Abstract
Introduction
and importance: Pelvic osteosarcoma is quite rare and is a challenging task for orthopedic surgeons. This aim of this study is to present the first case report using customized 3D-printed prosthesis in Vietnam.
Case presentation
57-year-old male was diagnosed with pelvic osteosarcoma. After neoadjuvant chemotherapy, we did limb-salvage surgery after partial pelvic resection. He had to undergo another surgery due to an infection complication that exposed part of the prosthesis. At 6 months follow-up, the patient's overall status was stable. VAS score when moving is 2/10. He can walk with one crutch. Patient is still being followed up and treated.
Clinincal discussion
Management of pelvic osteosarcoma remains a challenging task for orthopedic surgeons. Advancements in customized 3D-printed prosthesis have been applied in treatment of pelvic osteosarcoma. Despite the complications, the results are promising. We believe that this is a new and innovative route in surgery of pelvic osteosarcoma.
Conclusion
Using customized 3D-printed prosthesis is a good way for management of pelvic osteosarcoma.
Keywords: Osteosarcoma, Bone tumor, 3D printed, Pelvic replacement, Case report
Highlight
-
•
Osteosarcoma of pelvis is not common, account for 4–10% of all bone cancer.
-
•
Pelvis connect the lower limb with body by hip joint and support the intraabdominal organ.
-
•
It is very difficult to preserve the pelvis after wide resection.
-
•
Custom made 3D printed implant give us a chance to restore the pelvis anatomically and functionally.
1. Introduction
Osteosarcoma of the pelvis accounts for 4–10% of all bone cancers and it is a highly malignant tumor. The percentage of lesions located in the ilium, the periacetabular area and the sacroiliac joint are 37%, 23%, and 21%, respectively. The others can be seen in the ischium, sacrum and pubis. The disease is more common in the below 25-year-old and above 50-year-old groups [1].
The most effective treatment of pelvic osteosarcoma is a combination of chemotherapy and wide resection of the tumor [3]. The overall 5-year survival rate of osteosarcoma is 70–80%, however, according to Fuchs et al. (2008), pelvic osteosarcoma has a 5-year survival rate of only 38% [2].
Post-operative reconstruction of hip defect has been discussed for a long time, mostly applied to type II tumor in Enneking and Dunham classification, common techniques of reconstruction are allograft prosthesis composites reconstruction, allograft bone graft, saddle hip prosthesis or hip arthrodesis, nonetheless complication rates are relatively high. For that reason, novel techniques are still in development, one of the promising approaches is 3D printing for custom made prosthetics that has brought some optimistic results.
In this report, we present a case of pelvic reconstruction with a custom made 3D-printed prosthesis including a part of the ilium and hip joint. This is the first megaprosthesis replacement of the pelvis in Vietnam. This case report has been reported is compliant with the SCARE Guidelines 2020 [21].
2. Case presentation
2.1. Case description
Patient was a 57-year-old male, chief complaint of a left hip pain that had persisted for 9 months. Patient was biopsied and diagnosed with left pelvic osteosarcoma in a National Cancer Hospital. He was then given 4 cycles of neoadjuvant chemotherapy with IPE regimen: ifosfamide, cisplatin, epirubicin. Post neoadjuvant therapy outcomes were positive: pain was reduced, hip ROM was increased and MRI showed a tumor reduced in size.
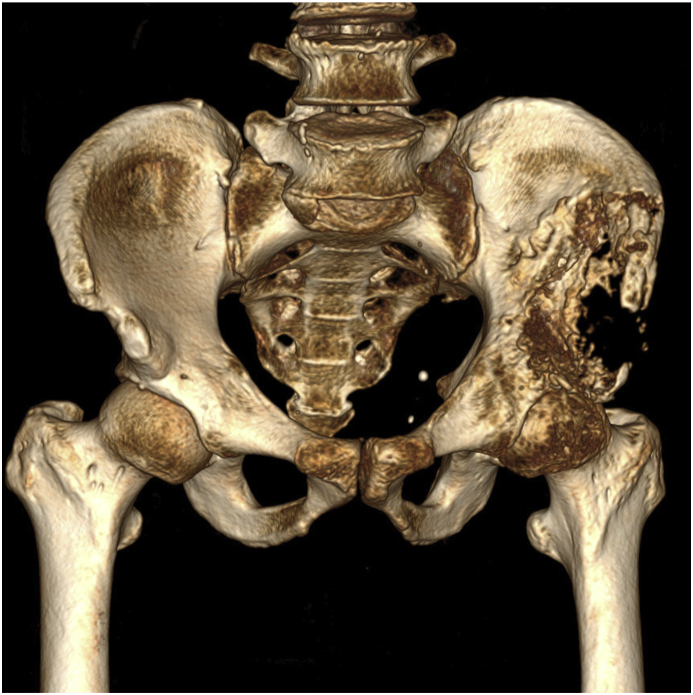
Patient had normal weight status (H:1.75 m, W:58Kg, BMI: 18,9Kg/m2). Clinical examination: there was no sign of systemic or local infection, patient was mild anemic, palpation showed a mid swollen, soft, immobile mass in the left ilium area, impinged hip movement. There were signs of femoral nerve compression, 3/5 quadriceps muscle strength, numbness on the anterolateral part of the thigh, normal hip passive ROM. Harris score was 27 which means poor hip function, patient was in wheelchair full time, his quality of life was affected significantly. Xray revealed an extensive destructive osteolytic in type 2 pelvic resection according to Enneking and Dunham (Fig. 1).
Fig. 1.
Pre-op pelvis in X Ray and CT.
CT and MRI results showed a 6 × 4x2cm tumor on patient's left ilium, sign of cortical destruction and muscles spread, angiogenesis in the center of the tumor, tumor had invaded part of the acetabular roof but not the femoral epiphysis and metaphysis. Further inspection on other organs showed no signs of metastasis (Fig. 2).
Fig. 2.
Pre - operation in CT 3D (mass 6 × 4x2cm in the left iliac wing, broke the shell and invaded the surrounding muscle mass. There is an increase in angiogenesis in the center of the tumor. Tumor partially invades into the acetabular roof).
2.2. Surgical planning and procedure
Using Radiant DICOM viewer (Medixant, Poland) from the patient's CT database, we planned the resection area of the tumor and acetabular positioning includes: acetabular diameter, inclination, anteversion, planned stem size and other variants. All data was sent to Chunli Zenda (Shanghai) for designing and manufacturing the custom-made 3D printed implant. From that design, we can plan the resection margins including the iliac crest (without the iliosacral joint); iliopubis ramus and ilioischial ramus margins (Fig. 3).
Fig. 3.
Planning resection area.
Patient was given general anesthesia and placed in a 45-degree left decubitus position and was secured to the operating table with back pads and girdle. We sanitized and prepped the patient's two legs and up to the xiphoid process. The chosen approach was the Mercedes Benz incision that combined the Smith-Petersen approach (to facilitate dissection to the ilium and the lesser pelvis) and Watson-Jones approach (to facilitate dissection to the hip joint and the ilioischial ramus).
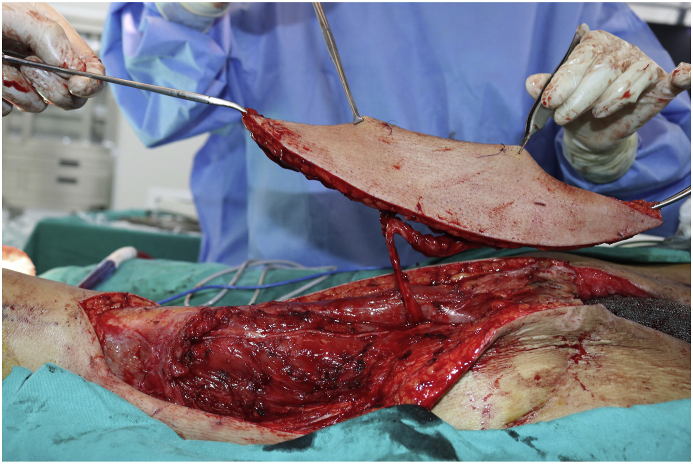
In tumor dissection phase, we dissected and exposed the tumor on the left iliac, overall assessment found a 8 × 6x4cm tumor which had invaded the roof of the acetabulum and groups of muscle attached to the pelvis (iliopsoas muscle, gluteus medius and gluteus minimus). After that, we continued dissecting the lateral part of the ilium, then the gluteus muscles were cut and coagulated 3cm from the tumor. We dissected and exposed the greater sciatic notch to identify and preserve the sciatic nerve, then we continued to the medial part of the iliac to expose and preserve the femoral neurovascular bundle. All of the iliacus was excised, and since the lateral femoral cutaneous nerve could not be separated from the tumor, it was resected as well.
In the stem placement phase, we performed a T-shaped capsulotomy to access the hip joint; the femoral neck was cut 1,5cm from the femoral saddle at 45-degree angle. Thorough inspection of the resected neck-head block found no sign of cancer invasion. The femoral canal was prepared and number 4 stem was inserted, we used Latitude cementless prosthesis from Meril (India).
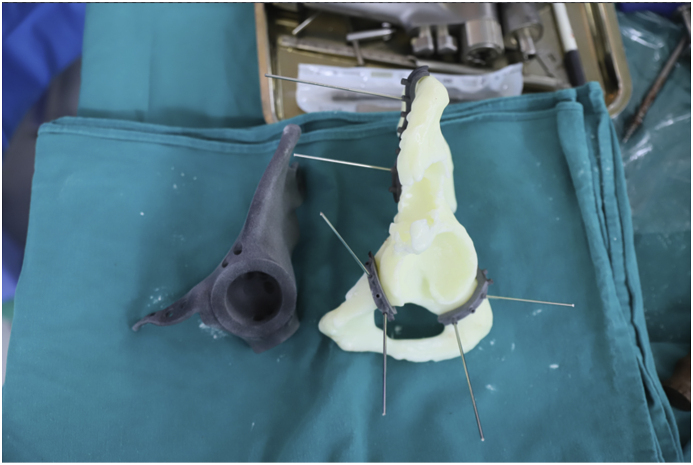
In tumor and pelvic resection phase, tumor and the affected pelvis (3 resection sites included the ilium, ischium and pubis) and surrounding tissue (gluteus medius and minimus, iliacus muscles) were resected en-bloc (Fig. 4). Custom made 3D printed saw guides were fixed to the resected margins on the pelvis and an oscillating saw was used to cut through bone. Frozen section of the resection margins was negative (Fig. 5).
Fig. 4.
Resected tumor with 3D model and implant.
Fig. 5.
Implant trial and 3D model with saw guides.
In the implant placement phase, the implant was designed and manufactured to fit in the pelvic defect, it was 3D-printed using titanium alloy with a rough surface, all the bone contact surfaces and the acetabulum were smooth with mounts and screw holes (Fig. 6). The acetabular size was 52mm, inclination and anteversion were 48° and 20°. Implant was secured to iliopubis ramus with a 4 holes mounting plate and to the ilioischial ramus and ilium with acetabular screws. A 52/32mm PE liner was placed, we used a 32mm offset +0 ceramic head, the hip joint was reduced and checked in all positions for stability and ROM. After that, the surgery field was irrigated carefully, two 400ml negative pressure drainages were placed. The joint capsule was sutured, the resected muscles was reattached with fiber wire suture to bone. The wound was closed anatomically.
Fig. 6.
Implant design.
Total surgery time was 380 mins, total blood loss was 1400ml. We had transfused 700ml of RBCs intraoperatively, post-operatively, and the patient was transfused with another 700ml of RBCs. There was no surgeon-induced fracture, the patient had normal anesthesia recovery and could move the left leg slightly.
2.3. Postoperation
At 24 hours post-op, the patient could sit up on bed. After 48 hours, he could walk with a support frame without weight-bearing on left leg (Fig. 7). Sign of femoral skin numbness had improved, however, he still had sensory loss at the innervation site of the lateral femoral cutaneous nerve. There was no sign of sciatic nerve injury.
Fig. 7.
(Day 2 post-operative the patient learned to walk with a support frame).
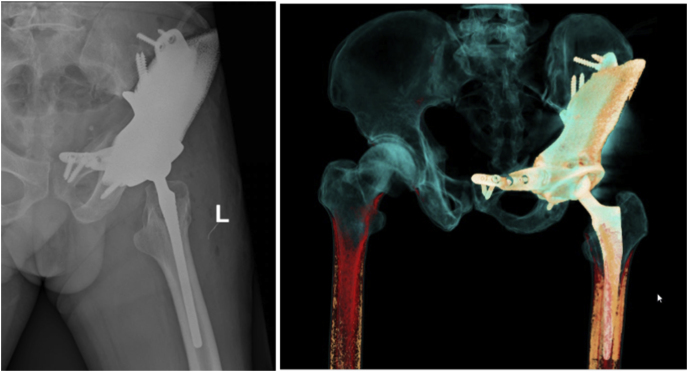
During the first 2 weeks, he started practicing muscle-strengthening exercise as range of motion in bed, and learned to walk with a support frame for 50 m each day (Fig. 7) [14]. He was discharged at day 10 without any complications of hemorrhage, infection, thrombus or fracture. VAS score at time of discharge was 4/10, active ROM: flex/extend: 70/0°, abduction: 10°, external rotation: 10°, quadriceps femoris muscle strength was 4/5, Harris hip score was 60 representing poor hip function. Post-op Xray result: because the implant could not be fitted properly so the acetabular inclination, anteversion and leg length were affected. Acetabular inclination and anteversion were 36° and 17°, legs length difference was 1cm (Fig. 8).
Fig. 8.
Postoperative X Ray and CT 3D.
Post-operative biopsy results showed more than 90% tumor necrosis, therefore the patient was indicated to continue the IPE neoadjuvant regimen for 3 more cycles. After 1 cycle chemotherapy after surgery, his white blood cells were lower lead to incision infection at the super lateral iliac crest then progressed to partial implant exposure. Management was divided into 2 phases: first, the patient was admitted for emergency surgery, we debrided thoroughly and placed a VAC, wound fluid was sent for microbial culture and antibiotic sensitivity testing, and the patient was prescribed with a combination of Ceftazidime and Gentamicin. Next, the defect was reconstructed with a pedicled tensor fasciae latae flap combined with vastus lateralis musculocutaneous flap one week later (Fig. 9). The flap healed well and the patient was discharged the following week.
Fig. 9.
Tensor fasciae latae flap combined with vastus lateralis musculocutaneous flap.
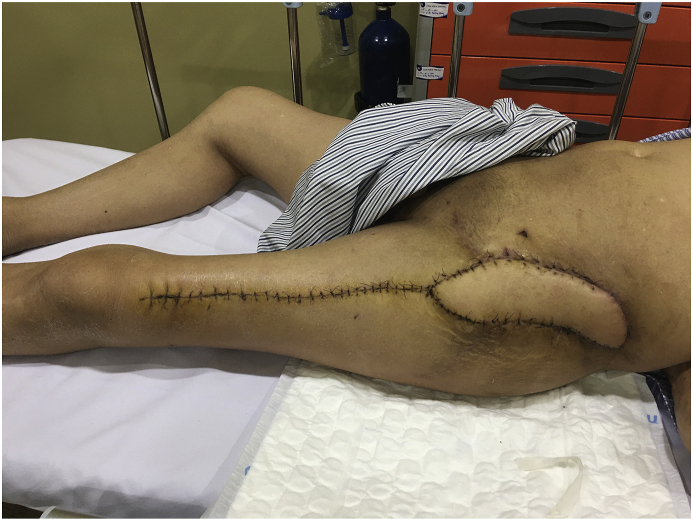
At 6 months follow-up, the patient's overall status was stable, incision healed completely (Fig. 10). VAS score when moving is 2/10. He can walk with one crutch. Active ROM: flexion/extension: 90/0°, abduction: 15°, adduction: 10°, external rotation: 20°, internal rotation: 10°. He was satisfied with this result. Patient is still being followed up and treated.
Fig. 10.
The incision healed completely.
3. Discussion
Osteosarcoma is the most common type of bone cancers nevertheless its incidence is much lower than other types of cancer in general. In the pelvis, osteosarcoma is the second most common cancer after chondrosarcoma. According to Meyers PA, pelvic osteosarcoma accounted for 10% of all osteosarcoma [4]. The disease is frequently seen in below 25 and above 55 groups, our patient was in the latter.
We approached the patient with multimodality therapy including chemotherapy and surgery, in line with Ozaki T (2003)'s plan [1]. Wide resection with negative margin is crucial in pelvic osteosarcoma and it depends on many factors such as: tumor size, metastasis, post neoadjuvant response of the tumor [5]. Type II tumor resection is one of the most challenging procedures in the pelvic area because of the multidimensional structures, numerous surrounding vessels and nerves in a tight and deep space. Post op outcomes were still poor [6]. Our patient required a type I + II + III resection followed by reconstruction with a custom-made 3D printed implant, this is a novel technique in managing this type of cancer which ameliorates a patient's quality of life as well as physical and mental wellness [7,8].
According to Wang, indications for surgery were: (1) confirmed malignant or invasive pelvic tumor without metastasis beyond control, (2) good response to neoadjuvant chemotherapy, (3) favorable surgical margin under limb salvage, and (4) no obvious invasion of the iliac vessels, sciatic nerve or femoral nerve. Contraindications for surgery includes (1) extensive invasion with poor response to chemotherapy, (2) nonstandard open biopsy leading to local tumor contamination, and (3) intolerance to surgical procedures due to poor general condition [9]. We think that in case of metastasis, surgical indication should be considered if a patient's health status fits surgical and anesthetic conditions because of remarkable benefits on patients physical and mental health from the procedure.
Many techniques have been used in post tumor resection hip reconstruction like: Harrington procedure, saddle prosthesis, Allograft prosthesis composite, modular hip joint or allograft [[17], [18], [19], [20]], …however, in Brown's analysis of over 1700 patients, complication rate was up to 50% which is fairly high, most complications were due to infection and prosthesis instability. However, with advancement in 3D printing, patient-specific instruments have seen some early positive results [10].
3D printing has been used by Kamada since the early 1980s and recently, the technology has been applied in Vietnam which helps surgeons practice on real size models prior to the surgeries, make precise implants, reduce surgery time, the models also make pre-operation explaining and demonstrating to patients and relatives much more straightforward.
Evrard showed that incorporating 3D printing in PSI helped reduce local recurrence, R0 resection margin rate was 88%, however mean operative time was 10 hours [11]. Identifying the correct resection border is one of the main keys in obtaining R0 margin both macroscopically and microscopically and also facilitates the implant placement [16]. Though our 3D printed PSI helped reduce surgery time, the implant placement was not precise because of soft tissue impingement and the saw guides did not have grooves so the saw tended to slip therefore we were unable to create a perpendicular cut on every plane.
Our 3D-printed implant design had a rough surface with multi holes for muscle reattachment and according to Peng Wei, this type of structure also helped with bone ingrown, reduce implant instability, diffuse pressure on implant and reduce implant's weight [7]. However, this type of structure also makes an adhesive surface for bacteria which brings higher risk of infection and makes it harder to debride. We had a really hard time debriding all of the necrotic infected tissue from the implant in the reoperation.
Although we had meticulously planned and calculated all the resection planes, fitting the implant to all 3 planes was still very challenging because all three sites: iliac crest, iliopubis and ilioischial resection site must be cut precisely, and this was also the cause of long surgery time. Since the implant could not fit perfectly, the patient's hip inclination, anteversion and leg length was different from pre-op calculation. Total surgery time was about 7 hours, amount of blood loss was 1400ml, lower than other researches 10 [11].
One of the main setbacks of 3D-printed PSI is that the implant is not available. The average time for designing and manufacturing the implant is 1 month, sometimes, it is too long for a patient to wait.
We used the incision combined Smith – Petersen and Watson – Jones approaches (Mercedes or T-shaped incision). The gluteus muscles on the outside and the pelvic muscles on the inside were dissected and released 3–5cm from the tumor. The sciatic nerve and femoral neurovascular bundle were exposed and preserved. However, we could not preserve the lateral femoral cutaneous nerve that was severed because of its adhesion to the tumor. According to Lackman, large incision helped open the surgical field on all four sides, which brought a large field of view made it easier for dissecting and approaching the tumor as well as other surrounding structures, moreover, this incision preserve the perfusion of the femoral artery to the anterior flap and superior gluteal artery to the posterior flap [12].
As we discussed, the implant did not fit properly, the outer part of the implant corresponding to the anterior superior iliac spine was pushed out about 1cm, therefore was only covered by skin and after neoadjuvant therapy, the spot got infected and the implant was exposed. It took two surgeries to completely cover the implant, the first one was to debride and place a VAC on the wound, the second followed next week to reconstruct the defect with fascia latae and vagus lateralis muscle flap. In his research, Wilson reported a complication rate of 49% and 37% of those needed reoperation [6]. According to Han's research, the complication rate was 50% mostly related to delayed wound healing and infection [13].
Patient was put on a rehabilitation program early under supervision. First day: he practiced sitting and passive motion on CPM. Second day: partial-weight-bearing walking with walking frame for 20 m. 3rd −10th day: practiced muscle strength and improved ROM. Patient was discharged on post-op day 10. Rehab objectives were to facilitate wound healing with stable stretch across the incision, maintain knee and ankle ROM as well as avoid stiffness of hip joint. Adele Wingrave emphasized that progressive rehabilitation helped restore normal knee and ankle's functionsβ as the opposite leg. [15].
At 6 months follow-up, the patient can walk with one crutch, Harris hip score was 72, there was no sign of local recurrence, and the implant was in the correct position. Our result was similar to Chen's report: Harris' score was 73 at 1 month post op, 79 after 6 months [8]. The limitation of our study is the short follow-up period. This is the initial results of a difficult and complicated surgery that needs more time to get satisfactory results.
4. Conclusion
Partial pelvic replacement with custom-made 3D-printed implant is an effective solution in managing osteosarcoma of the pelvis. This method not only helps restore pelvic function but also pelvic anatomy.
However, this is a complicated procedure with many challenges to overcome such as: risk of neurovascular injury, skin and soft tissue necrosis because of inappropriate coverage, delayed wound healing which eventually leads to incision infection, impair hip biomechanic. Therefore, this procedure should be performed in a large orthopedic center with carefully selected patients to achieve the best outcome.
Ethical approval
The procedures used in this study inhere to the tenets of the Declarations of Helsinki.
Sources of funding
We declare no funding for this study.
Author contribution
Dung Tran Trung: the main doctor conceived the original idea and operated the patients, revised manuscript.
Hieu Pham Trung, Tran Thiet Son, Pham Thi Viet Dung, Nguyen Van Truong: followed up, operated the patients, revised manuscript.
Tran Duc Thanh: followed up, summed up, revised manuscript.
Sang Nguyen Tran Quang, Nam Tu Tu: operated the patients, revised manuscript.
Nang Vo Sy Quyen, Nguyen Tien Dung: followed up, wrote manuscript.
Registration of research studies
Name of the registry:
Unique Identifying number or registration ID:
Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
Professor Dung Tran Trung MD, PhD.
OrcID: https://orcid.org/0000-0003-2015-3963.
Email: dungbacsy@dungbacsy.com.
Website: www.dungbacsy.com.
Mailing address: Center of Orthopedic and Sport Medicine, Vinmec Hospital, 458 Minh Khai, Khu đô thị Times City, Hai Bà Trưng, Hà Nội, Vietnam.
Tel: (+84) 4 3974 3556 Fax: (+84) 4 3974 3557.
Consent
We introduced the patient to sign informed consent and attached the manuscript.
Declaration of competing interest
We declare that we have no known competing financial interests or personal relationships with anyone that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102812.
Contributor Information
Dung Tran Trung, Email: dungbacsy@dungbacsy.com, http://www.dungbacsy.com.
Sang Nguyen Tran Quang, Email: sangntq@gmail.com.
Hieu Pham Trung, Email: phamtrunghieu@hmu.edu.vn.
Nam Vu Tu, Email: drtunam@hmu.edu.vn.
Nang Vo Sy Quyen, Email: dr.nandvn@gmail.com.
Thanh Tran Duc, Email: bsthanhyhn@gmail.com.
Nguyen Tien Dung, Email: dung91295@gmail.com.
Tran Thiet Son, Email: tranthietson@hmu.edu.vn.
Pham Thi Viet Dung, Email: phamthivietdung@hmu.edu.vn.
Nguyen Van Truong, Email: nguyenvantruong@hmu.edu.vn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Morris C.D. Pelvic bone sarcomas: controversies and treatment options. J Natl Compr Cancer Netw JNCCN. 2010;8(6):731–737. doi: 10.6004/jnccn.2010.0053. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs B., Hoekzema N., Larson D.R., Inwards C.Y., Sim F.H. Osteosarcoma of the pelvis: outcome analysis of surgical treatment. Clin. Orthop. 2009;467(2):510–518. doi: 10.1007/s11999-008-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyers P.A., Heller G., Healey J. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol Off J Am Soc Clin Oncol. 1992;10(1):5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Angelini A., Calabrò T., Pala E., Trovarelli G., Maraldi M., Ruggieri P. Resection and reconstruction of pelvic bone tumors. Orthopedics. 2015;38(2):87–93. doi: 10.3928/01477447-20150204-51. [DOI] [PubMed] [Google Scholar]
- 5.Ozaki T., Flege S., Kevric M. Osteosarcoma of the pelvis: experience of the cooperative osteosarcoma study group. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21(2):334–341. doi: 10.1200/JCO.2003.01.142. [DOI] [PubMed] [Google Scholar]
- 6.Wilson R.J., Freeman T.H., Halpern J.L., Schwartz H.S., Holt G.E. Surgical outcomes after limb-sparing resection and reconstruction for pelvic sarcoma: a systematic review. JBJS Rev. 2018;6(4) doi: 10.2106/JBJS.RVW.17.00072. [DOI] [PubMed] [Google Scholar]
- 7.Three-dimensional printed implant for reconstruction of pelvic bone after removal of giant chondrosarcoma: a case report - PubMed. Accessed July 2, 2021. https://pubmed.ncbi.nlm.nih.gov/32290744/. [DOI] [PMC free article] [PubMed]
- 8.Reconstruction of Bony Defects after Tumor Resection with 3D-Printed Anatomically Conforming Pelvic Prostheses through a Novel Treatment Strategy. Accessed July 2, 2021. https://www.hindawi.com/journals/bmri/2020/8513070/. [DOI] [PMC free article] [PubMed]
- 9.Wang B., Xie X., Yin J. Reconstruction with modular hemipelvic endoprosthesis after pelvic tumor resection: a report of 50 consecutive cases. PloS One. 2015;10(5) doi: 10.1371/journal.pone.0127263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown T.S., Salib C.G., Rose P.S., Sim F.H., Lewallen D.G., Abdel M.P. Reconstruction of the hip after resection of periacetabular oncological lesions: a systematic review. Bone Jt J. 2018;100-B(1 Supple A):22–30. doi: 10.1302/0301-620X.100B1.BJJ-2017-0548.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evrard R., Schubert T., Paul L., Docquier P.-L. Resection margins obtained with patient-specific instruments for resecting primary pelvic bone sarcomas: a case-control study. Orthop Traumatol Surg Res OTSR. 2019;105(4):781–787. doi: 10.1016/j.otsr.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Lackman R.D., Crawford E.A., Hosalkar H.S., King J.J., Ogilvie C.M. Internal hemipelvectomy for pelvic sarcomas using a T-incision surgical approach. Clin. Orthop. 2009;467(10):2677–2684. doi: 10.1007/s11999-009-0843-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han I., Lee Y.M., Cho H.S., Oh J.H., Lee S.H., Kim H.-S. Outcome after surgical treatment of pelvic sarcomas. Clin. Orthop. Surg. 2010;2(3):160–166. doi: 10.4055/cios.2010.2.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shehadeh A., El Dahleh M., Salem A. Standardization of rehabilitation after limb salvage surgery for sarcomas improves patients' outcome. Hematol Oncol Stem Cell Ther. 2013;6(3–4):105–111. doi: 10.1016/j.hemonc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Wingrave A., Jarvis H. The importance of the rehabilitation program following an internal hemipelvectomy and reconstruction with limb salvage - gait analysis and outcomes: a case study. Disabil. Rehabil. 2019;41(17):2066–2070. doi: 10.1080/09638288.2018.1457090. [DOI] [PubMed] [Google Scholar]
- 16.Wu S., Shi X., Zhou G., Lu M., Li C. Composite reconstruction of the hip following resection of periacetabular tumors: middle-term outcome. J. Arthroplasty. 2013;28:537–542. doi: 10.1016/j.arth.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Ueda T., Kakunaga S., Takenaka S., Araki N., Yoshikawa H. Constrained total hip megaprosthesis for primary periacetabular tumors. Clin. Orthop. Relat. Res. 2013;471:741–749. doi: 10.1007/s11999-012-2625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozaki T., Homann C., Hillmann A., Gosheger G., Lindner N., Winkelmann W. Implantaon of hemipelvic prosthesis aer re-secon of sarcoma. Clin. Orthop. Relat. Res. 2002;396:197–205. doi: 10.1097/00003086-200203000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Satcher R.L., Jr., O'Donnell R.J., Johnston J.O. Reconstruction of the pelvis after resection of tumors about the acetabulum. Clin. Orthop. Relat. Res. 2003;409:209–217. doi: 10.1097/01.blo.0000057791.10364.7c. [DOI] [PubMed] [Google Scholar]
- 20.de Meulemeester F.R., Taminiau A.H. Saddle prosthesis aer re-secon of a para-acetabular chondrosarcoma. A case report. Acta Orthop. Scand. 1989;60(3):363–364. doi: 10.3109/17453678909149295. [DOI] [PubMed] [Google Scholar]
- 21.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) Guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.