (a) MEKK immunoblot of DU145 and PC3 cells after infection with pSRαMEKKΔ-DA retrovirus or Neo control virus. Whole-cell lysates were prepared from cells 2 weeks after G418 selection. Equal protein loading was confirmed by protein assay and Ponceau S staining. (b) Change in cell number of prostate cancer cell lines stably expressing MEKKΔ-DA. After antibiotic selection, DU145, PC3, and LNCaP sublines were plated at 100,000 cells per 60-mm-diameter plate, and the cell numbers were calculated after 5 days in culture. Data are expressed as the percentage of cells on day 5 in the sublines (Neo or MEKKΔ-DA) compared with the parental line. Experiments were performed in duplicate, and this is one representative of three independently derived stable cell lines. (c) Cell cycle analysis of LNCaP cells stably expressing mutant MEKK isoforms. Subconfluent LNCaP cells growing in 10% FCS were permeabilized, stained with propidium iodide, and analyzed on a Becton Dickinson flow cytometer. There are no differences between MEKKΔ-DA-expressing cells and Neo control cells with regard to G1, S, and G2 peaks.
To determine whether MEKKΔ-DA functioned similarly in other prostate cancer cell lines, we extended our analysis to DU145 and PC3 cells, which differ from LNCaP because they do not express androgen receptor and do not require androgen for growth. DU145 and PC3 cells were infected with retrovirus expressing MEKKΔ-DA or Neo control, and sublines which expressed MEKKΔ-DA were derived by antibiotic selection (Fig. 2a). Unlike LNCaP cells, there was no difficulty in expanding MEKKΔ-DA-expressing DU145 and PC3 cells. To assess the effect of MEKKΔ-DA on PC3 and DU145 growth, each subline was plated at an equal density, and cell counts were determined after 5 days and compared to Neo control sublines. In contrast to LNCaP cells, MEKKΔ-DA expression did not impair the growth of PC3 or DU145 cells in three experiments with independently selected sublines (Fig. 2c). We then asked whether our difficulty in expanding the LNCaP cells which stably express MEKKΔ-DA was due to cell cycle arrest or an increase in cell death. Cell cycle analysis of propidium iodide-stained MEKKΔ-DA cells showed no differences in the percentage of cells in G1, S, or G2 compared with the Neo control (Fig. 2c). However, when the morphology of the cells was examined after staining with acridine orange, we noted changes in LNCaP cells stably expressing MEKKΔ-DA, such as cytoplasmic blebbing and detachment, that are suggestive of apoptosis.
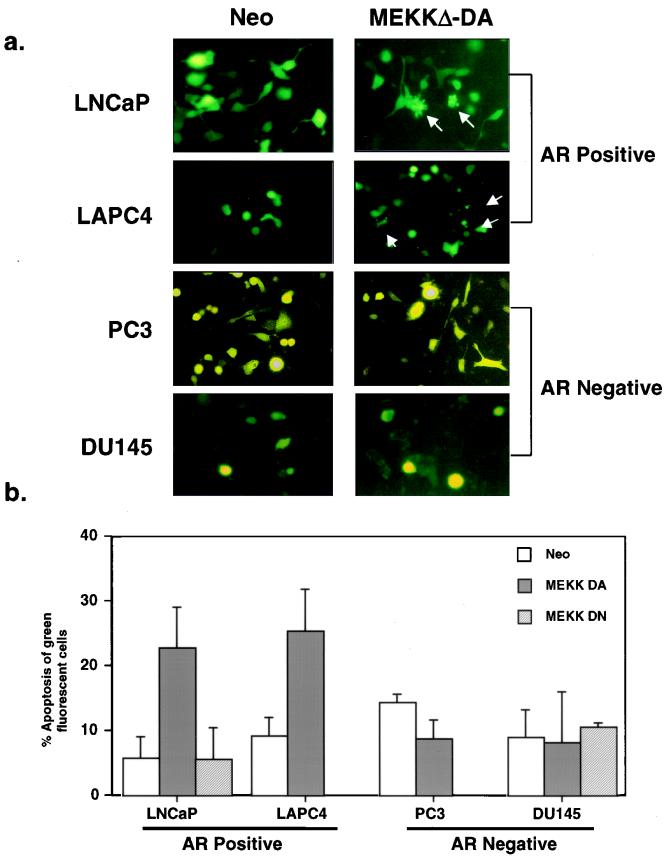
To determine whether MEKKΔ-DA induces apoptosis in LNCaP cells, a quantitative, short-term transient-transfection assay was utilized. LNCaP cells were transiently cotransfected with MEKKΔ-DA and a vector expressing GFP to visualize the morphology of the transfected cells. Approximately 25% of GFP-positive cells cotransfected with MEKKΔ-DA showed cytoplasmic blebbing, a morphologic feature of apoptosis, whereas GFP-positive cells cotransfected with control vector or kinase-inactive MEKKΔ-DN did not (Fig. 3). Our conclusion that MEKK1 induces apoptosis was confirmed independently by the demonstration of chromatin condensation in a high fraction of GFP-positive cells in plates transfected with MEKKΔ-DA but not with the control Neo vector (Fig. 3c). We conclude that the difficulty in expanding LNCaP cells expressing MEKKΔ-DA is most likely a result of the induction of apoptosis, a finding similar to those of earlier studies with fibroblasts and T cells (16, 25).
FIG. 3.
Effect of transient MEKKΔ-DA expression on apoptosis in androgen receptor-negative and androgen receptor-positive prostate cancer cell lines. (a) LNCaP cells were transiently transfected with GFP (0.4 μg) and cotransfected with Neo or MEKKΔ-DA (0.6 μg), and the total amount of transfected DNA (4 μg) was kept constant with Neo control vector. DU145, PC3, and LAPC4 cells were transfected with 3.6 μg of pCDNA3 Neo or MEKKΔ-DA and cotransfected with GFP (0.4 μg). Apoptotic cells demonstrate cytoplasmic blebbing (arrows). Cells were scored for apoptosis 48 h after transfection. The transfection efficiencies for each cell line are as follows: LNCaP Neo, 40 to 50%; MEKKΔ-DA, 40 to 50%; DU145 Neo, 20 to 30%; MEKKΔ-DA, 20 to 30%; PC3 Neo, 30 to 40%; MEKKΔ-DA, 30 to 40%; LAPC4 Neo, 20 to 30%; MEKKΔ-DA, 20 to 30%. (b) Graph represents three independent experiments in which 200 green fluorescent cells were counted and scored for cytoplasmic blebbing 48 h after transfection. For LNCaP cells, these experiments were also performed with transfected kinase-inactive MEKKΔ-DN (3 μg) which did not induce apoptosis. (c) LNCaP cells were transiently transfected with GFP (0.4 μg) and cotransfected with Neo or MEKKΔ-DA (0.6 μg), and the total amount of transfected DNA (4 μg) was kept constant with Neo control vector. DU145 cells were transfected with 3.6 μg of pCDNA3 Neo or MEKKΔ-DA and cotransfected with GFP (0.4 μg). At 48 h after transfection, cells were stained with the DNA dye Hoechst 33258, and GFP-positive cells were scored for chromatin condensation. There was no increase in chromatin condensation in DU145 cells transfected with Neo or MEKKΔ-DA. White arrows indicate GFP-positive cells, and yellow arrows indicate GFP-positive cells showing chromatin condensation.
Next, we analyzed the effect of MEKKΔ-DA expression on apoptosis in androgen receptor-negative DU145 cells and PC3 cells by using the transient-cotransfection assay described above. In contrast to LNCaP cells, there was no increase in the morphologic features of apoptosis in DU145 cells or PC3 cells expressing MEKKΔ-DA at 48 h after transfection (Fig. 3). We extended the analysis in PC3 and DU145 cells to 72 and 96 h after transient transfection with MEKKΔ-DA to look for delayed effects on apoptosis, but we continued to find no differences in apoptosis between Neo- and MEKKΔ-DA-transfected cells (data not shown). These data demonstrate that the ability of MEKKΔ-DA to impair growth or induce apoptosis is restricted to certain prostate cancer cell lines.
Because MEKKΔ-DA-induced apoptosis occurred in the androgen receptor-positive LNCaP cell line but not in two androgen receptor-negative prostate cell lines, we analyzed the effect of MEKKΔ-DA in another model of androgen receptor-positive prostate cancer developed in our laboratory (29). LAPC4 cells express wild-type androgen receptor (exons 2 to 8) and secrete PSA. Similar to LNCaP, transient transfection of MEKKΔ-DA induced apoptosis in LAPC4 cells (Fig. 3). These data suggest that androgen receptor-positive prostate cancer cells are sensitive to MEKKΔ-DA-induced apoptosis, whereas androgen receptor-negative cells are not.
MEKKΔ-DA-induced apoptosis is JNK independent but caspase dependent.
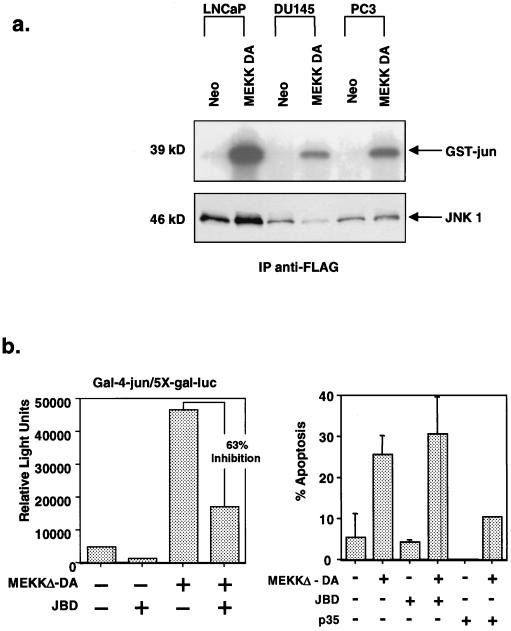
One reason for the failure of DU145 and PC3 cells to undergo apoptosis may be a defect in the ability of MEKKΔ-DA to activate the JNK pathway in these cells. To address this question, we tested the ability of MEKKΔ-DA to activate JNK and AP-1 transcriptional activity in androgen receptor-positive and androgen receptor-negative cell lines. To allow for differences in transfection efficiencies between prostate cancer cell lines, we transfected LNCaP, DU145, and PC3 cells with MEKKΔ-DA and FLAG-tagged JNK1 and performed an in vitro kinase assay with anti-FLAG immunoprecipitated JNK1 (Fig. 4a). As expected, an anti-FLAG immunoblot showed different levels of immunoprecipitated FLAG-JNK1 protein from the three cell lines, a finding consistent with distinct transfection efficiencies. However, JNK was activated four- to sixfold by MEKKΔ-DA in all three cell lines when adjusted to the level of immunoprecipitated JNK protein. Cotransfection of the 2X-TRE-luciferase reporter construct revealed similar findings of AP-1 activation in response to the transfection of MEKKΔ-DA in all three cell lines (data not shown). These data demonstrate that MEKKΔ-DA is capable of JNK activation in prostate cancer cell lines regardless of their sensitivity to MEKKΔ-DA-induced apoptosis.
FIG. 4.
Role of JNK activation in MEKKΔ-DA-induced apoptosis. (a) Comparison of JNK activation in prostate cancer cell lines in response to MEKKΔ-DA. LNCaP, DU145, and PC3 cells were transfected with FLAG-tagged JNK1 (2 μg) and cotransfected with Neo or MEKKΔ-DA (2 μg). The top panel shows a JNK assay in which 100 μg of total cellular protein was immunoprecipitated with anti-FLAG antibody and reacted with GST–c-jun. The bottom panel shows an anti-FLAG immunoblot. LNCaP cells have approximately sixfold-higher amount of transfected JNK1 than DU145 cells as determined by densitometry analysis. When corrected for this difference in transfected protein, MEKKΔ-DA-induced JNK activation is approximately four- to sixfold in all three cell lines. (b) Effect of JNK inhibition on MEKKΔ-DA-induced apoptosis in LNCaP cells. LNCaP cells were cotransfected with MEKKΔ-DA (0.6 μg) or Neo and pCDNA3-JBD (JNK1 inhibitor), p35 (caspase inhibitor), or vector control. (Left panel) Effect of transfected JBD on MEKKΔ-DA-induced c-jun transcriptional activity as measured by a 5X-Gal-luciferase reporter (0.4 μg) and gal4-jun (0.4 μg). (Right panel) Transfected cells were scored for apoptosis 48 h after transfection.
To directly test the role of the JNK pathway in MEKK-mediated apoptosis in androgen receptor-positive cell lines, we examined the effects of JNK inhibition in the transient-transfection assay. LNCaP cells were cotransfected with MEKKΔ-DA and JBD, a truncated form of JIP-1, a selective inhibitor of JNK1 (14). As suspected from related studies in other cell types, JBD inhibited MEKKΔ-DA-mediated activation of jun as measured by a gal4-jun reporter system, thus confirming the activity of JBD in LNCaP cells. However, JBD failed to block MEKKΔ-DA-mediated apoptosis, whereas cotransfection of the baculovirus-derived caspase inhibitor p35 did (57) (Fig. 4b). Taken together, these data indicate that MEKKΔ-DA-induced apoptosis is JNK independent but caspase dependent. This conclusion is in agreement with recent studies of MEKK function in fibroblasts (25, 51).
Modulation of androgen receptor function influences the sensitivity of MEKKΔ-DA-induced apoptosis.
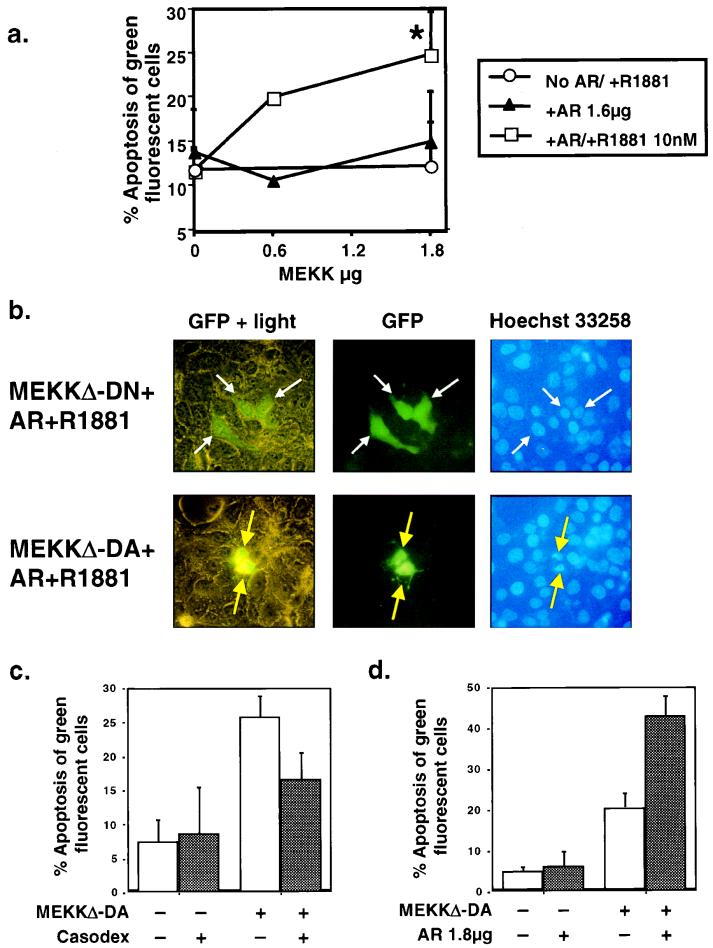
A major difference between the prostate cancer cell lines sensitive to MEKK1-induced apoptosis and those resistant to MEKK1-induced apoptosis is the presence of a functional androgen receptor pathway. The LNCaP and LAPC4 prostate cancer cell lines express the androgen receptor, whereas DU145 and PC3 do not. Based on these observations, we hypothesized that the androgen receptor pathway may be required for MEKK1-induced apoptosis in prostate cancer cells. We used three approaches to test this hypothesis: reconstitution of the androgen receptor pathway in androgen receptor-negative cells, pharmacologic inhibition of the androgen receptor pathway in androgen receptor-positive cells, and amplification of androgen receptor signaling in androgen receptor-positive cells. First, we reconstituted the androgen receptor pathway in DU145 cells by transfecting androgen receptor and treating the cells with androgen. Expression of wild-type androgen receptor with or without androgen did not induce significant levels of apoptosis (Fig. 5a). Expression of MEKKΔ-DA with the androgen receptor in the absence of ligand also did not result in apoptosis. However, the combination of MEKKΔ-DA, androgen receptor, and androgen did induce apoptosis in a dose-response manner, as increasing doses of MEKKΔ-DA induced more apoptosis with a fixed amount of androgen receptor (Fig. 5a). Kinase-inactive MEKKΔ-DN failed to induce apoptosis in this assay, indicating that kinase activity is required (Fig. 5b). These data demonstrate that reconstitution of the androgen receptor pathway rescues the apoptosis defect in DU145 cells and support the hypothesis that the androgen receptor pathway is required for MEKKΔ-DA-induced apoptosis in prostate cancer cells. The fact that additional ligand is required for MEKKΔ-DA-induced apoptosis in DU145 cells but not LNCaP cells may be a consequence of androgen receptor overexpression or cell-type differences.
FIG. 5.
Modulation of androgen receptor function alters the sensitivity of prostate cancer cells to MEKKΔ-DA-induced apoptosis. Reconstitution of the androgen receptor signaling pathway in DU145 cells. DU145 cells were cotransfected with MEKKΔ-DA, as indicated, and androgen receptor (AR) (1.8 μg) in the presence or absence of androgen R1881 (10 nM). This graph is an average of the results from eight independent experiments. The P value for the combined experiments is 0.002 as determined by the paired Student t test for MEKKΔ-DA plus androgen receptor plus R1881 versus MEKKΔ-DA plus androgen receptor. (b) Morphology of DU145 reconstituted with androgen receptor and androgen R1881 and cotransfected with MEKKΔ-DN (top row) or MEKKΔ-DA (bottom row) 48 h after transfection. White arrows indicate GFP-positive cells, and yellow arrows indicate GFP-positive cells showing chromatin condensation. (c) Effect of the androgen receptor antagonist Casodex on MEKKΔ-DA-induced apoptosis. Graph of LNCaP transfected with 0.6 μg of MEKKΔ-DA or Neo control vector and treated with the androgen receptor antagonist Casodex (10 μM) as indicated. Graph represents results of four independent experiments in which 200 green fluorescent cells were counted and scored for cytoplasmic blebbing; P = 0.004 as determined by the paired Student t test for MEKKΔ-DA versus MEKKΔ-DA plus Casodex. Cells were scored for apoptosis at 48 h after transfection. (d) Graph of LNCaP transfected with 0.6 μg of pCDNA3 containing MEKKΔ-DA or the empty vector and cotransfected with androgen receptor (1.8 μg) as indicated. Graph represents three independent experiments in which 200 green fluorescent cells were counted and scored for cytoplasmic blebbing at 48 h after transfection; P = 0.01 as determined by the paired Student t test for MEKKΔ-DA versus MEKKΔ-DA plus androgen receptor.
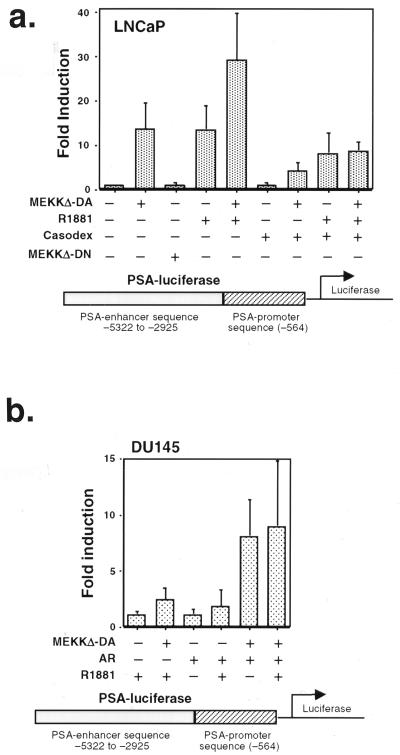
A corollary to the hypothesis that MEKKΔ-DA-induced apoptosis in prostate cancer cells requires functional androgen receptor is that blockade of androgen receptor signaling should protect against MEKKΔ-DA-induced apoptosis in androgen receptor-positive prostate cancer cells. We tested this hypothesis pharmacologically by using the androgen receptor antagonist casodex (49). To establish the activity of Casodex in our model, LNCaP cells were transfected with a reporter plasmid containing the promoter (P) and enhancer (E) of the androgen-dependent PSA gene fused to luciferase (PSA P/E-luc) (39). PSA is a prostate-specific, secreted kallikrein protein that is widely used as a serum marker to diagnose and monitor prostate cancer in patients (20). The promoter and enhancer both contain well-characterized androgen receptor binding sites which mediate androgen responsiveness (7, 44). Since the expression of PSA is androgen dependent, anti-androgen therapy causes a drop in PSA levels in serum, whereas relapse of androgen-independent cancer is heralded by a rise in PSA in serum. As expected, the androgen analog R1881 induced 13-fold activation of PSA P/E-luc in LNCaP cells (Fig. 6a) (39). Casodex partially inhibited PSA P/E-luc induction by ca. 40% (Fig. 6a, compare fourth and eighth columns). In the apoptosis experiments, the same concentration of Casodex partially inhibited MEKKΔ-DA-induced apoptosis by 40% and did not by itself induce apoptosis in parental LNCaP cells (Fig. 5c). The effect of Casodex was specific for androgen receptor-positive cells because Casodex had no effect on androgen receptor-independent, MEKKΔ-DA-mediated apoptosis of HEK293 cells (data not shown). Together with the androgen receptor reconstitution experiments, these data argue for a link between the androgen receptor and the MEKKΔ-DA pathway leading to apoptosis in prostate cancer cells.
FIG. 6.
MEKKΔ-DA increases the transcriptional activity of androgen receptor-regulated promoters. (a) Graph of PSA P/E-luc transcriptional activity in LNCaP cells. LNCaP cells were transfected with MEKKΔ-DA (0.6 μg) or MEKKΔ-DN or Neo control vector (3.6 μg) and cotransfected with a PSA P/E-luc reporter construct (0.4 μg). R1881 was added to a final concentration of 10 nM, and Casodex was added at a final concentration of 10 μM. This graph represents an average of six independent experiments; P = 0.009 as determined by the paired Student t test for MEKKΔ-DA compared to the control. (b) Graph of PSA P/E-luc transcriptional activity in DU145. DU145 were cotransfected with MEKKΔ-DA or Neo control vector (1.6 μg), androgen receptor (1.8 μg), and PSA-luc reporter (0.4 μg). R1881 was added to a final concentration of 10 nM. Luciferase activity was measured 48 h after transfection. This graph represents an average of six experiments; P = 0.01 for MEKKΔ-DA versus MEKKΔ-DA plus androgen receptor.
Since pharmacologic inhibition of androgen receptor function diminished MEKKΔ-DA-induced apoptosis, we reasoned that more androgen receptor expression in androgen receptor-positive cells may increase their sensitivity to MEKKΔ-DA-induced apoptosis. To test this hypothesis, LNCaP cells were transfected with wild-type androgen receptor in the presence or absence of MEKKΔ-DA (Fig. 5d). Overexpression of androgen receptor did not cause apoptosis above control levels. Coexpression of androgen receptor and MEKKΔ-DA induced apoptosis in over 40% of the cells, a significant increase compared to the expression of MEKKΔ-DA alone. These data indicate that overexpression of androgen receptor in cells with an intact androgen receptor pathway enhances MEKKΔ-DA-induced apoptosis.
One potential mechanism of MEKKΔ-DA-induced apoptosis in LNCaP cells is an alteration in the level of androgen receptor expression in androgen receptor-positive prostate cancer cells. To address this issue, androgen receptor expression was measured by immunoblot in LNCaP cells transfected with Neo or MEKKΔ-DA, LNCaP cells transfected with additional androgen receptor, and LNCaP cells stably infected with MEKKΔ-DA or control virus. No differences were seen in the endogenous expression of androgen receptor in LNCaP cells transiently or stably expressing MEKKΔ-DA (data not shown); therefore, MEKKΔ-DA does not regulate endogenous androgen receptor expression.
Activation of the MEKK1 pathway stimulates androgen-receptor regulated gene expression.
Activation of the tyrosine kinase receptors for KGF and IGF-1 or protein kinase A activation increases androgen receptor-mediated gene transcription in the absence of androgen, suggesting cross-talk with the androgen receptor pathway (11, 38). Because MEKKΔ-DA induces apoptosis in prostate cancer cells in an androgen receptor-dependent fashion, we hypothesized that MEKK1 signaling may also affect androgen receptor-mediated gene transcription. To test this hypothesis, LNCaP cells were transfected with the PSA P/E-luc reporter plasmid described above and cotransfected with MEKKΔ-DA in the presence or absence of androgen. Experiments were performed in medium containing charcoal-stripped serum to exclude potential effects of steroid hormones in FCS. MEKKΔ-DA activated the reporter 14-fold in the absence of androgen (Fig. 6a). Thus, the expression of MEKKΔ-DA results in androgen-independent PSA transcriptional activation that is similar in magnitude to the treatment of cells with androgen. The combination of MEKKΔ-DA and androgen led to further activation of PSA P/E-luc transcription (average fold induction of 30). To test whether transcriptional activation of PSA P/E-luc by MEKKΔ-DA required its kinase activity, LNCaP cells were transiently transfected with kinase inactive MEKKΔ-DN. MEKKΔ-DN had no effect on transcriptional activation, demonstrating that the kinase activity of MEKK1 is required for this effect.
We explored the role of the androgen receptor in MEKKΔ-DA induction of PSA transcriptional activity by using two complementary strategies. First, we asked if the androgen receptor antagonist Casodex inhibited MEKKΔ-DA activation of PSA P/E-luc in LNCaP cells. PSA P/E-luc activity in cells cotransfected with MEKKΔ-DA was reduced by Casodex from 14-fold to 4-fold (Fig. 6a, compare second and seventh columns). These results suggest that ligand-independent activation of the PSA promoter-enhancer by MEKKΔ-DA is mediated by the androgen receptor. To confirm this hypothesis we performed further experiments in androgen receptor-negative DU145 cells. The absence of endogenous androgen receptor expression in DU145 allowed us to study the effect of MEKKΔ-DA on the PSA promoter-enhancer in the presence or absence of transfected androgen receptor. Androgen induced activation of PSA P/E-luc a modest twofold when androgen receptor was included in the transfection (Fig. 6b), a result consistent with previous reports (55). Transfection of MEKKΔ-DA in the absence of androgen receptor also activated PSA P/E-luc twofold. However, the combination of androgen receptor and MEKKΔ-DA resulted in an average eightfold activation which was not enhanced further by androgen. In conjunction with the Casodex experiments, these data indicate that the effect of MEKKΔ-DA on PSA transcriptional activity requires the androgen receptor.
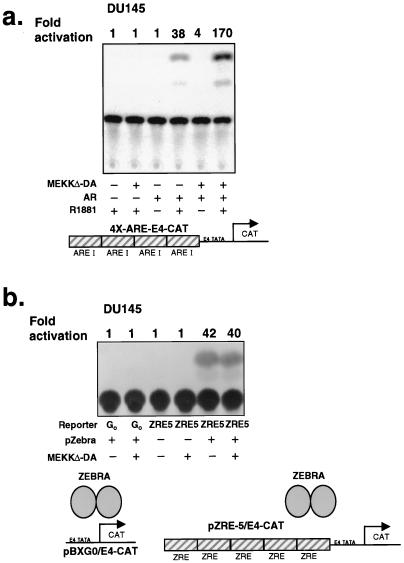
In addition to androgen receptor binding sites (AREs), the PSA promoter contains other transcription factor binding motifs, such as AP-1 recognition sites (46). Since MEKKΔ-DA activates transcription factors such as AP-1, a potential mechanism for cross-talk between MEKKΔ-DA signaling and the androgen receptor pathway is through cooperative effects between AP-1 sites and AREs in the PSA promoter and enhancer (8). Alternatively, MEKKΔ-DA-mediated induction of the PSA promoter may function solely through activation of the androgen receptor. We addressed this issue by examining the effect of MEKKΔ-DA on an artificial promoter consisting of four AREs multimerized upstream of the E4-CAT reporter gene (4X-ARE/E4-CAT) in DU145 cells. In the absence of transfected androgen receptor, neither MEKKΔ-DA nor androgen activated the 4X-ARE/E4-CAT reporter (Fig. 7a). Androgen activated the 4X-ARE/E4-CAT reporter 38-fold after reconstitution with androgen receptor. Cotransfection of androgen receptor and MEKKΔ-DA enhanced activation of the reporter from 38-fold to 170-fold in the presence of ligand. These effects are specific to AREs because MEKKΔ-DA had no effect on the parental E4-CAT reporter pBXG0, which lacks the AREs (Fig. 7b, lanes 1 and 2) or on the pZRE5-E4-CAT reporter in which the ARE sites were replaced with sites for the Epstein-Barr virus (EBV) transcription factor ZEBRA (Fig. 7b, lanes 3 and 4). These data indicate that the effect of MEKKΔ-DA on androgen receptor-mediated gene activation can be mediated through AREs in the absence of AP-1 sites. In contrast to the ligand-independent effects of MEKKΔ-DA in the context of the natural PSA promoter, ligand binding of androgen to androgen receptor is required to mediate the effect of MEKKΔ-DA on an artificial template containing only AREs. These differences may be a consequence of additional, ARE-independent effects of the PSA promoter. Alternatively, the effects of MEKK1 on these reporters, as well as apoptosis, may not be strictly correlated.
FIG. 7.
MEKKΔ-DA specifically increases the transcriptional activity of androgen receptor on a minimal promoter element. (a) Effect of MEKKΔ-DA on transcriptional activation of a promoter consisting of pure androgen response elements in DU145. A promoter consisting of four multimerized androgen response elements, 4X-ARE/E4-CAT (0.4 μg) was transfected into DU145 as in Fig. 6b. CAT production was analyzed by ELISA as described in Materials and Methods and by conventional CAT assay. ImageQuant software was used to analyze phosphorimager data for the conventional CAT assay. This is one representative experiment of four total. (b) Effect of MEKKΔ-DA on the transcriptional activation of a promoter consisting of ZEBRA response elements in DU145 cells. For these experiments, DU145 stably expressing androgen receptor or Neo-infected cells were transfected with the vectors as indicated: 0.8 μg of reporter plasmid, 0.8 μg of ZEBRA transcription factor, and 2.4 μg of MEKKΔ-DA or Neo vector control. The data shown were obtained with androgen receptor-expressing DU145 cells.
One potential explanation for the enhanced transcriptional activation of androgen receptor-regulated genes by MEKKΔ-DA is that MEKKΔ-DA is having nonspecific effects on the general transcription machinery. To test this possibility, we examined the effects of MEKKΔ-DA on another reporter system based on the EBV-derived transcription factor ZEBRA. This system is ideal for addressing the specificity of MEKKΔ-DA-induced transcriptional activation because the relationship between ZEBRA, its binding to core promoter elements, and the activation of the general transcription machinery have been carefully characterized (31). If MEKKΔ-DA acts nonspecifically, we would expect enhanced activation of pZRE5-E4-CAT in the presence of MEKKΔ-DA. However, MEKKΔ-DA had no effect on ZEBRA-mediated induction of pZRE5-E4-CAT (Fig. 7b, lanes 5 and 6). These data argue for specificity in the effects of MEKKΔ-DA on androgen receptor-mediated transcription.
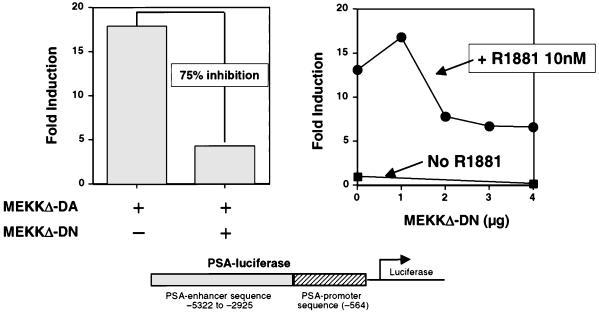
Based on our finding that MEKKΔ-DA-induced apoptosis of prostate cancer cells is dependent on androgen receptor signaling and that MEKKΔ-DA activates androgen receptor-dependent transcription, we sought to determine whether the MEKK signaling pathway plays a role in ligand-mediated activation of the androgen receptor in prostate cells. To test this possibility, we measured the effects of the dominant negative mutant, MEKKΔ-DN, on androgen-regulated gene expression (36). As expected, MEKKΔ-DA activated the PSA P/E-luc reporter in LNCaP cells. To validate the ability of MEKKΔ-DN to function as an MEKK antagonist, LNCaP cells were cotransfected with MEKKΔ-DA and MEKKΔ-DN (Fig. 8, left panel). MEKKΔ-DN inhibited MEKKΔ-DA-induced transcriptional activation of the PSA P/E-luc reporter between 50 and 75%. We then tested the ability of MEKKΔ-DN to inhibit androgen-mediated PSA P/E-luc activation. In four independent experiments, MEKKΔ-DN inhibited R1881-induced transcriptional activation of the PSA P/E-luc reporter in a dose-dependent fashion (Fig. 8, right panel). When similar experiments were performed with the 4X-ARE-CAT reporter in DU145 cells, we failed to see significant effects of MEKKΔ-DN on R1881-mediated activation of this reporter (data not shown). Therefore, the inhibitory effects of MEKKΔ-DN on the PSA P/E-luc reporter may be related to cis-acting elements which influence the outcome of androgen receptor activation in the context of a natural promoter.
FIG. 8.
Expression of MEKKΔ-DN inhibits androgen-mediated activation of PSA P/E-luc. (Left panel) Effect of MEKKΔ-DN on MEKKΔ-DA-induced PSA P/E-luc activity. LNCaP cells were transfected with MEKKΔ-DA (0.6 μg) and cotransfected with 3.6 μg (6:1 ratio) of MEKKΔ-DN as indicated. This is one representative experiment of three total, all with similar results. (Right panel) Effect of increasing amounts of MEKKΔ-DN on R1881-induced PSA P/E-luc activity. LNCaP cells were transfected with increasing amounts of MEKKΔ-DN as indicated in the presence of R1881 (10 nM). This is one representative experiment of four total, all with similar results.
DISCUSSION
Previous work on MEKK1 function has defined a role for this pathway in signaling involving the stress response (6, 13), NF-κB activation (30, 56), and integrin receptor engagement (5, 18). Results presented here provide evidence of a role in androgen receptor signaling in prostate cells. At a transcriptional level a constitutively active allele of MEKK1 stimulates natural and artificial androgen-responsive promoter templates in an androgen receptor-dependent fashion. In addition, transcriptional activation of the androgen receptor by androgen is impaired when a dominant negative mutant of MEKK1 is coexpressed. Taken together, these results suggest that the MEKK1 pathway plays a role in modulating the transcriptional response of the androgen receptor to ligands. Importantly, this cross-talk extends beyond the level of transcription to the biological response of cells to MEKK1 signaling. Consistent with previous reports in fibroblasts and T cells (16, 25), constitutive activation of MEKK1 induces apoptosis in prostate cancer cells. However, the apoptotic effect in prostate cells occurs only when the androgen receptor signaling pathway is intact. The evidence supporting this conclusion are the correlation of MEKKΔ-DA-induced apoptosis with androgen receptor expression, the ability of androgen receptor expression to restore the ability of MEKKΔ-DA to induce apoptosis in androgen receptor-negative prostate cancer cells, the potentiation of MEKKΔ-DA-induced apoptosis by overexpression of androgen receptor in androgen receptor-positive prostate cancer cells, and the partial inhibition of MEKKΔ-DA-induced apoptosis by androgen receptor blockade. In summary, our results establish a pattern of cross-talk between the MEKK1 and the androgen receptor pathways in prostate cells at a transcriptional and biological level.
The discovery of an interaction between the androgen receptor and MEKK1 signaling pathways adds to growing evidence that a number of different tyrosine and serine-threonine kinases can affect the function of steroid hormone receptors (4, 10, 11, 27). The molecular basis for each distinct example of cross-talk remains unknown and is the focus of much current research. A better understanding of this mechanism is likely to have important implications for hormone receptor regulation in cancer cells. In the case of MEKK1, its large size (196 kDa) and known ability to assemble in multiprotein complexes (12, 30, 56), as well as to interact with an array of signaling proteins (15, 54, 56), raise the possibility of a multiprotein signaling complex involving the androgen receptor in prostate cells. Alternatively, MEKK1 may activate a signaling cascade that indirectly leads to posttranslational modifications of the androgen receptor which affect its function, a possibility analogous to reported effects of the ERK pathway on the estrogen receptor (4, 24, 27, 58). It is also possible that MEKK1 affects coactivators, such as ARA-70 and GRIP-1 (22, 55), rather than androgen receptor itself or that it functions through transcription factors, such as c-jun (3, 43, 52), which act cooperatively with the androgen receptor to facilitate gene expression. More research is needed to sort through these various models.
MEKK1-induced apoptosis is known to occur in non-androgen-receptor-expressing cells such as fibroblasts (25), human embryonal kidney cells, and fibrosarcoma cells (51). In some settings, UV irradiation, chemotherapy, or tumor necrosis factor α are required to elicit the apoptotic phenotype, suggesting that MEKK1-induced apoptosis may require additional signals to initiate the apoptotic cascade. Our results would argue that androgen receptor signaling may be such a signal in prostate cells. This idea may seem paradoxical since androgen confers a survival and/or proliferative signal in prostate secretory epithelial cells. However, excess androgen receptor signaling in certain settings is detrimental to cell growth and survival. For example, androgen inhibits the growth of androgen receptor-positive LNCaP cells at high concentrations in vitro (48), and androgen receptor-negative PC3 cells transfected with a constitutively active androgen receptor have delayed growth compared with mock-transfected cells (33). Consistent with these reports, we find that excess androgen induces low levels of apoptosis in LNCaP cells in vitro (1). We hypothesize that excess stimulation of the androgen receptor signaling pathway, through MEKK1 activation or excess androgen, can lead to apoptosis of prostate cancer cells. This scenario is consistent with more extensively characterized signaling molecules such as the glucocorticoid receptor (17, 21) and c-Myc (1a, 45), which can induce either cell cycle progression or apoptosis in distinct cellular or environmental contexts.
In addition to the implications for hormone receptor signaling, our results offer potential insight into the mechanisms of prostate cancer progression. Anti-androgen therapy is the primary clinical treatment of metastatic prostate cancer and induces temporary remissions in the majority of patients. Eventually, prostate cancer cells regrow despite anti-androgen therapy, and the majority continue to express androgen receptor (40) and androgen-regulated genes such as PSA. This phenotype suggests that alternative, androgen-independent signaling pathways are utilized to activate the androgen receptor in these cells. Our observation that MEKK1 can substitute for androgen in androgen receptor-dependent transcription raises the possibility that this pathway may function in the progression to androgen independence. Further experiments with animal models and clinical material are required to address this hypothesis. Alternatively, the androgen receptor-dependent apoptotic function of activated MEKK1 in prostate cells might provide a therapeutic opportunity in androgen-independent prostate cancers. Because of its ability to sensitize cells to genotoxic stress (25, 51), expression of MEKK1 may be considered a strategy for cancer gene therapy.
ACKNOWLEDGMENTS
We thank Michael Carey and Yuriy Shostak for assistance and reagents used to perform 4X-ARE/E4-CAT experiments and the ZEBRA reporter assay. We thank David Chang for use of a fluorescent microscope and helpful discussions. We thank Michael Karin, Marco Marcelli, and Arie Belldegrun for providing necessary plasmids.
This work was supported by grants from the James S. McDonnell Foundation, the Margaret Early Trust, and CapCURE. M.T.A.-M. was supported by a Crohn’s and Colitis Foundation of America Career Development Award. A.C. was supported by a Howard Hughes Medical Institute Medical Student Research Fellowship.
REFERENCES
- 1.Abreu-Martin, M. T., and C. L. Sawyers. Unpublished data.
- 1a.Amati B, Land H. Myc-Max-Mad: a transcription factor network controlling cell cycle progression, differentiation and death. Curr Opin Genet Dev. 1994;4:102–108. doi: 10.1016/0959-437x(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 2.Brandstrom A, Westin P, Bergh A, Cajander S, Damber J E. Castration induces apoptosis in the ventral prostate but not in an androgen-sensitive prostatic adenocarcinoma in the rat. Cancer Res. 1994;54:3594–3601. [PubMed] [Google Scholar]
- 3.Bubulya A, Wise S C, Shen X Q, Burmeister L A, Shemshedini L. c-Jun can mediate androgen receptor-induced transactivation. J Biol Chem. 1996;271:24583–24589. doi: 10.1074/jbc.271.40.24583. [DOI] [PubMed] [Google Scholar]
- 4.Bunone G, Briand P A, Miksicek R J, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 5.Cardone M H, Salvesen G S, Widmann C, Johnson G, Frisch S M. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 1997;90:315–323. doi: 10.1016/s0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y R, Wang X, Templeton D, Davis R J, Tan T H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 7.Cleutjens K B, van der Korput H A, van Eekelen C C, van Rooij H C J, Faber P W, Trapman J. An androgen response element in a far upstream enhancer region is essential for high, androgen-regulated activity of the prostate-specific antigen promoter. Mol Endocrinol. 1997;11:148–161. doi: 10.1210/mend.11.2.9883. [DOI] [PubMed] [Google Scholar]
- 8.Cleutjens K B, van Eekelen C C, van der Korput H A, Brinkmann A O, Trapman J. Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J Biol Chem. 1996;271:6379–6388. doi: 10.1074/jbc.271.11.6379. [DOI] [PubMed] [Google Scholar]
- 9.Colombel M, Gil Diez S, Radvanyi F, Buttyan R, Thiery J P, Chopin D. Apoptosis in prostate cancer. Molecular basis to study hormone refractory mechanisms. Ann N Y Acad Sci. 1996;784:63–69. doi: 10.1111/j.1749-6632.1996.tb16228.x. [DOI] [PubMed] [Google Scholar]
- 10.Craft N, Shostak Y, Carey M, Sawyers C L. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 11.Culig Z, Hobisch A, Cronauer M V, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 12.Deak J C, Cross J V, Lewis M, Qian Y, Parrott L A, Distelhorst C W, Templeton D J. Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc Natl Acad Sci USA. 1998;95:5595–5600. doi: 10.1073/pnas.95.10.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 14.Dickens M, Rogers J S, Cavanagh J, Raitano A, Xia Z, Halpern J R, Greenberg M E, Sawyers C L, Davis R J. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 15.Fanger G R, Widmann C, Porter A C, Sather S, Johnson G L, Vaillancourt R R. 14-3-3 proteins interact with specific MEK kinases. J Biol Chem. 1998;273:3476–3483. doi: 10.1074/jbc.273.6.3476. [DOI] [PubMed] [Google Scholar]
- 16.Faris M, Kokot N, Latinis K, Kasibhatla S, Green D R, Koretzky G A, Nel A. The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by upregulating Fas ligand expression. J Immunol. 1998;160:134–144. [PubMed] [Google Scholar]
- 17.Feng Z, Marti A, Jehn B, Altermatt H J, Chicaiza G, Jaggi R. Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. J Cell Biol. 1995;131:1095–1103. doi: 10.1083/jcb.131.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisch S M, Vuori K, Kelaita D, Sicks S. A role for Jun-N-terminal kinase in anoikis; suppression by bcl-2 and crmA. J Cell Biol. 1996;135:1377–1382. doi: 10.1083/jcb.135.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadducci A, Genazzani A R. Steroid hormones in endometrial and breast cancer. Eur J Gynaecol Oncol. 1997;18:371–378. . (Review.) [PubMed] [Google Scholar]
- 20.Garnick M, Fair W. Prostate cancer: emerging concepts. Part II. Ann Intern Med. 1996;125:205–212. doi: 10.7326/0003-4819-125-3-199608010-00009. [DOI] [PubMed] [Google Scholar]
- 21.Helmberg A, Auphan N, Caelles C, Karin M. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J. 1995;14:452–460. doi: 10.1002/j.1460-2075.1995.tb07021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong H, Kohli K, Trivedi A, Johnson D L, Stallcup M R. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikonen T, Palvimo J J, Kallio P J, Reinikainen P, Janne O A. Stimulation of androgen-regulated transactivation by modulators of protein phosphorylation. Endocrinology. 1994;135:1359–1366. doi: 10.1210/endo.135.4.7925097. [DOI] [PubMed] [Google Scholar]
- 24.Jenster G, de Ruiter P E, van der Korput H A, Kuiper G G, Trapman J, Brinkmann A O. Changes in the abundance of androgen receptor isotypes: effects of ligand treatment, glutamine-stretch variation, and mutation of putative phosphorylation sites. Biochemistry. 1994;33:14064–14072. doi: 10.1021/bi00251a015. [DOI] [PubMed] [Google Scholar]
- 25.Johnson N L, Gardner A M, Diener K M, Lange-Carter C A, Gleavy J, Jarpe M B, Minden A, Karin M, Zon L I, Johnson G L. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J Biol Chem. 1996;271:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- 26.Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell. 1998;93:487–490. doi: 10.1016/s0092-8674(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 27.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 28.Kitsberg D I, Leder P. Keratinocyte growth factor induces mammary and prostatic hyperplasia and mammary adenocarcinoma in transgenic mice. Oncogene. 1996;13:2507–2515. [PubMed] [Google Scholar]
- 29.Klein K A, Reiter R E, Redula J, Moradi H, Zhu X L, Brothman A R, Lamb D J, Marcelli M, Belldegrun A, Witte O N, Sawyers C L. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3:402–408. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 30.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 31.Lehman A M, Ellwood K B, Middleton B E, Carey M. Compensatory energetic relationships between upstream activators and the RNA polymerase II general transcription machinery. J Biol Chem. 1998;273:932–939. doi: 10.1074/jbc.273.2.932. [DOI] [PubMed] [Google Scholar]
- 32.Lim D J, Liu X L, Sutkowski D M, Braun E J, Lee C, Kozlowski J M. Growth of an androgen-sensitive human prostate cancer cell line, LNCaP, in nude mice. Prostate. 1993;22:109–118. doi: 10.1002/pros.2990220203. [DOI] [PubMed] [Google Scholar]
- 33.Marcelli M, Haidacher S J, Plymate S R, Birnbaum R S. Altered growth and insulin-like growth factor-binding protein-3 production in PC3 prostate carcinoma cells stably transfected with a constitutively active androgen receptor complementary deoxyribonucleic acid. Endocrinology. 1995;136:1040–1048. doi: 10.1210/endo.136.3.7532576. [DOI] [PubMed] [Google Scholar]
- 34.Marcelli M, Tilley W, Wilson C, Griffin J, Wilson J, McPhaul M. Definition of the human androgen receptor gene structure permits the identification of mutations that cause androgen resistance: premature termination of the receptor protein at amino acid residue 588 causes complete androgen resistance. Mol Endocrinol. 1990;4:1105–1116. doi: 10.1210/mend-4-8-1105. [DOI] [PubMed] [Google Scholar]
- 35.McConkey D J, Greene G, Pettaway C A. Apoptosis resistance increases with metastatic potential in cells of the human LNCaP prostate carcinoma line. Cancer Res. 1996;56:5594–5599. [PubMed] [Google Scholar]
- 36.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 37.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nazareth L V, Weigel N L. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 39.Pang S, Dannull J, Kaboo R, Xie Y, Tso C-L, Michel K, deKernion J B, Belldegrun A S. Identification of a positive regulatory element responsible for tissue-specific expression of prostate-specific antigen. Cancer Res. 1997;57:495–499. [PubMed] [Google Scholar]
- 40.Prins G S, Sklarew R J, Pertschuk L P. Image analysis of androgen receptor immunostaining in prostate cancer accurately predicts response to hormonal therapy. J Urol. 1998;159:641–649. [PubMed] [Google Scholar]
- 41.Raitano A B, Halpern J R, Hambuch T M, Sawyers C L. The Bcr-Abl leukemia oncogene activates Jun kinase and requires Jun for transformation. Proc Natl Acad Sci USA. 1995;92:11746–11750. doi: 10.1073/pnas.92.25.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinikainen P, Palvimo J J, Janne O A. Effects of mitogens on androgen receptor-mediated transactivation. Endocrinology. 1996;137:4351–4357. doi: 10.1210/endo.137.10.8828495. [DOI] [PubMed] [Google Scholar]
- 43.Sato N, Sadar M D, Bruchovsky N, Saatcioglu F, Rennie P S, Sato S, Lange P H, Gleave M E. Androgenic induction of prostate-specific antigen gene is repressed by protein-protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP. J Biol Chem. 1997;272:17485–17494. doi: 10.1074/jbc.272.28.17485. [DOI] [PubMed] [Google Scholar]
- 44.Schurr E R, Henderson G A, Kmetec L A, Miller J D, Lamparski H G, Henderson D R. Prostate-specific antigen expression is regulated by an upstream enhancer. J Biol Chem. 1996;271:7043–7051. doi: 10.1074/jbc.271.12.7043. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y, Glynn J M, Guilbert L J, Cotter T G, Bissonnette R P, Green D R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992;257:212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- 46.Sun Z, Pan J, Balk S P. Androgen receptor-associated protein complex binds upstream of the androgen-responsive elements in the promoters of human prostate-specific antigen and kallikrein 2 genes. Nucleic Acids Res. 1997;25:3318–3325. doi: 10.1093/nar/25.16.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang D G, Porter A T. Target to apoptosis: a hopeful weapon for prostate cancer. Prostate. 1997;32:284–293. doi: 10.1002/(sici)1097-0045(19970901)32:4<284::aid-pros9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 48.van Steenbrugge G, van Uffelen C, Bolt J, Schroder F. The human prostatic cancer cell line LNCaP and its derived sublines: an in vitro model for the study of androgen sensitivity. J Steroid Biochem Mol Biol. 1991;40:207–214. doi: 10.1016/0960-0760(91)90184-7. [DOI] [PubMed] [Google Scholar]
- 49.Veldscholte J, Berrevoets C, Ris-Stalpers C, Kuiper G, Jenster G, Trapman J, Brinkmann A, Mulder E. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to anti-androgens. J Steroid Biochem Mol Biol. 1992;41:665–669. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 50.Widmann C, Gibson S, Johnson G L. Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J Biol Chem. 1998;273:7141–7147. doi: 10.1074/jbc.273.12.7141. [DOI] [PubMed] [Google Scholar]
- 51.Widmann C, Johnson N L, Gardner A M, Smith R J, Johnson G L. Potentiation of apoptosis by low dose stress stimuli in cells expressing activated MEK kinase 1. Oncogene. 1997;15:2439–2447. doi: 10.1038/sj.onc.1201421. [DOI] [PubMed] [Google Scholar]
- 52.Wise S C, Burmeister L A, Zhou X-F, Bubulya A, Oberfield J L, Birrer M J, Shemshedini L. Identification of domains of c-jun mediating androgen receptor transactivation. Oncogene. 1998;16:2001–2009. doi: 10.1038/sj.onc.1201697. [DOI] [PubMed] [Google Scholar]
- 53.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 54.Xu S, Cobb M H. MEKK1 binds directly to the c-Jun N-terminal kinases/stress-activated protein kinases. J Biol Chem. 1997;272:32056–32060. doi: 10.1074/jbc.272.51.32056. [DOI] [PubMed] [Google Scholar]
- 55.Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin M J, Christerson L B, Yamamoto Y, Kwak Y T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-I Tax protein binds to MEKK1 to stimulate IkappaB kinase activity and NF-kappaB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Q, Krebs J F, Snipas S J, Price A, Alnemri E S, Tomaselli K J, Salvesen G S. Interaction of the baculovirus anti-apoptotic protein p35 with caspases. Specificity, kinetics, and characterization of the caspase/p35 complex. Biochemistry. 1998;37:10757–10765. doi: 10.1021/bi980893w. [DOI] [PubMed] [Google Scholar]
- 58.Zhu X, Liu J P. Steroid-independent activation of androgen receptor in androgen-independent prostate cancer: a possible role for the MAP kinase signal transduction pathway? Mol Cell Endocrinol. 1997;134:9–14. doi: 10.1016/s0303-7207(97)00168-8. [DOI] [PubMed] [Google Scholar]